Abstract
Background
Self-reported sleep quality is poor in persons with human immunodeficiency virus (PWH), but prior studies commonly used nonspecific questionnaires, investigated only single sleep disorders, or lacked human immunodeficiency virus (HIV)-negative controls. We addressed these limitations in the Pharmacokinetics and Clinical Observations in People Over Fifty (POPPY) Sleep Substudy by assessing PWH and HIV-negative controls for insomnia, restless legs syndrome (RLS), and sleep apnea (SA).
Methods
Previously enrolled POPPY participants coenrolled in this substudy without regard to sleep symptoms. Participants completed validated sleep assessments including the Insomnia Severity Index questionnaire, International Restless Legs Syndrome Study Group questionnaire, and in-home, wrist-worn overnight oximetry. They also completed health-related quality of life questionnaires including 36-item Short Form (SF-36) and Patient-Reported Outcomes Measurement Information System (PROMIS) sleep questionnaires.
Results
We enrolled 357 PWH (246 >50 years of age; 111 between 18 and 50 years) and 126 HIV-negative controls >50 years of age. Among PWH, criteria were met by 21% for insomnia, 13% for RLS, and 6% for SA. Compared with HIV-negative controls, PWH had a higher risk of insomnia (adjusted odds ratio, 5.3; 95% confidence interval, 2.2–12.9) but not RLS or SA. Compared with PWH without insomnia, those with insomnia reported significantly worse scores on all SF-36 and PROMIS components; fewer than 30% reported previous diagnosis or treatment for insomnia.
Conclusions
Insomnia was more common in PWH, associated with worse health-related quality of life, and frequently undiagnosed. Further research should focus on the pathogenesis of insomnia in PWH and the development of effective screening and intervention strategies for this unique population.
Keywords: HIV, insomnia, patient reported outcomes measures, restless legs syndrome, sleep apnea syndromes
We used validated instruments to concurrently asses for insomnia, restless legs syndrome, and sleep apnea in 357 persons with HIV and 126 lifestyle-matched controls. Insomnia was far more common in HIV, associated with worse quality of life, and frequently undiagnosed.
Sleep disorders such as sleep apnea (SA), insomnia, and restless legs syndrome (RLS) are increasingly common as people age [1–3], and these sleep disorders can have substantial impact on quality of life (QoL). Studies also suggest that the presence of sleep disorders increases the risk for long-term adverse health consequences such as cardiovascular disease and cognitive decline [4, 5].
Persons with human immunodeficiency virus (PWH) are now increasingly older [6], and many studies have reported poor sleep quality in PWH [7–11]. However, sleep quality in PWH has most commonly been measured using nonspecific questionnaires such as the Pittsburgh Sleep Quality Index (PSQI), which do not identify specific sleep disorders a person might have.
Several studies have assessed populations of PWH for specific sleep disorders such as SA [12, 13] or insomnia [14–16]], but most were single-center studies, relatively small, focused on single sleep disorders, and often lacked human immunodeficiency virus (HIV)-negative controls. We simultaneously assessed for common sleep disorders (SA, insomnia, and RLS) in an established cohort of PWH and lifestyle-matched HIV-negative controls to estimate the prevalence of these sleep disorders, determine whether prevalence differs by HIV status, and test associations between sleep disorders and health-related QoL.
METHODS
Study Design and Participants
The Pharmacokinetics and Clinical Observations in People Over Fifty (POPPY) study is an observational cohort study that enrolled adult participants from 7 sites in England and 1 in Ireland [17]. Enrollment was specifically targeted to include PWH >50 years of age (“Older PWH”), along with demographically similar PWH 18–50 years of age (“Younger PWH”) and HIV-seronegative controls >50 years of age recruited from sexual health clinics and healthcare settings affiliated with the POPPY HIV sites (“Older HIV-Negative Controls”) such that the controls were considered “at risk” for HIV, but seronegative. For this POPPY-Sleep substudy, inclusion criteria required that participants were previously enrolled in the main POPPY study and be able to wear a fingertip oximetry device and wrist actigraph. The only exclusion criterion was an investigator’s judgment that the participant was unlikely to adhere to study procedures. Potential participants were identified without regard to sleep symptoms or previous sleep diagnoses.
Patient Consent Statement
All participants provided written informed consent. The protocol was approved by the UK National Health Service Health Research Authority and appropriate local ethics committees and institutional review boards.
Procedures
Participants completed a study visit to undergo consent and questionnaires, followed by a single night of in-home overnight pulse oximetry using a battery-powered, wrist-worn overnight fingertip pulse oximetry device (WristOx 3150; Nonin Medical, Plymouth, MN) with high-resolution sampling (1 sample per second) throughout a single night. Raw oximetry data were downloaded at the study site and electronically transferred to the Sleep Reading Center (Brigham and Women’s Hospital, Boston, MA) for central quality control review and retesting requests when <3 hours of artifact-free data were found. Recordings were annotated to identify periods of likely “lights off,” “lights on”, and artifact. After annotations, records were processed to generate various oxygen saturation metrics. This analysis focused on 4% oxygen desaturation index (ODI), scored as the number of times per hour that the oxygen saturation decreased by 4% or greater from a local baseline. We categorized participants with ODI ≥5/hour as having SA, because multiple studies have shown high predictive ability of overnight oximetry to discriminate the presence or absence of SA (C-statistics between 0.86 to 0.96) [18–20]. Sleep apnea can be caused by both airway obstruction events (ie, obstructive SA) and by central respiratory drive events (ie, central SA), but overnight oximetry does not distinguish between obstructive and central events, hence our use of the generic term “sleep apnea.”
Participants also completed questionnaires. The Insomnia Severity Index (ISI) is a validated, 7-item, self-reported questionnaire designed to detect insomnia [21]. A Likert scale of 0 to 4 points is used for each of the 7 items, for a total score range of 0–28. The ISI questions are directed to symptoms over the previous 2 weeks, and scores are interpreted as no insomnia (0–7), subthreshold insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28). In this analysis, we classified those reporting scores of 15 or higher as having insomnia.
The 2003 International Restless Legs Syndrome Study Group (IRLSSG) questionnaire for RLS assessment in epidemiologic studies is a 4-item questionnaire, with 3 dichotomous yes/no questions targeted at key RLS symptoms, and 1 question to assess the frequency of such symptoms [22]. Participants who responded yes to all 3 key symptoms were classified as RLS-positive.
Participants completed sleep-related QoL assessments using the Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires for sleep disturbance and sleep-related impairment 8-item short forms [23]. They also self-reported whether they had been previously diagnosed or treated for SA, insomnia, and RLS; formal review of medical records was not performed. Medical Outcomes Study, 36-item Short Form (SF-36) surveys were also used to calculate Physical Component Summary (PCS) and Mental Component Summary (MCS) scores [24, 25].
Statistical Analysis
We used Wilcoxon rank-sum, χ 2, or Fisher’s exact tests, as appropriate, to compare the 3 enrollment groups (older PWH, younger PWH, older HIV-negative controls) for clinical and demographic characteristics, as well as the presence of SA (ODI ≥5/hour), insomnia (ISI ≥15), and RLS (3 of 3 positive key RLS symptoms), the number of these conditions, self-reported diagnoses of these conditions, and self-reported previous or current treatment of these conditions.
In further analyses, we pooled the older and younger PWH together and compared the PWH participants to the older HIV-negative group. In these analyses, the association of HIV with each sleep disorder (SA, insomnia, and RLS) and having 2 or more sleep disorders were tested using logistic regression models, adjusting for age, sex, and ethnicity. We also tested the association between the presence and the number of these sleep disorders and scores from PROMIS and SF-36 questionnaires, using median regression, adjusted for age, sex, and ethnicity, stratified by HIV status. We also tested for an HIV interaction on these associations.
RESULTS
Between March 13, 2017 and July 31, 2018, we enrolled 483 participants with characteristics shown in Table 1. Median (interquartile range [IQR]) time from the main POPPY visit to the POPPY-Sleep substudy visit was 9.0 (IQR, 2.3–17.7) months. The older and younger PWH groups were generally similar, although the older group had larger median waist circumference (3.5 cm larger; P = .007) and lower nadir CD4+ T-cell count (91 cells/mm3 lower; P < .001). Comparing the older PWH and older HIV-negative controls, the older PWH included more men (87% vs 68%; P < .001), more men who have sex with men/homosexual persons (81% vs 52%; P < .001), fewer persons married or in a relationship (44% vs 60%; P = .003), less alcohol (78% vs 91%; P = .004), but more smoking (25% vs 14% current smokers; P = .03) and drug use (9% vs 2% injection drug use history; P = .005; 25% vs 14% recent recreational drug use; P = .02).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Median (IQR) or n (%) | Older PWH (n = 246) | Younger PWH (n = 111) | Older HIV, Controls (n = 126) | P a “Aging” | P b ”HIV” | P c |
|---|---|---|---|---|---|---|
| Gender | .18 | <.001 | .02 | |||
| Male | 213 (86.6%) | 90 (81.1%) | 85 (67.5%) | |||
| Female | 33 (13.4%) | 21 (18.9%) | 41 (32.5%) | |||
| Age [years] | 60 (56–65) | 46 (40–50) | 61 (57–66) | <.001 | .05 | <.001 |
| Ethnicity | .01 | .96 | .04 | |||
| Back African | 25 (10.2%) | 22 (20.0%) | 13 (10.3%) | |||
| White | 221 (89.8%) | 89 (80.2%) | 113 (89.7%) | |||
| Sexuality | .05 | <.001 | .002 | |||
| MSM/homosexual | 198 (80.5%) | 79 (71.2%) | 65 (51.6%) | |||
| Heterosexual | 48 (19.5%) | 32 (28.8%) | 61 (48.4%) | |||
| Educational attainmentd,e | .35 | .48 | .81 | |||
| Higher attainment | 179 (72.8%) | 86 (77.5%) | 96 (76.2%) | |||
| Lower attainment | 67 (27.2%) | 25 (22.5%) | 30 (23.8%) | |||
| Years of educationd | 15 (12–18) | 17 (13–19) | 16 (13–18) | .03 | .24 | .29 |
| Marital Statusd | .04 | .003 | <.001 | |||
| Single | 115 (46.7%) | 60 (54.1%) | 36 (28.6%) | |||
| Married/in a relationship | 107 (43.5%) | 48 (43.2%) | 75 (59.5%) | |||
| Divorced/widowed | 24 (9.8%) | 3 (2.7%) | 15 (11.9%) | |||
| Alcohol Consumptiond | .12 | .004 | .21 | |||
| Never consumed | 15 (6.1%) | 9 (8.1%) | 5 (4.0%) | |||
| Previous consumption | 39 (15.9%) | 9 (8.1%) | 6 (4.7%) | |||
| Current consumption | 192 (78.0%) | 93 (83.8%) | 115 (91.3%) | |||
| Smoking Statusd | .07 | .03 | .02 | |||
| Never smoked | 91 (37.1%) | 52 (46.9%) | 61 (48.4%) | |||
| Exsmoker | 92 (37.6%) | 28 (25.2%) | 47 (37.3%) | |||
| Current smoker | 62 (25.3%) | 31 (27.9%) | 18 (14.3%) | |||
| Ever injected drugsd | 23 (9.4%) | 10 (9.2%) | 2 (1.6%) | .96 | .005 | .008 |
| Recent use of recreational drugsd | 61 (24.8%) | 33 (29.7%) | 18 (14.3%) | .33 | .02 | .004 |
| Height [cm] | 174 (169–179) | 176 (169–181) | 172 (166–179) | .08 | .17 | .02 |
| Weight [kg] | 77.4 (69.6–88.0) | 79.0 (72.4–85.1) | 77.9 (69.9–90.0) | .37 | .62 | .68 |
| BMI [kg/m2] | 25.6 (23.5–28.8) | 25.2 (23.5–28.1) | 26.0 (23.8–29.7) | .77 | .17 | .15 |
| Waist circumference [cm] | 95.0 (89.0–102.0) | 91.5 (85.0–99.0) | 96.0 (88.5–103.0) | .007 | .89 | .02 |
| Neck circumference [cm] | 39.5 (37.5–42.0) | 39.0 (36.7–41.0) | 38.5 (36.0–41.3) | .09 | .02 | .61 |
| Current CD4+ countd [cells/µL] | 597 (470–780) | 610 (470–779) | n/a | .76 | n/a | n/a |
| Nadir CD4+ count [cells/µL] | 179 (80–270) | 270 (131–413) | n/a | <.001 | n/a | n/a |
| HIV RNA <40 copies/mLd | 224 (91.8%) | 99 (90.0%) | n/a | .58 | n/a | n/a |
| On antiretroviral treatment | 227 (92.2%) | 100 (90.1%) | .49 | |||
| Antiretroviral Regimen | ||||||
| NNRTI | 102 (44.9%) | 47 (47.0%) | n/a | .73 | ||
| Efavirenz | 35 (15.4%) | 18 (18.0%) | n/a | .56 | ||
| Nonefavirenz NNRTI | 67 (29.5%) | 29 (29.0%) | n/a | .92 | ||
| INSTI | 53 (23.4%) | 21 (21.0%) | n/a | .64 | ||
| Dolutegravir | 23 (10.1%) | 7 (7.0%) | n/a | .37 | ||
| Raltegravir | 23 (10.1%) | 7 (7.0%) | n/a | .37 | ||
| Elvitegravir | 7 (3.1%) | 7 (7.0%) | n/a | .11 | ||
| PI | 79 (34.8%) | 31 (31.0%) | n/a | .50 | ||
| NRTI | 193 (85.0%) | 90 (90.0%) | n/a | .22 | ||
| Other medicationsf | ||||||
| Sleep aids | 26 (10.6%) | 8 (7.2%) | 3 (2.4%) | .32 | .004 | .12 |
| Metal health medications | 33 (13.4%) | 19 (17.1%) | 7 (5.6%) | .36 | .02 | .005 |
| Analgesics | 43 (17.5%) | 22 (19.8%) | 12 (9.5%) | .60 | .04 | .02 |
Abbreviations: BMI, body mass index; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; iqr, interquartile range; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; PWH, persons with HIV; MSM, men whom have sex with men; n/a, not applicable; NRTI, nucleoside reverse-transcriptase inhibitor; RNA, ribonucleic acid.
aTest between older PWH and younger PWH.
bTest between older PWH and older HIV-negatives.
cTest between younger PWH and older HIV-negatives.
dAssessed at POPPY baseline study visit.
eHigher educational attainment defined as A-level, University, or Professional certification; lower as all others.
fMedications as reported by the participant for treatment of the specified row condition.
Among the 3 sleep disorders for which we assessed, study participants most commonly met validated criteria for insomnia, followed by RLS, then SA (Table 2). Criteria for at least 1 sleep disorder were met by 36% of PWH and 23% of the HIV-negative controls; few reported previous diagnosis or treatment. Less than 30% of those meeting ISI criteria for insomnia reported a prior diagnosis or treatment, whereas previous diagnosis or treatment for RLS and SA was rare (0% to 12%). All further references to insomnia, RLS, and SA will refer to these disorders per study protocol criteria as described in the methods, rather than self-reported diagnosis.
Table 2.
Prevalence of Sleep Disordersa
| Sleep Disorder | Older PWH (n = 219) | Younger PWH (n = 102) | Older HIV, Controls (n = 118) |
|---|---|---|---|
| Insomnia (ISI ≥15) | 46 (21.0%) | 23 (22.6%) | 6 (5.1%) |
| Medical history | 10/46 (21.7%) | 7/23 (30.4%) | 1/6 (16.7%) |
| Treatment history | 9/46 (19.6%) | 6/23 (26.1%) | 1/6 (16.7%) |
| Restless Legs Syndrome (3 of 3 cardinal symptoms on IRLSSG questionnaire) | 34 (15.5%) | 8 (7.8%) | 17 (14.4%) |
| Medical history | 1/34 (2.9%) | 0/8 (0.0%) | 2/17 (11.8%) |
| Treatment history | 0/34 (0.0%) | 0/8 (0.0%) | 2/17 (11.8%) |
| Sleep Apnea (ODI ≥5/hour) | 16 (7.3%) | 4 (3.9%) | 9 (7.6%) |
| Medical history | 0/16 (0.0%) | 0/4 (0.0%) | 1/9 (11.1%) |
| Treatment history | 0/16 (0.0%) | 0/4 (0.0%) | 0/9 (0.0%) |
| Number of Sleep Disordersb | |||
| 0 | 138 (63.0%) | 69 (67.6%) | 91 (77.1%) |
| 1 | 66 (30.1%) | 31 (30.4%) | 22 (18.6%) |
| 2+ | 15 (6.9%) | 2 (2.0%) | 5 (4.2%) |
Abbreviations: HIV, human immunodeficiency virus; IRLSSG, International Restless Legs Syndrome Study Group; ISI, Insomnia Severity Index; ODI, oxygen desaturation index; PWH, persons with HIV.
aProportions of study participants meeting criteria for insomnia (by Insomnia Severity Index [ISI] questionnaire ≥15), restless legs syndrome (by reporting 3 of 3 cardinal symptoms on International Restless Legs Syndrome Study Group [IRLSSG] questionnaire), and sleep apnea (by overnight oximetry testing ODI ≥5/hour), along with distribution of those meeting criteria for zero, 1, or at least 2 sleep disorders. Also shown are participant-reported medical history of previous diagnoses and self-reported current or previous treatment, among those meeting criteria for the sleep disorder.
bSee Supplemental Figure for further details of overlapping sleep disorders.
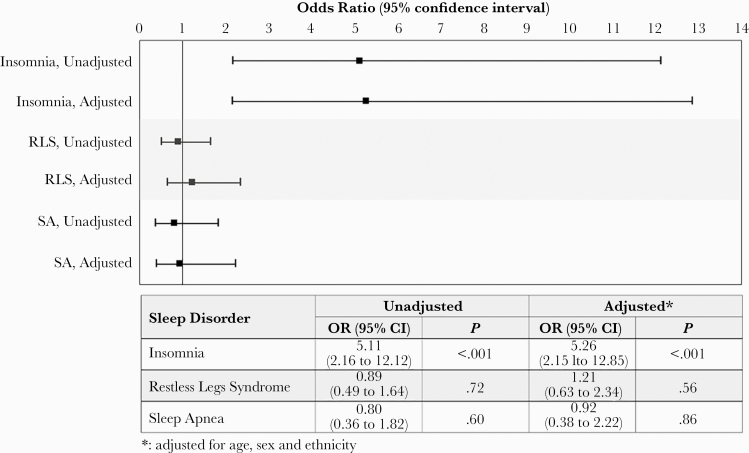
Persons with HIV had significantly higher odds of meeting criteria for insomnia (adjusted odds ratio [aOR], 5.26; 95% confidence interval [CI], 2.15–12.85), but not RLS or SA (Figure 1). Insomnia was associated with worse physical, mental, sleep disturbance, and sleep-related impairment scores in PWH, and with worse sleep disturbance and sleep-related impairment scores in HIV-negative controls (Table 3). Restless legs syndrome was not associated with any of these outcomes in either PWH or HIV-negative controls (Table 4). Sleep apnea was associated with worse mental health in PWH, but no other outcomes in PWH or HIV-negative controls (Table 5).
Figure 1.
Association between human immunodeficiency virus status and odds of meeting criteria for sleep disorders: insomnia (by Insomnia Severity Index questionnaire), restless legs syndrome ([RLS] by International Restless Legs Syndrome Study Group questionnaire), and sleep apnea ([SA] by overnight oximetry testing). Unadjusted and adjusted odds ratios ([OR]; 95% confidence interval [CI]) are shown.
Table 3.
Patient- Reported Outcomes and Insomniaa
| PWH (n = 321) | HIV-negative (n = 118) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Insomnia (n = 69) | No insomnia (n = 252) | Adjusted differenceb (95% CI) | P | Insomnia (n = 6) | No insomnia (n = 112) | Adjusted differenceb (95% CI) | P | P for interaction |
| SF-36 Physical Component Score | 44.1 (32.1, 52.3) | 52.3 (44.0, 56.6) | –7.9 (–12.2 to –3.6) | <.001 | 52.3 (46.2, 53.8) | 55.5 (52.5, 57.8) | – 3.2 (–8.4 to 2.1) | .24 | .43 |
| SF-36 Mental Component Score | 39.7 (31.6, 47.5) | 53.0 (43.6, 57.4) | –12.9 (–16.8 to –9.0) | <.001 | 46.0 (40.0, 51.6) | 55.0 (49.8, 58.5) | – 3.3 (–12.2 to 5.5) | .45 | .89 |
| PROMIS Sleep Disturbance Score | 60.4 (57.3, 63.7) | 49.0 (44.2, 54.3) | 11.4 (9.1 to 13.7) | <.001 | 62.6 (60.4, 64.9) | 46.7 (42.9, 52.2) | 15.1 (7.8 to 22.3) | <.001 | .37 |
| PROMIS Sleep-Related Impairment Score | 60.3 (57.2, 64.3) | 48.9 (43.6, 54.0) | 12.2 (9.9 to 14.6) | <.001 | 60.8 (58.2, 63.3) | 45.5 (40.1, 49.6) | 14.6 (6.9 to 22.3) | <.001 | .75 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus, PROMIS, Patient-Reported Outcomes Measurement Information System; PWH, persons with HIV; RLS, restless legs syndrome; SA, sleep apnea; SF-36, 36-item Short Form.
aAssociation of insomnia with patient-reported outcomes, stratified by HIV status and with P value tests for interactions by HIV status. Group values are shown as median (interquartile range) and differences as adjusted difference (95% CI). For SF-36 Physical Component and Mental Component Scores, lower scores indicate worse health-related quality of life. For patient-reported outcomes Measurement Information System (PROMIS) questionnaires for sleep disturbance and sleep-related impairment, higher scores indicate worse health-related quality of life.
bAdjusted for age, sex, and ethnicity.
Table 4.
Patient-Reported Outcomes and Restless Legs Syndromea
| PWH (n = 321) | HIV-negative (n = 118) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | RLS (n = 42) | No RLS (n = 279) | Adjusted differenceb (95% CI) | P | RLS (n = 17) | No RLS (n = 101) | Adjusted differenceb (95% CI) | P | P for interaction |
| SF-36 Physical Component Score | 48.2 (37.6, 54.5) | 51.6 (42.6, 56.4) | –1.8 (–7.1 to 3.4) | .49 | 56.3 (53.2, 57.9) | 54.9 (51.9, 57.5) | 1.8 (–5.2 to 1.5) | .27 | .30 |
| SF-36 Mental Component Score | 47.7 (38.6, 56.3) | 50.9 (40.5, 56.7) | –1.7 (–4.8 to 8.2) | .60 | 54.2 (46.6, 59.5) | 54.6 (49.8, 58.2) | –1.1 (–3.1 to 4.0) | .68 | .76 |
| PROMIS Sleep Disturbance Score | 54.3 (49.0, 59.4) | 51.2 (45.5, 57.3) | 2.1 (–1.8 to 6.0) | .28 | 50.1 (44.2, 53.3) | 47.3 (42.9, 52.8) | 2.9 (–2.1 to 8.0) | .25 | .93 |
| PROMIS Sleep-Related Impairment Score | 52.3 (47.3, 61.3) | 50.3 (43.6, 57.2) | 1.8 (–2.3 to 5.9) | .39 | 45.5 (41.4, 48.9) | 45.5 (41.4, 50.3) | –1.8 (–7.5 to 3.9) | .53 | .37 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus, PROMIS, Patient-Reported Outcomes Measurement Information System; PWH, persons with HIV; RLS, restless legs syndrome; SF-36, 36-item Short Form.
aAssociation of restless legs syndrome with patient-reported outcomes, stratified by HIV status and with P value tests for interactions by HIV status. Group values are shown as median (interquartile range) and differences as adjusted difference (95% CI). For SF-36 Physical Component and Mental Component Scores, lower scores indicate worse health-related quality of life. For patient-reported outcomes Measurement Information System (PROMIS) questionnaires for sleep disturbance and sleep-related impairment, higher scores indicate worse health-related quality of life.
bAdjusted for age, sex, and ethnicity.
Table 5.
Patient-Reported Outcomes and Sleep Apneaa
| PWH (n = 321) | HIV-negative (n = 118) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | SA (n = 20) | No SA (n = 301) | Adjusted differenceb (95% CI) | P | SA (n = 9) | No SA (n = 109) | Adjusted differenceb (95% CI) | P | P for interaction |
| SF-36 Physical Component Score | 50.8 (39.7, 53.4) | 51.3 (41.5, 56.3) | –1.4 (-8.8 to 6.1) | .72 | 53.5 (41.0, 56.3) | 55.3 (52.6, 57.8) | –2.9 (–7.3 to 1.5) | .19 | .97 |
| SF-36 Mental Component Score | 41.6 (35.6, 57.0) | 50.9 (40.5, 56.5) | –9.2 (–17.3 to –1.0) | .03 | 53.7 (45.2, 55.5) | 54.8 (49.6, 58.4) | –2.4 (–9.6 to 4.9) | .52 | .28 |
| PROMIS Sleep Disturbance Score | 51.2 (43.5, 57.3) | 52.2 (45.5, 57.3) | 0.0 (-5.4 to 5.4) | 1.00 | 52.2 (44.2, 53.3) | 47.9 (42.9, 53.3) | 3.1 (–4.6 to 10.8) | .42 | .33 |
| PROMIS Sleep- Related Impairment Score | 52.3 (43.6, 61.3) | 50.3 (45.5, 57.2) | 1.2 (–4.5 to 6.9) | .68 | 45.5 (41.4, 55.1) | 45.5 (41.4, 50.3) | 1.4 (–5.8 to 8.7) | .69 | .98 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus, PROMIS, Patient-Reported Outcomes Measurement Information System; PWH, persons with HIV; SA, sleep apnea; SF-36, 36-item Short Form.
aAssociation of sleep apnea with patient-reported outcomes, stratified by HIV status and with P value tests for interactions by HIV status. Group values are shown as median (interquartile range) and differences as adjusted difference (95% CI). For SF-36 Physical Component and Mental Component Scores, lower scores indicate worse health-related quality of life. For patient-reported outcomes Measurement Information System (PROMIS) questionnaires for sleep disturbance and sleep-related impairment, higher scores indicate worse health-related quality of life.
bAdjusted for age, sex, and ethnicity.
The number of participants meeting criteria for multiple sleep disorders was small (n = 17 PWH and n = 5 HIV-negative controls; Supplemental Figure), but among PWH, as the number of sleep disorders increased, physical, mental, and sleep-related QoL scores were all worse (Supplemental Table). Among HIV-negative controls, as the number of sleep disorders increased, sleep disturbance scores worsened, as well as sleep-related impairment and mental scores, although these last 2 were not statistically significant in this analysis with limited power.
DISCUSSION
We find that PWH most commonly meet validated questionnaire criteria for insomnia, followed by validated questionnaire criteria for RLS, then SA by objectively assessed overnight oximetry criteria. More importantly, when we compare the prevalence of these conditions to that in HIV-negative controls, we find that PWH are at substantially increased risk for insomnia (aOR, 5.26; 95% CI, 2.15–12.85), but not RLS or SA. Moreover, despite the high prevalence of insomnia in our PWH study participants (21% met criteria for insomnia), less than one third of these participants reported ever being diagnosed or treated for insomnia. These findings have several important clinical implications.
The finding that PWH are at higher risk for insomnia than HIV-negative controls has previously been suggested by other studies, although a 2014 systematic review was unable to assess insomnia prevalence in PWH, because none of the 19 publications that met their search criteria used validated insomnia instruments like the ISI [26]. A more recent single-center study administered the ISI questionnaire to 254 PWH, without HIV-negative controls, and found that insomnia prevalence by ISI ≥15 was 22% and did not vary by age [27]. Another recent study administered the Jenkins Sleep Problems Scale [28], which utilizes 4 questions directed at insomnia. In this study of 244 PWH and 244 HIV-negative controls recruited in 2014–2015, the prevalence of insomnia was 19% in PWH and 9% in the HIV-negative controls (P = .003) [29], which aligns very closely with our ISI-based prevalence estimates of 21% and 5%, respectively. That study also divided PWH into older and younger groups (mean ages of 47 and 63 years, which also aligns closely to our groupings of 46 and 60 years) and found no difference by age, which is also similar to our findings. As such, these emerging data indicate that PWH are at substantially increased risk for insomnia compared with HIV-negative controls and that the presence of insomnia in PWH is not related to aging.
More importantly, insomnia in PWH was associated with decrements in health-related QoL measures. The association between insomnia and worse PROMIS sleep measures is perhaps unsurprising, but we also observed strong associations between insomnia and worse SF-36 scores. Similar associations have been described in population-based samples [30] and recently in a cohort of 103 PWH, where a clinical sleep interview found a very high insomnia prevalence of 67%—much higher than the studies using ISI questionnaires—and associations between insomnia and worse health-related QoL [31]. The magnitude of the differences we observed in our cohort (7.9 points worse Physical and 12.9 points worse Mental Component scores) exceed the 3- to 5-point differences suggested as clinically important [32]. Our cross-sectional study design prevented determination of the impact of insomnia treatment on health-related QoL in PWH, but our data suggest that such intervention studies are needed.
The main treatment for insomnia, cognitive behavioral therapy for insomnia (CBT-I), is highly effective [33], but there are scant data regarding CBT-I in PWH [34], a population that may require tailored CBT-I due to biologic factors (eg, persistent neuroinflammation despite effective viral suppression), social factors (eg, high burden of stigma and mental health disorders), and polypharmacy. Insomnia in PWH may also be due to side effects of HIV antiretroviral treatment (ART), particularly with efavirenz [35] and the integrase strand transfer inhibitor class of ART [36].
Other than ART drugs, PWH may also have additional biologic reasons for insomnia. Despite effective ART, PWH can demonstrate persistent central nervous system inflammation [37] and systemic inflammation [38]. Such inflammation might disrupt normal sleep homeostasis. Persons with HIV may also have additional psychosocial risk factors for insomnia such as mood disturbance, substance use, and stigma. We recently showed that higher self-reported pain severity related to higher ISI scores [39]. An analysis of risk factors for insomnia in PWH was beyond the scope of this particular analysis, but such future analyses will be important to determine whether certain subgroups of PWH should be targeted for insomnia screening and treatment. From a clinical perspective, we emphasize that sedative or hypnotic drugs are not first-line treatments for insomnia. For PWH with insomnia, we believe that CBT-I should still be considered the first-line treatment, pending the results of ongoing research to determine how to most effectively deliver CBT-I to this unique population with potentially many opportunities for behavioral and lifestyle modifications to improve insomnia.
We are aware of only 2 published studies regarding RLS in PWH. In a single-center sample of 316 PWH enrolled in 2005–2007 in San Francisco, California (mean age 45 years and 48% with detectable HIV-ribonucleic acid), 8.2% met questionnaire crietria for RLS at baseline [40], which is nearly identical to the 7.8% we identified in our younger HIV sample with similar median age of 46 years. The study also included longitudinal follow-up of up to 2 years, during which an additional 3.2% met RLS criteria. Our study was only cross-sectional, but the higher prevalence of 15.5% in our older PWH group (median age 60 years) and the longitudinal San Francisco data suggest that RLS prevalence increases with aging in PWH, consistent with general population data.
The other study of RLS in PWH was a single-center, cross-sectional survey of 129 PWH (57% response rate, because 99 did not return the survey) and 100 healthy matched controls (response rate in controls not reported) in Munster, Germany. Thirty-three percent of the PWH (mean age 44 years, 82% on ART) met questionnaire criteria for RLS, compared with 7% of the age-matched HIV-negative controls [41]. Their data are contrary to our findings of no difference in RLS prevalence by HIV status, and their RLS prevalence in PWH far exceeds the 8% prevalence seen in similarly aged participants in both our study and the San Francisco study. Reasons for the discrepancy are not clear, but survey sampling methods can introduce survey response bias, in which symptomatic PWH may have been more likely to respond to the survey than those without symptoms. Nevertheless, we found that RLS was not related to health-related QoL in PWH, compared with the very strong associations between insomnia and these patient-centered outcomes. Given high demands on HIV clinician time and limited research resources, our data suggest that clinical and research efforts might benefit from prioritizing time and effort towards insomnia over RLS.
Sleep apnea has received recent attention in PWH, partially due to a rising prevalence of older age and obesity—2 major risk factors for SA—and data suggesting that SA might contribute to common HIV comorbidities such as cardiovascular disease [4]. Preliminary studies suggest that PWH have a high prevalence of SA symptoms and risk factors [13, 42], but few studies have performed objective testing for SA in PWH. The largest published study with such data that we are aware of came from the Baltimore site of the Multicenter AIDS Cohort Study (MACS). In a convenience sample (100% male, n = 99 PLWH and n = 60 HIV-negative controls) who underwent formal in-laboratory polysomnogram (PSG), SA prevalence in PWH was 72% [12]. Although this was a strikingly high prevalence of SA, uninfected controls had an even higher prevalence of SA (87%), again suggesting the possibility of sampling bias, where men with sleep symptoms may have been more likely to participate in the study than those without symptoms.
Only 6% of our PWH met criteria for SA. We note that our SA assessment was limited to overnight oximetry rather than the PSG assessments in MACS. Overnight oximetry may slightly underestimate SA severity [18, 19] and might also fail to identify some forms of SA such as upper airway resistance syndrome (UARS) in which mild forms of apneas and hypopneas can lead to cortical arousals and disrupted sleep without classic oxygen desaturation events. This would seem unlikely to explain the remarkably large difference in SA prevalence between the POPPY and MACS studies, but UARS is associated with insomnia [43], so we cannot exclude the potential for a higher prevalence of UARS in our cohort. Major risk factors for SA (eg, age, body mass index, neck circumference) were very similar in both cohorts, so these would not appear to explain the difference in SA prevalence. More importantly, both our POPPY study and the MACS study found no difference in SA prevalence by HIV status, suggesting that HIV does not uniquely lead to SA. Further longitudinal research remains important to determine whether the impact of SA in PWH differs from the impact in HIV-negative persons.
Strengths of our study include its multicenter enrollment of study participants from an ongoing cohort study, not selected on the basis of sleep symptoms, and its simultaneous assessment of multiple sleep outcomes. Some important limitations are also worth noting. Our criteria for insomnia, RLS, and SA are not diagnostic. The gold standard evaluation for insomnia is a standardized diagnostic clinical interview. Diagnosis for RLS requires further history and exclusion of other conditions (eg, neuropathy, pain syndromes). Sleep apnea was only assessed by overnight oximetry rather than more standard diagnostic sleep studies, and overnight oximetry has not been validated for SA diagnosis in PWH. Our cross-sectional assessment did not allow us to assess the long-term impact of insomnia, RLS, and SA on clinical outcomes.
CONCLUSIONS
More than 20% of PWH meet questionnaire criteria for insomnia, which is associated with significantly worse physical, mental, and sleep-related QoL. Persons with HIV are approximately 5 times more likely than matched HIV-negative controls to meet insomnia criteria, yet few report prior diagnosis and treatment for insomnia. Restless legs syndrome and SA were less common than insomnia, not associated with HIV status, and not as strongly associated with health-related QoL. Further research should focus on insomnia pathogenesis in PWH and development of effective screening and intervention strategies for PWH.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplemental Figure. Overlap between insomnia (by Insomnia Severity Index questionnaire), restless legs syndrome ([RLS] by International Restless Legs Syndrome Study Group questionnaire), and sleep apnea ([SA] by overnight oximetry testing) in persons with HIV (PWH) (n = 321) and HIV-negative individuals (n = 118).
Supplemental Table. Association of number of sleep disorders with outcomes in persons with HIV (PWH) and HIV-negative individuals, separately, with P value to assess the interaction with HIV.
Acknowledgments
The authors and study team thank the Pharmacokinetics and Clinical Observations in People Over Fifty (POPPY) Sleep Substudy participants for their contributions to our scientific understanding of sleep in human immunodeficiency virus. The Insomnia Severity Index questionnaire is licensed by Mapi Research Trust (Lyon, France), and a license agreement was completed for use in this study.
Author contributions. K. M. K. conceived the study. K. M. K., C. A. S., A. W., and S. R. designed the study. K. M. K. and S. R. obtained funding. A. W., P. W. G. M., J. A., E. B., M. B., N. D., L. H., F. A. P., and J. V. acquired the data. D. D. F. and C. A. S. performed the primary statistical analyses. K. M. K. drafted the manuscript. All authors critically revised the manuscript for important intellectual content and approved the final manuscript. All authors take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer. The views expressed in this article are those of the authors and do not reflect the views of the United States Government, the National Institutes of Health, the Department of Veterans Affairs, the funders, the sponsors, or any of the authors’ affiliated academic institutions. None of the funders nor sponsor had any input regarding the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial support. The POPPY Sleep Substudy was funded the National Heart Lung and Blood Institute (R01 HL131049). The parent POPPY study was primarily funded by investigator-initiated grants from BMS, Gilead Sciences, Janssen, MSD, and ViiV Healthcare. We acknowledge the use of the National Institute for Health Research (NIHR)/Wellcome Trust Clinical Research Facility at King’s College Hospital. The research is also funded by the National Institute for Health Research Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. S. R. was partially supported by the National Heart Lung and Blood Institute (R35 HL135818). This material is also the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Medical Center, Minneapolis, Minnesota.
Potential conflicts of interest. K. M. K. reports personal fees from GlaxoSmithKline and Nuvaira, Inc.; contracted clinical research support from Sanofi; all outside of the work reported here. C. A. S. reports personal fees from Gilead Sciences and ViiV; all outside of the work reported here. A. W. reports grants and personal fees from Gilead Sciences, ViiV healthcare, Janssen, and MSD; all outside of the work reported here. P. W. G. M. reports grants and/or personal fees from Gilead Sciences, MSD, ViiV Healthcare, and Janssen; all outside the work reported here. J. A. reports personal fees from Gilead Sciences and ViiV; all outside of the work reported here. M. B. has acted as a speaker or adviser to, has been an investigator for, or has received grants to her institution from Gilead, ViiV, Janssen, B. M. S., Teva, Cipla, Mylan, and MSD; all outside the work presented here. F. A. P. reports grants and/or personal fees from Gilead Sciences, ViiV, Janssen, and MSD; all outside of the work reported here. J. V. reports travel, research grants, and personal fees from Merck, Janssen Cilag, Piramal Imaging, ViiV Healthcare, and Gilead sciences; all outside of the work reported here. S. R. reports grants and personal fees from Jaxx Pharma and personal fees from Eisai Pharma; all outside of the work reported here. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Brewster GS, Riegel B, Gehrman PR. Insomnia in the older adult. Sleep Med Clin 2018; 13:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gulia KK, Kumar VM. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics 2018; 18:155–65. [DOI] [PubMed] [Google Scholar]
- 3. Chowdhuri S, Patel P, Badr MS. Apnea in older adults. Sleep Med Clin 2018; 13:21–37. [DOI] [PubMed] [Google Scholar]
- 4. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005; 365:1046–53. [DOI] [PubMed] [Google Scholar]
- 5. Blackwell T, Yaffe K, Laffan A, et al. ; Osteoporotic Fractures in Men (MrOS) Study Group. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS Sleep Study. Sleep 2014; 37:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubinstein ML, Selwyn PA. High prevalence of insomnia in an outpatient population with HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 19:260–5. [DOI] [PubMed] [Google Scholar]
- 8. Salahuddin N, Barroso J, Leserman J, et al. Daytime sleepiness, nighttime sleep quality, stressful life events, and HIV-related fatigue. J Assoc Nurses AIDS Care 2009; 20:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crum-Cianflone NF, Roediger MP, Moore DJ, et al. Prevalence and factors associated with sleep disturbances among early-treated HIV-infected persons. Clin Infect Dis 2012; 54:1485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee KA, Gay C, Portillo CJ, et al. Types of sleep problems in adults living with HIV/AIDS. J Clin Sleep Med 2012; 8:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gamaldo CE, Gamaldo A, Creighton J, et al. Evaluating sleep and cognition in HIV. J Acquir Immune Defic Syndr 2013; 63:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patil SP, Brown TT, Jacobson LP, et al. Sleep disordered breathing, fatigue, and sleepiness in HIV-infected and -uninfected men. PLoS One 2014; 9:e99258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goswami U, Baker JV, Wang Q, et al. Sleep apnea symptoms as a predictor of fatigue in an urban HIV clinic. AIDS Patient Care STDS 2015; 29:591–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee KA, Gay C, Pullinger CR, et al. Cytokine polymorphisms are associated with poor sleep maintenance in adults living with human immunodeficiency virus/acquired immunodeficiency syndrome. Sleep 2014; 37:453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faraut B, Malmartel A, Ghosn J, et al. Sleep disturbance and total sleep time in persons living with HIV: a cross-sectional study. AIDS Behav 2018; 22:2877–87. [DOI] [PubMed] [Google Scholar]
- 16. Jean-Louis G, Weber KM, Aouizerat BE, et al. Insomnia symptoms and HIV infection among participants in the Women’s Interagency HIV Study. Sleep 2012; 35:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagkeris E, Burgess L, Mallon PW, et al. Cohort profile: the pharmacokinetic and clinical observations in PeoPle over fiftY (POPPY) study. Int J Epidemiol 2018; 47:1391–1392e. [DOI] [PubMed] [Google Scholar]
- 18. Magalang UJ, Dmochowski J, Veeramachaneni S, et al. Prediction of the apnea-hypopnea index from overnight pulse oximetry. Chest 2003; 124:1694–701. [DOI] [PubMed] [Google Scholar]
- 19. Chung F, Liao P, Elsaid H, et al. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 2012; 114:993–1000. [DOI] [PubMed] [Google Scholar]
- 20. Kunisaki KM, Bohn OA, Wetherbee EE, Rector TS. High-resolution wrist-worn overnight oximetry has high positive predictive value for obstructive sleep apnea in a sleep study referral population. Sleep Breath 2016; 20:583–7. [DOI] [PubMed] [Google Scholar]
- 21. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001; 2:297–307. [DOI] [PubMed] [Google Scholar]
- 22. Allen RP, Picchietti D, Hening WA, et al. ; Restless Legs Syndrome Diagnosis and Epidemiology workshop at the National Institutes of Health; International Restless Legs Syndrome Study Group. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003; 4:101–19. [DOI] [PubMed] [Google Scholar]
- 23. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med 2011; 10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30:473–83. [PubMed] [Google Scholar]
- 25. Ware JE Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care 1995; 33:AS264–79. [PubMed] [Google Scholar]
- 26. Low Y, Goforth H, Preud’homme X, et al. Insomnia in HIV-infected patients: pathophysiologic implications. AIDS Rev 2014; 16:3–13. [PubMed] [Google Scholar]
- 27. Milinkovic A, Singh S, Simmons B, et al. Multimodality assessment of sleep outcomes in people living with HIV performed using validated sleep questionnaires. Int J STD AIDS 2020; 31:996–1003. [DOI] [PubMed] [Google Scholar]
- 28. Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol 1988; 41:313–21. [DOI] [PubMed] [Google Scholar]
- 29. Ding Y, Lin H, Zhou S, et al. Stronger association between insomnia symptoms and shorter telomere length in old HIV-infected patients compared with uninfected individuals. Aging Dis 2018; 9:1010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev 2010; 14:69–82. [DOI] [PubMed] [Google Scholar]
- 31. Rogers BG, Bainter SA, Smith-Alvarez R, et al. Insomnia, health, and health-related quality of life in an Urban clinic sample of people living with HIV/AIDS. Behav Sleep Med 2020:1–17. doi: 10.1080/15402002.2020.1803871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Samsa G, Edelman D, Rothman ML, et al. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics 1999; 15:141–55. [DOI] [PubMed] [Google Scholar]
- 33. Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: a meta-analysis. JAMA Intern Med 2015; 175:1461–72. [DOI] [PubMed] [Google Scholar]
- 34. Buchanan DT, McCurry SM, Eilers K, et al. Brief behavioral treatment for insomnia in persons living with HIV. Behav Sleep Med 2018; 16:244–58. [DOI] [PubMed] [Google Scholar]
- 35. Apostolova N, Funes HA, Blas-Garcia A, et al. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother 2015; 70:2693–708. [DOI] [PubMed] [Google Scholar]
- 36. Hill AM, Mitchell N, Hughes S, Pozniak AL. Risks of cardiovascular or central nervous system adverse events and immune reconstitution inflammatory syndrome, for dolutegravir versus other antiretrovirals: meta-analysis of randomized trials. Curr Opin HIV AIDS 2018; 13:102–11. [DOI] [PubMed] [Google Scholar]
- 37. Vera JH, Guo Q, Cole JH, et al. Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology 2016; 86:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Neuhaus J, Jacobs DR Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sabin CA, Harding R, Doyle N, et al. Associations between widespread pain and sleep quality in people with HIV. J Acquir Immune Defic Syndr 2020; 85:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hennessy MD, Zak RS, Gay CL, et al. Polymorphisms of interleukin-1 Beta and interleukin-17Alpha genes are associated with restless legs syndrome. Biol Res Nurs 2014; 16:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Happe S, Kundmüller L, Reichelt D, et al. Comorbidity of restless legs syndrome and HIV infection. J Neurol 2007; 254:1401–6. [DOI] [PubMed] [Google Scholar]
- 42. Kunisaki KM, Akgün KM, Fiellin DA, et al. Prevalence and correlates of obstructive sleep apnoea among patients with and without HIV infection. HIV Med 2015; 16:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guilleminault C, Kirisoglu C, Poyares D, et al. Upper airway resistance syndrome: a long-term outcome study. J Psychiatr Res 2006; 40:273–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.