ABSTRACT
The small GTPase Arf4-based ciliary membrane-targeting complex recognizes specific targeting signals within sensory receptors and regulates their directed movement to primary cilia. Activated Arf4 directly binds the VxPx ciliary-targeting signal (CTS) of the light-sensing receptor rhodopsin. Recent findings revealed that at the trans-Golgi, marked by the small GTPase Rab6, activated Arf4 forms a functional complex with rhodopsin and the Arf guanine nucleotide exchange factor (GEF) GBF1, providing positive feedback that drives further Arf4 activation in ciliary trafficking. Arf4 function is conserved across diverse cell types; however, it appears that not all its aspects are conserved across species, as mouse Arf4 is a natural mutant in the conserved α3 helix, which is essential for its interaction with rhodopsin. Generally, activated Arf4 regulates the assembly of the targeting nexus containing the Arf GAP ASAP1 and the Rab11a–FIP3–Rabin8 dual effector complex, which controls the assembly of the highly conserved Rab11a–Rabin8–Rab8 ciliary-targeting module. It was recently found that this module interacts with the R-SNARE VAMP7, likely in its activated, c-Src-phosphorylated form. Rab11 and Rab8 bind VAMP7 regulatory longin domain (LD), whereas Rabin8 interacts with the SNARE domain, capturing VAMP7 for delivery to the ciliary base and subsequent pairing with the cognate SNAREs syntaxin 3 and SNAP-25. This review will focus on the implications of these novel findings that further illuminate the role of well-ordered Arf and Rab interaction networks in targeting of sensory receptors to primary cilia.
Abbreviations: CTS: Ciliary-Targeting Signal; GAP: GTPase Activating Protein; GEF: Guanine Nucleotide Exchange Factor; RTC(s), Rhodopsin Transport Carrier(s); SNARE: Soluble N-ethylmaleimide-sensitive Factor Attachment Protein Receptor; TGN: Trans-Golgi Network.
KEYWORDS: Arf GTPases, Rab GTPases, membrane trafficking, cilia, sensory receptors, rhodopsin
Introduction
Primary cilia are ubiquitous organelles that originate from the basal bodies and arise in different shapes to fit their precise function in sensory receptor signalling. The exceptionally elaborate primary cilia of the retinal rod photoreceptor cells, the rod outer segments (ROS), concentrate the G-protein-coupled receptor rhodopsin and the accompanying phototransduction apparatus into thousands of light-sensitive membranous discs, creating a distinctive microenvironment that supports the optimal detection and propagation of the visual signal [1,2]. The light-sensitive ROS membranes are continuously subjected to photo-oxidative damage, necessitating their constant renewal to maintain photoreceptor homeostasis [3,4]. A major challenge during this process is the precise linkage of the ciliary cargo uptake into the Golgi-to-cilia-directed membrane carriers, with the assembly of the membrane trafficking machinery responsible for their ultimate fusion with the periciliary plasma membrane [3,5]. Through continuous remodelling, the conserved Arf4-based ciliary-targeting complex – containing the Arf GAP ASAP1 and the Rab11a–FIP3–Rabin8 dual effector complex, which activates a regulator of carrier fusion Rab8 – provides an ever-changing platform that guides the ciliary cargo to its final destination [6–21]. Two recent studies in retinal rod photoreceptors further delineated the assembly of the targeting complex, and revealed essential roles of the Arf GEF GBF1 and the R-SNARE VAMP7, in the initial and final stages of ciliary pathway, respectively [22,23].
Arf GTPases and their regulators
Arf4 belongs to the family of small GTPases that includes Arf, Arf-like (Arl) and Sar proteins [24]. Arfs regulate membrane trafficking, lipid metabolism, organelle morphology and cytoskeleton dynamics [25]. The mammalian Arfs consist of six isoforms (Arf1–Arf6); Arfs1–5 are associated with the Golgi and Arf6 functions at the plasma membrane [26]. Arf4, Arl3, Arl6 and Arl13b are also specifically implicated in membrane targeting to primary cilia, dysfunction of which is a known cause of human genetic diseases and syndromic disorders known as ciliopathies [6,26–30]. Arf GTPases function through the cycles of GTP binding and hydrolysis that are regulated by Arf guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) [26,31–35]. GEFs and GAPs control membrane association and signalling pathways of Arf GTPases through activation cascades and positive-feedback loops [36,37]. The prototypical Arf, Arf1, in its GDP-bound form associates with membranes through the N-terminal myristoyl group; however, GEF activation and GTP binding cause a conformational transition, termed the ‘myristoyl switch’ that tightly couples Arf1 activation with stable membrane association [38–43]. Unlike Arf1, GDP-bound Arf4 and Arf5 can stably associate with membranes without Arf GEF, using the N-terminal amphipathic helix and a specific residue in the conserved domain [44,45], and this difference may partially underlie their specific functions that differ from those of Arf1.
In their active, membrane-bound form, Arfs interact with multiple effectors such as coat proteins and lipid modifying enzymes [46]. Because Arfs have intrinsically low rate of GTP hydrolysis, inactivation and termination of Arf signalling is executed by Arf GAPs, [33,35], which in this way couple the proof-reading of cargo incorporation to budding of transport carriers [47]. Particularly, the Arf GAPs ASAP1 belongs to a family of multifunctional proteins containing pleckstrin–homology (PH), ankyrin repeats, proline-rich and SH3 domains, which serve as large scaffolds for the assembly of signalling complexes [33,35,48]. ASAP1 functions both as an Arf GAP and an Arf effector, because its N-terminal BAR (Bin/amphiphysin/Rvs) domain, together with the GAP domain, acts as a coincidence detector that senses the membrane phospholipid composition and the presence of GTP-Arf and responds by increasing membrane curvature, which may facilitate carrier budding [49,50].
Assembly of the Arf4-mediated ciliary membrane-targeting complex at the Golgi/TGN
The fundamental step that initiates the assembly of the rhodopsin ciliary-targeting complex involves activated Arf4, which interacts directly with the rhodopsin C-terminal VxPx ciliary-targeting signal (CTS) at the Golgi/TGN [51] (Figure 1). The specific GEF that activates Arf4 in transport to the cilia was recently identified as the Arf GEF GBF1 [22], which will be described in detail below. The activated Arf4 and rhodopsin next form a complex with the Arf GAP ASAP1 [6]. ASAP1 functions both as an Arf4 GAP and its effector, as evidenced by the Arf4 mutant deficient in ASAP1 hydrolysis that causes retinal degeneration, even though other Arf GAPs can mediate GTP hydrolysis [6]. ASAP1 also controls selective binding of Rab11a and the Rab11-Arf effector FIP3, which assists with GTP hydrolysis on Arf4 [6–8,52]. The Arf4-dependent Stage I of the complex assembly is completed by proofreading of the rhodopsin CTS VxPx coupled to Arf4 inactivation, permitting subsequent rhodopsin uptake into ciliary-targeted rhodopsin transport carriers (RTCs) (Figure 1).
Figure 1.
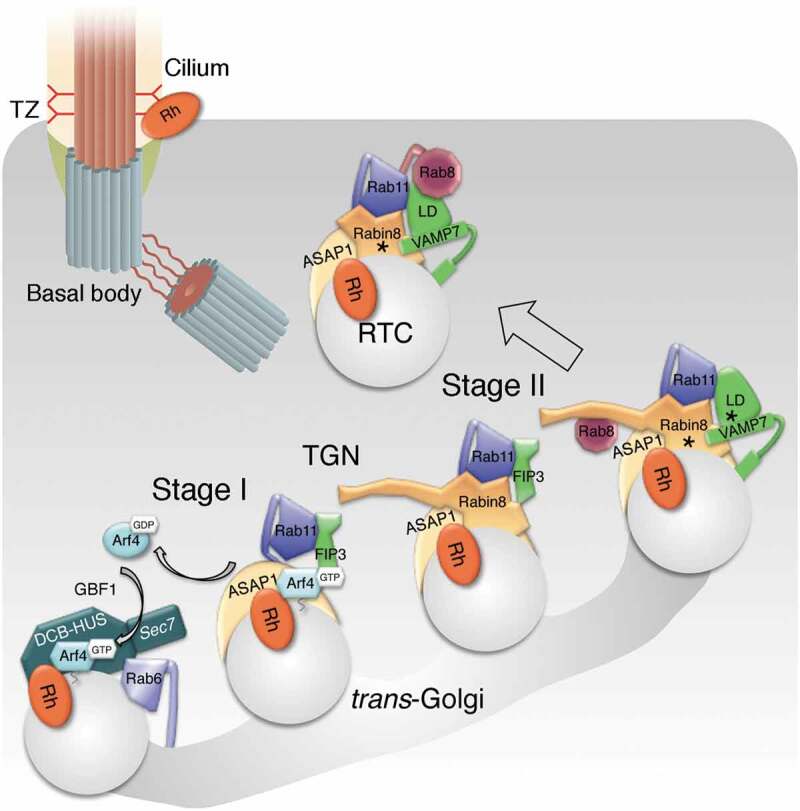
Model depicting the assembly and conversion of the Arf4-based rhodopsin ciliary-targeting complex at the Golgi/TGN
The assembly of the trafficking complex can be divided into two stages: the Arf4-dependent Stage I and the post-Arf4 Stage II. In Stage I, activated Arf4 recognizes the CTS VxPx of rhodopsin. Arf4, rhodopsin, and Rab6 cooperate to localize and activate the Arf GEF GBF1 at the trans-Golgi. Through positive feedback, Arf4 is further activated by GBF1. Coincidence detection of activated Arf4, acidic phospholipids and PIP2 recruits the Arf GAP ASAP1 to the Golgi/TGN, where it forms a complex with rhodopsin and Arf4, and selectively binds Rab11 and FIP3. Proofreading of CTS VxPx that regulates rhodopsin uptake into RTCs is coupled with GTP hydrolysis on Arf4 by ASAP1, assisted by FIP3, and followed by the departure of inactivated Arf4. In Stage II, ASAP1 serves as a platform for the assembly of the Rab11-FIP3-Rabin8 dual effector complex. Following GTP hydrolysis on Arf4, FIP3 departs the complex and the R-SNARE VAMP7 likely replaces it. Concurrently, Rabin8 activates GDP-bound Rab8. Rabin8 is phosphorylated by NDR2 kinase (asterisk), which increases its affinity for the Sec15 component of the exocyst membrane-tethering complex. VAMP7 is phosphorylated by the c-Src within the LD (asterisk) and activated for pairing with Q SNAREs syntaxin3 and SNAP-25, which ultimately drives RTC fusion with the periciliary plasma membrane.
In Stage II of the complex assembly, formation of nascent RTCs is coupled to the assembly of the membrane trafficking machinery responsible for their fusion, directing RTCs to the cilium (Figure 1). Following Arf4 inactivation and dissociation, ASAP1, Rab11a and FIP3 remain associated at the TGN where they recruit the Rab8 GEF Rabin8 [7,8]. This leads to the formation of a distinctive Rab11–FIP3–Rabin8 dual effector complex unique to Rab11, the ramifications of which in membrane trafficking have been reviewed previously [10]. Rab11–FIP3–Rabin8 complex assembles through multiple weak interactions that create a high-avidity complex, thus promoting cooperation between the two effectors in the execution of Rab11-directed functions [8–10]. Whereas Rab11 effector FIP3 stimulates the GAP activity of ASAP1 [52], the Rab11-mediated recruitment of Rabin8, the Rab8-specific GEF, initiates the Rab11-Rabin8–Rab8 ciliogenesis cascade [17–19]. Finally, the Rab11-Rabin8-Rab8 module directs Golgi-to-cilia trafficking, by capturing the RTC R-SNARE VAMP7 through direct binding of Rab8 and Rab11 to the VAMP7 regulatory longin domain (LD), and Rabin8 to its SNARE motif. With the R-SNARE aboard, RTCs are equipped for fusion via VAMP7 pairing with plasma membrane Q SNAREs syntaxin 3 and SNAP-25 [22].
The role of Arf4 in the ciliary membrane-targeting complex
Arf4 was identified as a regulatory protein that specifically binds the rhodopsin CTS VxPx [51]. In those experiments, the effects of blocking the action of Arf4 were functionally equivalent to blocking the rhodopsin CTS VxPx, mutations in which cause severe forms of autosomal dominant retinitis pigmentosa (adRP) [51,53,54]. Subsequent studies in transgenic frogs demonstrated that the expression of the Arf4I46D mutant, deficient in GTP hydrolysis by ASAP1, causes dysfunctional rhodopsin trafficking and rapid retinal degeneration [6], verifying a crucial role of the VxPx motif and the Arf4-based targeting complex in ciliary trafficking. Supporting this paradigm for sorting into transport vesicles, a specific CTS and Arf4 provide the directionality and increase the efficiency of ciliary transport of several sensory receptors [16,20,21,55,56]. However, the role of Arf4 in rhodopsin trafficking has been challenged using conditional knockout mouse retinas [57]. In our recently published study [23], we extensively addressed the issues of significance when comparing the data from the two published Arf4 in vivo models, the frog and the mouse, which are briefly summarized here: (i) Absence of a gene vs. a dominant negative action often have different effects, as exemplified by differences between rhodopsin mutant and knockout mouse models [58–62]. (ii) The volume of membrane trafficking in the frog eye exceeds by an order of magnitude that of the rodent rods [63]. (iii) Mouse models do not always recapture retinal membrane trafficking disease phenotype e.g. despite a relatively faithful manifestation of the hearing and balance disorders found in Usher syndrome, none of the Usher 1 mouse models undergo retinal degeneration [64]. Neither the frog nor mouse models are authentic representations of the human eye, but both aid in dissecting disease-related processes. The most likely explanation for the apparent discrepancy between the two models is that in the conditional mouse Arf4 knockout a compensatory mechanism allows rhodopsin trafficking to proceed, perhaps at a suboptimal level, because the mouse model system has low demands on membrane trafficking volumes.
Conversely, by magnifying the role of trafficking through its high volumes of membrane synthesis, the well-established frog model system revealed the stages and molecular machineries involved in the vectorial transport of ciliary cargo. Our research using the frog model has shown that the Arf4 α3 helix, encompassing the IQEAAEELQKML peptide, directly crosslinks to rhodopsin during membrane trafficking [51]. Notably, the human and frog Arf4 are 95% identical and anti-frog Arf4 antibody readily recognizes human Arf4 [8]. While reflecting on a potential compensatory mechanism in use in mouse Arf4 KO, we discovered that mouse is a natural mutant in the crucial Arf4 α3 helix (Figure 2). This is significant because the VxPx CTS of rhodopsin is surrounded by the conserved positively charged residues on the cytoplasmic surface of rhodopsin, which likely interact with the negatively charged residues in Arf4 α3 helix, resulting in an increase in affinity of the Arf4-Rhodopsin interaction probably without providing specificity (Figure 2(a,b). The specificity is likely provided by the essential hydrophobic C-terminal VxPx CTS that could interact with the neighbouring hydrophobic patch of Arf4 (Figure 2(a,b). As shown in Figure 2(c), the alignment of Arf4 sequences from several species reveals high conservation of the amino acid residues comprising Arf4 α3 helix. Compared to the frog, the human Arf4 has only conservative substitutions, whereas the bovine sequence is identical to that of the frog. In mouse and rat two acidic amino acid residues are changed to hydrophobic amino acids, creating a hydrophobic stretch instead of a negatively charged one (Figure 2(b)), which potentially conveys dissimilar functional advantage to rodent Arf4. This type of substitution, typically used in mutagenesis experiments to abolish protein-protein interactions, likely disrupts rhodopsin-Arf4 interactions. In fact, there is high likelihood that the mouse Arf4 either does not interact with rhodopsin, or does so with diminished affinity.
Figure 2.
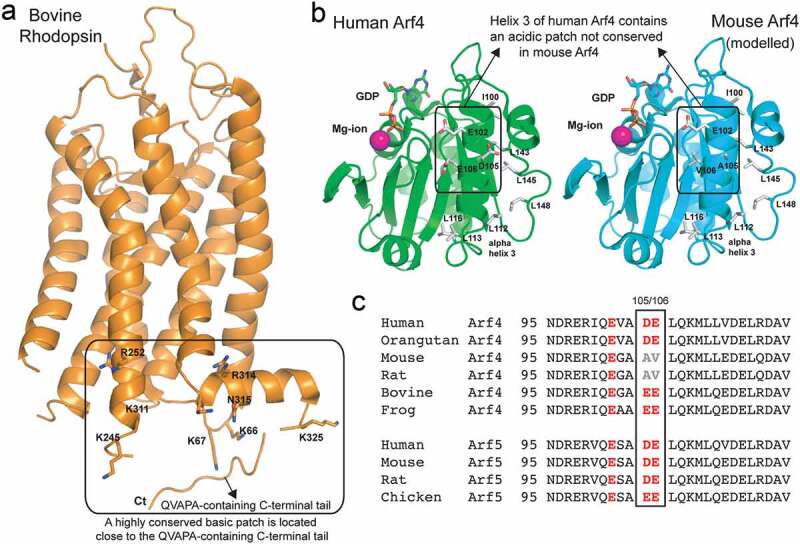
Arf4 α3 helix, which participates in rhodopsin interactions, is evolutionary conserved, with the exception of mouse and rat
(a) The crystal structure of bovine rhodopsin (PDB code 1F88) is shown in orange cartoon representation. Residues forming a basic patch near the C-terminal tail are shown in stick representation and labelled according to the bovine sequence. The position of the C-terminal QVAPA tail is indicated (note that the linker connecting the C-terminal tail to the rhodopsin core domain was not resolved in the crystal structure). (b) Crystal structure of human Arf4 (PDB code 1Z6X) is shown as a green cartoon representation and that of mouse Arf4 (modelled based on the human Arf4 structure) as a cyan cartoon representation. GDP (sticks) and a magnesium ion (pink ball) mark the GTPase active site of Arf4. Residues forming the acidic patch on human Arf4 and neighbouring hydrophobic residues are shown as sticks and labelled according to the sequence. (c) Sequence alignment of the region corresponding to helix 3 of Arf4 and Arf5 and the surrounding residues, from different species. The acidic residues substituted with hydrophobic residues in mouse and rat Arf4 are boxed.
Interestingly, unlike Arf4, Arf5 is 100% conserved within the species compared in Figure 2(c). Mouse Arf5 is a possible orthologue of human and frog Arf4, as it possesses the acidic residues potentially important for interactions with rhodopsin (Figure 2(c)). Arf4 and Arf5 could be interchangeable in mouse photoreceptors, but not in cells with high volumes of cargo transiting through the secretory pathway [57], where directionality and efficiency are of essence. Notably, GBF1 has high exchange activity on Arf5 [65] and its activation of both Arf4 and Arf5 initiates an activation cascade at the TGN [66]. If Arf5 indeed functions in mouse rod photoreceptors as the human and frog Arf4 orthologue, the distinct downstream functions of Arf4 vs. Arf5 may influence the subsequent steps in rhodopsin trafficking. They may consequently diverge from the highly conserved Arf4-mediated pathway that includes Rab11 and Rab8, which were also reported to be dispensable in the mouse KOs [67]. In contrast to that study, our study in transgenic frogs showed that the Rab8 T22N dominant-negative mutant, deficient in GTP binding, caused dysfunctional rhodopsin trafficking and rapid retinal degeneration, demonstrating an essential role for Rab8 in rhodopsin trafficking [12], consistent with the role of Rab8 as a regulator of carrier fusion with the periciliary plasma membrane, which is dysfunctional in human genetic diseases and ciliopathies [13–15,30,68–70]. These examples indicate that mouse knockout models addressing intracellular processes regulated by Arf and Rab GTPases are subject to further verification that can be accomplished by selecting for such studies the animal models in which these processes are conserved.
Activation of Arf4 by the Arf GEF GBF1
The spatiotemporally restricted activation of Arf4 through GTP binding is crucial to its function in complex assembly; however, available information about the signals that Arf GEFs recognize in order to activate Arfs is still scarce. These signals were examined in a recent study, which established that sensory receptor cargo, such as rhodopsin, directly promotes its own intracellular progression to the cilia by providing input to the specific large Arf GEF, GBF1, to activate a cognate Arf, Arf4, in ciliary trafficking [23]. In the likely scenario, this process starts with multiple random activation events that initially generate a small number of active Arf4 clusters at the Golgi/TGN. Next, a functional ternary complex – sensitive to the GBF1 inhibitor Golgicide A – is formed between GBF1, activated Arf4, and the ciliary cargo, rhodopsin [23], further activating Arf4 through the operation of an autocatalytic amplification mechanism (positive feedback) [71], building up levels of active Arf4, which leads to the assembly of the ciliary-targeting complex. The emergence of newly synthesized rhodopsin in the Golgi is essential for this process, as blocking of rhodopsin transit through the Golgi by cycloheximide abolishes all interactions between GBF1, Arf4 and rhodopsin, indicating the requirement of cargo influx for the complex formation in vivo [23].
GBF1 belongs to the BIG/GBF family of Golgi-localized large Arf GEFs, which comprises multifunctional scaffold proteins that contain the highly conserved Sec7 domain involved in the nucleotide-exchange activity. Arf GEFs are auto-inhibited in the cytosol [72]. Their membrane association and catalytic activity are controlled by cooperative allosteric regulation via coincidence detection by multiple regulatory domains: DCB (dimerization and cyclophilin binding) domain, a HUS domain (homology upstream of Sec7 domain) and HDS (homology downstream of Sec7) domains, which integrate direct inputs from membranes and multiple activated Arfs and Rabs [34,36]. For example, the prototypic Arf GEF, the yeast Sec7, is differentially regulated by two Arfs (Arl1 and Arf1) and two Rabs (YPT1/Rab1 and YPT31/32/Rab11), interacting with different regulatory domains and collectively mediating its TGN localization and allosteric activation [73–76].
It has been reported that both GBF1 and Arf4 function within the early Golgi, and at the TGN [6,7,44,77–83]. The Golgi localization of GBF1 is mediated by the HDS1 and HDS2 domains that appear to act as PIP-binding domains [84–87]. In photoreceptors, the N-terminal DCB-HUS domain of GBF1 directly interacts with Arf4 and with newly synthesised rhodopsin [23]. Notably, the DCB domain of GBF1 also directs Golgi localization through interactions with Golgi-associated Rabs, like Rab1b [88,89]. In support of this mode of action, our recent study revealed that in photoreceptors GBF1 nearly completely colocalizes with Rab6 at the trans-Golgi [23]. We thus hypothesized that Rab6 may control the trans-Golgi membrane association of GBF1.
Interaction of the Arf GEF GBF1 with the small GTPase Rab6
Rab6 is one of the most conserved Rab GTPases throughout evolution and the most abundant Rab protein associated with the Golgi complex. The two ubiquitous isoforms, Rab6A and Rab6A’, regulate transport in and out of the Golgi, including anterograde transport between the Golgi and the plasma membrane [90–92]. Rab6 was the first and the most abundant Rab GTPase identified as a potential regulator of rhodopsin trafficking [93]. Surprisingly, Rab6 function in rhodopsin trafficking is conserved in Drosophila photoreceptors as well, despite many differences between invertebrate and vertebrate photoreceptor cells [94–96]. The downstream effectors of Rab6 in photoreceptors are not known, thus we tested the notion that activated Rab6 controls trans-Golgi membrane association of GBF1. We discovered that GBF1 overlaps with Rab6 at the trans-Golgi significantly better than with GM130 at the cis-Golgi, as revealed by pixel colocalization analysis performed within the Golgi [23]. We thus examined their direct interaction and now demonstrate that GTPγS-activated Rab6, and the Rab6Q72L mutant directly and specifically interact with the DCB-HUS domain of GBF1 (Figure 3(a–c)). We additionally show, using an established retinal cell-free system [97,98], that the peptide mimicking the Rab6 switch 1 domain responsible for recruiting specific effectors (AA 37–52) [99] completely inhibits rhodopsin trafficking in vitro (Figure 3(d)). Peptides corresponding either to the highly homologous domain of Rab7 (AA 33–47), or to the Rab6 SF3 domain that is recognized by the Rab escort protein (REP) involved in prenylation [100] (AA 107–120)(Figure 3(e)) have no effect. Thus, a relevant binding partner and effector of Rab6 at the Golgi/TGN is another important regulator involved in RTC budding. We suggest that it is GBF1, which was previously reported as one of the top interactors of active Rab6 [101]. Several other known effectors link Rab6 to the molecular motors [102,103], including Myosin II, which, along with KIF20A, regulates fission of Rab6-positive carriers and exit from the Golgi [104,105]. Rab6 interacts specifically with the transport protein particle II (TRAPPII) complex, which is essential for ciliogenesis through its interaction with Rabin8 and activation of Rab11 [19,101,106]. One of the Rab6 effectors, Rab6IP1, directly links Rab6 and Rab11, whereas other effectors enable Rab6-postive carriers to acquire Rab8, which regulates their fusion [107–110]. In this context, photoreceptor Rab6 plays a crucial role in the Golgi exit of membrane cargo directed to the primary cilium, in part by concentrating GBF1 at Golgi exit sites where it could both sense the emerging cargo, and recruit and activate Arf4 for the ciliary cargo delivery via the Rab11-Rabin8-Rab8 pathway.
Figure 3.
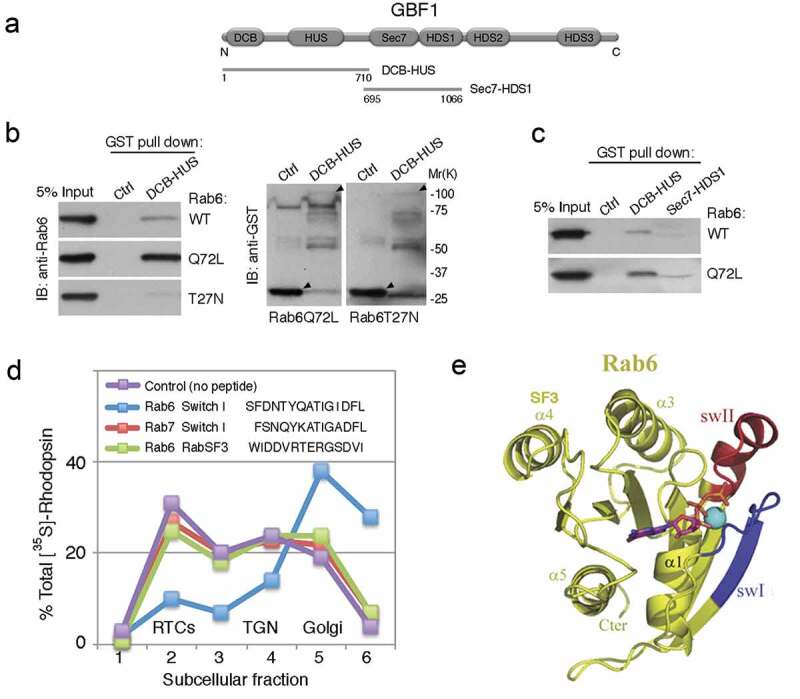
Assembly of the Arf4-mediated ciliary membrane-targeting complex at the trans-Golgi: the Arf GEF GBF1 is an effector of Rab6
(a) Schematic of GBF1. DCB-HUS (AA 1–710) and Sec7-HDS1 (AA 695–1066) are indicated. (b) GST-DCB-HUS, or GST (Ctrl), were incubated with recombinant human Rab6 bound to GTPγS, or with Rab6Q72L or T22N mutants. Bound Rab6 was detected by immunoblotting. The GST fusion proteins were detected with anti-GST antibody. Arrowheads point to the GST-fusion proteins used in pulldowns. (c) GST-DCB-HUS, GST-Sec7-HDS1, or GST (Ctrl), were incubated with Rab6 bound to GTPγS, or with Rab6Q72L mutant and bound Rab6 was detected as above. (d) Frog retinas were pulse-labelled for 60 min and retinal PNS was incubated for 30 min with 50 µM peptides, as indicated in the panel, prior to a 2 h cell-free chase; photoreceptor membranes were fractionated into Golgi, TGN and RTCs as described [6,98], and radiolabelled proteins analysed by SDS-PAGE and autoradiography ([35S]-Rh). The Rab6 effector peptide mimicking switch I arrested rhodopsin in the Golgi, where Rab6 and GBF1 are localized [23,93] and potently and specifically inhibited its uptake into RTCs. The Rab7 switch I and the Rab6 SF3 peptide had no effect. (e) The ribbon model of Rab6. Switch I is coloured blue, and switch II is red. Modified from ref [110]. SF3 domain is indicated.
RTC fusion: Rabs and their effectors provide framework for SNARE assembly
Activation of Rab8 by Rabin8 renders nascent RTCs competent for fusion with the periciliary plasma membrane, because Rab8 regulates the final stages of polarized membrane traffic, fusion of ciliary-targeted carriers and ciliogenesis [7,11–15,30,68]. Phosphorylation of T72 of the switch II region of Rab8 by the Parkinson’s disease kinase LRRK2 results in a failure of Rabin8-mediated activation of Rab8 [111] and consequently inhibits cilium formation [112,113]. Rabin8 is phosphorylated at S272 by NDR2 kinase (also known as STK38L), which regulates the switch in binding specificity of Rabin8 from PS to the Sec15 component of the exocyst complex that mediates carrier tethering at the periciliary plasma membrane [114]. Mutations in NDR2 affecting Rabin8 function cause canine retinal degeneration corresponding to human ciliopathy Leber congenital amaurosis (LCA) characterized by early-onset blindness [115,116], highlighting the central role of the Rab11-Rabin8-Rab8 pathway.
The fusion event through which RTCs deliver rhodopsin to the ciliary base is driven by the pairing of plasma membrane SNAREs syntaxin 3 and SNAP-25, regulated by omega-3 docosahexaenoic acid (DHA) [117]. The long sought after RTC R-SNARE that pairs with syntaxin 3 and SNAP-25 is now identified as VAMP7 or TI-VAMP [22]. Overall, VAMP7 is known to regulate fusion of transport carriers with the plasma membrane; plasma membrane expansion and neurite outgrowth; Golgi homeostasis and Golgi-to-plasma membrane transport; selective apical exocytosis and polarity, as well as ciliogenesis [118–128]. VAMP7 possesses a N-terminal regulatory LD, which is generally involved in binding small GTPases [129–131]. The closed auto-inhibited conformation of VAMP7, in which LD folds back onto the SNARE motif, is stabilized in part by tyrosine 45 in the LD [119,132]. Vps9-ankyrin repeat protein/Ankrd27 (VARP) stabilizes VAMP7 LD in the closed conformation substantially diminishing SNARE complex formation [133]. Phosphorylation at tyrosine 45 by c-Src kinase activates VAMP7 Q-SNARE binding and complex assembly [134]. In photoreceptors, c-Src phosphorylation occurs at the Golgi, indicating that VAMP7 is trafficking to the cilium in its activated form [22].
During RTC formation and trafficking, VAMP7 interacts with the Rab11-Rabin8-Rab8 trafficking module. Rab11 and Rab8 bind VAMP7 LD, whereas Rabin8 interacts with the SNARE domain [22]. FIP3 regulates VAMP7 access to Rab11, as the interactions between FIP3 and VAMP7 with Rab11 are mutually exclusive. Rabin8 and VARP directly interact in ciliary trafficking of VAMP7, and their respective affinities for VAMP7 may be regulated by its c-Src phosphorylation and activation. To better understand interactions of VAMP7 with its numerous partners, we wanted to visualize the topography of the putative Rab11-Rabin8-VAMP7 complex. We modelled it by superposition of the structure of the SRP receptor [130] as a model for small GTPase-LD interaction, onto the structure of Rab11-Rabin8-FIP3 complex [9] and VAMP7-VARP complex [133] (Figure 4). This model predicts that VAMP7 LD binds to the canonical effector-binding site of Rab11a and overlaps with FIP3. Strikingly, if VAMP7 bound to Rab11-Rabin8 complex is in the inactive closed conformation, in which the VAMP7 SNARE domain folds over the LD [133], the arginine (R150) at the zero layer would point straight towards and clash with the most C-terminal α5 helix of Rabin8 [22]. We postulate that phosphorylation by c-Src kinase induces conformational change in VAMP7 to remove the potential steric hindrance and allow for binding to Rabin8. This, in turn would enable the Rab11-Rabin8-Rab8 module to capture VAMP7 for delivery to the ciliary base, and subsequent pairing with the cognate SNAREs syntaxin 3 and SNAP-25, to regulate the fusion event through which RTCs deliver rhodopsin in the final stages of ciliary-directed trafficking.
Figure 4.
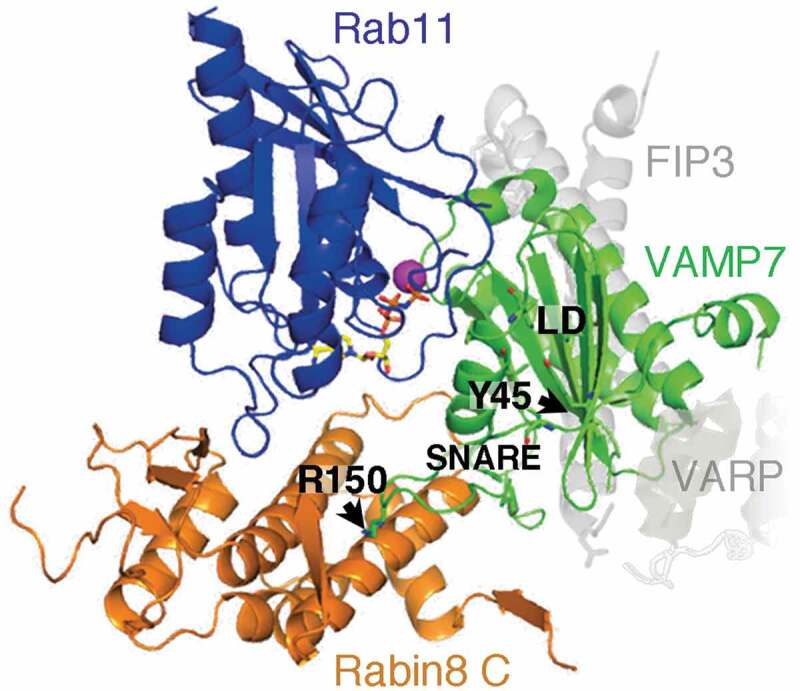
A model of the putative Rab11-Rabin8-VAMP7 complex
Model was generated with PyMOL, using the structure of the SRP receptor (PDB code: 2FH5) [130], Rab11-Rabin8-FIP3 complex (PDB code: 4UJ3) [9], and VAMP7–VARP complex (PDB code: 4B93) [133]. Positions of FIP3 and VARP in the crystalized complexes are indicated in light gray. VAMP7 SNARE domain, LD, and residues Y45 and R150 are indicated. Modified from reference [22]. Conformational change of VAMP7 associated with c-Src phosphorylation at Y45 is predicted to rearrange the SNARE domain and reposition R150 at the zero layer, thus optimizing binding of activated VAMP7 to Rabin8.
Concluding remarks
Communication between Arf and Rab GTPases via regulatory protein and effector networks ultimately leads to the directional delivery of ciliary membrane cargo. Ciliary sensory receptors, such as rhodopsin, are active participants in these processes through direct interactions with select components of the ciliary trafficking complexes. While these interactions are highly evolutionary conserved, the species conservation is unclear in mouse models of ciliary trafficking in photoreceptor cells. Future research will reveal the level of conservation of the Arf4-based ciliary-targeting complex in the assembly of the membrane trafficking machinery fundamental to the biogenesis of cilia and cilia-derived sensory organelles, and its involvement in ciliopathies and other degenerative diseases.
Acknowledgments
We thank Cathy Jackson, Bruno Goud and Thierry Galli for proving reagents and for valuable discussions in the course of these studies.
Funding Statement
This work was supported by the NIH [grant number EY-12421 to DD] and by the Novo Nordisk Foundation [grant number NNF15OC0014164 to EL].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Palczewski K. Chemistry and biology of vision. J Biol Chem. 2012;287:1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arshavsky VY, Burns ME.. Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem. 2012;287:1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res. 2014;38:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pearring JN, Salinas RY, Baker SA, et al. Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res. 2013;36:24–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deretic D. Crosstalk of Arf and Rab GTPases en route to cilia. Small GTPases. 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mazelova J, Astuto-Gribble L, Inoue H, et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. Embo J. 2009;28:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang J, Morita Y, Mazelova J, et al. The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. Embo J. 2012;31:4057–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang J, The Arf DD. Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J Cell Sci. 2015;128:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vetter M, Stehle R, Basquin C, et al. Structure of Rab11-FIP3-Rabin8 reveals simultaneous binding of FIP3 and Rabin8 effectors to Rab11. Nat Struct Mol Biol. 2015;22:695–702. [DOI] [PubMed] [Google Scholar]
- [10].Vetter M, Wang J, Lorentzen E, et al. Novel topography of the Rab11-effector interaction network within a ciliary membrane targeting complex. Small GTPases. 2015;6:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Deretic D, Huber LA, Ransom N, et al. Rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci. 1995;108:215–224. [DOI] [PubMed] [Google Scholar]
- [12].Moritz OL, Tam BM, Hurd LL, et al. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell. 2001;12:2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ojeda Naharros I, Gesemann M, Mateos JM, et al. Loss-of-function of the ciliopathy protein Cc2d2a disorganizes the vesicle fusion machinery at the periciliary membrane and indirectly affects Rab8-trafficking in zebrafish photoreceptors. PLoS Genet. 2017;13:e1007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bachmann-Gagescu R, Phelps IG, Stearns G, et al. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum Mol Genet. 2011;20:4041–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Omori Y, Zhao C, Saras A, et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. [DOI] [PubMed] [Google Scholar]
- [16].Ezratty EJ, Pasolli HA, Fuchs E. A Presenilin-2-ARF4 trafficking axis modulates Notch signaling during epidermal differentiation. J Cell Biol. 2016;214:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Feng S, Knödler A, Ren J, et al. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem. 2012;287:15602–15609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Knodler A, Feng S, Zhang J, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci U S A. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Westlake CJ, Baye LM, Nachury MV, et al. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A. 2011;108:2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lodowski KH, Frisby CL, Kennaway DJ, et al. Signals governing the trafficking and mistrafficking of a ciliary GPCR, rhodopsin. J Neurosci. 2013;33:13621–13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Follit JA, Hu Y-C, Bhattacharyya T, et al. Arf4 is required for Mammalian development but dispensable for ciliary assembly. PLoS Genet. 2014;10:e1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kandachar V, Tam BM, Moritz OL, et al. An interaction network between the SNARE VAMP7 and Rab-GTPase within a ciliary membrane-targeting complex. J Cell Sci. 2018;131:jcs222034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang J, Fresquez T, Kandachar V, et al. The Arf GEF GBF1 and Arf4 synergize with the sensory receptor cargo, rhodopsin, to regulate ciliary membrane trafficking. J Cell Sci. 2017;130:3975–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kahn RA, Cherfils J, Elias M, et al. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol. 2006;172:645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Donaldson JG. Arfs, phosphoinositides and membrane traffic. Biochem Soc Trans. 2005;33:1276–1278. [DOI] [PubMed] [Google Scholar]
- [26].Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Seixas E, Barros M, Seabra MC, et al. Rab and Arf proteins in genetic diseases. Traffic. 2013;14:871–885. [DOI] [PubMed] [Google Scholar]
- [28].Wiens CJ, Tong Y, Esmail MA, et al. The Bardet-Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signalling. J Biol Chem. 2010;285:16218–16230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Q, Hu J, Ling K. Molecular views of Arf-like small GTPases in cilia and ciliopathies. Exp Cell Res. 2013;319:2316–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nachury MV, Loktev AV, Zhang Q, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. [DOI] [PubMed] [Google Scholar]
- [31].Casanova JE. Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8:1476–1485. [DOI] [PubMed] [Google Scholar]
- [32].Bui QT, Golinelli-Cohen MP, Jackson CL. Large Arf1 guanine nucleotide exchange factors: evolution, domain structure, and roles in membrane trafficking and human disease. Mol Genet Genomics. 2009;282:329–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nie Z, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–1211. [DOI] [PubMed] [Google Scholar]
- [34].Nawrotek A, Zeghouf M, Cherfils J. Allosteric regulation of Arf GTPases and their GEFs at the membrane interface. Small GTPases. 2016;7:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 2004;16:401–413. [DOI] [PubMed] [Google Scholar]
- [36].Stalder D, Antonny B. Arf GTPase regulation through cascade mechanisms and positive feedback loops. FEBS Lett. 2013;587:2028–2035. [DOI] [PubMed] [Google Scholar]
- [37].Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Franco M, Chardin P, Chabre M, et al. Myristoylation-facilitated binding of the G protein ARF1GDP to membrane phospholipids is required for its activation by a soluble nucleotide exchange factor. J Biol Chem. 1996;271:1573–1578. [DOI] [PubMed] [Google Scholar]
- [39].Pasqualato S, Renault L, Arf CJ, et al. Sar proteins: a family of GTP-binding proteins with a structural device for ‘front-back’ communication. EMBO Rep. 2002;3:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Randazzo PA, Terui T, Sturch S, et al. The myristoylated amino terminus of ADP-ribosylation factor 1 is a phospholipid- and GTP-sensitive switch. J Biol Chem. 1995;270:14809–14815. [DOI] [PubMed] [Google Scholar]
- [41].Goldberg J. Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 1998;95:237–248. [DOI] [PubMed] [Google Scholar]
- [42].Antonny B, Beraud-Dufour S, Chardin P, et al. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. [DOI] [PubMed] [Google Scholar]
- [43].Liu Y, Kahn RA, Prestegard JH. Dynamic structure of membrane-anchored Arf*GTP. Nat Struct Mol Biol. 2010;17:876–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chun J, Shapovalova Z, Dejgaard SY, et al. Characterization of class I and II ADP-ribosylation factors (Arfs) in live cells: GDP-bound class II Arfs associate with the ER-Golgi intermediate compartment independently of GBF1. Mol Biol Cell. 2008;19:3488–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Duijsings D, Lanke KH, van Dooren SH, et al. Differential membrane association properties and regulation of class I and class II Arfs. Traffic. 2009;10:316–323. [DOI] [PubMed] [Google Scholar]
- [46].Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. [DOI] [PubMed] [Google Scholar]
- [47].Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic. 2007;8:1465–1475. [DOI] [PubMed] [Google Scholar]
- [48].Brown MT, Andrade J, Radhakrishna H, et al. ASAP1, a phospholipid-dependent arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol. 1998;18:7038–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Nie Z, Hirsch DS, Luo R, et al. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol. 2006;16:130–139. [DOI] [PubMed] [Google Scholar]
- [50].Luo R, Ahvazi B, Amariei D. Kinetic analysis of GTP hydrolysis catalysed by the Arf1-GTP-ASAP1 complex. Biochem J. 2007;402:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Deretic D, Williams AH, Ransom N, et al. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci U S A. 2005;102:3301–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Inoue H, Ha VL, Prekeris R, et al. Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell. 2008;19:4224–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Berson EL, Rosner B, Weigel-DiFranco C, et al. Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci. 2002;43:3027–3036. [PubMed] [Google Scholar]
- [54].Aleman TS, Cideciyan AV, Sumaroka A, et al. Retinal laminar architecture in human retinitis pigmentosa caused by Rhodopsin gene mutations. Invest Ophthalmol Vis Sci. 2008;49:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Follit JA, Li L, Vucica Y, et al. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol. 2010;188:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ward HH, Brown-Glaberman U, Wang J, et al. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell. 2011;22:3289–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pearring JN, San Agustin JT, Lobanova ES, et al. Loss of Arf4 causes severe degeneration of the exocrine pancreas but not cystic kidney disease or retinal degeneration. PLoS Genet. 2017;13:e1006740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Concepcion F, Chen J. Q344ter mutation causes mislocalization of rhodopsin molecules that are catalytically active: a mouse model of Q344ter-induced retinal degeneration. PLoS One. 2010;5:e10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Concepcion F, Mendez A, Chen J. The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision Res. 2002;42:417–426. [DOI] [PubMed] [Google Scholar]
- [60].Li T, Snyder WK, Olsson JE, et al. Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci U S A. 1996;93:14176–14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Humphries MM, Shaffer LG. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. [DOI] [PubMed] [Google Scholar]
- [62].Lem J, Krasnoperova NV, Calvert PD, et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Natl Acad Sci U S A. 1999;96:736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Besharse JC. Photosensitive membrane turnover: differentiated membrane domains and cell-cell interaction. In: Adler R, Farber D, editors. The retina: a model for cell biological studies. New York: Academic Press; 1986. p. 297–352. [Google Scholar]
- [64].Williams DS, Bernard J-B, Calabrese A, et al. Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res. 2008;48:433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Claude A, Sowerby PJ, Lui WW, et al. GBF1: A novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J Cell Biol. 1999;146:71–84. [PMC free article] [PubMed] [Google Scholar]
- [66].Lowery J, Szul T, Styers M, et al. The Sec7 guanine nucleotide exchange factor GBF1 regulates membrane recruitment of BIG1 and BIG2 guanine nucleotide exchange factors to the trans-Golgi network (TGN). J Biol Chem. 2013;288:11532–11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ying G, Gerstner CD, Frederick JM, et al. Small GTPases Rab8a and Rab11a are dispensable for rhodopsin transport in mouse photoreceptors. PLoS One. 2016;11:e0161236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yoshimura S, Egerer J, Fuchs E, et al. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol. 2017;18:533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Murga-Zamalloa CA, Atkins SJ, Peranen J, et al. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet. 2010;19:3591–3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jackson CL. GEF-effector interactions. Cell Logist. 2014;4:e943616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cherfils J, Melancon P. On the action of Brefeldin A on Sec7-stimulated membrane-recruitment and GDP/GTP exchange of Arf proteins. Biochem Soc Trans. 2005;33:635–638. [DOI] [PubMed] [Google Scholar]
- [73].McDonold CM, Fromme JC. Four GTPases differentially regulate the Sec7 Arf-GEF to direct traffic at the trans-golgi network. Dev Cell. 2014;30:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Richardson BC, Halaby SL, Gustafson MA, et al. The Sec7 N-terminal regulatory domains facilitate membrane-proximal activation of the Arf1 GTPase. Elife. 2016;5:e12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Richardson BC, McDonold CM, Fromme JC. The Sec7 Arf-GEF is recruited to the trans-Golgi network by positive feedback. Dev Cell. 2012;22:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Halaby SL, Fromme JC. The HUS box is required for allosteric regulation of the Sec7 Arf-GEF. J Biol Chem. 2018;293:6682–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Szul T, Garcia-Mata R, Brandon E, et al. Dissection of membrane dynamics of the ARF-guanine nucleotide exchange factor GBF1. Traffic. 2005;6:374–385. [DOI] [PubMed] [Google Scholar]
- [78].Szul T, Grabski R, Lyons S, et al. Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking. J Cell Sci. 2007;120:3929–3940. [DOI] [PubMed] [Google Scholar]
- [79].Kawamoto K, Yoshida Y, Tamaki H, et al. GBF1, a guanine nucleotide exchange factor for ADP-ribosylation factors, is localized to the cis-Golgi and involved in membrane association of the COPI coat. Traffic. 2002;3:483–495. [DOI] [PubMed] [Google Scholar]
- [80].Zhao X, Claude A, Chun J, et al. GBF1, a cis-Golgi and VTCs-localized ARF-GEF, is implicated in ER-to-Golgi protein traffic. J Cell Sci. 2006;119:3743–3753. [DOI] [PubMed] [Google Scholar]
- [81].Garcia-Mata R, Szul T, Alvarez C, et al. ADP-ribosylation factor/COPI-dependent events at the endoplasmic reticulum-Golgi interface are regulated by the guanine nucleotide exchange factor GBF1. Mol Biol Cell. 2003;14:2250–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nakai W, Rotelli MD, Brown BR, et al. ARF1 and ARF4 regulate recycling endosomal morphology and retrograde transport from endosomes to the Golgi apparatus. Mol Biol Cell. 2013;24:2570–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ben-Tekaya H, Kahn RA, Hauri HP, et al. ADP Ribosylation factors 1 and 4 and group VIA phospholipase <ins style=“background: springGreen”>A2 regulate morphology and intraorganellar traffic in the endoplasmic reticulum–golgi intermediate compartment. Mol Biol Cell. 2010;21:4130–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bouvet S, Golinelli-Cohen MP, Contremoulins V, et al. Targeting of the Arf-GEF GBF1 to lipid droplets and Golgi membranes. J Cell Sci. 2013;126:4794–4805. [DOI] [PubMed] [Google Scholar]
- [85].Meissner JM, Bhatt JM, Lee E, et al. The ARF guanine nucleotide exchange factor GBF1 is targeted to golgi membranes through a PIP-binding domain. J Cell Sci. 2018;131:jcs210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Pocognoni CA, Viktorova EG, Wright J, et al. Highly conserved motifs within the large Sec7 ARF guanine nucleotide exchange factor GBF1 target it to the Golgi and are critical for GBF1 activity. Am J Physiol Cell Physiol. 2018;314:C675–C689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Quilty D, Chan CJ, Yurkiw K, et al. The Arf-GDP-regulated recruitment of GBF1 to Golgi membranes requires domains HDS1 and HDS2 and a Golgi-localized protein receptor. J Cell Sci. 2018;132:jcs208199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Monetta P, Slavin I, Romero N, et al. Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol Biol Cell. 2007;18:2400–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Alvarez C, Garcia-Mata R, Brandon E, et al. COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol Biol Cell. 2003;14:2116–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Goud B, Zahraoui A, Tavitian A, et al. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990;345:553–556. [DOI] [PubMed] [Google Scholar]
- [91].Grigoriev I, Splinter D, Keijzer N, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13:305–314. [DOI] [PubMed] [Google Scholar]
- [92].Martinez O, Schmidt A, Salaméro J, et al. The small GTP-binding protein rab6 functions in intra-Golgi transport. J Cell Biol. 1994;127:1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Deretic D, Papermaster DS. Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci. 1993;106:803–813. [DOI] [PubMed] [Google Scholar]
- [94].Satoh T, Nakamura Y, Satoh AK. Rab6 functions in polarized transport in Drosophila photoreceptors. Fly (Austin). 2016;10:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Shetty KM, Kurada P, O’Tousa JE. Rab6 regulation of rhodopsin transport in Drosophila. J Biol Chem. 1998;273:20425–20430. [DOI] [PubMed] [Google Scholar]
- [96].Iwanami N, Nakamura Y, Satoh T, et al. Rab6 Is required for multiple apical transport pathways but not the basolateral transport pathway in drosophila photoreceptors. PLoS Genet. 2016;12:e1005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Deretic D. Rhodopsin trafficking in photoreceptors using retinal cell-free system. Methods Enzymol. 2000;315:77–88. [DOI] [PubMed] [Google Scholar]
- [98].Deretic D, Mazelova J. Assay for in vitro budding of ciliary-targeted rhodopsin transport carriers. Methods Cell Biol. 2009;94:241–257. [DOI] [PubMed] [Google Scholar]
- [99].Khan AR, Menetrey J. Structural biology of Arf and Rab GTPases’ effector recruitment and specificity. Structure. 2013;21:1284–1297. [DOI] [PubMed] [Google Scholar]
- [100].Pereira-Leal JB, Strom M, Godfrey RF, et al. Structural determinants of Rab and Rab Escort Protein interaction: rab family motifs define a conserved binding surface. Biochem Biophys Res Commun. 2003;301:92–97. [DOI] [PubMed] [Google Scholar]
- [101].Gillingham AK, Sinka R, Torres IL, et al. Toward a comprehensive map of the effectors of rab GTPases. Dev Cell. 2014;31:358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Matanis T, Akhmanova A, Wulf P, et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4:986–992. [DOI] [PubMed] [Google Scholar]
- [103].Echard A, Jollivet F, Martinez O, et al. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279:580–585. [DOI] [PubMed] [Google Scholar]
- [104].Miserey-Lenkei S, Chalancon G, Bardin S, et al. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol. 2010;12:645–654. [DOI] [PubMed] [Google Scholar]
- [105].Miserey-Lenkei S, Bousquet H, Pylypenko O, et al. Coupling fission and exit of RAB6 vesicles at Golgi hotspots through kinesin-myosin interactions. Nat Commun. 2017;8:1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Thomas LL, Fromme JC. GTPase cross talk regulates TRAPPII activation of Rab11 homologues during vesicle biogenesis. J Cell Biol. 2016;215:499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Miserey-Lenkei S, Waharte F, Boulet A, et al. Rab6-interacting protein 1 links Rab6 and Rab11 function. Traffic. 2007;8:1385–1403. [DOI] [PubMed] [Google Scholar]
- [108].Grigoriev I, Yu KL, Martinez-Sanchez E, et al. Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr Biol. 2011;21:967–974. [DOI] [PubMed] [Google Scholar]
- [109].Shibata S, Kawanai T, Hara T, et al. ARHGEF10 directs the localization of Rab8 to Rab6-positive executive vesicles. J Cell Sci. 2016;129:3620–3634. [DOI] [PubMed] [Google Scholar]
- [110].Recacha R, Boulet A, Jollivet F, et al. Structural basis for recruitment of Rab6-interacting protein 1 to Golgi via a RUN domain. Structure. 2009;17:21–30. [DOI] [PubMed] [Google Scholar]
- [111].Steger M, Tonelli F, Ito G, et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Dhekne HS, Yanatori I, Gomez RC, et al. A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. Elife. 2018;7:e40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Steger M, Diez F, Dhekne HS, et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Chiba S, Amagai Y, Homma Y, et al. NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. Embo J. 2013;32:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Berta AI, Boesze-Battaglia K, Genini S, et al. Photoreceptor cell death, proliferation and formation of hybrid rod/S-cone photoreceptors in the degenerating STK38L mutant retina. PLoS One. 2011;6:e24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Goldstein O, Kukekova AV, Aguirre GD, et al. Exonic SINE insertion in STK38L causes canine early retinal degeneration (erd). Genomics. 2010;96:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Mazelova J, Ransom N, Astuto-Gribble L, et al. 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci. 2009;122:2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chaineau M, Danglot L, Galli T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 2009;583:3817–3826. [DOI] [PubMed] [Google Scholar]
- [119].Martinez-Arca S, Alberts P, Zahraoui A, et al. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol. 2000;149:889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Molino D, Nola S, Lam SM, et al. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein in membrane domains transport and homeostasis. Cell Logist. 2015;5:e1025182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Danglot L, Chaineau M, Dahan M, et al. Role of TI-VAMP and CD82 in EGFR cell-surface dynamics and signaling. J Cell Sci. 2010;123:723–735. [DOI] [PubMed] [Google Scholar]
- [122].Ghosh D, Pinto S, Danglot L, et al. VAMP7 regulates constitutive membrane incorporation of the cold-activated channel TRPM8. Nat Commun. 2016;7:10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Burgo A, Proux-Gillardeaux V, Sotirakis E, et al. A molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev Cell. 2012;23:166–180. [DOI] [PubMed] [Google Scholar]
- [124].Pocard T, Le Bivic A, Galli T, et al. Distinct v-SNAREs regulate direct and indirect apical delivery in polarized epithelial cells. J Cell Sci. 2007;120:3309–3320. [DOI] [PubMed] [Google Scholar]
- [125].Galli T, Zahraoui A, Vaidyanathan VV, et al. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lafont F, Verkade P, Galli T, et al. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci U S A. 1999;96:3734–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Vogel GF, Klee KMC, Janecke AR, et al. Cargo-selective apical exocytosis in epithelial cells is conducted by Myo5B, Slp4a, Vamp7, and Syntaxin 3. J Cell Biol. 2015;211:587–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Szalinski CM, Labilloy A, Bruns JR, et al. VAMP7 modulates ciliary biogenesis in kidney cells. PLoS One. 2014;9:e86425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].De Franceschi N, Gilis F, Dogné S, et al. Longin and GAF domains: structural evolution and adaptation to the subcellular trafficking machinery. Traffic. 2014;15:104–121. [DOI] [PubMed] [Google Scholar]
- [130].Schlenker O, Hendricks A, Sinning I, et al. The structure of the mammalian signal recognition particle (SRP) receptor as prototype for the interaction of small GTPases with Longin domains. J Biol Chem. 2006;281:8898–8906. [DOI] [PubMed] [Google Scholar]
- [131].Levine TP, Daniels RD, Wong LH, et al. Discovery of new Longin and Roadblock domains that form platforms for small GTPases in Ragulator and TRAPP-II. Small GTPases. 2013;4:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Vivona S, Liu CW, Strop P, et al. The longin SNARE VAMP7/TI-VAMP adopts a closed conformation. J Biol Chem. 2010;285:17965–17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Schafer IB, Hesketh GG, Bright NA, et al. The binding of Varp to VAMP7 traps VAMP7 in a closed, fusogenically inactive conformation. Nat Struct Mol Biol. 2012;19:1300–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Burgo A, Casano AM, Kuster A, et al. Increased activity of the vesicular soluble N-ethylmaleimide-sensitive factor attachment protein receptor TI-VAMP/VAMP7 by tyrosine phosphorylation in the Longin domain. J Biol Chem. 2013;288:11960–11972. [DOI] [PMC free article] [PubMed] [Google Scholar]


