Abstract
Background
The aim of this study was to investigate the impact of anti-HBc (HBcAb) positivity on the progression of liver fibrosis (Fibrosis-4 score >3.25) in the Italian cohort of HIV-infected individuals naïve to antiretroviral treatment (ICONA).
Methods
All patients with FIB-4 <3.25 at baseline were evaluated prospectively: 6966 people with HIV (PWH) were screened and classified based on hepatitis B virus (HBV) and hepatitis C virus (HCV) serology.
Results
Patients who were HBcAb+/HCV-/HBs antigen (HBsAg)- and HCV+/HBcAb+/HBsAg- or HBsAg+/HBcAb+/HCV- had CD4+ cell counts below the nadir and significantly higher prevalence of AIDS diagnosis at baseline than the other groups (P < .0001). A Cox regression model adjusted for age, HIV transmission mode, country of birth, and alcohol consumption showed a higher relative risk (HR) of progression to FIB-4 >3.25 in HCV+/HBcAb+/HBsAg- patients (HR, 7.2; 95% CI, 3 8–13.64).
Conclusions
HBcAb+ contributes to liver damage in HIV+/HCV+/HBcAb+/HBsAg- subjects. A careful monitoring for signs of previous HBV infection is needed in this kind of patients.
Keywords: anti-HBc, HBV, HIV/HBV coinfection, liver fibrosis, OBI
Although recent data show a reduction in liver-related and AIDS-related mortality among antiretroviral (highly active antiretroviral therapy [HAART])-treated patients diagnosed with HIV infection in the 2000s [1], coinfection with HIV and hepatitis B virus (HBV) remains a challenge for clinicians. In HBV-endemic areas of the world (Asian and African countries), but also in European cohorts of coinfected people, HIV/HBV coinfection is characterized by accelerated progression to cirrhosis and hepatocellular carcinoma [2, 3]. Published data show that recovery of CD4 count is slowed down in HIV/HBV-coinfected people with chronic hepatitis B (CHB), who also show reduced HBs antigen (HBsAg) and HBe antigen (HBeAg) seroclearance compared with monoinfected HBV patients [4]. Furthermore, data on the clinical impact of resolved HBV infection, specifically the presence of anti-HBc (HBcAb) with or without anti-HBs (HBsAb) antibodies, in HIV-positive patients are lacking. The presence of HBV-DNA in serum or in liver tissue, in the absence of signs of active infection, has been demonstrated in anti-HBc-positive patients and has been defined as occult hepatitis B (OBI) [5]. OBI is observed most frequently in bone marrow/organ recipients and in people undergoing immunosuppressive treatments and chemotherapy, probably linked to compromised host defenses and, consequently, deregulation of HBV replication control. The role of OBI in HBV-monoinfected patients is recognized as a risk factor for the progression of liver disease, fibrosis, end-stage liver disease (ESLD), and hepatocellular carcinoma (HCC) [6–8]. In HBV-endemic countries, OBI is quite common in HIV-infected patients [9], and some studies have reported a trend toward increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in OBI individuals with HIV [10, 11]. Data on the efficacy of HAART regimens containing HBV-active drugs in suppressing HBV-DNA levels in PWH with OBI are limited and not always consistent. On the other hand, detectable levels of HBV-DNA and the emergence of mutated HBV strains are reported in HIV patients with OBI on HAART [9, 12].
With the ultimate aim of understanding the influence of HBcAb positivity on the outcome of liver damage, the evolution of severe liver fibrosis was evaluated in coinfected patients from the Italian Cohort of Antiretroviral-Naïve HIV Patients (ICONA) Foundation.
METHODS
Study Population
This analysis includes prospectively collected data of HIV-infected individuals enrolled in the ICONA cohort. The ICONA Foundation is an observational cohort of HIV-infected individuals who were antiretroviral naïve at the time of enrollment. This cohort was set up in January 1997 and currently consists of >14 000 patients from 50 Italian infectious disease units. The ICONA Foundation study has been approved by the institutional review board (IRB) of all participating centers; sensitive data from patients are seen only in aggregate form. All patients signed a consent form to participate in ICONA, in accordance with the ethical standards of the committee on human experimentation and the Helsinki Declaration (1983 revision). Demographic and sociobehavioral data, initiation and discontinuation dates of each antiretroviral drug, HIV viral load and CD4 cell count every 3 ± 6 months, AIDS-defining diseases according to the Centers for Disease Control and Prevention (CDC) criteria, and non-HIV-related diseases and death were recorded for all enrolled patients. In particular, alcohol consumption information was collected by physician interviews at study enrollment and at subsequent clinical visits during follow-up (a unit of alcohol in Italy is defined as containing 14 g of pure alcohol, which corresponds to 125 mL of wine, 330 mL of a can of beer, and 40 mL of liquor). Hematochemical data, including liver function parameters, were also available at 3 ± 6 month intervals. Further details are available at http://www.fondazioneicona.org/.
End Point and Inclusion Criteria
Patients belonging to the ICONA Foundation Study Cohort with a mild or moderate fibrosis level at baseline (defined as Fibrosis-4 score [FIB-4] <3.25, corresponding to F0–F2 METAVIR) were prospectively evaluated to investigate the influence of isolated HBcAb positivity on the risk of occurrence of advanced liver fibrosis (defined at the time of the first of 2 consecutive values of FIB-4 >3.25, corresponding to F3-F4 METAVIR) after the date of their first available HBV serologic test (baseline and time 0 for the analysis). Patients who were never tested for HBV or already showed an FIB-4 >3.25 at baseline were excluded. Five groups of PWH patients were defined according to the results of their HBV and HCV serology tests at baseline: (A) HCV-/HBsAg-/HBcAb-; (B) HCV+/HBsAg-/HBcAb-; (C) HBcAb+/HCV-/HBsAg-; (D) HCV+/HBcAb+/HBsAg-; (E) HBsAg+/HBcAb+/HCV-. There were an additional 62 patients with the HBsAg+/HBcAb+/HCV+ profile who were excluded from this analysis.
Statistical Analysis
The characteristics of the study population were described and compared across the 5 exposure groups with the Kruskal-Wallis or Pearson chi-square test as appropriate. Standard survival analysis of the time to advanced liver fibrosis by means of Kaplan-Meier curves and Cox regression models with time-fixed covariates measured at baseline was performed. The following factors were included as covariates in the model and were identified as possible confounders of the association between the exposure of interest and the risk of advanced liver fibrosis: age, mode of HIV transmission, and nation of birth. In a separate model, we further adjusted for alcohol consumption, which was determined by physician interviews at study enrollment and at subsequent clinical visits (at least every 6 months) during follow-up [14]. The model assumptions have been described by means of a direct acyclic graph (DAG), which was built using DAGitty, version 2.3 (http://www.daggity.net/). With this DAG, controlling for age, mode of HIV transmission, and nation of birth was sufficient to block all the backdoor pathways from exposure to outcome. We have performed a sensitivity analysis with an alternative end point using the threshold of 1.45 instead of 3.25 for FIB-4 elevation (results are shown in Supplementary Table 1 and Supplementary Figure 1).
To evaluate whether the risk of liver fibrosis in group HBcAb+/HCV-/HBsAg- (C) varied according to the type of treatment used at the time of the serology test, we performed an additional survival analysis using a weighted marginal structural Cox regression model. The simulated intervention was treatment with tenofovir disoproxil fumarate/tenofovir alafenamide (TDF/TAF)– or lamivudine (LMV)-based regimens vs TDF/TAF- or LMV-sparing regimens. To increase statistical power, we evaluated the end points of FIB-4 elevation >3.25 (using a single value above the threshold) and >1.45 (2 consecutive values above this lower threshold). The set of variables included to construct the weights were the time-fixed factors described above (age, mode of HIV transmission, and nation of birth) as well as the most recent HIV viremia and CD4 cell count values. Indeed, in this analysis of the association between anti-HBV treatment and outcome, HIV viremia and CD4 cell count are time-varying confounders affected by prior treatment that could not be correctly controlled for in a standard unweighted Cox regression model.
Patients Consent Statement
The ICONA study has been approved by the institutional review boards of all the participating centers. Data are collected prospectively from the date of entry in the cohort until the last available follow-up for all patients who agree to participate and sign consent forms, in accordance with the ethical standards of the Committee on Human Experimentation and the Helsinki Declaration. Demographic, clinical, and laboratory data and information on therapies are retrospectively collected and recorded in anonymous form.
RESULTS
Overall Description of the Study Population
Among the 6966 patients with >1 serologic HBV test result included in the study (Table 1), 1242 (23.6) were female. Patients acquired HIV predominantly through sexual contacts (42% homosexual and 41.9% heterosexual). Patients with a history of injecting drug use accounted for 9.6%. The median age of the study population (interquartile range [IQR]) was 38 (31–45) years. Interestingly, almost 19% of patients were of foreign nationality. Regarding participants’ viroimmunological status at baseline, patients had a median viral load of HIV (IQR) of 4.32 (2.88–5.00) log10 cp/mL and a median CD4 cell count (IQR) of 429/mm3 (258–618/mm3). The CD4 cell count nadir at baseline (IQR) was 355 (190–531) cell/mm3. Six hundred eighty-one patients (9.8%) had an AIDS-defining diagnosis before baseline, while 1169 (18.9%) showed a baseline CD4 cell count ≤200 cells/mm3.
Table 1.
Main Characteristics of HIV-Infected Patients by Baseline HBV and HCV Serology Group—FIB-4 Analysis
| Characteristics | HBV/HCV Serology | ||||||
|---|---|---|---|---|---|---|---|
| HCV/HBsAg-/HBcAb | HCV+/HBsAg-/HBcAb- | HCV-/HBsAg-/HBcAb+ | HCV+/HBsAg-/HBcAb+ | HCV-/HBsAg+/HBcAb+ | P Valuea | Total | |
| n = 5264 | n = 490 | n = 533 | n = 312 | n = 367 | n = 6966 | ||
| Gender, No. (%) | <.001 | ||||||
| Female | 1242 (23.6) | 188 (38.4) | 121 (22.7) | 60 (19.2) | 76 (20.7) | 1687 (24.2) | |
| Mode of HIV transmission, No. (%) | <.001 | ||||||
| Injecting drug use | 109 (2.1) | 281 (57.5) | 18 (3.4) | 244 (78.7) | 13 (3.6) | 665 (9.6) | |
| Homosexual contacts | 2434 (46.5) | 80 (16.4) | 219 (41.5) | 27 (8.7) | 156 (43.0) | 2916 (42.1) | |
| Heterosexual contacts | 2340 (44.5) | 110 (22.4) | 258 (48.4) | 34 (10.9) | 174 (47.4) | 2916 (41.9) | |
| Other/unknown | 354 (6.8) | 18 (3.7) | 33 (6.3) | 5 (1.6) | 20 (5.5) | 430 (6.2) | |
| Nationality, No. (%) | <.001 | ||||||
| Not Italian | 955 (18.1) | 40 (8.2) | 184 (34.5) | 24 (7.7) | 112 (30.5) | 1315 (18.9) | |
| AIDS diagnosis, No. (%) | <.001 | ||||||
| Yes | 455 (8.6) | 55 (11.2) | 72 (13.5) | 45 (14.4) | 54 (14.7) | 681 (9.8) | |
| Calendar year of baseline | <.001 | ||||||
| Median (IQR) | 2012 (2009–2015) | 2009 (2003–2013) | 2012 (2008–2015) | 2005 (2003–2011) | 2012 (200–2016) | 2012 (2008–2015) | |
| Age, y | <.001 | ||||||
| Median (IQR) | 37 (30–44) | 39 (33–45) | 42 (35–50) | 41 (36–46) | 39 (33–46) | 38 (31–45) | |
| CD4 count, cells/mm3 | <.001 | ||||||
| Median (IQR) | 432 (263–618) | 446 (295–636) | 385 (200–563) | 435 (272–640) | 423 (223–642) | 429 (258–618) | |
| CD4 count nadir, cells/mm3 | <.001 | ||||||
| Median (IQR) | 368 (207–544) | 347 (170–500) | 293 (131–460) | 291 (172–501) | 323 (144–510) | 355 (190–531) | |
| CD8 count, cells/mm3 | .205 | ||||||
| Median (IQR) | 877 (618–1230) | 825 (641–1206) | 870 (599–1251) | 952 (685–1357) | 830 (626–1188) | 874 (624–1229) | |
| HIV-RNA, log10 copies/mL | <.001 | ||||||
| Median (range) | 4.38 (0.00–8.00) | 3.90 (0.00–7.04) | 4.30 (0.00–7.00) | 3.66 (0.00–6.36) | 4.36 (0.00–7.00) | 4.32 (0.00–8.00) | |
| Median (IQR) | 4.38 (3.18–5.03) | 3.90 (1.91–4.82) | 4.30 (2.61–5.06) | 3.66 (1.95–4.69) | 4.36 (1.90–4.96) | .027 | 4.32 (2.88–5.00) |
| CD4 count, No. (%) | <.001 | ||||||
| ≤200 cells/mm3 | 861 (18.6) | 69 (15.3) | 120 (25.2) | 44 (14.8) | 75 (23.6) | 1169 (18.9) | |
| Time from HIV diagnosis to date of HBV serology, mo | <.001 | ||||||
| Median (IQR) | 2 (0, 23) | 50 (2, 130) | 2 (0, 35) | 106 (18, 190) | 2 (0, 32) | 3 (0, 35) | |
| Antivirals started, No. (%) | |||||||
| Zidovudine | 321 (6.1) | 71 (14.5) | 47 (8.8) | 58 (18.6) | 29 (7.9) | 526 (7.6) | |
| Lamivudine | 679 (12.9) | 116 (23.7) | 90 (16.9) | 78 (25.0) | 59 (16.1) | 1022 (14.7) | |
| Abacavir | 291 (5.5) | 30 (6.1) | 31 (5.8) | 13 (4.2) | 15 (4.1) | 380 (5.5) | |
| Tenofovir | 1481 (28.1) | 100 (20.4) | 179 (33.6) | 55 (17.6) | 124 (33.8) | 1939 (27.8) | |
| Emtricitabine | 1515 (28.8) | 85 (17.3) | 178 (33.4) | 49 (15.7) | 123 (33.5) | 1950 (28.0) | |
| TAF | 92 (1.7) | 3 (0.6) | 7 (1.3) | 2 (0.6) | 8 (2.2) | 112 (1.6) | |
| Rilpivirine | 177 (3.4) | 7 (1.4) | 15 (2.8) | 3 (1.0) | 11 (3.0) | 213 (3.1) | |
| Stribild | 138 (2.6) | 4 (0.8) | 25 (4.7) | 5 (1.6) | 19 (5.2) | 191 (2.7) | |
| Triumeq | 94 (1.8) | 4 (0.8) | 8 (1.5) | 1 (0.3) | 8 (2.2) | 115 (1.7) | |
| Genvoya | 49 (0.9) | 2 (0.4) | 4 (0.8) | 1 (0.3) | 3 (0.8) | 59 (0.8) | |
| Dolutegravir | 269 (5.1) | 12 (2.4) | 24 (4.5) | 4 (1.3) | 31 (8.4) | 340 (4.9) | |
| Elvitegravir | 187 (3.6) | 6 (1.2) | 29 (5.4) | 6 (1.9) | 21 (5.7) | 249 (3.6) | |
| Raltegravir | 227 (4.3) | 13 (2.7) | 19 (3.6) | 5 (1.6) | 19 (5.2) | 283 (4.1) | |
| Follow-up time, mo | <.001 | ||||||
| Median (IQR) | 40 (13–75) | 48 (16–100) | 35 (13–75) | 49 (14–121) | 36 (9–75) | 40 (13–78) | |
| Alcohol use, No. (%) | .003 | ||||||
| None | 1927 (36.6) | 160 (32.7) | 203 (38.1) | 106 (34.0) | 131 (35.7) | 2527 (36.3) | |
| Moderate | 1265 (24.0) | 112 (22.9) | 108 (20.3) | 67 (21.5) | 64 (17.4) | 1616 (23.2) | |
| Hazardous | 336 (6.4) | 35 (7.1) | 36 (6.8) | 35 (11.2) | 25 (6.8) | 467 (6.7) | |
| Unknown | 1736 (33.0) | 183 (37.3) | 186 (34.9) | 104 (33.3) | 147 (40.1) | 2356 (33.8) | |
Abbreviations: HBcAb, anti-HBc; HBsAg, HBs antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; TAF, tenofovir alafenamide.
aChi-square or Kruskal-Wallis test.
Study Population Stratified by Exposure Group
Table 1 shows the number and characteristics of the study population stratified by baseline exposure group: 5264 patients (61%) were HIV monoinfected (group A); 490 (5.7%) were HIV+ HCV+/HBsAg-/HBcAb coinfected (group B); 533 (7.6%) were HIV+ HBcAb+/HCV-/HBsAg- (group C); 312 (4.4%) were HIV+ HCV+/HBcAb+/HBsAg- (group D); and 367 (4%) were HIV+ HBsAg+/HBcAb+/HCV- (group E) patients. Overall, HBcAb+/HCV-/HBsAg- (group C), HCV+/HBsAg-/HBcAb- (group B), HCV+/HBcAb+/HBsAg- (group D), and HBsAg+/HBcAb+/HCV- coinfected patients (group E) had a significantly higher prevalence of AIDS diagnosis at baseline than HIV-monoinfected patients (group C 72 [13.5%], group B 55 [11%], group D 45 [14.4%], and group E 54 [15%] vs group A 455 [9%]; P < .001). Patients with previous HBV infection, with or without hepatitis C antibody (HCVAb) positivity (groups D and C, respectively), were older than those in the other groups (group C median age [IQR], 42 [35–50] years; group D median age [IQR], 41 [36–46] years; vs group A median age [IQR], 37 [30–44] years; group B median age [IQR], 39 [33–45] years; and group E median age [IQR], 39 [33–46] years; P < .0001). Among HCV-positive patients, those who were HBcAb negative or positive (groups B and D, respectively) were more frequently injecting drug users (group B 281 [57.5%] and group D 244 [78.7%] vs group A 109 [2.1%], group C 18 [3.4%], and group E 13 [3.6%]; P < .001) and had a longer follow-up time than other populations (group B median [IQR], 48 [16–100] months; and group D median [IQR], 49 [14–121] months; vs group A median [IQR], 40 [13–75] months; group C median [IQR], 35 [13–75] months; and group E median [IQR], 36 [9–75] months; P < .001). HBsAg+/HBcAb+/HCV- and HBcAb+/HCV-/HBsAg- patients (groups E and C) were more frequently of foreign origin (P < .001). Interestingly, HBsAg+/HBcAb+/HCV- patients (group E) and HBcAb+/HCV-/HBsAg- patients (group C) had lower median CD4 counts (group C median [IQR], 385 [200–563]; group E median [IQR], 423 [223–642]; vs group A median [IQR], 432 [263–618]; group B median [IQR], 446 [295–636]; and group D median [IQR], 435 [272–640]; P < .0001) and a more frequent CD4 cell baseline value <200 cells/mm3 than patients in the other groups (group C 120 [25.2%] and group E 75 [23.6%] vs group A 861 [18.6%], group B 69 [15.3%], and group D 44 [14.8%]; P < .001).
Liver Fibrosis Evolution During Follow-up
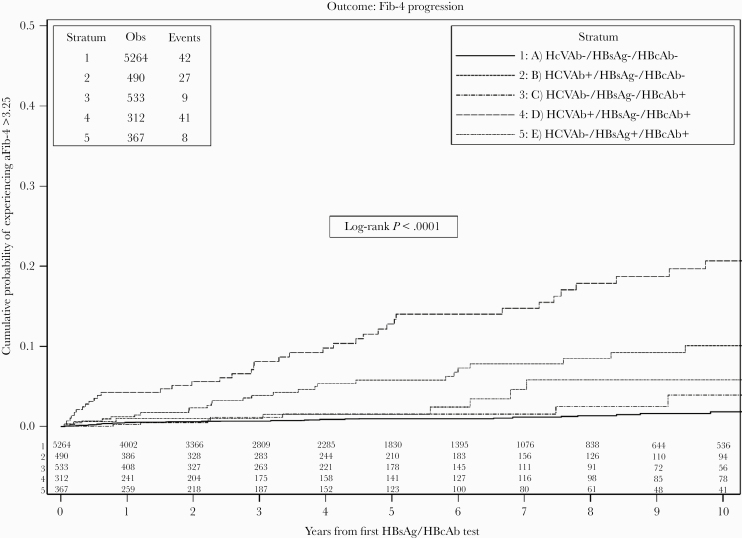
Overall, 180 patients developed an FIB-4 >3.25 during the 10-year follow-up period, and the risks calculated according to the exposure group were as follows: group A 42/5264 (0.8%), group B 27/490 (5.5%), group C 9/533 (1.6%), group D 41/312 (13.1%), and group E 8/367 (2.1%). The unweighted Kaplan-Meier estimates of the probability of experiencing an FIB-4 >3.25 at 3, 7, and 10 years from baseline are reported in Figure 1. When compared with HIV-monoinfected patients, the probability of developing an FIB-4 >3.25 at 3, 7, and 10 years was higher in groups of HCV+/HBsAg-/HBcAb- or HCV+/HBcAb+/HBsAg- patients (groups B and D; 3-, 7-, and 10-year percentages of FIB-4 >3.5: group B, 4.2%; 95% CI, 2.1%–6.4%; 8.5%, 95% CI, 5.0%–11.9%; and 11.1%; 95% CI, 6.6%–15.5%; group D, 7.6%; 95% CI, 4.3%–10.9%; 15.5%; 95% CI, 10.3%–20.6%; 20.7%; 95% CI, 14.4%–27.0%), with higher values in group D.
Figure 1.
KM plot of time to a FIB-4 >3.45 (confirmed value) stratified by HBV serology status. Abbreviations: FIB-4, Fibrosis-4; HBcAb, anti-HBc; HBsAg, HBs antigen; HCVAb, hepatitis C antibody; KM, Kaplan-Meier.
From fitting a Cox regression model after controlling for age, risk factor for HIV transmission, country of origin at baseline, and alcohol consumption (Table 2), still using HIV monoinfection as the comparator group, evidence for a significantly higher risk of progression to FIB-4 >3.25 was found in group B (HCV+; adjusted HR, 3.88; 95% CI, 2.13–7.08; P < .001), in group D (HCV+/HBcAb+; adjusted HR, 7.20; 95% CI, 3.80–13.64; P < .001), and in group E (HBsAg+; adjusted HR, 2.48; 95% CI, 1.16–5.32; P = .008, respectively). In the unadjusted analysis, a higher risk of progression to FIB-4 >3.25 was also shown for group C (HBcAb+; HR, 2.13; 95% CI, 1.04–4.37; P = .040); however, the association was largely attenuated after controlling for age, risk of HIV transmission, nationality, and alcohol consumption (adjusted HR, 1.68; 95% CI, 0.81–3.49; P = .162).
Table 2.
Hazard Ratios of FIB-4 Elevation >3.25 From Fitting a Cox Regression Model
| Exposure Group | Unadjusted HR (95% CI) | P Value | Adjusteda HR (95% CI) |
P Value |
|---|---|---|---|---|
| Group A (HCVAb-/HBsAg-/HBcAb-) | 1 | 1 | ||
| Group B (HCVAb+/HBsAg-/HBcAb-) | 5.82 (3.59–9.46) | <.0001 | 3.88 (2.13–7.08) | <.001 |
| Group C (HCVAb-/HBsAg-/HBcAb+) | 2.13 (1.04–4.37) | .040 | 1.68 (0.81–3.49) | .162 |
| Group D (HCVAb+/HBsAg-/HBcAb+) | 13.12 (8.51–20.24) | <.001 | 7.20 (3.80–13.64) | <.001 |
| Group E (HCVAb-/HBsAg+/HBcAb+) | 2.79 (1.31–5.94) | .008 | 2.48 (1.16–5.32) | .019 |
Abbreviations: HBcAb, anti-HBc; HBsAg, HBs antigen; HCVAb, hepatitis C antibody; HR, hazard ratio.
aAdjusted for age, mode of HIV transmission, nation of birth, and level of alcohol consumption.
Risk of Liver Fibrosis According to the Presence or Absence of Anti-HBV Drugs in the HAART Composition at Baseline
Given the potential effect of anti-HBV drugs (LMV, TDF/TAF) in preventing liver fibrosis, we simulated a trial comparing LMV-, TDF-, or TAF-based with LMV-, TDF-, or TAF-sparing regimens in the subset of participants belonging to group C (HBcAb+/HCV-/HBsAg-) (Table 3A and 3B). As expected, because of confounding by indication, participants treated with TDF/TAF- or LMV-based regimens were found to be at higher risk of developing liver fibrosis. This bias was, however, attenuated, especially for the end point of elevation >1.45 in the weighted analysis, suggesting a 15% reduction in risk of moderate elevation in people treated with anti-HBV drugs (weighted HR, 0.85; 95% CI, 0.32–2.30; P = .76). Overall, although the analysis is likely to be underpowered, there was little evidence to support that treating patients with HAART-containing anti-HBV drugs had a large impact on risk of fibrosis in this group.
Table 3.
A, Relative Hazards of Developing a FIB-4 >3.25 From Fitting a Weighted Cox Regression Model
| Relative Hazards (95% CI) of FIB-4 >3.25 | ||||
|---|---|---|---|---|
| HAART at Baseline | Undjusted | Adjusteda | ||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| TDF/TAF- or LMV-sparing | 1.00 | 1.00 | ||
| TDF/TAF- or LMV-based | 1.26 (0.65–2.44) | .501 | 1.13 (0.48–2.64) | .775 |
B, Relative Hazards of Developing a FIB-4 >1.45 From Fitting a Weighted Cox Regression Model
| Relative Hazards (95% CI) of FIB-4 >1.45 | ||||
|---|---|---|---|---|
| HAART at Baseline | Undjusted | Adjusteda | ||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| TDF/TAF- or LMV-sparing | 1.00 | 1.00 | ||
| TDF/TAF- or LMV-based | 1.38 (0.77–2.47) | .279 | 0.85 (0.32–2.30) | .756 |
Abbreviations: HAART, highly active antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; LMV, lamivudine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
aAdjusted for age, gender, mode of HIV transmission, nation of birth, current CD4 count, and HIV-RNA using inverse probability of weighting.
DISCUSSION
In the ICONA cohort, a high risk of FIB-4 >3.25 liver fibrosis progression was demonstrated in all groups of HIV/HBV- and HCV-coinfected patients; however, higher values were detected in the group of HCV+/HBcAb+/HBsAg- subjects (HR, 7.20; 95% CI, 3.80–13.64), followed by the HCV+/HBsAg-/HBcAb- group (HR, 3.88; 95% CI, 2.13–7.08), than in the other groups. The use of anti-HBV active drugs (LMV, TDF, or TAF) in HAART compositions did not seem to significantly influence liver fibrosis evolution in the subset of HBcAb-positive participants.
ESLD, as the result of severe liver fibrosis, is the leading non-AIDS cause of death among PWH [13]. Several clinical studies [14–16] have reported that HIV alone increases the evolution of hepatic fibrosis in monoinfected patients, and the persistence of HIV viremia, even at low levels, has been associated with an increase in inflammation and consequent induction of hepatocyte apoptosis [17–19]. In the course of HIV infection, a series of contributing factors have been associated with hepatic injury (such as drugs and alcohol) [20, 21]; however, HCV and HBV chronic hepatitis have a central role. In particular, PWH with active HBV and HCV infections are at a 3.73 and 6.66 times higher risk, respectively, of liver-related death [22].
Little is known about the influence of HBcAb positivity on hepatic liver damage in the course of HIV infection. Commonly, HBcAb is the sign of a past/resolved infection; however, it could be the only marker of OBI, a condition characterized by the persistence of intrahepatic cccHBV-DNA and the occasional reappearance of HBV-DNA viremia [22]. Liver-related outcomes among HIV/HCV-infected patients with CHB have been reported by a number of studies [23–25], and it is known that liver-related deaths occur more frequently among patients with triple HIV/HBV/HCV chronic infections; however, very little is known about HIV/HCV-infected patients with signs of a previous HBV infection.
Bhattacharya and colleagues, in a large cross-sectional study on a cohort of 44 180 HIV-infected American veterans (Veterans Ageing Cohort Study–Virtual Cohort [VACS-VC]), found that the isolated anti-HBc pattern was associated with advanced hepatic fibrosis in HIV/HCV-coinfected patients but not in HIV infection without concomitant HCV infection [26]. This result is consistent with our findings, and occasional HBV viremia could contribute to the increase in liver damage in HCV-coinfected subjects. As early as 2009, Morsica and co-authors, analyzing the data of coinfected patients of the ICONA cohort, found that 15% of HIV/HbcAb-positive subjects had detectable HBV-DNA in their plasma, and 22% of these had no active drugs against HBV in their HAART composition [27]. The study concluded that it was advisable to monitor HBV-DNA blood levels in this category of subjects to avoid, over the years, the occurrence of liver damage as a result of active HBV replication. Poor control of the progression of liver damage despite the use of anti-HBV active drugs was also reported by several French studies on HIV patients with active chronic HBV infection [28–30]. Moreover, we did not find any significant protective effect on fibrosis evolution in the group of HCV+/HBcAb+/HBsAg- patients receiving HAART-containing anti-HBV drugs compared with those receiving HAART without anti-HBV drugs.
In our analysis, a lower risk of severe hepatic fibrosis was demonstrated in the population of HIV/HbcAb-positive patients, which also seems to indicate a limited benefit of the inclusion of LMV, TDF, or TAF treatment in HAART.
Before drawing final conclusions, a number of limitations need to be mentioned: first, the lack of HBV-DNA viremia levels, which limited the possibility of assessing the impact of true OBI rather than potential OBI; second, the characterization of HCV coinfection took place only via the serological detection of antibodies against HCV rather than HCV viremia data. Moreover, information on anti-HCV treatments (IFN-based or direct-acting antiviral–based) during follow-up was lacking; third, although the alcohol abuse data were collected in the ICONA database via physician interviews at study enrollment and subsequent clinical visits, patients underreporting use (due to social desirability and fear of the impact on antiretroviral therapy initiation) is possible, so residual confounding bias due to misclassification of alcohol consumption is possible. Also, participants have been compared on the basis of their serology status and antiretroviral treatment received at the time of their first HBV serology test, ignoring the fact that participants’ status and treatment might have changed over follow-up.
In conclusion, in our cohort of HIV-infected patients seen for care in Italy, coinfection with hepatitis viruses was associated with an increased risk of severe liver fibrosis development compared with HIV monoinfection. The association was particularly strong in the HIV/HCV-coinfected group with signs of resolved HBV infection (HBcAb positive). Our data suggest that anti-HBc positivity contributes to liver damage in HCV+/HBcAb+/HBsAg- HIV-positive subjects. These findings reinforce the need for careful monitoring for signs of previous HBV infection in HIV/HCV-positive patients and suggest that underlying active HBV replication (OBI) may contribute to liver damage in these patients.
Supplementary Material
Acknowledgments
Icona Fundation Study Group. Board of Directors: A. d’Arminio Monforte (President), A. Antinori (Vice-President), M. Andreoni, A. Castagna, F. Castelli, R. Cauda, G. Di Perri, M. Galli, R. Iardino, G. Ippolito, A. Lazzarin, G.C. Marchetti, G. Rezza, F. von Schloesser, P. Viale. Scientific Secretary: A. d’Arminio Monforte, A. Antinori, A. Castagna, F. Ceccherini-Silberstein, A. Cozzi-Lepri, E. Girardi, A. Gori, S. Lo Caputo, F. Maggiolo, C. Mussini, M. Puoti, C.F. Perno. Steering Committee: A. Antinori, F. Bai, A. Bandera, S. Bonora, M. Borderi, A. Calcagno, M.R. Capobianchi, A. Castagna, F. Ceccherini-Silberstein, S. Cicalini, A. Cingolani, P. Cinque, A. Cozzi-Lepri, A. d’Arminio Monforte, A. Di Biagio, R. Gagliardini, E. Girardi, N. Gianotti, A. Gori, G. Guaraldi, G. Lapadula, M. Lichtner, A. Lai, S. Lo Caputo, G. Madeddu, F. Maggiolo, G. Marchetti, E. Merlini, C. Mussini, S. Nozza, C.F. Perno, S. Piconi, C. Pinnetti, M. Puoti, E. Quiros Roldan, R. Rossotti, S. Rusconi, M.M. Santoro, A. Saracino, L. Sarmati, V. Spagnuolo, V. Svicher, L. Taramasso. Statistical and Monitoring Team: A. Cozzi-Lepri, I. Fanti, L. Galli, P. Lorenzini, A. Rodanó, M. Macchia, A. Tavelli. Community Advisory Board: A. Bove, A. Camposeragna, M. Errico, M. Manfredini, A. Perziano, V. Calvino. Biological Bank INMI: F. Carletti, S. Carrara, A. Di Caro, S. Graziano, F. Petroni, G. Prota, S. Truffa. Participating Physicians and Centers: Italy: A. Giacometti, A. Costantini, V. Barocci (Ancona); G. Angarano, L. Monno, E. Milano (Bari); F. Maggiolo, C. Suardi (Bergamo); P. Viale, V. Donati, G. Verucchi (Bologna); F. Castelnuovo, C. Minardi, E. Quiros Roldan (Brescia); B. Menzaghi, C. Abeli (Busto Arsizio); L. Chessa, F. Pes (Cagliarti); B. Cacopardo, B. Celesia (Catania); J. Vecchiet, K. Falasca (Chieti); A. Pan, S. Lorenzotti (Cremona); L. Sighinolfi, D. Segala (Ferrara); P. Blanc, F. Vichi (Firenze); G. Cassola, M. Bassetti, A. Alessandrini, N. Bobbio, G. Mazzarello (Genova); M. Lichtner, L. Fondaco (Latina); P. Bonfanti, C. Molteni (Lecco); A. Chiodera, P. Milini (Macerata); G. Nunnari, G. Pellicanò (Messina); A. d’Arminio Monforte, M. Galli, A. Lazzarin, G. Rizzardini, M. Puoti, A. Castagna, E.S. Cannizzo, M.C. Moioli, R. Piolini, D. Bernacchia, A. Poli, C. Tincati (Milano); C. Mussini, C. Puzzolante (Modena); C. Migliorino, G. Lapadula (Monza); V. Sangiovanni, G. Borgia, V. Esposito, G. Di Flumeri, I. Gentile, V. Rizzo (Napoli); A.M. Cattelan, S. Marinello (Padova); A. Cascio, M. Trizzino (Palermo); D. Francisci, E. Schiaroli (Perugia); G. Parruti, F. Sozio (Pescara); C. Lazzaretti, R. Corsini (Reggio Emilia); M. Andreoni, A. Antinori, R. Cauda, A. Cristaudo, V. Vullo, R. Acinapura, S. Lamonica, M. Capozzi, A. Mondi, A. Cingolani, M. Rivano Capparuccia, G. Iaiani, A. Latini, G. Onnelli, M.M. Plazzi, G. De Girolamo, A. Vergori (Roma); M. Cecchetto, F. Viviani (Rovigo); G. Madeddu, A. De Vito (Sassari); B. Rossetti, F. Montagnani (Siena); A. Franco, R. Fontana Del Vecchio (Siracusa); C. Di Giuli (Terni); P. Caramello, G. Di Perri, S. Bonora, G.C. Orofino, M. Sciandra (Torino); A. Londero (Udine); V. Manfrin, G. Battagin (Vicenza); G. Starnini, A. Ialungo (Viterbo).
Financial support. The ICONA Foundation is supported by unrestricted grants from Gilead Sciences, Janssen, MSD, and ViiV Healthcare. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. Vincenzo Malagnino reports a grant from Gilead and payment for lectures and consulting fron Janssen-Cilag, outside the submitted work; Carlotta Cerva has nothing to disclose; Antonella Cingolani has nothing to disclose; Francesca Ceccherini-Silberstein has nothing to disclose; Alessandra Vergori reports grants from Gilead Sciences, grants and personal fees from Janssen-Cilag, and personal fees from MSD, outside the submitted work; Gianluca Cuomo has nothing to disclose; Carlo Federico Perno has nothing to disclose; Massimo Puoti has nothing to disclose; Antonella d’Arminio Monforte has nothing to disclose; Alessandro Cozzi Lepri has nothing to disclose; Massimo Andreoni reports personal fees from Merck, personal fees from Gilead, personal fees from Abbvie, personal fees from Angelini SpA, and personal fees from Janssen-Cilag, outside the submitted work; Loredana Sarmati reports personal fees from Merck, personal fees from Gilead, personal fees from Abbvie, and personal fees from Angelini SpA, outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. The results of the present article were partially presented at CROI 2019, 4–7 March 2019, Seattle (WA, USA), as a poster presentation (Abstract #0607).
Contributor Information
ICONA Foundation Study Group:
A d’Arminio Monforte, A Antinori, M Andreoni, A Castagna, F Castelli, R Cauda, G Di Perri, M Galli, R Iardino, G Ippolito, A Lazzarin, G C Marchetti, G Rezza, F von Schloesser, P Viale, A d’Arminio Monforte, A Antinori, A Castagna, F Ceccherini-Silberstein, A Cozzi-Lepri, E Girardi, A Gori, S Lo Caputo, F Maggiolo, C Mussini, M Puoti, C F Perno, A Antinori, F Bai, A Bandera, S Bonora, M Borderi, A Calcagno, M R Capobianchi, A Castagna, F Ceccherini-Silberstein, S Cicalini, A Cingolani, P Cinque, A Cozzi-Lepri, A d’Arminio Monforte, A Di Biagio, R Gagliardini, E Girardi, N Gianotti, A Gori, G Guaraldi, G Lapadula, M Lichtner, A Lai, S Lo Caputo, G Madeddu, F Maggiolo, G Marchetti, E Merlini, C Mussini, S Nozza, C F Perno, S Piconi, C Pinnetti, M Puoti, E Quiros Roldan, R Rossotti, S Rusconi, M M Santoro, A Saracino, L Sarmati, V Spagnuolo, V Svicher, L Taramasso, A Cozzi-Lepri, I Fanti, L Galli, P Lorenzini, A Rodanó, M Macchia, A Tavelli, A Bove, A Camposeragna, M Errico, M Manfredini, A Perziano, V Calvino, F Carletti, S Carrara, A Di Caro, S Graziano, F Petroni, G Prota, S Truffa, A Giacometti, A Costantini, V Barocci, G Angarano, L Monno, E Milano, F Maggiolo, C Suardi, P Viale, V Donati, G Verucchi, F Castelnuovo, C Minardi, E Quiros Roldan, B Menzaghi, C Abeli, L Chessa, F Pes, B Cacopardo, B Celesia, J Vecchiet, K Falasca, A Pan, S Lorenzotti, L Sighinolfi, D Segala, P Blanc, F Vichi, G Cassola, M Bassetti, A Alessandrini, N Bobbio, G Mazzarello, M Lichtner, L Fondaco, P Bonfanti, C Molteni, A Chiodera, P Milini, G Nunnari, G Pellicanò, A d’Arminio Monforte, M Galli, A Lazzarin, G Rizzardini, M Puoti, A Castagna, E S Cannizzo, M C Moioli, R Piolini, D Bernacchia, A Poli, C Tincati, C Mussini, C Puzzolante, C Migliorino, G Lapadula, V Sangiovanni, G Borgia, V Esposito, G Di Flumeri, I Gentile, V Rizzo, A M Cattelan, S Marinello, A Cascio, M Trizzino, D Francisci, E Schiaroli, G Parruti, F Sozio, C Lazzaretti, R Corsini, M Andreoni, A Antinori, R Cauda, A Cristaudo, V Vullo, R Acinapura, S Lamonica, M Capozzi, A Mondi, A Cingolani, M Rivano Capparuccia, G Iaiani, A Latini, G Onnelli, M M Plazzi, G De Girolamo, A Vergori, M Cecchetto, F Viviani, G Madeddu, A De Vito, B Rossetti, F Montagnani, A Franco, R Fontana Del Vecchio, C Di Giuli, P Caramello, G Di Perri, S Bonora, G C Orofino, M Sciandra, A Londero, V Manfrin, G Battagin, G Starnini, and A Ialungo
References
- 1. van Welzen BJ, Smit C, Boyd A, et al. Decreased all-cause and liver-related mortality risk in HIV/hepatitis B virus coinfection coinciding with the introduction of tenofovir-containing combination antiretroviral therapy. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lemoine M, Thursz MR. Battlefield against hepatitis B infection and HCC in Africa. J Hepatol 2017; 66:645–54. [DOI] [PubMed] [Google Scholar]
- 3. Thornton AC, Jose S, Bhagani S, et al. ; UK Collaborative HIV cohort (UK CHIC) steering committee Hepatitis B, hepatitis C, and mortality among HIV-positive individuals. AIDS 2017; 31:2525–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh KP, Crane M, Audsley J, et al. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS 2017; 31:2035–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol 2008; 49:652–7. [DOI] [PubMed] [Google Scholar]
- 6. Shi Y, Wu YH, Wu W, et al. Association between occult hepatitis B infection and the risk of hepatocellular carcinoma: a meta-analysis. Liver Int 2012; 32:231–40. [DOI] [PubMed] [Google Scholar]
- 7. Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat 2014; 21:153–62. [DOI] [PubMed] [Google Scholar]
- 8. Cohen Stuart JW, Velema M, Schuurman R, et al. Occult hepatitis B in persons infected with HIV is associated with low CD4 counts and resolves during antiretroviral therapy. J Med Virol 2009; 81:441–5. [DOI] [PubMed] [Google Scholar]
- 9. Ryan K, Anderson M, Gyurova I, et al. High rates of occult hepatitis B virus infection in HIV-positive individuals initiating antiretroviral therapy in Botswana. Open Forum Infect Dis 2017; 4:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Opaleye OO, Oluremi AS, Atiba AB, et al. Occult hepatitis B virus infection among HIV positive patients in Nigeria. J Trop Med 2014; 2014:796121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chadwick D, Doyle T, Ellis S, et al. Occult hepatitis B virus coinfection in HIV-positive African migrants to the UK: a point prevalence study. HIV Med 2014; 15:189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saha D, Pal A, Sarkar N, et al. Occult hepatitis B virus infection in HIV positive patients at a tertiary healthcare unit in eastern India. PLoS One 2017; 12:e0179035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farahani M, Mulinder H, Farahani A, Marlink R. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS 2017; 28:636–50. [DOI] [PubMed] [Google Scholar]
- 14. Kong L, Cardona Maya W, Moreno-Fernandez ME, et al. Low-level HIV infection of hepatocytes. Virol J 2012; 9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kovari H, Ledergerber B, Battegay M, et al. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis B or C virus co-infection. Clin Infect Dis 2010; 50:502–11. [DOI] [PubMed] [Google Scholar]
- 16. Babu CK, Suwansrinon K, Bren GD, et al. HIV induces TRAIL sensitivity in hepatocytes. PLoS One 2009; 4:e4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sacchi P, Cima S, Corbella M, et al. Liver fibrosis, microbial translocation and immune activation markers in HIV and HCV infections and in HIV/HCV co-infection. Dig Liver Dis 2015; 47:218–25. [DOI] [PubMed] [Google Scholar]
- 18. Page EE, Nelson M, Kelleher P. HIV and hepatitis C coinfection: pathogenesis and microbial translocation. Curr Opin HIV AIDS 2011; 6:472–7. [DOI] [PubMed] [Google Scholar]
- 19. Bilal U, Lau B, Lazo M, et al. Interaction between alcohol consumption patterns, antiretroviral therapy type, and liver fibrosis in persons living with HIV. AIDS Patient Care STDS 2016; 30:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pessione F, Degos F, Marcellin P, et al. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology 1998; 27:1717–22. [DOI] [PubMed] [Google Scholar]
- 21. Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166:1632–41. [DOI] [PubMed] [Google Scholar]
- 22. Chang JJ, Mohtashemi N, Bhattacharya D. Significance and management of isolated hepatitis B core antibody (anti-HBc) in HIV and HCV: strategies in the DAA era. Curr HIV/AIDS Rep 2018; 15:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonacini M, Louie S, Bzowej N, Wohl AR. Survival in patients with HIV infection and viral hepatitis B or C: a cohort study. AIDS 2004; 18:2039–45. [DOI] [PubMed] [Google Scholar]
- 24. Arribas JR, González-García JJ, Lorenzo A, et al. Single (B or C), dual (BC or BD) and triple (BCD) viral hepatitis in HIV-infected patients in Madrid, Spain. AIDS 2005; 19:1361–5. [DOI] [PubMed] [Google Scholar]
- 25. Sollima S, Caramma I, Menzaghi B, et al. Chronic coinfection with hepatitis B and hepatitis C viruses in an Italian population of HIV-infected patients. J Acquir Immune Defic Syndr 2007; 44:606–7. [DOI] [PubMed] [Google Scholar]
- 26. Bhattacharya D, Tseng CH, Tate JP, et al. Isolated hepatitis B core antibody is associated with advanced hepatic fibrosis in HIV/HCV infection but not in HIV infection alone. J Acquir Immune Defic Syndr 2016; 72:e14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morsica G, Ancarani F, Bagaglio S, et al. ; HepaICONA and the ICONA Study Groups Occult hepatitis B virus infection in a cohort of HIV-positive patients: correlation with hepatitis C virus coinfection, virological and immunological features. Infection 2009; 37:445–9. [DOI] [PubMed] [Google Scholar]
- 28. Boyd A, Bottero J, Miailhes P, et al. Liver fibrosis regression and progression during controlled hepatitis B virus infection among HIV-HBV patients treated with tenofovir disoproxil fumarate in France: a prospective cohort study. J Int AIDS Soc 2017; 20:21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Malagnino V, Bottero J, Miailhes P, et al. Hepatitis B virus genotype G and liver fibrosis progression in chronic hepatitis B and human immunodeficiency virus coinfection. J Med Virol 2019; 91:630–41. [DOI] [PubMed] [Google Scholar]
- 30. Klein MB, Althoff KN, Jing Y, et al. ; North American AIDS Cohort Collaboration on Research and Design of IeDEA; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA Risk of end-stage liver disease in HIV-viral hepatitis coinfected persons in North America from the early to modern antiretroviral therapy eras. Clin Infect Dis 2016; 63:1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.