ABSTRACT
The Arabidopsis transcription factor Myeloblastosis protein 75 (MYB75, AT1G56650) is a well-established transcriptional activator of genes required for anthocyanin and flavonoid production, and a repressor of lignin and other secondary cell wall biosynthesis genes. MYB75 is itself tightly regulated at the transcriptional, translational and post-translational levels, including protein phosphorylation by Arabidopsis MAP kinases Examination of the behavior of different phosphovariant versions of MYB75 in vitro and in vivo revealed that overexpression of the MYB75T131E phosphovariant had a particularly marked effect on global changes in gene expression suggesting that phosphorylated MYB75 could be involved in a broader range of functions than previously recognized. Here, we describe a range of distinct developmental phenotypes observed among Arabidopsis lines expressing various phosphovariant forms of MYB75. Expression of either MYB75T131E or MYB75T131A phosphovariants, from the endogenous MYB75 promoter, in Arabidopsis myb75− mutants (Nossen background), resulted in severely impaired germination rates, and developmental arrest at early seedling stages. Arabidopsis plants overexpressing MYB75T131E from a strong constitutive Cauliflower mosaic virus (CaMV35S) promoter displayed slower development, with delayed bolting, flowering and onset of senescence. Conversely, MYB75T131A -overexpressing lines flowered and set seed earlier than either Col-0 WT controls or other MYB75-overexpressors (MYB75WT and MYB75T131E). Histochemical analysis of mature stems also revealed ectopic vessel development in plants overexpressing MYB75; this phenotype was particularly prominent in the MYB75T131E phosphovariant. These data suggest that MYB75 plays a significant role in plant development, and that this aspect of MYB75 function is influenced by its phosphorylation status.
KEYWORDS: Arabidopsis thaliana, Myeloblastosis protein 75, mitogen activated protein kinase, phosphorylation, germination, flowering time, bolting time, vascular development
Introduction
Transcriptional regulators are a vital part of the cellular circuitry that controls gene expression, and ultimately the biochemical, developmental and physiological changes that occur in living organisms. Many transcription factors characterized over the years have been initially identified as regulators of a specific biological trait for which distinct phenotypes could be easily identified. However, an increasing body of evidence shows extensive interconnectivity between different signaling pathways, and multifunctionality of transcriptional regulators. A prominent example of such multifunctionality is HY5, initially identified as a suppressor of hypocotyl elongation during de-etiolation, but now known to be a global regulator of light-responsive genes and a driver of photomorphogenesis.3–5 Other examples include Enhancer of Glabra 3 (EGL3, AT1G63650) and Glabra 3 (GL3, AT5G41315), which regulate anthocyanin biosynthesis in concert with MYB75,6–8 and, independently of this function also play a role in epidermal cell fate determination.9–14 The involvement of MYB75 in multiple, seemingly unrelated biological processes can be in part attributed to its ability to form complexes with different transcriptional regulators. Formation of the ternary complex MYB75-EGL3/GL3/TT8-TTG1 is required for anthocyanin biosynthesis,7 while association of MYB75 and KNAT7 (AT1G62990) is needed to suppress expression of lignin and other cell wall biosynthesis genes.15,16 Thus, the ability of MYB75 to control the expression of specific genes depends on the presence of appropriate binding partners, which is ultimately dictated by the cellular milieu in which the protein is found.
We earlier demonstrated that MYB75 can physically interact with a large number of Arabidopsis MAP kinases, suggesting that this transcription factor may be involved in multiple MPK signaling cascades.1 While total anthocyanin production was similar in Arabidopsis plants overexpressing MYB75T131E and MYB75T131A phosphovariants, MYB75T131E was found to be a more potent driver of global gene expression changes in Arabidopsis, compared to MYB75T131A, and MYB75T131E protein displayed more rapid turnover under certain conditions.1 These findings suggest that phosphorylation at Threonine 131 influences the ability of MYB75 to drive expression of downstream genes and, through control of protein turnover, could play a role in spatial-temporal distribution of the protein, thus determining the cellular milieu where MYB75 is found.1
When analyzing the properties of the Arabidopsis lines expressing various MYB75 phosphovariants we consistently observed developmental and physiological changes in certain plant lines which affected their viability, suggesting MYB75 function has a profound influence on plant development. In this report we describe some of the phenotypes we observed among Arabidopsis lines expressing different phosphovariants of MYB75; phenotypes which do not appear to be directly related to MYB75’s role in regulating anthocyanin biosynthesis, the function canonically associated with this protein.
Materials and methods
Site-directed mutagenesis
Phosphovariant (phosphomimic and phosphonull) versions of MYB75 were created by replacing each threonine found in the putative MPK target site with either alanine or glutamic acid, using site-directed mutagenesis as described in previous work.1
Plant material and transgenic lines
Arabidopsis seeds (Col-WT and Nossen WT) were purchased from Lehle seeds, 1102 South Industrial Blvd. Suite D, Round Rock, Texas 78681, United States, Phone: 1-(512) 388–3945 (512) 388–3974 Website: http://www.arabidopsis.com.
All transgenic Arabidopsis lines used in this study were generated in our laboratory. To create transgenic lines where each MYB75 phosphovariant (MYB75T126A, MYB75T126E, MYB75T131A, MYB75T131E, MYB75T126/131A and MYB75T126/131E) was expressed from the endogenous MYB75 promoter, cDNA version of each MYB75 phosphovariant was cloned into the gateway entry vector pENTR2B, behind an endogenous MYB75 promoter, encompassing 2742bp of genomic sequence upstream of the MYB75 translational start site. The MYB75pr:MYB75 cDNA constructs were transferred into the binary GATEWAY® vector pEARLEY-GATE301, by recombination based cloning, using LR Clonase II (Thermo Fisher Scientific). The resulting constructs were subsequently used to transform myb75− (Nossen) mutant plants, using Agrobacterium-mediated floral transformation as described previously.1 Successfully transformed T1 plants were isolated on Murashige and Skoog (½MS) agar plates containing 50 µg/mL kanamycin as the selection agent.
Arabidopsis lines overexpressing different phosphovariants of MYB75 (full gene version, of MYB75WT, MYB75T131A and MYB75T131E), with an N-terminal 3x hemagglutinin (HA)-epitope tag, under the control of a constitutive Cauliflower mosaic virus (CaMV35S) promoter, were generated using GATEWAY® recombination-based cloning, as described previously.1 Successfully transformed T1 plants were isolated on Murashige and Skoog (½MS) agar plates containing 30 µg/mL hygromycin as the selection agent.
Plant growth conditions
Arabidopsis seeds were surface sterilized, plated on Petri dishes containing ½ MS medium with 0.8% plant agar, and vernalized for 3 days at 4ᵒC. After vernalization, the plates were placed under light fluence of ~30 µmol m-2 s − 1 and a 16 h light/8 h dark photoperiod, at 21°C and grown under these conditions until they were transferred to soil. Between 7 and 12 days after germination, seedlings were planted in soil and transferred to a growth chamber with a light fluence of ~100-120 µmol m-2 s − 1, a photoperiod of 16 h light/8 h dark, and temperature of 21°C.
To evaluate the ability of each MYB75 phosphovariant to drive anthocyanin production and to assess MYB75 promoter functionality, in MYB75pr:MYB75 cDNA lines, 6-week-old transgenic plants were exposed to continuous light for 48 h (fluence of ~100-120 µmol m-2 s − 1).
Anthocyanin quantification
Anthocyanin quantification was performed as previously described,17 using mature rosette leaves from 6-week-old Arabidopsis plants, expressing different MYB75 phosphovariants from the endogenous MYB75 promoter, in a myb75− mutant background (Nossen ecotype). Fresh, healthy plant tissue (1–2 g) was collected into microcentrifuge tubes and fresh weight was recorded for each sample, which was immediately frozen in liquid nitrogen and ground to a powder. The lyophilized tissue was immediately re-suspended in 1 mL extraction solution (49.5% methanol, 49.5% distilled water, 1% acetic acid), the samples were incubated at 45°C for 4 h, centrifuged at 25000 g and the cleared supernatant was transferred to a cuvette. Absorbance was measured at λ = 530 nm and λ = 650 nm, and total anthocyanin quantity was determined using the equation Qanthocyanin = [A530- (A650x0.25)]/mass(g). Each sample consisted of tissue from 3–4 plants belonging to an individual line. The number of samples used for each genotype were as follows: four samples for each Nossen WT and myb75− controls; six samples for MYB75T126A genotype (one sample for each line); six samples for MYB75T131A genotype (one sample for each line); three samples for MYB75T126/131A genotype (one sample for each line).
Detection of recombinant MYB75 expression
RNA extraction from Arabidopsis plants (mature rosette leaves and stem sections) was performed using TRIzol® reagent (Thermo Fischer Scientific), according to manufacturer’s protocol. RNA quality was evaluated by running 5 µL of RNA on 2% agarose gel, and the RNA concentration was determined spectrophotometrically using NanoDrop® (Thermo Fischer Scientific). Reverse transcription was performed using SuperscriptII (Invitrogen) to obtain total cDNA.
In transgenic Arabidopsis lines where each phosphovariant was expressed from the endogenous MYB75 promoter (myb75− mutant background), the primers used to detect MYB75 transcripts were F-5ʹ TCCTAGAGGAAAGCCAAGAGG and R-5ʹ- CTAATCAAATTTCACAGTCTCTCCATCG, while Actin1 (AT3G18780) reference gene was detected with the forward F-5ʹ CCACCTGAAAGGA AGTACAGTG and reverse R-5ʹ GTGAACGATTCCTGGACCTG primers. Semi-quantitative RT-PCR was performed using MangoMix™ (Bioline), by running the PCR reaction for 25 cycles. The resulting bands were resolved by gel electrophoresis (1% agarose gel) and relative band intensity was quantified using ImageJ software: the relative amount of MYB75 cDNA in each sample was obtained by evaluating MYB75 band intensity, normalized to Actin1 signal. As for anthocyanin quantification, each sample consisted of tissue from 3–4 plants belonging to an individual line. The number of samples used for each genotype were as follows: four samples for each Nossen WT and myb75− controls; five samples for MYB75T126A genotype (one sample for each line); six samples for MYB75T131A genotype (one sample for each line); three samples for MYB75T126/131A genotype (one sample for each line).
Expression of recombinant 3xHA:MYB75 in Arabidopsis plants overexpressing different phosphovariants of MYB75 from the constitutive 35S promoter, (35Spr:3xHA:MYB75 gene) was detected using the forward primer F-5ʹ CAGTGCAGCGCTGTTATCACAAG, which binds to the HA-tag portion of the construct, and the reverse primer R-5ʹ- CTAATCAAATTTCACAGTCTCTCCATCG, which binds to a region of MYB75 coding sequence. PCR reaction was run for 35 cycles, using the MangoMix™ (Bioline), in conjunction with the primers listed above and the PCR products were analyzed by gel electrophoresis (1% agarose gel).
Scoring germination
Germination rate in MYB75pr:MYB75 cDNA lines was scored in homozygous T3 plants, including six independent MYB75pr:MYB75T131AcDNA and four MYB75pr:MYB75T131EcDNA lines, along with myb75− mutant and Nossen WT controls. Seeds were surface sterilized, plated on ½ MS-agar plates and vernalized for 3 days at 4ᵒC, then placed under light fluence of ~30 µmol m-2 s − 1 and 16 h light/8 h dark photoperiod, at 21ᵒC. Ten days after exposure to light, the germination percentage was scored for each plate by counting the number of unresponsive seeds and the number of germinated plants. Plants which were counted as germinated included any seed that showed signs of an emerging seedling. Student T-test was performed to evaluate statistical significance of differences in the average germination rates between different genotypes.
Flowering time of Arabidopsis plants overexpressor different recombinant MYB75 phosphovariants
Heterozygous T2 Arabidopsis plants carrying 35Spr:3xHA:MYB75 gene constructs were used in this assay, including three lines for 35Spr:3xHA:MYB75WT (L11, L12, L14), and 35Spr:3xHA:MYB75T131A; (L1, L22, L23), and four lines for 35Spr:3xHA:MYB75T131E; (L2, L4, L5, L9). Seeds of transgenic lines, along with Col-0 WT were germinated simultaneously on Petri dishes containing ½MS agar medium, supplemented with 30 µg/mL hygromycin (except for Col-0 WT controls). Seedlings were grown on petri plates for ten days, at which point they were transferred to soil in a growth chamber (see plant growth conditions). Individuals representative of each line were photographed on the same day, when plants were 5 weeks old and again at 7 weeks, to capture differences in timing of flowering and other developmental transitions, between different phosphovariant genotypes.
Histochemical analysis of stem cross-sections
The lower 5 cm of the stems of 7- and 8-week-old Arabidopsis plants were hand-sectioned. Lignin staining was performed by submerging stem sections in phloroglucinol (Sigma) solution (2% w/v phloroglucinol in 95% ethanol) and adding concentrated HCl dropwise. After 5 min incubation in phloroglucinol-HCL, the sections were washed three times in distilled water, mounted on slides (in distilled water) and imaged immediately. Additional stem sections were stained with toluidine blue by submerging the sections in 0.02% toluidine blue solution for 1–2 min and rinsing in distilled water three times. The toluidine-blue stained sections were then mounted on slides in distilled water and imaged immediately. All imaging was performed using a Leica Mecatron Precision DMR compound microscope, with a mounted digital camera, Canon EOS Rebel T5.
Detection of recombinant 3xHA-tagged MYB75 protein in Arabidopsis stems
The lower ~5 cm of Arabidopsis stems were collected from 7-week-old plants expressing 3xHA-tagged MYB75. The stems were ground to a fine powder and the lyophilized material was immediately resuspended in 2xSDS loading buffer. SDS PAGE and Western blot detection of MYB75 was performed as described previously1 using a 1:1000 dilution of a primary anti-HA antibody (high affinity rat monoclonal, clone 3F10, Roche), and a 1:5000 dilution of a secondary antibody conjugated with Horseradish peroxidase (HRP) (goat anti-rat IgG-HRP, Santa Cruz Biotech Sc-2032). Chemiluminescence signal detection was performed using ECL Western blot reagent (Pierce ™ ECL plus) and recorded on photosensitive film (Bioflex Blue Lite EC, Mandel Scientific).
Results
Complementation of anthocyanin biosynthesis in Arabidopsis myb75− null mutants, by different MYB75 phosphovariants
Our initial approach to understanding how phosphorylation might affect MYB75 function, was to create MYB75 phosphovariant lines by transforming Arabidopsis myb75− null mutants (Nossen background) with each MYB75 phosphovariant cDNA, under the control of the endogenous MYB75 promoter. In this complementation experiment the ability of each MYB75 phosphovariant to drive anthocyanin production was tested simultaneously with promoter functionality, by stressing the adult transgenic plants with continuous light (a known driver of MYB75 expression), for 48 h before measuring total anthocyanin content in mature rosette leaves. Complementation of the anthocyanin deficiency typical of myb75− null mutant plants, by each phosphovariant, was evaluated by comparing total anthocyanin levels in transgenic lines to the levels found in Nossen wild type plants, grown under the same conditions. Analysis of T2 plants generated with each phosphovariant revealed that MYB75T126A -expressing plants grown under light stress were able to accumulate average anthocyanin levels, comparable to those seen in Nossen wild type plants. In contrast, plants carrying MYB75T131A and MYB75T126/131A phosphovariants showed only marginal levels of anthocyanin production, which did not differ significantly from those seen in the parental myb75− mutant phenotype, deficient in anthocyanin production (Figure 1a). Although these data would suggest that certain phosphovariant forms of MYB75 fail to drive anthocyanin production, when we examined anthocyanin levels in individual lines, as well as recombinant MYB75 gene expression, it became apparent that lines which failed to produce anthocyanins had only negligible expression of recombinant MYB75 (Figure 1b–d). These data suggest that phosphorylation status at T126 does not affect the ability of MYB75 to drive anthocyanin expression, since all MYB75T126A lines which expressed recombinant MYB75 were able to complement anthocyanin deficiency in the myb75− mutant phenotype. Importantly, all lines where T131 was mutated, (including MYB75T131A and MYB75T126/131A) were displaying very low levels of recombinant MYB75 expression (Figure 1).
Figure 1.
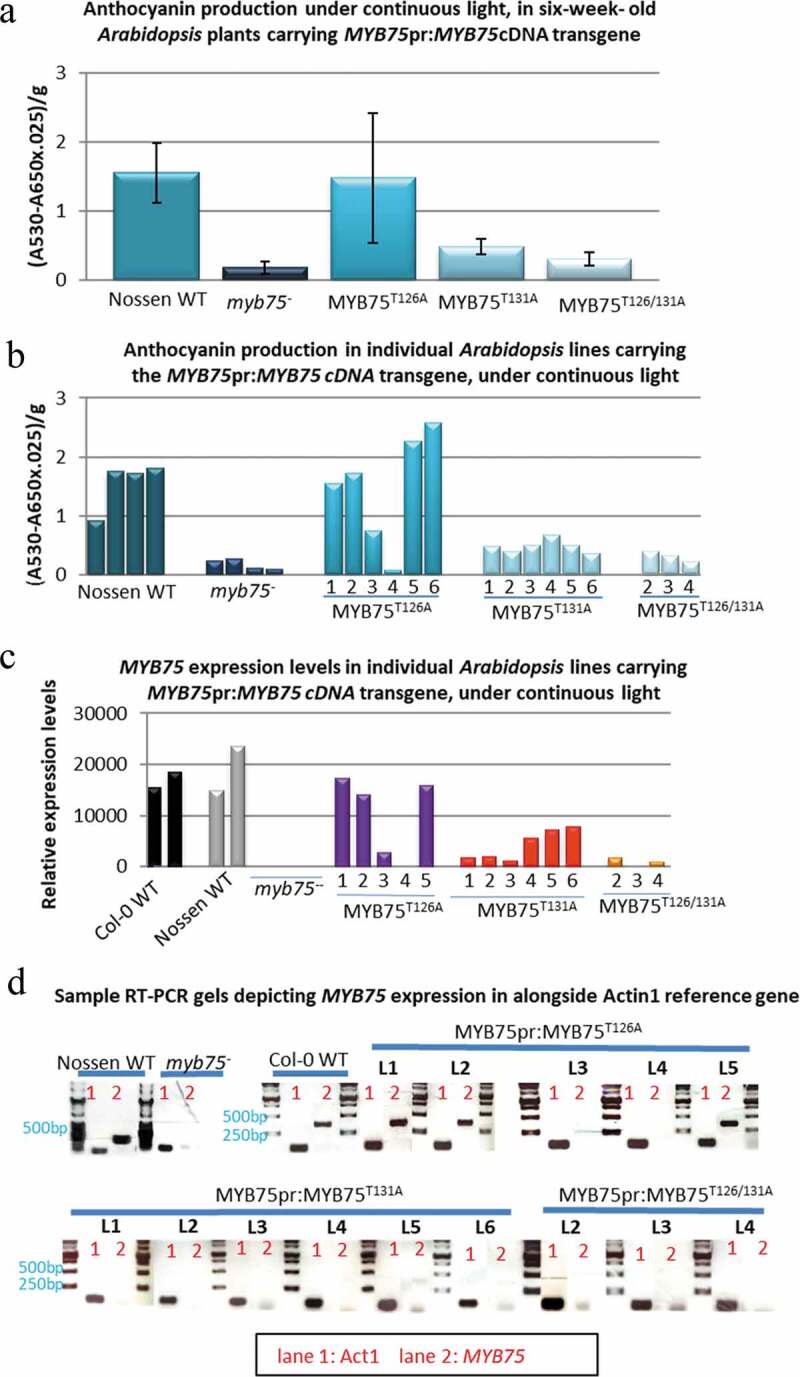
Complementation of anthocyanin biosynthesis in Arabidopsis myb75− null mutants (Nossen), by different MYB75 phosphovariants, driven by the endogenous MYB75 promoter. The ability of each phosphovariant to complement anthocyanin production in myb75− mutants was evaluated in 6-week-old heterozygous T2 plants, exposed to continuous light, for over 48 h. Total anthocyanin (average for each genotype) was quantified as described in materials and methods, error bars represent ±SD. (a) Anthocyanin production was recovered in MYB75pr:MYB75T126A genotype, but not in MYB75pr:MYB75T131A or MYB75pr:MYB75T126/131A mutants. (b) Examination of anthocyanin levels in individual lines revealed that only lines 1,2,5 and 6 from MYB75pr:MYB75T126A genotype produced anthocyanin levels comparable to Nossen WT. (c) Gene expression analysis, by semi-quantitative RT PCR revealed that transgenic lines which showed anthocyanin complementation were expressing recombinant MYB75 at levels comparable to wild type plants, while lines that failed to produce WT levels of anthocyanin had negligible levels of recombinant MYB75 expression. (d) Sample gels of RT-PCR results depicting expression of MYB75 in different MYB75pr:MYB75 lines, along with Nossen WT, myb75−, and Col-0 WT controls. Lane1-Actin1 control, Lane2-MYB75.
Impaired germination in Arabidopsis plants expressing MYB75T131A and MYB75T131E from the endogenous MYB75 promoter
An initial screen of large numbers (approximately 100 in total) of T1 plants expressing either MYB75T131A or MYB75T131E from the endogenous MYB75 promoter established that expression of MYB75 phosphovariants in which T131 was mutated to either alanine (T131A) or glutamic acid (T131E), were failing to complement the low anthocyanin phenotype of the myb75− parent plants because the transgenic lines displayed very low levels of MYB75 transgene expression. One possible explanation for this is that strong expression of either phosphovariant is detrimental to early developmental stages, such as embryogenesis or germination, in these transgenic lines, resulting in artificial selection of plants that express only low levels of recombinant MYB75. Having noted that MYB75pr:MYB75T131A and MYB75pr:MYB75T131E plants showed low germination rates, we wanted to quantify this phenomenon, in order to test our hypothesis that MYB75 expression and phosphorylation status can have an impact on early development. For this purpose we used seeds from homozygous T3 parent plants, carrying MYB75pr:MYB75 cDNA (MYB75T131A and MYB75T131E phosphovariants), in a myb75− mutant background (Nossen ecotype), including six independent MYB75pr:MYB75T131A and four independent MYB75pr:MYB75T131E lines. Both Nossen WT and myb75− mutants showed germination rates close to 100%. In contrast, lines carrying either MYB75pr:MYB75T131A or MYB75pr:MYB75T131E transgene displayed severely impaired germination (26.8% and 62%, respectively; Figure 2). It is challenging to assess this phenotype in the absence of MYB75pr:MYB75WT transgenic control, nevertheless when the two phosphovariants are compared to each other, it is apparent that germination is significantly lower in MYB75T131A-expressing plants than in MYB75T131E lines, suggesting that phosphonull MYB75T131A has a greater negative effect on germination than the phosphomimic MYB75T131E version. These low germination rates support the idea that our failure to isolate transgenic lines expressing MYB75T131A and MYB75T131E was caused by artificial selection for plants that express lower levels of these recombinant MYB75 phosphovariants. In addition to low germination rates, many plants from these transgenic lines displayed arrested development at early stages and did not survive past the seedling stage (data not shown). This phenomenon was very challenging to quantify, as there was no clear phenotype, timing or percentage of developmental arrest in these seedlings; nevertheless, our observations suggested that these transgene variants could be contributing to developmental abnormalities at the embryonic and early seedling stages, and prompted further examination of developmental phenotypes in plants expressing different MYB75 phosphovariants.
Figure 2.
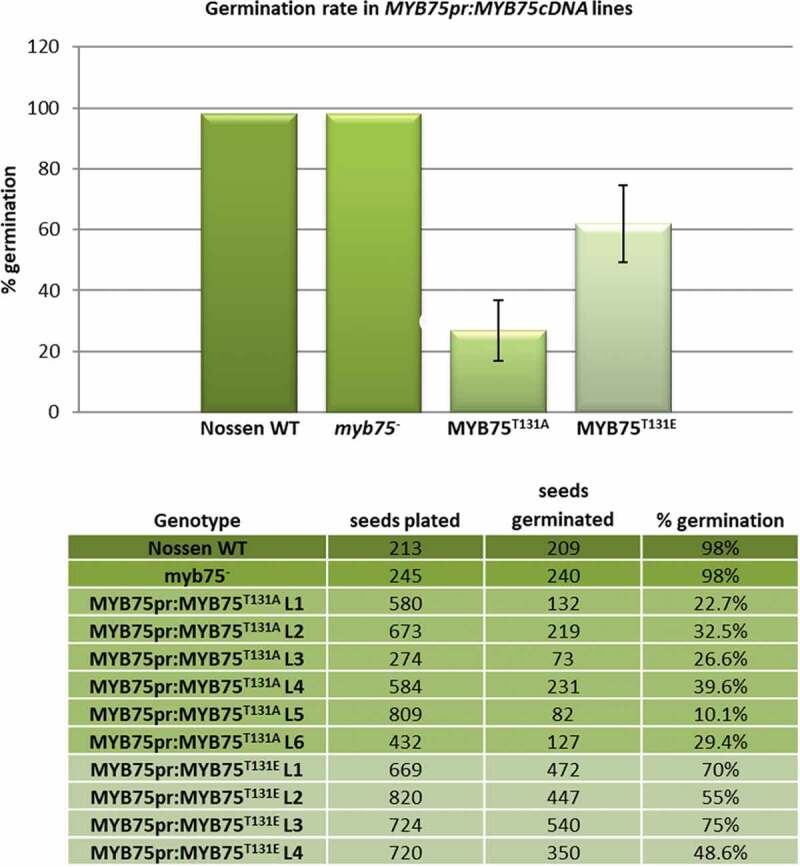
Impaired germination rates in MYB75pr:MYB75T131A and MYB75pr:MYB75T131E transgenic plants. Seeds from homozygous T3 plants were plated on ½ MS agar medium along with myb75− and Nossen WT controls. After vernalization, the plates were placed under light fluence of ~30 µmol m-2 s − 1 and a 16 h light/8 h dark photoperiod, at 21°C. Germination rate was scored ten days after transferring the plates into light. Percentage germination was determined for each line by counting the total number of seeds and the total number of germinated plants. Percentages of germinated plants were averaged for each genotype, error bars represent ±SD. Germination rates were 26.8% (SD = 9.99) for MYB75pr:MYB75T131A plants and 62% (SD = 12.62) for MYB75pr:MYB75T131E. These data indicate that MYB75pr:MYB75T131A plants have a significantly lower germination rate than either Nossen WT, myb75− or MYB75pr:MYB75T131E transgenic lines. T test <0.05
Overall our approach, of expressing MYB75 from the endogenous promoter did not yield results which could help us understand how phosphorylation affects MYB75 function, since not all phosphovariant lines could be isolated. For this reason, we shifted our approach to using overexpressor lines, where the full-length MYB75 gene is driven by a strong constitutive 35S promoter, as described in our previous work.1 Furthermore, we narrowed our focus to the single phosphorylation site T131, as in vitro phosphorylation data indicated that this is the primary site for phosphorylation by MPK3, MPK4, MPK6 and MPK11.1
Flowering time and senescence are delayed in Arabidopsis plants over-expressing MYB75T131E
Arabidopsis plants overexpressing different MYB75 phosphovariants (35Spr:3xHA:MYB75WT, 35Spr:3xHA:MYB75T131A, and 35Spr:3xHA:MYB75T131E) were grown side by side with Col-0 WT, in the same growth chamber under regular photoperiod, 16 h light/8 h dark (see materials and methods). Wild type Col-0 plants began to bolt and flower after four-five weeks of growth. Transgenic 35Spr:MYB75WT plants showed differences in flowering time between different lines; L11 displayed flowering time comparable to Col-0 WT plants, while L12 and L14 produced an inflorescence stem and flowers before Col-0 WT of the same age (Figure 3a). This apparent acceleration in development persisted after bolting, as 35Spr:MYB75WT L12 and L14 displayed abundant formation of lateral branches and flowers, at a stage when Col-0 WT and 35Spr:MYB75WT L11 plants had only developed the main inflorescence (Figure 3a). Similar developmental acceleration was observed in 35Spr:MYB75T131A L1, L22 and L23. Early bolting, flowering and development of numerous lateral branches was consistently observed in all 35Spr:MYB75T131A plants, with no observable developmental differences between lines of this genotype. All 35Spr:MYB75T131E plants displayed a delay in bolting and flowering compared to Col-0 WT (Figure 3a). The developmental delay observed in 35Spr:MYB75T131E lines was also evident in later stages of development. At 7 weeks of age Col-0 WT as well 35Spr:MYB75WT and 35Spr:MYB75T131A lines ceased to flower, had formed mature siliques, and were beginning to show signs of senescence manifested as yellowing of the rosette leaves, and desiccating siliques. On the other hand, all 35Spr:MYB75T131E lines displayed abundant flowers at 7 weeks, with only a few young siliques forming at this stage, and healthy green tissue throughout the plant, with no signs of senescence (Figure 3c). Despite the variability between individual lines in 35Spr:MYB75WT plants, a clear trend emerged between the phosphovariant plant lines: development was accelerated in plants over-expressing MYB75T131A and retarded in those over-expressing MYB75T131E (Figure 3b).
Figure 3.

Developmental differences in bolting and flowering time as well as onset of senescence in plants over-expressing different phosphovariants of MYB75 (WT, T131A and T131E) from a constitutive CAMV35S promoter, in the Col-0 WT background. (a) Among 35Spr:MYB75WT lines, L 11 bolted and flowered at a rate comparable to Col-0 WT (untransformed control), while L12 and L14 displayed early bolting and flowering compared to Col-0 WT controls. All 35Spr:MYB75T131A lines bolted and flowered early compared to Col-0 WT. Conversely, 35Spr:MYB75T131E plants showed delayed bolting and flowering. (b) The T131A mutation led to earlier bolting and flowering, while T131E mutation appears to slow down the transition from vegetative to reproductive growth. (c) Furthermore, 35Spr:MYB75T131E lines displayed delayed maturation and onset of senescence compared to all recombinant genotypes analyzed and Col-0 WT controls
Ectopic vessel development in stems of mature Arabidopsis plants over-expressing different MYB75 phosphovariants
In addition to regulating anthocyanin production within MYB/bHLH/WDR complexes, MYB75 has also been shown to interact with KNAT7, a member of the Knotted Arabidopsis Thaliana Protein family.15 KNAT7 activity negatively affects lignin deposition and secondary cell wall thickness in interfascicular fibers16,19 and appears to act synergistically with MYB75 in inflorescence stems and the seed coat by down-regulating lignin biosynthesis.16 Because of this involvement of MYB75 in cell wall development, we wanted to determine if phosphorylation status of MYB75 at T131 had an impact on vascular architecture of Arabidopsis stems. Stem sectioning experiments were performed on 7-week-old and 8-week-old 35Spr:MYB75 plants, in which we examined the distribution of vascular and supporting tissues in the stem cross-sections.
At 8-weeks-old, the developmentally delayed 35Spr:MYB75T131E lines had formed mature siliques and were beginning to show signs of senescence, while Col-0 WT, 35Spr:MYB75WT and 35Spr:MYB75T131A plants had reached full senescence: rosettes leaves and siliques were beginning to desiccate, but the stems were still green. All plants were watered in the usual cycle, to ensure that the stems did not dry out, which could lead to vascular collapse. The lower portions of the main inflorescence stems (approximately 5 cm from the base) were hand-sectioned, and the stem sections were stained with phloroglucinol-HCl. As expected, wild type Arabidopsis stems showed a normal tissue organization, with vessel elements appearing as clusters of cells with dark reddish-brown-staining cell walls, restricted to the vascular bundles (Figure 4a). On the other hand, transgenic plants overexpressing MYB75 showed what appear to be vessel-like cells in the interfascicular region, with dark reddish-brown staining walls, and a larger cell diameter compared to the surrounding fibers (Figure 4b-d). In 35Spr:MYB75WT stems the phenotype was relatively mild, with only a few isolated dark cell files in the interfascicular region, made up of cells with a slightly larger diameter than the surrounding fibers (Figure 4b). In 35Spr:MYB75T131A plants, these darker staining cells were more prominent, with a diameter comparable to vessel elements found in the vascular bundles; however, only a few such cells, or very small cell clusters, could be seen in the interfascicular region (Figure 4c). In contrast, in plants over-expressing the phosphomimic MYB75T131E, abundant development of vessel elements could be observed in the interfascicular region, with large cell clusters, stretching between the vascular bundles. In some cases, these prominent clusters of large, dark-staining cells spanned the entire length of the interfascicular arc (Figure 4d).
Figure 4.
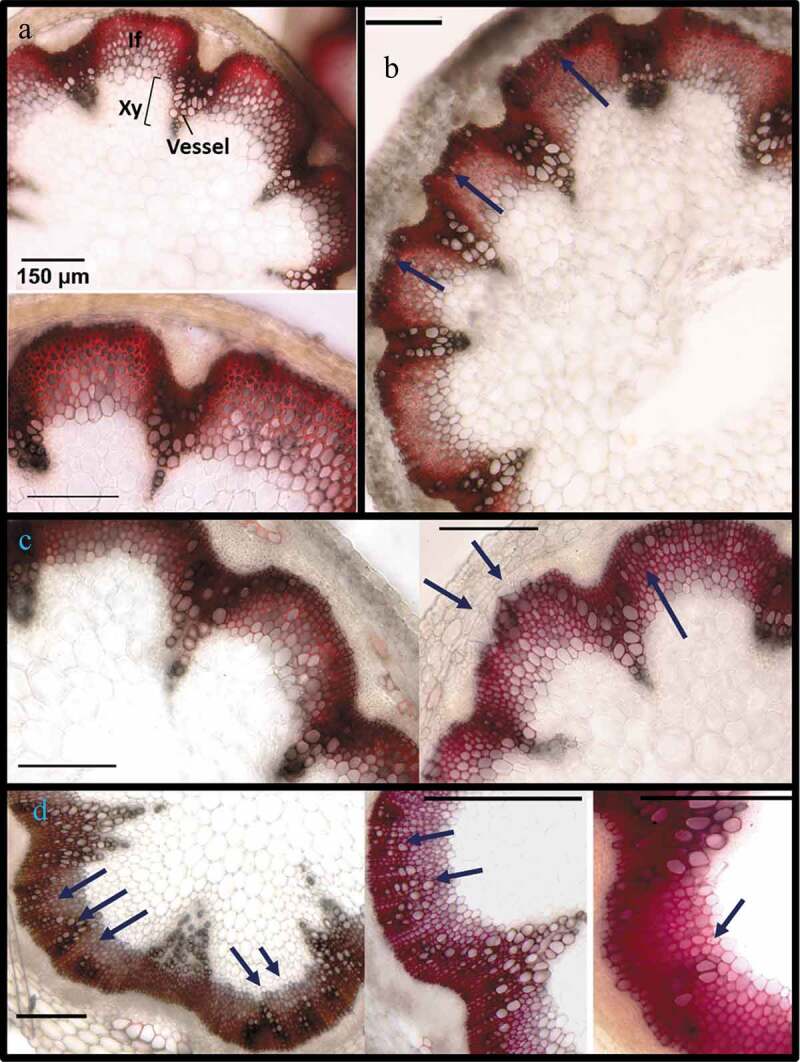
Ectopic vessel development in stems of 8-week-old Arabidopsis plants over-expressing different phosphovariants of MYB75. Stems from 8-week-old plants were harvested and the bottom 5 cm of each stem was hand sectioned and stained in phloroglucinol-HCl. (a) Stem sections from Col-0 WT plants display normal distribution of vessels, within vascular bundles, where xylem vessels can be seen, as large perforated cells. In phloroglucinol-HCl stained sections, vessel walls appear dark reddish-brown, while interfascicular fibers are bright red/fuchsia arcs between the vascular bundles. (b) 35Spr:MYB75WT lines displayed relatively normal stem tissue organization, with a few large cells resembling vessel elements at the periphery of the interfascicular arc. (c) In 35Spr:MYB75T131A plants, large isolated cells or short cell files, resembling vessels can be seen in the interfascicular region. (d) In 35Spr:MYB75T131E plants prominent clusters of vessels could be seen in the interfascicular region
These stem development phenotypes showed some variability within the population of each phosphovariant genotype; some of the transgenic stems examined did not show any abnormal tissue distribution at all. However, about 50% of the stems sectioned in each genotype (35Spr:MYB75WT, 35Spr:MYB75T131A and 35Spr:MYB75T131E) showed at least some ectopic vessel production, with ~25-30% of the stems examined for each transgenic line displaying more extreme phenotypes, with prominent vessel cell-files in the interfascicular region of MYB75WT and MYB75T131A plants (Figure 4b, c) and extensive vessel clusters, spanning the entire interfascicular arc in MYB75T131E stems (Figure 4d). These results indicate that over-expression of MYB75 can lead to ectopic differentiation in the interfascicular region of cells that appear to be vessels, and that over-expression of the MYB75T131E phosphovariant is more effective at driving this process.
At 8 weeks of age, Arabidopsis plants can undergo secondary growth in their lower stems.21,22 Signs of this secondary growth were observed in all genotypes, including Col-0 WT, with the appearance of cells around the periphery of the stem, with dark staining cell walls, reminiscent of vessel elements (Figure 4a). To better distinguish this normal secondary growth, present in all genotypes, from what we believed to be ectopic vessel formation in MYB75 overexpressing lines, we performed a second round of sectioning using younger plants, (7-weeks-old), in hopes of capturing ectopic vessel development before the onset of secondary growth. In addition to phloroglucinol-HCl staining, we used toluidine blue stain, which offers a better color contrast between vessels and fibers. In toluidine blue-stained sections, interfascicular fiber walls appear bright turquoise, whereas the xylem vessels are greenish gray.
Histochemical analysis of stems from 7-week-old plants indicates that at this stage in development Col-0 WT plants do not show significant signs of secondary growth (Figure 5a) Furthermore, ectopic vessel differentiation was not observed in 35Spr:MYB75WT plants (Figure 5a, b), and only a mild phenotype was seen in 35Spr:MYB75T131A plants (Figure 5a). On the other hand, 35Spr:MYB75T131E plants showed prominent ectopic vessels developing as bundles or cell files in the interfascicular arcs (Figure 5a, b). In toluidine blue-stained cross-sections, the vascular bundles appear greenish gray, in contrast to the bright blue-green interfascicular fibers (Figure 5b). Cell clusters with greenish gray walls can be seen in the interfascicular arcs of 35Spr:MYB75T131E stems, but not in the 35Spr:MYB75WT plants (Figure 5b). As in the phloroglucinol-stained sections, these unusual cells have a larger diameter than the surrounding interfascicular fiber cells and are similar in appearance to vessels found in the vascular bundles. We therefore concluded that these are, in fact, ectopic vessel elements that develop in the interfascicular region of MYB75 over-expressing transgenic plants, most prominently in 35Spr:MYB75T131E lines. Due to the limited availability of heterozygous T2 plants, about a dozen plants were used for each genotype in each round of sectioning. Nevertheless, within this sample pool 35Spr:MYB75T131E plants consistently showed more prominent ectopic vessel development than the other genotypes. Western blot analysis and RT-PCR confirmed that the transgene was being expressed and that the corresponding recombinant protein was being produced in the stems of 35Spr:MYB75 plants (Figure 5c).
Figure 5.
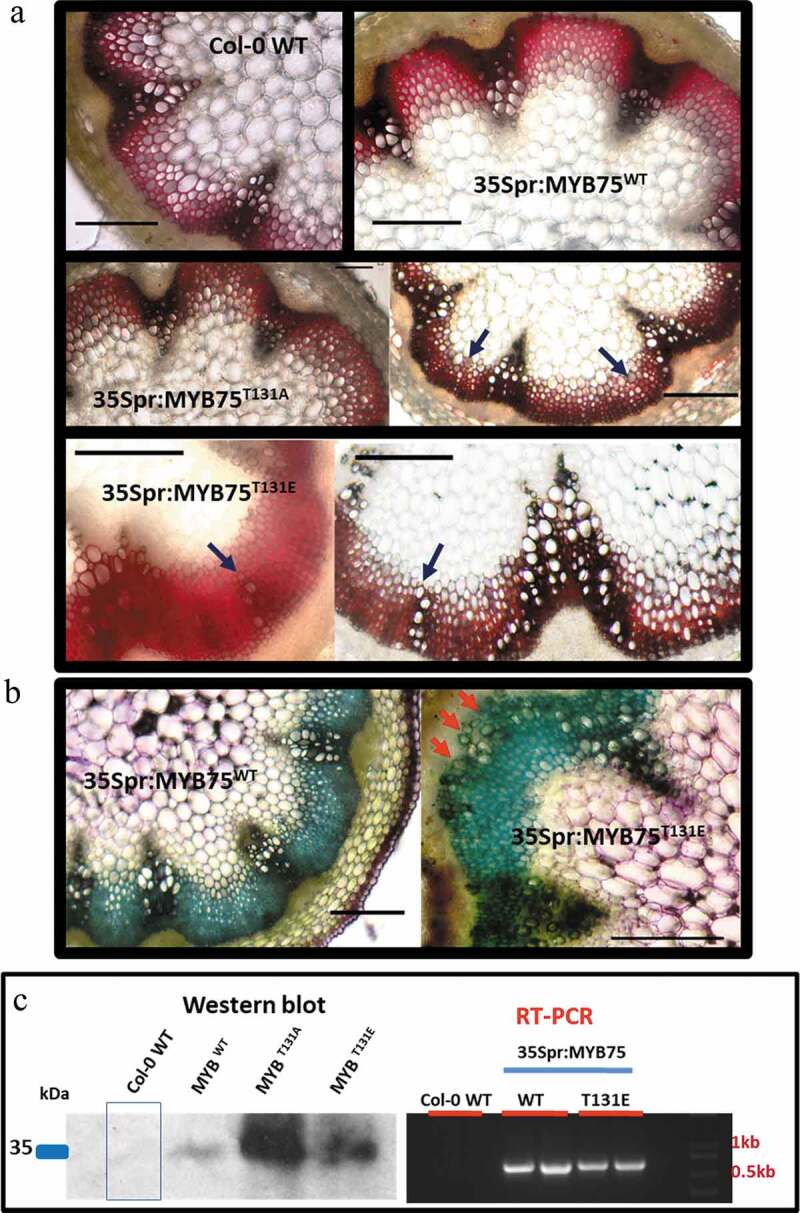
Ectopic vessel development in stems of 7-week-old Arabidopsis plants over-expressing different phosphovariants of MYB75. Stems were harvested from 7-week-old plants and the bottom 5 cm of each stem was hand sectioned and stained in phloroglucinol-HCl or toluidine blue. Neither (a), Col-0 WT nor 35Spr:MYB75WT plants showed any signs of ectopic vessels in stem sections at this stage, while 35Spr:MYB75T131A plants displayed a mild phenotype, with isolated cells or small clusters of cells resembling vessel elements in the interfascicular arc. Conversely 35Spr:MYB75T131E display prominent cell files of vessels in the interfascicular region. (b) The presence of vessel elements in the interfascicular region of 35Spr:MYB75T131E plants was confirmed using toluidine blue stain; prominent clusters of vessel elements appear as large olive green staining cells in the interfascicular arc of 35Spr:MYB75T131E plants, but not observed in 35Spr:MYB75WT lines. (c) Western blot and gene expression analysis confirms that recombinant MYB75 is expressed in the stems at this stage
Discussion
Although flavonoids and anthocyanins are considered ‘secondary’ metabolites in plants, typically associated with stress response, emerging research suggests that these compounds have a profound impact on development. Anthocyanins and some flavonoids are sunscreens, as such their levels and composition in the epidermis can influence how much visible light reaches the photosynthetic machinery, which can ultimately affect light harvesting, photosynthesis and photomorphogenesis.23 Flavonols also have an established role in development, due to their ability to modulate auxin flow,24 and thereby influence global gene expression and organ differentiation. The influence of flavonols on development is well exemplified in the analysis of transparent testa mutants, which are deficient in different flavonoid biosynthesis enzymes and regulatory proteins. These mutants display a range of phenotypic changes in root and shoot architecture, including changes in the number, position and initiation site of root hairs, and lateral roots, inflorescence height and number, and changes in silique density.25 Experiments employing DBPA (diphenylboric acid 2‐aminoethylester) staining unveiled cell-specific distribution patterns of quercetin and kaempferol derivatives concentrated in transition zones and actively growing tissues, of Arabidopsis seedlings and mature plants.26,27 Flavonols, including quercetin, kaempferol and their glycoside derivatives, can negatively impact auxin flow24,26 by directly binding to auxin ABCB1 and ABCB19 transporters.18,28 Additionally, flavonols can influence the localization of PIN efflux channels, which mediate directional auxin transport, and drive auxin canalization required for vascular differentiation.29 Crucially, changes in spatial-temporal distribution of specific flavonol molecular species correlated with alterations in auxin canalization and direction of auxin flow,26 a primary driving mechanism for development and organogenesis.
Two transcriptional regulators and interacting partners of MYB75, Teosinte branched 1 cycloidea and PCF transcription factor 3 (TCP3, AT1G53230) and Squamosa Promoter binding protein-like 9 (SPL9, AT2G42200), have an established role in developmental processes connected to flavonoid metabolism and the hormone auxin. TCP3 positively regulates complex formation between MYB75, TT8/GL3/EGL3 and TTG1, leading to increased anthocyanin biosynthesis, and can also bind MYB11 (AT3G62610), MYB12 (AT2G47460) and MYB111 (AT5G49330), enhancing their ability to drive transcription of early flavonoid biosynthesis genes.30,31 In Arabidopsis plants over-expressing TCP3, increased levels of flavonols coincide with inhibition of auxin-response as well as reduced abundance and altered localization of PIN1 auxin efflux channels.31 TCP3-overexpressing plants showed widespread developmental abnormalities such as changes in seed color and size, high seedling mortality, altered vessel development, organ shape, root growth, reduced apical dominance and more.31 Crucially, these phenotypes were abolished when TCP3-overexpressors were crossed with tt4 mutants, lacking a functional chalcone synthase enzyme, essential for flavonoid biosynthesis, suggesting flavonoid overaccumulation was the underpinning mechanism of these phenotypes.31 In contrast to TCP3, SPL9 interferes with MYB75-bHLH-TTG1 complex formation, and therefore negatively impacts anthocyanin production.20 SPL9 over-expression promotes flavonol and inhibits anthocyanin accumulation: in this manner spatial-temporal distribution of SPL9 modulates the proportion of anthocyanins to flavonols in developing stems. The relative proportions of these classes of compounds in Arabidopsis stems can affect certain aspects of development, including time of floral transition, in which SPL proteins have been implicated.20,32
Neither myb75− null mutant33 nor the PAP-1D overexpressor34 have ever been reported to display any developmental phenotype analogous to the phosphovariant mutants we have analyzed. The unique aspect of our findings is that the expression of either the MYB75T131A or MYB75T131E phosphovariant appears to have greater impact on development than the absence of MYB75 (myb75−) or the overexpression of wild type protein. It remains to be seen whether MYB75 is influencing these various developmental processes directly, or more indirectly, through control of spatial-temporal distribution of specific flavonol biochemical species. Nevertheless, the phenotypes we described above clearly demonstrate that this protein, and its phosphorylation status, play a vital role in development.
Germination
In this study we examined germination rate, in MYB75pr:MYB75 plants, as this was an easily quantifiable phenotype; however, the high seedling mortality and developmental arrest among these lines point to developmental perturbations beyond simply failure to germinate.
According to gene-expression data in the eFP Browser, Arabidopsis plants display the highest levels of MYB75 expression in senescing leaves.35,36 On the other hand, a recent analysis of the Arabidopsis embryonic transcriptome found that MYB75 expression levels spike at the early torpedo and bent cotyledon stages.37 An earlier study, which employed laser capture microdissection to analyze tissue-specific gene expression in the Arabidopsis embryos, revealed a detailed picture of the MYB75 expression pattern: at the heart stage, MYB75 expression levels were elevated in cotyledons, compared to other organs, while at the torpedo stage MYB75 expression was concentrated in the root apical meristem.38 These highly specific spatial-temporal distribution patterns suggest that MYB75 plays a role in embryonic development, and that the low germination rate and high seedling mortality observed in MYB75pr:MYB75cDNA lines (T131A and T131E) could be a result of altered MYB75 function or protein distribution.
MYB75 (along with MYB123/TTG2) participates in flavonoid biosynthesis during embryogenesis, where proanthocyanidins are the end product.39 Proanthocyanidins (PA’s) accumulate in different parts of the developing seed coat, including the micropyle, chalaza and the endothelium (an inner layer of cells in the seed coat, enclosing the developing embryo).40 Recent research indicates that seed coat development can influence cellularization of the endosperm, which has a direct impact on embryogenesis.41 The authors suggest that part of this mechanism involves modulation of auxin fluxes in the endosperm by flavonoid accumulation in the endothelium.41 Although the impact of flavonols on auxin transport is typically not lethal in adult plants, at early stages of development small perturbations in auxin flow can have catastrophic effects.42 Since auxin plays a central role in embryogenesis and seedling development, it is possible that perturbations in auxin flow, driven by changes in flavonoid profiles, led to developmental abnormalities in MYB75 phosphomutant lines, and in some cases arrest at these early developmental stages. Developmental arrest and death at early seedlings stages, as well as developmental abnormalities, including fused cotyledons, wavy leaves and irregular vasculature, have been documented in Arabidopsis plants overexpressing TCP3, and linked to altered flavonol production and auxin flow.31 A great deal more research is needed in order to explain our findings, such as determining how each phosphovariant version of MYB75 protein is distributed in the embryo. It would be important to assess impact of MYB75 phosphorylation on proanthocyanidin production in the seed coat, and on the expression of downstream genes, associated with these early stages of development. Nevertheless, our finding showing that MYB75 phosphorylation status has an impact on germination is congruent with previous research which suggests that flavonoids play an important role in early plant development.
Flowering time
The transition from vegetative to flowering stage is a complex process, driven by a vast regulatory network that integrates signals from multiple plant hormones and environmental cues such as light, photoperiod, temperature, and the circadian clock. In order to interpret how each MYB75 phosphovariant might be affecting this process, we can look at the parallels between our observations and phenotypes described in certain transparent testa mutants, deficient in various enzymes and regulatory proteins required for flavonoid biosynthesis. Inflorescence elongation rates were faster in mutants which accumulate flavonols in the root-shoot junction, including transparent testa 8 (tt8, AT4G09820) transparent testa glabra 1 (ttg1, AT5G24520) and transparent testa glabra 2 (ttg2, At2G37260), and slower in those that did not, namely transparent testa 4 (tt4, AT5G13930), transparent testa 6 (tt6, At3G51240) and transparent testa 7 (tt7, AT5G07990).26 Lesions in the tt6 and tt7 genes resulted in an early bolting phenotype.26
Changes in bolting time among transparent testa mutants would suggest that the acceleration and delay in bolting times observed in MYB75T131A and MYB75T131E-overexpressing plants, respectively, are likely to be at least in part caused by changes in distribution of flavonoid molecular species throughout the plant, at these stages of development. However, besides bolting time, MYB75 phosphomutant-overexpressing plants displayed changes in developmental timing across all stages of the mature plant development, including flowering time, and onset of senescence, which suggest more profound systemic changes in the plant’s responses to developmental cues. Delayed senescence in 35Spr:MYB75T131E lines is congruent with our previous findings showing that the expression of photosynthesis-related genes is increased in these plants.1 Upregulation of these photosynthesis genes is typically associated with photomorphogenesis, and active chloroplast development in green tissues. Conversely these genes are down-regulated during senescence, therefore their upregulation in 35Spr:MYB75T131E plants, implicates this phosphovariant in suppression of senescence.
Overexpression of MYB75T131E was earlier shown to influence a much wider spectrum of genes than MYB75WT or MYB75T131A, including those genes that can impact global gene expression changes.1 For instance, Histones H3.1 and H2A.11/H2A.Z, upregulated in 35Spr:MYB75T131E plants, and ATNAC3 (OSR1/ATNAC3, AT3G29035), a global regulator of senescence was downregulated in these lines.1 Decreased expression of the NAC3 (OSR1) transcription factor could account for the increased expression of photosynthetic genes exclusively observed in 35Spr:MYB75T131E plants, since an inverse correlation has been observed between NAC3 expression levels and chlorophyll content,43 which is dependent on expression of photosynthesis- and chloroplast-associated genes.
Vascular development
As a suppressor of biosynthesis of lignin and other secondary cell wall components, MYB75 has an established role in vascular development.15,16 Since polar auxin flow and canalization can be influenced by flavonols, MYB75 may also play a role in vessel development through control of flavonoid metabolism. To interpret our observations, we look to similar phenotypes reported in the literature. The pin1pin2 double mutants (defective in auxin efflux channels), as well as plants treated with the auxin transport inhibitor, NPA, display disorganized vessel positioning, with numerous xylem vessels differentiating in the interfascicular regions, outside their normal vascular bundles.44 Canalization and drainage of auxin is an essential part of vascular differentiation and continuity. The absence or blockage of auxin efflux carriers results in failure to canalize auxin, leading to spilling of auxin into neighboring cells, which can result in their reprogramming and differentiation into vessel elements. Therefore, the phenotypes that we observed can potentially be a result of reduced polar auxin flow, driven by increased intracellular flavonol levels. All 35Spr:MYB75 plants had elevated flavonol levels compared to Col-0 WT plants,1 and these lines also showed some degree of ectopic vessel formation. Ectopic vessel formation was most pronounced, and apparent in earlier stages of development, only in 35Spr:MYB75T131E lines, possibly because expression of this phosphovariant has a greater positive impact on flavonol production.1
Conclusions
Over the last few decades, our growing understanding of how plants perceive and respond to their environment has been steadily eroding the boundary that distinguishes primary from secondary metabolism, as we uncover the crucial role many secondary metabolites play in development. The relationship between MYB75, development and the hormone auxin is intuitive, yet not well defined. However, our observations are not without precedent since mutations in other transcriptional regulators of flavonoid metabolism (tt8, ttg1 and ttg2) also lead to developmental changes,26 with phenotypes that share features with our 35Spr:MYB75 lines. Spatial-temporal distribution of MYB75 in its various states of phosphorylation is another key question that needs to be addressed, since different phosphorylation states can affect MYB75 protein stability,1,2 and as a result may influence protein distribution. Additional experiments are needed to understand how different phosphovariants of MYB75 are distributed across different tissues and organs throughout development, and how this affects localization of specific flavonoid biochemical species, and in turn auxin canalization and development.
We cannot exclude the possibility that MYB75 has additional transcriptional targets that have not been characterized in the literature, and that the developmental phenotypes we observed result from a combination of changes in auxin transport and differential regulation of gene expression by each phosphovariant. For this reason, it would be important carry out more in-depth studies of global gene expression patterns in these phosphovariant lines across developmental time. Such an undertaking would allow us to look for relationships between genes whose expression was affected differently in each phosphovariant, and the phenotypes we describe here. Additional work is required to fully understand the nature and underlying mechanisms of the developmental perturbations observed in this study. One possible approach is crossing Arabidopsis plants expressing different MYB75 phosphovariants to plants expressing cell-specific reporters, such as enhancer trap lines expressing Green Fluorescent Protein (GFP) in developing vascular tissues, which would enable visualization of vascular perturbations from embryonic to mature stages of plant development.45
The relationship between MYB75 and photosynthetic gene expression described in our previous work1 is particularly interesting, since MYB75 is directly connected to light-driven signals through transcriptional regulation by HY5, and MYB75 protein turnover mediated by COP/SPA complexes in the dark.46–48 The regulation of anthocyanin production through MYB75 is an important ‘dimmer switch’ that dictates how much light reaches the photosynthetic machinery. A recent study depicting MYB75 phosphorylation by MPK4, showed that far-red light was responsible for MPK3, MPK4 and MPK6 activation,2 implicating these MPKs in light signal transduction and photomorphogenesis.49 These observations reinforce the idea that MYB75, being a downstream target of these MPKs could be implicated in light signal transduction.
Understanding the interplay between secondary metabolism and development is of vital importance since crops and medicinal plants are often bred for characteristics (such as color, flavor, as well as nutritional and medicinal value) conveyed by what we consider to be secondary metabolites. High disease susceptibility and low environmental resilience among economically important crops have often been the outcome of irresponsible breeding, aimed at enhancing a single characteristic that is of commercial interest. A better understanding of the relationships between various biochemical and developmental pathways can yield new perspectives that help inform crop breeding programs and eventually lead to more sustainable agricultural practices.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- 1.Kreynes AE, Yong Z, Xiao-Min L, Wong DCJ, Castellarin SD, Ellis BE.. Biological impacts of phosphomimic AtMYB75. Planta. 2020;251(60). PMID:32030477. doi: 10.1007/s00425-020-03350-0. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Wang W, Gao J, Yin K, Wang R, Wang C, Petersen M, Mundy J, Qiu J-L. MYB75 phosphorylation by MPK4 is required for light-induced anthocyanin accumulation in Arabidopsis. Plant Cell 2016;28(11):1–14. PMID:27811015. doi: 10.1105/tpc.16.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang C-S J, Li Y-H, Chen L-T, Chen W-C, Hsieh W-P, Shin J, Jane W-N, Chou S-J, Hu J-M, Somerville S, et al. LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J 2008;54(2):205–219. PMID: 18182030. doi: 10.1111/j.1365-313X.2008.03401.x. [DOI] [PubMed] [Google Scholar]
- 4.Cluis CP, Mouchel CF, Hardtke CS. The Arabidopsis transcription factor HY5 integrates light and hormone signalling pathways. Plant J 2004;38:332–347. PMID:15078335. doi: 10.1111/j.1365-313X.2004.02052.x. [DOI] [PubMed] [Google Scholar]
- 5.Gangappa SN, Botto JF. The multifaceted role of HY5 in growth and development. Mol Plant 2016;9(10):1353–1369. PMID:27435853. doi: 10.1016/j.molp.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshar B, Lepiniec L. TT2, TT8 and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 2004;39(3):366–380. PMID: 15255866. doi: 10.1111/j.1365-313X.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. Regulation of the anthocyanin biosynthesis pathway by the TTG1/bHLH/MYB transcriptional complex in Arabidopsis seedlings. Plant J 2008;53(5):814–827. PMID:18036197. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann MI, Heim MA, Weisshaar B, Uhrig JF. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/Blike bHLH proteins. Plant J 2004;40(1):22–34. PMID:15361138. doi: 10.1111/j.1365-313X.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 9.Bernhardt C, Zhao M, Gonzalez A, Lloyd A, Schiefelbein J. The bHLH genes GL3 and EGL3 participate in an intercellular regulatory circuit that controls cell patterning in the Arabidopsis root epidermis. Development 2005;132(2):291–298. PMID:15590742. doi: 10.1242/dev.01565. [DOI] [PubMed] [Google Scholar]
- 10.Schellmann S, Hulskamp M. Epidermal differentiation: trichomes in Arabidopsis as a model system. Int J of Dev Biol 2005;49(5–6):579–584. PMID:16096966. doi: 10.1387/ijdb.051983ss. [DOI] [PubMed] [Google Scholar]
- 11.Serna L. Epidermal cell patterning and differentiation throughout apical-basal axis of the seedling. J of Exp Bot 2005;56(418):1983–1989. PMID:15967776. doi: 10.1093/jxb/eri213. [DOI] [PubMed] [Google Scholar]
- 12.Serna L, Martin C. Trichomes: different regulatory networks lead to convergent structures. Trends Plant Sci 2006;11(6):274–280. PMID:16697247. doi: 10.1016/j.tplants.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Song SK, Ryu KH, Kang YH, Song JH, Choo YH, Schiefelbein J, Lee MM. Cell fate in Arabidopsis root epidermis is determined by competition between WEREWOLF and CAPRICE. Plant Physiol 2011;157(3):1196–1208. PMID:21914815. doi: 10.1104/pp.111.185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsay NA, Glover BJ. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci 2005;10(2):63–70. PMID:15708343. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Bhargava A, Mansfield SD, Hall HC, Douglas CJ, Ellis BE. MYB75 functions in the regulation of secondary cell wall formation in Arabidopsis inflorescence stem. Plant Physiol 2010;154(3):1428–1438. PMID:20807862. doi: 10.1104/pp.110.162735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhargava A, Ahad A, Wang S, Mansfield D, Haughn GW, Douglas CJ, Ellis BE. The interacting MYB75 and KNAT7 transcription factors modulate secondary cells wall deposition both in stems and seed coat of Arabidopsis. Planta 2013;237(5):1199–1211. PMID:23328896. doi: 10.1007/s00425-012-1821-9. [DOI] [PubMed] [Google Scholar]
- 17.Mehrtens F, Kranz H, Bednarek P, The WB. Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid. Plant Physiol 2005;138:1083–1096. PMID: 15923334. doi: 10.1104/pp.104.058032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchard R, Bailly A, Blakeslee JJ, Oehring SC, Vincenzetti V, Lee OR, Paponov I, Palme K, Mancuso S, Murphy AS, et al. Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis P-glycoproteins. JBC 2006;281(41):30603–30612. PMID:16887800. doi: 10.1074/jbc.M604604200. [DOI] [PubMed] [Google Scholar]
- 19.Li E, Bhargava A, Qiang W, Friedmann MC, Forniers N, Savidge RA, Johnson LA, Mansfield SD, Ellis BE, Douglas CJ. The Class II KNOX gene KNAT7 negatively regulates secondary wall formation in Arabidopsis and is functionally conserved in Populus. New Phytol 2012;194(1):102–115. PMID:22236040. doi: 10.1111/j.1469-8137.2011.04016.x. [DOI] [PubMed] [Google Scholar]
- 20.Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011;23::1512–1522. PMID:21487097. doi: 10.1105/tpc.111.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altamura MM, Possenti M, Matteucci A, Baima S, Ruberti I, Morelli G. Development of the vascular system in the inflorescence stem of Arabidopsis. New Phytol. 2001;151:381–389. doi: 10.1046/j.0028-646x.2001.00188.x. [DOI] [Google Scholar]
- 22.Ragni L, Greb T. Secondary growth as a determinant for plant shape and form. Seminars in Cell and Dev Biol 2018;79:58–67. PMID:28864343. doi: 10.1016/j.semcdb.2017.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. Anthocyanin in vegetative tissues: a proposed unified function in photoprotection. New Phytol. 2002;155:349–361. doi: 10.1046/j.1469-8137.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Munday GL. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 2001;126(2):524–535. PMID:11402184. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buer CS, Djordjevic MA. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J Exp Bot 2009;60(3):751–763. PMID:19129166. doi: 10.1093/jxb/ern323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buer CS, Kordbacheh F, Truong TT, Hocart CH, Djordjevic MA. Alterations of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 2013;238(1):171–189. PMID: 23624937. doi: 10.1007/s00425-013-1883-3. [DOI] [PubMed] [Google Scholar]
- 27.Peer WA, Brown DE, Tague BE, Muday GK, Taiz L, Murphy AS. Flavonoid accumulation pattern in transparent testa mutants of Arabidopsis. Plant Phys 2001;126(2):536–548. PMID:11402185. doi: 10.1104/pp.126.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailly A, Sovero V, Vincenzetti V, Santelia D, Bartnik D, Koenig BW, Mancuso S, Martinoia E, Geisler M. Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. JBC 2008;283(31):21817–21826. PMID:18499676. doi: 10.1074/jbc.M709655200. [DOI] [PubMed] [Google Scholar]
- 29.Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007;130(6):1044–1056. PMID:17889649. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Stracke R, Ishihara H, Huep G, Mehrtens F, Niehaus K, Weisshaar B. Differential regulation of closely related R2R3-MYB transcription factors control flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 2007;50(4):660–677. PMID:17419845. doi: 10.1111/j.1365-313X.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Zachgo S. TCP3 interacts with R2R3-MYB proteins, promotes flavonoid biosynthesis and negatively regulates the auxin response in Arabidopsis thaliana. Plant J 2013;76:901–913. PMID:24118612. doi: 10.1111/tpj.12348. [DOI] [PubMed] [Google Scholar]
- 32.Wang J-W, Czech B, Weigel D. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 2009;138(4):738–749. PMID: 19703399. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires MYB75/PAP1 gene. Plant Physiol 2005;139:1840–1852. PMID:16299184. doi: 10.1104/pp.105.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 2000;12(12):2383–2393. PMID:11148285. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schӧlkopf B, Weigel D, Lohmann JU. A gene expression map of Arabidopsis thaliana development. Nat Genet 2005;37(5):501–506. PMID: 15806101. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 36.Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLOS One 2007;2(8):e718. PMID:17684564. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann F, Schon MA, Nodine MD. The embryonic transcriptome of Arabidopsis thaliana. Plant Reprod 2019;32:77–91. PMID:30610360. doi: 10.1007/s00497-018-00357-2. [DOI] [PubMed] [Google Scholar]
- 38.Casson S, Spencer M, Walker K, Lindsey K. Laser capture microdissection for the analysis of gene expression during embryogenesis of Arabidopsis. Plant J 2005;42(1):111–123. PMID: 15773857. doi: 10.1111/j.1365-313X.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 39.Sharma SB, Dixon RA. Metabolic engineering of proanthocyanidins by ectopic expression of transcription factors in Arabidopsis thaliana. Plant J 2005;44(1):62–75. PMID:16167896. doi: 10.1111/j.1365-313X.2005.02510.x. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 2015;20(3):176–185. PMID: 25577424. doi: 10.1016/j.tplants.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Doughty J, Aljabri M, Scott RJ. Flavonoids and the regulation of seed size in Arabidopsis. Biochem Soc Trans 2014;42(2):364–369. PMID:24646245. doi: 10.1042/BST20140040. [DOI] [PubMed] [Google Scholar]
- 42.Mӧller B, Weijers D. Auxin control of embryo patterning. Cold Spring Harb Perspect Biol 2009;1(5):a001545. PMID:20066117. doi: 10.1101/cshperspect.a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balazadeh S, Kwasniewski M, Caldana C, Mehrnia M, Zanor MI, Xue GP, Mueller-Roeber B. ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol Plant 2011;4(2):346–360. PMID:21303842. doi: 10.1093/mp/ssq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibañes M, Fabregas N, Chory J, Caño-Delgado AI. Brassinosteroid signalling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. PNAS 2009;106(32):13630–13635. PMID:19666540. doi: 10.1073/pnas.0906416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ckurshumova W, Koizumi K, Chatfield SP, Sanchez-Buelna SU, Gangaeva AE, McKenzie R, Berleth T. Tissue-specific GAL4 expression patterns as a resource enabling targeted gene expression, cell type-specific transcript profiling and gene function characterization in the Arabidopsis vascular system. Plant Cell Physiol 2009;50(1):141–150. PMID:19068493. doi: 10.1093/pcp/pcn180. [DOI] [PubMed] [Google Scholar]
- 46.Osterlund MT, Wei N, Deng XW. The roles of photoreceptor systems and the COP1-targeted destabilization of HY5 in light control of Arabidopsis seedling development. Plant Physiol 2000;124(4):1520–1524. PMID:11115869. doi: 10.1104/pp.124.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saijo Y, Sullivan JA, Wang H, Yang J, Shen Y, Rubio V, Ma L, Hoecker U, Deng XW. The COP1–SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev 2003;17(21):2642–2647. PMID:14597662. doi: 10.1101/gad.1122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maier A, Schrader A, Kokkelink L, Flake C, Welter B, Iniesto E, Rubio V, Uhrig JF, Hülskamp M, Hoeker U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J 2013;74(4):638–651. PMID:23425305. doi: 10.1111/tpj.12153. [DOI] [PubMed] [Google Scholar]
- 49.Arsovski AA, Galstyan A, Guseman JM, Nemhauser JL. Photomorphogenesis. Arabidopsis Book. 2012;10:e0147. doi: 10.1199/tab.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]


