Abstract
It has been demonstrated that activated mast cells (MCs) are enriched in Kaposi sarcoma (KS) tumors and contribute to the inflammatory microenvironment. Mechanisms driving MC activation, however, are incompletely understood. We sought to understand whether immunoglobulin E (IgE), a potent activator of MCs, was associated with KS incidence and severity. In a cross-sectional study of untreated human immunodeficiency virus (HIV)–infected adults with or without KS in Uganda, we found that patients with KS had higher plasma IgE levels than those without KS. After adjustment for age, sex, CD4+ T-cell count, and HIV RNA levels, there was a dose-response relationship between plasma IgE levels and the presence and severity of KS. Higher eosinophil counts were also associated with IgE levels, and plasma interleukin 33 concentrations were higher in individuals with KS. These findings suggest that IgE-driven atopic inflammation may contribute the pathogenesis of KS. Therapies targeting IgE-mediated MC activation thus might represent a novel approach for treatment or prevention of KS.
Keywords: Immunoglobulin E (IgE), mast cell, KSHV, IL-33, Kaposi sarcoma, HIV, Africa
Activated mast cells (MCs) are enriched in Kaposi sarcoma (KS) tumors and contribute to the inflammatory microenvironment. We demonstrated that immunoglobulin E and interleukin 33, potent activators of MCs, are associated with KS in human immunodeficiency virus–infected adults in Uganda, suggesting atopic inflammation may be a driver of KS.
Kaposi sarcoma (KS) is the most common cancer in people living with human immunodeficiency virus (HIV) worldwide. In sub-Saharan Africa. where there is a high prevalence of both HIV and KS-associated herpesvirus (KSHV), KS is among the most common cancers in the region [1]. All variants of KS exhibit the same unusual multicentric inflammation-driven, angioproliferative tumor of endothelial cell origin [2]. The requisite inflammation predates the formation of detectable lesions, underscoring that inflammation is essential to disease pathogenesis [3–6]. Although the inflammatory nature of KS is well appreciated from a clinical perspective, there is a lack of understanding of mechanisms governing inflammation in this tumor microenvironment, which hampers the development of pathogenesis-specific therapeutic targets.
Identification of factors associated with inflammation that increase the risk of developing KS has long been sought in an effort to understand the drivers of tumor establishment and progression. Prior research showed an increased risk for the development of classic KS in patients with a history of asthma, allergies, and topical corticosteroid use [7]. More recently, KSHV seropositivity was significantly associated with antibody levels to 2 Plasmodium falciparum malaria antigens in Kenya [8] and was found to be associated with coinfection with multiple parasitic infections, including malaria, hookworm, and mansonellosis in Ugandan women [9]. Asthma, allergy, inflammatory dermatitides requiring topical steroids, and parasitic infections all share the feature of significantly elevated levels of circulating immunoglobulin E (IgE) antibodies (reviewed in [10]). IgE is a potent activator of highly inflammatory tissue resident mast cells (MCs) and eosinophils, both of which contribute to the pathogenesis of allergic diseases and are central to the immune response involved in parasite control.
Our group has demonstrated that MCs are enriched and extensively activated in KS lesions, that they support productive infection with KSHV, and that the levels of secreted MC-derived products (ie, a measure of the degree of MC activation in patients with KS) were significantly elevated in HIV-infected Ugandan adults with KS [11]. Importantly, contravening MC activation and antagonizing the proinflammatory MC products histamine and cysteinyl leukotrienes led to the rapid and durable regression of KS lesions in a patient with aggressive AIDS-associated KS [11]. In the current study, we extend those findings to assess the upstream signals associated with MC activation in this population.
Given the high burden of parasitic infections in sub-Saharan Africa and the known relationship with KSHV infection in this population [7, 9, 12], we sought to understand the contribution of IgE-mediated inflammation to the development and severity of KS. We hypothesized that IgE, a potent activator of MCs, is increased in patients with KS. In a study of KSHV-seropositive HIV-infected adults in sub-Saharan Africa, we measured plasma levels of IgE and assessed their association with KS.
METHODS
Overall Design
In a cross-sectional study of antiretroviral therapy (ART)–untreated HIV-infected adults (≥18 years old) in Uganda, we assessed the association between plasma IgE levels and KS.
Study Population
We included in this study, individuals with newly diagnosed KS. These were all the individuals enrolled in the Antiretrovirals for Kaposi’s Sarcoma (ARKS) study based at the Infectious Diseases Institute, Kampala, Uganda [13]. The ARKS study recruited ART-untreated individuals with a KS diagnosis based on histopathology and a few persons with oral lesions that were very characteristic of KS but were difficult to biopsy.
We also included individuals without a KS diagnosis, derived from the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort. UARTO consecutively enrolled individuals before initiating ART at the Immune Suppression Syndrome Clinic at Mbarara Regional Referral Hospital in southwestern Uganda. For the present study, we included all UARTO participants without KS (based on patient report and review of medical records at the Immune Suppression Syndrome Clinic), who were enrolled between April 2007 and February 2011, were KSHV antibody positive, and had available cryopreserved plasma specimens.
Measurements
Demographic characteristics and medical history were collected using interviewer-administered questionnaires. For all measurements, the same data collection tools were used for all participants, irrespective of KS diagnosis. In those with KS, the number of lesions, the anatomic sites with KS lesions, and the presence of edema were documented at enrollment.
Laboratory measurements for all participants were performed in the same laboratories, using cryopreserved plasma samples obtained before ART initiation. Total plasma IgE was measured by means of enzyme-linked immunosorbent assay (eBioscience). The assay was performed according to the manufacturer’s instructions. Briefly, 96-well plates were coated with capture antibody overnight at 4°C. The next day, plates were washed 2 times and blocked for 2 hours at room temperature (RT). After 2 washes, samples were added at dilutions ranging from 1:10 to 1:500. Purified IgE was used as standard, ranging in concentration from 7.8 to 500 ng/mL. Samples and standards were left on plates for 2 hours at RT with shaking. After 2 hours, detection antibody was added to plates and incubated for 1 hour at RT with shaking. Plates were washed 4 times, and substrate was added for 15 minutes at RT. Reaction was stopped with 2N sulfuric acid and the optical densities of wells were read at 450 nm.
Concentrations of IgE in plasma were extrapolated from a graph of standard optical densities versus concentrations. Plasma levels of interleukin 33 (IL-33) were measured with enzyme-linked immunosorbent assay (Peptrotech). The assay was performed according to the manufacturer’s instructions. Samples were diluted 1:2 to 1:5 before being added to the plates. Purified recombinant IL-33 was used as standard, ranging in concentration from 32 to 4000 pg/mL. Plasma HIV RNA levels were measured using Amplicor HIV Monitor (version 1.5) or the Cobas Taqman HIV-1 (version 1.0) assays (Roche). CD4+ T-cell counts were assessed with flow cytometry (FACSCalibur; Becton Dickinson). Antibodies to KSHV were measured with 2 enzyme immunoassays [14, 15] and 1 direct immunofluorescence assay [16, 17], and the results were interpreted using a previously described algorithm [17]. Plasma KSHV DNA was determined by quantitative polymerase chain reaction for open reading frame 25 (lower limit of detection, 66 copies/mL). Eosinophil counts was measured using flow cytometry (Beckman Coulter).
Statistical Analysis
We compared plasma IgE levels between patients with KS and those without KS, using Mann-Whitney U tests. Logistic regression models were used to examine the association between plasma IgE levels and KS. We adjusted for confounding by age, sex, and severity of HIV infection as measured by CD4+ T-cell count and plasma HIV RNA load. We used linear regression to assess the association between the extent of KS and plasma IgE levels and adjusted for age, sex, CD4+ T-cell count, and plasma HIV RNA load. Variables were used as restricted cubic splines, categorized to accommodate for nonlinearity, or log-transformed to nonnormality when required. We also assessed for goodness of fit of the models, using the Hosmer-Lemeshow test, and for the presence of multiplicative interaction, using P values <.05 to guide reporting. In a random sample of the study population, we compared plasma IL-33 levels among patients with KS and those without KS, using Mann-Whitney U tests. All analyses were performed using SAS (version 9.4) and Stata (version 13.1) software.
RESULTS
Characteristics of the Study Population
A total of 416 ART-untreated HIV-infected adults with KSHV coinfection were included in these analyses. Of these, 224 had KS, and 192 did not (Table 1). The median (interquartile range [IQR]) age was 33 (28–39) years for individuals with KS and 34 (28–41) years for those without KS. Women comprised 44% of those with and 61% of those without KS. Compared with those without KS, a higher proportion of individuals with KS had very low CD4+ T-cell counts (<50/µL) (34% vs 9%, respectively) and a lower proportion had low plasma HIV RNA levels (≤10 000 copies/mL) (3% vs 14%, respectively).
Table 1.
Characteristics of the Study Participants
| Participants, %a | ||
|---|---|---|
| Characteristic | With KS (n = 224) | Without KS (n = 192) |
| Age, median (IQR), y | 33 (28–39) | 34 (28–41) |
| Female sex | 44 | 61 |
| Detectable plasma KSHV DNA | 56 | 19 |
| CD4+ T-cell count | ||
| <50/µL | 34 | 9 |
| 51–100/µL | 11 | 10 |
| 101–200/µL | 25 | 36 |
| 201–350/µL | 16 | 33 |
| >350/µL | 14 | 12 |
| Plasma HIV RNA, copies/mL | ||
| ≤10 000 | 3 | 14 |
| 10 001–50 000 | 12 | 23 |
| 50 001–100 000 | 14 | 16 |
| 100 001–500 000 | 53 | 31 |
| >500 000 | 18 | 16 |
| Plasma IgE quartile (level, ng/mL) | ||
| Quartile 1 (20–656) | 19 | 32 |
| Quartile 2 (657–1962) | 20 | 31 |
| Quartile 3 (1963–5559) | 26 | 23 |
| Quartile 4 (5560–140 480) | 35 | 14 |
Abbreviations: HIV, human immunodeficiency virus; IgE, immunoglobulin E; IQR, interquartile range; KS, Kaposi sarcoma; KSHV, KS-associated herpesvirus.
aData represent % of participants unless otherwise specified.
Significant Elevation of Plasma IgE Levels in KS
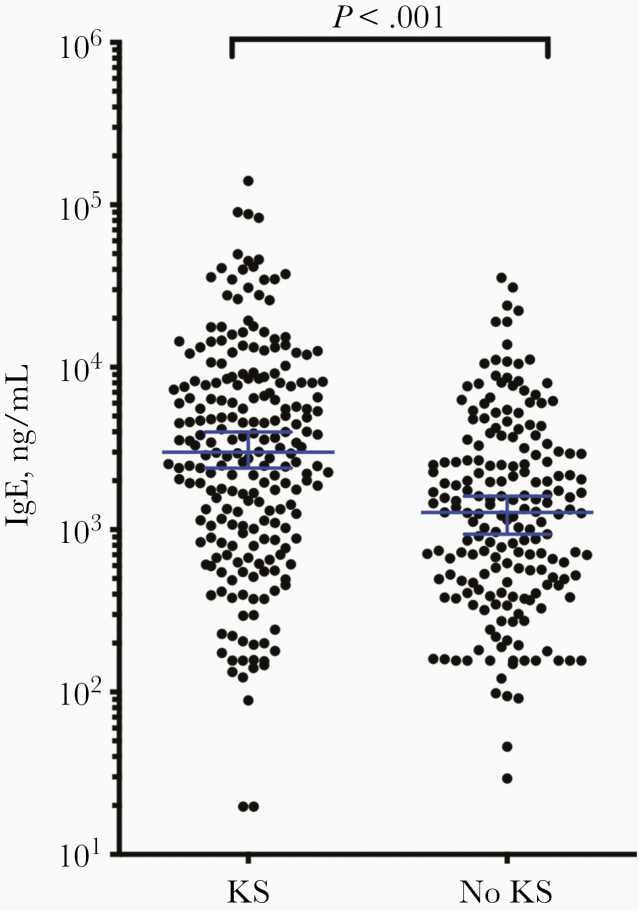
The median (IQR) plasma IgE level in the study population was 1962 (656–5559) ng/mL, higher than previously reported in both HIV-infected and non–HIV-infected Africans [18]. Individuals with KS had significantly higher plasma IgE levels (median [IQR], 2995 [983–8100] ng/mL) than those without KS (1272; 472–2936) ng/mL; P < .001) (Table 1 and Figure 1). Among patients with KS, 35% had levels of IgE that were >5560 ng/mL (quartile 4), compared with only 14% of those without KS. In contrast, 32% of patients without KS had levels of IgE <656 ng/mL (quartile 1), compared with only 19% of patients with KS.
Figure 1.
Distribution of plasma immunoglobulin E (IgE) levels in patients with or without Kaposi sarcoma (KS). Commercial enzyme-linked immunosorbent assay was used to measure IgE levels in 224 patients with KS and 192 without KS; those with KS had significantly higher IgE levels (P < .001). Horizontal lines represent medians with 95% confidence intervals.
In logistic regression analysis we found that higher plasma IgE levels were associated with KS in both unadjusted and adjusted analyses. Compared with individuals with the lowest levels of plasma IgE (quartile 1), those with the highest levels (quartile 4) had 4.2 times the odds of KS (95% confidence interval [CI], 1.6–6.2; P = .001) (Table 2). These findings remained consistent in adjusted analyses: compared with individuals with the lowest levels of plasma IgE (quartile 1), those with the highest levels (quartile 4) had 3.2 times the odds of KS (95% CI, 1.6–6.2; P = .001; P for trend = .002) after we adjusted for age, sex, CD4+ T-cell count, and plasma HIV RNA levels. The association between plasma IgE and KS was not modified by plasma HIV RNA load, CD4+ T-cell count, age or sex (P for interaction = .27, .29, .60, and .15, respectively). Given the possible confounding effect of plasma interleukin 6 levels, we repeated the analyses while also adjusting for plasma interleukin 6, and our observation of an association between plasma IgE levels and KS remained (P for trend = .04) [19–21].
Table 2.
Unadjusted and Adjusted Logistic Regression Analyses of Characteristics Associated With Kaposi Sarcoma
| Unadjusted Analysis | Adjusted Analysisa | |||
|---|---|---|---|---|
| Characteristic | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Age, per 10 y | 0.83 (.66–1.03) | .09 | 0.72 (.54–.94) | .02 |
| Sex | ||||
| Male | Reference | … | Reference | … |
| Female | 0.50 (.34–.74) | .001 | 0.51 (.32–.81) | .005 |
| CD4+ T-cell count | ||||
| <50/µL | Reference | … | Reference | … |
| 51–100/µL | 0.29 (.13–.64) | .002 | 0.40 (.18–.93) | .03 |
| 101–200/µL | 0.19 (.10–.36) | <.001 | 0.25 (.13–.48) | <.001 |
| 201–350/µL | 0.13 (.067–.25) | <.001 | 0.20 (.097–.42) | <.001 |
| >350/µL | 0.32 (.15–.66) | .002 | 0.65 (.27–1.6) | .34 |
| HIV RNA, copies/mL | ||||
| ≤10 000 | Reference | … | Reference | … |
| 10 001–50 000 | 2.5 (.91–6.9) | .08 | 2.2 (.73–6.5) | .17 |
| 50 001–100 000 | 4.5 (1.6–12.4) | .004 | 3.1 (1.0–9.6) | .048 |
| 100 001–500 000 | 8.6 (3.4–22.0) | <.001 | 6.0 (2.1–17.1) | .001 |
| >500 000 | 5.9 (2.2–16.2) | .001 | 3.4 (1.1–10.6) | .04 |
| Plasma IgE quartile (level, ng/mL) | ||||
| Quartile 1 (20–656) | Reference | … | Reference | … |
| Quartile 2 (657–1962) | 1.1 (.65–2.0) | .67 | 1.1 (.60–2.1) | .75 |
| Quartile 3 (1963–5559) | 2.0 (1.2–3.5) | .01 | 1.8 (.97–3.4) | .06 |
| Quartile 4 (5560–140 480) | 4.2 (2.3–7.6) | <.001 | 3.2 (1.6–6.2) | .001 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IgE, immunoglobulin E; OR, odds ratio.
aAdjusted for all variables in the table.
No Correlation Between Plasma KSHV DNA and Plasma IgE Levels in Patients with KS
The proportions of participants with detectable KSHV DNA in plasma were 56% for those with KS and 19% for those without KS. These findings are similar to some prior reports from the region [22] but differ from findings in other studies that reported higher rates of KSHV detection in the plasma of HIV-infected patients with KS [23, 24]. We examined whether the association between plasma IgE levels and KS was due to differences in the proportions of those with detectable plasma KSHV DNA between study participants with KS and those without KS. In analyses where we adjusted for age, sex, CD4+ T-cell count, plasma HIV RNA levels, and plasma KSHV DNA, the association between higher plasma IgE levels and KS persisted (data not shown). Compared with individuals with the lowest levels of plasma IgE (quartile 1), those with the highest levels (quartile 4) had 3.0 times the odds of KS (95% CI, 1.5–6.2; P = .002; P for trend = .009).
Correlation Between Severity of KS and Plasma IgE Levels
Among study participants with KS, the extent of KS ranged from mild to widespread. The number of mucocutaneous sites with KS lesions ranged from 1 to 16; 78% of KS patients had 1–49 lesions, and 22% had ≥50 lesions; 50% had edema, and 59% had ≥3 sites with edema (Table 3). In unadjusted analyses, having more anatomic sites with KS lesions and having >49 mucocutaneous KS lesions were associated with higher plasma IgE levels (Table 4). After adjustment for age, sex, CD4+ T-cell count, and plasma HIV RNA load, compared with individuals with 1–49 lesions, those with ≥ 50 lesions had 23% higher plasma IgE levels (P = .06), and compared with individuals with the fewest anatomic sites with KS lesions (quartile 1; 1–3 sites) those with most sites (quartile 4; 11–16 sites) had 27% higher levels of plasma IgE (P = .054). The AIDS Clinical Trials Group tumor stage was not associated with plasma IgE levels (Table 4).
Table 3.
Characteristics of Study Participants With Kaposi Sarcoma
| Characteristic | Participants With KS, % (n = 224) |
|---|---|
| Eosinophil count by quartile | |
| Quartile 1 (0–80/µL) | 26 |
| Quartile 2 (81–185/µL) | 24 |
| Quartile 3 (186–460/µL) | 25 |
| Quartile 4 (461–3020/µL) | 25 |
| Anatomic sites with KS lesions by quartile | |
| Quartile 1 (1–3 sites) | 29 |
| Quartile 2 (4–6 sites) | 25 |
| Quartile 3 (7–10 sites) | 21 |
| Quartile 4 (11–16 sites) | 25 |
| No. of KS lesions | |
| 1–49 | 78 |
| ≥50 | 22 |
| Edema present | 50 |
| No. of sites with edema | |
| 1–2 | 41 |
| 3–4 | 51 |
| 5–6 | 8 |
| ACTG tumor stage T1 | 71 |
Abbreviations: ACTG, AIDS Clinical Trials Group; KS, Kaposi sarcoma.
Table 4.
Multivariable Linear Regression Analysis for Determinants of Plasma Immunoglobulin E Elevation in Patients With Kaposi Sarcoma (n = 224)
| Unadjusted Analysis | Adjusted Analysisa | |||
|---|---|---|---|---|
| Characteristic | Change in Log10-Transformed Plasma IgE Levels, Mean (95% CI) | P Value | Change in Log10-Transformed Plasma IgE Levels, Mean (95% CI) | P Value |
| Eosinophils count by quartile | ||||
| Quartile 1 (0–80/µL) | Reference | … | Reference | … |
| Quartile 2 (81–185/µL) | 0.44 (.20–.67) | <.001 | 0.41 (.17–.64) | .001 |
| Quartile 3 (186–460/µL) | 0.59 (.36–.82) | <.001 | 0.53 (.29–.76) | <.001 |
| Quartile 4 (461–3020/µL) | 0.76 (.53–.99) | <.001 | 0.71 (.48–.95) | <.001 |
| No. of KS lesions | ||||
| 1–49 | Reference | … | Reference | … |
| ≥ 50 | 0.24 (.025–.46) | .03 | 0.21 (–.09 to .42) | .06 |
| Anatomic sites with KS lesions by quartile | ||||
| Quartile 1 (1–3 sites) | Reference | … | Reference | … |
| Quartile 2 (4–6 sites ) | 0.10 (–.14 to .34) | .42 | 0.024 (–.21 to .26) | .84 |
| Quartile 3 (7–10 sites) | –0.015 (–.27 to .24) | .91 | –0.069 (–.32 to .18) | .59 |
| Quartile 4 (11–16 sites) | 0.31 (.063–.55) | .01 | 0.24 (–.0041 to .48) | .054 |
| Edema | ||||
| Absent | Reference | … | Reference | … |
| Present | –0.080 (–.26 to .10) | .38 | –0.12 (–.30 to .058) | .18 |
| No. of sites with edema | … | |||
| 1–2 | Reference | … | Reference | … |
| 3–4 | –0.10 (–.35 to .14) | .41 | –0.16 (–.40 to .083) | .20 |
| 5–6 | 0.29 (–.16 to .74) | .21 | 0.39 (–.055 to .84) | .08 |
| ACTG tumor stage | ||||
| T0 (low risk) | Reference | … | Reference | … |
| T1 (high risk) | 0.003 (–.20 to .20) | .98 | –0.014 (–.21 to .18) | .89 |
Abbreviations: ACTG, AIDS Clinical Trials Group; CI, confidence interval; IgE, immunoglobulin E; KS, Kaposi sarcoma.
aAdjusted for age, sex, CD4+ T-cell count, and plasma human immunodeficiency virus RNA load.
Correlation Between Eosinophil Counts and Plasma IgE Levels
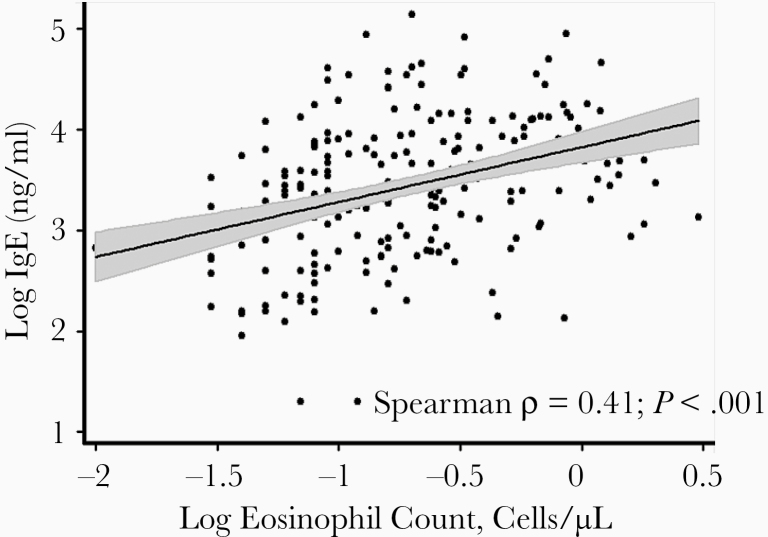
We next examined the association between eosinophil counts and plasma IgE levels in those with KS in our study population. The median (IQR) eosinophil count in the individuals with KS was 185/µL (80–460/µL), consistent with reports in HIV-infected individuals in the region [25]. There was a positive correlation between eosinophil count and plasma IgE levels (Figure 2) (Spearman correlation coefficient ρ = 0.41; P < .001). We also observed a dose-response association between higher eosinophil counts and plasma IgE levels in both unadjusted and adjusted analyses (Table 4). Plasma IgE levels in individuals with the highest eosinophil counts (fourth quartile), were twice the levels in those with the lowest eosinophil counts (first quartile) (P < .001, after adjustment for age, sex, CD4+ T-cell count, and plasma HIV RNA load).
Figure 2.
Scatterplot of plasma immunoglobulin E (IgE) levels versus eosinophil counts in study participants with Kaposi sarcoma (KS). Data show a positive correlation between eosinophil counts and plasma IgE levels (Spearman ρ = .41; P < .001).
Elevated Plasma IL-33 Levels in Patients with KS
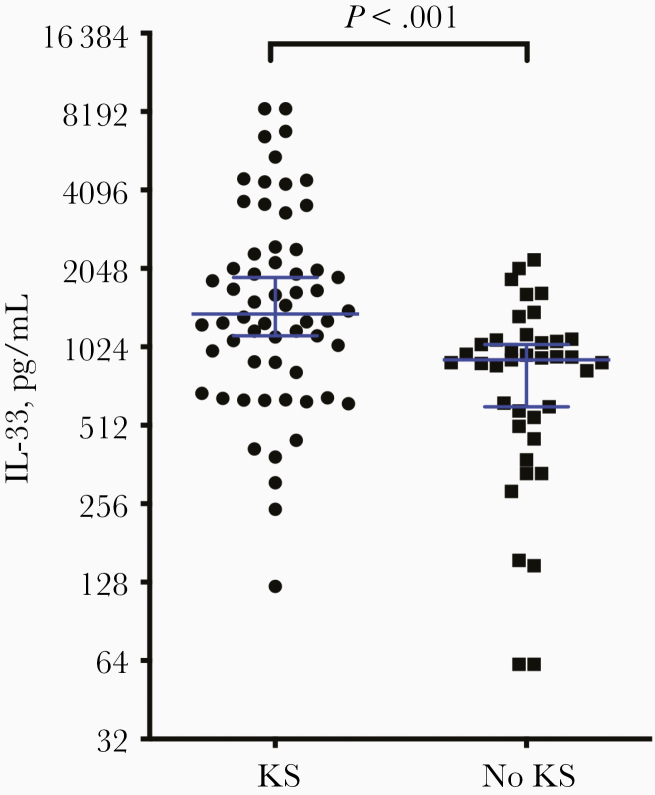
Increasing evidence suggests that the alarmin IL-33 promotes IgE production in an antigen-independent positive feedback loop [26, 27]. IL-33 also lowers the threshold for activation in response to IgE, amplifying the innate immune response. We obtained plasma IL-33 measurements in 99 individuals (60 with and 39 without KS) from our study population. Among them, individuals with KS had higher IL-33 levels (median [IQR], 1371 [836–8397] ng/mL) than those without KS (913 [508–2210] ng/mL; P < .001) (Figure 3). There was no correlation between IgE and IL-33 levels (Spearman ρ = 0.039; P = .77).
Figure 3.
Plasma interleukin 33 (IL-33) levels were measured with enzyme-linked immunosorbent assay in 99 patients, 60 with and 39 without Kaposi sarcoma (KS). Patient with KS had significantly higher levels of IL-33 than those without KS (P < .001). Horizontal lines represent medians with 95% confidence intervals.
DISCUSSION
As one hallmark of cancer, inflammation is known to be essential for the selection, proliferation, and survival of malignant cells. Chronic inflammation associated with KS is so central to tumor establishment and progression that directly targeting inflammation represents a promising treatment strategy. In the current study of ART-untreated HIV-infected Ugandan adults, higher plasma IgE levels were associated with KS independent of plasma HIV load, CD4+ T-cell count, age, and sex. A greater extent of KS and higher eosinophil counts were associated with higher levels of plasma IgE in this population. Our findings provide evidence that atopic inflammation is an important pathogenic mechanism for KS.
IgE normally comprises a small fraction of circulating immunoglobulin ranging from 0 to 400 ng/mL (or approximately 0.05% of total immune globulin) [27]. The majority of the Ugandan adults in the current study had circulating IgE levels above the normal range, which were independently associated with KS. Given that IgE in circulation has a much shorter half-life than other immunoglobulin types, these data suggest that these individuals have sustained production of IgE. Unlike unbound antibody, IgE bound to FcεR1 on MCs, and basophils can persist for weeks or months [28]. Thus, the levels of total IgE in patients with KS (including circulating and cell associated) may actually be underestimated in the current study.
Although not assessed here, the cell-associated fraction of IgE may be of critical importance in KS progression, because recognition of cognate ligands by FcεR1-bound IgE leads to activation and degranulation of MCs. Furthermore, monomeric IgE on the surface of MCs has been found to mediate significant effects, which include MC proliferation, survival, and activation of inflammatory pathways, in addition to, and independent of, degranulation [29–31]. Thus, elevation of IgE in our Ugandan patients may promote both increased numbers and activation of MCs in KS lesions. One principal driver of B-cell class switch to IgE is interleukin 13 (IL-13) [32], and IL-13 polymorphisms are associated with an increased risk in classic KS [33]. Future studies are planned to assess whether these described IL-13 polymorphisms also increase the risk of developing epidemic KS.
Consistent with prior reports, we observed an association between eosinophil counts and plasma IgE levels among individuals with KS. These data, together with findings of previous studies that showed eosinophilia during parasitic infections, support the hypothesis that parasitic infection is an important driver of the elevations in IgE, IL-33, and eosinophils and that, collectively, these derangements may be contributing to KS progression by the activation of proinflammatory MCs. A number of studies have established that MCs are more numerous and are extensively activated in response to parasitic infection [34–36]. Moreover, MCs are known to mediate parasitic gut expulsion and limit tissue burden in models of hookworm, Trichinella spiralis, and Strongyloides infections [34, 35, 37, 38]. In addition, parasite infection, in an analogous murine gamma herpesvirus infection model, has been shown to reactivate murine gamma herpesvirus 68 infection via T-helper (Th) 2–driven activation of viral gene expression. The Th2 cytokines interleukin 4 and IL-13 stimulated activation of viral genes in vivo, suggesting that direct reactivation of KSHV infection by Th2-inducing cytokines would also promote KS disease through modulation of viral gene expression [39].
In a subset of our study population, we also demonstrated that plasma IL-33 levels were significantly elevated in patients with KS, compared with those without KS. IL-33 is a proinflammatory Th2-inducing cytokine of the interleukin 1 cytokine family. It is released by many cell types, including fibroblasts, endothelial cells, and MCs, and it is known to be elevated in patients with allergy and asthma [40]. As an alarmin, IL-33 mediates many functions of the innate immune system, including potentiating the activation of ST2+ MCs, both independent of, and in response to, IgE cross-linking [41–44]. Binding ST2, IL-33 mediates increased production of IgE via interleukin 4–driven B-cell class switch [26] and can potentiate IgE-induced anaphylactic shock [45]. IL-33 activation of human MCs promotes maturation and enhanced survival [42, 46, 47]. Together, the elevation of IL-33 may further exacerbate the known activation of MCs in patients with KS [11]. Further study will be required to elucidate the mechanism driving the observed elevation in IL-33.
Despite dramatic improvements in the last 20 years, clinical outcomes for patients with KS often remain unsatisfactory. Patients with HIV with good immunological and virological responses to ART continue to have an increased lifetime risk of developing KS and often respond poorly to cytotoxic chemotherapy agents [48–50]. In developing countries, particularly in sub-Saharan Africa, economic constraints often hamper access to both antiretrovirals and the cytotoxic agents preferred for KS chemotherapy. Therefore, to meaningfully address the public health problem of KS, new therapeutic approaches must be identified that that are well tolerated, inexpensive, and compatible with lifelong administration. It is notable that agents that antagonize MCs are included on the World Health Organization’s list of essential medicines, including loratidine (a type I histamine receptor antagonist), ranitidine (a type II histamine receptor antagonist), and vitamin C (a MC stabilizer). Thus, the combination of MC-targeted agents might provide economically feasible augmentation to the treatment of KS in resource-limited settings where KS incidence is highest.
Notes
Acknowledgments. We thank Shelia Dollard for quantifying the Kaposi sarcoma–associated herpesvirus DNA in our samples.
Financial support. The work was supported by a Young Investigator Pilot Award from the AIDS and Cancer Specimen Resource, National Cancer Institute (grant UM1 CA181255 to Mike McGrath, and subaward grant 7854SC to C. A. K.), the National Institute of Allergy and Infectious Diseases (grant R03 AI122221 to C. A. K. and grants U54 CA190153, P30 AI027763, R01 MH054907, and R01 CA119903 to J. M.), and the Mexican National Council of Science and Technology (fellowship to A. B. G.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 20th International Workshop on Kaposi’s Sarcoma Herpesvirus (KSHV) and Related Agents, Berlin, Germany, 25–28 July 2017; Second Annual International Herpesvirus Workshop, Ghent, Belgium, 29 July to 2 August 2017; 16th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies, Bethesda, Maryland, 23–24 October 2017.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Ganem D. KSHV infection and the pathogenesis of Kaposi’s sarcoma. Annu Rev Pathol 2006; 1:273–96. [DOI] [PubMed] [Google Scholar]
- 3. Ensoli B, Stürzl M. Kaposi’s sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev 1998; 9:63–83. [DOI] [PubMed] [Google Scholar]
- 4. Nakamura S, Salahuddin SZ, Biberfeld P, et al. Kaposi’s sarcoma cells: long-term culture with growth factor from retrovirus-infected CD4+ T cells. Science 1988; 242:426–30. [DOI] [PubMed] [Google Scholar]
- 5. Salahuddin SZ, Nakamura S, Biberfeld P, et al. Angiogenic properties of Kaposi’s sarcoma-derived cells after long-term culture in vitro. Science 1988; 242:430–3. [DOI] [PubMed] [Google Scholar]
- 6. Ensoli B, Barillari G, Gallo RC. Cytokines and growth factors in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Immunol Rev 1992; 127:147–55. [DOI] [PubMed] [Google Scholar]
- 7. Goedert JJ, Vitale F, Lauria C, et al. ; Classical Kaposi’s Sarcoma Working Group Risk factors for classical Kaposi’s sarcoma. J Natl Cancer Inst 2002; 94:1712–8. [DOI] [PubMed] [Google Scholar]
- 8. Oluoch PO, Oduor CI, Forconi CS, et al. Kaposi sarcoma-associated herpesvirus infection and endemic Burkitt’s lymphoma. J Infect Dis 2020; 222:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wakeham K, Webb EL, Sebina I, et al. Risk factors for seropositivity to Kaposi sarcoma-associated herpesvirus among children in Uganda. J Acquir Immune Defic Syndr 2013; 63:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paparo SB, Guaragna MA, Albanesi M. High IgE levels in patients affected by psoriasis: review of the literature and personal observations. Clin Ter 2014; 165:91–3. [DOI] [PubMed] [Google Scholar]
- 11. Ayers LW, Barbachano-Guerrero A, McAllister SC, et al. Mast cell activation and KSHV infection in Kaposi sarcoma. Clin Cancer Res 2018; 24:5085–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nalwoga A, Cose S, Wakeham K, et al. Association between malaria exposure and Kaposi’s sarcoma-associated herpes virus seropositivity in Uganda. Trop Med Int Health 2015; 20:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin JN, Laker-Oketta M, Walusana V, et al. Randomized trial of protease inhibitor-based antiretroviral therapy for Kaposi’s sarcoma in Africa. Presented at: 21st Conference on Retroviruses and Opportunistic Infections; March 3–6, 2014, Boston, MA. [Google Scholar]
- 14. Pau CP, Lam LL, Spira TJ, et al. Mapping and serodiagnostic application of a dominant epitope within the human herpesvirus 8 ORF 65-encoded protein. J Clin Microbiol 1998; 36:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spira TJ, Lam L, Dollard SC, et al. Comparison of serologic assays and PCR for diagnosis of human herpesvirus 8 infection. J Clin Microbiol 2000; 38:2174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dollard SC, Nelson KE, Ness PM, et al. Possible transmission of human herpesvirus-8 by blood transfusion in a historical United States cohort. Transfusion 2005; 45:500–3. [DOI] [PubMed] [Google Scholar]
- 17. Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet 1996; 348:858–61. [DOI] [PubMed] [Google Scholar]
- 18. Messele T, Brouwer M, Girma M, et al. Plasma levels of viro-immunological markers in HIV-infected and non-infected Ethiopians: correlation with cell surface activation markers. Clin Immunol 2001; 98:212–9. [DOI] [PubMed] [Google Scholar]
- 19. Nakahata T, Toru H. Cytokines regulate development of human mast cells from hematopoietic progenitors. Int J Hematol 2002; 75:350–6. [DOI] [PubMed] [Google Scholar]
- 20. Desai A, Jung MY, Olivera A, et al. IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3. J Allergy Clin Immunol 2016; 137:1863–71 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kikuchi T, Ishida S, Kinoshita T, et al. IL-6 enhances IgE-dependent histamine release from human peripheral blood-derived cultured mast cells. Cytokine 2002; 20:200–9. [DOI] [PubMed] [Google Scholar]
- 22. Whitby D, Howard MR, Tenant-Flowers M, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet 1995; 346:799–802. [DOI] [PubMed] [Google Scholar]
- 23. Campbell TB, Borok M, White IE, et al. Relationship of Kaposi sarcoma (KS)-associated herpesvirus viremia and KS disease in Zimbabwe. Clin Infect Dis 2003; 36:1144–51. [DOI] [PubMed] [Google Scholar]
- 24. Duprez R, Kassa-Kelembho E, Plancoulaine S, et al. Human herpesvirus 8 serological markers and viral load in patients with AIDS-associated Kaposi’s sarcoma in Central African Republic. J Clin Microbiol 2005; 43:4840–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chorba TL, Nkengasong J, Roels TH, et al. Assessing eosinophil count as a marker of immune activation among human immunodeficiency virus-infected persons in sub-Saharan Africa. Clin Infect Dis 2002; 34:1264–6. [DOI] [PubMed] [Google Scholar]
- 26. Komai-Koma M, Brombacher F, Pushparaj PN, et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naïve mice. Allergy 2012; 67:1118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lawrence MG, Woodfolk JA, Schuyler AJ, Stillman LC, Chapman MD, Platts-Mills TA. Half-life of IgE in serum and skin: consequences for anti-IgE therapy in patients with allergic disease. J Allergy Clin Immunol 2017; 139:422–8 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oettgen HC. Fifty years later: emerging functions of IgE antibodies in host defense, immune regulation, and allergic diseases. J Allergy Clin Immunol 2016; 137:1631–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalesnikoff J, Huber M, Lam V, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity 2001; 14: 801–11. [DOI] [PubMed] [Google Scholar]
- 30. Kashiwakura J, Otani IM, Kawakami T. Monomeric IgE and mast cell development, survival and function. Adv Exp Med Biol 2011; 716:29–46. [DOI] [PubMed] [Google Scholar]
- 31. Bax HJ, Keeble AH, Gould HJ. Cytokinergic IgE action in mast cell activation. Front Immunol 2012; 3:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poulsen LK, Hummelshoj L. Triggers of IgE class switching and allergy development. Ann Med 2007; 39:440–56. [DOI] [PubMed] [Google Scholar]
- 33. Brown EE, Fallin D, Ruczinski I, et al. Associations of classic Kaposi sarcoma with common variants in genes that modulate host immunity. Cancer Epidemiol Biomarkers Prev 2006; 15:926–34. [DOI] [PubMed] [Google Scholar]
- 34. Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun 1983; 41:445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol 1996; 8:559–67. [DOI] [PubMed] [Google Scholar]
- 36. Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol 2007; 19:31–8. [DOI] [PubMed] [Google Scholar]
- 37. Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med 2000; 192:1849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fukao T, Yamada T, Tanabe M, et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol 2002; 3:295–304. [DOI] [PubMed] [Google Scholar]
- 39. Reese TA, Wakeman BS, Choi HS, et al. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science 2014; 345:573–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Y, Wang W, Lv Z, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol 2018; 200:2253–62. [DOI] [PubMed] [Google Scholar]
- 41. Ho LH, Ohno T, Oboki K, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcεRI signals. J Leukoc Biol 2007; 82:1481–90. [DOI] [PubMed] [Google Scholar]
- 42. Iikura M, Suto H, Kajiwara N, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest 2007; 87:971–8. [DOI] [PubMed] [Google Scholar]
- 43. Saluja R, Khan M, Church MK, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy 2015; 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One 2010; 5:e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pushparaj PN, Tay HK, H’ng SC, et al. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci U S A 2009; 106:9773–8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Xu K, Williams RM, Holowka D, Baird B. Stimulated release of fluorescently labeled IgE fragments that efficiently accumulate in secretory granules after endocytosis in RBL-2H3 mast cells. J Cell Sci 1998; 111(pt 16):2385–96. [DOI] [PubMed] [Google Scholar]
- 47. Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol 2007; 179:2051–4. [DOI] [PubMed] [Google Scholar]
- 48. Krown SE, Lee JY, Dittmer DP, Consortium AM. More on HIV-associated Kaposi’s sarcoma. N Engl J Med 2008; 358:535–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nguyen HQ, Magaret AS, Kitahata MM, Van Rompaey SE, Wald A, Casper C. Persistent Kaposi sarcoma in the era of highly active antiretroviral therapy: characterizing the predictors of clinical response. AIDS 2008; 22:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Robey RC, Bower M. Facing up to the ongoing challenge of Kaposi’s sarcoma. Curr Opin Infect Dis 2015; 28:31–40. [DOI] [PubMed] [Google Scholar]