Abstract
Tamsulosin, an alpha-1 adrenoreceptor antagonist, has been used as a primary option for medical treatment of benign prostate hyperplasia. An open-label, single-dose, randomized, three-treatment, three-period, three sequence crossover study was conducted to evaluate the pharmacokinetics (PKs) of 0.2 and 0.4 mg tamsulosin hydrochloride (HCl) in the fed versus the fasted state. Subjects were randomly assigned to three sequences and received one of the following treatments at each period: tamsulosin HCl 0.2 or 0.4 mg in the fed state with a high-fat meal, or tamsulosin HCl 0.4 mg in the fasted state. Blood samples for the PK analysis were collected at pre-dose and up to 48 h post-dose. The PK parameters were calculated by a non-compartmental method. The geometric mean ratio (GMR) and its 90% confidence intervals (CIs) of the plasma maximum concentration (Cmax) and area under concentration curve from time zero to last measurable concentration (AUClast) were calculated. Twenty-two subjects completed the study. The systemic exposure of tamsulosin 0.4 mg decreased approximately 9% in the fed state compared to the fasted state, and the time to reach peak concentration was slightly delayed in the fed state. The dose normalized GMR and its 90% CIs of Cmax and AUClast for 0.2 and 0.4 mg tamsulosin in the fed state were within 0.8 and 1.25 range. Systemic exposure of tamsulosin was decreased in the fed condition compared to the fasted condition. Linear PK profiles were observed between 0.2 and 0.4 mg tamsulosin in the fed state.
Trial Registration
ClinicalTrials.gov Identifier: NCT02529800
Keywords: Tamsulosin, Bioavailability, Pharmacokinetics
INTRODUCTION
Benign prostate hyperplasia (BPH) is one of the common diseases in aging males, affecting nearly half of men aged over 50 years and nearly 90% of men over 80 years in general populations [1]. The most common complication of BPH is acute urinary retention that diminishes quality of life. However, sometimes BPH can lead to more severe complications such as urinary tract infections, formation of bladder stones and damage to the bladder wall and kidneys [2]. The medical treatments with alpha-blockers or 5-alpha-reductase inhibitors are first-line therapy for the medical treatment of BPH at present [3]. Alpha-blockers relax the smooth muscles of the prostate, therefore improving urinary flow and low urinary tract symptoms [4].
Tamsulosin, a first subtype-selective alpha-1 antagonist, is one of the preferred alpha- blockers due to its relatively lower rate of adverse events (AEs) compared with other alpha-blockers [4]. Tamsulosin is known to exhibit linear pharmacokinetic (PK) characteristics between 0.1 to 1.0 mg [5,6]. The majority of the absorbed tamsulosin is distributed in the extracellular fluids of the body and mostly metabolized by hepatic enzymes such as CYP3A4 and CYP2D6 [5]. The oral absorption of tamsulosin hydrochloride (HCl) has been known to be food-sensitive. When tamsulosin is administered with food, the absorption rate and the maximum plasma concentration (Cmax) are decreased compared to its administration in the fasted state. However, the overall plasma exposure of tamsulosin, represented by the area under the plasma concentration-time curve (AUC), is similar regardless of food intake [5,6]. To decrease the Cmax related AEs, such as orthostatic hypotension, it is recommended tamsulosin be administered after a meal as per the drug label [6].
The standard treatment regimen of tamsulosin for BPH is different between Western and Asian countries, and the difference in body weight between ethnic groups is one of the reasons for it [4,7]. Currently, a initial daily dose of 0.4 mg tamsulosin is recommended in western countries. In some Asian countries, such as Korea and China, a lower dose (0.2 mg) is initially used, and the dose is titrated according to the patients' drug responses [8,9,10]. However, the clinical evidence for using 0.2 mg tamsulosin as a standard regimen for the treatment of BPH in Asian population is insufficient, and 0.4 mg tamsulosin was more effective than 0.2 mg tamsulosin for the initial medication therapy of BPH in several recent studies conducted in Korea [11,12]. For these reasons, Hanmi Tams Caps. 0.4 mg (Hanmi Pharmaceutical Co., Seoul, Korea), a tamsulosin 0.4 mg formulation, was developed for the initial treatment of BPH for improving medical adherence.
Based on these understandings, the purpose of the present study was to evaluate the PK characteristics including the effect of food on the oral bioavailability and tolerability profile of a novel tamsulosin 0.4 mg formulation. Moreover, this study also aimed to compare the PK characteristics and tolerability of novel tamsulosin 0.4 mg formulation versus the existing tamsulosin 0.2 mg formulation in healthy male subjects at fed states.
METHODS
Study design and subjects
This study was an open-label, single-dose, sequence randomized, three-way crossover design. Hanmi Tams Cap. 0.4 mg (tamsulosin HCl 0.4 mg capsule) and Hanmi Tams Cap. 0.2 mg (tamsulosin HCl 0.2 mg capsule) were used as the test and reference drugs, respectively, and both of the drugs were manufactured by Hanmi Pharmaceutical Co., Ltd (Seoul, Republic of Korea). Eligible subjects were randomized into three treatment sequences (Fig. 1) and received a single dose of the following three treatments in a crossover manner: test drug with food (A) or test drug without food (B), or reference drug with food (C). There were 7 days of washout between each treatment period, which was longer than five times of the terminal elimination half-life (t1/2) of tamsulosin [5,6]. When subjects received the study drug with food (i.e., treatment A and C), all of the subjects had to ingest a high-fat meal within 20 minutes prior to receiving the study drug in accordance with the regulatory guidance of food effect studies [13]. Blood samples were collected for PK analysis, and tolerability was assessed throughout the study. The tolerability was assessed by monitoring AEs, clinical laboratory tests, vital signs, and 12-lead electrocardiograms (ECG).
Figure 1. Study design.
0.2 mg, single administration of tamsulosin 0.2 mg; 0.4 mg, single administration of tamsulosin 0.4 mg.
A total of 24 healthy Korean male subjects aged 19–50 years with a body mass index (BMI) of 18.0 to 27.0 kg/m2 were enrolled in this study, and their eligibility was assessed by physical examination, clinical laboratory tests, 12-lead ECG, and medical histories. Participants were provided a written informed consent form before the start of any study-related procedures. All the subjects were not allowed to take any medication, alcohol, smoking, caffeine-containing food, or grapefruit containing food during the entire period of study. And the subjects had to avoid irregular activities such as excessive exercise.
This clinical study (NCT number 02529800) was approved by the Institutional Review Board at Seoul National University Hospital, Seoul, Republic of Korea (IRB number 1507-148-692), and by the Ministry of Food and Drug Safety of Republic of Korea. All of the study-related procedures were conducted in accordance with the principles of the Declaration of Helsinki and ICH Good Clinical Practice.
Blood sample collection and analysis
The plasma samples for the PK analysis were collected at 0 (i.e., pre-dose), 1, 2, 3, 4, 5, 6, 8, 12, 24, 36, and 48 h after dosing in each period. The plasma samples were collected using EDTA-K2 containing tubes and stored at −70°C until bioanalysis.
The plasma samples of tamsulosin were measured by a validated assay method using Liquid chromatography (Shimadzu UFLC, Shimadzu, Japan) coupled with tandem mass spectrometry (TQ5500, ABSCIEX, USA). The assay method consisted of liquid/liquid extraction with 50% acetonitrile, reversed-phase chromatography, and atmospheric pressure chemical ionization (APCI) detection. Tamsulosin HCl was calibrated and quantified using tamsulosin-d4 HCl as an internal standard. Briefly, the internal standard was added to 0.2 mL of a plasma sample and then extracted under alkaline conditions. The extract was centrifuged, and the supernatant was evaporated. The residue was dissolved in an aqueous mobile phase consisting of acetonitrile and ammonium formate 1 mM (75/25;v/v), and an aliquot of dissolved residues was injected into the LC-MS/MS system. The analytical procedure in human plasma was linear over a concentration range from 0.1 to 40 ng/mL. The in-study imprecision for the quality control samples was less than 15%, and the accuracy range was within 85% to 115%.
PK data assessment
A non-compartmental method was used to evaluate the following PK parameters of tamsulosin using the WinNonlin® version 7.0 Software (Certara, Princeton, NJ, USA): AUC from time 0 to the last quantifiable concentration (AUClast), AUC from 0 to infinity (AUCinf), Cmax, time to reach Cmax (Tmax), t1/2, apparent clearance (CL/F), and apparent volume of distribution (Vz/F).
Statistical analysis
The demographic characteristics of the subjects, tolerability data, and PK parameters were summarized using descriptive statistics. A one-way analysis of variance (ANOVA) model was applied to detect differences in the age, height, weight, and BMI between the sequences. A general linear mixed effects-model was developed using log-transformed data to compare the PK parameters (AUClast, AUCinf, and Cmax) between the three treatments. The geometric mean ratio (GMR) and its 90% confidence interval (CI) between the treatment groups were estimated using the model. Statistical analysis was performed using the SAS software version 9.4 (SAS institute, Cary, NC, USA)
RESULTS
Demographic characteristics
A total of 24 subjects were enrolled and assigned to 1 of the 3 sequence groups (Fig. 1). Among them, 1 subject each in sequence group 1 and 3 dropped out of the study, and a total of 22 subjects completed the study as planned. The mean ± standard deviation values of the demographic characteristics of the enrolled subjects were 28.0 ± 6.5 years for age, 1.75 ± 0.06 m for height, 71.4 ± 7.9 kg for weight, and 23.4 ± 2.0 kg/m2 for BMI. All demographic characteristics in each sequence group were not statistically different (Table 1).
Table 1. Demographic characteristics of the subjects.
| Parameters | Sequence 1 (n = 8) | Sequence 2 (n = 8) | Sequence 3 (n = 8) | p-value* |
|---|---|---|---|---|
| Age (yr) | 27 ± 7.0 | 29 ± 9.0 | 29 ± 2.0 | 0.7959 |
| Height (cm) | 174.1 ± 8.3 | 174.4 ± 6.1 | 175.3 ± 4.5 | 0.9275 |
| Weight (kg) | 73.0 ± 7.9 | 71.2 ± 9.2 | 69.9 ± 7.1 | 0.7569 |
| BMI (kg/m2) | 24.1 ± 2.3 | 23.3 ± 2.0 | 22.7 ± 1.6 | 0.3944 |
Data are presented as the mean ± standard deviation.
*Analysis of variance.
Comparison of the PK characteristics between the tamsulosin 0.2 and 0.4 mg
The Cmax of tamsulosin was about 2-fold increased as the tamsulosin dose was increased from 0.2 to 0.4 mg, and Tmax and the terminal slopes were similar between the fed groups (Figs. 2 and 3). The GMRs (90% CI) for dose-normalized AUClast, AUCinf, and Cmax of the tamsulosin 0.4 to 0.2 mg were 1.02 (0.96–1.09), 0.99 (0.93–1.06), and 1.21 (1.09–1.34), respectively (Table 2). The other PK parameters, including CL/F, Vz/F, Tmax and t1/2, were also comparable between the tamsulosin 0.2 and 0.4 mg dose groups (Table 2).
Figure 2. Mean plasma concentration-time profiles of tamsulosin (A) in linear scale and (B) in semi-log scale after single oral administration of tamsulosin 0.2 mg (filled triangles) and tamsulosin 0.4 mg under fed conditions (filled circles) and tamsulosin 0.4 mg under fasting conditions (blank circles). Error bars denote the standard deviations.
Figure 3. Comparison of dose-normalized (A) Cmax and (B) AUClast between the tamsulosin 0.2 mg and tamsulosin 0.4 mg capsule under fed condition.
Cmax, maximum plasma concentration; AUClast, area under the curve from the time of administration to last measurable concentration.
Table 2. Comparison of the pharmacokinetic parameters of tamsulosin 0.2 and 0.4 mg capsules in the fed state.
| Pharmacokinetic parameters | Tamsulosin 0.4 mg under fed state (n = 22) | Tamsulosin 0.2 mg under fed state (n = 22) | Geometric mean ratio (90% confidence interval) |
|---|---|---|---|
| Tmax (h) | 5.00 [3.00–10.00] | 6.00 [4.00–10.00] | |
| Cmax (μg/L) | 13.43 ± 4.11 | 5.51 ± 1.76 | 2.41 (2.18–2.68) |
| AUClast (h·μg/L) | 185.94 ± 56.30 | 90.76 ± 25.01 | 2.04 (1.93–2.18) |
| AUCinf (h·μg/L) | 199.61 ± 66.57 | 100.30 ± 29.67 | 1.98 (1.86–2.11) |
| Cmax/dose (/L) | 0.034 ± 0.010 | 0.028 ± 0.009 | 1.21 (1.09–1.34) |
| AUClast/dose (h/L) | 0.46 ± 0.14 | 0.45 ± 0.13 | 1.02 (0.96–1.09) |
| AUCinf/dose (h/L) | 0.50 ± 0.17 | 0.50 ± 0.15 | 0.99 (0.93–1.06) |
| Vz/F (L) | 34.35 ± 11.11 | 39.10 ± 13.87 | |
| CL/F (L/h) | 2.22 ± 0.76 | 2.22 ± 0.82 | |
| t1/2 (h) | 11.19 ± 3.02 | 12.56 ± 2.32 |
Data are presented as the mean ± standard deviation except for Tmax which is expressed as the median [minimum-maximum].
Cmax, maximum plasma concentration; Tmax, time of maximum plasma concentration; AUClast, area under the curve from the time of administration to last measurable concentration; AUCinf, AUC from administration to infinity based on the last concentration; Cmax/dose, dose-normalized maximum plasma concentration; AUClast/dose, dose-normalized AUClast; AUCinf/dose, dose-normalized AUCinf; Vz/F, apparent volume of distribution during the terminal phase; CL/F, apparent clearance; t1/2, terminal elimination half-life.
Effect of food on the PK characteristics of tamsulosin 0.4 mg
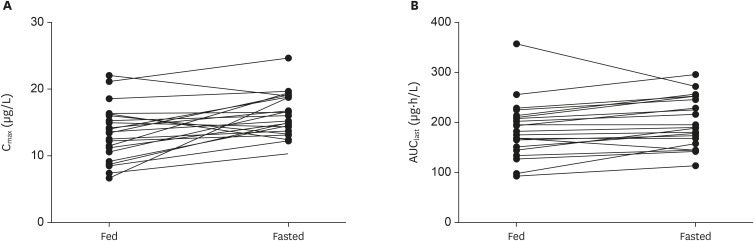
The Cmax of tamsulosin was about 20% lower when the tamsulosin 0.4 mg was administered with a high-fat meal compared to administration under the fasting condition, but the overall plasma concentration of tamsulosin was comparable between those 2 conditions (Figs. 2 and 4). The GMRs (90% CI) for the AUClast, AUCinf, and Cmax of the fed condition to the fasted condition were 0.91 (0.86–0.97), 0.93 (0.87–0.99) and 0.80 (0.72–0.89), respectively (Table 3). The plasma concentration-time profile of tamsulosin during the absorption process was different between the fed and fasted condition, although those profiles during the elimination process were similar (Fig. 2) The median Tmax was prolonged about 0.5 h when the tamsulosin 0.4 mg was administered with a high-fat meal compared to the administration under fasting condition, but the t1/2 was comparable between the fed and fasted condition (Table 3).
Figure 4. Comparison of (A) Cmax and (B) AUClast of tamsulosin after oral administration of tamsulosin 0.4 mg with or without food.
Cmax, maximum plasma concentration; AUClast, area under the curve from the time of administration to last measurable concentration.
Table 3. Effect of food on the pharmacokinetic parameters of the tamsulosin 0.4 mg capsule.
| Pharmacokinetic parameters | Tamsulosin 0.4 mg under fed state (n = 22) | Tamsulosin 0.4 mg under fasted state (n = 22) | Geometric mean ratio (90% confidence interval) |
|---|---|---|---|
| Tmax (h) | 5.00 [3.00–10.00] | 4.50 [2.00–6.00] | |
| Cmax (μg/L) | 13.43 ± 4.11 | 16.15 ± 3.26 | 0.80 (0.72–0.89) |
| AUClast (h·μg/L) | 185.94 ± 56.30 | 201.08 ± 48.12 | 0.91 (0.86–0.97) |
| AUCinf (h·μg/L) | 199.61 ± 66.57 | 211.87 ± 53.51 | 0.93 (0.87–0.99) |
| Vz/F (L) | 34.35 ± 11.11 | 30.99 ± 6.68 | |
| CL/F (L/h) | 2.22 ± 0.76 | 2.01 ± 0.54 | |
| t1/2 (h) | 11.19 ± 3.02 | 10.93 ± 1.95 |
Data are presented as the mean ± standard deviation except for Tmax which is expressed as the median [minimum-maximum].
Cmax, maximum plasma concentration; Tmax, time of maximum plasma concentration; AUClast, area under the curve from the time of administration to last measurable concentration; AUCinf, AUC from administration to infinity based on the last concentration; Cmax/dose, dose-normalized maximum plasma concentration; AUClast/dose, dose-normalized AUClast; AUCinf/dose, dose-normalized AUCinf; Vz/F, apparent volume of distribution during the terminal phase; CL/F, apparent clearance; t1/2, terminal elimination half-life.
Tolerability
A single administration of 0.2 or 0.4 mg tamsulosin in the fed condition or 0.4 mg tamsulosin in the fasting condition generally was well-tolerated by the subjects. During this clinical study, 13 of the 24 subjects (54.2%) reported a total of 17 AEs. All the AEs were assessed as mild in severity and no serious AE was reported. The most common AEs were dizziness, and there were no subjects with hypotension, which is a well-known adverse drug reaction of tamsulosin [14]. There were no clinically significant changes in vital signs, clinical laboratory tests, 12-lead ECGs, and physical exams.
DISCUSSION
This study was the first study that evaluated the PK and tolerability profile of a novel tamsulosin 0.4 mg formulation in Korean. In this study, the plasma concentration of a tamsulosin 0.4 mg capsule was about 2-fold higher compared to the conventional tamsulosin 0.2 mg capsule, and both were well tolerated in those healthy subjects. The mean plasma concentrations-time profiles of tamsulosin were similar between the tamsulosin 0.2 and 0.4 mg capsules, which exhibited first-order elimination profiles (Fig. 2). The GMRs and 90% CI for the dose normalized AUClast and AUCinf of tamsulosin 0.4 to 0.2 mg were within the conventional bioequivalence criteria, and the Tmax and t1/2 were similar between the dose groups. And both dose groups were well tolerated by the subjects based on the AEs, vital signs, clinical laboratory tests, 12-lead ECGs, and physical exams.
The oral absorption of tamsulosin was delayed, and the relative bioavailability was decreased when the tamsulosin 0.4 mg capsules were administered with food compared to its administration in the fasted state. In this study, the GMRs for Cmax and AUClast were 20% and 9% lower, respectively, and the median Tmax was about 0.5 h delayed when the tamsulosin 0.4 mg capsule was administered in the fed state compared to the fasted state (Fig. 2 and Table 3). Because the elimination profiles and t1/2 were similar regardless of food consumption, we can conclude that the oral absorption and distribution process of tamsulosin were mainly affected by the food rather than the elimination processes [15]. Those effects of food on tamsulosin's PK is corresponding to the known PK characteristics of tamsulosin, although the relative bioavailability was much more decreased (24 percent on average) in the previous clinical study [6]. Because food can decrease the Cmax of tamsulosin, thereby reducing concentration-dependent AEs, tamsulosin should be administered after a meal [4,6,14].
The study methods were appropriate to evaluate the PK characteristics of the tamsulosin 0.4 mg capsule. The study was performed by a randomized 3-period, 3-treatment crossover design to adjust for the inter-subject variability when comparing the treatment effects. The number of subjects was sufficient to summarize the PK parameters of the study drugs, and the blood sampling time points were appropriate to describe the plasma concentration profiles of tamsulosin. Because the ratio of AUClast to AUCinf was more than 80% for all the subject, the overall PK sampling period was sufficient for evaluation of the overall drug exposure [16]. The washout period in this study (7 days) was longer than 5 times of the tamsulosin half-life, which was sufficient to eliminate the carryover effect [16]. Pre-dose drug concentrations of the subsequent periods were all below the lower limit of quantification.
This study was done in young healthy males to minimize potential confounding factors, although the typical BPH patient population are elderly males. Hepatic drug clearance can be decreased in elderly subjects due to the decrement of cardiac output and to the reduction of liver volume [17]. However, the PK parameters of the tamsulosin 0.4 mg capsule in our young subjects were similar to those of elderly subjects in a previous study [18]. Therefore, our study result will be applicable to typical elderly BPH patients.
In conclusion, the systemic exposure to tamsulosin 0.4 mg capsule was about 2-fold higher when compared to the existing 0.2 mg tamsulosin capsule, and the oral bioavailability was decreased when administered with food. The 0.4 mg tamsulosin capsule was tolerated in the healthy subjects. Therefore, the 0.4 mg tamsulosin capsule could be used as an alternative therapeutic option for 2 capsules of tamsulosin 0.2 mg to enhance compliance in the patients.
Footnotes
Reviewer: This article was reviewed by peer experts who are not TCP editors.
Conflicts of Interest: - Authors: Jung J and Kim YI are employees of Hanmi Pharmaceutical Co., Ltd. The other authors report no conflicts of interest in this work.
- Reviewers: Nothing to declare
- Editors: Nothing to declare
- Conceptualization: Yu KS.
- Formal analysis: Kim YK, Oh J.
- Funding acquisition: Jung J, Kim YI, Yu KS.
- Investigation: Kim YK, Oh J, Yu KS.
- Project administration: Jung J, Kim YI, Yu KS.
- Resources: Jung J, Kim YI.
- Supervision: Kim YK, Oh J, Yu KS.
- Validation: Ban MS.
- Visualization: Ban MS.
- Writing - original draft: Ban MS.
- Writing - review & editing: Kim YK, Kim B, Oh J, Yu KS.
References
- 1.Napalkov P, Maisonneuve P, Boyle P. Worldwide patterns of prevalence and mortality from benign prostatic hyperplasia. Urology. 1995;46(Suppl A):41–46. doi: 10.1016/s0090-4295(99)80249-0. [DOI] [PubMed] [Google Scholar]
- 2.Barkin J. Benign prostatic hyperplasia and lower urinary tract symptoms: evidence and approaches for best case management. Can J Urol. 2011;18(Suppl):14–19. [PubMed] [Google Scholar]
- 3.Yeo JK, Choi H, Bae JH, Kim JH, Yang SO, Oh CY, et al. Korean clinical practice guideline for benign prostatic hyperplasia. Investig Clin Urol. 2016;57:30–44. doi: 10.4111/icu.2016.57.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilde MI, McTavish D. Tamsulosin. A review of its pharmacological properties and therapeutic potential in the management of symptomatic benign prostatic hyperplasia. Drugs. 1996;52:883–898. doi: 10.2165/00003495-199652060-00012. [DOI] [PubMed] [Google Scholar]
- 5.Franco-Salinas G, de la Rosette JJ, Michel MC. Pharmacokinetics and pharmacodynamics of tamsulosin in its modified-release and oral controlled absorption system formulations. Clin Pharmacokinet. 2010;49:177–188. doi: 10.2165/11317580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Boehringer Ingelheim. Flomax (tamsulosin hydrochloride) [package insert] Ridgefield (CT): Boehringer Ingelheim; 2007. [Google Scholar]
- 7.Dunn CJ, Matheson A, Faulds DM. Tamsulosin: a review of its pharmacology and therapeutic efficacy in the management of lower urinary tract symptoms. Drugs Aging. 2002;19:135–161. doi: 10.2165/00002512-200219020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Park CH, Chang HS, Oh BR, Kim HJ, Sul CK, Chung SK, et al. Efficacy of low-dose tamsulosin on lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a nonblind multicentre Korean study. Clin Drug Investig. 2004;24:41–47. doi: 10.2165/00044011-200424010-00005. [DOI] [PubMed] [Google Scholar]
- 9.Lee E. Comparison of tamsulosin and finasteride for lower urinary tract symptoms associated with benign prostatic hyperplasia in Korean patients. J Int Med Res. 2002;30:584–590. doi: 10.1177/147323000203000606. [DOI] [PubMed] [Google Scholar]
- 10.Li NC, Chen S, Yang XH, Du LD, Wang JY, Na YQ, et al. Efficacy of low-dose tamsulosin in Chinese patients with symptomatic benign prostatic hyperplasia. Clin Drug Investig. 2003;23:781–787. doi: 10.2165/00044011-200323120-00003. [DOI] [PubMed] [Google Scholar]
- 11.Kim JJ, Han DH, Sung HH, Choo SH, Lee SW. Efficacy and tolerability of tamsulosin 0.4 mg in Asian patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia refractory to tamsulosin 0.2 mg: a randomized placebo controlled trial. Int J Urol. 2014;21:677–682. doi: 10.1111/iju.12412. [DOI] [PubMed] [Google Scholar]
- 12.Chung JH, Oh CY, Kim JH, Ha US, Kim TH, Lee SH, et al. Efficacy and safety of tamsulosin 0.4 mg single pills for treatment of Asian patients with symptomatic benign prostatic hyperplasia with lower urinary tract symptoms: a randomized, double-blind, phase 3 trial. Curr Med Res Opin. 2018;34:1793–1801. doi: 10.1080/03007995.2018.1447451. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration. Assessing the effects of food on drugs in INDs and NDAs – clinical pharmacology considerations. Silver Spring (MD): Food and Drug Administration; 2019. [Google Scholar]
- 14.Michel MC, Korstanje C, Krauwinkel W. Cardiovascular safety of tamsulosin modified release in the fasted and fed state in elderly healthy subjects. Eur Urol Suppl. 2005;4:9–14. [Google Scholar]
- 15.Koziolek M, Alcaro S, Augustijns P, Basit AW, Grimm M, Hens B, et al. The mechanisms of pharmacokinetic food-drug interactions - a perspective from the UNGAP group. Eur J Pharm Sci. 2019;134:31–59. doi: 10.1016/j.ejps.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. Bioavailability and bioequivalence studies submitted in NDAs or INDs — general considerations (draft guidance) Silver Spring (MD): Food and Drug Administration; 2014. [Google Scholar]
- 17.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. doi: 10.1046/j.1365-2125.2003.02007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolzt M, Fabrizii V, Dorner GT, Zanaschka G, Leufkens P, Krauwinkel WJ, et al. Pharmacokinetics of tamsulosin in subjects with normal and varying degrees of impaired renal function: an open-label single-dose and multiple-dose study. Eur J Clin Pharmacol. 1998;54:367–373. doi: 10.1007/s002280050477. [DOI] [PubMed] [Google Scholar]