ABSTRACT
Triple-negative breast cancer (TNBC) is the most aggressive type of breast cancer and still lacks available targeted therapy options. Thioridazine hydrochloride (Thi-hyd), a common phenothiazine antipsychotic drug, has shown great tumor-suppressive potential in several types of cancer cells. In this study, we explored the anti-tumor effects of Thi-hyd on TNBC. Thi-hyd significantly inhibited the viability and migration of TNBC cells in vitro. PI3K/AKT signaling pathway was verified to be related to Thi-hyd-induced tumor inhibition. G0/G1 cell cycle arrest and apoptosis were observed after treatment with Thi-hyd. The cell cycle arrest was accompanied by down-regulation of CDK4/cyclin D1 and up-regulation of p21 and p27. The apoptosis was accompanied by mitochondrial dysfunction. In vivo, Thi-hyd significantly suppressed the growth of tumors showing a 63.73% inhibition rate in tumor weight. Spontaneous lung metastasis was also efficiently prevented by Thi-hyd treatment, with a 72.58% inhibition rate. Immunohistological analysis showed increased Ki67 expression and reduced cleaved caspase-3 expression in Thi-hyd-treated mice. No apparent side effects were observed according to blood test and H&E staining results. Collectively, our results show that Thi-hyd can be used as a potential drug for TNBC treatment and set the basis for its clinical evaluation and investigation.
Abbreviations
CCK8: Cell Counting Kit-8; CDK: cyclin-dependent kinase; DRD2: dopamine D2 receptor; ERK1/2: extracellular signal-regulated kinase 1/2; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; H&E: hematoxylin and eosin; MMP: membrane potential; NAC: N-acetyl-L-cysteine; PI: Propidium iodide; Rh123: rhodamine-123; ROS: reactive oxygen species; TBST: tris-buffered saline containing 0.1% Tween 20 TNBC: Triple-negative breast cancer; Thi-hyd: Thioridazine hydrochloride.
KEYWORDS: Thioridazine hydrochloride, antipsychotic agent, triple-negative breast cancer, PI3K/AKT pathway
Introduction
Breast cancer is the second leading cause of mortality in women. About 500,000 people die of breast cancer every year worldwide [1,2]. Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer [3,4], with 15–20% of breast cancer cases diagnosed as TNBC [5,6]. TNBC is characterized by the lack of expression of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2; thus, cytotoxic chemotherapy is still the major treatment for this type of breast cancer [7]. In addition, its rate of metastasis, particularly to the lung, is very high, which is closely related to the prognosis and short survival [8]. Thus, there is an urgent need to develop new therapeutic interventions for preventing and treating metastases of patients with TNBC. Recently, drug repurposing has been shown as a cost-effective and time-saving method for identifying novel drug therapies to manage tumors [9].
Thioridazine hydrochloride (Thi-hyd), which contains a thioridazine, is a common phenothiazine antipsychotic drug used for schizophrenia in the clinical setting [10]. In advanced tumors, it is applied for the treatment of tumor-linked sweating and depression [11] and was also reported to be effective for cancer therapy. In glioblastoma, thioridazine was identified among 79 drugs according to 356 glioblastoma gene signatures from public databases and shown to significantly inhibit glioma stem cells and induce autophagy in glioblastoma cell lines [12]. Another study revealed that thioridazine induces autophagy in glioma cells, and Wnt/β-catenin signaling was shown to be involved in this process through whole gene expression screening [13]. In melanoma, thioridazine remarkably retarded tumor growth and reduced tumor vasculature [14]. Similar effects were also observed in gastric cancer, prostate cancer, and lymphoma [15–17]. In breast cancer, thioridazine was reported to enhance dexamethasone responses [18]. Moreover, the self-renewal of basal-like morphology TNBC cells was verified to be targeted by thioridazine [7].
In this study, we explored the molecular targets of Thi-hyd and its role on the subcutaneous 4T1 breast tumor mouse model showing spontaneous lung metastasis.
Methods
Reagents and antibodies
Thi-hyd was obtained from AstaTech Biochem Corp. (Cat#AT230916, Chengdu, China). For in vitro experiments, Thi-hyd was initially prepared as a 40-mM stock solution in dimethyl sulfoxide, while for in vivo experiments, it was dissolved in ethanol/Cremophor EL/0.9% saline at 12.5:12.5:75 v/v. Cell Counting Kit-8 (CCK8) was obtained from MedChem Express (Cat#HY-K0301). Annexin V-PE/7-AAD Apoptosis Detection Kit (Cat#556,547) and Matrigel (Cat#354,234) were obtained from BD Biosciences. Propidium iodide (PI; Cat#P4170), rhodamine-123 (Rh123; Cat#R8004), and the reactive oxygen species (ROS) inhibitor N-acetyl-L-cysteine (NAC; Cat#A7250) were obtained from Sigma. The caspase inhibitor Z-VAD-FMK (Cat#KGA8254) was obtained from KeyGen BioTECH. H2DCFDA (Cat#D399) was obtained from Thermo Fisher Scientific. Cell Signaling Technology provided the primary antibodies against phosphorylated-AKT (p-AKT; Cat#4060s), AKT (Cat#4658s), p-extracellular signal-regulated kinase (p-ERK1/2; Cat#4370), ERK1/2 (Cat#4695s), cyclin-dependent kinase 2 (CDK2; Cat#2546p), cyclin D1 (Cat#2978), p27 (Cat#3698s), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cat#97,166). Abcam provided the primary antibodies against CDK4 (Cat#ab108357), cyclin E (Cat#ab211342), p21 (Cat#ab109199), Ki67 (Cat#ab16667), and cleaved caspase-3 (Cat#ab2302).
Cell lines and cell culture
All TNBC cell lines were obtained from American Type Culture Collection. MDA-MB-231 and 4T1 cells lines were maintained in DMEM (Gibco) and RPMI-1640 (Gibco), respectively, containing 10% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin at 37 °C in a humidified atmosphere with 5% CO2. MDA-MB-231 cells were authenticated by short tandem repeat analysis in Shanghai Biowing Applied Biotechnology Co. Ltd.
Cell viability assay
The viability of breast cells was determined by CCK8 assay. Briefly, 2000–5000 cells were seeded into 96-well plates. Medium containing the indicated dose of Thi-hyd (0, 1.25, 5, 10, 20, and 40 μM) was added to each well 12 hours later. After 48 or 72 hours of treatment, the cells were stained with 10% CCK8 solution. The absorbance was read at 450 nm, and the viability index was normalized to that of untreated control cells.
Clonogenic assay
4T1 and MDA-MB-231 cells (600–1000 cells/plate) were seeded into 6-well plates and incubated with various concentrations of Thi-hyd (0, 5, 10, 20, and 40 μM) for one week. Then, the cells were fixed with 4% paraformaldehyde and stained with 0.5% crystal violet. Colonies that contained more than 50 cells were counted.
Cell migration assay
4T1 cells (4 × 104/plate) were suspended in 200 μL serum-free medium with indicated doses of Thi-hyd on the upper chamber, while the lower chamber was filled with 600 μL of culture medium containing 20% fetal bovine serum. Then, the cells were incubated at 37 °C with 5% CO2 for 24 hours. Cells remaining in the upper chamber were removed using cotton swabs, and those that had migrated to the surface of the lower chamber were fixed with 4% paraformaldehyde for 10 minutes, stained with 0.5% crystal violet, and finally captured by a microscope.
Cell cycle analysis
4T1 and MDA-MB-231 cells were seeded into six-well plates and incubated with the indicated dose of Thi-hyd for 24 hours. After that, the cells were collected and fixed with 70% ice-cold ethanol overnight at 4 °C. Then, cells were stained with 50 μg/mL PI dye-containing 20 μg/mL RNAse for 30 minutes at room temperature, and processed by NovoCyte Flow Cytometer (ACEA Biosciences, Inc.).
Morphological analysis of cells and nuclei
Morphological changes in cells were captured by light microscope after 48 hours of treatment with 5 µM Thi-hyd. After that, nuclei were stained with Hoechst33258 (10 μg/mL) and imaged by a fluorescence microscope.
Apoptosis assay
The cells were treated with 0, 10, and 15 µM Thi-hyd for 48 hours. After that, they were collected and stained with Annexin V/PE and 7-AAD (BD Pharmingen™) according to the manufacturer’s protocol. Then, the samples were processed by NovoCyte Flow Cytometer (ACEA Biosciences, Inc.). Annexin V positive cells were considered as apoptotic.
Measurement of ROS content and changes in mitochondrial membrane potential (MMP)
Cells were incubated with Thi-hyd for 24 hours and subsequently analyzed by flow cytometry. Before analyses, cells were incubated in the dark at room temperature for 30 minutes with 10 μM DCFH-DA for measuring ROS content or 5 μg/mL Rh123 for measuring MMP changes. Then, cells were digested into a single-cell suspension. After washing with PBS three times, the fluorescence intensity was monitored by NovoCyte Flow Cytometer (ACEA Biosciences, Inc.).
Western blotting
Subsequent to the 24 hours of treatment with Thi-hyd, cells were lysed in RIPA lysis buffer containing protease and phosphatase inhibitors on ice. Then, samples were denatured in the buffer and boiled at 100°C for 10 minutes. Proteins were loaded and resolved using SDS-PAGE, and transferred onto PVDF membranes (Millipore, Billerica, MA). After blocking with 5% nonfat milk in tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h at room temperature, the blots were probed overnight at 4°C with primary antibodies. After washing with TBST three times, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature at 1:10,000 dilution. Blots were developed using an enhanced chemiluminescence detection kit (Millipore, Billerica, MA). GAPDH was used as an internal control.
Xenograft tumor formation
Six-week-old female Balb/c mice were housed in standard housing conditions. After one week of adaption, 2 × 105 4T1 cells in serum-free culture medium were injected to the right-back flank region of mice. Six days later, the mice were randomly divided into two groups: those injected with vehicle (control) and those injected with 10 mg/kg Thi-hyd . Thi-hyd and vehicle were intraperitoneally injected into mice every 3 days. Bodyweight was also recorded during the process. After 18 days of treatment, blood was collected from the eye before the sacrifice. Then, the mice were sacrificed, and the tumor tissue and major organs (heart, spleen, and kidney) were isolated and immediately fixed with 4% polyoxymethylene. All animal-related procedures were approved by the institutional animal care and treatment committee of Sichuan University.
Immunohistological analysis of tumor sections
The harvested tumor tissues that were embedded in paraffin were sectioned into 5-μm sections and rehydrated. To reduce nonspecific background, the sections were incubated with 3% hydrogen peroxide for 10 minutes and 5% goat serum for 5 minutes. Then, the sections were incubated with cleaved caspase-3 or Ki67 antibodies at 4°C overnight, followed by incubation with biotinylated goat anti-rabbit secondary antibody and streptavidin-biotin complex at 37°C. Diaminobenzidine peroxide solution was then used to reveal the staining.
Toxicity evaluation
The behavior of mice was monitored every day to assess any side effects of Thi-hyd. After 18 days of treatment, blood was collected from the eye to detect changes in blood cells and biochemistry. Harvested tissues (heart, spleen, and kidney) from 4T1-xenografted mice were stained with hematoxylin and eosin (H&E) to evaluate morphological changes.
Statistical analysis
All data in figures are represented as average ± standard deviation or average ± standard error of the mean. Student’s unpaired t-test and one-way analysis of variance were used to determine significant differences among groups. GraphPad Prism 8 was used for statistical analysis. Significant p values are shown as * p < 0.05, ** p < 0.01, *** p < 0.001.
Results
Thi-hyd inhibits the viability and migration of TNBC cells
As shown in Figure 1(b), Thi-hyd attenuated the viability of 4T1 and MDA-MB-231 cells in a concentration and time-dependent manner. The IC50 values in the two cell lines were 9.87 μM and 18.70 μM, respectively, after 72 hours of treatment with Thi-hyd. The inhibition of viability was visually assessed by colony formation assay. As shown in Figure 1(c), Thi-hyd not only reduced the number but also the size of colonies. ERK1/2 and AKT, the key molecular players of two different signaling pathways involved in TNBC cell growth were detected by Western Blot [19,20]. The levels of p-AKT significantly decreased after treatment with Thi-hyd, while those of p-ERK1/2 relatively increased compared with the levels of total ERK1/2 (Figure 1(d)). Next, we determined the effect of Thi-hyd on TNBC cell migration by conducting transwell migration assays. As expected, Thi-hyd obviously inhibited the migration of 4T1 cells (Figure 1(e)).
Figure 1.
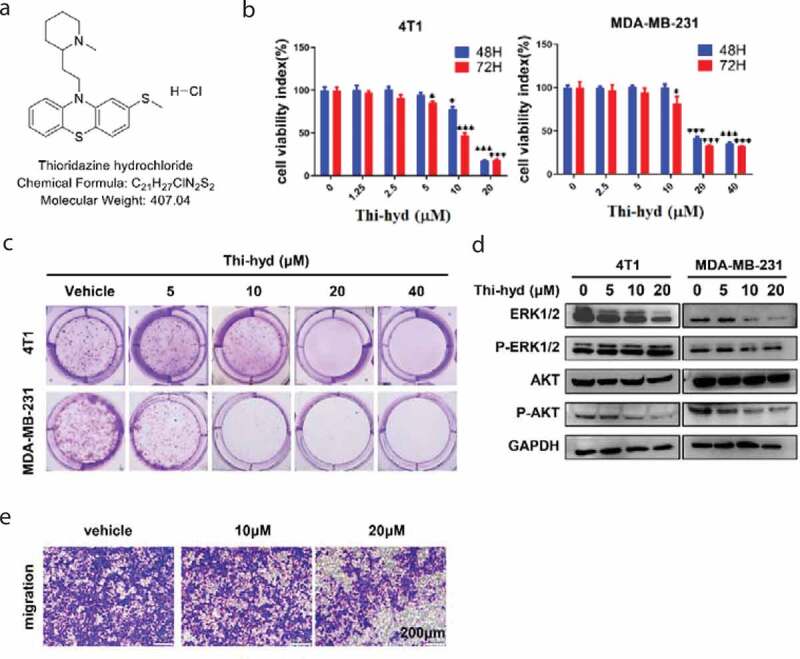
Thi-hyd reduced the viability and migration of TNBC cells. (a) The structure of Thi-hyd. (b) Cell viability was reduced by Thi-hyd in a dose-dependent manner. The cell viability was detected by CCK-8 assay after 48 hours or 72 hours of treatment with indicated dose of Thi-hyd. Results were expressed as average ± S.D. (n = 6, one way ANOVA, *p < 0.05, ***p < 0.001) (c) Cell colony formation was inhibited by Thi-hyd in a dose-dependent manner. The colonies of 4T1 and MAD-MB-231 cells were stained with crystal violet following 6–8 days treatment of different dose of Thi-hyd (0μM, 5 μM, 10 μM, 20 μM, 40 μM). (d) Thi-hyd inhibited the phosphorylation of AKT instead of ERK1/2 in TNBC cells. Cells were treated with various concentrations of Thi-hyd for 24 hours. The phosphorylation of the AKT and ERK1/2 was determined by western blotting. GAPDH was used as internal control. (e) Thi-hyd inhibited the migration of 4T1 cells. 4T1 cells were seeded on the up chamber of transwells and treated with various concentrations of Thi-hyd for 24 hours. Then the cells were dried and stained with crystal violet. The representative picture from 3 replicates in each group was shown in this figure. Scale bar, 200 μm
Thi-hyd arrests TNBC cells on the G0/G1 phase
Previous studies have shown that cell viability inhibition is always accompanied by cell cycle arrest [21,22]. An obvious G0/G1 phase arrest of TNBC cells was observed in our study (Figure 2(a,b)). The proportion of 4T1 cells increased from 38.72 ± 1.36% in vehicle-treated cells to 46.77 ± 1.67% in cells treated with 15 μM Thi-hyd, while that of MDA-MB-231 cells increased from 52.95 ± 0.15% to 64.98 ± 0.51%, respectively (Figure 2(c,d)). The levels of the CDK2/cyclin E and CDK4/cyclin D1 complexes are correspondingly reduced upon G0/G1 phase arrest, as indicated by previous studies [23,24]. We found that CDK4 and cyclin D1 levels were obviously decreased, while those of CDK2 and cyclin E were not changed (Figure 2(e,f)). These results indicated that Thi-hyd could effectively induce G0/G1 arrest by inhibiting CDK4/cyclin D1 but not CDK2/cyclin E expression levels. Moreover, p21 and p27, which conversely mediate the assembly and nuclear import of CDK-cyclin complexes [25,26], were up-regulated in MDA-MB-231 but not in 4T1 cells (Figure 2(e,f)).
Figure 2.
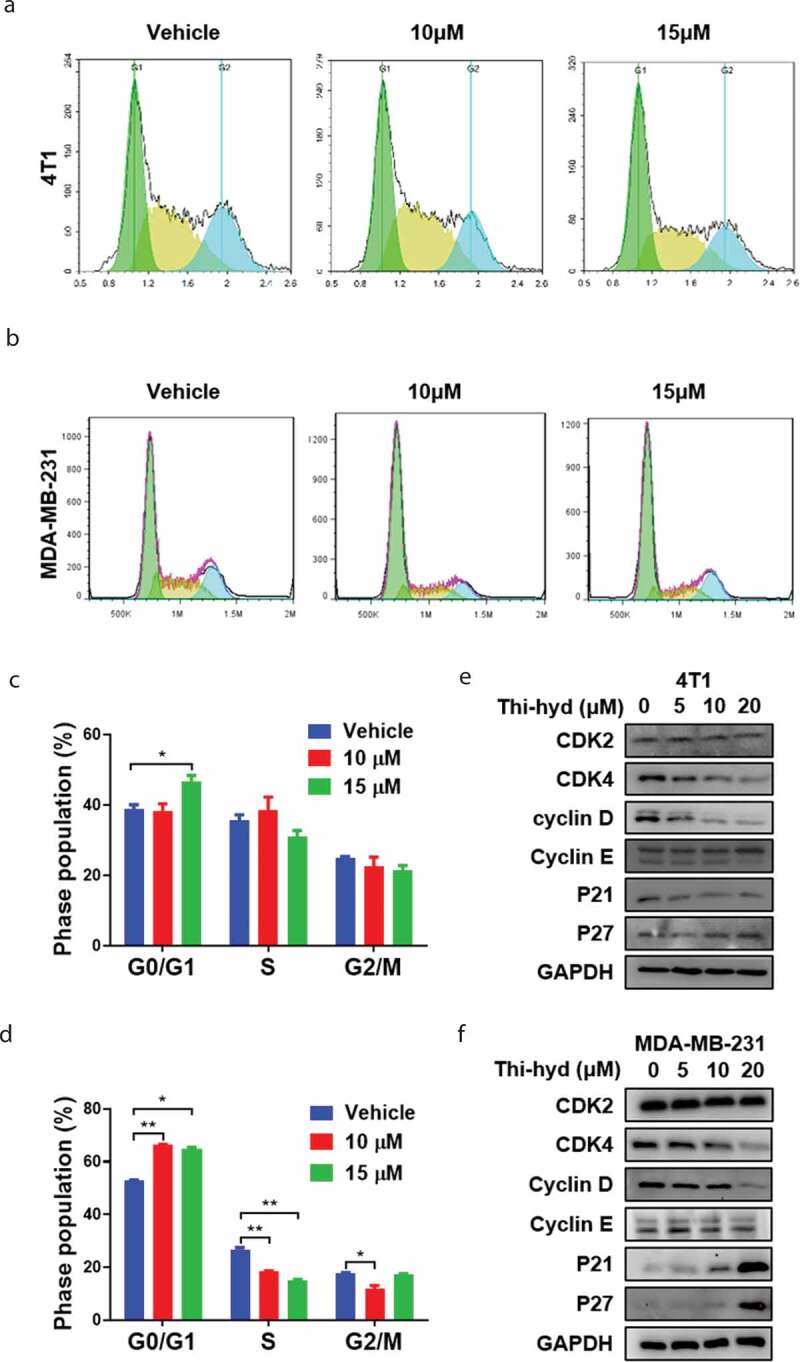
Thi-hyd induced G0/G1 arrest in TNBC cells. (a) and (b) 4T1 and MDA-MB-231 cells were cultured with indicated dose of Thi-hyd for 24 hours. Then the cells were fixed with cold ethanol and stained with PI solution, followed by flow cytometer analysis. The representative picture from 3 replicates in each group was shown in the figure. (c)and (d) Cell cycle distribution was quantified as a histogram. Results were presented as average ± S.D. (n = 3, student’s t test, *p < 0.05, **p < 0.01) (e) and (f) Thi-hyd’s effect on the G0/G1 cell cycle-related proteins. 4T1 (e) and MDA-MB-231 (f) cells were treated with various concentrations of Thi-hyd for 24 hours. The protein levels were determined by western blotting. GAPDH was used as internal control
Thi-hyd induces TNBC cell apoptosis and mitochondrial dysfunction
To explore whether the observed inhibition of viability was associated with cell apoptosis, cell morphology and nuclei changes were observed by microscopy. We observed more abnormal morphologies and nuclei contractions in Thi-hyd-treated cells than in untreated cells (Figure 3(a,b)). Notably, the proportion of apoptotic cells increased in a concentration-dependent manner (Figure 3(c)). These results indicated that Thi-hyd effectively induced apoptosis in TNBC cells.
Figure 3.
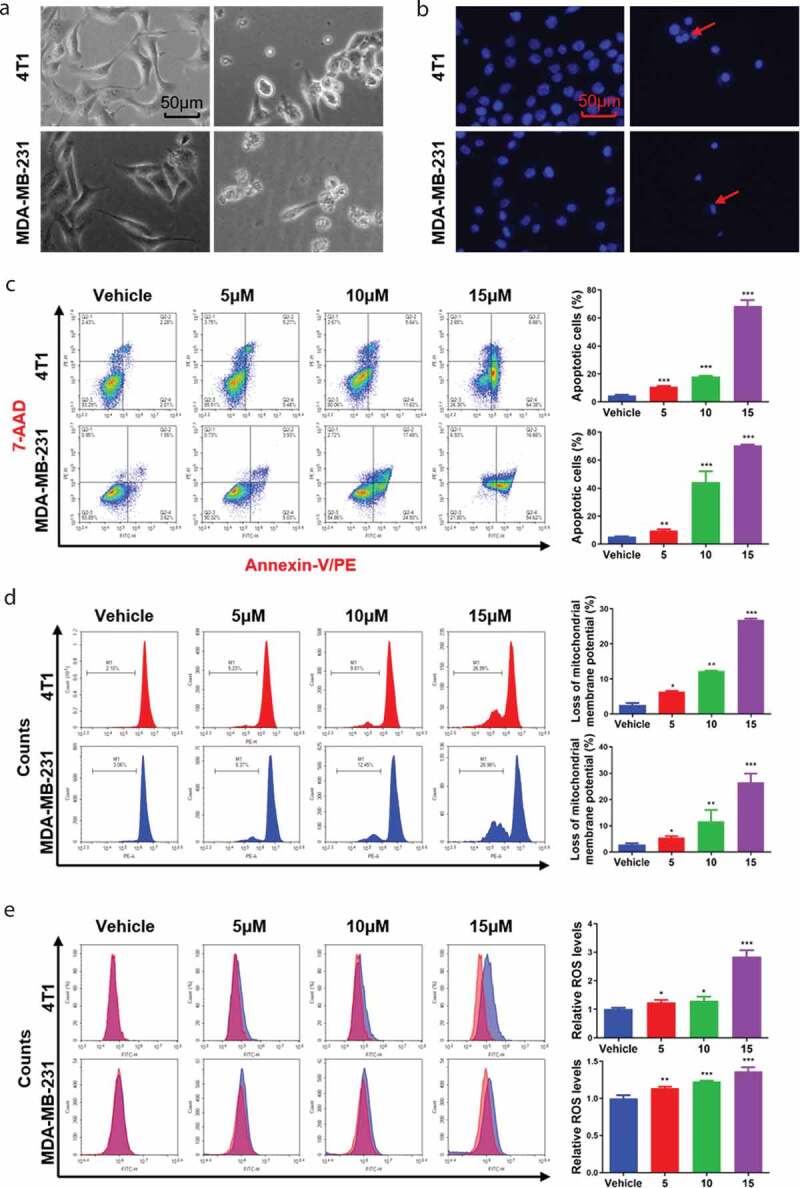
Thi-hyd induced the apoptosis of TNBC cells and accompanied with dysfunction of mitochondria. (a) Cell morphologies of TNBC cells were changed after 48 hours of treatment with 5 μM Thi-hyd. Scale bar, 50 μm. (b) Thi-hyd induced condensation of nuclei in TNBC cells. TNBC cells were treated with 5 μM Thi-hyd for 48 hours. Then, the nuclei were stained with Hoechst 33258 and captured by a fluorescence microscope. The red arrows indicated condensed nuclei. Scale bar, 50 μm. (c) Thi-hyd induced apoptosis of TNBC cells. TNBC cells were cultured with the indicated dose of Thi-hyd for 48 hours. Then the cells were stained with PE-Annexin V and 7-AAD, followed by flow cytometer analysis. The ratio of apoptosis cells was quantified as histogram on the right of each cell line. (d) Thi-hyd decreased the ΔΨm in TNBC cells. Cells were cultured with the indicated dose of Thi-hyd for 48 hours. Then the cells were stained with RH123, followed by flow cytometer analysis. The loss of ΔΨm was quantified as histogram on the right of each cell line. (e) Thi-hyd elevated the ROS level in TNBC cells. Cells were cultured with the indicated dose of Thi-hyd for 24 hours. Then the cells were stained with 10 μM DCFH-DA, followed by flow cytometer analysis. The relative ROS levels were quantified as histogram on the right of each cell line. All quantitative data shown above were presented as average ± S.D. (n= 3, one way ANOVA, *p< 0.05, **p< 0.01, ***p< 0.001)
Apoptosis is always accompanied with dysfunction in mitochondria, known as mitochondrial-mediated apoptosis [27,28], and is characterized by the generation of ROS and loss of MMP (ΔΨm). In our study, treatment of TNBC cells with Thi-hyd induced MMP dysfunction, represented as a reduction in the average intensity of Rh123 fluorescence (Figure 3(d)). Relative to the fluorescence in control 4T1 cells (2.75%), that of TNBC cells treated with 5, 10, and 15 μM Thi-hyd was reduced by 5.49%, 11.69%, 26.57%, respectively (6.34%, 12.27%, and 26.89% reduction, respectively, in MDA-MB-231 cells). In addition, we found a significant increase in ROS levels in cells treated with Thi-hyd (Figure 3(e)). Representative curves obtained from cells treated with Thi-hyd showed a significant increase in the average intensity of DCFH-DA fluorescence, indicating excessive ROS generation. Compared with the DCFH-DA fluorescence in control 4T1 cells, that in cells treated with 5, 10, and 15 μM of Thi-hyd was higher by 1.23, 1.30, and 2.84 fold, respectively (increase of 1.14, 1.23, and 1.37 fold, respectively, in MDA-MB-231 cells). To further confirm the role of ROS and caspases in the Thi-hyd-induced cell apoptosis, we pretreated cells with the respective inhibitors, and then incubated them with Thi-hyd. As anticipated, treatment with either a ROS inhibitor (NAC) or a caspase inhibitor (Z-VAD-FMK) largely blocked cell apoptosis caused by Thi-hyd (Figure S1).
Thi-hyd inhibits tumor growth and lung metastasis in a subcutaneous 4T1-xenograft tumor model in mice
The in vivo anti-tumor efficacy of Thi-hyd was evaluated in a mouse breast-tumor model with spontaneous lung metastasis induced by subcutaneous 4T1 xenograft. At the endpoint of the experiment, tumors were isolated and weighed (Figure 4(a)). Thi-hyd displayed a good anti-tumor potential with a statistical significance. Treatment with Thi-hyd resulted in an apparent shrinking of the tumors, with a 63.73% inhibition rate in tumor weight (Figure 4(b)). Moreover, there was no obvious body weight loss (Figure 4(c)) during the treatment. To investigate the mechanisms of tumor inhibition in vivo, the tumors were sectioned and stained with Ki67 and cleaved caspase-3. The expression of Ki67 obviously decreased, while that of cleaved caspase-3 increased after Thi-hyd treatment, indicating that Thi-hyd inhibits tumor growth by inhibiting proliferation and inducing apoptosis (Figure 4(d,e)). Spontaneous lung metastasis readily developed in the subcutaneous 4T1 breast tumor model, as shown in the control group (Figure 4(f)). Treatment with Thi-hyd efficiently prevented this metastasis by 72.58% (Figure 4(g)).
Figure 4.
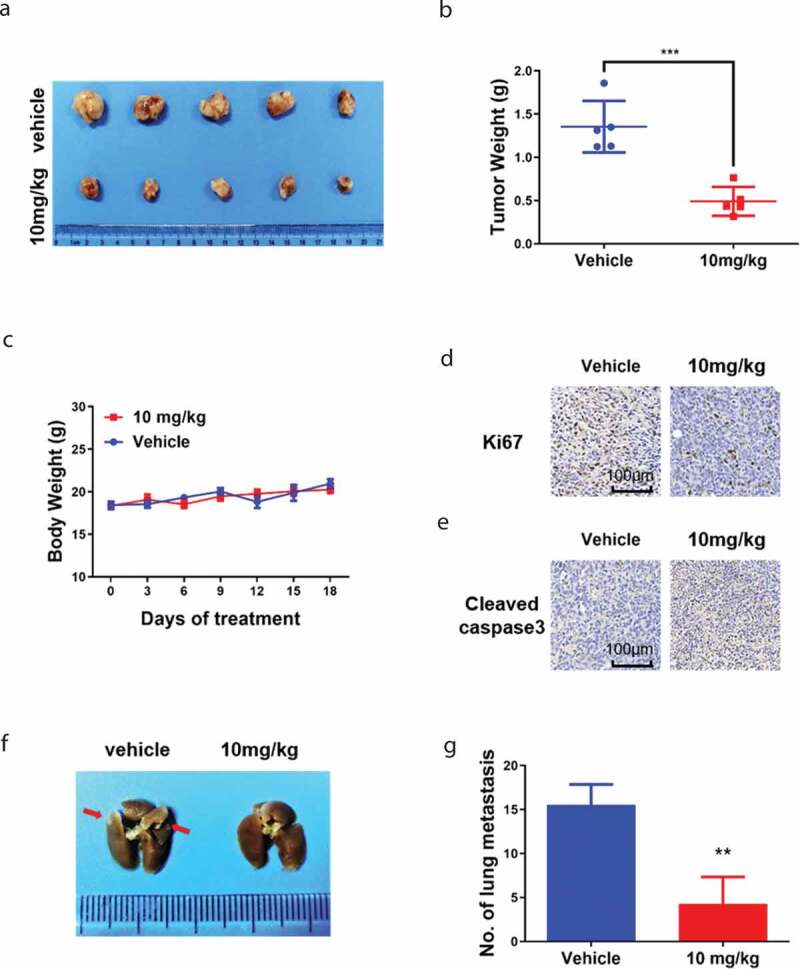
Thi-hyd inhibited tumor growth and lung metastasis in the 4T1 subcutaneous tumor model. 4T1 cells (2 × 105 cells/mice) were injected subcutaneously in the right dorsal flank of BALB/c mice. 10 mg/kg Thi-hyd was given to the mice by i.p. every three days when the tumors reached the average volume of 30–40 mm3. (a) Isolated tumors from each group after 18 days treatment of Thi-hyd. (b) The tumor weights were plotted as average ± S.D. (n = 5, Student’s unpaired t-test, ***p < 0.001) (c) The changes of body weights were monitored during the progress of treatment. (d) Immunohistochemical staining of Ki67 in 4T1 tumor. Scale bar, 100 μm. (e) Immunohistochemical staining of cleaved-caspase 3 in 4T1 tumor. Scale bar, 100 μm. (F) Thi-hyd inhibited lung metastasis of 4T1 tumors. Lungs isolated from sacrificed mice were fixed in 4% paraformaldehyde for at least 24 hours and the metastasis nodes were counted under a stereoscope. The representative picture of the lung was shown from five mice of each group. The red arrows indicated the metastasis nodes. (g) The metastasis tumor nodes in the lung were quantified as a histogram. Quantitative data were expressed as average ± S.D. (n = 5, Student’s unpaired t-test, **p < 0.01)
Thi-hyd is well-tolerated in mice
To assess the safety of Thi-hyd, we analyzed blood collected from the eye; no abnormal values were observed in Thi-hyd-treated group (Figure 5(a,b)). In addition, no visible lesions were observed by H&E staining in major organs, further indicating the safety of Thi-hyd in mice (Figure 5(c)).
Figure 5.
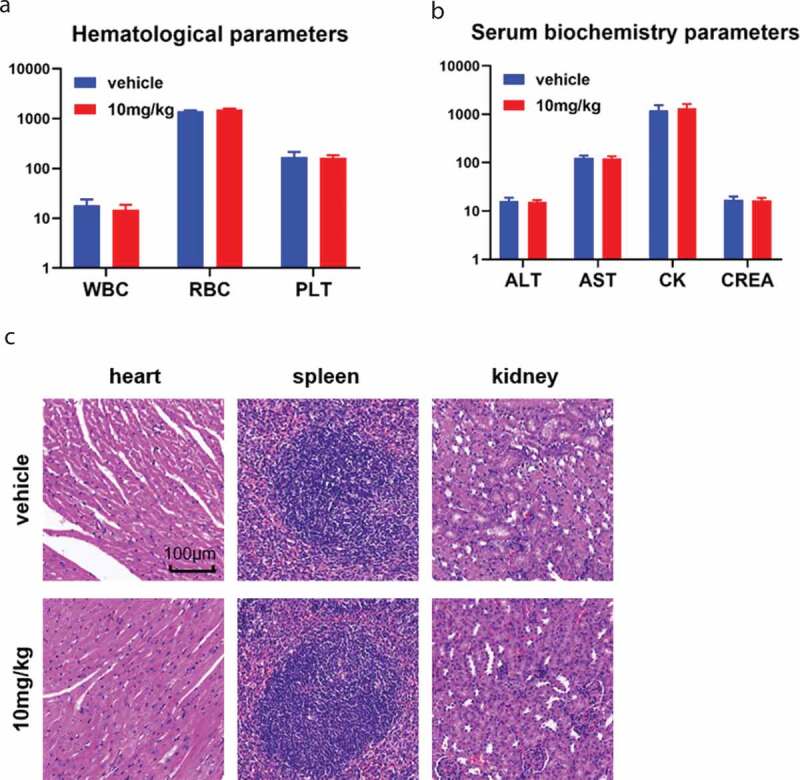
Thi-hyd showed good tolerability in mice. (a) and (b) Eye-ball blood was collected before the sacrifice of mice. No apparent difference was observed in hematological parameters (WBC, white blood cell, 109/L; RBC, red blood cell, 109/L; PLT, platelet, 109/L) and serum biochemistry parameters (ALT, alanine aminotransferase, U/L; AST, aspartate aminotransferase, U/L; CK, creatinine kinase, U/L; CREA, creatinine, μM/L) (c) No visible pathological changes were observed in the heart, spleen, and kidney of mice by H&E staining. Scale bar, 100 μm
Discussion
In this study, we investigated the anti‑tumor efficacy of Thi-hyd on TNBC both in vivo and in vitro. In vitro results indicated that Thi-hyd significantly inhibits TNBC cells. These results were confirmed using two breast tumor cell lines, 4T1 and MDA-MB-231, isolated from mice and humans, respectively. Thi-hyd not only inhibited the proliferation but also the migration of tumor cells. PI3K/AKT signaling was identified as the target of Thi-hyd in both MDA-MB-231 and 4T1 cells. Further analysis elucidated that the inhibition of TNBC cell proliferation was associated with mitochondria-related apoptosis and G0/G1 arrest, which was accompanied with the down-regulation of CDK4/cyclin D1. In vivo, Thi-hyd significantly reduced tumor growth by inducing apoptosis and inhibiting proliferation. Thi-hyd not only inhibited the growth of subcutaneous tumors but also lung metastasis. Good tolerability of the drug was indicated by H&E staining and blood sample analysis.
Thi-hyd is the hydrochloride salt form of thioridazine. Similar effects of Thi-hyd and thioridazine have been observed in patients with schizophrenia for the same target in dopamine receptor D2. Previous studies have shown that thioridazine targets cancer stem cells in patients with glioma [12]. Thioridazine was also shown to inhibit proliferation and induce apoptosis in leukemia, lung, ovarian, and breast cancers [17,29–31]. Consistently, our results showed that Thi-hyd inhibits TNBC cell proliferation and induces apoptosis in a concentration-dependent manner. Tegowski et al showed that 5–10 µM thioridazine causes cell cycle arrest in TNBC cell lines [32]. In agreement, our results indicated that Thi-hyd induces cell cycle arrest at 10 µM.
MAPK/ERK1/2 and PI3K/AKT pathways are both important intracellular pathways, demonstrated to regulate growth, motility, and survival of tumor cells [33,34]. Aberrant activation of MAPK/ERK1/2 and PI3K/AKT pathways contributes to tumor development. In this study, we found significant suppression of p-AKT in Thi-hyd-treated cells, while p-ERK1/2 was overexpressed relative to total ERK1/2 upon Thi-hyd treatment. Activated ERK1/2 was reported to induce apoptosis of cells [35]. In previous studies, ERK1/2 was observed to be associated with the intrinsic or extrinsic apoptotic pathway depending on different types and injuries of cells. In this study, we also observed an increased ratio of apoptosis which might be related to the activated ERK1/2.
PI3K/AKT pathway is important for cell cycle control [35]. Cyclin D levels were reported to be regulated by upstream signals such as PI3K/AKT and Wnt/β‐catenin [36]. CDK4/cyclin D complex is required for the progression from G1 to S phase, while CDK2/cyclin E complex drives cancer cells through the G1/S checkpoint [23]. Consistently with previous studies, CDK4/cyclin D leves significantly decreased after treatment with Thi-hyd; however, no apparent changes were observed in CDK2/cyclin E levels.
Prior to this study, the anti-tumor effects of thioridazine on breast cancer had been explored by Yin and colleagues [31]. In that study, a tumor inhibition rate of 55% was achieved after 25 days of treatment with 32 mg/kg thioridazine in a 4T1 xenograft tumor model. Similar anti-tumor effects of thioridazine were also verified in an MDA-MB-231 xenograft tumor model by Goyette et al [9]. In this study, treatment with 10 mg/kg Thi-hyd resulted in significant shrinking of the tumors, achieving a 63.73% inhibition rate in tumor weight. Moreover, Thi-hyd treatment efficiently prevented lung metastasis by 72.58%.
The main limitation of our study is that the precise targets and anti-tumor mechanism of Thi-hyd were not clearly elucidated. In a previous study, thioridazine was reported to act as a dopamine D2 receptor (DRD2) antagonist [32]. Another study showed that thioridazine induces SiHa cell apoptosis and significantly downregulates DRD2 expression [37]. Thioridazine was also shown to have a high affinity to muscarinic receptors [38], which could be considered as a candidate target for this drug. Thus, further studies are warranted to explore the mechanism underlying thioridazine’s anti-tumor effects.
In conclusion, our study provided convincing results for the anti-tumor effects of Thi-hyd on TNBC. Moreover, our results demonstrated that Thi-hyd inhibits spontaneous lung metastasis in the subcutaneous 4T1 breast tumor model. Our study suggests that Thi-hyd can be used as a drug for the treatment of TNBC and sets the basis for further evaluation and investigation of Thi-hyd effects in clinical settings.
Supplementary Material
Funding Statement
This study was funded by grants from the National Natural Science Foundation of China [31800773], China Postdoctoral Science Foundation [2018M633369], Sichuan Science and Technology Program [2019YJ0063], and The Youth Science Foundation of West China Hospital of Stomatology [WCHS-201703] to M.L.
Supplementary material
Supplemental data for this article can be accessed here.
Author contributions
Yanlin Song and Lu Li designed and performed experiments, analyzed data, and prepared manuscript. Jiao Chen, Hongli Chen, Bomiao Cui, Yun Feng, Ping Zhang, and Qiangsheng Zhang assisted with experiments’ execution, data collection, and analysis. Min Luo designed experiments, interpreted data, and prepared the manuscript.
Data availability statement
All data that support the findings of this study are available on request from the corresponding author.
Disclosure statement
The authors report no conflict of interest.
References
- [1].Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. [DOI] [PubMed] [Google Scholar]
- [2].Gallo S, Sangiolo D, Carnevale Schianca F, et al. Treating breast cancer with cell-based approaches: an overview. Expert Opin Biol Ther. 2017;17(10):1255–1264. [DOI] [PubMed] [Google Scholar]
- [3].Huang R, Li J, Pan F, et al. The activation of GPER inhibits cells proliferation, invasion and EMT of triple-negative breast cancer via CD151/miR-199a-3p bio-axis. Am J Transl Res. 2020;12(1):32–44. [PMC free article] [PubMed] [Google Scholar]
- [4].Deepak KGK, Vempati R, Nagaraju GP, et al. Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153:104683. [DOI] [PubMed] [Google Scholar]
- [5].Giuli MV, Giuliani E, Screpanti I, et al. Notch signaling activation as a hallmark for triple-negative breast cancer subtype. J Oncol. 2019;2019:8707053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hunakova L, Horvathova E, Gronesova P, et al. Triorganotin isothiocyanates affect migration and immune check-point receptors in human triple-negative breast carcinoma MDA-MB-231 cells. Anticancer Res. 2019;39(9):4845–4851. [DOI] [PubMed] [Google Scholar]
- [7].Tegowski M, Fan C, Baldwin AS.. Selective effects of thioridazine on self-renewal of basal-like breast cancer cells. Sci Rep. 2019;9(1):18695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Iriondo O, Liu Y, Lee G, et al. TAK1 mediates microenvironment-triggered autocrine signals and promotes triple-negative breast cancer lung metastasis. Nat Commun. 2018;9(1):1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Goyette MA, Cusseddu R, Elkholi I, et al. AXL knockdown gene signature reveals a drug repurposing opportunity for a class of antipsychotics to reduce growth and metastasis of triple-negative breast cancer. Oncotarget. 2019;10(21):2055–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fenton M, Rathbone J, Reilly J, et al. Thioridazine for schizophrenia. Cochrane Database Syst Rev. 2007;2007(3):CD001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhukovsky DS. Fever and sweats in the patient with advanced cancer. Hematol Oncol Clin North Am. 2002;16(3):579–88, viii. [DOI] [PubMed] [Google Scholar]
- [12].Cheng HW, Liang YH, Kuo YL, et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015;6(5):e1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chu CW, Ko HJ, Chou CH, et al. Thioridazine enhances P62-mediated autophagy and apoptosis through Wnt/β-catenin signaling pathway in glioma cells. Int J Mol Sci. 2019;20(3):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jiang X, Chen Z, Shen G, et al. Psychotropic agent thioridazine elicits potent in vitro and in vivo anti-melanoma effects. Biomed Pharmacother. 2018;97:833–837. [DOI] [PubMed] [Google Scholar]
- [15].Mu J, Xu H, Yang Y, et al. Thioridazine, an antipsychotic drug, elicits potent antitumor effects in gastric cancer. Oncol Rep. 2014;31(5):2107–2114. [DOI] [PubMed] [Google Scholar]
- [16].Lee SI, Roney MSI, Park JH, et al. Dopamine receptor antagonists induce differentiation of PC-3 human prostate cancer cell-derived cancer stem cell-like cells. Prostate. 2019;79(7):720–731. [DOI] [PubMed] [Google Scholar]
- [17].Spengler G, Molnar J, Viveiros M, et al. Thioridazine induces apoptosis of multidrug-resistant mouse lymphoma cells transfected with the human ABCB1 and inhibits the expression of P-glycoprotein. Anticancer Res. 2011;31(12):4201–4205. [PubMed] [Google Scholar]
- [18].Li J, Yao QY, Xue JS, et al. Dopamine D2 receptor antagonist sulpiride enhances dexamethasone responses in the treatment of drug-resistant and metastatic breast cancer. Acta Pharmacol Sin. 2017;38(9):1282–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He J, Wink S, de Bont H, et al. FRET biosensor-based kinase inhibitor screen for ERK and AKT activity reveals differential kinase dependencies for proliferation in TNBC cells. Biochem Pharmacol. 2019;169:113640. [DOI] [PubMed] [Google Scholar]
- [20].He Q, Xue S, Tan Y, et al. Dual inhibition of Akt and ERK signaling induces cell senescence in triple-negative breast cancer. Cancer Lett. 2019;448:94–104. [DOI] [PubMed] [Google Scholar]
- [21].Wang L, Liu D, Wu X, et al. Long non-coding RNA (LncRNA) RMST in triple-negative breast cancer (TNBC): expression analysis and biological roles research. J Cell Physiol. 2018;233(10):6603–6612. [DOI] [PubMed] [Google Scholar]
- [22].Yang K, Yao Y. Mechanism of GPER promoting proliferation, migration and invasion of triple-negative breast cancer cells through CAF. Am J Transl Res. 2019;11(9):5858–5868. [PMC free article] [PubMed] [Google Scholar]
- [23].Chang H, Li J, Cao Y, et al. Bufadienolides from Venenum Bufonis inhibit mTOR-mediated cyclin D1 and retinoblastoma protein leading to arrest of cell cycle in cancer cells. Evid Based Complement Alternat Med. 2018;2018:3247402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee HL, Lin CS, Kao SH, et al. Gallic acid induces G1 phase arrest and apoptosis of triple-negative breast cancer cell MDA-MB-231 via p38 mitogen-activated protein kinase/p21/p27 axis. Anticancer Drugs. 2017;28(10):1150–1156. [DOI] [PubMed] [Google Scholar]
- [25].Li Y, Huang J, Zeng B, et al. PSMD2 regulates breast cancer cell proliferation and cell cycle progression by modulating p21 and p27 proteasomal degradation. Cancer Lett. 2018;430:109–122. [DOI] [PubMed] [Google Scholar]
- [26].Liu Q, Cao Y, Zhou P, et al. A inhibits cell proliferation by inducing G0/G1 phase cell cycle arrest and induces apoptosis in breast cancer cells. Biomol Ther. 2018;26(3):328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41(1):11–22. [DOI] [PubMed] [Google Scholar]
- [28].Tan PP, Zhou BH, Zhao WP, et al. Mitochondria-mediated pathway regulates C2C12 cell apoptosis induced by fluoride. Biol Trace Elem Res. 2018;185(2):440–447. [DOI] [PubMed] [Google Scholar]
- [29].Qian G, Dai L, Yu T. Thioridazine sensitizes cisplatin against chemoresistant human lung and ovary cancer cells. DNA Cell Biol. 2019;38(7):718–724. [DOI] [PubMed] [Google Scholar]
- [30].Park MS, Dong SM, Kim BR, et al. Thioridazine inhibits angiogenesis and tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian cancer xenografts. Oncotarget. 2014;5(13):4929–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yin T, He S, Shen G, et al. Dopamine receptor antagonist thioridazine inhibits tumor growth in a murine breast cancer model. Mol Med Rep. 2015;12(3):4103–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tegowski M, Fan C, Baldwin AS. Thioridazine inhibits self-renewal in breast cancer cells via DRD2-dependent STAT3 inhibition, but induces a G1 arrest independent of DRD2. J Biol Chem. 2018;293(41):15977–15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen S, Wang Y, Zhang J-H, et al. Long non-coding RNA PTENP1 inhibits proliferation and migration of breast cancer cells via AKT and MAPK signaling pathways. Oncol Lett. 2017;14(4):4659–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhu X, Qiu J, Zhang T, et al. MicroRNA-188-5p promotes apoptosis and inhibits cell proliferation of breast cancer cells via the MAPK signaling pathway by targeting Rap2c. J Cell Physiol. 2020;235(3):2389–2402. [DOI] [PubMed] [Google Scholar]
- [35].Cagnol S, Chambard J-C. ERK and cell death: mechanisms of ERK-induced cell death - apoptosis, autophagy and senescence. Febs J. 2010;277(1):2–21. [DOI] [PubMed] [Google Scholar]
- [36].Deng W, Wang Y, Zhao S, et al. MICAL1 facilitates breast cancer cell proliferation via ROS-sensitive ERK/cyclin D pathway. J Cell Mol Med. 2018;22(6):3108–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mao M, Yu T, Hu J, et al. Dopamine D2 receptor blocker thioridazine induces cell death in human uterine cervical carcinoma cell line SiHa. J Obstet Gynaecol Res. 2015;41(8):1240–1245. [DOI] [PubMed] [Google Scholar]
- [38].Johnson DE, Nedza FM, Spracklin DK, et al. The role of muscarinic receptor antagonism in antipsychotic-induced hippocampal acetylcholine release. Eur J Pharmacol. 2005;506(3):209–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available on request from the corresponding author.


