ABSTRACT
Immunotherapy has shown limited success in prostate cancer; this may be partially explained by its immunosuppressive tumor microenvironment (TME). Although androgen-deprivation therapy (ADT), the most common treatment for prostate cancer, initially promotes a robust T cell infiltrate, T cell responses are later attenuated. Based on the castration-sensitive Myc-CaP model, we developed an antigen-specific system to study CD8 T cell tolerance to prostate tumors. This model is unique in that CD8 T cells recognize a bona-fide tumor antigen (Her-2/neu), rather than an overexpressed xenogenic antigen like chicken ovalbumin or influenza hemagglutinin. Using this novel model, we demonstrate robust tolerance that is not alleviated by TLR agonists or ADT. This model may serve as a novel and useful tool to further interrogate methods by which to augment anti-tumor cancer immune responses to prostate cancer.
Significance
Prostate cancer is a leading cause of cancer-related death in men worldwide, with an estimated 33,000 deaths projected in the U.S. in 2020. Although primary (localized) tumors can be cured by surgery or radiation, approximately 40% of patients eventually develop recurrent disease. While initially responsive to androgen-deprivation, many patients with recurrent prostate cancer eventually progress to a more advanced disease state known as metastatic castration-resistant prostate cancer (mCRPC); this is the lethal phenotype. These studies describe a novel androgen-responsive murine cell line that expresses a bona-fide tumor antigen (Her-2/neu). Pre-clinical work with this model shows robust and antigen-specific CD8 T cell tolerance, providing a novel preclinical model to study CD8 T cell tolerance to prostate tumors.
KEYWORDS: CD8, tolerance, neoantigen, prostate, Her-2/Neu, androgen-deprivation therapy, castration-resistance
Introduction
Although cancer immunotherapy is a rapidly evolving treatment option for many tumor types,1 responses in prostate cancer have been limited.2–4 For example, two large randomized phase III trials of immune checkpoint blockade with anti-CTLA-4 (ipilimumab) failed to meet their primary endpoint,5,6 as did a recent large randomized phase III trial of the PSA-directed vaccine known as ProstVac VF.7 Responses to anti-PD-1 have been reported, but these predominantly occur in a subset of patients with mutations in DNA damage repair (DRD) pathways.8,9 Yet, the FDA-approved therapeutic vaccine sipuleucel-T provides a survival advantage, supporting the potential of immunotherapy for the treatment of advanced prostate cancer.4,10 Antigens expressed only in the tumor, neoantigens, are by definition not susceptible to central tolerance. However, multiple peripheral tolerance mechanisms attenuate or otherwise prevent responses to antigens presented in a non-inflammatory context. Immunotherapy must overcome these peripheral tolerance mechanisms in order to mount an effective adaptive anti-tumor immune response.11–15 Several studies showed T cell tolerance in mice with prostate-specific expression of a model or viral antigen.12,16-19 For example, using a model in which influenza hemagglutinin is over-expressed in the prostate gland and in autochthonous prostate tumors of transgenic adenocarcinoma of mouse prostate (TRAMP) mice, we showed CD4 T cell tolerance that could be transiently mitigated by androgen-deprivation therapy (ADT).12 Peripheral tolerance to this continuously expressed antigen was further supported by studies in double transgenic mice in which cytolytic activity of hemagglutinin-specific CD8 T cells was restored after adoptively transferring hemagglutinin-specific CD8 T cells from TRAMP tumor-bearing hosts into tumor-free hosts.16 Additional models support T cell tolerance to prostate-restricted expression of ovalbumin in prostate gland of probasin ovalbumin expressing transgenic (POET-1) mice17 and influenza virus in autochronous prostate tumors of TRAMP mice.18 In each of these models, antigens were expressed using the androgen-driven rat probasin promoter where antigen levels can be abrogated through androgen deprivation. Thus, it is not known whether similar tolerance mechanisms exist to an antigen whose expression is independent of androgen signaling.
To understand peripheral tolerance to a bona-fide cancer antigen and to investigate whether immunotherapy interventions can break CD8 peripheral tolerance, we developed a murine prostate cancer cell line (Myc-CaP/Neu) that expresses the rat Her-2/neu protein which is immunogenic in a breast cancer model.20,21 In addition, rat Her-2/neu-specific CD8 T cells from TCR transgenic mice are available, and these facilitate antigen-specific interrogation of T cell tolerance.22 Using these tools, we found that the rat Her-2/neu protein was successfully processed and the immunodominant peptide (RNEU420-429) was presented to transgenic CD8 T cells. We further investigated whether peripheral tolerance was induced and whether tolerance could be mitigated by TLR-agonists or ADT.
Results
Generation of Neu-expressing Myc-CaP cells
To study antigen-specific CD8 T cell responses to androgen-deprivation therapy (ADT) sensitive prostate cancer, we introduced a model tumor antigen for which an antigen-specific T cell expressing a transgenic TCR has been generated.22 The model was based on the Myc-CaP cell line,23 which was derived from a transgenic prostate cancer model driven by prostate-specific overexpression of the MYC oncogene24 – a gene commonly up-regulated in invasive prostate cancer patients.25 Myc-CaP cells originate from the immunocompetent FVB/N strain. To model a bona-fide cancer antigen, Myc-CaP cells were transduced with a lentivirus encoding rat Her-2/neu (pWPXL-Neu; Figure 1(a,b)). This construct included the immunodominantepitope previously shown to bind to the class I MHC molecule H-2Dq.26 Transduced tumor cells were sorted to >99% purity (Figure 1(c)) and rat Her-2/neu (RNEU) expression was confirmed by immunofluorescence (Figure 1(d)).
Figure 1.
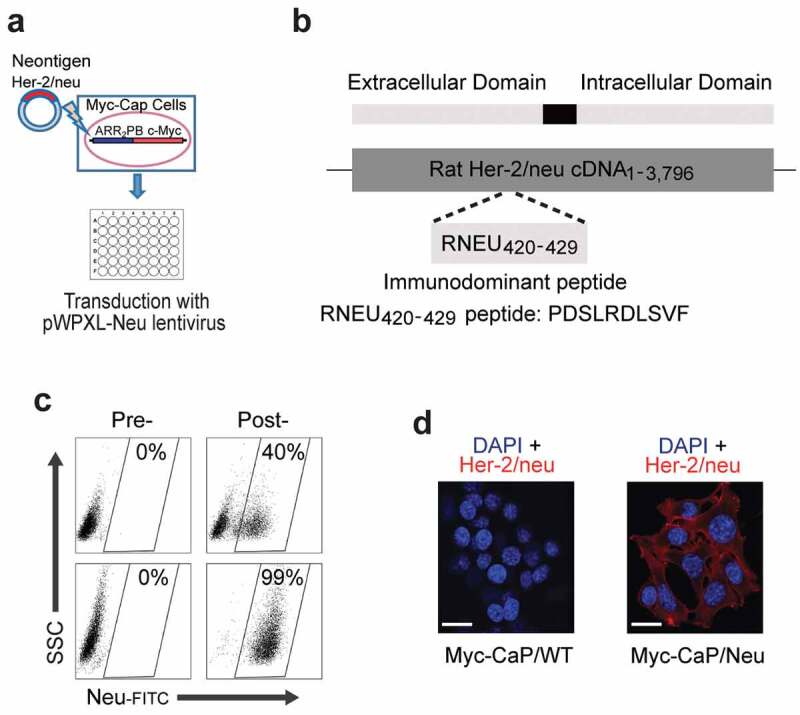
Generation of the Myc-CaP/Neu Cell Line.
(a) Schematic for the transduction of the Her-2/neu neoantigen into the androgen-responsive Myc-CaP cell line. (b) The extracellular rat Her-2/neu (neu) cDNA fragment containing the immunodominant MHC-I epitope recognized by the FVB/N-derived T cell clone TCRVβ4 (RNEU420-429 peptide: PDSLRDLSVF)26 was ligated into the vector pWPXL. (c) Sorting strategy to isolate Myc-CaP cells based on their expression of the rat neu antigen. Myc-CaP cells pre- and post-transduction with lentivirus containing RNEU420-429 peptide (top,) and pre- and post-sorting for neu-expressing cells (Myc-CaP/Neu; bottom). (d) Fluorescent detection of Her-2/neu in formalin-fixed WT Myc-CaP and Myc-CaP/Neu tumor cells grown on poly-D-Lysine-coated coverslips. Expression of the antigen on tumor cells was evaluated with CF640-labeled αHer-2/neu antibody (red); nuclei were counterstained with DAPI (blue). Scale bar = 100 μm.
RNEU-specific cytotoxic CD8 T cell responses to Myc-CaP/Neu tumor cells
Granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates the recruitment of dendritic cells and augments tumor antigen presentation.20,27 Thus, GM-CSF has been used as a component of therapeutic cancer vaccines to stimulate anti-tumor immunity in preclinical models28 as well as in multiple clinical trials.29,30 To determine whether CD8 T cells recognize RNEU420-429 in Myc-CaP/Neu cells, we performed vaccination studies using a vaccine (GVAX) comprised of irradiated Myc-CaP/Neu cells co-administered with GM-CSF secreting bystanders. As a readout for Her-2/neu expression, CFSE-labeled RNEU-specific CD8 T cells from Thy1.2+ donor mice were adoptively transferred 24 hours post-vaccination into Thy1.1+ recipient FVB/NJ mice (Figure 2(a,b)). Five days post-transfer, RNEU-specific CD8 T cells recovered from the inguinal lymph nodes (ILNs) and spleens of vaccinated recipient mice had undergone significant division (Figure 2(c)). In contrast, RNEU-specific CD8 T cells recovered from ILNs and spleens of naïve recipients had not undergone significant division (Figure 2(c)); these data support antigen expression and subsequent recognition. Intracellular staining for canonical effector cytokines (TNFα, IFNγ, GzB, and IL-2) confirmed T cell activation (Figure 2(d)). We next tested whether adoptively transferred Her-2/neu-specific CD8 T cells could recognize well-established Her-2/neu-expressing tumors (Figure 3(a–c)). As shown in Figure 3, implanted Myc-CaP/Neu tumors induced proliferation of adoptively transferred RNEU-specific CD8 T cells in tumor-draining lymph nodes (TDLNs) and spleens, while Myc-CaP/WT tumors did not (Figure 3(d)). To evaluate the functional capacity of RNEU-specific CD8 T cells recovered from TDLNs and spleens, we performed intracellular staining for TNFα, IFNγ, GzB, and IL-2. In line with our previous observations (Figure 2(d)), a fraction of RNEU-specific CD8 T cells isolated from Myc-CaP/Neu tumor-bearing recipients expressed these cytotoxic effector cytokines (Figure 3(e)).
Figure 2.
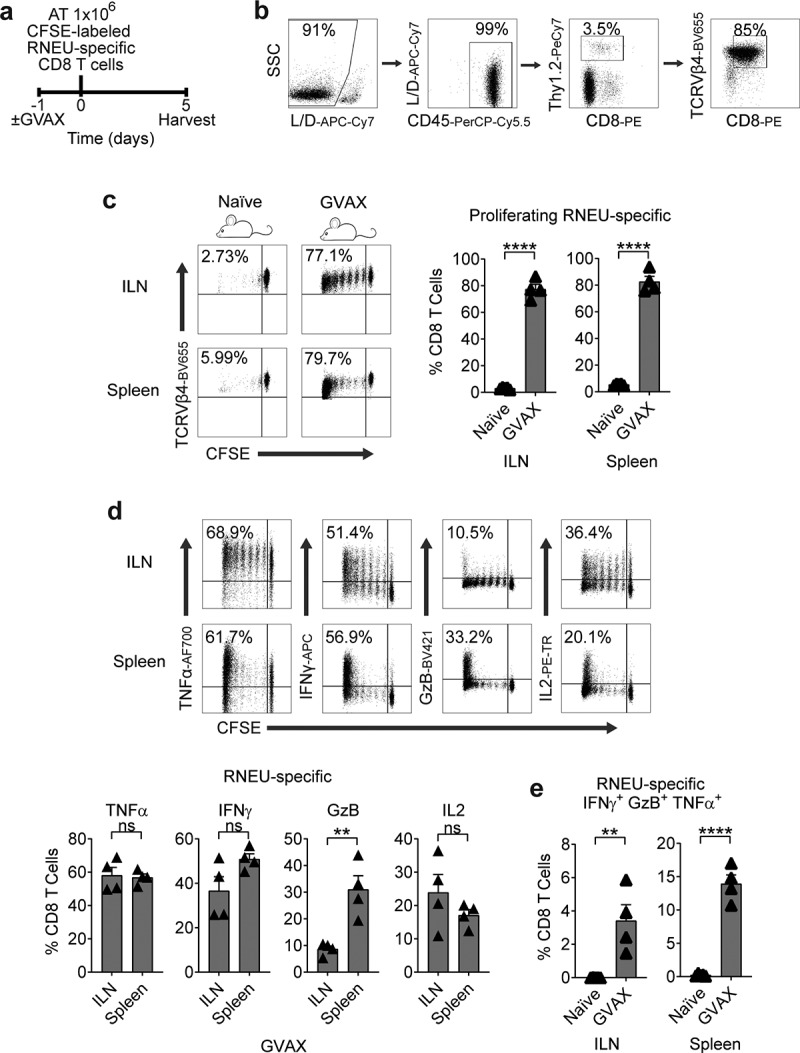
GVAX Vaccination Induces a Systemic Cytotoxic CD8 T Cell Response to Rat-Neu Neoantigen.
(a) Treatment scheme for the neu-expressing (GM-CSF–secreting) vaccination (GVAX) group. One day after the vaccination, 1 × 106 high-avidity CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells were adoptively transferred (AT) into mice. On day 5, inguinal LNs (ILNs) and spleens were harvested and analyzed by flow cytometry. (b) Gating strategy to profile RNEU-specific CD8 T cells by flow cytometry. FVB/N-derived T cell clone TCRVβ4 was gated based on CD45+CD8+Thy1.2+. (c) Percentages of proliferating TCRVβ4+ CD8 T cells in indicated tissues (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). (d) Percentages of cytokine production by RNEU-specific TCRVβ4+ CD8 T cells (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). (e) Percentages of polyfunctional RNEU-specific TCRVβ4+ IFNγ+GzB+TNFα+ CD8 T cells in indicated tissues (n ≥ 4 per group, repeated x 2). Proliferating TCRVβ4+ CD8 T cells, and their cytokine production, were calculated as fraction of TCRVβ4+ CD8 T cells.
Figure 3.
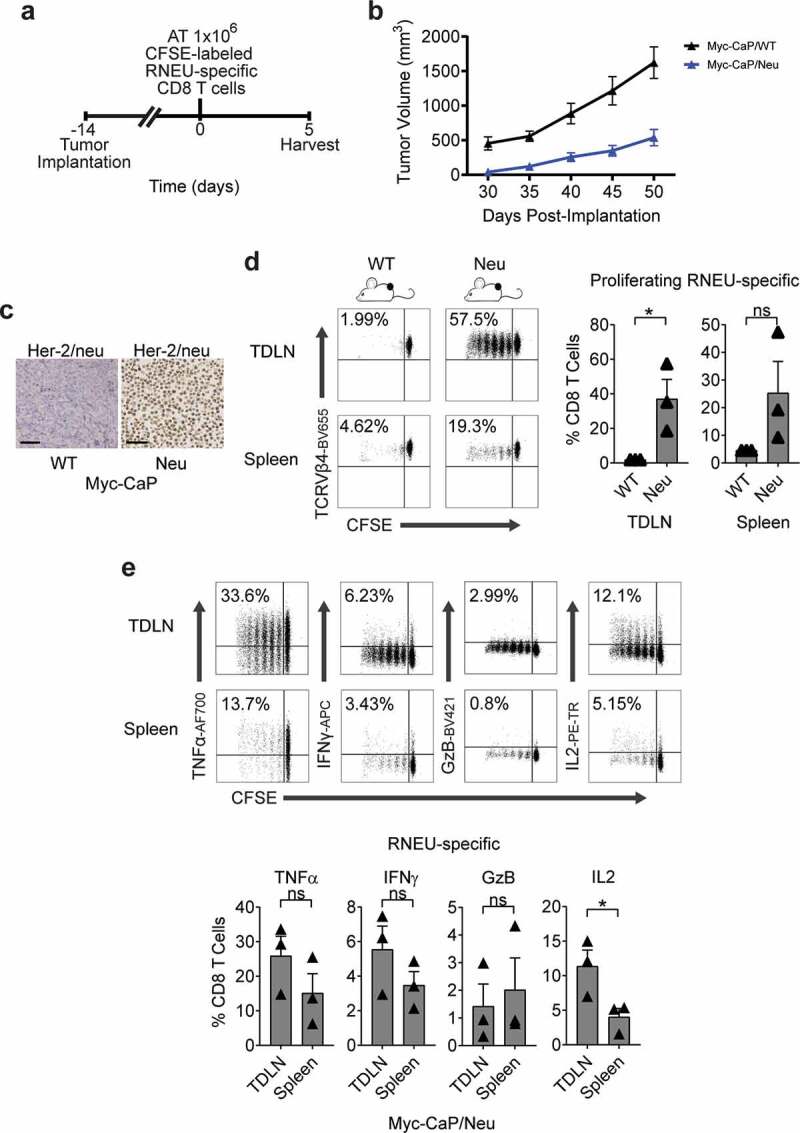
Established Myc-CaP Tumors Maintain Her-2/neu Expression and Induce a Systemic Cytotoxic CD8 T Cell Response to Rat-Neu Neoantigen.
(a) Treatment scheme for tumor implantation with either 1 × 106 Myc-CaP/WT or Myc-CaP/Neu cells. Fourteen days after tumor implantation, 1 × 106 high-avidity CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells were adoptively transferred (AT) into the mice. On day 5, tumor-draining lymph nodes (TDLNs) were harvested and analyzed by flow cytometry. (b) Tumor growth curves of mice from Myc-CaP/WT and Myc-CaP/Neu tumor-bearing mice. Average tumor volume (±s.e.m.) for each experimental group. (c) Her-2/neu expression on indicated murine allografts (representative immunohistochemistry; repeated x 2). Scale bar = 50 μm. (d) Percentages of proliferating TCRVβ4+ CD8 T cells in indicated tissues (representative flow plots and quantification; n ≥ 3 per group, repeated x 2). (e) Percentages of cytokine production by RNEU-specific TCRVβ4+ CD8 T cells in indicated tissues (representative flow plots and quantification; n ≥ 3 per group, repeated x 2). Proliferating TCRVβ4+ CD8 T cells, and their cytokine production, were calculated as fraction of TCRVβ4+ CD8 T cells.
Established tumors suppress antigen-specific CD8 T responses induced by vaccination
We next tested whether vaccination with Myc-CaP/Neu cells + GM-CSF producing bystander (GVAX) would affect tumor antigen-specific CD8 T cell responses in the setting of a suppressive TME (Figure 4(a–c)). Here we found that RNEU-specific CD8 T cells recovered from vaccinated Myc-CaP/Neu tumor-bearing recipients divided less and exhibited a lower percentage of TNFα, IFNγ, and IL2 cytokines producing RNEU-derived CD8 T cells than those harvested from vaccinated, non-tumor bearing (naïve) recipients (Figure 4(d,e)). These data suggest that recognition of the Her-2/neu peptide (RNEU420-429: PDSLRDLSVF) appeared to be tolerogenic in the context of a suppressive TME, and that vaccination may not be sufficient to mitigate this tolerance.
Figure 4.
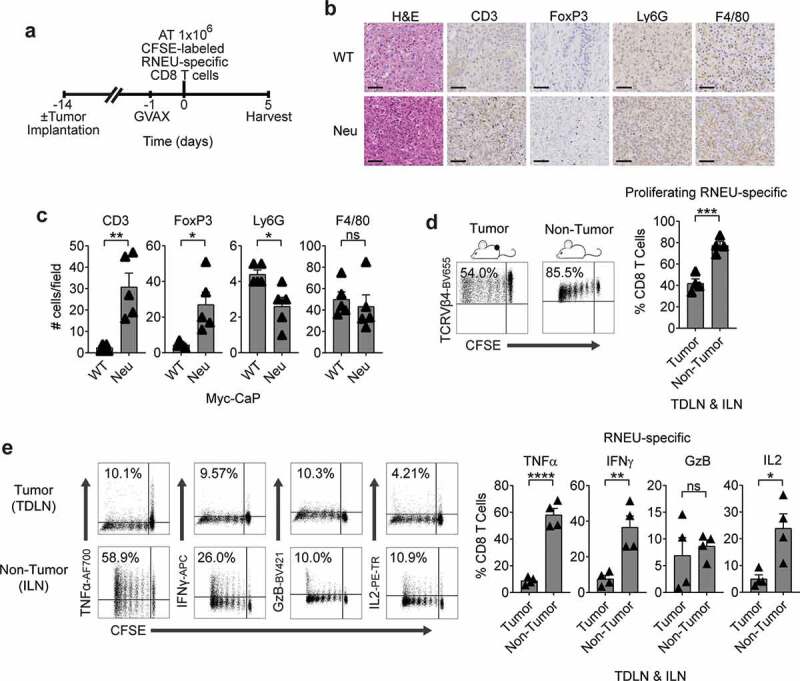
Myc-CaP/Neu Tumors Attenuate The RNEU-specific CD8 T Cell Response Induced by Vaccination with Her-2/neu Expressing Cells.
(a) Treatment scheme for tumor implantation with 1 × 106 Myc-CaP/Neu cells. One day after the neu-expressing (GM-CSF–secreting) vaccine (GVAX) was administrated, 1 × 106 high-avidity CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells were adoptively transferred (AT) into the mice. On day 5, tumor-draining lymph nodes (TDLNs; tumor) and inguinal LNs (ILNs; non-tumor) were harvested and analyzed by flow cytometry. (b) Tumor-infiltrating lymphocytes (TIL; CD3), regulatory T cells (FoxP3), myeloid-derived suppressor cells (Ly6 G), and macrophages (F4/80) of indicated murine allografts (representative immunohistochemistry; repeated x 2). Scale bar = 50 μm. (c) Counts of immune cells in tumor microenvironment (TME). (d) Percentages of proliferating TCRVβ4+ CD8 T cells in TDLNs and ILNs (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). (e) Percentages of cytokine production by RNEU-specific TCRVβ4+ CD8 T cells in TDLNs and ILNs (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). Proliferating TCRVβ4+ CD8 T cells, and their cytokine production, were calculated as fraction of TCRVβ4+ CD8 T cells. ILNs and TDLNs were isolated from naïve and tumor-bearing mice, respectively. Whole-cell vaccination (GVAX) was prepared as described in materials and methods.
TLR agonists do not mitigate antigen-specific CD8 T cell tolerance to a tumor antigen
The TLR3 agonist Poly I:C is a synthetic polyinosinic-polycytidylic double-stranded RNA widely used as a vaccine adjuvant due to its ability to induce DC maturation and TH1 polarization.31,32 Thus, we tested whether Poly I:C would affect the induction of tolerance in dividing RNEU-specific CD8 T cells isolated from Myc-CaP/Neu tumor-bearing recipient mice (Figure 5(a)). We found that IP treatment with Poly I:C 24 hours prior to the adoptive transfer of RNEU-specific CD8 T cells did not mitigate the induction of peripheral tolerance in TDLNs of Myc-CaP/Neu tumor-bearing recipient mice (Figure 5(b–d)). As a positive control, we tested whether IP Poly I:C treatment was able to induce a cytotoxic effector response in RNEU-specific T cells harvested from the ILN of naïve recipients; indeed this was the case (Supp. Fig. 2). So, while Her-2 specific T cells may achieve cytolytic potential with Poly I:C, recognition of peripherally expressed tumor antigen in the context of the TME renders them tolerant.
Figure 5.
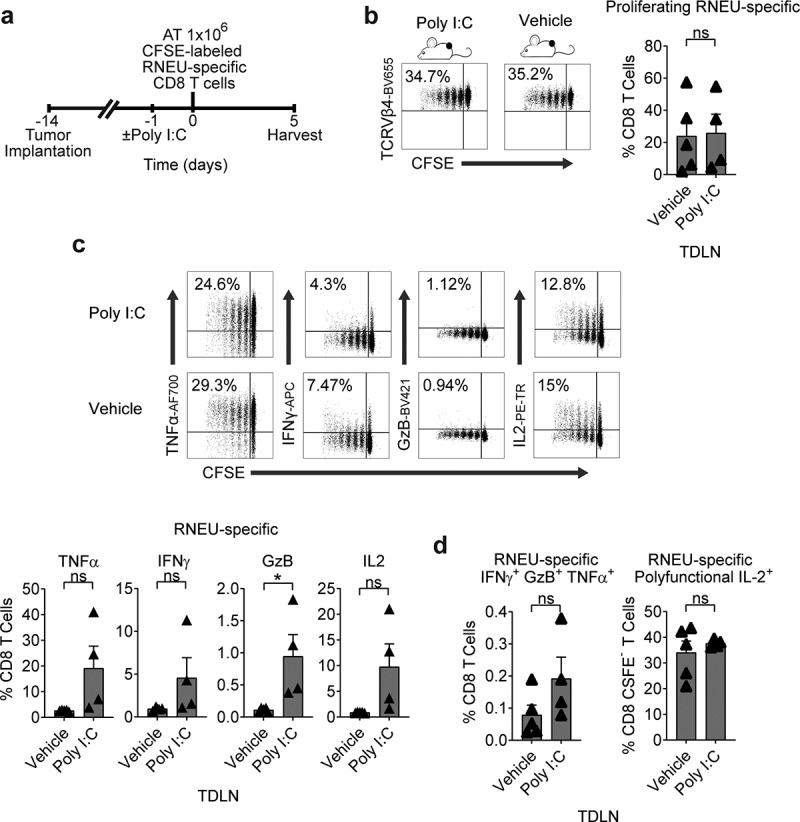
Tumor-Induced RNEU-specific CD8 T Cell Tolerance is Maintained After Stimulation with a TLR3 Agonist.
(a) Treatment scheme for tumor implantation with 1 × 106 Myc-CaP/Neu cells. One day after Poly I:C IP administration, 1 × 106 high-avidity CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells were adoptively transferred (AT) into the mice. On day 5, tumor-draining lymph nodes (TDLNs) were harvested and analyzed by flow cytometry. (b, c) Percentages of proliferating TCRVβ4+ CD8 T cells in TDLNs (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). (d) Percentages of cytokine production by RNEU-specific TCRVβ4+ CD8 T cells (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). (e) Percentages of polyfunctional TCRVβ4+ CD8+ IFNγ+GzB+TNFα+ in TDLNs (n ≥ 4 per group, repeated x 2). Proliferating TCRVβ4+ CD8 T cells, and their cytokine production, were calculated as fraction of TCRVβ4+ CD8 T cells.
Androgen-deprivation therapy (ADT) does not mitigate antigen-specific CD8 T cell tolerance to a tumor antigen
Our prior work showed that ADT transiently mitigates CD4 T cell tolerance to a model antigen expressed under the androgen-regulated probasin promoter. To test whether a similar effect occurred in the context of a tumor antigen whose expression is not driven by an androgen-regulated promoter, we implanted Myc-CaP/Neu tumors in WT recipients and then treated them with ADT prior to adoptive transfer of CFSE-labeled RNEU-specific CD8 T cells (Figure 6(a)). Consistent with prior data,33,34 over time, ADT increased the infiltration of immune cells with the potential to suppress CD8 T cell responses – Tregs, PMN-MDSCs, and macrophages (Figure 6(b,c)). ADT (7 or 24 d) prior to adoptive transfer did not significantly increase the percentage of dividing RNEU-specific CD8 T cells relative to intact (vehicle; non-castrated) recipients (Figure 6(d)), and did not increase cytokine secretion by RNEU-specific CD8 CTLs (Figure 6(e)). These data support the robustness of CD8 T cell tolerance to this prostate-tumor restricted antigen and suggest that prior results using influenza hemagglutinin driven by the androgen-responsive probasin promoter as a model antigen may reflect mitigation of tolerance by transient antigen loss.12
Figure 6.
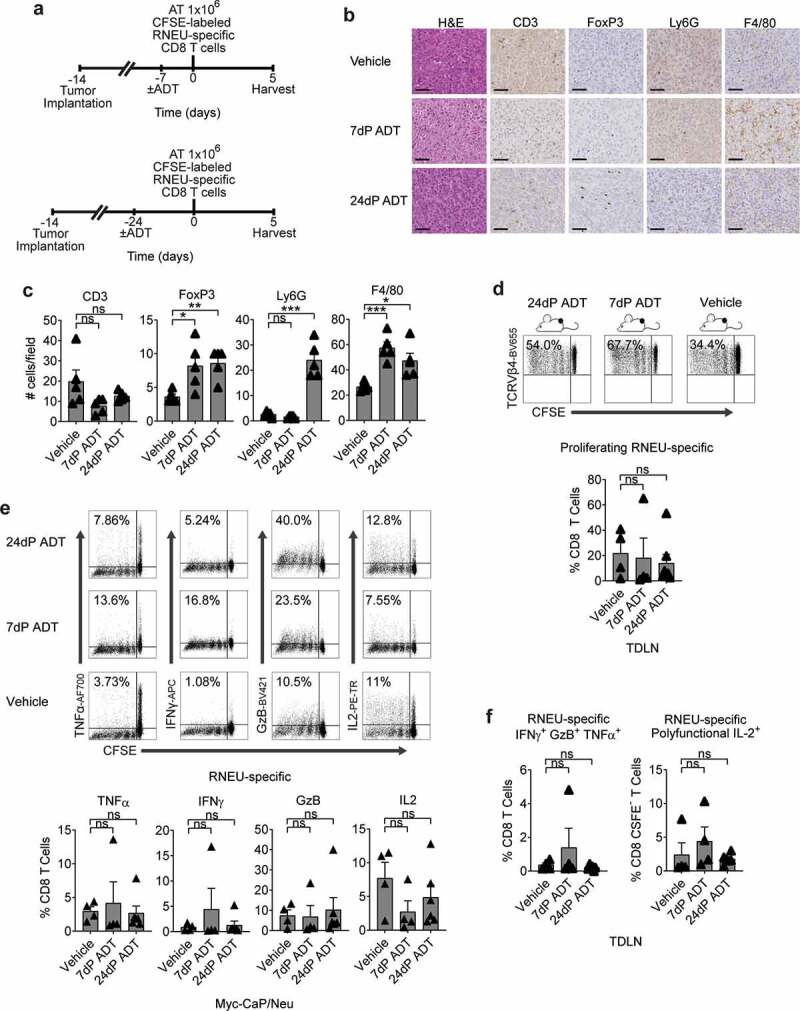
Androgen-Deprivation Therapy (ADT) Does Not Significantly Attenuate Tumor-Induced RNEU-specific CD8 T Cell Tolerance.
(a) Treatment scheme for tumor implantation with 1 × 106 Myc-CaP/Neu cells. Seven or twenty-four days after androgen-deprivation (ADT) was administrated, 1 × 106 high-avidity CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells were adoptively transferred (AT) into the mice. On day 5, tumor-draining lymph nodes (TDLNs) were harvested and analyzed by flow cytometry. (b) Tumor-infiltrating lymphocytes (TIL; CD3), regulatory T cells (FoxP3), myeloid-derived suppressor cells (Ly6 G), and macrophages (F4/80) of indicated murine allografts (representative immunohistochemistry; repeated x 2). Scale bar = 50 μm. (c) Counts of immune cells in tumor microenvironment (TME). (d) Percentages of proliferating TCRVβ4+ CD8 T cells in TDLNs (representative flow plots and quantification; n ≥ 4 per group, repeated x 2). (e) Percentages of cytokine production by RNEU-specific TCRVβ4+ CD8 T cells (representative flow plots; repeated x 2). (f) Percentages of polyfunctional TCRVβ4+ CD8+ IFNγ+GzB+TNFα+ in TDLNs (n ≥ 4 per group, repeated x 2). Proliferating TCRVβ4+ CD8 T cells, and their cytokine production, were calculated as fraction of TCRVβ4+ CD8 T cells. IL-2 production was evaluated as a fraction of polyfunctional IFNγ+GzB+TNFα+ TCRVβ4+ CD8 T cells.
Discussion
We previously showed that androgen-derivation therapy (ADT) has an additive effect when combined with immunotherapeutic interventions.33,34 However, the lack of models to study antigen-specific CD8 T cell responses to neoantigens expressed in an androgen-independent manner has limited understanding of the cellular and molecular mechanisms involved in antigen-specific immune responses to prostate cancer antigens that are not androgen-regulated. In this study, we developed a model to study antigen-specific CD8 T cell peripheral tolerance in an implantable, androgen-responsive murine cell line in which expression of a bona-fide cancer antigen is uncoupled from androgen receptor signaling. We found that tumor cells expressing Her-2/neu maintain their immunogenicity in vivo, and recruit RNEU-specific CD8 T cells. Furthermore, we found this recognition led to a reduction of effector cytokine production in the context of a suppressive TME. This tolerance was robust and was not significantly mitigated by either the TLR-agonist Poly I:C or by ADT. These data are consistent with clinical data showing that the use of immunotherapy has generally met with limited clinical success in prostate cancer.3
These data are potentially discordant with our prior work using a variant of the TRAMP model that expresses influenza hemagglutinin under the control of the androgen-responsive, prostate-specific minimal rat probasin promoter.35 There, we found that ADT results in de novo presentation of a prostate-restricted antigen in TDLN when castration (ADT) is performed 1 day prior to adoptive transfer of antigen-specific CD4 T cells. In those studies, we also showed that CD4 T cell proliferation was diminished when ADT was performed 10 d prior to adoptive transfer.12 Thus, those results are likely consistent with the notion that persistent expression of tissue antigen is important in the establishment of peripheral tolerance. Consistent with this, studies from another group showed that ADT dramatically decreased probasin-driven expression of ovalbumin in POET-1 mice.17 Here, in the presence of both antigen and antigen-specific CD8 T cells, we found more profound tolerance – that was not significantly mitigated by ADT. The differences between the prior models and the current one are possibly related to how the T-cell recognized antigen is expressed; in prior studies, antigen expression was driven by the androgen-responsive probasin promoter and was thus transiently decreased after ADT. By contrast, here the bona-fide tumor antigen Her-2/neu was constitutively expressed in an androgen-insensitive manner – with persistent presentation likely driving the more robust tolerance seen in the new model.
Supporting persistent tolerance, we observed continued Treg infiltration even late after ADT (25dP ADT; at onset of castration-resistance), supporting a long-lived tolerogenic mechanism. These data are consistent with our prior studies in which we used the androgen-responsive murine prostate cancer cell line Myc-CaP, and found that the initial pro-inflammatory infiltrate (apparent in the early post-ADT period) was subsequently followed by an infiltration of Tregs into the TME that diminished late after ADT.33 Indeed, anti-CTLA-4 treatment prior to ADT resulted in Treg depletion and delayed tumor growth in that model,33 suggesting that tumor-infiltrating Tregs may be an important mechanism of primary resistance to immunotherapy. These data align with the observation that CD8 T infiltration was accompanied by a proportional influx of Tregs in prostate cancer patients upon neoadjuvant ADT treatment.36 Also consistent with prior data,34 we found that PMN-MDSCs infiltrated tumors as castration-resistance emerges, suggesting their suppressive role may be important in the development of acquired resistance to immunotherapy. In line with this hypothesis, we and others have demonstrated that blocking PMN-MDSC trafficking into the tumor augments responses to immune checkpoint blockade.34,37 In addition, the rationale for targeting these cells in combination with other immune therapeutic interventions has also been supported by studies using TRAMP mice38,39 and recently reviewed.40 Further studies characterizing tumor-infiltrating Tregs and PMN-MDSCs in the novel model presented here may aid in the development of new therapeutic approaches to overcome immunotherapy resistance in prostate cancer, including metastatic prostate cancer. Although metastasis has not been observed in MYC driven Myc-CaP tumors,24 a potential limitation of this model, the TME of prostate tumors in bone, the most clinical relevant metastatic niche, can be studied by direct intratibial injection of Myc-CaP/Neu tumor cells41 followed by adoptive transfer of CFSE-labeled RNEU-specific CD8 T cells.
In summary, we report a novel cell line/adoptive CD8 T cell transfer model to study antigen-specific T cell tolerance to prostate cancer. Although the interventions explored here were insufficient to significantly break tolerance, this system may serve as a useful tool to further interrogate methods by which to augment antigen-specific CD8 T cell anti-tumor responses.
Materials and methods
Cell lines
Myc-CaP cells derived from spontaneous prostate cancer in c-Myc transgenic mice23,24 were a generous gift from Dr. Charles Sawyers. Myc-CaP cells were transduced with viral particles containing the rat Her-2/neu transcript and isolated by FACS sorting based on Her-2/neu expression to establish Myc-CaP/Neu cells. Myc-CaP and Myc-CaP/Neu cells were cultured in DMEM as previously described.23 All cell lines were cultured in 1% penicillin/streptomycin media at 37°C, 5% CO2.
Mouse strains
Seven-week-old FVB/NJ Thy1.1 male mice were purchased from The Jackson Laboratory (JAX stock #001800). FVB/N Thy1.2+ mice were created by backcrossing the Thy1.2 allele onto the FVB/N background for 10 generations. These mice were then crossed to FVB/N clone 100 transgenic mice carrying T cells specific for MHC I (H-2Dq) rat-neu immunodominant peptide (RNEU420-429).26 Breeding pairs of clone 100 transgenic mice and FVB/N Thy1.2 labeled mice were kindly transferred from the Laboratory of Dr. Elizabeth M. Jaffee at Johns Hopkins to Columbia University. Experimental animals were bred in-house and phenotyped with TCRVβ4 and Thy1.2 staining as previously described.42 Mice were acclimated for at least 1 week before any experimental procedures were performed. Animals were kept in specific pathogen-free facilities in either Johns Hopkins University School of Medicine or Columbia University Medical Center. All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the Johns Hopkins University School of Medicine and Columbia University Medical Center.
Her-2/Neu transfection
Rat-neu cDNA was cloned from pSV2-neu-N (gift from Bob Weinberg; Addgene plasmid # 10917)43 and ligated into the pWPXL vector (Supp. Fig. 1) to replace EGFP sequence (gift from Didier Trono; Addgene plasmid # 12257); pMD2.G and psPAX2 plasmids were used as envelope and packing systems. All plasmids were transfected into 293 T using lipofectamine 2000 (Invitrogen) and 293 T cell supernatants were collected 48 hours post-transfection and used to transduce Myc-CaP cells. Viral load was titrated to mimic different multiplicity of infections (MOI 5–50) using appropriate volumes of medium with lentivirus containing polybrene (Sigma-Aldrich). The same parental Myc-CaP cells were transduced with a murine GM-CSF lentivirus using the same method for inserting the rat Her-2/neu containing the RNEU420-429 peptide (PDSLRDLSVF) to create Myc-CaP/Neu cells. Successfully transduced cells were isolated based on their Her-2/neu expression by FACS for Myc-CaP/Neu and based on their GM-CSF expression levels by ELISA for GM-CSF secreting bystanders. Efficiency was evaluated 24 hours after transduction in both cases.
Adoptive transfer (AT) experiments
High-avidity RNEU-derived CD8 T cells were isolated untouched from the spleens of 8-week old male clone 100 transgenic mice using CD8a immunomagnetic negative selection beads (Miltenyi Biotec). CD8 T cells were then labeled with CFSE (Invitrogen) and resuspended in PBS. One million CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells were adoptively transferred intravenously into 8-week old male FVB/NJ mice (JAX stock #001800) – that, unlike most strains, express the congenic marker Thy1.1.42 On days 5 and 7, LNs (draining or inguinal) and spleens were harvested. CFSE dilution of the adoptively transferred Thy1.2+ RNEU-derived CD8 CTLs was measured by flow cytometry. To maximize the identification of adoptively transferred CFSE-labeled RNEU420–429-specific CD8 T cells, we first gated on the congenic marker Thy1.2 and next gated on the clonotypic Vβ chain, Vβ4.
Toll-like receptor 3 (TLR3) stimulation experiments
Naïve mice received a low molecular weight (LMW) synthetic polyinosinic-polycytidylic acid (Poly I:C) intraperitoneally (IP) at a dose of 100 μg/mouse (InvivoGen). Twenty-four hours later, mice received an intravenous injection of the high-avidity CFSE-labeled Thy1.2+ RNEU420–429-specific CD8 T cells.
Tumor allografts
Eight-week-old male FVB/NJ mice were subcutaneously inoculated with either Myc-CaP or Myc-CaP/Neu (1 × 106 cells/mouse) in the right flank. Tumor diameters were measured with an electronic caliper every 3 d as indicated and the tumor volume was calculated using the formula: [longest diameter × (shortest diameter)2]/2.
Vaccination (GVAX) experiments
Naïve and tumor-bearing mice received a whole-cell GM-CSF vaccine composed of a mixture of irradiated Myc-CaP cells expressing GM-CSF adjuvant (3x106) and Myc-CaP/Neu cell expressing Rat Her-2/neu protein (GVAX). The two types of cells were harvested, washed in PBS, and irradiated at 30,000 rads using a γ irradiator (GammaCell 1000 irradiator), and administered subcutaneously (SC) in equal aliquots in the remaining three limbs (50 µL volume).
Androgen-deprivation treatment (ADT) experiments
Myc-CaP/Neu tumor-bearing mice received androgen-deprivation therapy (ADT) 2 weeks after tumor implantation. ADT was administered via subcutaneous (SC) injection of degarelix acetate (a GnRH receptor antagonist; Ferring Pharmaceuticals Inc.) at a dosage of 0.625 mg/100 μl H2O/25 g body weight.
Flow cytometry
Single-cell suspensions of tumor-draining lymph nodes (TDLNs) and spleens were homogenized mechanically with the back of a syringe. Cells were Fc-blocked with purified rat anti-mouse CD16/CD32 (Clone: 2.4 G2, Becton Dickinson BD) for 15 minutes at RT. Dead cells were discriminated using the LIVE/DEAD (L/D) near-IR dead cell stain kit (Thermo Fisher) and samples were stained for the extracellular and intracellular markers. The following antibodies were used: Her-2/neu (7.16.4), anti-mouse IgG2a (RMG2a-62), CD45 (30 F-11), Thy1.2 (53–2.1), TCRVβ4 (KT4), CD8 (53–6.7), IFN-γ (XMG1.2), TNF-α (MP6-XT22), IL-2 (JES6-5H4), and GZB (GB11). For intracellular staining, cells were fixed and permeabilized by using BD Perm/Wash (BD Biosciences) at room temperature for 45 minutes. For intracellular cytokine staining, cells were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 4 hours in the presence of protein transport inhibitor cocktail (eBiosciences). Gates of cytokines were determined using fluorescence minus one (FMO) controls. Staining was visualized by fluorescence-activated cell sorting (FACS) analysis using a BD FACS LSR II (BD Biosciences) and analyzed using FlowJo® (Flowjo LLC). Polyfunctional CD8 T cells are defined as CD45+Thy1.2+TCRVβ4+CD8+IFNγ+TNFα+GzB+.Proliferation was assessed using the CellTrace CFSE Cell Proliferation Kit (Invitrogen).
Immunohistochemical staining (IHC)
Tumor samples were fixed with 10% formalin (Fisher Scientific, Pittsburgh, PA) for 24 hours before paraffin embedding and sectioning. Sections were stained with hematoxylin and eosin (H&E), and antibodies against mouse Ly6 G (1A8; BD Pharmingen), FoxP3 (D6O8 R; Cell Signaling), CD3 (SP7; Spring Bioscience), and F4/80 (BM8; eBioscience), and Her-2/neu (7.16.4; Emdmillipore). All images were acquired on a Leica SCN 400 system with high throughput 384 slide autoloader (SL801) and a 40X objective; files were processed with Aperio ImageScope v12.3.1.6002. Marker-positive cell counts were obtained from 5 random 40X fields per histological section and results were averaged over the number of counted fields.
Immunocytochemistry (ICC)
Manual fluorescent staining was performed using company protocols. Briefly, tumor cells were grown on poly-D-Lysine coated coverslips for 24 hrs and fixed with 10% neutral-buffered formalin for 30 min at RT. Samples were permeabilized with 0.2% TBS-Tween 20 for 10 min at RT, then blocked with mouse IgG blocking reagent for 60 min at RT, followed by 2.5% of normal goat serum (Vector) for 5 min at RT. Primary antibody for Her-2/neu was diluted 1:100 in renaissance background reducing diluent (Biocare Medical) and incubated overnight at 4°C. After washing off the primary antibody, the slides were incubated 10 min at RT with peroxidase micropolymer for detecting mouse primary antibodies on mouse tissue (Vector M.O.M.). Tyramide CF640 R (Biotium) was used to visualize Her-2/neu staining. After washing off the primary antibody, the slides were incubated 15 min at RT with HRP secondary antibody (Vector). All images were acquired on a Nikon A1RMP confocal microscope using a 60X objective. Image analysis was performed using ImageJ.
Statistical analysis
Statistical analysis was performed using Prism 7 (GraphPad). Unpaired two-tailed t-tests or Wilcoxon test were conducted and considered statistically significant at p-values ≤0.05 (*), 0.01 (**), 0.001 (***) and 0.0001 (****).
Supplementary Material
Acknowledgments
We thank members of the Drake Lab for discussion and insightful comments; Dr. E. M. Jaffee for kindly providing clone 100 transgenic mice and Thy1.2-labeled mice; T. Nirschl and J. Leatherman for administrative assistance; H. Zhang, C. Lu and the Cell Sorting Core Facilities at Johns Hopkins and Columbia University for help with sorting; T. Swayne, E. Munteanu, and the Confocal and Specialized Microscopy Shared Resource at Columbia University for help with microscopy; and Z.J. Kerner for his help revising the manuscript. This study was supported by U.S. Department of Defense (W81XWH-13-1-0369), U.S. National Institutes of Health National Cancer Institute (R01: CA127153), the Patrick C. Walsh Fund, the OneInSix Foundation, and the Prostate Cancer Foundation. Research reported in this publication was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, National Institutes of Health under awards S10OD020056. IHC staining and image collection for this work were performed in the Molecular Pathology Shared Resource and the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, supported by NIH grant #P30 CA013696 (National Cancer Institute). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
This work was supported by the U.S. National Institutes of Health National Cancer Institute [CA127153]; U.S. Department of Defense [W81XWH-13-1-0369]; the Patrick C. Walsh Fund; the OneInSix Foundation; the Prostate Cancer Foundation.
Disclosure of potential conflicts of interest
C.G.D. has stock or ownership interests in Compugen, Harpoon, Kleo, and Tizona Therapeutics, and has served as a consultant for Agenus, Dendreon, Janssen Oncology, Eli Lilly, Merck, AstraZeneca, MedImmune, Pierre Fabre, Genentech, and Genocea Biosciences. L.a.E. has stock or ownership interests in Aduro, and has served as a consultant for AbbVie, Amgen, AstraZeneca, Bayer, Celgene, Chugai, Gritstone, Medimmune, Molecuvax, Macrogenics, Peregrine, Replimune, Syndax, and Vaccinex.
Authors’ contributions
Conception and design: Z.A. Lopez-Bujanda, C.G. Drake
Development of methodology: Z.A. Lopez-Bujanda, C.G. Drake
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Z.A. Lopez-Bujanda, M.G. Chaimowitz, T.D. Armstrong, C.G. Drake
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): Z.A. Lopez-Bujanda, C.G. Drake
Writing and/or revision of the manuscript: Z.A. Lopez-Bujanda, C.G. Drake
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J.B. Foote, L.A. Emens, T.D. Armstrong
Study supervision: C.G. Drake
All authors reviewed and approved the manuscript
Supplementary material
Supplemental material for this article can be accessed on the publisher’s website.
References
- 1.Siegel RL, Miller KD, Jemal a. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–9. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao W, Drake CG. Immunotherapy for Prostate Cancer: An Evolving Landscape. In: Laurence Zitvogel & Guido Kroemer, editors. Oncoimmunology: a practical guide for cancer immunotherapy. Berlin, Heidelberg: Springer; 2018. p. 593–606. [Google Scholar]
- 4.Cordes LM, Gulley JL, Madan RA. Perspectives on the clinical development of immunotherapy in prostate cancer. Asian J Androl. 2018;20:253–259. doi: 10.4103/aja.aja_9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJM, Krainer M, Houede N, Santos R, Mahammedi H, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, Ganju V, Polikoff J, Saad F, Humanski P, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017;35:40–47. doi: 10.1200/JCO.2016.69.1584. [DOI] [PubMed] [Google Scholar]
- 7.Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J, et al. Results of PROSPECT: a randomized phase 3 trial of PROSTVAC-V/F (PRO) in men with asymptomatic or minimally symptomatic metastatic, castration-resistant prostate cancer. J Clin Oncol. 2018;36:5006. doi: 10.1200/JCO.2018.36.15_suppl.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudadi K, Suzman DL, Anagnostou V, Fu W, Luber B, Wang H, Niknafs N, White JR, Silberstein JL, Sullivan R, et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget. 2018;9:28561–28571. doi: 10.18632/oncotarget.25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, Vaishampayan U, Berger R, Sezer A, Alanko T, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol. 2020;38:395–405. doi: 10.1200/JCO.19.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Morgan DJ, Kreuwel HT, Sherman LA. Antigen concentration and precursor frequency determine the rate of CD8+ T cell tolerance to peripherally expressed antigens. J Immunol. 1999;163:723–727. [PubMed] [Google Scholar]
- 12.Drake CG, Doody ADH, Mihalyo MA, Huang C-T, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–249. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 14.Emens LA. Cancer vaccines: on the threshold of success. Expert Opin Emerg Drugs. 2008;13:295–308. doi: 10.1517/14728214.13.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson SR, Yuan J, Teague RM. Targeting CD8+ T-cell tolerance for cancer immunotherapy. Immunotherapy. 2014;6:833–852. doi: 10.2217/imt.14.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno TC, Rothwell C, Grosso JF, Getnet D, Yen HR, Durham NM, Netto G, Pardoll DM, Drake CG. Anti-tumor effects of endogenous prostate cancer-specific CD8 T cells in a murine TCR transgenic model. Prostate. 2012;72:514–522. doi: 10.1002/pros.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees JR, Charbonneau B, Swanson AK, Jensen R, Zhang J, Matusik R, Ratliff TL. Deletion is neither sufficient nor necessary for the induction of peripheral tolerance in mature CD8+ T cells. Immunology. 2006;117:248–261. doi: 10.1111/j.1365-2567.2005.02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci USA. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer-Weaver KA, Watkins SK, Anderson MJ, Draper LJ, MalyguineA, Alvord WG, Greenberg NM, Hurwitz AA. Immunity to murine prostatic tumors: continuous provision of T-cell help prevents CD8 T-cell tolerance and activates tumor-infiltrating dendritic cells. Cancer Res. 2009;69:6256–6264. doi: 10.1158/0008-5472.CAN-08-4516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Reilly RT, Gottlieb MB, Ercolini AM, Machiels JP, Kane CE, Okoye FI, Muller WJ, Dixon KH, Jaffee EM. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–3576. [PubMed] [Google Scholar]
- 21.Sunay MM, Foote JB, Leatherman JM, Edwards JP, Armstrong TD, Nirschl CJ, Hicks J, Emens LA. Sorafenib combined with HER-2 targeted vaccination can promote effective T cell immunity in vivo. Int Immunopharmacol. 2017;46:112–123. doi: 10.1016/j.intimp.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black CM, Armstrong TD, Jaffee EM. Apoptosis-regulated low-avidity cancer-specific CD8(+) T cells can be rescued to eliminate HER2/neu-expressing tumors by costimulatory agonists in tolerized mice. Cancer Immunol Res. 2014;2:307–319. doi: 10.1158/2326-6066.CIR-13-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson PA, Ellwood-Yen K, King JC, Wongvipat J, LeBeau MM, Sawyers CL. Context-dependent hormone-refractory progression revealed through characterization of a novel murine prostate cancer cell line. Cancer Res. 2005;65:11565–11571. doi: 10.1158/0008-5472.CAN-05-3441. [DOI] [PubMed] [Google Scholar]
- 24.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 25.Koh CM, Bieberich CJ, Dang CV, Nelson WG, Yegnasubramanian S, De Marzo AM. MYC and Prostate Cancer. Genes Cancer. 2010;1:617–628. doi: 10.1177/1947601910379132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ercolini AM, Machiels JPH, Chen YC, Slansky JE, Giedlen M, Reilly RT, Jaffee EM. Identification and characterization of the immunodominant rat HER-2/neu MHC class I epitope presented by spontaneous mammary tumors from HER-2/neu-transgenic mice. J Immunol. 2003;170:4273–4280. doi: 10.4049/jimmunol.170.8.4273. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto C, Kohara H, Inoue H, Narusawa M, Ogawa Y, Hirose-Yotsuya L, Miyamoto S, Matsumura Y, Yamada K, Takahashi A, et al. Therapeutic vaccination based on side population cells transduced by the granulocyte-macrophage colony-stimulating factor gene elicits potent antitumor immunity. Cancer Gene Ther. 2017;24:165–174. doi: 10.1038/cgt.2016.80. [DOI] [PubMed] [Google Scholar]
- 28.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arlen PM, Mohebtash M, Madan RA, Gulley JL. Promising novel immunotherapies and combinations for prostate cancer. Future Oncol. 2009;5:187–196. doi: 10.2217/14796694.5.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 31.Martins KA, Bavari S, Salazar AM. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev Vaccines. 2015;14:447–459. doi: 10.1586/14760584.2015.966085. [DOI] [PubMed] [Google Scholar]
- 32.Garzon-Muvdi T, Theodros D, Luksik AS, Maxwell R, Kim E, Jackson CM, Belcaid Z, Ganguly S, Tyler B, Brem H, et al. Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma. Oncotarget. 2018;9:20681–20697. doi: 10.18632/oncotarget.25061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen YC, Ghasemzadeh A, Kochel CM, Nirschl TR, Francica BJ, Lopez-Bujanda ZA, Carrera Haro MA, Tam A, Anders RA, Selby MJ, et al. Combining intratumoral Treg depletion with androgen deprivation therapy (ADT): preclinical activity in the Myc-CaP model. Prostate Cancer Prostatic Dis. 2018;21:113–125. doi: 10.1038/s41391-017-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Bujanda ZA, Haffner MC, Chaimowitz MG, Chowdhury N, Venturini NJ, Obradovic A, Hansen CS, Jacków J, Sfanos KS, Bieberich CJ, et al. Castration-mediated IL-8 promotes myeloid infiltration and prostate cancer progression. bioRxiv. 2019;651083. doi: 10.1101/651083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obradovic AZ, Dallos MC, Zahurak ML, Partin AW, Schaeffer EM, Ross AE, Allaf ME, Nirschl TR, Liu D, Chapman CG, et al. T-cell infiltration and adaptive treg resistance in response to androgen deprivation with or without vaccination in localized prostate cancer. Clin Cancer Res. 2020;26:3182–3192. doi: 10.1158/1078-0432.CCR-19-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci Transl Med. 2014;6:237ra267. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 39.Jayaprakash P, Ai M, Liu a, Budhani P, Bartkowiak T, Sheng J, Ager C, Nicholas C, Jaiswal AR, Sun Y, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest. 2018;128:5137–5149. doi: 10.1172/JCI96268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Bono JS, Guo C, Gurel B, De Marzo AM, Sfanos KS, Mani RS, Gil J, Drake CG, Alimonti A. Prostate carcinogenesis: inflammatory storms. Nat Rev Cancer. 2020;20:455–469. doi: 10.1038/s41568-020-0267-9. [DOI] [PubMed] [Google Scholar]
- 41.Corey E, Quinn JE, Bladou F, Brown LG, Roudier MP, Brown JM, Buhler KR, Vessella RL. Establishment and characterization of osseous prostate cancer models: intra-tibial injection of human prostate cancer cells. Prostate. 2002;52:20–33. doi: 10.1002/pros.10091. [DOI] [PubMed] [Google Scholar]
- 42.Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, Jaffee EM, Armstrong TD. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS One. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bargmann CI, Hung MC, Weinberg RA. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


