Abstract
Background
The global crisis caused by the outbreak of severe acute respiratory syndrome caused by the SARS-CoV-2 virus, better known as COVID-19, brought the need to improve the population's immunity. The foods rich in polysaccharides with immunomodulation properties are among the most highly rated to be used as immune response modulators. Thus, the use of polysaccharides obtained from food offers an innovative strategy to prevent serious side effects of viral infections.
Scope and approach
This review revisits the current studies on the pathophysiology of SARS-CoV-2, its characteristics, target cell interactions, and the possibility of using polysaccharides from functional foods as activators of the immune response. Several natural foods are explored for the possibility of being used to obtain polysaccharides with immunomodulatory potential. And finally, we address expectations for the use of polysaccharides in the development of potential therapies and vaccines.
Key findings and conclusions
The negative consequences of the SARS-CoV-2 pandemic across the world are unprecedented, thousands of lives lost, increasing inequalities, and incalculable economic losses. On the other hand, great scientific advances have been made regarding the understanding of the disease and forms of treatment. Polysaccharides, due to their characteristics, have the potential to be used as potential drugs with the ability to modulate the immune response. In addition, they can be used safely, as they have no toxic effects, are biocompatible and biodegradable. Finally, these biopolymers can still be used in the development of new therapies and vaccines.
Keywords: Functional foods, Polysaccharides, Immunomodulation, COVID-19, Vaccines
Graphical abstract
1. Introduction
At the end of 2019, a new viral outbreak was identified, and after preliminary studies some of its main characteristics were identified, leading researchers to consider the possibility of a new pandemic, especially considering the high rate of infection associated with the high incidence of pneumonia, acute respiratory distress syndrome (ARDS) and death. Clinical and pathophysiological studies carried out in Wuhan province, China, have identified the coronavirus (SARS-CoV-2) as the infectious agent responsible for serious respiratory complications and damage to multiple organs. Although the true origin of the virus is still unknown, it is believed based on recent studies (Andersen, Rambaut, Lipkin, Holmes, & Garry, 2020) that it occurred from zoonotic transmission.
The coronavirus SARS-CoV-2 is transmitted from human to human and has spread with frightening frequency. Currently, the virus is distributed throughout the world, affecting millions of people, causing human, economic and social losses. As reported by the World Health Organization (OMS), severe acute respiratory syndrome (SARS-CoV-2) or COVID-19, has become a global health pandemic, affecting all people, regardless of social class, creed or religion (Tay, Poh, Rénia, MacAry, & Ng, 2020). Daily reports provided by all countries in the world show that the number of infected and dead has grown continuously. Good news during this crisis comes from the considerable number of people healed and the international effort to develop vaccines and new drugs capable of mitigating the side effects of the disease and reducing human losses (Tay et al., 2020).
Worldwide, several research groups are engaged in the search for new drugs and vaccines to combat SARS-CoV-2 and its adverse effects. However, although vaccine development is ideal, it still requires a lot of effort and investment. Recent studies (Long et al., 2020; Weitz et al., 2020; Wen et al., 2020), show that the immune system plays a crucial role in the body's response to viral infection. Therefore, promising new therapeutic interventions should somehow improve the hosts' immune response. The immune system is the focus of some of the therapeutic interventions being developed. In particular, the adaptive immune system, which needs to be, stimulated with immunoregulatory agents or immune system activators. The polysaccharides that are present in some foods have biologically active properties, such as the ability to activate immune functions, inducing the production of antioxidant, anti-inflammatory and immune function regulating substances (Fu, Belwal, Cravotto, & Luo, 2020; Yockey, Lucas, & Iwasaki, 2020).
In the midst of the current crisis caused by the pandemic of COVID-19, the consumption of healthy foods, especially those with functional properties, has become a necessity, mainly as a way to prevent and improve the immune system (Galanakis, 2020). One of the current perspectives is to apply new technologies to obtain biologically active compounds from natural sources and waste (Galanakis, 2018). Technologies such as electro-osmotic dehydration, high hydrostatic pressure, ultrasound-assisted extraction, supercritical fluid technology, and pulsed electric field, among countless others have been applied in the extraction of polyphenols, polysaccharides and various molecules of high commercial value (Zinoviadou et al., 2015). The technologies for extracting added value compounds are one of the most relevant steps in the process of prospecting molecules, so it is important that the reader is up to date with the new trends on this subject. As a result, the following articles are suggested for more information (Galanakis, 2013, 2015).
In addition, it is important to emphasize that food supply has always been a global concern, especially considering the worrying scenarios of global economic slowdown, trade war and obvious obstacles caused by the very peculiarities of the current moment. Considering this scenario, where the consumption of healthy foods is more than necessary, important concerns about food security arise, mainly with the real possibility of transmission of COVID-19 through the food supply chain. Therefore, new security strategies and measures, in addition to detection technologies for SARS-CoV-2 are still a prevalent need. Readers can search for more information on relevant issues related to food security, food supply chain and SARS-CoV-2 detection strategies in articles published in Trends in Food Science & Technology by Rizou, Galanakis, Aldawoud, & Galanakis (2020).
Polysaccharides are complex molecules, composed of monosaccharides, united by glycidic bonds, with different branched grains, composition of monosaccharides, molecular weight and structural conformation. Polysaccharides are present in all living organisms, are part of structural biochemistry and have relevant functions in the cellular environment (Ng et al., 2020). Natural foods such as mushrooms, yeasts, fruits, algae and cereals have polysaccharides in their chemical composition, these biopolymers can be obtained and purified to be used as potent substances in the activation of the immune system, especially the adaptive system, as shown by recent studies (Mohan et al., 2020; Wang, Zhang, et al., 2020; Zhang, Zeng, et al., 2020). Also, these biopolymers have interesting biological properties as an antioxidant activity (Su & Li, 2020; Wu, Luo, Yao, & Yu, 2020; Yuan et al., 2020), anti-inflammatory (de Lacerda Bezerra et al., 2018; Xiong et al., 2017), antiviral (Wang, Wang, et al., 2020), biocompatible, biodegradable and non-toxic (He, Chen, et al., 2020), interesting biological properties, which can be used in innovative therapies. Thus polysaccharides can be used as an activating agent of the immune system, reducing the damage caused by infectious agents such as SARS-CoV-2.
Given the current scenario, the use of foods rich in immunomodulator polysaccharides is a viable option to increase immunity and reduce risks associated with SARS-CoV-2 contamination. Also, understanding the biology resulting from the SARS-CoV-2 host-pathogen will provide relevant information on the treatment and management of the disease. Although the use of polysaccharides as a therapy strategy is not yet a reality, the application as supplements is a rational possibility, based on all scientific evidence on the immunomodulatory potential of these biopolymers (Wang, Zhang, et al., 2020; Zhang, Zeng, et al., 2020).
Here, we review the literature on the pathophysiology of SARS-CoV-2, its characteristics, interactions with target cells, and the possibility of using polysaccharides obtained from functional foods as activators of the immune response. We highlight several natural foods, rich in polysaccharides, making a survey by categories, in order to contextualize the reader about the diversity of food sources with promising potential. However, the focus is on the extracted polysaccharides and not on the food. Therefore, the implications of the specific molecular characteristics of polysaccharides that can be used for promising therapeutic interventions are discussed in detail. In addition, we discussed how polysaccharides produce a crucial adaptive immune response to inform the development of antibodies and in detail the main mechanisms of modulation of the immune system. Finally, to complement the discussion, a contextualized dialogue is opened about the potential of polysaccharides to be applied as efficient and safe platforms in the development of vaccines and potential immunity-boosting therapies.
2. Understanding the disease, its characteristics, and clinical evolution
A severe type of pneumonia caused by the new coronavirus in 2019 in China has become known worldwide as COVID-19. Currently, the disease is expanding worldwide, is highly infectious, lethal, and still poorly comprehended. The world health organization (OMS) recently declared that the outbreak of the new coronavirus is a global health emergency and needs the engagement of government organizations, civil society, and the military, all of which are committed to reducing contamination and mortality rates (Zhu et al., 2020).
Recently, an article published in The Lancet by Zhou, Yu, et al. (2020), showed the main characteristics of COVID-19 pneumonia, through clinical results obtained from patients in the year 2019–2020 in Wuhan, China. The report describes the epidemiological, clinical, laboratory, radiological characteristics, as well as the treatment and clinical results, however, of adult patients only. Common symptoms at the beginning of the disease were fever, cough, and fatigue. Less common symptoms were sputum production, headache, hemoptysis, and diarrhea. In severe cases the disease progressed to inflammation of the lungs, causing pneumonia. The main symptoms in these cases were acute respiratory distress syndrome, acute cardiac injury, and secondary infections that in most cases caused severe pulmonary complications and deaths in several patients.
The SARS-CoV-2 virus has been known to the medical community and infectologist for many years, however, until 2019, there were no reports of infections in humans, until the first cases of the outbreak of COVID-19 were reported in China. It is known that viruses of the coronavirus family can cause disease and illness in both humans and animals. Four types of coronaviruses known as (human coronavirus 229E, NL63, OC43, and HKU1), infect the respiratory tract and cause symptoms considered moderate (Gorbalenya et al., 2020). However, there are three coronaviruses that cause severe respiratory tract complications they are the severe acute respiratory syndrome coronavirus (SARS-CoV), Middle Eastern respiratory syndrome coronavirus (MERS-CoV) and (SARS-CoV-2) (Gorbalenya et al., 2020). Among these, SARS-CoV-2 is a virus belonging to the genus betacoronavirus, with much genetic similarity to SARS-CoV. Also, SARS-CoV-2 has a 98% similarity to the bat coronavirus RaTG13 and the scaly anteater coronavirus (Andersen et al., 2020; Zhou, Yang, et al., 2020).
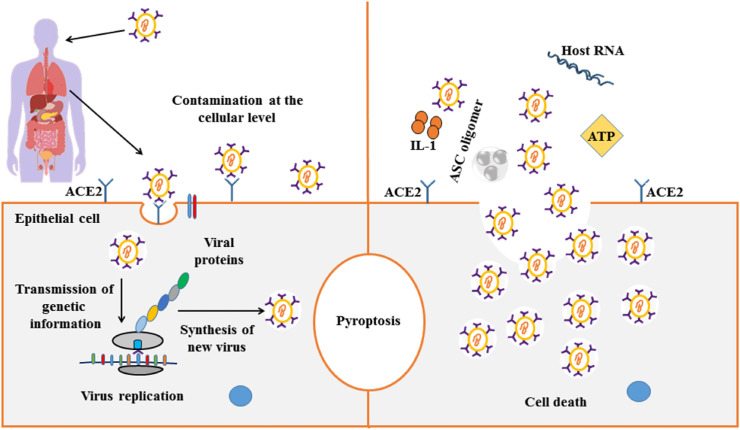
SARS-CoV-2, like other coronaviruses, is transmitted through respiratory droplets or other fluids, however, these other pathways are not proven. The (Fig. 1 ) summarizes the main events that occur during the onset of SARS-CoV-2 infection. After infection, the virus undergoes a 4–5 day incubation period, before the onset of symptoms (Guan et al., 2020). Generally, patients at the time of hospitalization have difficulty breathing, muscle pain, headache, nausea and bloody cough, less common symptoms such as fever and dry cough also occur (Chan et al., 2020; Chen, Wu, et al., 2020). After the incubation period, on average 5–6 days, the viral load of SARS-CoV-2 reaches its peak, interestingly earlier than that of SARS-CoV, which reaches its viral load around 10 days after the start of the symptoms (Pan, Zhang, Yang, Poon, & Wang, 2020). In severe cases, on average 8–9 days after the onset of symptoms, the disease progresses to acute respiratory discount syndrome, which in some cases may lead to multiple failures in the function of the lungs and other organs (Chen, Zhou, et al., 2020).
Fig. 1.
Summary of onset of major events associated with SARS-CoV-2 infection. After contamination by SARS-CoV-2, the virus starts a race for cells that express the angiotensin-converting enzyme 2 of the surface receptors (ACE2) and TMPRSS2. After the virus enters the host cell, the process of viral replication and release of viral proteins begins, causing the host cell to undergo pyroptosis, releasing damage as associated molecular patterns, including ATP, nucleic acids, and ASC oligomers.
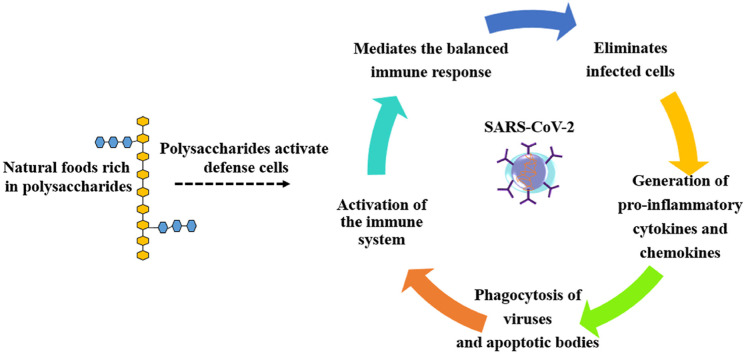
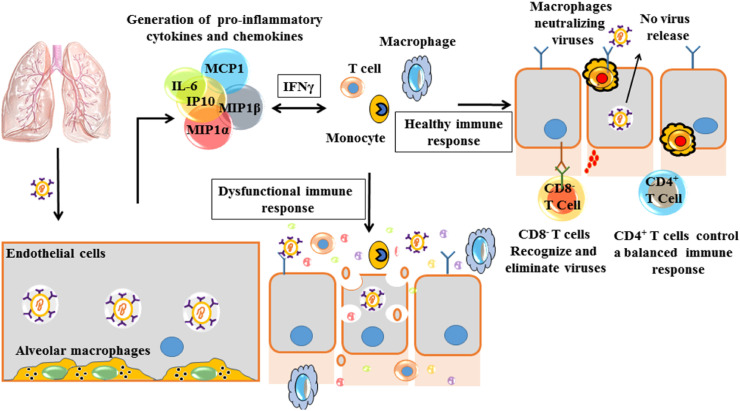
Pathophysiological studies of acute SARS-CoV-2 infection (Ghinai et al., 2020; Yu et al., 2020a; Zhou, Yu, et al., 2020), showed aggressive inflammatory responses, leading to severe airway complications. The (Fig. 2 ) summarizes the main events associated with inflammatory responses, the disease progression for clinical cases of pneumonia and what a balanced immune response would look like. Therefore, in addition to viral contamination, the organism must deal with a strong immune response from the host, accompanied by what experts call the ‘‘storm’’ of pro-inflammatory compounds. The organisms' strong response to infection is accompanied by serious complications to multiple organs. Also, associated with risk factors such as autoimmune diseases, cancer, old age, spinal cord injuries and other morbidities, the clinical condition of patients with SARS-CoV-2 may worsen (He, Fang, et al., 2020; Kujawski et al., 2020; Li, Hu, Yu, & Ma, 2020; Stillman, Capron, Alexander, Di Giusto, & Scivoletto, 2020).
Fig. 2.
Induction of inflammatory process and production of an immune response. The patterns are recognized by neighboring epithelial cells, endothelial cells, and alveolar macrophages, which initiate the production of pro-inflammatory cytokines and chemokines (including IL-6, IP-10, inflammatory protein macrophage 1a (MIP1α), MIP1β and MCP1). Immediately monocytes, macrophages, and T cells are attracted to pro-inflammatory proteins, initiating localized inflammation, with the production of IFNγ by T cells. In some situations, an additional accumulation of immune cells in the lungs can occur, causing a storm of pro-inflammatory cytokines, which damage the pulmonary infrastructure and other organs. A balanced immune response occurs as follows: the initial inflammation attracts specific T cells, which can attack the virus and eliminate infected cells. The production of neutralizing antibodies helps to block viral infection, reducing the associated risks. Finally, alveolar macrophages initiate a coordinated attack on neutralized viruses and apoptotic cells and eliminate them by phagocytosis. The process reduces viral load and damage to the lungs, resulting in faster recovery.
The severe acute respiratory syndrome (SDRA) observed in patients with SARS-CoV-2 is characterized by low blood oxygen level, drop in blood pressure and difficulty breathing. Due to the negative effects of low oxygenation of blood and organs, some patients end up acquiring other diseases such as infections caused by fungi, bacteria and even other viruses, resulting in the worsening of the clinical picture (Li, Chen, et al., 2020). About 70% of cases of deaths from SARS-CoV-2 result from the worsening clinical picture of respiratory failure. Also, the storm of cytokines released by the immune system in response to the infection (viral infection and/or secondary infections), results in the worsening of sepsis symptoms (symptoms: include fever, difficulty breathing, low blood pressure, fast heart rate, and mental confusion), responsible for more than 28% of deaths from SARS-CoV-2. In these cases, the condition of uncontrolled inflammation causes multiple damage, mainly to the organs of the heart, liver, kidney and respiratory system, making it very difficult for patients to recover (Chu et al., 2005; Li, Pei, et al., 2020).
One of the great current challenges is to understand the role of the immune system in the face of SARS-CoV-2 infection. So far we know that the protective effects of immunity against SARS-CoV-2 suffer widespread failures. However, the real causes and mechanisms by which the immune response is compromised in individuals with SARS-CoV-2 are still unclear. In addition, the picture of infections is very varied, as has been discussed previously, several types of patients can be observed, from asymptomatic patients to severe cases, with a wide spectrum of impaired vital functions. However, we know that immunity remains the Holy Grail of COVID-19 research. Although the immunity of individuals appears to have a central role, we know that there is still some mechanism to be identified that acts as an immune response imbalance system. This has been observed and shows that even patients with good immunity can progress to severe clinical conditions, although the highest mortality rate can be observed in patients with comorbidities and advanced age (Ghinai et al., 2020). The central role of immunity against SARS-CoV-2 infection is no different than that seen for other viral infections. As already described by Gorbalenya et al. (2020), immunity acts directly, initially controlling and managing an innate immune response against viral infection, then, if innate immunity manages to overcome the infection, a new immune response is achieved, that is, the acquired immune response. The innate immune response acts directly after the identification of an infectious process, by stimulating the natural mechanisms of protection against the penetration of pathogens, such as the skin, enzymes and antiviral peptides. However, in several cases, of individuals with SARS-CoV-2 infection, the immune system itself already suffers serious damage and its functionality is impaired, even at the beginning of the innate immune response. What has been the subject of many discussions about, what are the mechanisms for activating an inadequate immune response? Therefore, in these cases, the majority of infected individuals have several clinical signs of inflammation and, in several cases, processes of hyperinflammation and serious impairment of vital organs. On the other hand, Individuals who manage to overcome all infections properly, that is, with the immune system acting in a balanced way, obtain the acquired immune response. Although it is not clear how long the immune response acquired due to SARS-CoV-2 infection can prevent new infections, we know that the immune system after the infection has recovered is prepared to deal with possible new infections in a more balanced way (Yu et al., 2020a). To understand in more detail about immune system failures in patients infected with SARS-CoV-2, we will address the recent findings by Lucas et al. (2020), in Box 1 .
Box 1. Immune system failures in patients infected with SARS-CoV-2.
Lucas et al. (2020), in a study published on August 17 by the journal Nature, performs extensive analyzes on the immune response of patients infected with COVID-19, using a longitudinal study methodology. The study evaluated 113 people hospitalized with COVID-19 and a similar number of healthy patients as a control. Blood plasma molecules were monitored in both groups, in particular, immune cells such as peripheral blood mononuclear cells, CD4 T cells, CD8 T cells, and B cells. The longitudinal study allowed researchers to draw important conclusions about the evolution of phenomena in the immune system. Therefore, a summary of these conclusions is presented below:
-
•
The authors identified that some immunological signaling molecules, known as cytokines, are expressed in people with moderate or severe clinical conditions of COVID-19.
-
•
The level of cytokines, such as IFN-λ, increased dramatically in patients who progressed to more severe clinical conditions.
-
•
Pro-inflammatory cytokines such as TNF-α, increased dramatically and correlated positively with viral load in the nasal passages.
-
•
Some cytokines not associated with the defense system against viral infections, such as IL-5 that aid in the defense against parasites and allergic reactions are regulated positively according to the disease progression.
-
•
The levels of CD4 and CD8 T cells, which are responsible for the immune response against viral infections, have been drastically reduced in both patients with moderate disease and in critically ill patients.
Based on these results, the authors divide patients into three groups based on the course of clinical evolution and the severity of the disease. Therefore, we have patients with moderate, severe and very severe clinical conditions:
-
•
Patient with moderate clinical condition: in general, these patients after infection have low levels of inflammatory markers and an increase in the level of proteins associated with tissue repair.
-
•
Patients with severe and very severe clinical condition: these patients have high levels of IFN-α, IL-1Ra and proteins associated with T H 1-, T H 2- and T H, even after the final phase of viral replication.
Lucas and his collaborators fill an important gap in understanding the role of the immune system in the face of COVID-19 infection. This study provides an understanding of the signature of the immune response and helps to clarify how the disease has progressed to severe cases. However, the role of the immune system in the face of COVID-19 infection is not yet clear. However, this study helps to clarify the basic principles by which the immune system has suffered cascades of failures that result in severe illness. Therefore, new efforts should focus on understanding the reasons for failures and how they can be avoided.
Alt-text: Box 1
3. Functional foods rich in biologically active polysaccharides
Polysaccharides are distributed throughout nature, from microorganisms to complex organisms. We functional foods such as fruits, vegetables, mushrooms, algae, herbs and plants in general, these biopolymers are found in abundance. In fact, in recent years, a great effort has been made by researchers worldwide to understand the importance and distribution of polysaccharides in natural foods. Also, considerable efforts have been made to establish a well-founded understanding of the influence of polysaccharides on human health. Thus, in this topic, we will address the main polysaccharides obtained from natural foods, with special emphasis on their structural properties that may attribute some biological potential. Meanwhile, (Table 1 ), summarizes some functional foods rich in biologically active polysaccharides. Although this topic deals exclusively with functional foods rich in biologically active polysaccharides, we must make it clear that the role of this article is to demonstrate the relevance of polysaccharides isolated from these edible natural sources.
Table 1.
Some functional foods rich in biologically active polysaccharides.
| Source | Polysaccharides/characteristics | Biological activity | Observations | References |
|---|---|---|---|---|
| Cuminum cyminum | →4)-Galp-(1→, →3)-Galp-(1→, →2)-Arap-(1→ and →2)-Arap-(1→ glycosidic linkages Mw: 191.4–512.2 × 103 g/mol |
Modulation of the immune system | Polysaccharides induced RAW264.7 cells to release nitric oxide and express inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-12. In addition to activating NK-92 cells to produce TNF-α, IFN-γ, perforin, granzyme B, NKG2D, and FasL | Tabarsa et al. (2020) |
| Pouteria campechiana | →4)-α-d-Glc (1→ and →6)-α-d-Glc (1→ Mw: 67,900 Da |
Antioxidant activity | Pouteria campechiana, also known as yellow sapota, is a fruit originally from tropical regions. In Latin America, the fruit can be found from Mexico to Brazil | Ma et al. (2020) |
| Nephelium longanum | two specific glycosidic linkages of α-Araf-(1→ and →5)-α-Araf-(1→ Mw: 1.47 × 105 Da |
Protector of the intestinal barrier | Exhibited an ability to increase the expression of ZO-1, claudin-1, occludine and E-cadherin mRNA in differentiated Caco-2 cells | Bai et al. (2020) |
| Cabernet Sauvignon wine | Complex of several heteropolysaccharides | Anti-inflammatory activity | The treatment reduced leukocyte migration inhibited pro-inflammatory cytokines and increased production of IL-10 anti-inflammatory cytokines | de Lacerda Bezerra, Caillot, de Oliveira, Santana-Filho, and Sassaki (2019) |
| Terminalia chebula | Amylopectin with backbone (1 → 4)-α-Glc(p) backbone branched at C6/C2. Side chains were composed of (1 → 4)-β-Gal(p) substituted with α-Ara(f), β-GalUA(p), β-GalUA(p)-Me, and α-Rham(p) Mw: 534.9 kDa |
Antioxidant activity | Terminalia chebula, commonly known as black or chebulic mirobalane, is a species of Terminalia, native to southern Asia from India and Nepal | Jeong, Lee, Kim, and Baek (2019) |
| Morinda citrifolia | Homogalacturonan and rhamnogalacturonan. | Anti-inflammatory activity | The polysaccharide was able to inhibit leukocyte migration to the site of inflammation and further reduced the tests for inflammatory nociception | Sousa et al. (2018) |
| Ilex asprella | IAPS-1 was elucidated as 1,6-linked α-d-glucopyranosyl. IAPS-2, the backbone is composed of 1, 4-linked α-d-glucose, galactose and rhamnose, and branched chains consists of arabinose, rhamnose and galacturonic |
Immunoregulatory activity | The polysaccharides increase the secretion of important inflammatory cytokines in macrophages, such as TNF-α, IL-1β, IL-12 | Meng et al. (2018) |
| Meretrix meretrix | α (1 → 4) -glucan branched with Man or Gal. | Immunoregulatory activity | Splenocyte proliferation and cytokine secretion | Wang, Chen, Li, Di, and Wu (2018) |
3.1. Polysaccharides obtained from mushrooms
Mushrooms are fungi, that is, eukaryotic organisms, which includes them in the Eukarya domain (classification above kingdom). Mushroom is the common name given to the fruiting of some fungi of the phyla Basidiomycota and Ascomycota. They have a fruitful body composed of a base (stape) and cap (also called pileus). These microorganisms reproduce sexually by the joining of hyphae, however other reproductive forms can also be observed, and for this reason they are considered sexually promiscuous. Mushrooms contain a wide variety of shapes, colors, and sizes, and can also be found in various habitats (Halbwachs & Simmel, 2018).
Currently, several species of edible mushrooms are known, and only a part of the known edible mushrooms is commercialized. Edible mushrooms are rich in essential amino acids, minerals, proteins, and biologically active polysaccharides. Mushrooms are mainly eaten in Asian countries, however, in recent years some edible mushrooms have been popularized, such as Pleurotus ostreatus, Boletus edulis, Lentinula edodes (Shiitake), Ganoderma spp (Reishi), Trametes versicolor, Leafy grifola (Maitake), Agaricus bisporus and Agaricus subrufescens, being found in markets, almost worldwide (Ruthes, Smiderle, & Iacomini, 2016).
In a recent report, Barbosa, dos Santos Freitas, da Silva Martins & de Carvalho Junior (2020) show that edible mushrooms of the genus Pleurotus spp, have a diversity of polysaccharides, in particular heteropolysaccharides and glucans. These biopolymers have significant differences in monosaccharide composition, molecular weight distribution, structural conformation, and types of chemical bonds. Also, it has been shown that polysaccharides from mushrooms of the genus Pleurotus spp have important biological activities such as antitumor, antioxidant, anti-inflammatory, immunomodulatory, antiviral, antimicrobial and antidiabetic activities. Finally, it was shown that polysaccharides, due to their biological potential, are being used in the development of new technologies such as the production of selenized polysaccharides and vaccines.
Ruthes et al. (2016), in a literature review article showed that mushrooms are rich in heteropolysaccharides. Several edible and medicinal mushrooms were explored in the review article, with an emphasis on structural characteristics and biological effects. The authors show that mushrooms have several different types of heteropolysaccharides, that is, polysaccharides with more than one type of monomer in the monosaccharide chain. The study shows that hybrid mushrooms also produce heteropolysaccharides. Also, heteropolysaccharides have important biological activities, such as anti-tumor, antioxidant, anti-inflammatory, and immunomodulatory activity.
Mushrooms are the main foods with a high content of polysaccharides, mainly polysaccharides with antioxidant and immunomodulatory activity. As demonstrated in a study by Barbosa et al. (2020b) using a supercritical binary hot water and CO2 system for the first time, it was possible to obtain polysaccharide-rich fractions from the edible mushroom Pleurotus ostreatus. The study revealed that the extracts were a mixture of several polysaccharides, including glucans. The antioxidant activity of the extracts in the DPPH models showed a radical reduction potential of up to 80%. The antioxidant potential of the extracts has also been confirmed in studies with a cell model, which revealed the potential of the extracts in protecting cells from oxidative damage induced by hydrogen peroxide. Another study demonstrated that the mushroom Phallus atrovovatus has high concentrations of polysaccharides, mainly fractions of β-glucan and α-glucan. The polysaccharides obtained from this mushroom are known to have immune system modulating activity, as fractions of up to 50 μg of the polysaccharides have been shown to significantly reduce the activity of myeloperoxidase (MPO), as well as reduced cytokine secretion TNF-α, IL- 6 and IL-10, that is, anti-inflammatory activity (Chaiyama et al., 2020).
Finally, in a report recently published in Trends in Food Science and Technology, Mingyi, Belwal, Devkota, Li, and Luo (2019), showed that mushroom polysaccharides are biomolecules functional. The authors show that antioxidant, immunomodulatory and anticancer activities are the most significant activities of polysaccharides. The article presents the current scenario on the use of mushroom polysaccharides in foods, medicines, cosmetics and looks at applications of mushroom polysaccharides in the development of functional food for the coming years.
3.2. Polysaccharides obtained from herbs
The use of plants and herbs for medicinal purposes undoubtedly begins at the origin of human civilization. Human beings have always sought natural herbal resources to treat their illnesses. According to the bibliographic review article of Thakur et al. (2012), Rasayana is an important class of Ayurvedic herbs, which have been used for thousands of years as rejuvenators and tonics. The use of Ayurvedic herbs to treat diseases is very popular, however, the chemical guiding principle associated with bioactivities is not yet clear. However, the article shows that the polysaccharides present in these herbs have relevant biological activities such as antioxidant, antitumor, and immunomodulatory. All the studies evaluated by the authors suggest that polysaccharides contribute to the maintenance of physiological homeostasis, which is the guiding principle of Rasayana therapy.
In a similar article, Jin, Zhao, Huang, Xu, and Shang (2012), report that the root of Angelica sinensis (Oliv.) Diels, well-known Chinese herb properties herbal-medicines, has been used for millennia as a tonic, hematopoietic agent and anti-inflammatory. Modern phytochemical and pharmacological studies, conducted with the herb show that polysaccharide is one of the main active ingredients and responsible for several important biological activities, such as hematopoiesis, immunomodulation, antitumor, antioxidant, radioprotection, and hypoglycemic activity.
Kakar et al. (2020), reports in a review article the structural characteristics, extraction techniques and biological activities of polysaccharides isolated from Cyclocarya paliurus (Batalin) Iljinskaja. The leaves of this plant have important chemical compounds, especially polysaccharides, and have been used in the production of teas and food for millennia. Several studies evaluated by the authors, explore various polysaccharide extraction techniques, and a set of modern spectroscopy techniques associated with others such as chromatography and nuclear magnetic resonance have been applied to structural polysaccharide elucidation. Finally, the author shows the main biological activities associated with the purified and modified polysaccharides of Cyclocarya paliurus (Batalin) Iljinskaja, and includes anti-cancer, anti-inflammatory, antioxidant, antimicrobial, anti-hyperlipidemic and anti-diabetic activities.
3.3. Polysaccharides obtained from fruits
Fruits are one of the most consumed foods in the world and can be consumed in many ways, in natura, dehydrated, in jams, juices, sweets, cakes, cookies, and many others. Currently, more than 53 fruits have been characterized for the presence of polysaccharides, as shown in an updated report produced by Mohan et al. (2020). In the same study, the authors updated readers on the main techniques for extracting and characterizing polysaccharides from tropic and subtropical fruits. The authors showed that fruit polysaccharides have relevant biological activities such as antioxidants, immunomodulatory, anti-diabetic, anti-cancer, anti-tumor, anti-glycation, hepatoprotective effects, anti-inflammatory effects, and anti-microbial activities.
Song et al. (2019), identified a new polysaccharide from Chinese wild fruits (Passiflora foetida) and demonstrated that it was a heteropolysaccharide with structure of → 1) -α-D-Manp → 1,2) -β-D-Manp- linked 1,2,6) -β-D-Manp residues and side chains consisted of → 1) -β-D-Galp, → 1,4) -α-D-Manp, → 1, 4) -β-D -Glcp, → 1,3) -α-D-Galp, → 1,6) -β-D-Manp, → 1,6) -β-D-Galp, → 1,2,3) -β-D -Manp and → 1,3,6) -β-D-Galp residues. This new polysaccharide has Mannose as its main monosaccharide, and has the potential to increase the immune system.
In another study, Peng et al. (2019), isolated and characterized two new water-soluble polysaccharides from Citrus medica L. var. sarcodactyl. The new polysaccharides have groups of sulfates in their structure, which explains much of their characteristics as solubility. The low molecular weight fraction has a triple helix conformation, while the high molecular weight fraction is more complex. The authors demonstrate that the composition of polysaccharides rich in arabinose, mannose, glucose and sulfate groups may be closely related to immunomodulatory activity. It has been shown that the fraction with the highest percentage of sulfate groups forces stereochemical exposure of hydroxyl groups present in the polysaccharide molecule, favoring coupling to cellular receptors, and therefore increasing immunomodulatory activity. Nagarajan et al. (2019) demonstrated that lycopene-pectin complexes can be obtained from the water-induced pink guava decanter. The complex has high antioxidant activity, mainly associated with lycopene molecules in the Trans configuration. These studies show that fruits are rich in polysaccharides, in particular polysaccharide complexes such as pectin linked to various secondary compounds.
3.4. Polysaccharides obtained from algae
Edible seaweed is a delicacy, found in various dishes, such as soups, Sushi, encapsulated, dehydrated, among others. The main edible algae come from saltwater since most freshwater algae are toxic. Among the edible algae, the Sargassum fusiform stands out, mainly due to the huge amount of scientific work already produced. There are so many scientific works that Zhang, Zhang, Tang, and Mao (2020), gathered these researches in a bibliographic review article. The authors update readers on the main characteristics of polysaccharides found in the Sargassum fusiform seaweed. The main polysaccharides found are alginic acid, fucoidan, and laminarane. Polysaccharides have relevant biological properties including antioxidant, anti-tumor, promoting immunity, and anti-aging, stimulating bone growth, decreasing blood glucose, anti-clotting, anti-virus, anti-bacteria, and anti-fatigue, promoting growth and development and protection of the skin. Also, extraction, isolation, purification and characterization techniques are addressed by the authors.
A similar study conducted by Sanjeewa et al. (2018) sulfated polysaccharides obtained from the edible seaweed Sargassum spp are explored. In the review the bioactive properties of sulfated polysaccharides applications for improving human health are reported. Also, anticoagulant, antioxidant, anticancer, antibacterial and antiviral activities of sulfated polysaccharides is updated. The article addresses the use of sulfated polysaccharides in an updated way, focusing on the development of natural products, to fill the knowledge gap between research studies and industrial application.
With the purpose of order to fill in the gaps on the use of algae for the production of polysaccharides and the relationship with the digestive tract, microbial catabolism, and prebiotic potential, Zheng, Chen, and Cheong (2020), summarize the latest findings in the literature. The authors show that seaweed contains unique polysaccharides, with totally different structural properties when compared to terrestrial plant polysaccharides. Seaweed polysaccharides are not digestible by digestive enzymes in humans. However, when these biopolymers reach the gastric and intestinal systems, they can selectively increase the activities of some populations of beneficial bacteria in the intestinal flora. Also, they stimulate the organism with a series of biological activities such as anticancer, antioxidant, immunomodulatory and antidiabetic.
In a study published in the journal Trends in Food Science and Technology, Zhong et al. (2020), reports the current advances in research on the structural, functional and chemical characteristics of Enteromorpha polysaccharides. The algae of the genus Enteromorpha produce several types of polysaccharides with relevant biological activities. However, the relationship between structure and function of polysaccharides is not yet fully elucidated. A peculiar characteristic of sulfated polysaccharides is that they have lower molecular weights and exhibited excellent immuno-relevant and antioxidant activities.
3.5. Polysaccharides obtained from cereals
Cereal grains and pseudocereals have attractive nutritional properties, mainly due to the presence of biopolymers such as starch, dietary fiber and polysaccharide complex linked with polyphenol molecules. Although cereal grains such as rice, barley and sorghum have highly branched and nutritionally relevant starch polysaccharides, these biopolymers are poorly active in improving immunity, since most of them are consumed by the body and used in energy production (Jimenez, Lobo, & Sammán, 2019). On the other hand, pseudocereal grains such as Amaranth (Amaranthus spp.), Buckwheat (Fagopyrum esculentum and F. tataricum) and quinoa (Chenopodium quinoa), are rich in dietary fiber, polysaccharides linked to metal ions such as iron and zinc, plus polysaccharide-protein complexes (Jimenez et al., 2019). Pseudocereals are but interesting for obtaining polysaccharides when compared to cereal grains, mainly due to the vast chains of polysaccharide complexes with biomolecules, metal ions and polyphenols. Although both types of grains are nutritionally important, we will look at the intrinsic properties of polysaccharides and their relationship to increased immunity.
Lamothe, Srichuwong, Reuhs, and Hamaker (2015) isolated a fraction of insoluble fiber from A. caudatus, and demonstrated that the fraction was composed of homogalacturonans divided into interspersed chains, with units of ramnogalacturonane and side chains of xyloglucans, disaccharides, trisaccharides and cellulose. Zhang, Wang, Tan, and Zhang (2020), identified several types of polysaccharides in Quinoa seeds, especially pectins and insoluble fibers. It was identified in the fraction of insoluble fiber homogalacturonans interspersed with ramnogalacturonan units and side chains of xyloglucans, trisaccharides and cellulose, a structure equivalent to that described by Lamothe et al. (2015) from A. caudatus. But for a comprehensive understanding of polysaccharides obtained from pseudo-cereals, it is worthwhile for the reader to consult the review article written by Zhu (2020). In this article the author summarizes the relevant aspects about the chemical structure, physical properties, biological functions and food uses of polysaccharides obtained from pseudocereals. In addition, comparative observations between grain polysaccharides and fiber fractions from common sources, such as fruits and vegetables, reveal many similarities, mainly in their composition and potential immunomodulatory activities.
4. Polysaccharides with properties to modulate the immune system
The polysaccharide potential in modulating the immune system will be the focus of this topic. Also, the polysaccharide immunomodulatory potential will be used as an indication of the ability of these biopolymers to stimulate the body to improve the immune response to viral infections, especially by SARS-CoV-2. Therefore, for a better understanding of the reader on the subject, we will initially address important aspects of inflammatory immunopathogenesis related to SARS-CoV-2 infection. Then, the main polysaccharides with immunomodulatory activity will be addressed.
After infection of the cells by SARS-CoV-2 and therefore the destruction of lung cells, the body initiates a local immune response, recruiting defense cells such as macrophages and monocytes that immediately respond to infection, releasing cytokines, substances that modulate the immune response. The released cytokines stimulate the defense cells, adaptive T and B cells, which respond to infection, attacking contaminated cells and viruses (Yang, Yu, et al., 2020, p. P497). In most cases, this process is capable of reducing infection, however, it depends on how the organism will behave in the face of the underlying biochemical phenomena. However, in some cases, the body reacts differently, initiating a dysfunctional immune response, which leads to a chain reaction, and causes serious complications to the lungs and even systemic pathologies, which can lead to death (He, Fang, et al., 2020).
The virus replicates continuously in the epithelial cells of the airways, causing high levels of pyroptosis, a type of programmed cell death. This is probably the trigger for the localized inflammatory response. Subsequently, IL-1β, a cytokine released during pyroptosis increases considerably, attracting an immediate inflammatory response. Using pattern recognition receptors (PRRs), cells such as alveolar macrophages and alveolar epithelial cells identify receptors for pathogens such as viral RNA, and damage-associated molecular patterns (DAMPs). Thus these cells initiate localized inflammation, with increased cytokines and pro-inflammatory chemokines IL-6, IFNγ, MCP1, and IP-10 in the blood (Wrapp et al., 2020). In response to the high level of pro-inflammatory substances, defense cells such as monocytes and T lymphocytes initiate a coordinated attack at the sites of inflammation. In most cases, recruited cells can respond adequately to the immune system and patients recover. However, in other cases, still poorly understood, patients exhibited higher blood plasma levels of IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), IP-10, MCP1, macrophage inflammatory protein 1a (MIP1α) and tumor necrosis factor (TNF). The mechanism by which the SARS-CoV-2 virus subverts the antiviral response is still unknown, but it is known that the cytokine storm has side effects on the body, leading to septic shock and multiple organ failure. Also, adults, the elderly and people with morbidities have lower levels of defense cells when compared to children, therefore, the inflammatory response can be harmful (Wrapp et al., 2020).
Over 50% of children under the age of 18 have mild symptoms and some are asymptomatic, although the viral load is high. Around 6% develop severe symptoms, however, in most cases; these children have some associated morbidity. The balance between defense cells and the mechanisms of the production of pro-inflammatory substances are crucial for compression about the mechanisms involved in immune response in children. Although, a complete picture of the factors critical to the development of inflammatory responses remains somewhat undefined. But, it is known that stimulation of the immune system with substances that modulate the immune response helps to adapt the body before serious infections, helping in a more controlled response (He, Fang, et al., 2020). Therefore, although polysaccharides are not a therapy proposed during infection, mainly due to the few targeted studies, they can modulate an adequate immune response beforehand, preparing the body to face infection in a balanced way.
4.1. Polysaccharides activate immune responses
Polysaccharides stimulate immune cells by means of specific receptors, for example in necrophagous and macrophage cells by means of bonds between functional groups of polysaccharides and molecular groups on the cell surface. From the moment the polysaccharides bind to membrane receptors in the defense cells, the signaling pathways are activated, and a cycle of biochemical processes begins that leads to the positive regulation of gene expression in the ribosomes, initiating the protein production. In cells such as macrophages, polysaccharides activate the protein pathways, which in turn are activated by mitogens (MAPKs) and others such as the nuclear factor (NF-kB), stimulating the immune response control processes. Macrophages can initiate an immune response in several ways, but it is common for polysaccharides to stimulate the production of nitric oxide and pro-inflammatory cytokines (Barbosa, dos Santos Freitas, da Silva Martins & de Carvalho Junior, 2020). Another relevant aspect about the modulating activity of polysaccharides can be found in studies on gene expression. Some polysaccharides stimulate the production and expression of messenger RNA (mRNA) during the synthesis of nitric oxide and pro-inflammatory cytokines. In addition, at the genetic level, some signaling proteins such as p38, p65, p50, signal regulated kinase and JNK are activated by polysaccharides. Polysaccharides obtained from these functional foods significantly increase the expression of messenger RNA (mRNA), and cytokines, both by active regulation of receptors such as TLR2 and TLR4 (Barbosa, dos Santos Freitas, da Silva Martins & de Carvalho Junior, 2020). Recently, studies conducted by Shen et al. (2017), with polysaccharides from wheat, show that they can possibly induce cytokine expression through the MAP38 p38 signaling pathway mediated by the toll-like receptor 4. Another study conducted by Deng, Fu, Shang, Chen, and Xu (2018) with Dictyophora indusiata demonstrated that the Dectin-1 receptor on the surface of macrophages is activated by β-D-glucan, composed of a main chain of (1 → 3) -β-glucan with side chains of residues (1 → 6) -β-glucan and molecular weight of 650 kDa. It was confirmed that the polysaccharide activates macrophages and increases their phagocytic activity; in addition, the MAPK pathway is activated and promotes nuclear translocation of NF-kB. In short, these results shed light on the molecular pathways involved in macrophage immunomodulation activity. In addition to macrophages, other cells are activated and stimulated by polysaccharides, and assist in modulating the immune system, among these cells, they can highlight T cells and dendritic cells. Wang, Hwang, et al. (2020) isolated a polysaccharide from the algae Pyropia yezoensis and demonstrated that the lipopolysaccharide has protective effects on immunity through the activation of tooth cells. It has been identified that tooth cells are modulated by expressing expression of CD86, CD40 and MHC-II. Likewise, polysaccharides can also modulate the secretion of IL-12 by dendritic cells and increase the proliferation of T lymphocytes and the levels of CD4 + and CD8-T cells.
Another edible and medicinal fungus, Ganoderma lucidum has polysaccharides that modulate the immune response. Li, Gu, et al. (2020) showed the effects of the structure-bioactivity relationship of two purified and characterized fractions of Ganoderma lucidum polysaccharides. Applying modern purification techniques and molecular characterization, the authors conclude that the GLP-1 fraction is a d-galactoglucan (composed of →6)-β-d-Glcp-(1→, →6)-α-d-Galp-(1→, and →3)-β-d-Glcp-(1→ residues), while the GLP-2 fraction is a β-D-glucan (→6)-β-d-Glcp-(1→ and →3)-β-d-Glcp-(1→ residues). The authors showed that the GLP-1 fraction produced a better immune response, protecting the spleen and the tino, in addition to promoting the production of immunoglobulin A (IgA). Zheng, Gu, et al. (2020) also obtained several polysaccharide fractions from the edible mushroom Leccinum crocipodium (Letellier.) and assess the immunomodulatory potential. Three fractions of different molecular weight and degree of homogeneity were obtained, purified, and characterized. Cell viability studies show that these biopolymers improve immunomodulatory activities in macrophage RAW 264.7 cells.
Polysaccharides modulate the immune response in several ways, increasing macrophage phagocytosis, production of ROS (reactive oxygen species), NO (nitrous oxide), the release of cytokines (IL-6, TNF-α) and by activation of signaling pathways such as NF-κB, toll-like 4 (TLR4), type A hijacker receptor (SRA) and glucan receptor (GR). In the study conducted by Wu et al. (2020) A neutral polysaccharide, obtained from Kushui rose residues, showed immunomodulatory activity, increasing macrophage phagocytosis, production of ROS, NO, IL-6, TNF-α by the activation of signaling pathways NF-κB. Huang et al. (2020), isolated and purified a new polysaccharide from the roots of Millettia Speciosa Champ. The polysaccharide increased pinocytic capacity and promoted the secretion of NO and cytokines in RAW 264.7 cells. Macrophage production was mediated by signaling receptors such as toll-like 4 (TLR4), type A hijacker (RAS) and glucan (GR). Finally, Hu et al. (2019) isolated and purified a homogeneous polysaccharide, with bioactive properties of the lotus root. It has been shown that the polysaccharide induces macrophage activation through the PI3K/Akt and MAPKs signal pathways, which had not yet been reported. Also, the polysaccharide promoted the secretion of TNF-α and interleukin-2 in artificially induced immunosuppressive mice.
To conclude the subject, although the theme is not exhausted, it is important to emphasize that in vivo studies have also revealed the potential of polysaccharides in modulating the immune system. The L. edodes mushroom has several polysaccharides, including β-glucans, which have different biological properties. Recently Zou, Duan, and Xu (2019) demonstrated using tumor models in vivo that L. edodes β-glucan positively regulated the level of CD4 + T cells in lymphoid organs. In addition, neutrophil infiltration has been demonstrated at sites where tumor cells were concentrated. The study is conclusive in stating that the modulation of the defense system was activated by the polysaccharide, mainly by the interaction with T cells, and finally the elimination of neutrophils was found.
A β-1,3/1,6-glucan from Durvillaea Antarctica inhibits tumor proliferation and regulates immune expression in vivo studies. According to the studies by Su et al. (2019) the β-1,3/1,6-glucan increases the phagocytic activity of macrophages and the secretion of cytokines and chemokines, modulating the immune response, reducing tumor cells and increasing immune cells.
The authors found that the molecular weight influences the immunomodulatory capacity, as already demonstrated in other studies with edible mushrooms (Ferreira, Passos, Madureira, Vilanova, & Coimbra, 2015; Jin et al., 2017). Chen et al. (2020) also shows that the lower molecular weight fraction was more effective in regulating immunity in mice. The authors show that the lower molecular weight polysaccharides have simpler structural conformation and can pass through the cell barrier with less problems. However, polysaccharides with large molecular weight also have immunomodulatory activity but have slower mechanisms of activation of the immune system.
4.2. Polysaccharides activate immunosuppression pathways
The immune response is the main factor between the functional balance of the cells and the possible mechanisms of functional unbalance, which can lead to complex clinical conditions. In cases of viral infections, the appropriate immune response helps to balance the level of pro-inflammatory substances and the cell recovery cycle. However, an exaggerated immune response is at the center of numerous problems such as organ damage and failure of vital functions (Barbosa, dos Santos Freitas, da Silva Martins & de Carvalho Junior, 2020). It is easy to understand how the immune system is important, making a brief example. Individuals with clinical allergies have several imbalances in the immune system, in which case the immune system cannot accurately identify foreign bodies and therefore the immune response is compromised. In cases of people infected with COVID-19, especially in severe cases, the role of the immune system is evident. In these cases, as discussed in the previous topic, the immune response is so exaggerated that complex hyperinflammation is observed, in addition to serious side effects, such as hypercoagulation and multiple organ failure (Wrapp et al., 2020). As already highlighted in the previous topic, there are several polysaccharides that stimulate the immune system through indirect ways, mainly by regulating the expression of pro-inflammatory substances. These polysaccharides are ideal for use in long-term immunity-enhancing therapies. However, these polysaccharides could not be used in patients with severe cases of COVID-19, as they could help to worsen the conditions of hyperinflammation. However, there are polysaccharides, which activate immunosuppression pathways, and could be used to stimulate the production of anti-inflammatory substances, which can be applied in the treatment of severe cases. From now on, we will take a targeted approach to the polysaccharides that activate the immunosuppression pathways.
Inflammatory processes can start in many ways, however the endotoxin-mediated pathway (LPS) is the, but well elucidated. This is a molecule composed of two highly complex parts, one of lipid and the other of carbohydrate, a type of glucan. Endotoxin (LPS) molecules are present on the outer membrane of cells and are responsible for strong immune system responses in healthy organisms. Cells such as macrophages, B lymphocytes, monocytes and dendritic cells, when they come into contact with an LPS, either by binding to the TLR4 receptor, or other, promote an inflammatory response such as fever, vasodilation due to the production of nitric oxide and secretion of eicosanoids (prostanoids, leukotrienes and lipoxins). Defense cells like macrophages are the most active in the immune system, although we have other defense cells, macrophages are usually cells, but agile when stimulated (Wrapp et al., 2020). Therefore, considering macrophages as a study model, we can evaluate how the cycle of stimuli and responses that induces the defense system to produce an inflammatory response. Next, we will evaluate how polysaccharides manage to modulate an immunosuppressive response, controlling the flow of pro-inflammatory substances production and regulating the ways of maintaining the oxide-reducing balance.
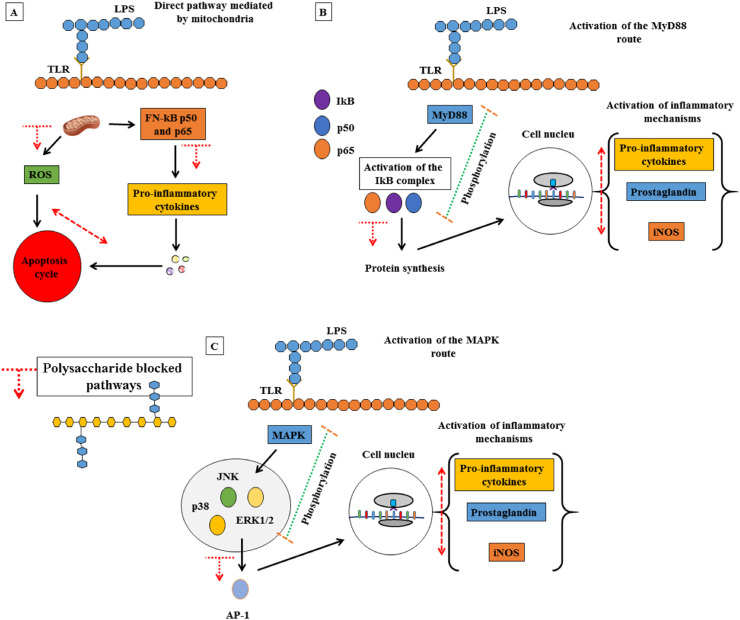
Extracellular stimuli lead to the activation of three pathways, namely the MAP Kinase pathway (Mitogen Activated Protein Kinases), the MyD88 protein pathway and the direct pathway with mitochondria activation, both of which induce the production of inflammation, proliferation, differentiation and apoptosis. The MAPk activation pathway initiates the regulation of a series of biological processes, especially through the action of the kinase JNK, p38 and ERK 1/2. At the end, the signaling kinases activate the AP-1 protein (activator protein 1), responsible for the regulation of gene expression. The stimulus of the TRL4 receptor can activate the domain of the MyD88 protein, which in turn activates IκB kinase, an enzyme complex responsible for the propagation of the cellular response to inflammation (Wrapp et al., 2020). The IkB kinase complex induces a cascade of signal transduction mediated by phosphorylation processes until activation of the nuclear factor kappa B (NF-kB), a protein complex that performs functions as a transcription factor of genetic material and induction of protein synthesis. Finally, signaling of the direct pathway mediated by mitochondria can also occur, which initiates the production of reactive oxygen species (ROS), initiating an apoptosis cycle. On the other hand, the ROS pathway can also induce the nuclear factor kappa B (NF-kB) signaling pathway, which has functions as a transcription factor for genetic material and induction of protein synthesis. Therefore, as already observed in case of serious infections by COVID-19, where the side effects of hyperinflation are clear, the inhibition of inflammatory responses mediated by macrophages or other cells of the immune system, must suppress or control the signaling pathways (Barbosa, dos Santos Freitas, da Silva Martins & de Carvalho Junior, 2020). All the mechanisms described can be seen in (Fig. 3 ), in addition to the possible polysaccharide signaling mechanisms that contribute to the immunosuppression of the pro-inflammatory substances production pathways.
Fig. 3.
Main pathways of inflammation mediated by the immune system and possible polysaccharide signaling mechanisms that contribute to the immunosuppression of pro-inflammatory substances production pathways. A) Direct route mediated by mitochondria; B) MyD88 protein signaling pathway; C) MAPK protein signaling pathway. In addition, the main polysaccharide-mediated immunosuppression pathways are shown with red arrows. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Anti-inflammatory activity has been reported by several researches, as shown below. Especially the polysaccharides that have in their structure monomers of Gal, Glc, GalA and Rha, revealed potent anti-inflammatory activity in both in vitro and in vivo models. As highlighted earlier, the endotoxin-mediated pathway (LPS) is best elucidated in studies on the onset of inflammatory processes. Therefore, polysaccharides that block pro-inflammatory signaling pathways are essential as possible treatments for cases of COVID-19. For example, mixing three fractions of mannan-like polysaccharides isolated from wines inhibited the production of inflammatory cytokines (TNF-α and IL-1β) and the mediator (NO) in RAW 264.7 cells stimulated with LPS (de Lacerda Bezerra et al., 2018). An isolated and purified fraction of polysaccharide obtained from the edible mushroom Phellinus linteus inhibits the production of pro-inflammatory substances in a cell and animal model. It was demonstrated based on the study directed by the LPS pathway that the polysaccharide fraction significantly reduced the expression of inflammatory cytokines in the colon tissues, and reduced the expression of inflammatory cytokines, inhibiting the MAPK pathway, as well as the translocations of NF-κB and AP-1 (Hu et al., 2018). In another study, conducted by Sousa et al. (2018), with Morinda citrifolia Linn (Noni) identified a polysaccharide composed of homogalacturonan and rhamnogalacturonan. Studies have shown that the fraction has anti-inflammatory activity, and even helps to reverse inflammatory parameters such as edema, leukocyte migration and nociception. Finally, in a study conducted by Tong et al. (2018) isolated a polysaccharide from Bupleurum chinense capable of reducing lung injury in a model of acute pneumonia through inhibition of neutrophil recruitment pathways mediated by P-selectin. Another polysaccharide, now obtained from Scutellaria baicalensis is one of the many polysaccharides that have the potential to be used as a drug in the treatment of hyperinflammatory conditions by suppressing the signaling of the NF-κB pathway and activating the NLRP3 inflammasome (Cui et al., 2019). Although the topic is not exhausted, these are the main polysaccharide-mediated immune system modulation activities.
5. Polysaccharides as potential platforms for vaccine development
When a virus infects the human body, the first step in the evolution of the biological cycle is the attachment of the virus to a host cell, through target receptors. The work done with SARS-CoV serves as a reference for understanding how SARS-CoV-2 reacts when infecting a cell. It has been shown that the virus has a preference for airway epithelial cells, alveolar epithelial cells, vascular endothelial cells and macrophages in the lung (Wrapp et al., 2020). When the virus attacks these cells, the cells immediately express the target receptor of the host of the angiotensin-converting enzyme 2 (ACE2). As SARS-CoV-2 uses the same input receptor, there is a reduction in the expression of ACE2 in lung cells. ECA2 reduction after a viral infection can result in dysfunction of the renin-angiotensin system, influencing blood pressure, and electrolyte balance in the cell. Understanding the relationship between target cell interactions is important for the development of therapies. Therapeutic development against SARS-CoV-2 passes through the target host ACE2 receptor (Shang et al., 2020).
As previously described, polysaccharides can modulate the immune response to infections. Therefore, the use of polysaccharides for vaccine production has been reported as a possibility. Recent studies (Han et al., 2019; Liu et al., 2016), show that polysaccharides can be used as cheap and efficient platforms in the development of vaccine formulations. We believe that more in-depth studies can be conducted to apply polysaccharides in the production of vaccines against SARS-CoV-2.
As of April 2020, 115 SARS-CoV-2 vaccine candidates were being produced globally. Of the 115 vaccine candidates, 78 are confirmed as active and the other 37 are privately owned, without many public details (Curtis, Sparrow, Ghebreyesus, & Netea, 2020; Thanh Le et al., 2020). Some of these vaccine production projects are in the exploratory phase, while others are already more advanced, already in clinical development. As an example we have mRNA-1273 from Moderna, Ad5-nCoV from CanSino Biologicals, INO-4800 from Inovio and LV-SMENP-DC and aAPC specific to pathogens from Shenzhen Geno-Immune Medical Institute. There are many candidates for vaccine production and include, from LNP encapsulated mRNA vaccine that encodes protein S, Type 5 adenovirus vector that expresses protein S, DNA plasmid encoding protein S delivered by electroporation and aAPCs modified with a lentiviral vector that express synthetic minigene based on selected viral protein domains (Yamey et al., 2020).
Although all vaccine projects are concentrated on biomolecules such as RNA, DNA, attenuated virus, and solid particles, we believe that natural polysaccharides could play a crucial role in the development of oral vaccines. In fact, polysaccharides are viable platforms for vaccine development. As reported in the bibliographic review article written by (Barbosa, dos Santos Freitas, da Silva Martins & de Carvalho Junior, 2020), polysaccharides can bind to cell receptors more easily than other biomolecules, initiating a series of biochemical reactions that lead to the production of antibodies. Thus, polysaccharides could be used as adjuvants in the development of vaccines against SARS-CoV-2.
The idea of using natural polysaccharides as platforms for vaccine development has grown in academia, especially in the past ten years. Several research groups have explored the potential of polysaccharides to transport antigens and counteract side effects in antiviral vaccines (Barbosa & de Carvalho Junior, 2020). Now, during a COVID-19 pandemic, platforms for vaccine development have been the focus of numerous researches. Although there is no natural polysaccharide being used as a platform for vaccines against COVID-19, it is evident from the studies that will be evaluated, that polysaccharides are efficient, low-cost, biodegradable and biocompatible platforms.
Polysaccharides like chitosan and glucans are being used in several technologies to transport antigens. For example, nanosheet of chitosan/calcium phosphates has been used successfully to transport internalized exogenous antigens by dendritic cells. The use of the chitosan/calcium system has increased antigen cross-presentation, indicating that it can be used as an efficient vaccine carrier (Pei et al., 2019). Several complexes of chitosan and functionalized nanoparticles have been used as adjuvants in an inactivated vaccine for Newcastle disease. The study shows that cellular immunity levels increased and there was a significant reduction in viral load, as observed by the expression of cytokines, lymphocyte proliferation, percentages of CD4 + and CD8 + T lymphocytes (Yang, Xing, et al., 2020). In a study conducted by Zhang et al. (2017) conjugated chitosan oligosaccharide vaccines increase the immune response to porcine circovirus type 2 (PCV2) vaccines. Conjugates have been shown to promote the proliferation of T lymphocytes, initiating a mixed immune response, including increased antibody production and regulated pro-inflammatory cytokine secretion.
Yang, Yang, Yang, and Wang (2015), isolated an alkali polysaccharide from the stems of Physalis alkekengi L, an exotic edible fruit. The structural analysis of the purified fraction indicates that the structure is formed by (1 → 3) -linked Glc, (1 → 3) -linked Gal, (1 → 2) -linked Xyl, (1 → 2) -linked Ara, and (1 → 2) -linked Rha residues, with a molecular weight of 31 kDa. The polysaccharide was used as an immune booster in a DNA vaccine, and the results show significantly increased IgG antibody response, but specifically higher IgG1 titers, as well as IgG2b. Although polysaccharides have relevant properties to be used as platforms for vaccine development, in-depth studies on membrane receptors, types of interactions, and mechanisms of modulating activity are still needed. In addition, there are still problems for large-scale polysaccharide production, especially those from natural sources. However, we believe that soon, more in-depth studies should elucidate profound aspects of the use of polysaccharides in vaccines and their potential in clinical studies. Perhaps, in the future, polysaccharides could be used to develop platforms for vaccines against COVID-19 and other infectious diseases.
6. Concluding remarks
Severe acute respiratory syndrome caused by the SARS-CoV-2 virus originating in China is a serious disease, with a high rate of transmissibility and mortality. In a short time, the disease spread across the world through infected people, thus increasing the spread of the disease. The disease has caused major changes in the world's routine, isolating people in their homes, destabilizing the world economy, and increasing mortality rates worldwide. Also, serious problems with the collapse of hospital networks and the economy in different countries have increased social insecurity and contributed to the reduction of people's physical and mental health. The disease, although known in several aspects about its immunopathogenesis, biology, and forms of transmission, is not yet fully understood as to the conditions under which the virus subverts the immune response.
Polysaccharides obtained from functional foods have interesting structural properties that contribute to a diversity of biological potentialities. The review showed that polysaccharides can modulate an immune response by various biochemical pathways. Also, these biopolymers can be found in natural and edible sources such as mushrooms, plants, algae, fruits, and other matrices. The potential of natural polysaccharides for disease prevention is clear, as demonstrated by several updated research reports. Foods rich in these biopolymers can be used safely since they have no toxic effects are biocompatible and biodegradable. In addition, polysaccharides obtained and purified from these food sources may be the key in the development of new drugs and immunity-boosting therapies.
Although several studies, some well advanced, and research groups distributed around the world are committed to the development of vaccines and drugs for the treatment of SARS-CoV-2, the disease continues to progress exponentially worldwide. Thus, new ways of preventing and stimulating efficient immune responses must be tested. In this context, polysaccharides can play a crucial role. Based on several studies carried out in the last 5 years, we concluded that natural polysaccharides can be used as platforms for the production of vaccines, acting as coadjuvants, since they can easily bind to various cell receptors. Finally, we conclude that although the use of isolated polysaccharides is ideal due to bioavailability, we emphasize that the consumption of foods rich in biologically active polysaccharides is also useful. Even though the biological effects are not equivalent to those of the isolated polysaccharides, it can still help people to increase immunity, reducing the risk of a complicated clinical condition if infected with SARS-CoV-2.
Declaration of competing interest
The author declare no conflict of interest.
Acknowledgment
Jhonatas Rodrigues Barbosa thanks the Federal University of Para (UFPA) for the space for scientific development.
References
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nature Medicine. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Huang F., Zhang R., Dong L., Jia X., Liu L.…Zhang M. Longan pulp polysaccharides relieve intestinal injury in vivo and in vitro by promoting tight junction expression. Carbohydrate Polymers. 2020;229:115475. doi: 10.1016/j.carbpol.2019.115475. [DOI] [PubMed] [Google Scholar]
- Barbosa J.R., de Carvalho Junior R.N. Occurrence and possible roles of polysaccharides in fungi and their influence on the development of new technologies. Carbohydrate Polymers. 2020;246:116613. doi: 10.1016/j.carbpol.2020.116613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa J.R., dos Santos Freitas M.M., da Silva Martins L.H., de Carvalho Junior R.N. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. Carbohydrate Polymers. 2020;229:115550. doi: 10.1016/j.carbpol.2019.115550. [DOI] [PubMed] [Google Scholar]
- Barbosa J.R., dos Santos Freitas M.M., de Oliveira L.C., da Silva Martins L.H., de Almada-Vilhena A.O., de Oliveira R.M.…de Carvalho Junior R.N. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chemistry. 2020;330:127173. doi: 10.1016/j.foodchem.2020.127173. [DOI] [PubMed] [Google Scholar]
- Chaiyama V., Keawsompong S., LeBlanc J.G., de LeBlanc A.D.M., Chatel J.M., Chanput W. Action modes of the immune modulating activities of crude mushroom polysaccharide from Phallus atrovolvatus. Bioactive Carbohydrates and Dietary Fibre. 2020;23:100216. doi: 10.1016/j.bcdf.2020.100216. [DOI] [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J.…Tsoi H.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H.…Zhang X. Clinical and immunological features of severe and moderate coronavirus disease 2019. Journal of Clinical Investigation. 2020;130(5):2620–2629. doi: 10.1172/jci137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y.…Yu T. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. The Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F.…Lai T.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney International. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L., Wang W., Luo Y., Ning Q., Xia Z., Chen J.…Tan W. Polysaccharide from Scutellaria baicalensis Georgi ameliorates colitis via suppressing NF-κB signaling and NLRP3 inflammasome activation. International Journal of Biological Macromolecules. 2019;132:393–405. doi: 10.1016/j.ijbiomac.2019.03.230. [DOI] [PubMed] [Google Scholar]
- Curtis N., Sparrow A., Ghebreyesus T.A., Netea M.G. Considering BCG vaccination to reduce the impact of COVID-19. The Lancet. 2020;395:1545–1546. doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Fu H., Shang J., Chen J., Xu X. Dectin-1 mediates the immunoenhancement effect of the polysaccharide from Dictyophora indusiata. International Journal of Biological Macromolecules. 2018;109:369–374. doi: 10.1016/j.ijbiomac.2017.12.113. [DOI] [PubMed] [Google Scholar]
- Ferreira S.S., Passos C.P., Madureira P., Vilanova M., Coimbra M.A. Structure–function relationships of immunostimulatory polysaccharides: A review. Carbohydrate Polymers. 2015;132:378–396. doi: 10.1016/j.carbpol.2015.05.079. [DOI] [PubMed] [Google Scholar]
- Fu X., Belwal T., Cravotto G., Luo Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrasonics Sonochemistry. 2020;60:104726. doi: 10.1016/j.ultsonch.2019.104726. [DOI] [PubMed] [Google Scholar]
- Galanakis C.M. Food and bioproducts processing short communication emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food and Bioproducts Processing. 2013;91:575–579. doi: 10.1016/j.fbp.2013.01.004. [DOI] [Google Scholar]
- Galanakis C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends in Food Science & Technology. 2015;42(1):44–63. doi: 10.1016/j.tifs.2014.11.005. [DOI] [Google Scholar]
- Galanakis C.M. Phenols recovered from olive mill wastewater as additives in meat products. Trends in Food Science & Technology. 2018;79:98–105. doi: 10.1016/j.tifs.2018.07.010. [DOI] [Google Scholar]
- Galanakis C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods. 2020;9(4):523. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K.…Fricchione M.J. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. The Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J., Drosten C., Gulyaeva A.A.…Ziebuhr J. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X.…Du B. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbwachs H., Simmel J. Some like it hot, some not–Tropical and arctic mushrooms. Fungal Biology Reviews. 2018;32(3):143–155. doi: 10.1016/j.fbr.2018.04.001. [DOI] [Google Scholar]
- Han B., Xu K., Liu Z., Ge W., Shao S., Li P.…Zhang Z. Oral yeast-based DNA vaccine confers effective protection from Aeromonas hydrophila infection on Carassius auratus. Fish & Shellfish Immunology. 2019;84:948–954. doi: 10.1016/j.fsi.2018.10.065. [DOI] [PubMed] [Google Scholar]
- He W., Chen L., Chen L., Yuan G., Fang Y., Chen W.…Li L. COVID-19 in persons with haematological cancers. Leukemia. 2020:1–9. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Fang J., Guo Q., Wang M., Li Y., Meng Y., et al. Advances in antiviral polysaccharides derived from edible and medicinal plants and mushrooms. Carbohydrate Polymers. 2020;229:115548. doi: 10.1016/j.carbpol.2019.115548. [DOI] [PubMed] [Google Scholar]
- Huang Z., Zeng Y.J., Chen X., Luo S.Y., Pu L., Li F.Z.…Lou W.Y. A novel polysaccharide from the roots of Millettia Speciosa champ: Preparation, structural characterization and immunomodulatory activity. International Journal of Biological Macromolecules. 2020;145:547–557. doi: 10.1016/j.ijbiomac.2019.12.166. [DOI] [PubMed] [Google Scholar]
- Hu W., Jiang Y., Xue Q., Sun F., Zhang J., Zhou J.…Shen T. Structural characterisation and immunomodulatory activity of a polysaccharide isolated from lotus (Nelumbo nucifera Gaertn.) root residues. Journal of Functional Foods. 2019;60:103457. doi: 10.1016/j.jff.2019.103457. [DOI] [Google Scholar]
- Hu T., Lin Q., Guo T., Yang T., Zhou W., Deng X.…Luo F. Polysaccharide isolated from Phellinus linteus mycelia exerts anti-inflammatory effects via MAPK and PPAR signaling pathways. Carbohydrate Polymers. 2018;200:487–497. doi: 10.1016/j.carbpol.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Jeong H.K., Lee D., Kim H.P., Baek S.H. Structure analysis and antioxidant activities of an amylopectin-type polysaccharide isolated from dried fruits of Terminalia chebula. Carbohydrate Polymers. 2019;211:100–108. doi: 10.1016/j.carbpol.2019.01.097. [DOI] [PubMed] [Google Scholar]
- Jimenez M.D., Lobo M., Sammán N. 12th IFDC 2017 Special Issue–Influence of germination of quinoa (Chenopodium quinoa) and amaranth (Amaranthus) grains on nutritional and techno-functional properties of their flours. Journal of Food Composition and Analysis. 2019;84:103290. doi: 10.1016/j.jfca.2019.103290. [DOI] [Google Scholar]
- Jin W., Zhang W., Liu G., Yao J., Shan T., Sun C., et al. The structure-activity relationship between polysaccharides from Sargassum thunbergii and anti-tumor activity. International Journal of Biological Macromolecules. 2017;105:686–692. doi: 10.1016/j.ijbiomac.2017.07.089. [DOI] [PubMed] [Google Scholar]
- Jin M., Zhao K., Huang Q., Xu C., Shang P. Isolation, structure and bioactivities of the polysaccharides from Angelica sinensis (oliv.) Diels: A review. Carbohydrate Polymers. 2012;89(3):713–722. doi: 10.1016/j.carbpol.2012.04.049. [DOI] [PubMed] [Google Scholar]
- Kakar M.U., Naveed M., Saeed M., Zhao S., Rasheed M., Firdos S.…Dai R. A review on structure, extraction, and biological activities of polysaccharides isolated from Cyclocarya paliurus (Batalin) Iljinskaja. International Journal of Biological Macromolecules. 2020;156:420–429. doi: 10.1016/j.ijbiomac.2020.04.022. [DOI] [PubMed] [Google Scholar]
- Kujawski S.A., Wong K.K., Collins J.P., Epstein L., Killerby M.E., Midgley C.M.…Beer K. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nature Medicine. 2020;26 doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- de Lacerda Bezerra I., Caillot A.R.C., de Oliveira A.F., Santana-Filho A.P., Sassaki G.L. Cabernet Sauvignon wine polysaccharides attenuate sepsis inflammation and lethality in mice. Carbohydrate Polymers. 2019;210:254–263. doi: 10.1016/j.carbpol.2019.01.025. [DOI] [PubMed] [Google Scholar]
- de Lacerda Bezerra I., Caillot A.R.C., Palhares L.C.G.F., Santana-Filho A.P., Chavante S.F., Sassaki G.L. Structural characterization of polysaccharides from cabernet franc, cabernet sauvignon and sauvignon blanc wines: Anti-inflammatory activity in LPS stimulated RAW 264.7 cells. Carbohydrate Polymers. 2018;186:91–99. doi: 10.1016/j.carbpol.2017.12.082. [DOI] [PubMed] [Google Scholar]
- Lamothe L.M., Srichuwong S., Reuhs B.L., Hamaker B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chemistry. 2015;167:490–496. doi: 10.1016/j.foodchem.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Li D., Chen Y., Liu H., Jia Y., Li F., Wang W.…Zeng R. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: Insights from ERS-COVID-19 study. Signal Transduction and Targeted Therapy. 2020;5(1):1–3. doi: 10.1038/s41392-020-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Gu F., Cai C., Hu M., Fan L., Hao J., et al. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. International Journal of Biological Macromolecules. 2020;143:806–813. doi: 10.1016/j.ijbiomac.2019.09.141. [DOI] [PubMed] [Google Scholar]
- Li Y., Hu Y., Yu J., Ma T. Retrospective analysis of laboratory testing in 54 patients with severe-or critical-type 2019 novel coronavirus pneumonia. Laboratory Investigation. 2020:1–7. doi: 10.1038/s41374-020-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Zhou G., Ren C., Xu K., Yan Q., Li X.…Zhang Z. Oral administration of myostatin-specific recombinant Saccharomyces cerevisiae vaccine in rabbit. Vaccine. 2016;34:2378–2382. doi: 10.1016/j.vaccine.2016.03.036. [DOI] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K.…Wang D.Q. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine. 2020:1–4. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Lucas C., Wong P., Klein J., Castro T.B., Silva J., Sundaram M.…Takahashi T. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020:1–9. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.S., Liu H., Han C.R., Zeng S.J., Xu X.J., Lu D.J., et al. Extraction, characterization and antioxidant activity of polysaccharide from Pouteria campechiana seed. Carbohydrate Polymers. 2020;229:115409. doi: 10.1016/j.carbpol.2019.115409. [DOI] [PubMed] [Google Scholar]
- Meng F., Li Q., Qi Y., He C., Wang C., Zhang Q. Characterization and immunoregulatory activity of two polysaccharides from the root of Ilex asprella. Carbohydrate Polymers. 2018;197:9–16. doi: 10.1016/j.carbpol.2018.05.066. [DOI] [PubMed] [Google Scholar]
- Mingyi Y., Belwal T., Devkota H.P., Li L., Luo Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends in Food Science & Technology. 2019;92:94–110. doi: 10.1016/j.tifs.2019.08.009. [DOI] [Google Scholar]
- Mohan K., Muralisankar T., Uthayakumar V., Chandirasekar R., Revathi N., Abirami R.G.…Seedevi P. Trends in the extraction, purification, characterisation and biological activities of polysaccharides from tropical and sub-tropical fruits-A comprehensive review. Carbohydrate Polymers. 2020:116185. doi: 10.1016/j.carbpol.2020.116185. [DOI] [PubMed] [Google Scholar]
- Nagarajan J., Krishnamurthy N.P., Ramanan R.N., Raghunandan M.E., Galanakis C.M., Ooi C.W. A facile water-induced complexation of lycopene and pectin from pink guava byproduct: Extraction, characterization and kinetic studies. Food Chemistry. 2019;296:47–55. doi: 10.1016/j.foodchem.2019.05.135. [DOI] [PubMed] [Google Scholar]
- Ng J.Y., Obuobi S., Chua M.L., Zhang C., Hong S., Kumar Y.…Ee P.L.R. Biomimicry of microbial polysaccharide hydrogels for tissue engineering and regenerative medicine–A review. Carbohydrate Polymers. 2020:116345. doi: 10.1016/j.carbpol.2020.116345. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L., Wang Q. Viral load of SARS-CoV-2 in clinical samples. The Lancet Infectious Diseases. 2020;20(4):411–412. doi: 10.1016/s1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei M., Liang J., Zhang C., Wang X., Zhang C., Ma G., et al. Chitosan/calcium phosphates nanosheet as a vaccine carrier for effective cross-presentation of exogenous antigens. Carbohydrate Polymers. 2019;224:115172. doi: 10.1016/j.carbpol.2019.115172. [DOI] [PubMed] [Google Scholar]
- Peng B., Luo Y., Hu X., Song L., Yang J., Zhu J.…Yu R. Isolation, structural characterization, and immunostimulatory activity of a new water-soluble polysaccharide and its sulfated derivative from Citrus medica L. var. sarcodactylis. International Journal of Biological Macromolecules. 2019;123:500–511. doi: 10.1016/j.ijbiomac.2018.11.113. [DOI] [PubMed] [Google Scholar]
- Rizou M., Galanakis I.M., Aldawoud T.M., Galanakis C.M. Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends in Food Science & Technology. 2020;102:293–299. doi: 10.1016/j.tifs.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthes A.C., Smiderle F.R., Iacomini M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydrate Polymers. 2016;136:358–375. doi: 10.1016/j.carbpol.2015.08.061. [DOI] [PubMed] [Google Scholar]
- Sanjeewa K.A., Kang N., Ahn G., Jee Y., Kim Y.T., Jeon Y.J. Bioactive potentials of sulfated polysaccharides isolated from brown seaweed Sargassum spp in related to human health applications: A review. Food Hydrocolloids. 2018;81:200–208. doi: 10.1016/j.foodhyd.2018.02.040. [DOI] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H.…Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:1–4. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T., Wang G., You L., Zhang L., Ren H., Hu W.…Zhao X. Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food & Nutrition Research. 2017;61(1):1344523. doi: 10.1080/16546628.2017.1344523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Zhu M., Hao H., Deng J., Li M., Sun Y.…Huang R. Structure characterization of a novel polysaccharide from Chinese wild fruits (Passiflora foetida) and its immune-enhancing activity. International Journal of Biological Macromolecules. 2019;136:324–331. doi: 10.1016/j.ijbiomac.2019.06.090. [DOI] [PubMed] [Google Scholar]
- Sousa S.G., Oliveira L.A., de Aguiar Magalhães D., de Brito T.V., Batista J.A., Pereira C.M.C.…da Silva D.A. Chemical structure and anti-inflammatory effect of polysaccharide extracted from Morinda citrifolia Linn (Noni) Carbohydrate Polymers. 2018;197:515–523. doi: 10.1016/j.carbpol.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Stillman M.D., Capron M., Alexander M., Di Giusto M.L., Scivoletto G. COVID-19 and spinal cord injury and disease: Results of an international survey. Spinal Cord Series and Cases. 2020;6(1):1–4. doi: 10.1038/s41394-020-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Li L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydrate Polymers. 2020;229:115407. doi: 10.1016/j.carbpol.2019.115407. [DOI] [PubMed] [Google Scholar]
- Su F., Song Q., Zhang C., Xu X., Li M., Yao D.…Yang J. A β-1, 3/1, 6-glucan from Durvillaea Antarctica inhibits tumor progression in vivo as an immune stimulator. Carbohydrate Polymers. 2019;222:114993. doi: 10.1016/j.carbpol.2019.114993. [DOI] [PubMed] [Google Scholar]
- Tabarsa M., You S., Yelithao K., Palanisamy S., Prabhu N.M., Nan M. Isolation, structural elucidation and immuno-stimulatory properties of polysaccharides from Cuminum cyminum. Carbohydrate Polymers. 2020;230:115636. doi: 10.1016/j.carbpol.2019.115636. [DOI] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F. The trinity of COVID-19: Immunity, inflammation and intervention. Nature Reviews Immunology. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M., Weng A., Fuchs H., Sharma V., Bhargava C.S., Chauhan N.S.…Bhargava S. Rasayana properties of Ayurvedic herbs: Are polysaccharides a major contributor. Carbohydrate Polymers. 2012;87(1):3–15. doi: 10.1016/j.carbpol.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Thanh Le T.A.Z., Kumar A., Gomez Roman R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nature Reviews Drug Discovery. 2020;10–10 doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- Tong H., Wu S., Song K., Liu J., Song X., Zhang X.…Wu M. Characterization of a P-selectin-binding moiety from Bupleurum chinense polysaccharide and its antagonistic effect against P-selectin-mediated function. Carbohydrate Polymers. 2018;196:110–116. doi: 10.1016/j.carbpol.2018.05.035. [DOI] [PubMed] [Google Scholar]
- Wang L., Chen L., Li J., Di L., Wu H. Structural elucidation and immune-enhancing activity of peculiar polysaccharides fractioned from marine clam Meretrix meretrix (Linnaeus) Carbohydrate Polymers. 2018;201:500–513. doi: 10.1016/j.carbpol.2018.08.106. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hwang J.Y., Park H.B., Yadav D., Oda T., Jin J.O. Porphyran isolated from Pyropia yezoensis inhibits lipopolysaccharide-induced activation of dendritic cells in mice. Carbohydrate Polymers. 2020;229:115457. doi: 10.1016/j.carbpol.2019.115457. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang W., Hou L., Qin L., He M., Li W., et al. A sulfated glucuronorhamnan from the green seaweed Monostroma nitidum: Characteristics of its structure and antiviral activity. Carbohydrate Polymers. 2020;227:115280. doi: 10.1016/j.carbpol.2019.115280. [DOI] [PubMed] [Google Scholar]
- Wang N., Zhang X., Wang S., Guo Q., Li Z., Liu H., et al. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydrate Polymers. 2020;227:115314. doi: 10.1016/j.carbpol.2019.115314. [DOI] [PubMed] [Google Scholar]
- Weitz J.S., Beckett S.J., Coenen A.R., Demory D., Dominguez-Mirazo M., Dushoff J.…Rodriguez-Gonzalez R. Modeling shield immunity to reduce COVID-19 epidemic spread. Nature Medicine. 2020:1–6. doi: 10.1038/s41591-020-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y.…Dong L. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discovery. 2020;6(1):1–18. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O.…McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Luo M., Yao X., Yu L. Purification, structural characterization, and antioxidant activity of the COP-W1 polysaccharide from Codonopsis tangshen Oliv. Carbohydrate Polymers. 2020;236:116020. doi: 10.1016/j.carbpol.2020.116020. [DOI] [PubMed] [Google Scholar]
- Xiong Q., Hao H., He L., Jing Y., Xu T., Chen J.…Yuan J. Anti-inflammatory and anti-angiogenic activities of a purified polysaccharide from flesh of Cipangopaludina chinensis. Carbohydrate Polymers. 2017;176:152–159. doi: 10.1016/j.carbpol.2017.08.073. [DOI] [PubMed] [Google Scholar]
- Yamey G., Schäferhoff M., Hatchett R., Pate M., Zhao F., McDade K.K. Ensuring global access to COVID-19 vaccines. The Lancet. 2020;395:1405–1406. doi: 10.1016/S0140-6736(20)30763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xing R., Liu S., Qin Y., Li K., Yu H., et al. Chitosan, hydroxypropyltrimethyl ammonium chloride chitosan and sulfated chitosan nanoparticles as adjuvants for inactivated Newcastle disease vaccine. Carbohydrate Polymers. 2020;229:115423. doi: 10.1016/j.carbpol.2019.115423. [DOI] [PubMed] [Google Scholar]
- Yang J., Yang F., Yang H., Wang G. Water-soluble polysaccharide isolated with alkali from the stem of physalis alkekengi l.: Structural characterization and immunologic enhancement in DNA vaccine. Carbohydrate Polymers. 2015;121:248–253. doi: 10.1016/j.carbpol.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y.…Wang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;395(10223):P497–P506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey L.J., Lucas C., Iwasaki A. Contributions of maternal and fetal antiviral immunity in congenital disease. Science. 2020;368(6491):608–612. doi: 10.1126/science.aaz1960. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Zhang J., Xiao C., Harqin C., Ma M., Long T.…Zhao L. Structural characterization of a low-molecular-weight polysaccharide from Angelica pubescens Maxim. f. biserrata Shan et Yuan root and evaluation of its antioxidant activity. Carbohydrate Polymers. 2020;236:116047. doi: 10.1016/j.carbpol.2020.116047. [DOI] [PubMed] [Google Scholar]
- Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X.…Chen S. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. The Lancet Infectious Diseases. 2020;20:559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Jia P., Cheng G., Jiao S., Ren L., Ji S.…Du Y. Enhanced immune response to inactivated porcine circovirus type 2 (PCV2) vaccine by conjugation of chitosan oligosaccharides. Carbohydrate Polymers. 2017;166:64–72. doi: 10.1016/j.carbpol.2017.02.058. [DOI] [PubMed] [Google Scholar]
- Zhang J., Litvinova M., Wang W., Wang Y., Deng X., Chen X.…Wu Q. 2020. Evolving epidemiology of novel coronavirus diseases 2019 and possible interruption of local transmission outside hubei province in China: A descriptive and modeling study. MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Wang L., Tan B., Zhang W. Dietary fibre extracted from different types of whole grains and beans: A comparative study. International Journal of Food Science and Technology. 2020;55(5):2188–2196. doi: 10.1111/ijfs.14472. [DOI] [Google Scholar]
- Zhang Y., Zeng Y., Cui Y., Liu H., Dong C., Sun Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydrate Polymers. 2020;235:115969. doi: 10.1016/j.carbpol.2020.115969. [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhang X., Tang Y., Mao J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydrate Polymers. 2020;228:115381. doi: 10.1016/j.carbpol.2019.115381. [DOI] [PubMed] [Google Scholar]
- Zheng L.X., Chen X.Q., Cheong K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. International Journal of Biological Macromolecules. 2020;152:344–354. doi: 10.1016/j.ijbiomac.2020.02.168. [DOI] [PubMed] [Google Scholar]
- Zheng T., Gu D., Wang X., Shen X., Yan L., Zhang W.…Fan J. Purification, characterization and immunomodulatory activity of polysaccharides from Leccinum crocipodium (Letellier.) Watliag. International Journal of Biological Macromolecules. 2020;148:647–656. doi: 10.1016/j.ijbiomac.2020.01.155. [DOI] [PubMed] [Google Scholar]
- Zhong R., Wan X., Wang D., Zhao C., Liu D., Gao L.…Capanoglu E. Polysaccharides from marine Enteromorpha: Structure and function. Trends in Food Science & Technology. 2020;99:11–20. doi: 10.1016/j.tifs.2020.02.030. [DOI] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W.…Chen H.D. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z.…Guan L. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. The lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydrate Polymers. 2020:116819. doi: 10.1016/j.carbpol.2020.116819. [DOI] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J.…Niu P. A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoviadou K.G., Galanakis C.M., Brnčić M., Grimi N., Boussetta N., Mota M.J.…Barba F.J. Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Research International. 2015;77:743–752. doi: 10.1016/j.foodres.2015.05.032. [DOI] [Google Scholar]
- Zou S., Duan B., Xu X. Inhibition of tumor growth by β-glucans through promoting CD4+ T cell immunomodulation and neutrophil-killing in mice. Carbohydrate Polymers. 2019;213:370–381. doi: 10.1016/j.carbpol.2019.03.006. [DOI] [PubMed] [Google Scholar]