Abstract
Amyloid beta peptides (Aβ1-42) have been found to be associated with the cause of Alzheimer's disease (AD) and dementia. Currently, methods for detecting Aβ1-42 are complicated and expensive. The present study is aimed at developing an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) to detect Aβ1-42 by using a polyclonal antibody from alpaca, an application used in urine samples. The serum was collected from the alpaca after immunizing it with Aβ1-42 at 500 μg/injection 5 times. The ic-ELISA was developed and showed a half-maximal inhibitory concentration (IC50) of 103.20 ng/ml. The limit of detection (LOD) was 0.39 ng/100 μl. The cross-reactivity was tested with Aβ1-40 and 8 synthesized peptides that had sequence similarities to parts of Aβ1-42. The cross-reactivity of Aβ1-40 and peptide 1 (DAEFRHDSGYE) was 55% and 69.4%, respectively. The ic-ELISA was applied to analyze Aβ1-42 in the urine and precipitated protein urine samples. This method can be used for detecting a normal level of total soluble Aβ (approximately 1 ng in 5 mg of precipitated urine protein) and can be used for detecting the early stages of AD. It is considered to be an easy and inexpensive method for monitoring and diagnosing AD.
1. Introduction
Alzheimer's disease (AD) is an age-related chronic degenerative disease that damages the nervous system. It is mostly found in the elderly as a progressive neurodegenerative disease that predominantly affects cognitive functions [1]. The increasing number of AD patients is becoming an issue of serious concern due to the concomitant rise in medical and social costs. AD can be caused by several reasons, such as genetic causes, aging, malnutrition, immune system dysfunction, infectious agents, severe brain damage, congenital diseases (i.e., hypertension and diabetes), and the environment (i.e., exposure to pesticides or aluminum) [2, 3].
Amyloid beta peptides (Aβ) are neurotrophic and neurotoxic proteins that are used as a biomarker for detecting AD. Extracellular Aβ can lead to synapse loss and oxidative stress. Intracellular Aβ can increase the production of free radical species (ROS), causing increased levels of neurotoxicity and the death of neurons [4, 5]. Aβ is formed after sequential cleavage of the amyloid precursor protein (APP) by β-secretase, which removes the soluble APPβ (sAPPβ) and leaves a 99 amino acid C-terminal APP fragment (C99) in the cell membrane. Then, C99 is serial cleaved by γ-secretase at the ε-, ζ-, and γ-sites in the transmembrane domain (TMD), which releases the APP intracellular domain (AICD) into the cytosol and the 37-43 amino acid Aβ species into the extracellular space [6]. Aβ molecules can aggregate to form flexible soluble oligomers that may exist in several forms [7]. Aβ is the major constituent of the fibrils deposited into senile plaques and cerebral blood vessels of patients with AD. For Aβ levels under normal conditions, the daily excretion of intact soluble amyloid beta in the urine represents less than 1% of the circulating pool [8].
Several genetic, cell biology, biochemical, and animal studies support the concept that Aβ plays a central role in the development of AD pathology [9, 10]. The most common isoforms are Aβ1-40 and Aβ1-42 [11]. Aβ1-42 and Aβ1-43 are the major constituents of senile plaques and neurofibrillary tangles that occur in the hippocampus, neocortex, and amygdala of patients with AD [12]. Aβ1-42 is a good marker for the early stages of AD and is often detectable in several biological samples such as CSF, saliva, and urine in normal stages before the patient shows symptoms of AD, suggesting it is involved in the cause of AD [13–15].
Brain imaging techniques, which are crucial in the assessment of patients with AD, include fluoro-D-glucose integrated with positron emission tomography (FDG-PET) and amyloid positron emission tomography (amyloid PET) imaging [16, 17]. However, there are still many limitations of these methods, including cost, potential complications, the need for a specialist, and the requirement for high-performance equipment. While these conventional methods for identifying AD by detecting Aβ1-42 are still required and useful, they cannot detect the early stages of AD.
The immunoassay is a simple, economical technique with high sensitivity and specificity [18, 19] that can be developed and applied to biological samples. In the present day, immunoassays for human Aβ have been developed and can be purchased from pharmaceutical companies throughout the world. The most commercial enzyme-linked immunosorbent assay (ELISA) test kits are developed based on sandwich-ELISA, which requires two antibodies to detect the target antigen. They are used for serum, plasma, tissue homogenates, and cerebrospinal fluid (CSF). The sensitivity of commercial kits is a LOD between 4.73 and 1,000 pg/ml and a detection range between 7.8 and 480,000 pg/ml for all Aβ. Aβ1-42 can be present at a lower concentration, where the LOD is between 4.73 and 9.38 pg/ml. Conventional antibodies such as polyclonal antibodies (pAb) and monoclonal antibodies (mAb) from rodents are used in the test kits. The price of one test kit is in the range of 395-875 dollars for 96 tests [20], which is far too expensive to be widely used.
This study was conducted to develop ELISA for detecting Aβ1-42 in urine samples in which Aβ is found as a normal component, and the monomeric Aβ level is related to the clinical dementia rating score (CDR) [21].
2. Materials and Methods
2.1. Preparation of the Antibody
A single alpaca was raised at Nanchang (Jiangxi Province, People's Republic of China) and immunized subcutaneously 5 times at 2-week intervals with a 500 μg/injection of Aβ1-42 to produce antibodies against Aβ1-42. The immunogen was prepared with Aβ1-42, which was purchased from Thermo Fisher (USA), dissolved in phosphate-buffered saline (PBS) pH 7.2, and gently mixed with an equal volume of Freund's Complete Adjuvant (Sigma-Aldrich, USA) to make an emulsion for the first immunization and Freund's Incomplete Adjuvant (Sigma-Aldrich, USA) to make an emulsion for the 2nd, 3rd, 4th, and 5th immunization. The blood was collected from the jugular vein of the alpaca before the 4th and 5th injection and 1 week after the last immunization. The 5th serum was used to develop the method in this study. The protocol of the experiment was approved by the animal ethical committee of South China Agricultural University, Guangdong, Guangzhou, China, and the study has been conducted in accordance with the guidelines for the animal screening of antiserum.
2.2. Indirect ELISA for the Titration of the Alpaca Antiserum
An indirect ELISA was used to determine the optimal dilution factor of the antibodies that were later used in ic-ELISA procedure [22, 23]. Standard Aβ1-42 was serially diluted into coating buffer (carbonate buffer, pH 9.6), producing 11 concentrations of Aβ1-42, and coated on Maxisorp microwell plates (Thermo Fisher Scientific, Denmark) at 4°C for 16 hours overnight. After incubation, the plate was washed 4 times with 400 μl/well PBST (PBS with 0.05% Tween) by a microplate washer (Thermo Fisher Scientific, Finland) and dried by patting with paper towels. After washing, the plate was blocked with 200 μl/well of 1% skim milk in PBS as a blocking solution and incubated for 1 hour at 25°C. Then, the blocking solution was discarded, and the plate was dried by patting with paper towels. Alpaca antiserum, which was prepared with 8 dilutions from 1 : 1,000 to 1 : 32,000, was added to the wells and incubated for 1 hour at 25°C. Then, the plate was washed as in the previous washing procedure. In the next step, 100 μl of goat anti-llama IgG labeled with horseradish peroxidase (1 : 5,000 in PBST) (Abcam, USA) was added to each well and incubated for 1 hour at 25°C. Then, the washing procedure was repeated. For determination, 100 μl of TMB substrate (12.5 ml citrate buffer pH 4, 200 μl stock TMB, and 50 μl of 1% H2O2) was added and rested in the dark at 25°C for 10 min. The reaction was stopped with 2 M H2SO4. The optical density (OD) was measured at 450 nm in a multiscan spectrophotometer (Tecan, Austria) to evaluate the optimal dilution of the alpaca serum and the concentration of coating [24].
2.3. Indirect Competitive ELISA for Quantification of Aβ1-42
The ic-ELISA assay was performed with alpaca antiserum (1 : 1,000 in PBST), and the competition between the free standard Aβ1-42 and the coated antigen (Aβ1-42) was observed in order to determine the concentration of the free standard. The microwell plate was coated at 4°C for 16 hours overnight with 100 μl/well of 3 concentrations of coating antigen (Aβ-42, 2-4 μg/ml in coating buffer). The plate was then washed and blocked as described above. An equal volume of standard Aβ-42 was added to the alpaca antiserum in a u-shaped plate at 25°C for 1 hour. After the blocking solution was discarded and dried, the mixture of standard Aβ1-42 and alpaca antiserum was added in triplicate to the wells of each coating antigen. After the plate was incubated for 1 hour at 25°C and washed, anti-llama IgG labeled with horseradish peroxidase (1 : 5,000 in PBST) and TMB substrate were added following the ELISA protocol, as described above.
The specificity and sensitivity of this method were measured with alpaca antiserum (1 : 1,000 in PBST), and the competition between the free standard Aβ1-42 with the coated antigen (Aβ1-42) was observed. The microwell plate was coated at 4°C for 16 hours overnight with 100 μl/well of 2 μg/ml Aβ1-42 in coating buffer. Twelve concentrations of competitive Aβ1-42 were prepared (0-4,000 ng/ml, twofold dilution), and ic-ELISA was performed with 1 : 1,000 (v/v) dilution of alpaca antiserum, as described above.
The calibration curve was obtained and calculated as IC50. The limit of detection (LOD) was calculated by using the formula [25] as follows:
| (1) |
where B0 is the optical density (OD) of blank (no Aβ1‐42) and SD is the standard deviation of B0.
2.4. Assessment of the Cross-Reactivity of Aβ1-42 ic-ELISA
Standard Aβ1-40, Aβ1-42, and 8 synthesized Aβ peptides (Aβ1-8, Aβ7-15, Aβ14-21, Aβ20-27, Aβ26-33, Aβ32-39, Aβ35-42, and Aβ36-43) were tested in the ic-ELISA assay as described above. Calibration curves were obtained. The cross-reactivity (CR) values were calculated by using the formula [26] as follows:
| (2) |
where IC50 is the half-maximal inhibitory concentration.
2.5. Preparation of the Urine Protein
A pool of urine was prepared from 10 healthy donors (5 male and 5 female) and used in this study. Due to the small amount of Aβ1-42 in urine, the concentration of the urine proteins was increased by a precipitation technique. Urine protein was prepared by the salting out technique, which is the most common method used to precipitate proteins. Ammonium sulfate ((NH4)2SO4) was used to compress the solvation layer and increase the protein–protein interactions. The increasing salt concentration caused the protein to separate and fall to the bottom of the sample solution by aggregation and precipitation activities [27]. The precipitation was performed with 31.77 g of ammonium sulfate added to 100 ml urine (to reach 50% saturation) and well-mixed by a magnetic stirrer for 1 hour on ice. Then, the mixture was centrifuged 4,000 rpm for 1 hour at 4°C. The supernatant was discarded; then, the protein pellet was resuspended in 1 ml PBS buffer (pH 7.2) and kept at 4°C.
2.6. Assessment of the Matrix Effect on Aβ1-42 ic-ELISA Calibration Graph
The effects of urine and precipitated urine protein on the performance of the ic-ELISA were assessed. Twelve dilutions of each urine sample (1 : 1-1 : 5,120 in PBS) and precipitated urine protein (0.001-4.56 mg/ml) were determined by ic-ELISA, as described above. The nonmatrix effect was determined by the same value OD of the sample dilution and the OD of the PBS sample.
Calibration curves of the Aβ1-42 in urine and precipitated urine protein were obtained. Twelve concentrations of Aβ1-42 (0–8,000 ng/ml) were spiked into 1 : 160 urine in PBS and 0.456 mg/ml precipitated urine protein, and then, ic-ELISA was performed as described above. LODs were calculated as described above.
The correlation curves were obtained by comparing the concentration of spiked Aβ1-42 samples and the detected concentration in the calibration curves.
3. Results
3.1. Development of ic-ELISA
To develop the ic-ELISA for the detection of Aβ1-42, alpaca antiserum was tested using indirect ELISA in the first step to determine the optimal concentration of coating antigen and the optimal dilution of antiserum. Six concentrations of Aβ1-42 in coating buffer were coated on the wells, and six serial dilution ratios from 1 : 1,000 to 1 : 32,000 of alpaca antiserum were investigated. The 1 : 1,000 dilution showed the highest binding sensitivity among all coating antigen concentrations (Table 1). Then, sensitivity of the indirect ELISA was tested with 15 concentrations of Aβ1-42. The half-maximal effective concentration (EC50) of a 1 : 1,000 (v/v) dilution of alpaca antiserum was 928.40 ng/ml as shown in Figure 1(a).
Table 1.
Results of the ELISA checker-board titration test of the binding of 6 concentrations of Aβ1-42 and 6 serially diluted antiserums by the indirect ELISA method.
| Antiserum (1 : X) | OD at 450 nm | |||||
|---|---|---|---|---|---|---|
| Concentration of coated Aβ1-42 (ng/ml) | ||||||
| 4000 | 2000 | 1000 | 500 | 250 | 125 | |
| 1,000 | 1.053 | 0.938 | 0.852 | 0.74 | 0.644 | 0.525 |
| 2,000 | 0.929 | 0.799 | 0.686 | 0.56 | 0.443 | 0.368 |
| 4,000 | 0.821 | 0.622 | 0.475 | 0.379 | 0.272 | 0.217 |
| 8,000 | 0.659 | 0.441 | 0.342 | 0.235 | 0.178 | 0.146 |
| 16,000 | 0.456 | 0.278 | 0.203 | 0.149 | 0.113 | 0.099 |
| 32,000 | 0.293 | 0.171 | 0.13 | 0.098 | 0.081 | 0.073 |
Figure 1.
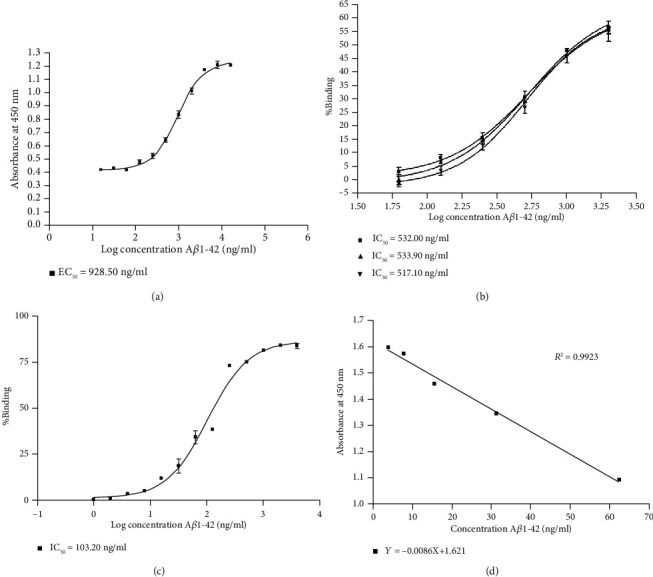
Development of Aβ1-42 detection. (a) Indirect ELISA calibration curves for Aβ1-42 using 1 : 1,000 (v/v) dilution of alpaca antiserum. (b) Optimization of coating antigen using indirect competitive ELISA. The percent binding showed a competition percentage of 6 concentrations of competitive Aβ1-42 (62, 125, 250, 500, 1,000, and 2,000 ng/ml) to a 1 : 1,000 (v/v) dilution of alpaca antiserum at 3 concentrations of coating antigen (■ = 2,000, ▲ = 3,000, and ▼ = 4,000 ng/ml of Aβ1-42 in coating buffer). (c) ic-competitive ELISA calibration curves for Aβ1-42 using a 1 : 1000 (v/v) dilution of alpaca antiserum. The calibration curve was obtained using analyte standards prepared in PBS buffer. (d) ic-competitive ELISA calibration curves obtained by OD.
As the second step, the ic-ELISA was used to optimize the concentration of coating antigen (Aβ1-42). Three concentrations of Aβ1-42, prepared at 2,000, 3,000, and 4,000 ng/ml in the coating buffer, were used as the coating antigen, and ic-ELISA was performed with a 1 : 1,000 (v/v) dilution of alpaca antiserum, as described above. The coated concentration of 2,000 ng/ml Aβ1-42 was selected since it showed the same half-maximal inhibitory concentration (IC50) as the above concentrations (Figure 1(b)).
For the third step, the sensitivity of this method was determined. The IC50 of ic-ELISA was 103.20 ng/ml as shown in Figure 1(c). The LOD of this method was 0.39 ng/100 μl, calculated by the formula and OD calibration curve shown in Figure 1(d).
3.2. Cross-Reactivity of the Assay
The specificity of the developed ic-ELISA was assessed by measuring the cross-reactivity toward the same structural proteins as Aβ1-42, using Aβ1-40, and 8 synthesized Aβ peptides (GenScript, China) in the test (the sequence of peptides is shown in Table 2). The data within a range of 12 concentrations of the Aβ1-42 standard (0–4,000 ng/ml, twofold dilution in PBST) were used for the calculation of cross-reactivity. Aβ7-15, Aβ14-21, Aβ20-27, Aβ26-33, Aβ32-39, Aβ35-42, and Aβ36-43 showed no detectable cross-reactivity. However, Aβ1-8 showed 69.40% and Aβ1-40 showed 55.00% cross-reactivity as shown in Table 2, suggesting both can have some contribution to the measurement of the Aβ1-42 level with this method.
Table 2.
Cross-reactivity of synthesized peptides and two beta amyloid proteins.
| Sample | Sequence | IC50 (ng/ml) | % cross-reaction |
|---|---|---|---|
| Aβ1-42 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA | 103.20 | 100.00 |
| Aβ1-8 | DAEFRHDS | 148.80 | 69.40 |
| Aβ1-40 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV | 187.70 | 55.00 |
| Aβ7-15 | DSGYEVHHQ | >4000 | - |
| Aβ14-21 | HQKLVFFA | >4000 | - |
| Aβ20-27 | FAEDVGSN | >4000 | - |
| Aβ26-33 | SNKGAIIG | >4000 | - |
| Aβ32-39 | IGLMVGGV | >4000 | - |
| Aβ35-42 | MVGGVVIA | >4000 | - |
| Aβ36-43 | VGGVVIAT | >4000 | - |
3.3. Matrix Effect on the Aβ1-42 ic-ELISA
The developed ic-ELISA was performed by using a 1 : 1,000 (v/v) dilution of alpaca antiserum in 12 diluted samples (1 : 1–1 : 5120 in PBS) of pooled urine and 12 diluted samples of precipitated urine protein (urine protein = 0.001‐4.56 mg/ml) that were used as the matrix antigen of competition with coated Aβ1-42. The results had no matrix effect OD at 1 : 160 for the pooled urine, as shown in Figure 2(a), and 0.456 mg/ml for the urine protein, as shown in Figure 2(b). The calibration curve was obtained using analyte standard Aβ1-42 prepared in 1 : 160 diluted pool urine and 0.456 mg/ml precipitated urine protein. The results showed an IC50 = 115.90 ng/ml for 1 : 160 pooled urine, as shown in Figure 3(a), and 579.00 ng/ml for precipitated urine protein, as shown in Figure 3(b). The LOD of this method was 16.83 ng/ml for the pooled urine sample and 139.60 ng/ml for the precipitated urine protein. The correlation of the detected and spiked concentrations of Aβ1-42 in the samples by using the ic-ELISA calibration curve showed R2 = 0.9973 for pooled urine (in the range 7.80-1000.00 ng/ml Aβ1-42) as shown in Figure 3(c) and R2 = 0.9918 for the precipitated urine protein (in the range 15.63-1000.00 ng/ml Aβ1-42) as shown in Figure 3(d).
Figure 2.
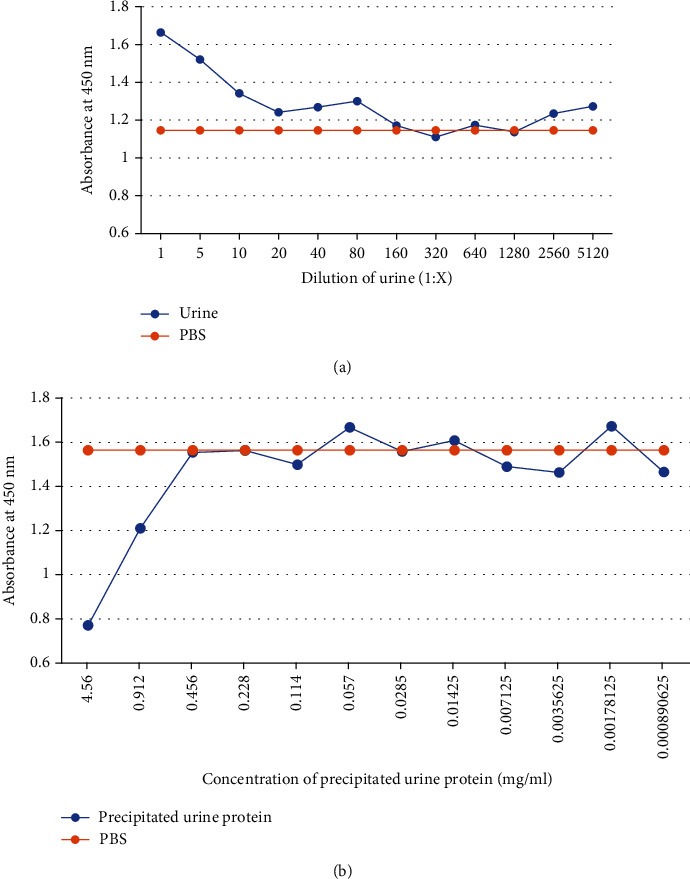
Observation of the urine matrix effect using ic-ELISA. (a) The curve was obtained using analyte 12 dilutions of pooled urine prepared in PBS buffer (1 : 1–1 : 5120). (b) The curve was obtained using analyte 12 concentrations of precipitated urine protein prepared in PBS buffer (0.001-4.56 mg/ml).
Figure 3.
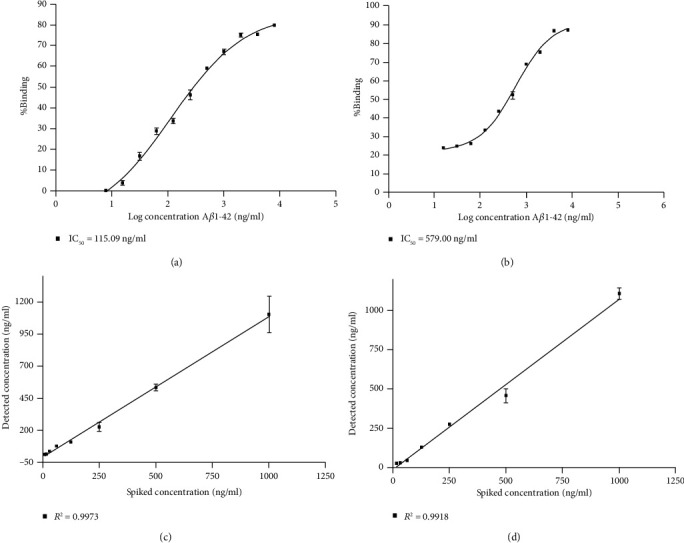
The ic-ELISA calibration curves for Aβ1-42 using a 1 : 1000 (v/v) dilution of alpaca antiserum were obtained using analyte standards prepared in 0.456 mg/ml precipitated urine. (c) The correlation curve of the detected and spiked concentrations of Aβ1-42 in 1 : 160 diluted urine. (d) The correlation curve of the detected and spiked concentrations of Aβ1-42 in 0.456 mg/ml precipitated urine.
4. Discussion
We propose a new alternative method for the detection of Aβ in human biological samples, an ic-ELISA for the detection of Aβ1-42 in urine samples that we have developed. Most commercial ELISA test kits for Aβ in humans are developed based on sandwich-ELISA, which requires two antibodies for detection, and only a few commercial test kits can detect Aβ in urine. One advantage of our method is that it only requires one specific antibody and can be detected in urine samples. Another advantage of using a single antibody is reducing nonspecific bounding since other substances may affect each antibody. The commercial kits have a LOD between 4.73 and 1,000 pg/ml, and their detection range is between 7.8 and 480,000 pg/ml for all Aβ. Aβ1-42 shows a lower concentration, where LOD is usually between 4.73 and 9.38 pg/ml. Our method shows that the concentration of the LOD for Aβ1-42 is higher than the commercial kit at 3.90 ng/ml (390 pg/well), based on the levels of Aβ1-42 in human biological samples that are regularly monitored at relatively low levels: 750 pg/ml for CSF samples [28] and 17.65 pg/ml for serum samples [29]. So, we are not able to use the commercial methods to detect Aβ1-42 in these samples.
However, our method showed 69.4% and 55% cross-reaction to the synthesized Aβ1-8 and Aβ1-40, respectively, as expected, since Aβ1-40 has a sequence close to Aβ1-42. Synthesized Aβ1-8 also has a sequence present at the beginning of every Aβ. The LOD of this method cannot detect Aβ1-42 in urine samples. However, due to the high cross-reactivity, the method can be developed to detect total Aβ in urine and could be used to detect total Aβ in other biological samples.
In urine samples, the LOD was 16.83 ng/ml and 139.60 ng/ml for precipitated urine protein. Since urine contains inorganic ions (K +, Na +, Cl −, and Ca2 +), organic molecules such as creatinine, urea, and uric acid, and more than 1,500 proteins, mainly extracellular, membranes, cell membranes, and cell debris, the higher LOD may come from the matrix in urine, which can interrupt the binding of antigens with antibodies used in the ELISA assays [30]. Another limitation of commercial ELISA kits is the price; most are expensive and cannot be routinely used for early detection in people. The yields of pAb from rodents are limited due to the size of the animal. In this study, the antibody was produced in an alpaca, whose blood can be collected in larger quantities, which allows our method to be developed at a lower cost.
A previous study reported that Aβ is removed from circulation by the capillary beds of the kidneys, liver, and gastrointestinal tract, as well as through the skin and excreted in the urine [31]. According to studies from Takata et al., Aβ has a correlation with the clinical dementia rate (CDR) of people with AD and mild cognitive impairment (MCI), with an increasing value of Aβ in mild AD (CDR 0.5 to 1) and a decreasing value in more severe AD (CDR 2 to 3), since aggregated Aβ plaques might not be able to be eliminated via normal clearance routes in severe cases. The detection sensitivity of their method was 40 pg/ml, which still cannot be quantified [21]. Since our new ic-ELISA method can detect this amount of Aβ in urine samples, it can be used as a preliminary assessment for AD.
Aβ peptides are a normal component of human urine as identified in the study of Ghiso et al. [8], who conducted immunoprecipitation experiments with anti-Aβ antibodies followed by detection and identification by immunoblots and MALDI mass spectrometry. For Aβ levels in normal donors (proteinuria < 5 mg/dl), the excretion of soluble Aβ was calculated at 0.81 ± 0.26 ng/5 mg of urinary proteins (13 ± 4 ng/24 h) [8]. Therefore, at this Aβ level, our method could measure the level of monomer Aβ in the urine. By applying urine sample preparation methods, such as precipitation techniques to increase the concentration of Aβ in the urine sample, we can use 1 dl of urine to prepare 5 mg urine protein, which can reach approximately 1 ng of Aβ in normal donors. In addition, from the urine matrix tests, our method found an effect of the pooled urine at under 1 : 160 dilution (urine protein < 32.25 ng in 100 μl sample). This means that it is not possible to use this method to determine Aβ in urine samples without a sample pretreatment process. However, since precipitated urine protein has shown nonmatrix effect results at 0.456 mg/ml (urine protein = 0.05 mg in 100 μl sample) and unidentified inhibition results of 4.56 and 0.91 mg/ml (urine protein = 0.46 and 0.09 mg in 100 μl sample), it is possible to develop this method to detect amyloid in urine samples in combination with appropriate urine protein sample preparation.
The serum and CSF sample collection requires invasive methods and can be harmful to participants, while urine samples can be easily and not harmfully collected. The results can be corrected referencing the concentration of Aβ against urine creatinine and reported as microgram per milliliter urine or microgram per gram creatinine, as reported in several previous studies.
Most of the immunoassay methods were developed based on conventional antibodies and have some limitations, i.e., the lack of specificity of pAb and complicated production of mAb [32]. However, the production of a single-domain antibody (sdAb) from a phage display technique could provide more sensitivity, specificity, and stability of the antibody. In addition, a large number of antibodies can be produced by these methods [33]. An sdAb is an antibody fragment consisting of a single monomeric variable antibody domain, unlike a whole antibody, which can selectively bind to a very specific antigen. The sdAb could be produced from both conventional antibodies [34] and heavy chain antibodies from camelids [35].
In our study, an alpaca was immunized with Aβ1-42 to produce antibodies. For our novel ic-ELISA for Aβ, pAb was applied due to its convenience in the development of this method of examination, but sdAb for Aβ can be developed in the future.
5. Conclusions
The developed ic-ELISA indicated sensitivity for Aβ1-42 and peptides having a similar structure. Aβ1-40 showed 55% cross-reactivity, and the synthesized Aβ1-8, which consists of the same beginning sequence for all Aβ, showed 69.4% cross-reactivity. In addition, the method showed no cross-reactivity to other synthesized Aβ peptides, which have the same sequence as Aβ1-42 from different segments of the sequence. We can presume that the binding site of the antibody has the same sequence as Aβ1-8 (DAEFRHD). The assay, as described in this study, has great potential to detect Aβ1-42 protein in cases of high-risk and early stages of AD, with an easy and rapid method when applied to a urine sample. Moreover, this assay can be widely used at a low cost and applied to other types of biological samples.
Acknowledgments
We gratefully acknowledge the support from the Research Institute for Health Science, Chiang Mai University, 50200, Thailand, and Guangdong Provincial Key Laboratory of Food Quality and Safety, College of Food Science, South China Agricultural University, Guangzhou, 510642, People's Republic of China.
Data Availability
All data were shown in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Schachter A. S., Davis K. L. Alzheimer’s disease. Dialogues in Clinical Neuroscience. 2000;2(2):91–100. doi: 10.31887/DCNS.2000.2.2/asschachter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan D., Zhang Y., Liu L., Yan H. Pesticide exposure and risk of Alzheimer’s disease: a systematic review and meta-analysis. Scientific Reports. 2016;6(1):p. 3222. doi: 10.1038/srep32222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong A. R. What causes Alzheimer’s disease. Folia Neuropathologica. 2013;3(3):169–188. doi: 10.5114/fn.2013.37702. [DOI] [PubMed] [Google Scholar]
- 4.Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 5.Ghasemi E., Azad N. G., Tondar M., Parirokh P., Aval F. Tracking the footprint of pesticides in Alzheimer’s disease. Journal of Systems and Integrative Neuroscience. 2015;1(1):14–19. doi: 10.15761/JSIN.1000104. [DOI] [Google Scholar]
- 6.Harald S., Akio F., Shinji T., Masayasu O. Making the final cut: pathogenic amyloid-β peptide generation by γ-secretase. Cell Stress. 2018;2(11):292–310. doi: 10.15698/cst2018.11.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haass C., Selkoe D. J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid β-peptide. Nature Reviews. Molecular Cell Biology. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 8.Ghiso J., Calero M., Matsubara E., et al. Alzheimer’s soluble amyloid β is a normal component of human urine. FEBS Letters. 1997;408(1):105–108. doi: 10.1016/S0014-5793(97)00400-6. [DOI] [PubMed] [Google Scholar]
- 9.Hardy J., Duff K., Hardy K. G., Perez-Tur J., Hutton M. Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nature Neuroscience. 1998;1(5):355–358. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- 10.Ghiso J., Frangione B. Amyloidosis and Alzheimer’s disease. Advanced Drug Delivery Reviews. 2002;54(12):1539–1551. doi: 10.1016/S0169-409X(02)00149-7. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann T., Bieger S. C., Brühl B., et al. Distinct sites of intracellular production for Alzheimer’s disease Aβ40/42 amyloid peptides. Nature Medicine. 1997;3(9):1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 12.Roher A. E., Lowenson J. D., Clarke S., et al. Beta-amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proceedings of the National Academy of Sciences. 1993;90(22):10836–10840. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schirinzi T., Di Lazzaro G., Sancesario G. M., et al. Levels of amyloid-beta-42 and CSF pressure are directly related in patients with Alzheimer’s disease. Journal of Neural Transmission. 2017;124(12):1621–1625. doi: 10.1007/s00702-017-1786-8. [DOI] [PubMed] [Google Scholar]
- 14.Lee M., Guo J. P., Kennedy K., McGeer E. G., McGeer P. L. A method for diagnosing Alzheimer’s disease based on salivary amyloid-β protein 42 levels. Journal of Alzheimer's Disease. 2017;55(3):1175–1182. doi: 10.3233/JAD-160748. [DOI] [PubMed] [Google Scholar]
- 15.Sabbagh M. N., Shi J., Lee M., et al. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: preliminary findings. BMC Neurology. 2018;18(1):p. 155. doi: 10.1186/s12883-018-1160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson K. A., Fox N. C., Sperling R. A., Klunk W. E. Brain imaging in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2012;2(4, article a006213) doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toledo J. B., Shaw L. M., Trojanowski J. Q. Plasma amyloid beta measurements - a desired but elusive Alzheimer’s disease biomarker. Alzheimer's Research & Therapy. 2013;5(2):p. 8. doi: 10.1186/alzrt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan S. D., Patel K. R. Enzyme immunoassay and enzyme-linked immunosorbent assay. Journal of Investigative Dermatology. 2013;133(9):1–3. doi: 10.1038/jid.2013.287. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt S. D., Nixon R. A., Mathews P. M. ELISA method for measurement of amyloid-beta levels. Methods in Molecular Biology. 2005;299:279–297. doi: 10.1385/1-59259-874-9:279. [DOI] [PubMed] [Google Scholar]
- 20.Biocompare. Excellent Technical Reproducibility - Beta-Amyloid ELISA Kit. Biocompare; 2020. https://www.biocompare.com/Search-ELISA-Kits/?search=Amyloid%2C+beta+42. [Google Scholar]
- 21.Takata M., Nakashima M., Takehara T., et al. Detection of amyloid β protein in the urine of Alzheimer’s disease patients and healthy individuals. Neuroscience Letters. 2008;435(2):126–130. doi: 10.1016/j.neulet.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Wiederschain G. Y. The ELISA guidebook. Biochemistry (Moscow) 2009;74(9):1058–1058. doi: 10.1134/S000629790909017X. [DOI] [Google Scholar]
- 23.Crowther J. R. ELISA. Theory and practice. Methods in Molecular Biology. 1995;42:1–218. doi: 10.1385/0-89603-279-5:1. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt S. D., Mazzella M. J., Nixon R. A., Mathews P. M. Aβ measurement by enzyme-linked immunosorbent assay. Methods in Molecular Biology. 2012;849:507–527. doi: 10.1007/978-1-61779-551-0_34. [DOI] [PubMed] [Google Scholar]
- 25.Linnet K., Kondratovich M. Partly nonparametric approach for determining the limit of detection. Clinical Chemistry. 2004;50(4):732–740. doi: 10.1373/clinchem.2003.029983. [DOI] [PubMed] [Google Scholar]
- 26.Ruprich J., Ostrý V. Immunochemical methods in health risk assessment: cross reactivity of antibodies against mycotoxin deoxynivalenol with deoxynivalenol-3-glucoside. Central European Journal of Public Health. 2008;16(1):34–37. doi: 10.21101/cejph.a3455. [DOI] [PubMed] [Google Scholar]
- 27.Englard S., Seifter S. [22] precipitation techniques. Methods in Enzymology. 1990;182:285–300. doi: 10.1016/0076-6879(90)82024-V. [DOI] [PubMed] [Google Scholar]
- 28.Brothers H. M., Gosztyla M. L., Robinson S. R. The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease. Frontiers in Aging Neuroscience. 2018;10:p. 118. doi: 10.3389/fnagi.2018.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zecca C., Tortelli R., Panza F., et al. Plasma β-amyloid1–42 reference values in cognitively normal subjects. Journal of the Neurological Sciences. 2018;391:120–126. doi: 10.1016/j.jns.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Chatziharalambous D., Lygirou V., Latosinska A., et al. Analytical performance of ELISA assays in urine: one more bottleneck towards biomarker validation and clinical implementation. PLoS One. 2016;11(2, article e0149471) doi: 10.1371/journal.pone.0149471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Y., Bu X. L., Liu Y. H., et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathologica. 2015;130(4):487–499. doi: 10.1007/s00401-015-1477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lipman N. S., Jackson L. R., Trudel L. J., Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR Journal. 2005;46(3):258–268. doi: 10.1093/ilar.46.3.258. [DOI] [PubMed] [Google Scholar]
- 33.De Meyer T., Muyldermans S., Depicker A. Nanobody-based products as research and diagnostic tools. Trends in Biotechnology. 2014;32(5):263–270. doi: 10.1016/j.tibtech.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Holt L. J., Herring C., Jespers L. S., Woolven B. P., Tomlinson I. M. Domain antibodies: proteins for therapy. Trends in Biotechnology. 2003;21(11):484–490. doi: 10.1016/j.tibtech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Pardon E., Laeremans T., Triest S., et al. A general protocol for the generation of nanobodies for structural biology. Nature Protocols. 2014;9(3):674–693. doi: 10.1038/nprot.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were shown in the manuscript.


