Abstract
Introduction: Polycystic ovary syndrome (PCOS) is a common endocrine disorder affecting women of reproductive age. The aim of this study was to determine the variations in the clinical presentation and frequency of metabolic syndrome (MetS) in women with PCOS.
Methods: This cross-sectional study was conducted at the Baqai Institute of Diabetology and Endocrinology, Baqai Medical University, Karachi, Pakistan, from April 2019 to March 2020. Women attending the endocrine clinic who satisfied the Rotterdam criteria of PCOS and agreed to participate in the study were included. Detailed personal and family history of menstrual cycle, hirsutism, diabetes, hypertension, dyslipidemia and obesity was noted along with measurement of vitals, anthropometric measures and calculation of the body mass index. Physical examination performed for signs of hyperandrogenism, insulin resistance and biochemical and hormonal evaluation was also carried out in recruited participants. Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) version 20 (IBM Corp., Armonk, NY).
Results: A total of 153 participants with mean age of 27.2±8.13 years were included in this study. Regarding clinical presentation, menstrual irregularity (oligomenorrhea 39.85%, amenorrhea 38.9%), followed by hirsutism 52.3%, was the most common presentation. Polycystic appearance of ovaries was noted in 33.3% of our study participants. MetS was identified in 46.4% participants (obesity was noted at the highest frequency at 82.4% followed by dyslipidemia at 56.2%).
Conclusion: We observed a high frequency of MetS in females presenting with PCOS. There is a need to evaluate women with PCOS for various components of MetS to prevent potential complications.
Keywords: pcos, metabolic syndrome, clinical presentation
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders that affects 5%-10% women of reproductive age [1,2]. It is clearly heterogeneous with speculative etiology, causing a wide range of reproductive, metabolic, endocrine and psychological effects. Among them, ovulatory dysfunction, menstrual irregularities, infertility, hyperandrogenism, increased insulin level, obesity, obstructive sleep apnea, nonalcoholic fatty liver disease, eating and mood disorders, cardiovascular disease (CVD) and an increased risk of type 2 diabetes mellitus (T2DM) are significant factors [3,4]. However, the most common presenting problem observed in PCOS is menstrual cycle disturbances (oligo/amenorrhea), hirsutism, infertility, dyslipidemia and metabolic disturbances due to insulin resistance (IR) [5]. Hyperinsulinemia, with consequent IR, has a significant role to play in its pathogenesis [6]. The diagnosis of PCOS is based on the presence of at least two of the three criteria, namely, chronic anovulation, hyperandrogenism (clinical or biological) and polycystic ovaries on ultrasound, according to Rotterdam criteria [2], which are widely used for the diagnosis of PCOS [6], in addition to excluding all other potential causes.
The prevalence of metabolic syndrome (MetS) is increasing throughout the world [7]. MetS and PCOS share a bidirectional relationship and common pathogenic factors that predispose women with PCOS to an increased risk of developing MetS [8]. Women with PCOS have a fivefold increase risk of developing MetS compared with women without PCOS, suggesting PCOS alone is an independent risk factor for MetS [9]. In one study, the reported prevalence of MetS was 44.6% in PCOS women [10]. MetS is a constellation of disorders that include abdominal obesity, impaired glucose tolerance/DM, hypertension and/or dyslipidemia [11]. MetS predispose patients with PCOS to high risk of CVD, T2DM and gynecological cancer [11]. PCOS and MetS share a bidirectional relationship and both conditions produce profound effect on fertility, reproductive biology and oxidative stress-induced vascular complications [8].
In a study conducted in Karachi, Pakistan, the reported prevalence of MetS in women with PCOS was 35.6%, which was significantly greater than the control group [12]. This substantial risk of increased morbidity and mortality has long-term implications for health of women with PCOS [13]. Data regarding the association of MetS and PCOS is scarce in our part of the world; therefore, our objective was to determine the clinical presentation and frequency of MetS in women presenting with PCOS at a tertiary care hospital [4].
Materials and methods
This cross-sectional study was conducted at the Baqai Institute of Diabetology and Endocrinology (BIDE), Baqai Medical University, Karachi, Pakistan, over a period of one year from April 1, 2019, to March 31, 2020. All 13- to 45-year-old females attending the outpatient department satisfying the Rotterdam criteria of PCOS (defined as the presence of two of the following: oligo/anovulation, hyperandrogenism and/or polycystic ovaries on ultrasound) were recruited through non-probability consecutive sampling after taking informed verbal consent. Other causes of hyperandrogenism and menstrual irregularity were excluded by doing relevant lab investigations. Ethical approval was taken from the Institutional Review Board of BIDE.
Detailed personal, menstrual and family history of hypertension, diabetes, dyslipidemia and obesity (MetS) was taken on a predesigned proforma. MetS by definition comprises three or more of the following: (1) central obesity with waist circumference ≥80 cm (WHO cutoff for Asian women), (2) systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or taking antihypertensive medication, (3) fasting plasma glucose ≥100 mg/dl or previously diagnosed type 2 diabetes, (4) plasma high-density lipoprotein (HDL) cholesterol <50 mg/dl, (5) plasma triglycerides >150 mg/dl (as per American Heart Association/National Heart, Lung, and Blood Institute [AHA/NHLBI] criteria) [14]. Detailed physical examination was done including the measurement of blood pressure, pulse, waist circumference, and BMI. Signs of insulin resistance (acanthosis nigricans, skin tags) and signs of hyperandrogenism (hirsutism, acne, alopecia, clitoromegaly) were noted. The Ferriman-Gallwey score was used to assess the hirsutism (score >8 was considered positive). The biochemical and hormonal evaluation, including fasting plasma glucose, insulin, lipid profile, thyroid stimulating hormone, follicle stimulating hormone, luteinizing hormone, prolactin, estradiol, testosterone, 17-hydroxyprogesterone, dehydroepiandrosterone level, was carried out. The homeostatic model assessment of insulin resistance (HOMA-IR) was used to calculate insulin resistance: HOMA-IR = glucose fasting (mg/dl) x fasting insulin (µUI/ml) ÷ 405; values above 1.5 are indicative of insulin resistance. Pelvic ultrasound was performed for the evaluation of ovarian morphology.
Data analysis was done using the Statistical Package for the Social Sciences (SPSS) version 20 (IBM Corp., Armonk, NY) to compute mean, standard deviation, and percentages.
Results
A total of 153 participants with a mean age of 27.2±8.13 years were included in this study. The mean age of menarche was 12.68±1.2 years while the mean BMI was found to be 31.68±7.37 (kg/m2). The baseline characteristics and biochemical parameters are mentioned in Table 1.
Table 1. Baseline characteristics and biochemical parameters of study participants.
HOMA-IR, homeostatic model assessment of insulin resistance; FSH, follicle stimulating hormone; LH, luteinizing hormone; TSH, thyroid stimulating hormone; IQR, interquartile range.
Data is presented as mean±SD or n (%) or median (IQR).
| Parameters | Mean±SD or N (%) or median (IQR) |
| N | 153 |
| Age (years) | 27.2±8.13 |
| Age at menarche (years) | 12.68±1.2 |
| Marital status | |
| Single | 65 (43.9%) |
| Married | 83 (55.1%) |
| BMI (kg/m2) | 31.68±7.37 |
| Waist circumference (cm) | 86.71±16.72 |
| Systolic blood pressure (mmHg) | 116.37±15.14 |
| Diastolic blood pressure (mmHg) | 78.83±11.26 |
| Fasting insulin | 15 (10-24) |
| Fasting blood sugar (mg/dl) | 96.35±29.4 |
| HOMA-IR | 4.71±4.11 |
| FSH | 5.3 (3.7-8.7) |
| LH | 7.4 (4.9-14.4) |
| Estradiol | 77 (33.4-155) |
| Testosterone | 13.5 (1-57.6) |
| TSH | 2.6±2.0 |
| Prolactin | 21.5±14.17 |
The most common clinical presentation of women with PCOS was menstrual irregularity; oligomenorrhea was reported in 61 (39.85%) and amenorrhea in 44 (38.9%), followed by hirsutism that was noted in 80 (52.3%) while acne and alopecia were found in 33 (21.5%) and 24 (15.6%), respectively. Moreover, infertility was present in 50 (32.6%) women, whereas 51 (33.3%) women had polycystic morphology of ovaries on ultrasound. The family history of diabetes, hypertension, dyslipidemia and obesity was present in 85 (55.6%), 65 (42.5%), 22 (14.4%), and 44 (28.8%) participants, respectively, as mentioned in Table 2.
Table 2. Clinical presentations of study participants.
PCOM, polycystic ovarian morphology.
Data is presented as n (%).
| Parameters | Frequency (n) | Percentage |
| N | 153 | - |
| Menstrual cycle irregularity | 89 | 58.2 |
| Dysmenorrhea | 41 | 26.7 |
| Oligomenorrhea | 61 | 39.8 |
| Amenorrhea | 44 | 28.7 |
| Dysfunctional uterine bleeding | 17 | 11.2 |
| Infertility | 50 | 32.6 |
| Hirsutism | 80 | 52.3 |
| Alopecia | 24 | 15.6 |
| Acne | 33 | 21.5 |
| Polycystic ovaries on ultrasound (PCOM) | 51 | 33.3 |
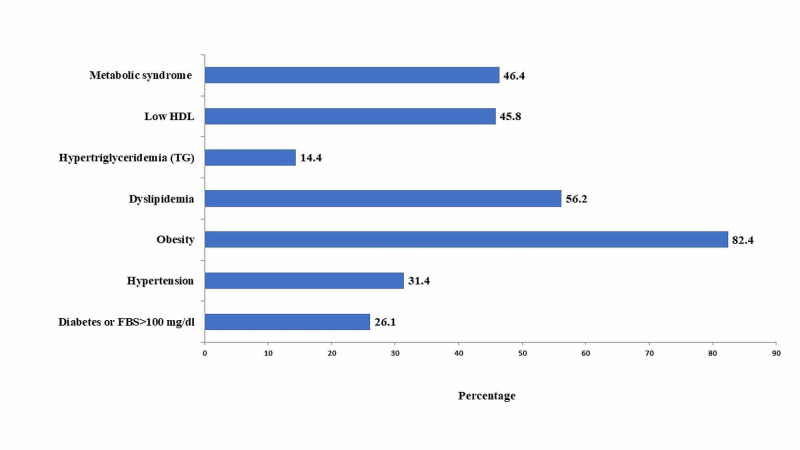
The frequency and percentages of MetS and its individual parameters are shown in Figure 1. Out of 153 women with PCOS, 71 (46.4%) participants fulfilled the criteria of MetS. The obesity was noted in the highest frequency, in 126 (82.4%), followed by dyslipidemia, in 86 (56.2%), whereas 48 (31%) females had hypertension and 40 (26%) were either diagnosed with diabetes or impaired glucose tolerance.
Figure 1. Frequency of metabolic syndrome in study participants.
HDL, high-density lipoprotein; FBS, fasting blood sugar.
Age, BMI, waist circumference and blood pressure had statistically significant association in PCOS women with MetS. The comparison among PCOS women with and without MetS in terms of baseline characteristics and lab parameters is shown in Table 3.
Table 3. Comparison of different variables in PCOS women with and without MetS.
PCOS, polycystic ovary syndrome; MetS, metabolic syndrome; HOMA, homeostatic model assessment; FSH, follicle stimulating hormone; LH, luteinizing hormone; TSH, thyroid stimulating hormone; IQR, interquartile range.
Data is presented as mean±SD or n (%) or median (IQR). P-value <0.05 was considered to be statistically significant.
| Variables | Without MetS | With MetS | P-value |
| N | 82 | 71 | - |
| Age (years) | 25.63±7.5 | 29.01±8.5 | 0.01 |
| Age at menarche (years) | 12.82±1.21 | 12.51±1.17 | 0.146 |
| Marital status | |||
| Single | 37 (44.21%) | 28 (39.4%) | 0.291 |
| Married | 40 (48.7%) | 43 (60.6%) | |
| Not known | 5 (6.1%) | 0 (0%) | |
| BMI (kg/m2) | 28.64±7.12 | 34.89±6.2 | <0.0001 |
| Waist circumference (cm) | 75.97±17.99 | 93.29±11.9 | <0.0001 |
| Systolic blood pressure (mmHg) | 110.3±12.55 | 122.43±15.16 | <0.0001 |
| Diastolic blood pressure (mmHg) | 74.16±8.62 | 83.9±11.64 | <0.0001 |
| HOMA | 4.47±3.58 | 4.88±4.48 | 0.693 |
| Fasting blood glucose (mg/dl) | 89.73±10.57 | 101.06±36.85 | 0.073 |
| Fasting insulin | 14 (9.2-19.8) | 16.5 (10.6-25.25) | 0.505 |
| FSH | 5.4 (4.2-8.7) | 5.1 (3.5-8.5) | 0.592 |
| LH | 7.2 (5.1-21.0) | 7.5 (4.8-12.6) | 0.367 |
| Estradiol | 74 (31.2-80) | 165 (35-242) | 0.222 |
| Testosterone | 20 (1-62.25) | 7.5 (1.75-39.75) | 0.782 |
| TSH | 2.4±1.76 | 2.7±2.28 | 0.616 |
| Prolactin | 24.43±17.15 | 19.65±11.86 | 0.254 |
As far as the individual component of MetS is concerned, diabetes was more prevalent in family members of women with PCOS followed by hypertension, obesity and dyslipidemia as shown in Figure 2.
Figure 2. The components of metabolic syndrome in family members of women with PCOS.
PCOS, polycystic ovary syndrome.
Discussion
The present study demonstrated a high frequency of MetS in women with PCOS. Among the different parameters of MetS, obesity and dyslipidemia were the two most frequent components in our study participants, while diabetes and hypertension were reported more in family members of the recruited women. The most common presenting complaint was menstrual irregularity (oligomenorrhea, amenorrhea) followed by hirsutism in our study participants.
The manifestation of MetS and clustering of its component varies in relation to studied population, ethnicity, and criteria applied to identify the MetS [12,15]. We observed MetS in almost half of our study participants; a similar finding was observed by Ehrmann et al., who reported a prevalence of 33.4% [15]. However, in contrast to our finding, MetS was noted only in 11.9% participants in another study [16].
Metabolic derangements are more obvious in obese as compared to non-obese PCOS counterparts [9]. Two-thirds of our study participants were obese; a similar prevalence was reflected in other studies [1,17]. In agreement with our observation, Anjum et al. [12] and Ehrmann et al. [15] observed that MetS and its distinct components are particularly common among females with increased BMI. However, Akram and Roohi [18] and Shanmugham et al. [19] reported obesity in only one-third of their study participants; this may be explained by the difference in the prevalence of obesity in different parts of the world [1]. The reported prevalence of obesity in Pakistan was 62.1% as per the second National Diabetes Survey of Pakistan (NDSP) during year 2016-2017 [20]. This high prevalence of obesity may be responsible for the increased frequency of MetS in our study population.
Women with PCOS, especially obese females, frequently displayed atherogenic dyslipidemia [10]. More than half of our study participants had dyslipidemia; the same was noted in another study [21]. In our study, low HDL contributed more as compared to high triglycerides to dyslipidemia, whereas Celik et al. [22] revealed significant high triglyceride levels in PCOS women as compared to controls. This disparity in data is enlightened by variation in the life style, dietary habits, prevalence of diabetes and obesity in different communities [10].
One third of our study participants were hypertensive, and similar finding was observed in another study [23]. In contrast to our finding, another study reported the frequency of hypertension in 10.7% of study participants [18]. Women with PCOS are at an increased risk of developing dysglycemia due to IR [5]. One fourth of our study participants had either impaired fasting plasma glucose or diabetes. Similar findings were revealed by other studies as well [24,25]. However, in contrast to our findings, Amato et al. noted impaired fasting glucose/T2DM in 12% of women with PCOS [26]; the observed diversity may be due to the variance in the prevalence of diabetes in different parts of the world.
The increased occurrence of different components of MetS among family members of women with PCOS pointed towards the genetic and environmental factors behind its pathogenesis. The significantly increased prevalence of components of MetS in the family history of our study participants is comparable with other studies also [18,27,28].
Menstrual cycle irregularities are noted early because of their monthly occurrence and may be the reason for being the common presenting complaint to health care professionals. In this study, menstrual irregularity was the main presenting complaint in the majority of study participants. This was also reflected in other studies [1,19,26]. In contrast to this finding, a meta-analysis revealed infertility and hirsutism as common presenting problem in females with PCOS [2].
Our study demonstrated hirsutism in almost half of PCOS women, and the same frequency was perceived by Amato et al. [26]. However, in contrast to our observation, Najem et al. [17] and Shanmugam et al. [19] found hirsutism in above 90% of study participants, whereas several other studies concluded that hirsutism affects 65%-75% of Southeast Asian women [1,21]. Statistics advocated that androgen level and hair follicle sensitivity to androgen are responsible for variations in the severity of the hirsutism seen in women with PCOS belonging to different ethnicities [5]. The number of women seeking medical advice regarding hirsutism varies widely depending upon the socio-cultural norms and acceptability in society.
Furthermore, a lot of women use herbal products and home remedies for hirsutism that may result in the variation of reported frequency of hirsutism.
Studies postulated that IR and compensatory hyperinsulinemia are common pathogenic factors for both PCOS and MetS [26]. In our study, IR was noted in two-thirds of women with PCOS and this finding is consistent with the research performed by Yu and Wangl [21]. Several other case control studies reported statistically significant high fasting insulin levels and HOMA-IR in women with PCOS [11,22,24]. However, this observation was contradictory to a study that showed IR in only one third participants [29]. The variation in genetic and environmental factors may be the reason behind the difference in severity of IR in different studied population [26]. Polycystic ovarian morphology (PCOM) on ultrasound was observed in one third of females with PCOS. In contrast to our findings, other studies demonstrated PCOM in more than 90 % of participants [19]. It is a well-known fact that ovarian androgenic dysfunction ranges from subclinical hyperandrogenemia in normal-variant PCOM to severe ovarian hyperandrogenism in most classic PCOS [3]. Moreover, polycystic ovaries are common in anovulatory women due to several causes and are not necessarily associated with PCOS [30].
Strength and limitation
This study provides local data on MetS in women with PCOS that would be helpful for further studies; however, this being a single-center study is the main limitation. Further studies are required to validate our findings.
Conclusions
The frequency of MetS is high in females presenting with PCOS, and therefore, metabolic surveillance should be considered in such women to reduce the risk of potential complications.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study. Institutional Review Board, Baqai Institute of Diabetology and Endocrinology issued approval Ref:/BIDE/IRB/SANJUM/08/19/022
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Thyroid disorders in polycystic ovarian syndrome subjects: a tertiary hospital based cross-sectional study from Eastern India. Sinha U, Sinharay K, Saha S, Longkumer TA, Baul SN, Pal SK. Indian J Endocrinol Metab. 2013;17:304–309. doi: 10.4103/2230-8210.109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - part 1. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. Endocr Pract. 2015;21:1291–1300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 3.The polycystic ovary morphology - polycystic ovary syndrome spectrum. Rosenfield RL. J Pediatr Adolesc Gynecol. 2015;28:412–419. doi: 10.1016/j.jpag.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metabolic syndrome, diet and exercise. De Sousa SM, Norman RJ. Best Pract Res Clin Obstet Gynaecol. 2016;37:140–151. doi: 10.1016/j.bpobgyn.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Clinical characteristics of polycystic ovary syndrome in Indian women. Ramanand SJ, Ghongane BB, Ramanand JB, Patwardhan MH, Ghanghas RR, Jain SS. Indian J Endocrinol Metab. 2013;17:138–145. doi: 10.4103/2230-8210.107858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Rotterdam criteria for polycystic ovary syndrome: evidence-based criteria? Wang R, Mol BW. Hum Reprod. 2017;32:261–264. doi: 10.1093/humrep/dew287. [DOI] [PubMed] [Google Scholar]
- 7.Frequency of the metabolic syndrome in type 2 diabetic subjects attending the diabetes clinic of a tertiary care hospital. Imam SK, Shahid SK, Hassan A, Alvi Z. https://pubmed.ncbi.nlm.nih.gov/17571479/ J Pak Med Assoc. 2007;57:239–242. [PubMed] [Google Scholar]
- 8.Thyroid disease and female reproduction. Poppe K, Velkeniers B, Glinoer D. Clin Endocrinol (Oxf) 2007;66:309–321. doi: 10.1111/j.1365-2265.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 9.Cardiovascular risks and metabolic syndrome in Hong Kong Chinese women with polycystic ovary syndrome. Cheung LP, Ma RC, Lam PM, et al. Hum Reprod. 2008;23:1431–1438. doi: 10.1093/humrep/den090. [DOI] [PubMed] [Google Scholar]
- 10.Prevalence of non-alcoholic fatty liver disease in women with polycystic ovary syndrome and its correlation with metabolic syndrome. Romanowski MD, Parolin MB, Freitas AC, Piazza MJ, Basso J, Urbanetz AA. Arq Gastroenterol. 2015;52:117–123. doi: 10.1590/S0004-28032015000200008. [DOI] [PubMed] [Google Scholar]
- 11.Polycystic ovary syndrome and metabolic syndrome. Ali AT. https://pubmed.ncbi.nlm.nih.gov/26265416/ Ceska Gynekol. 2015;80:279–289. [PubMed] [Google Scholar]
- 12.Prevalence of metabolic syndrome in Pakistani women with polycystic ovarian syndrome. Anjum N, Zohra S, Arif A, Azhar A, Qureshi M. http://pjbmb.org.pk/images/Current_Issue/03.pdf Pak J Biochem Mol Biol. 2013;46:97–100. [Google Scholar]
- 13.Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte-endothelium interactions in PCOS. Bañuls C, Rovira-Llopis S, de Marañon AM, et al. Metabolism. 2017;71:153–162. doi: 10.1016/j.metabol.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Discordance of metabolic syndrome and abdominal obesity prevalence according to different criteria in Andean highlanders: a community-based study. Herrera-Enriquez K, Narvaez-Guerra O. Diabetes Metab Syndr. 2017;11:0. doi: 10.1016/j.dsx.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN, PCOS/Troglitazone Study Group. J Clin Endocrinol Metab. 2006;91:48–53. doi: 10.1210/jc.2005-1329. [DOI] [PubMed] [Google Scholar]
- 16.Hypertriglyceridemic waist is associated with impaired glucose tolerance in polycystic ovary syndrome. Lerchbaum E, Schwetz V, Giuliani A, Obermayer-Pietsch B. Nutr Metab Cardiovasc Dis. 2013;23:0. doi: 10.1016/j.numecd.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and biochemical characteristics of polycystic ovary syndrome in Benghazi-Libya: a retrospective study. Najem F, Elmehdawi R, Swalem A. Libyan J Med. 2008;3:71–74. doi: 10.4176/080122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endocrine correlates of polycystic ovary syndrome in Pakistani women. Akram M, Roohi N. https://www.jcpsp.pk/archive/2015/Jan2015/07.pdf. J Coll Physicians Surg Pak. 2015;25:22–26. [PubMed] [Google Scholar]
- 19.Prevalence of thyroid dysfunction in patients with polycystic ovarian syndrome: a cross sectional study. Shanmugham D, Natarajan S, Karthik A. Int J Reprod Contracept Obstet Gynecol. 2018;7:3055–3059. [Google Scholar]
- 20.Prevalence of diabetes, pre-diabetes and associated risk factors: second National Diabetes Survey of Pakistan (NDSP), 2016-2017. Basit A, Fawwad A, Qureshi H, Shera AS, NDSP Members. BMJ Open. 2018;8:0. doi: 10.1136/bmjopen-2017-020961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subclinical hypothyroidism in PCOS: impact on presentation, insulin resistance, and cardiovascular risk. Yu Q, Wang JB. Biomed Res Int. 2016;2016:2067087. doi: 10.1155/2016/2067087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Is subclinical hypothyroidism contributing dyslipidemia and insulin resistance in women with polycystic ovary syndrome? Celik C, Abali R, Tasdemir N, Guzel S, Yuksel A, Aksu E, Yılmaz M. Gynecol Endocrinol. 2012;28:615–618. doi: 10.3109/09513590.2011.650765. [DOI] [PubMed] [Google Scholar]
- 23.Obesity, arterial hypertension, metabolic disorders, and polycystic ovary syndrome. (Article in Spanish) Quiñónez CZ, Silva RR, Torres JJ. https://pubmed.ncbi.nlm.nih.gov/11006648/ Ginecol Obstet Mex. 2000;68:317–322. [PubMed] [Google Scholar]
- 24.Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. Legro RS, Kunselman AR, Dodson WC, Dunaif A. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 25.Analysis of clinical characteristics in large-scale Chinese women with polycystic ovary syndrome. Shi Y, Guo M, Yan J, et al. https://pubmed.ncbi.nlm.nih.gov/18063948/ Neuro Endocrinol Lett. 2007;28:807–810. [PubMed] [Google Scholar]
- 26.Lower insulin sensitivity differentiates hirsute from non-hirsute Sicilian women with polycystic ovary syndrome. Amato MC, Galluzzo A, Merlino S, Mattina A, Richiusa P, Criscimanna A, Giordano C. Eur J Endocrinol. 2006;155:859–865. doi: 10.1530/eje.1.02290. [DOI] [PubMed] [Google Scholar]
- 27.Metabolic syndrome in patients with polycystic ovary syndrome in Iran. Zahiri Z, Sharami SH, Milani F, Mohammadi F, Kazemnejad E, Ebrahimi H, Heirati SF. Int J Fertil Steril. 2016;9:490–496. doi: 10.22074/ijfs.2015.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metabolic syndrome, hypertension, and hyperlipidemia in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Yilmaz B, Vellanki P, Ata B, Yildiz BO. Fertil Steril. 2018;109:356–364. doi: 10.1016/j.fertnstert.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prevalence and clinical profile of insulin resistance in young women of poly cystic ovary syndrome: a study from Pakistan. Tabassum R, Imtiaz F, Sharafat S. Pak J Med Sci. 2013;29:593–596. doi: 10.12669/pjms.292.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Adams J, Polson DW, Franks S. Br Med J (Clin Res Ed) 1986;293:355–359. doi: 10.1136/bmj.293.6543.355. [DOI] [PMC free article] [PubMed] [Google Scholar]