Abstract
Murine infections with most Coccidioides spp. strains are lethal by 3 weeks, limiting the study of immune responses. Coccidioides posadasii, strain 1038 (Cp1038), while slowly lethal, resulted in protracted survival of C57BL/6 (B6) mice. In resistant (B6D2)F1/J mice, lung fungal burdens stabilized by week 4 without progression through week 16, better modeling human coccidioidal infections after their immunologic control. Immunodeficient tumor necrosis factor (Tnf) α knockout (KO) and interferon (Ifn) γ receptor 1 (Ifn-γr1) KO mice survived a median of 22.5 and 34 days, compared with 70 days in B6 mice (P = .001 and P < .01, respectively), though 14-day lung fungal burden studies showed little difference between Ifn-γr1 KO and B6 mice. B6 mice showed peak concentrations of key inflammatory lung cytokines, including interleukin 6, 23, and 17A, Tnf-α, and Ifn-γ, only after 4 weeks of infection. The slower progression in B6 and the acquired fungal burden stability in B6D2 mice after Cp1038 infection greatly increases the array of possible immunologic studies.
Keywords: Coccidioides, mice, chronic, interferon γ, TNF-α, B6D2, survival, cytokines
Using a slower growing strain of Coccidioides posadasii results in a chronic model of experimental murine coccidioidal infection which more precisely mimics naturally acquired human infection.
Experimental infection of mice is the most commonly used disease model to study coccidioidomycosis, a systemic fungal infection caused by the species Coccidioides posadasii and Coccidioides immitis. This model is used to study new antifungal drug therapies, vaccine candidates, and host responses to infection. The model can be manipulated by infecting mice via lungs (intranasally, intratracheally, or aerosol), intraperitoneally, intravenously, intrathecally/intracranially, or subcutaneously [1]. For immunology research, inbred strains, especially C57BL/6 (B6) mice, are used extensively, in part because the majority of genetically modified mice are on this background. B6 mice are very susceptible to infection, and 50 arthroconidia of C. posadasii, strain Silveira, and C. immitis, strain RS, are lethal within 2–3 weeks after intranasal infection [2].
Because the interval between spherule rupture at 5 days and death at 14–16 days is so short, there is little opportunity to study the adaptive immune response that is likely critical to control infections in humans and other species [3]. This makes extrapolating findings in mice to humans extremely difficult, since conditions in humans, even in those with immune deficiencies, seldom progress as rapidly as in mice. Previously, Shubitz et al [4] infected outbred Swiss-Webster mice, which constrained granulomas and fungal burden and survived ≥56 days. By contrast, in the same study, 60% of the most resistant inbred mice tested, DBA/2NHsd, also survived 56 days but had high lung fungal burdens (LFBs) and extensive lung lesions. A chronic disease model in inbred mice after respiratory coccidioidal infection would be a major advance to study the effects of immunosuppression and immunodeficiency on coccidioidal disease.
Host genetics play an important role in human susceptibility to coccidioidomycosis as several inborn immunodeficiencies, such as interleukin 12 receptor, signal transducer and activator of transcription (STAT) 4, STAT1, STAT3, and interferon (Ifn) γ receptor 1 mutations, have been identified in humans with severe to fatal coccidioidal infection [5–9]. Immunosuppression due to broad-spectrum and targeted immunotherapeutics to treat inflammatory and neoplastic diseases and prevent transplant rejection also can result in severe coccidioidomycosis [8, 10]. A less fulminant infection model in mice would allow more careful study of the effects of both inherited and induced immunodeficiencies on the host response to disease, including the potential to protect these populations by vaccination. A slower, chronic model would also allow measurement of immune function from time points during the early innate stages through the adaptive phase of immunity to better describe the mechanisms of failure that lead to death or the host’s inability to control infection without the chronic application of antifungal medication [11].
Cox and Magee [12] reported that an isolate of C. posadasii, recovered from a patient in Arizona and designated RMSCC1038 (Cp1038), was of reduced virulence in BALB/c mice, as indicated by extended survival of ≥45 days after intranasal infection, but no further characterization was provided. In the current work, we extend that initial observation with studies of survival, quantitative LFBs, and cytokine profiles of normal and immunodeficient inbred strains of mice after infection with the reduced-virulence Cp1038. Our findings demonstrate that with this strain a greater range of immunologic studies are now possible.
MATERIALS AND METHODS
Fungal Strains
C. posadasii strains Silveira and RMSCC 1038 (Cp1038) were grown on 2× glucose yeast extract agar (GYE) (2% glucose, 1% yeast extract, and 1.5% agar) at 30°C for 4 and 12 weeks, respectively, and arthroconidia were harvested and enumerated using a hemocytometer, as described elsewhere [13]. Cp1038 was filtered through Pellon Thermolam Plus to remove hyphal elements. Viability was determined by growth of 10-fold serial dilutions on 2× GYE at 37°C for 3 and up to 7 days for strains Silveira and Cp1038, respectively. Viable suspensions for animal inoculation were diluted in isotonic saline. All manipulation of live fungus was performed at biosafety level 3 with University of Arizona Institutional Biosafety Committee approval.
Mice and Infections
Female C57BL/6J (B6), B6D2F1/J (B6D2), B6.129S7-Ifngr1tm1Agt/J (IFN-γ receptor 1 [Ifn-γr1] knockout [KO]) (stock 003288), and B6.129S-Tnftm16kl/J (tumor necrosis factor [Tnf] α KO) (stock 005540) mice, 6–8 weeks old, were purchased from Jackson Laboratories [14, 15]. Ifn-γr1 KO mice from Jackson were also bred in house, and both male and female mice were used. Mice were housed according to PHS standards; all procedures were approved by the Institutional Animal Care and Use Committee of the University of Arizona. Mice were housed and manipulated at animal biosafety level 3 for the duration of the infection studies.
Groups of 5–8 mice were infected intranasally under ketamine-xylazine anesthesia with approximately 50 arthroconidia in 30 µL of isotonic sterile saline, as described elsewhere [16]. Mice for fungal burden studies were euthanized at prespecified time points, or earlier if moribund (defined as weight loss >20%, inactivity and ruffled condition, or dehydration 7%–10% based on skin turgor). For survival studies, mice were observed for up to 16 weeks, and moribund animals were euthanized as necessary.
For all studies, LFBs were quantitated by serial dilution of homogenized tissue, as described elsewhere [16]. Where spleen fungal burdens are reported, they were homogenized with serial dilutions and enumerated as for the lungs. For most studies, spleens were qualitatively cultured whole on GYE plates at 35°C–37°C for up to 7 days and reported positive or negative for fungal growth to assess dissemination. For immunologic studies, groups of mice were euthanized at prespecified time points, and lungs were collected for further analysis.
Cytokine Analysis
Lungs of B6 mice infected with 50 arthroconidia of Cp1038 were collected at 2, 4, 5, and 6 weeks after infection; mice for controls were given saline intranasally when the other groups were infected, and they were euthanized at the week 6 time point. Left lungs were weighed, minced, and incubated for 24 hours in 2.5 mL of RPMI 1640 medium with 10% fetal calf serum at 37°C and 5% carbon dioxide, in 6-well tissue culture plates. Supernatants were centrifuged, filtered through a 0.8-µm filter to sterilize, and stored at −80°C until analysis. Cytokines were assayed using the LEGENDplex Mouse Inflammation Panel 13-plex cytometric bead array (BioLegend), according to the manufacturer’s instructions. Concentrations were determined using standard curves and reported as concentration per gram of lung.
Lung Histopathology
B6 mice were infected intranasally with approximately 500 arthroconidia of Cp1038 because it requires a high starting dose to detect developing spherules on slide sections at early time points without reviewing hundreds of sections of lung. Two mice were euthanized on each of days 3, 4, 5, 6, 7, and 14 after infection. Lungs were fixed with 4% paraformaldehyde for 24 hours and then transferred into 70% ethanol and processed and embedded routinely. Pairs of step sections (5 µm thick) were cut every 20 µm; one slide was stained with hematoxylin-eosin (HE) and the other immunohistochemically stained with a Coccidioides-specific polyclonal goat antibody to Ag2/PRA (PRA), as described elsewhere [4]. Historical slides of B6 mice infected with Silveira were shown for comparison, because we have an extensive library of HE- and PRA-stained slides. In those studies, mice were infected intranasally with 50 arthroconidia of Silveira [4].
Statistical Analysis
Fungal burden and survival data were assessed using the Shapiro-Wilk test for normality and all were determined to be not normal. Groups were log-transformed and analyzed by means of nonparametric Kruskal-Wallis or Mann-Whitney tests to compare groups in each study. Survival curves were compared using log-rank tests. Results were considered significant at P ≤ .05. Cytokine significance was determined using a 2-step process. Each cytokine was subjected to a Kruskal-Wallis test for variance and adjusted for multiple comparisons using a Bonferroni adjustment. Cytokines that were significant in this first analysis were then log-transformed and compared with the control at each time point, using a Mann-Whitney rank order test corrected for multiple comparisons. Results were considered significant at P ≤ .05.
RESULTS
In Vitro and In Vivo Growth of Cp1038
For production of arthroconidia to inoculate mice, Cp1038 grew more slowly than Silveira on medium routinely used to grow Coccidioides spp. in our laboratory and it also produced fewer mature arthroconidia. The yield from Silveira cultures after 4 weeks was approximately 1 × 108 arthroconidia per 10-cm petri dish, while the yield from Cp1038 after 12 weeks was a log lower. When postmortem organ cultures were performed to quantitate fungal burden, Cp1038 colonies appeared later on the culture plates and cultures needed to be incubated up to 7 days to finalize counts, compared with only 3 days for Silveira. Under all in vitro growth conditions, Cp1038 grew slower than Silveira.
To understand the growth of Cp1038 in vivo, lung sections of B6 mice infected with 500 arthroconidia were examined at early time points to describe the spherules and host inflammation. Examinations were perform only at 14 days or earlier, because B6 mice are frequently moribund by day 14 with florid infiltration of spherules and endospores into all lung fields after infection with 50 arthroconidia of Silveira [4]. On days 3 and 4, abnormalities consisted of sparse (1–4 per slide), tiny clusters of primarily neutrophilic inflammation in a peribronchial or perivascular location but no spherules seen with HE or PRA stain. Inflammatory sites remained microscopic and sparse on days 5 and 7 but were ≥1 mm and grossly visible on day 14 (Figure 1). Inflammatory cells in the lesions consisted of neutrophils, macrophages, and a few lymphocytes.
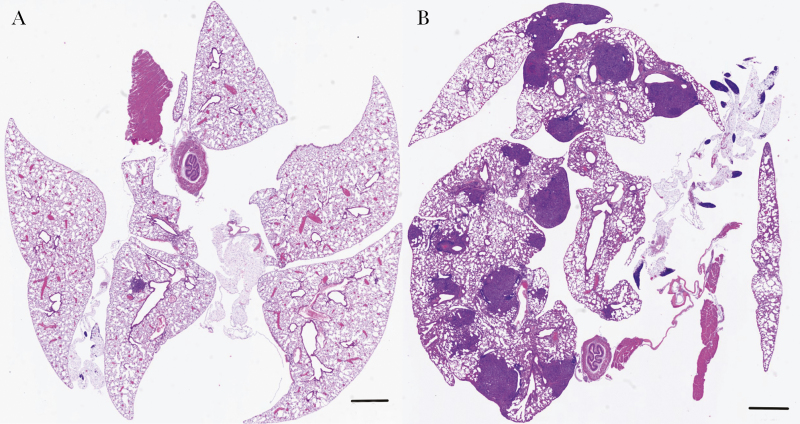
Figure 1.
Low-magnification images of the entire lungs of B6 mice infected with 500 arthroconidia of Cp1038 (hematoxylin-eosin stain; MoticEasyScan digital slide scanner; bars represent 1 mm). A, On day 7 after infection, there are very few small lesions, which stain dark purple owing to the high number of neutrophils. B, By day 14, lung lesions are significantly increased in number and size, and they are grossly visible at postmortem examination, approximately 1 mm in diameter.
On days 5 and 7, the PRA stain detected low numbers of small (approximately 10–15-µm) spherules and endospores within the inflammatory lesions (Figure 2A and B) On day 14, the spherules of Cp1038 remained primarily in the 15–20-µm size range and relatively sparse, with typical pyogranulomatous inflammation (Figure 2C), in stark contrast to the innumerable, large spherules of Silveira observed on day 14 after infection with 50 arthroconidia (Figure 2D). Our group has previously reported that spherules of Silveira can be seen in mice with PRA stain by day 3 after infection, that they enlarge to ≥50 µm in diameter by day 4–5 after infection, and that they rupture multitudinous endospores [4, 17]. Slow growth of Cp1038 occurred both in vitro and in the mice.
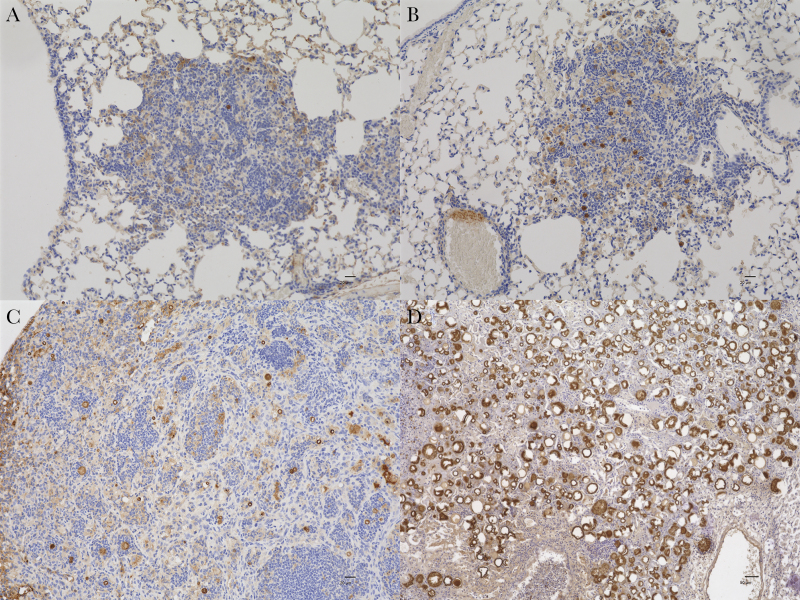
Figure 2.
A, B, In lungs of B6 mice, Cp1038 spherules, stained dark red or brown, are sparse in lesions on days 5 (A) and 7 (B) after infection and are approximately 10–15 µm in diameter. C, On day 14 after infection, the abundance of spherules is modestly increased, and some spherules reach a diameter of approximately 20 µm (A–C, PRA stain, original magnification ×200; scale bars = 20 µm). D, For comparison, a B6 mouse from a study infected with 50 arthroconidia of Silveira had large, floridly abundant spherules on day 14 after infection (PRA stain, original magnification ×100; scale bar = 50 µm).
Fungal Burden and Survival in Mice Infected with Cp1038
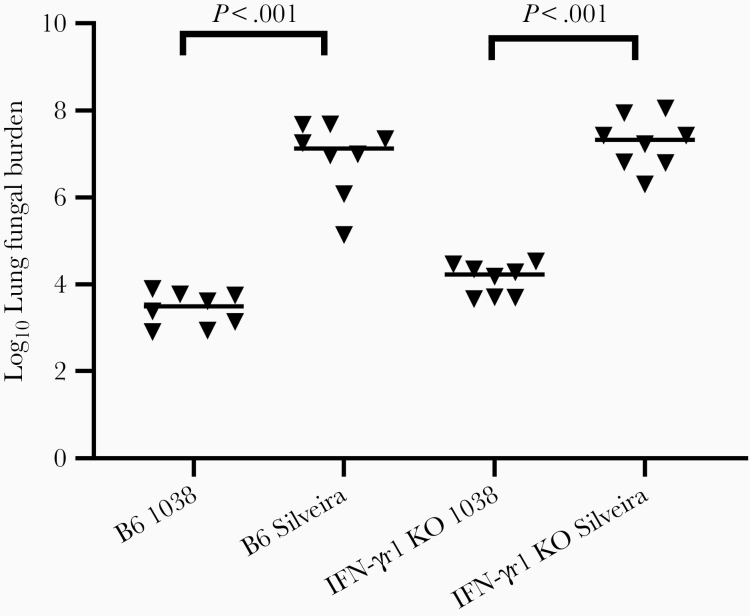
Previously, differences in disease progression using immunodeficient mice were difficult to detect with Silveira infection due to the short disease course. IFN-γ has been implicated in the resistance to CM, and TNF-α inhibitors as immunosuppressant therapy are associated with severe coccidioidomycosis [10]. Because it is produced by both innate and adaptive immune cells, we tested the growth of Cp1038 in Ifn-γr1 KO mice as well as B6. The slower growth of Cp1038 in mouse lungs predicted lower LFBs at day 14, which were indeed 3 logs lower in both B6 and Ifn-γr1 KO mice (n = 8 per group) infected with Cp1038 compared with Silveira (P < .001 for both strains of mice) (Figure 3). The mean LFBs for B6 (2 × 107) and Ifn-γr1 KO (3.6 × 107) mice infected with Silveira were also significantly different (P = .02).
Figure 3.
Lung fungal burden (LFB) on day 14 after infection of C57BL/6 and interferon γ receptor 1 (Ifn-γr1) knockout (KO) mice after intranasal infection with 32 or 34 arthroconidia of Cp1038 or Silveira, respectively (n = 8 per group). LFBs are approximately 1000-fold lower with Cp1038 in both wild-type and the immunodeficient mice (P < .001 for both comparisons). Differences in LFB were significant between the 2 mouse strains for Silveira (P = .02) but not for Cp1038 (P > .99) infection (Kruskal-Wallis).
Some Silveira-infected mice of both strains were removed early because of moribundity, and LFBs were typical of fatal infections. In addition, splenic dissemination (not quantitated) occurred in all mice infected with Silveira. Mean LFBs for B6 (3.6 × 103) and Ifn-γr1 (1.7 × 104) KO mice infected with Cp1038 were not significantly different (P > .99) at 2 weeks, all the mice were clinically normal with no weight loss, and less than half had splenic dissemination (4 Ifn-γr1 KO and 2 B6; not quantitated). These findings clearly demonstrate the time limitations for studies with virulent fungal strains and the potential for longer studies to reveal differences in disease progression and immunologic responses.
Two immunodeficient strains of mice predicted to have altered outcomes to coccidioidal infection, Ifn-γr1 KO and Tnf-α KO [20-24], were tested for extended times. Groups of 8 TNF-α KO or IFN-γr1 KO mice and normal B6 controls were monitored for 10-week survival in separate studies. In the Tnf-α KO study, instead of fungal burden, 3 Tnf-α KO mice had lungs fixed for histopathology at the time of moribundity and the B6 mice with matching numbers were euthanized on the same day for comparison. B6 mice euthanized for histopathologic comparisons were not included in survival analysis.
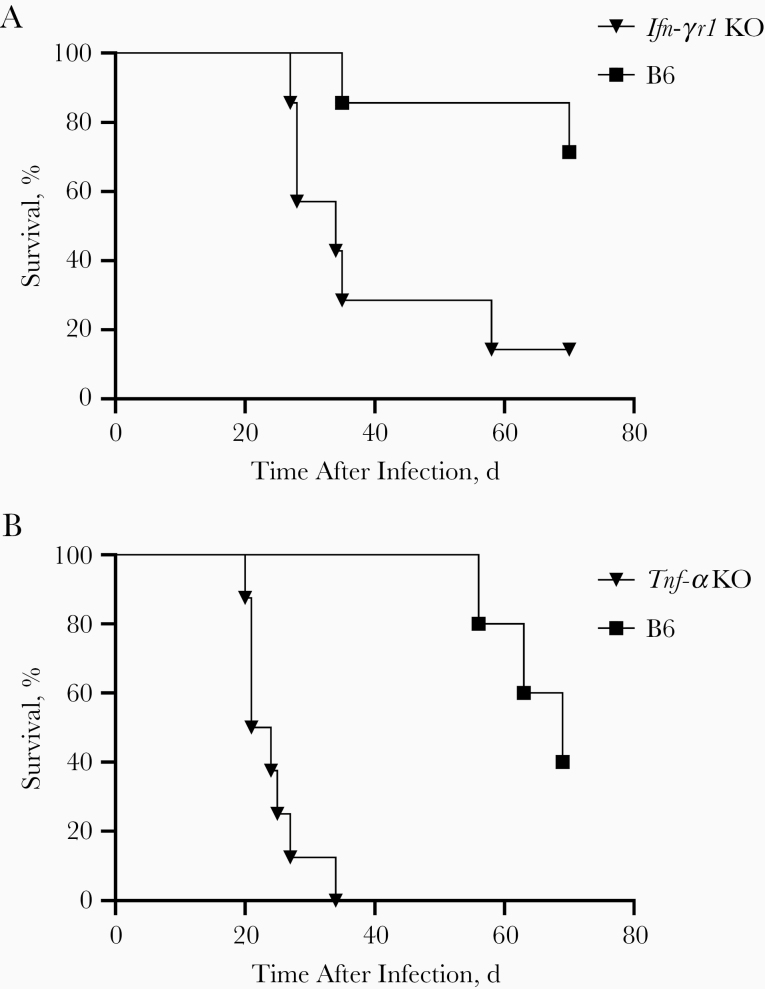
Mice in the Tnf-α KO and Ifn-γr1 KO studies received 43 or 52 arthroconidia of Cp1038 intranasally, respectively. Both immunodeficient strains of mice died significantly sooner than B6 mice (Figure 4). The median survival times were 34 days for Ifn-γr1 KO mice and 22.5 days for Tnf-α KO mice, compared with 70 days for B6 mice in both studies (P = .01 and P < .001, respectively). This confirms the postulated role of both IFN-γ and Tnf-α in coccidioidomycosis infections in mice. Some deaths before 70 days in B6 mice, as well as the extent of disease detected after death (mean LFBs in B6 mice, log 5.7 and log 6.5 in the Inf-γr1 KO and Tnf-α KO studies, respectively), showed that Cp1038 is progressive even in immunologically normal B6 mice, but the slower rate of this infection provides opportunities for an array of studies in normal and immunodeficient mice that were never possible with the most common laboratory Coccidioides infections.
Figure 4.
Survival of interferon γ receptor 1 (Ifn-γr1) knockout (KO) (A) and tumor necrosis factor (Tnf) α KO mice (B) compared with wild-type B6 mice after intranasal infection with 43 or 52 arthroconidia of Cp1038, respectively (n = 5–8 mice per group). Immunodeficient mice died significantly earlier than wild-type B6 mice (Ifn-γr1 KO, P = .01; Tnf-α KO, P < .001), with biologically relevant separation of survival between strains (log-rank test).
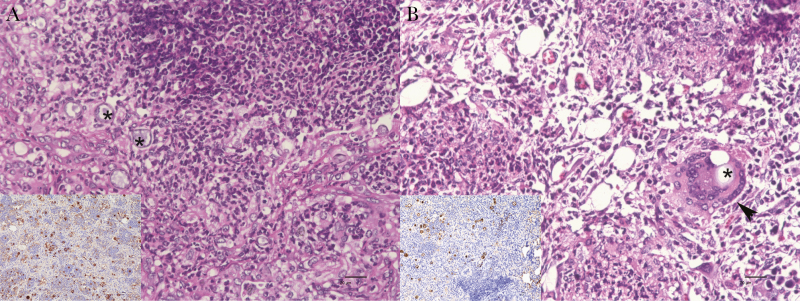
In comparing histopathologic findings in Tnf-α KO mice and nonmoribund B6 mice euthanized on the same day, Figure 5A shows that Tnf-α KO mice do not form classic granulomas in response to the coccidioidal infection. The lesions were characterized by extensive, dense pyogranulomatous inflammation and little or no fibrosis. In contrast, B6 mice had discrete granulomas with borders, characterized by pyogranulomatous inflammation of lower inflammatory cell density, more macrophages than neutrophils, and multinucleate giant cells, a hallmark of granuloma formation, with spherule ingestion (Figure 5B). There were also more spherules in the Tnf-α KO than in B6 mice (Figure 5, insets).
Figure 5.
Lungs of tumor necrosis factor (Tnf) α knockout (KO) and B6 mice 25 days after infection with 43 arthroconidia of Cp1038 intranasally. A, Dense pyogranulomatous inflammation of predominantly neutrophils and macrophages, as well as numerous spherules, infiltrate the lungs in the Tnf-α KO mice; there is little structure or evidence of fibrosis. Asterisks indicate 2 of the dozen or so visible spherules in the field shown. B, Inflammation in B6 mice is characteristic of granuloma formation, with reduced density of neutrophilic inflammation, epithelioid macrophages, and multinucleate giant cells (arrowhead). At least 1 spherule (asterisk) is engulfed by the giant cell in this field of view. Insets in A and B show significantly fewer spherules in B6 mice (hematoxylin-eosin stain, original magnification ×400 [scale bar = 20 µm]; inset: PRA stain, original magnification ×200 [scale bar = 20 µm]).
Chronic, Stable Fungal Burden in Resistant B6D2 Mice
DBA/2 mice are known to be more resistant to coccidioidal infection, and resistance is the dominant trait in DBA/2 mice crossed with more susceptible strains [4, 18, 19]. We therefore postulated that offspring of B6 mice crossed with DBA/2 mice (B6D2) and infected with Cp1038 would exhibit a chronic or arrested infection, more closely approximating that of most humans. To investigate this, groups of B6D2 mice were infected with approximately 50 arthroconidia of Cp1038 intranasally and groups euthanized at either 4 or 10 weeks to compare LFBs, splenic dissemination, and survival. Mice in both groups survived to planned termination, and analysis of LFBs at 4 and 10 weeks showed static disease (median [range], log 4.61 [4.11–4.64] and log 4.44 [4.11–4.75], respectively). Dissemination based on fungal growth from spleens (not quantitated for this study) was present in all 5 mice euthanized at 4 weeks and 3 of 5 euthanized at 10 weeks, although all mice were outwardly healthy.
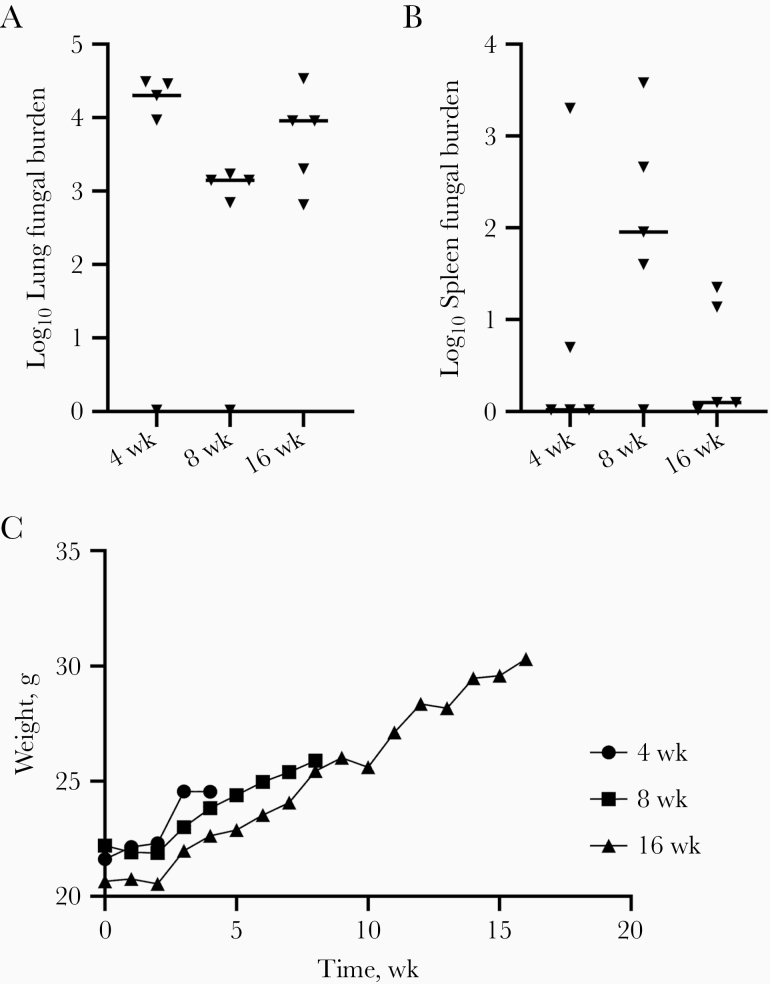
To extend observations, groups of 5 B6D2 mice were infected as before and euthanized 4, 8, or 16 weeks later to determine lung and spleen fungal burdens. All mice survived to their scheduled termination, and lung and spleen fungal burdens did not differ significantly between the groups of mice (lung, P = .11; spleen, P = .22; Kruskal-Wallis test) (Figure 6A and 6B). Furthermore, mice at all time points remained clinically healthy, and mean group weights increased throughout the study (Figure 6C). The static LFB of approximately 104 colony-forming units in B6D2 mice was consistent across both studies between 4 and 16 weeks after infection, and the frequent finding of dissemination in the spleen did not result in progression to death in the time frame studied. The ultimate fate of these mice if they were observed longer is unknown, but at least until 16 weeks, Cp1038 seemed to produce a chronic, static infection that may approximately model arrested pulmonary disease in humans.
Figure 6.
A, B, Lung (A) and spleen (B) fungal burdens in B6D2 mice (n = 5 per group) infected with 58 arthroconidia intranasally of Cp1038 and euthanized 4, 8, or 16 weeks after infection. There were no significant difference between the fungal burdens over time (lung, P = .11; spleen, P = .22; Kruskal-Wallis test). C, Mean group weights charted over time showed weight gains throughout, and mice remained healthy.
Peak Lung Cytokines
Cytokine patterns usually offer clues regarding host immunity, and once again we postulated that the slower progression of Cp1038 infection would reveal important interactions of innate and adaptive immune response in coccidioidomycosis that are impossible to measure in a 14-day infection model. In unpublished data, we have shown very little cytokine response in lungs or peripheral blood mononuclear cells of mice infected with Silveira in the first 2 weeks. In Cp1038-infected mice studied for 6 weeks, 11 cytokines showed changes in patterns from uninfected control mice, with maximal differences observed at 4 weeks or later for the majority of them (Table 1). The statistically significant changes are notes in Table 1, though most cytokines showed changes at later time points (4–6 weeks).
Table 1.
Cytokine Alterations During Cp1038 Infection
| Cytokine | Cytokine Level, Median (Maximum–Minimum), pg/g Lung Tissue | ||||
|---|---|---|---|---|---|
| Uninfected | Week 2 | Week 4 | Week 5 | Week 6 | |
| TNF-⍺ | 8505 (9772–3782) | 10 797 (17 713–8288) | 29 203 (60 623–11 237) | 11 320 (82 055–41) | 45 802 (118 117–3098) |
| IL-1⍺ | <1.3 LOD (<1.3–1234) | 2972 (3596–1774)a | 22 577 (29 165–2609)b | 26 292 (29 165–14 504)a | 20 364 (30 100–2022) |
| IL-1β | 1538 (1773–1206) | 1333 (1542–661) | 1661 (5483–993) | 4520 (7888–2436) | 2530 (6988–600) |
| IL-23 | <4.2 LOD (<4.2–<4.2) | <4.2 (1475–<4.2) | 6326 (10 164–<4.2) | 2535 (5189–1740) | 1412 (2521–<4.2) |
| MCP-1 | 55 239 (63 056–48 920) | 51 419 (65 628–25 626) | 39 420 (91 082–19 351) | 6826 (12 181–3110)a | 14 809 (23 045–7345) |
| GM-CSF | 22 661 (30 537–14 005) | 43 975 (63 419–24 935) | 19 882 (43 825–9228) | 3076 (12 087–849)a | 9117 (13 551–5728) |
| IFN-β | 176 147 (217 878–72 299) | 61 506 (110 102–35 924) | 106 309 (199 120–44 928) | 19 288 (32 180–5139)a | 52 164 (76 054–23 309) |
| IL-6 | 252 102 (310 612–199 122) | 381 933 (494 675–191 489) | 255 359 (448 848–182 647) | 79 278 (140 224–46 324)a | 166 889 (199 945–89 619) |
| IL-10 | 7464 (8543–6506) | 7028 (8747–4215) | 5528 (10 967–4476) | 2579 (5026–1366) | 3602 (4490–1998) |
| IL-17A | <1.8 LOD (<1.8–<1.8) | 597 (2148–287) | 6058 (33 146–<1.8) | 1584 (9086–1114) | 7139 (10 988–2839) |
| IFN-ɣ | <0.8 LOD (<0.8–<0.8) | <0.8 (<0.8–<0.8) | 425 (9028–<0.8) | 5389 (11 077–302) | 1579 (7823–<0.8) |
| IL-27 | 8218 (9903–7062) | 7571 (10 257–4366) | 10 802 (13 740–6819) | 15 178 (16 217–6054) | 9175 (19 327–4563) |
| IL-12p70 | <0.7 LOD (<0.7–<0.7) | <0.7 LOD (<0.7–<0.7) | <0.7 LOD (<0.7–<0.7) | <0.7 LOD (<0.7–<0.7) | <0.7 LOD (<0.7–<0.7) |
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; IL-1α (etc), interleukin 1α (etc); LOD, limit of detection; MCP-1, monocyte chemoattractant protein 1; TNF, tumor necrosis factor.
aSignificant at P = .04.
bSignificant at P = .02.
Levels of the innate cytokine granulocyte-macrophage colony-stimulating factor rose quickly and were elevated at 2 weeks after infection, while other innate cytokines showed significant decreases at 5 weeks after infection (interleukin 6, 10, and 23 and monocyte chemoattractant protein 1; P ≤ .05). Whether this is related to pathogen suppression or host down-regulation remains to be explored. Although differences did not reach significance, peak concentrations for interleukin 6, 10, 23 and monocyte chemoattractant protein 1 occurred 4 weeks after infection. Elevations in cytokines typically associated with successful adaptive host responses to coccidioidal infection occurred at 4 (Ifn-γ) or 6 (interleukin 17A and Tnf-α) weeks after infection. Most of the peak cytokine responses, and nearly all of the significant changes detected in this study, occurred after mice in an acute model would have died. These results provide additional evidence that the slower Cp1038 infection model is likely to enhance studies to model human immune responses in mice.
DISCUSSION
A better understanding of coccidioidomycosis has been hampered by the lack of an animal model that recapitulates the usual chronic course of the human disease. Here we described the slower growth and pathogenicity of a relatively unstudied patient isolate, Cp1038, and its extended disease course in both inbred B6 and B6D2 hybrid mice. This provides a useful alternative to the rapidly fatal murine model of coccidioidomycosis, such as that caused by Silveira. B6 mice survived approximately 4 times as long as after infection with Silveira, and death was not seen in the resistant B6D2 mice even at 4 months.
The slower disease progression of Cp1038 in B6 mice allowed the use of KO mice to detect important immunologic phenotypes. We were able to demonstrate that the absence of Ifn-γr1 or Tnf-α in B6 mice resulted in a worse outcome (Figure 4), something that was not readily apparent when comparing fungal burdens at 14 days with either Cp1038 or Silveira (Figure 3). Elsewhere, we have shown that both a homozygous and heterozygous mutation in Stat4 resulted in greater susceptibility to Cp1038 infection [9]. That the immune responses in B6 mice required several weeks to develop (Table 1) may explain why the consequence of the Ifn-γr1 deficiency, the Tnf-α deficiency, or the dominant negative effect of the Stat4 mutation could be detected only with a more protracted course of infection such as Cp1038 produces. To the extent that other aspects of murine immune responses require weeks to become evident, experimental coccidioidal infection with strain Cp1038 may provide an avenue to study and better understand immune responses in ways not previously available.
Our studies also discovered that a Cp1038 respiratory infection in B6D2 mice resulted in a nonlethal, relatively low, and stable fungal burden for ≥16 weeks (Figure 6). This is remarkably similar to the course of coccidioidal infections in most humans [25]. It is surprising that mice survived despite frequent dissemination to the spleen. Although clinically apparent disseminated coccidioidal infection is typically progressive [11], asymptomatic human disseminated infection has been identified occasionally in the prostate [26] and in the eye in 9% of patients with otherwise uncomplicated and untreated pulmonary coccidioidomycosis [27].
It is possible that hybrids between genetically modified B6 and DBA/2 mice in this model will allow studies of heterozygous gene mutations, such as the previously described human STAT1 gain-of-function or the STAT4 immunodominant mutations [8, 9]. Moreover, the static infection model in B6D2 mice may provide insights into the effects of various immunosuppressive therapies, such as corticosteroids, anti-TNF, or other biological response modifiers on the likelihood of progressive infection if the immunosuppression is present at the time of infection or of reactivation if it begins after infection is already stabilized [28–30]. These and perhaps other experimental questions are now possible to address using Cp1038 in those studies.
Notes
Acknowledgments. We thank Edward Bedrick, PhD, for assistance with statistical analysis and Sharon Dial for input on the histopathology descriptions.
Financial support. This work was supported by the National Institutes of Health (grants 5-U01-AI122275 and 5-R01-AI132140).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Clemons KV, Capilla J, Stevens DA. Experimental animal models of coccidioidomycosis. Ann N Y Acad Sci 2007; 1111:208–24. [DOI] [PubMed] [Google Scholar]
- 2. Shubitz LF, Powell DA, Trinh HT, et al. Viable spores of Coccidioides posadasii Δcps1 are required for vaccination and provide long lasting immunity. Vaccine 2018; 36:3375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sun SH, Cole GT, Drutz DJ, Harrison JL. Electron-microscopic observations of the Coccidioides immitis parasitic cycle in vivo. J Med Vet Mycol 1986; 24:183–92. [PubMed] [Google Scholar]
- 4. Shubitz LF, Dial SM, Perrill R, Casement R, Galgiani JN. Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect Immun 2008; 76:5553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sampaio EP, Hsu AP, Pechacek J, et al. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 2013; 131:1624–34.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-γ receptor 1 deficiency. Clin Infect Dis 2009; 49:e62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Odio CD, Lee-Milligan K, McGowan K, et al. Endemic mycoses in patients with STAT3-mutated hyper-IgE (Job) syndrome. J Allergy Clin Immunol 2015; 136:1411–3.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 2017; 23:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Powell DA, Shubitz LF, Hsu A, et al. 2888. STAT4 mutation in three generations with disseminated coccidioidomycosis (DCM) also exhibits increased susceptibility to coccidioidal infection in transfected mice. Open Forum Infect Dis 2019; 6:S77–8. [Google Scholar]
- 10. Blair JE, Ampel NM, Hoover SE. Coccidioidomycosis in selected immunosuppressed hosts. Med Mycol 2018; 57:S56–63. [DOI] [PubMed] [Google Scholar]
- 11. Galgiani JN, Ampel NM, Blair JE, et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 2016; 63:e112–46. [DOI] [PubMed] [Google Scholar]
- 12. Cox RA, Magee DM. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev 2004; 17:804–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huppert M, Sun SH, Gross AJ. Evaluation of an experimental animal model for testing antifungal substances. Antimicrob Agents Chemother 1972; 1:367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang S, Hendriks W, Althage A, et al. Immune response in mice that lack the interferon-gamma receptor. Science 1993; 259:1742–5. [DOI] [PubMed] [Google Scholar]
- 15. Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 1996; 184:1397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shubitz LF, Yu JJ, Hung CY, et al. Improved protection of mice against lethal respiratory infection with Coccidioides posadasii using two recombinant antigens expressed as a single protein. Vaccine 2006; 24:5904–11. [DOI] [PubMed] [Google Scholar]
- 17. Donovan FM, Shubitz L, Powell D, Orbach M, Frelinger J, Galgiani JN. Early events in coccidioidomycosis. Clin Microbiol Rev 2019; 33:e00112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirkland TN, Fierer J. Inbred mouse strains differ in resistance to lethal Coccidioides immitis infection. Infect Immun 1983; 40:912–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox RA, Kennell W, Boncyk L, Murphy JW. Induction and expression of cell-mediated immune responses in inbred mice infected with Coccidioides immitis. Infect Immun 1988; 56:13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergstrom L, Yocum DE, Ampel NM, et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor α antagonists. Arthritis Rheum 2004; 50:1959–66. [DOI] [PubMed] [Google Scholar]
- 21. Magee DM, Cox RA. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun 1995; 63:3514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fierer J, Walls L, Eckmann L, Yamamoto T, Kirkland TN. Importance of interleukin-10 in genetic susceptibility of mice to Coccidioides immitis. Infect Immun 1998; 66:4397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woelk CH, Zhang JX, Walls L, et al. Factors regulated by interferon gamma and hypoxia-inducible factor 1A contribute to responses that protect mice from Coccidioides immitis infection. BMC Microbiol 2012; 12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. del Pilar Jiménez-A M, Viriyakosol S, Walls L, et al. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of dectin-1 (Clec7a). Genes Immun 2008; 9:338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galgiani JN, Blair JE, Ampel NM, Thompson GR. Treatment for early, uncomplicated coccidioidomycosis: what is success? Clin Infect Dis 2019; 70:2008–12. [DOI] [PubMed] [Google Scholar]
- 26. Yurkanin JP, Ahmann F, Dalkin BL. Coccidioidomycosis of the prostate: a determination of incidence, report of 4 cases, and treatment recommendations. J Infect 2006; 52:e19–25. [DOI] [PubMed] [Google Scholar]
- 27. Rodenbiker HT, Ganley JP. Ocular coccidioidomycosis. Surv Ophthalmol 1980; 24:263–90. [DOI] [PubMed] [Google Scholar]
- 28. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004; 38:1261–5. [DOI] [PubMed] [Google Scholar]
- 29. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology 2017; 152:1297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blair JE. Approach to the solid organ transplant patient with latent infection and disease caused by Coccidioides species. Curr Opin Infect Dis 2008; 21:415–20. [DOI] [PubMed] [Google Scholar]