ABSTRACT
Aeromonas hydrophila (A. hydrophila) can cause a number of diseases in both human and animals. A. hydrophila-related infections in aquaculture cause severe economic losses every year throughout the world. The emergence of antibiotic resistance that is due to the abuse of antibiotics has limited the application of antibiotics. Thus, novel approaches are needed to combat with treatment failure of antibiotics caused by resistant bacterial strains. Aerolysin plays a critical role in the pathogenesis of A. hydrophila and has been considered as a novel target for developing drugs based on anti-virulence strategies. Here, we reported that luteolin, a natural product with no anti-A. hydrophila activity, could reduce aerolysin-induced hemolysis by inhibiting aerolysin activity. The binding mode was simulated by molecular docking and dynamics simulation. Then the main binding sites were confirmed by fluorescence quenching assays. We found that luteolin could hindered the formation of functional heptamer of aerolysin according to the results of the oligomerization assay. Moreover, luteolin could protect A549 cells from aerolysin mediated cell death and increase the survival rate of A. hydrophila-infected channel catfish. These findings suggest a novel approach to developing drugs fighting against A. hydrophila, and luteolin can be a promising drug candidate for treatment of A. hydrophila-associated infections.
KEYWORDS: Aeromonas hydrophila, aerolysin, luteolin, anti-virulence, molecular dynamics
Introduction
Aeromonas hydrophila (A. hydrophila) is a freshwater-dwelling zoonotic bacterium, which can cause infections in aquatic animals, terrestrial animals, and human [1]. The bacterium can be isolated from aquatic environment, drinking water, , and even wastewater [2]. Diseases caused by pathogenic A. hydrophila in freshwater fish species result in millions of dollars of economic losses worldwide, which has affected the healthy development of freshwater aquaculture [1]. A. hydrophila was first reported as a human pathogen in 1968 [3]. Since then, incidence of infections caused by pathogenic A. hydrophila has been on the rise because the bacterium can transmit to human through fish, water, or seafood exposed to A. hydrophila [4]. Thus, A. hydrophila has become an emerging foodborne pathogen to human, and which associated with various infectious diseases from slight skin infections to life-threatened bacteremia [5,6]. The use of antibiotics is the main approach of controlling diseases caused by bacterial pathogens since which were introduced into aquaculture industry. However, antibiotic resistance has emerged because of the overuse of antibiotics, which has limited the application of antibiotics in fighting bacterial infections in aquaculture [7]. Treatment failure by antibiotics has led to call for novel drugs or strategies against resistant A. hydrophila infections.
A. hydrophila harbors a number of virulence factors and toxins, including enterotoxin, cytotoxin, and toxins with hemolytic activities, which contribute to the pathogenesis of the bacterium [8]. Aerolysin, purified from A. hydrophila, is a well-characterized pore-forming toxin with multiple activities, such as hemolytic, enterotoxic and cytotoxic activities [9]. Aerolysin is secreted as a soluble precursor (named proaerolysin) into supernatant and has no biological activity [10]. Maturation of proaerolysin to aerolysin with activity must cleave the flexible loop of 43-residue at C-terminus by furin, trypsin, or other proteases [11]. Aerolysin can insert into target cell membrane after forming heptamer with a transmembrane pore [12,13]. The channel pore of the heptamer disrupts the membrane permeability barrier of cells and results in cell death [14]. Aerolysin has become a virulence marker of A. hydrophila because of the importance of the toxin [15]. Therefore, agents with anti- aerolysin activity might be useful for developing anti-A. hydrophila drugs.
Luteolin (Figure 1(a)), a natural flavonoid compound, has been isolated from a number of herbal medicine including Perilla frutescens, Dendranthema indicum and Lonicera japonica Thunb. Luteolin exhibits a certain amount of biological activities, such as anticancer, anti-inflammatory, antioxidant and neuroprotective effects [16]. In the present study, we found that luteolin could significantly reduce the pathogenesis of A. hydrophila by decreasing the activity of aerolysin. The mechanism of inhibitory effect was determined by molecular dynamics simulations and confirmed by fluorescence quenching assay.
Figure 1.
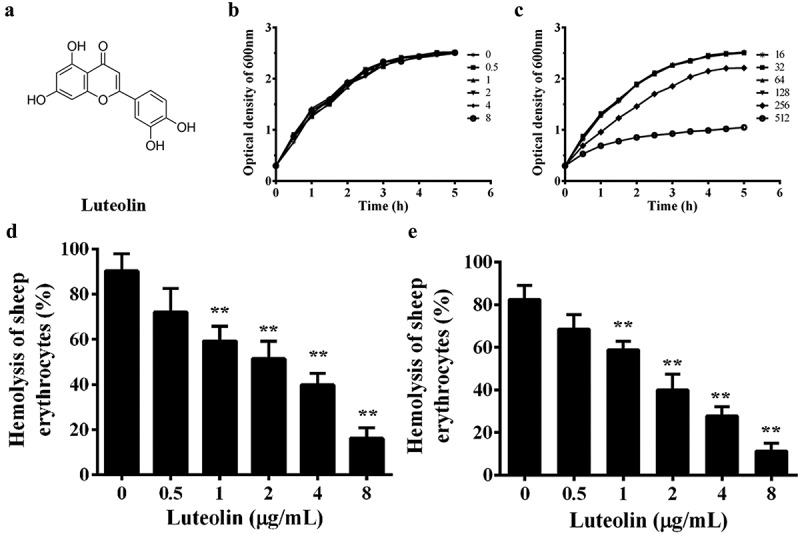
Luteolin decreased the AerA-mediated hemolytic activity. (a), structure of luteolin; (b), growth curves of XS-91-4-1 co-cultured with luteolin at concentrations ranging from 0 to 8 μg/mL; (c), growth curves at concentrations ranging from 16 to 512 μg/mL; (d), luteolin decrease the hemolysis of supernatants of A. hydrophila; E, hemolytic activity of purified AerA with a series of luteolin. Data presented in Figure 1(d and e) were the mean value ± standard divisions (SD) of three independent assays. *, p < 0.05 and **, p < 0.01 when compared with the 0.1% Triton X-100 group
Materials and methods
Micro-organism and reagents
A. hydrophila strain XS-91-4-1, isolated from diseased Hypophthalmichthys molitrix, was kindly supplied by Prof. Aihua Li at the Institute of Hydrobiology, Chinese Academy of Sciences. Luteolin was obtained from National Institutes for Food and Drug Control (Beijing, China), and enrofloxacin was obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Both drugs were dissolved in DMSO to prepare stock solutions at concentrations of 40,960 μg/mL.
Determination of minimal inhibitory concentrations
Broth micro-dilution method was used to determine the minimal inhibitory concentrations (MICs) under the instructions of Clinical and Laboratory Standards Institute (CLSI) [17]. Drugs were diluted by 2-fold methods with MHB medium to obtain concentrations from 512 μg/mL to 2 μg/mL for luteolin, while from 32 μg/mL to 0.125 μg/mL for enrofloxacin. Following addition of 5 × 105 CFU/mL bacterial suspensions to each well, the plates were incubated for 18–20 h at 28°C. MIC values were determined with respect to measurement of the first well with no bacterial growth.
Growth curves
Bacterial culture was cultured in 100 mL fresh BHI medium at 28°C until the optical density at 600 nm (OD600nm) reached to 0.3. Then, the culture was aliquoted into 12 100-ml flasks and luteolin at indicated concentrations (ranging from 0 to 512 μg/mL) was added to each flask. The mixtures were further incubated for 5 h at 28°C. The impact of luteolin on A. hydrophila was monitored by recording the OD600nm values by a spectrophotometer every 30 min.
Hemolytic activity assays
Impact of luteolin on hemolytic activities was determined by purified aerolysin (AerA) and bacterial supernatants co-cultured with indicated concentrations of luteolin according to previous studies [18,19]. For hemolytic activity of bacterial supernatants, the supernatants were centrifuged when the OD600nm reached 1.5 of A. hydrophila co-cultured with luteolin. Then, 100 μL of trypsin-treated supernatant and 25 μL of sheep erythrocyte were added into 875 μL of hemolytic buffer to obtain the reaction system. For activity of purified AerA, the hemolysis system at a 1 mL volume was composed of 5 μg/mL AerA, 5 × 106 defibrinated sheep red blood cells and indicated concentrations of luteolin in hemolytic buffer, then the reaction system was incubated at 37°C for 15 min. The activities of hemolysis in different groups were evaluated by values of OD543nm. Cells treated with 0.1% Triton X-100 served as the 100% hemolysis group.
Immunoblotting for aerolysin
Bacterial supernatants were collected by centrifugation when the OD600nm of co-cultures of A. hydrophila and luteolin was 1.5. Proteins in the supernatants were transferred onto a PVDF membrane following sodium dodecyl sulfate (SDS)-polyacrylamide (12%) gel electrophoresis. The protein levels were determined by ECL detection regents after the membrane was blocked with slim milk and reacted with antibodies.
Molecular docking
Molecular docking assay was performed using MOE v2018.0101 [20]. The 2D structure of luteolin was prepared by molecule and protein was built module in MOE, while energy minimization method was used to converting 3D structure of luteolin. The X-ray structure of AerA was obtained from Protein Data Bank (PDB ID: 1PRE). Luteolin was chosen as ligand and protein as receptor. The binding site for molecular docking was identified by Site Finder module in MOE, the surrender residues are: ASN178, ASP311, VAL312, SER313, TYR314, PHE343, VAL344, ILE345, GLY346, PRO347, TYR348, LYS349, SER353, SER354, ILE355, ARG356, LEU393, ARG394 PRO395, VAL396, and ARG397. The AMBER10: EHT force field and Reaction Field (R-field) implicit solvation model were selected before docking. The docking process adopted a flexible “induced fit” mode, the side chain of the amino acid binding pocket could be optimized and adjusted according to the ligand conformation, and the weight of restraining side chain rotation was set to 10. The binding mode of protein and molecule were first sorted by the London dG scoring function, and the first 20 conformations were reevaluated by further energy optimization and GBVI/WSA dG method. The best combination mode in the final ranking was chosen and all-atom, explicit water molecular dynamics simulation was performed.
Molecular dynamics (MD) simulation
The MD simulation was used to optimize the structure of the compound obtained by docking luteolin and AerA. The MMFF94x force field was selected to generate luteolin parameters [21]. The hydrogen atom of luteolin was optimized by Gaussian09 software package under HF/6-31 g*. And HF/6-31 g* was used to calculate the charge, and then the restrained electrostatic potential (RESP) fitting method was used to fit the electrostatic potential [22,23]. The sodium/chloride counterion was added to the complex to neutralize the system and solvated it in the rectangular water box of TIP3P to form a 10 Å solvent layer between the edge of the water box and the surface of the solute.
AMBER16 was used for performing MD simulations [24]. Covalent bonds involving hydrogen atoms were limited by the AMBER GAFF, FF14SB force fields and the SHAKE algorithm with 2 fs time step. Long-range electrostatic interactions were handled by the Particle mesh Ewald (PME) method. Before the heating step, two minimization steps were carried out for each solvation system. In the case where all heavy atoms were constrained by 50 kcal/(mol·Å2), the smallest first 4000 cycles were performed, while solvent molecules and hydrogen atoms moved freely. Then, unconstrained minimization was performed, including 2,000 steepest descent minimum cycles and 2,000 conjugate gradient minimization cycles. The entire system was heated from 0 K to 300 K at a constant volume at 50 ps using Langevin kinetics firstly and then equilibrate at a constant pressure of 1 atm for 400 ps. A 10 kcal/(mol·Å2) weak constraint was set up to constrain all heavy atoms in the heating step. In the production step, the NPT (constant composition, pressure, and temperature) set was simulated with periodic boundary dynamics of the entire system at 1 atmosphere and a constant pressure of 300 K. In the production phase, a 100 ns simulation was performed. The MM-GBSA method was used to determine the binding-free energy of the complex.
Mutagenesis of the proaerolysin (pAerA)protein
The plasmids encoding D7A-pAerA and D311A-pAerA were conducted according to our previous study [18]. Primer pairs used for D7A-pAerA were 5ʹ- GAGCCCGTCTATCCAGCGCAGCTGCGCCTGTTCTC-3ʹ (forward) and 5ʹ- GAGAACAGGCGCAGCTGCGCTGGATAGACGGGCTC (reverse). Primer pairs used for D311A-pAerA were 5ʹ- CTATGAATTCAAAGCCGCGGTCAGCTATGACCTGAC-3ʹ (forward) and 5ʹ- GTCAGGTCATAGCTGACCGCGGCTTTGAATTCATAG (reverse). Plasmids were transformed into BL21(DE3) competent cells and were overexpressed by induction of IPTG. After purification, the mutations were mixed with trypsin to obtain the activity. After stopping the reaction by trypsin/chymotrypsin inhibitor, proteins were stored at −80°C until use.
Analysis of the binding energy of WT-AerA and its mutants
As reported previously, the fluorescence quenching assays were carried out to evaluate the binding constants of luteolin to AerA and its mutants [25,26]. Briefly, a 3 mL solution of AerA (1 μM) was titrated by successive additions of aliquot amount of luteolin stock solution to obtain final concentrations of luteolin ranging from 2 μM to 64 μM. For fluorescence spectrofluorimetry determination, the excitation and emission wavelength was set to 280-nm with a 5-nm band-passand 345-nm with a 10-nm band-pass, respectively. The data were analyzed by Stern-Volmer and van’t Hoff equations.
Inhibition of forming heptamer
Heptamer was induced according to a previous study [27]. Briefly, proaerolysin was digested by trypsin at room temperature for 10 min to obtain activated aerolysin. Then, the same mol ratio of AerA and luteolin as used in hemolytic assay was used to create a mixture with the storage buffer to a total volume of 20 μL. The mixtures were incubated at 37°C for15 min, and 1 μL of 1 M Hepes was added to the reaction systems to induce oligomerization. The mixtures were further incubated at 4°C for 1 h and loaded onto an 8% SDS-PAGE gel for electrophoresis. The ratio of heptamer and monomer was calculated by Image Lab (Bio-Rad, USA) and statistical significance was analyzed.
Influence of luteolin on cell viability
Human lung epithelial (A549) cells were cultured in DMEM with 10% fetal bovine serum at 37°C with 5% CO2 in a humidified incubator. After digested by trypsin, cells at a density of 1.5 × 105 were seeded into each well of a 96-well plate. Then, AerA and luteolin at indicated concentrations were added to each well and incubated for 24 h. Lactate dehydrogenase (LDH) release and live/dead cell staining were used to assess cell viability after different treatments. LDH activities were determined by cell supernatants with different treatments on a microplate reader (Bio-Rad, USA). Images of cells stained by live/dead regents were captured by a fluorescence microscope (Olympus, Japan).
Experimental therapeutics
Animal studies were carried out under the guidance of the Animal Welfare and Research Ethics Committee of Yangtze River Fisheries Research Institute (Permit No. 2,019,052,203). Channel catfish (200 ± 10 g) were divided into 3 groups and each group contained 10 fish. Before infection, fish were maintained in 100-L glass aquaria tanks for 15 days. Bacterial culture was centrifuged to obtain the bacterial cells when the bacterium reached to logarithmic growth phase in BHI medium at 28°C. The concentration of bacterial cells was then adjusted to approximately 1.5 × 108 CFU/mL after washed with sterile PBS for 3 times. Fish were injected with 100 μL of bacterial suspension intraperitoneally to establish the infection model. Then, fish in treated group were given 50 mg/mL luteolin 6 h post-infection at 12-h intervals for a total of 6 doses. Fish in the positive group were exposed to the bacterium and administered with sterile PBS, while in the negative group were administered with sterile PBS only. The survival rate of each group was recorded and the experimental setup was monitored for 8 days. For histological examination of kidney, 5 fish in each group were sacrificed, then kidneys were removed and fixed in 10% formalin immediately. These tissues were embedded in paraffin wax and stained with hematoxylin-eosin for light microscopy.
Statistical analysis
The significance of the results of hemolytic activity and LDH release assays was analyzed by independent Student’s t-test. Mortality data were calculated by the Kaplan-Meier analysis, and the significance were compared by log-rank test. A p value less than 0.05 was considered to be statistically significant.
Results
Luteolin decrease the hemolysis of AerA
The MIC value of luteolin against A. hydrophila XS-91-4-1 was higher than 256 μg/mL, while enrofloxacin was 4 μg/mL. The growth curves showed that the growth of the strain was inhibited at concentrations higher than 128 μg/mL (Figure 1(c)), but no influence was observed at concentrations ranging from 0 to 128 μg/mL luteolin (Figure 1(b and Figure 1c)). These results demonstrated that luteolin showed no inhibitory effect on bacterial growth under our experimental concentrations. But we found that luteolin could significantly decrease the hemolytic activity of bacterial supernatant when co-cultured with A. hydrophila at indicated concentrations (Figure 1(c)). The results inferred that luteolin could inhibit the expression or activity of AerA in the supernatants. Western-blot assay was carried out to determine the influence of luteolin on the production of AerA in supernatants. We found that luteolin had no evident effect on the amount of AerA (Figure 2). Then the hemolytic assays were performed using purified AerA. As shown in Figure 1(d), luteolin could reduce the hemolytic activity of AerA directly. The percentage of hemolysis was reduced to 11.25 ± 3.66% when added with 8 μg/mL luteolin (Figure 1(d)). These results illustrated that luteolin could decrease the activity of AerA without affecting the growth of A. hydrophila.
Figure 2.
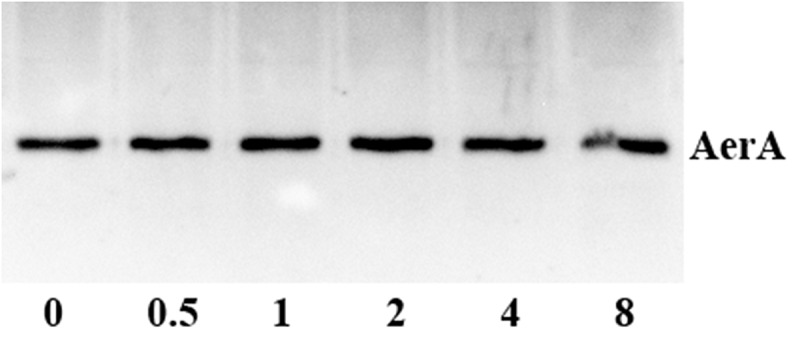
Detection of pAerA levels in bacterial supernatants with different concentrations of luteolin by Western-blot
Molecular dynamics results
The stable complex of luteolin and AerA was established by all-atom, explicit water MD simulations. As shown in Figure 3, the root means square deviation (RMSD) of the backbone atoms of AerA is less than 8.0 angstrom and that of Luteolin is less than 2.5 angstrom, and the system achieves equilibrium within the simulation time (no drastic fluctuation occur on the value of RMSD), which suggest that the force field and simulation protocol are adequate. Moreover, system balance was observed within simulation time. These findings indicated that adequate force field and simulation protocol could be provided. The final stable complex of luteolin with AerA was shown in Figure 4. Hydrogen bonds were provided by the oxygen atoms of hydroxyl groups in benzopyran and benzene ring of luteolin, of which form hydrogen bonds with oxygen atom of Asp7, Thr87, and Asp 311, respectively.
Figure 3.
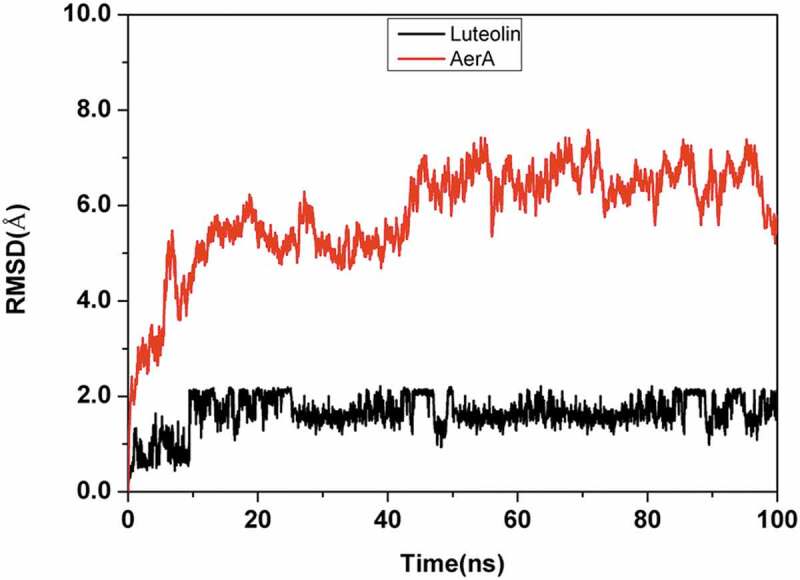
System flexibility analysis of complex luteolin with AerA
Figure 4.
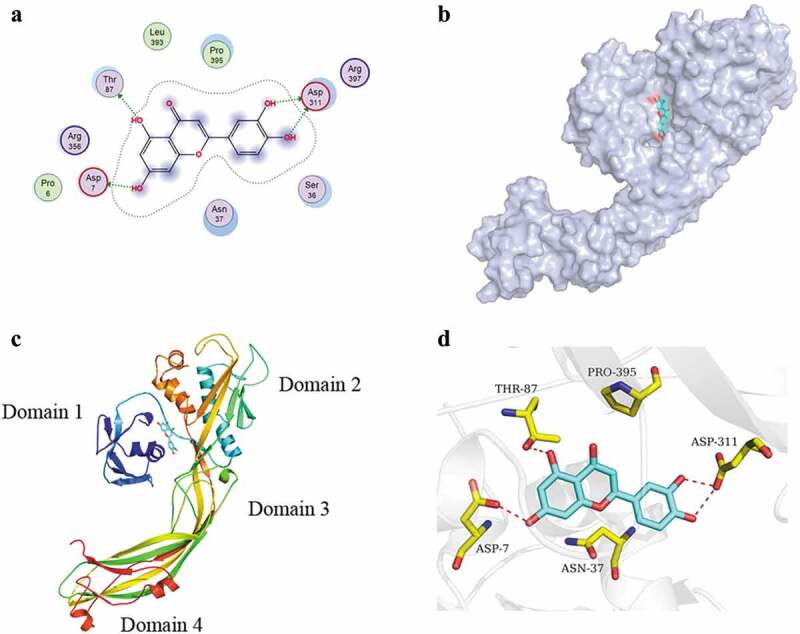
AerA complex with luteolin. (a) Binding mode of AerA with luteolin; (b) The predicted binding model of luteolin on the pocket of AerA; (c) a ribbon representation of the AerA-luteolin complex; (d) The 3D binding mode of AerA with luteolin
As shown in Table 1, the MM-GBSA assay was performed to determine the binding energy (∆Gtotal) of luteolin with AerA. ∆Gtotal represent the contribution to the binding free energy. ∆Evdw and ∆Eele represent the VdW and electrostatic interactions contributions. ∆Gpolar and ∆Gnonpolar represent the polar and nonpolar solvation energy contributions. As shown in Table 1, the main contributor of luteolin-AerA binding was ∆Eelec, indicating that the binding was managed by electrostatic interactions to large extent. ∆Gpolar was positive and unfavorable for the binding, while ∆Gnonpolar was negative and favorable. The binding-free energy of luteolin-AerA complex was calculated to be −11.82 kcal/mol. Energy composition analysis suggested that residues Asp7, Asp311, Thr87, and Asn37 were the main binding residues of the complex binding energy (Figure 5).
Table 1.
Binding energy of luteolin-AerA complex calculated by MM-GBSA method
| Contribution | Energy (kcal/mol) |
|---|---|
| ∆Evdw | −21.86 ± 0.35 |
| ∆Eele | −25.63 ± 0.75 |
| ∆Gpolar | 39.01 ± 0.61 |
| ∆Gnonpolar | −3.34 ± 0.03 |
| ∆Gtotal | −11.82 ± 0.33 |
Figure 5.
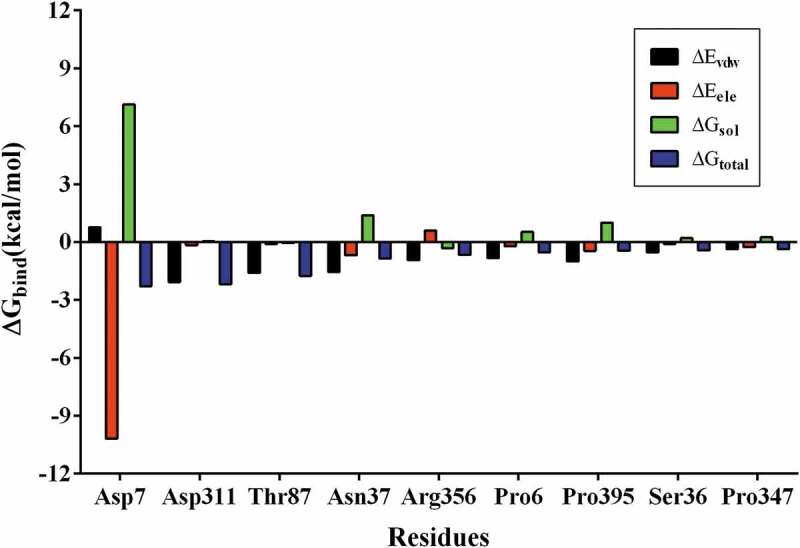
Decomposition of binding energy of the ranked top 9 residues in the complex
Mechanism of luteolin against AerA activity
The main binding sites of AerA-luteolin complex have been predicted by molecular dynamics. The fluorescence quenching and oligomerization assays were performed to calculate the binding constants (KA) and the number of binding sites (n). As shown in Table 2, the KA of WT-AerA, Asp7A-AerA and Asp311A-AerA mixed with luteolin were calculated, which confirmed the results of molecular dynamics. Furthermore, we found that indicated concentrations of luteolin could reduce the amount of heptamer by oligomerization assay in a dose-dependent manner (Figure 6). Moreover, we found that the ratio of heptamer and monomer was significantly reduced when WT-AerA incubated with luteolin at a mol ratio higher than 1:50 (Supplemental Figure 1). The results revealed that luteolin could reduce the hemolysis of AerA by hindering the formation of heptamer, Asp7 and Asp311 were confirmed to be the main binding sites of luteolin with AerA.
Table 2.
Relationship of the values of binding energy (ΔGbind) and binding constants (KA)
| WT-AerA | D7A | D311A | |
|---|---|---|---|
| ΔGbind (kcal/mol) | −11.82 | −2.28 | −2.19 |
| KA(1 × 104)L ‧ mol−1 | 14.10 | 12.40 | 8.80 |
| n | 1.0157 | 0.9984 | 0.9838 |
Figure 6.
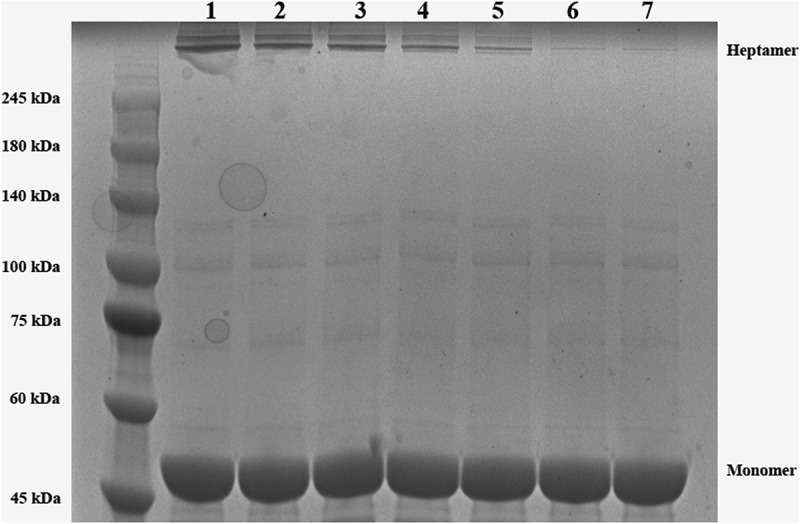
Influence of luteolin on the heptamer formation of AerA. Coomassie blue staining was used to detect the amount of heptamer after separated by SDS-PAGE. Lane 1, WT-AerA; Lane 2, WT-AerA with luteolin (1:50, mol/mol); Lane 3, WT-AerA with luteolin (1:100, mol/mol); Lane 4, WT-AerA with luteolin (1:150); Lane 5, WT-AerA with luteolin (1:200); Lane 6, WT-AerA with luteolin (1:250); Lane 7, WT-AerA with luteolin (1:300)
Luteolin decreases AerA induced the cell injury
According to previous study, AerA could lead to cell injury of a number of mammalian cells [28]. Our previous studies indicated that A549 and Vero cells could be used as target cells to evaluate the effects of natural compounds to AerA induced cell injury [18,19]. As shown in Figure 7(a), untreated cells were shown green, indicating that cells were live. AerA treated cells showed the increase of red fluorophore (Figure 7(b)), indicating a large increase of dead cells. However, cells added with AerA and then 2 μg/mL luteolin showed a significant decrease in cell death (Figure 7(c)). In addition, the extent of cell injury induced by AerA after treatment with luteolin was determined. As expected, luteolin could significantly attenuate cell injury at concentrations higher than 1 μg/mL (Figure 7(d)). Taken together, these results revealed that luteolin might provide a protective effect against A. hydrophila infections.
Figure 7.
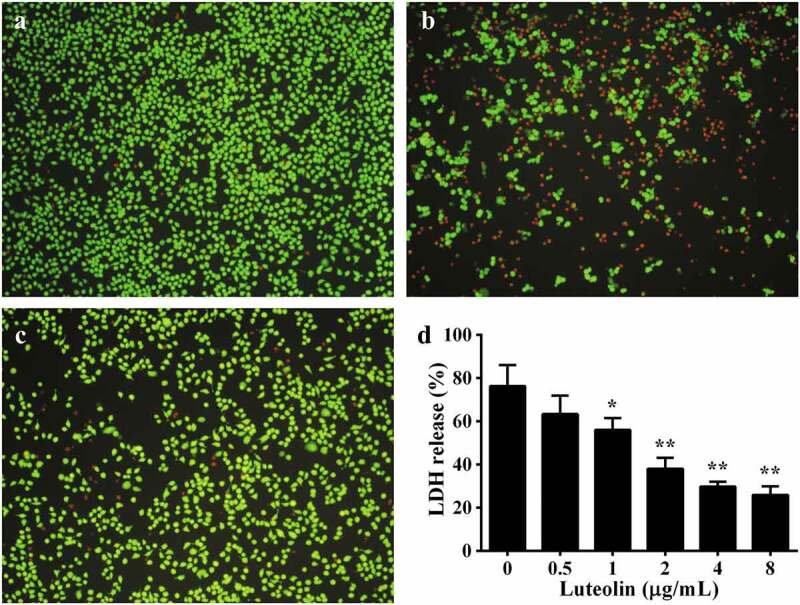
Luteolin reduced AerA mediated cell injury. A live/dead regent was used to stain A549 cells with different treatments. Live cells were stained with green, while death cells were red, fluorescence were shot by a fluorescence microscope. A, negative control (cells without any treatment); B, positive control (AerA treated cells); C, cells added with AerA plus 2 μg/mL luteolin; D, A549 cells LDH release after treatment with AerA and indicated concentrations of luteolin. Data were presented as mean value ± SD of three independent experiments. *, p < 0.05 and **, p < 0.01 when compared with the positive group
Luteolin increase the suvival of A. hydrophila infected channel catfish
According to the results of in vitro assays, luteolin can decrease the activity of AerA by blocking the formation of heptamer and decrease AerA induced cell injury, indicating that luteolin can be identified as a potent drug against A. hydrophila infections in fish and other animals. As shown in Figure 8, deaths occurred in 24 h post-infection, the survival rate in the positive group was exhibited to 10%, while 80% in luteolin treated group after a luteolin course of 3 days. All fish injected with sterile PBS survived for 8 days (Figure 8). Moreover, kidneys were obtained to evaluate the injury caused by A. hydrophila infection. Kidneys in the positive group showed hyaline degeneration and necrosis (Figure 9A1, black arrow) in renal tubular epithelia; Bleeding (Figure 9A2, blue arrow) and macrophages infiltration (Figure 9A1, red arrow) were observed in renal interstitial. The findings indicated that fish in the positive group exhibited severe renal injury. However, kidney in the luteolin treated group showed macrophages infiltration in renal interstitial only (Figure 9(b), red arrow), indicating that the renal injury was repaired. Kidney in the negative group showed slightly macrophages infiltration in renal interstitial (Figure 9(c), red arrow). Taken together, these results demonstrated that luteolin could decrease the renal injuries in kidneys of channel catfish infected with A. hydrophila.
Figure 8.
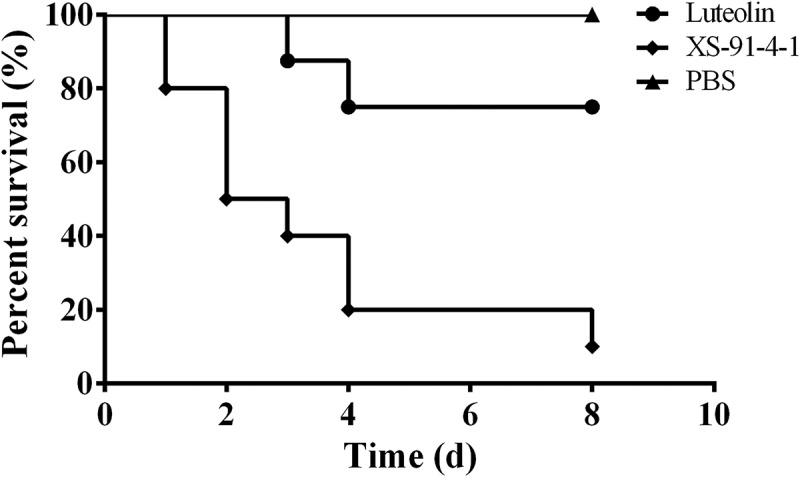
Luteolin increase the survival rate of channel catfish post infection. Infected fish were treated with luteolin or sterile PBS 6 hs post infection, and deaths were recorded for 8 days. The survival rate of fish treated with luteolin was significantly increased compared with positive control group (p = 0.009)
Figure 9.
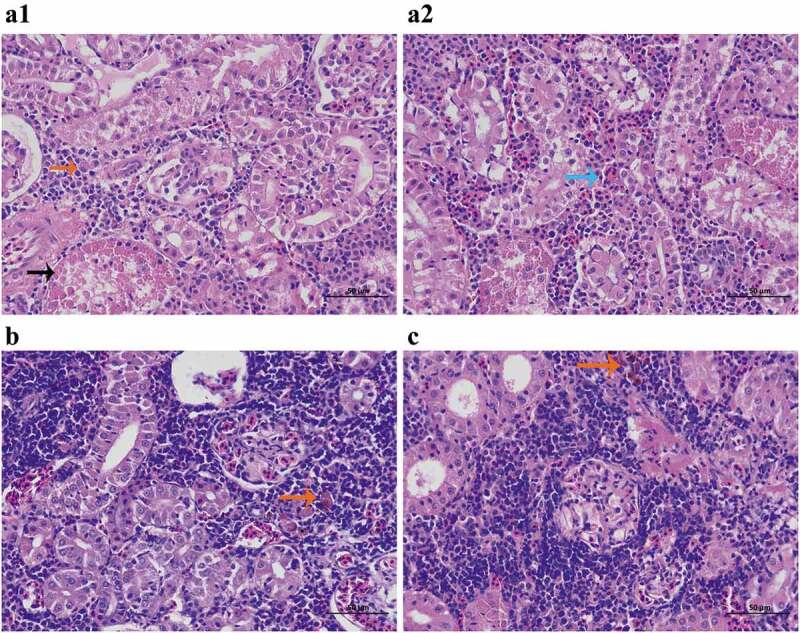
Luteolin alleviated the renal injuries mediated by A. hydrophila infection. A1 and A2 were kidney from positive group, hyaline degeneration and necrosis (A1, black arrow), Bleeding (A2, blue arrow) and macrophages infiltration (A1, red arrow) were observed; B represented kidney from luteolin treated group, macrophages infiltration (B, red arrow) was observed; while C was kidney from negative group, macrophages infiltration (C, red arrow) was observed
Discussion
It is known that freshwater aquaculture plays an important role in global food security [29]. Fish has become one of the most important sources of protein in China and perhaps other countries [30]. Therefore, China has been the largest producer and consumer of aquaculture in the world, counting for approximately 60% of the global productions [31]. However, the expansion of breeding scale results in the emergence of fish diseases and other problems [32]. Among of which, bacterial infections have become the main obstruction to freshwater farming [33]. Epidemic disease caused by A. hydrophila has been reported, which may result in millions of dollars’ economic losses worldwide [34]. Antibiotics have been extensively used in aquaculture for preventing or treating bacterial infections, including A. hydrophila infections [35]. Although the discovery and application of antibiotics decreased the loses caused by bacterial infections, several problems including antibiotic resistance and pollution have been observed [36]. Moreover, the emergence of antibiotic resistance leads to treatment failure. Therefore, solutions are urgently demanded for dissolving the growing number of infectious diseases caused by antibiotic-resistant bacterial pathogens [37].
Pore-forming toxins (PFTs), secreted by a large number of important bacterium, have been found that contributing to the virulence of bacterium [38]. PFTs are unique targets for identifying drugs battling with resistant bacterial pathogen infections because the presence of PFTs is nearly universal [38]. Qiu et al. demonstrated that baicalin could reduce the activity of α-hemolysin of Staphylococcus aureus (S. aureus) through binding to α-toxin and hindering the formation of functional heptamer, which resulted in the decrease of pathogenicity of S. aureus to mice [39]. Myricetin, a natural flavonoid compound, has been reported to be a candidate for the treatment of Streptococcus suis by abolishing the activity of suilysin via inhibiting oligomer formation [40]. Lu et al. found that betulin attenuated the virulence of Listeria monocytogenes in a mice infection model by suppressing the oligomerization process of listeriolysin O (LLO) [41]. Taken together, these findings demonstrated that PFTs could be promising targets developing novel drugs against bacterial infections. Previous studies have shown that tannic acid and rosmarinic acid can inhibit the hemolytic activity of A. hydrophila by inhibiting the quorum sensing system, resulting in the reduction of bacterial pathogenesis [42,43]. Moreover, T. Chakraborty et al. found that the 50% lethal dose of A. hydrophila strain deleted AerA was much higher than the wild type strain [44]. These findings indicated that AerA is critical for the virulence of A. hydrophila. Thus, AerA has been employed as a target identifying anti-A. hydrophila drugs.
The purpose of traditional antibiotics is to kill the bacteria or to suppress the bacterial growth by inhibiting their essential functions [45]. Although the modes of actions have been proven to be highly effective, the selective pressure has led to the emergence of antibiotic resistance [45]. Thus, compounds without anti-bacterial activities have been used for screening drugs to avoid the induction of resistance. Here we demonstrated that luteolin, had no influence on bacterial growth under our experimental concentrations, could decrease the hemolytic activity of AerA. Difference with our previous studies on thymol and magnolol, luteolin could directly neutralize the activity of AerA rather than affecting the production of AerA [19,46]. Moreover, indolo[3,2-b] quinolones have been identified as good candidates for anti-virulence drugs by inhibiting the activity of AerA like hemolysin of A. sobria. However, the mechanism and major binding sites were not determined by the authors [47]. Molecular dynamics (MD) simulations, a promising tool for drug identification, have been widely applied to determine the binding modes and binding energies of ligand–target interactions. Moreover, the increasing knowledge of structure and pore assembly of AerA makes it possible to clarify the mechanism of luteolin against the activity of AerA [48,49]. Our previous study confirmed that Arg356, Pro347 and Asn 37 were major binding sites of morin-AerA complex [18]. Here, we found that Asp7 and Asp311 were major sites when determined by the same methods with morin. Although the binding sites were different, the two drugs shared the same mode of action by hindering the formation of heptamer. Previous study showed that the conformation of AerA would change in the process of forming heptamer, including the rotation of domain 1 and movement of domain 4 with respect to domain 3 [50]. Taken our results together, these findings indicated that the binding of luteolin to AerA limited the movement of Asp7 and Asp 311 residues in conformation, which resulted in the barrier of forming heptamer. Of which might be the mechanism of luteolin inhibiting formation of heptamer. To obtain the results of oligomerization assay, the concentration of AerA used in the study was increased to 1 mg/mL, about 200-fold higher than the luteolin used in hemolytic activity assay. Thus, the mol ratio of luteolin and AerA was used to determine the concentration of luteolin in oligomerization assay. As expected, the production of heptamer was decreased following the addition of luteolin. Different with previous studies, Asp7 and Asp311 did not involve in the formation of heptamer directly (Supplemental Figure 2), we found that both the mutants could form heptamer in the absence of luteolin. However, there were still a certain amount of heptamer of the mutants in the presence of luteolin compared with WT-AerA plus luteolin (Supplemental Figure 2). The results indicated that AerA mutated Asp7 or Asp311 led to a decrease of binding ability.
A previous study showed that luteolin could serve as a potent anti-virulent agent against Vibrio harveyi mediated infections by inhibiting the quorum sensing system [51]. Qiu et al. found that luteolin could reduce the product of α-toxin of S. aureus by inhibiting the agr locus [52]. Wang et al. reported that luteolin could reduce the LLO activity by binding to the hly mRNA coding region directly and their results demonstrated that luteolin could be used as a lead compound against Listeria monocytogenes infections [53]. These findings indicated that luteolin might be a novel agent against bacterial infections targeting the virulence. To the best of our knowledge, the present study is the first report of luteolin on the pathogenicity of A. hydrophila. Overall, a new approach was developed in identifying drugs by inhibiting the activity of PFTs. Moreover, luteolin is a promising candidate against A. hydrophila-mediated infections.
Supplementary Material
Acknowledgments
We thank Dr. Zhihong Liu from Sun Yat-sen University for kindly providing modeling software mentioned in the study.
Funding Statement
This work is supported by the National Nature Science Foundation of China (No. 31702368) and National Key R&D Program of China (No. 2019YFD0900104).
Disclosure statement
No conflict of interest declared.
Authors’ contributions
J.D. designed the experiments. J.D. and L.S.Z. carried out the experiments, collected data, and wrote this manuscript. Y.T.L., Y.B.Y., X.H.A. and N.X. checked and revised the manuscript. Q.H.Y., J.D. and S.Z. contributed data analysis.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Hossain MJ, Sun D, McGarey DJ, et al. An Asian origin of virulent Aeromonas hydrophila responsible for disease epidemics in United States-farmed catfish. mBio. 2014;5:e00848–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Janda JM, Abbott SL.. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Von Graevenitz A, Mensch AH. The genus aeromonas in human bacteriology; report of 30 cases and review of the literature. N Engl J Med. 1968;278:245–249. [DOI] [PubMed] [Google Scholar]
- [4].Grim CJ, Kozlova EV, Ponnusamy D, et al. Functional genomic characterization of virulence factors from necrotizing fasciitis-causing strains of Aeromonas hydrophila. Appl Environ Microbiol. 2014;80:4162–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chang CY, Thompson H, Rodman N, et al. Pathogenic analysis of Aeromonas hydrophila septicemia. Ann Clin Lab Sci. 1997;27:254–259. [PubMed] [Google Scholar]
- [6].Reines HD, Cook FV. Pneumonia and bacteremia due to Aeromonas hydrophila. Chest. 1981;80:264–267. [DOI] [PubMed] [Google Scholar]
- [7].Yao ZJ, Li WX, Lin Y, et al. Proteomic analysis reveals that metabolic flows affect the susceptibility of aeromonas hydrophila to antibiotics. Sci Rep. 2016;6. DOI: 10.1038/srep39413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chopra AK, Houston CW, Peterson JW, et al. Cloning, expression, and sequence analysis of a cytolytic enterotoxin gene from Aeromonas hydrophila. Can J Microbiol. 1993;39:513–523. [DOI] [PubMed] [Google Scholar]
- [9].Wang G, Clark CG, Liu C, et al. Detection and characterization of the hemolysin genes in Aeromonas hydrophila and Aeromonas sobria by multiplex PCR. J Clin Microbiol. 2003;41:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Song TY, Toma C, Nakasone N, et al. Aerolysin is activated by metalloprotease in Aeromonas veronii biovar sobria. J Med Microbiol. 2004;53:477–482. [DOI] [PubMed] [Google Scholar]
- [11].Wuethrich I, Peeters JGC, Blom AEM, et al. Site-specific chemoenzymatic labeling of aerolysin enables the identification of new aerolysin receptors. Plos One. 2014;9. DOI: 10.1371/journal.pone.0109883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Diep DB, Nelson KL, Raja SM, et al. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J Biol Chem. 1998;273:2355–2360. [DOI] [PubMed] [Google Scholar]
- [13].van der Goot FG, Pattus F, Wong KR, et al. Oligomerization of the channel-forming toxin aerolysin precedes insertion into lipid bilayers. Biochemistry. 1993;32:2636–2642. [DOI] [PubMed] [Google Scholar]
- [14].Parker MW, van der Goot FG, Buckley JT. Aerolysin--the ins and outs of a model channel-forming toxin. Mol Microbiol. 1996;19:205–212. [DOI] [PubMed] [Google Scholar]
- [15].Zhang D, Pridgeon JW, Klesius PH. Expression and activity of recombinant proaerolysin derived from Aeromonas hydrophila cultured from diseased channel catfish. Vet Microbiol. 2013;165:478–482. [DOI] [PubMed] [Google Scholar]
- [16].Nabavi SF, Braidy N, Gortzi O, et al. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res Bull. 2015;119:1–11. [DOI] [PubMed] [Google Scholar]
- [17].CLSI . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically-eighth edition: approved standard M07-A8. Wayne, PA, USA: CLSI; 2009. [Google Scholar]
- [18].Dong J, Liu Y, Xu N, et al. Morin protects channel catfish from aeromonas hydrophila infection by blocking aerolysin activity. Front Microbiol. 2018;9:2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dong J, Zhang L, Liu Y, et al. Thymol protects channel catfish from aeromonas hydrophila infection by inhibiting aerolysin expression and biofilm formation. Microorganisms. 2020;8. DOI: 10.3390/microorganisms8050636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Inc. CCG . Molecular operating environment (MOE), v2018.0101. Canada: Chemical Computing Group Inc.; 2018. [Google Scholar]
- [21].Halgren TA. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem. 1996;17:490–519. [Google Scholar]
- [22].Firisch MJ, Trucks HB, Schlegel GE, et al. Gaussian 09. Gaussian: Inc., Wallingford, CT; 2016. [Google Scholar]
- [23].Wang J, Cieplak P, Kollman PA. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 2000;21:1049–1074. [Google Scholar]
- [24].Case DA, Cheatham III TE, Darden T, et al. The Amber biomolecular simulation programs. J Comput Chem. 2005;26:1668–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jurasekova Z, Marconi G, Sanchez-Cortes S, et al. Spectroscopic and molecular modeling studies on the binding of the flavonoid luteolin and human serum albumin. Biopolymers. 2009;91:917–927. [DOI] [PubMed] [Google Scholar]
- [26].Ibrahim N, Ibrahim H, Kim S, et al. Interactions between antimalarial indolone-N-oxide derivatives and human serum albumin. Biomacromolecules. 2010;11(12):3341–3351. [DOI] [PubMed] [Google Scholar]
- [27].Iacovache I, Degiacomi MT, Pernot L, et al. Dual chaperone role of the C-terminal propeptide in folding and oligomerization of the pore-forming toxin aerolysin. PLoS Pathog. 2011;7:e1002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abrami L, Fivaz M, Glauser PE, et al. Sensitivity of polarized epithelial cells to the pore-forming toxin aerolysin. Infect Immun. 2003;71:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang Q, Li Z, Gui JF, et al. Paradigm changes in freshwater aquaculture practices in China: moving towards achieving environmental integrity and sustainability. Ambio. 2018;47:410–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mo WY, Man YB, Wong MH. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: needs and challenge. Sci Total Environ. 2018;613-614:635–643. [DOI] [PubMed] [Google Scholar]
- [31].Wang JJ, Beusen AHW, Liu XC, et al. Aquaculture production is a large, spatially concentrated source of nutrients in Chinese freshwater and coastal seas. Environ Sci Technol. 2020;54:1464–1474. [DOI] [PubMed] [Google Scholar]
- [32].Wang QC, Ji W, Xu Z. Current use and development of fish vaccines in China. Fish Shellfish Immunol. 2020;96:223–234. [DOI] [PubMed] [Google Scholar]
- [33].Singh V, Chaudhary DK, Mani I, et al. Development of diagnostic and vaccine markers through cloning, expression, and regulation of putative virulence-protein-encoding genes of Aeromonas hydrophila. J Microbiol. 2013;51:275–282. [DOI] [PubMed] [Google Scholar]
- [34].Chen N, Jiang JJ, Gao XJ, et al. Histopathological analysis and the immune related gene expression profiles of mandarin fish (Siniperca chuatsi) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2018;83:410–415. [DOI] [PubMed] [Google Scholar]
- [35].Kummerer K. Antibiotics in the aquatic environment--a review--part I. Chemosphere. 2009;75:417–434. [DOI] [PubMed] [Google Scholar]
- [36].Wright GD. Bacterial resistance to antibiotics: enzymatic degradation and modification. Adv Drug Deliv Rev. 2005;57:1451–1470. [DOI] [PubMed] [Google Scholar]
- [37].Cotter PD, Ross RP, Hill C. Bacteriocins - a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11:95–105. [DOI] [PubMed] [Google Scholar]
- [38].Los FC, Randis TM, Aroian RV, et al. Role of pore-forming toxins in bacterial infectious diseases. Microbiol Mol Biol Rev. 2013;77:173–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Qiu J, Niu X, Dong J, et al. Baicalin protects mice from Staphylococcus aureus pneumonia via inhibition of the cytolytic activity of alpha-hemolysin. J Infect Dis. 2012;206:292–301. [DOI] [PubMed] [Google Scholar]
- [40].Niu X, Sun L, Wang G, et al. Investigation of the inhibition effect and mechanism of myricetin to Suilysin by molecular modeling. Sci Rep. 2017;7:11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu GJ, Xu L, Zhang P, et al. Betulin efficiently suppresses the process of an experimental Listeria monocytogenes infection as an antagonist against listeriolysin O. Fitoterapia. 2019;139. DOI: 10.1016/j.fitote.2019.104409 [DOI] [PubMed] [Google Scholar]
- [42].Rama Devi K, Srinivasan R, Kannappan A, et al. In vitro and in vivo efficacy of rosmarinic acid on quorum sensing mediated biofilm formation and virulence factor production in Aeromonas hydrophila. Biofouling. 2016;32:1171–1183. [DOI] [PubMed] [Google Scholar]
- [43].Patel B, Kumari S, Banerjee R, et al. Disruption of the quorum sensing regulated pathogenic traits of the biofilm-forming fish pathogen Aeromonas hydrophila by tannic acid, a potent quorum quencher. Biofouling. 2017;33:580–590. [DOI] [PubMed] [Google Scholar]
- [44].Chakraborty T, Huhle B, Hof H, et al. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect Immun. 1987;55:2274–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3:541–548. [DOI] [PubMed] [Google Scholar]
- [46].Dong J, Ding H, Liu Y, et al. Magnolol protects channel catfish from Aeromonas hydrophila infection via inhibiting the expression of aerolysin. Vet Microbiol. 2017;211:119–123. [DOI] [PubMed] [Google Scholar]
- [47].Takahashi E, Fujinami C, Kuroda T, et al. Indolo[3,2-b]quinoline derivatives suppressed the hemolytic activity of beta-pore forming toxins, aerolysin-like hemolysin produced by aeromonas sobria and alpha-hemolysin produced by staphylococcus aureus. Biol Pharm Bull. 2016;39:114–120. [DOI] [PubMed] [Google Scholar]
- [48].Degiacomi MT, Iacovache I, Pernot L, et al. Molecular assembly of the aerolysin pore reveals a swirling membrane-insertion mechanism. Nat Chem Biol. 2013;9:623–629. [DOI] [PubMed] [Google Scholar]
- [49].Parker MW, Buckley JT, Postma JP, et al. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature. 1994;367:292–295. [DOI] [PubMed] [Google Scholar]
- [50].Iacovache I, De Carlo S, Cirauqui N, et al. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat Commun. 2016;7:12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sowndarya J, Farisa Banu S, Madhura G, et al. Agro food by-products and essential oil constituents curtail virulence and biofilm of Vibrio harveyi. Microb Pathog. 2019;135:103633. [DOI] [PubMed] [Google Scholar]
- [52].Qiu J, Li H, Meng H, et al. Impact of luteolin on the production of alpha-toxin by Staphylococcus aureus. Lett Appl Microbiol. 2011;53:238–243. [DOI] [PubMed] [Google Scholar]
- [53].Wang J, Liu S, Liu B, et al. Luteolin inhibits listeriolysin O translation by directly targeting the coding region of the hly mRNA. Front Microbiol. 2019;10:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


