Abstract
Suppressors of cytokine signaling (SOCS) exhibit diverse anti-inflammatory effects. Since ROS acts as a critical mediator of inflammation, we have investigated the anti-inflammatory mechanisms of SOCS via ROS regulation in monocytic/macrophagic cells. Using PMA-differentiated monocytic cell lines and primary BMDMs transduced with SOCS1 or shSOCS1, the LPS/TLR4-induced inflammatory signaling was investigated by analyzing the levels of intracellular ROS, antioxidant factors, inflammasome activation, and pro-inflammatory cytokines. The levels of LPS-induced ROS and the production of pro-inflammatory cytokines were notably down-regulated by SOCS1 and up-regulated by shSOCS1 in an NAC-sensitive manner. SOCS1 up-regulated an ROS-scavenging protein, thioredoxin, via enhanced expression and binding of NRF-2 to the thioredoxin promoter. SOCS3 exhibited similar effects on NRF-2/thioredoxin induction, and ROS downregulation, resulting in the suppression of inflammatory cytokines. Notably thioredoxin ablation promoted NLRP3 inflammasome activation and restored the SOCS1-mediated inhibition of ROS and cytokine synthesis induced by LPS. The results demonstrate that the anti-inflammatory mechanisms of SOCS1 and SOCS3 in macrophages are mediated via NRF-2-mediated thioredoxin upregulation resulting in the downregulation of ROS sig-nal. Thus, our study supports the anti-oxidant role of SOCS1 and SOCS3 in the exquisite regulation of macrophage activation under oxidative stress.
Keywords: Inflammasome, Reactive oxygen species (ROS), Suppressors of cytokine signaling (SOCS), Thioredoxin, TLR4 signal
INTRODUCTION
Inflammation is a front-line defense mechanism triggered by exposure to various antigens and involves primarily macrophages, neu-trophils and dendritic cells. These cells recognize pathogens with pattern recognition receptors such as TLRs, perform phagocytosis, and regulate the activation of other cells by producing in-flammatory cytokines (1).
The generation of reactive oxygen species (ROS) is a key feature of phagocytic cells via oxidative burst, which mediates many aspects of inflammatory reactions (2). While ROS are essential for anti-microbial defense via production of pro-inflammatory cytokines, they have been implicated in diverse inflammatory and autoimmune diseases (3, 4). ROS regulation is thus considered important in immune homeostasis. Indeed, ROS broadly participate in the modulation of immune responses via activation of cellular signaling pathways involving tyrosine kinases, tyrosine phosphatases, mitogen-activated protein kinases (MAPK), and NF-κB (5, 6). However, the biological mechanisms underlying these processes are not always clear (7).
Lipopolysacharride (LPS) is a principal active agent, which induces a strong inflammatory response by stimulating monocytes and macrophages. LPS is recognized by TLR4, which then triggers MyD88- and TRIF-dependent pathways, leading to the activation of transcription factors NF-κB and IFN regulatory factor 3 (IRF3), respectively, resulting in the production of inflammatory cytokines including TNF-α, IL-1, IL-6 and IFNs (8, 9).
Suppressor of cytokine signaling 1 (SOCS1) was first characterized as a regulator of IFN-γ-induced inflammatory responses, via inhibition of Jak/STAT pathways (10). SOCS1 expression is increased in macrophages following LPS exposure, and represents a negative feed-back mechanism of LPS signaling (11, 12). The inhibition of NF-κB, as well as the downregulation of MyD88-associated adaptor (MAL) and TRAF6 by SOCS1 via SOCS box-mediated protein degradation have been suggested as possible modes of SOCS action regulating the LPS response (13). However, the molecular mechanisms underlying SOCS1 regulation of pro-inflammatory cytokine production appear rather complex involving both direct and indirect mechanisms (14).
We have previously reported the inhibitory action of SOCS1 on ROS-mediated signaling pathways during T cell apoptosis in response to TNF-α and oxidative stress (15). The anti-apoptotic activity of SOCS1 was demonstrated using both over-expression and knockdown systems, via protection of protein tyrosine phosphatases as well as direct interaction with Jaks required for the apoptosis signaling. SOCS1 was induced as a protective response to ROS-generating apoptotic stimuli, which represented an intracellular defense mechanism against oxidative stress (15).
Since ROS also acts as a critical mediator of inflammatory signaling, we have investigated the anti-inflammatory mechanism of SOCS action in macrophages during LPS/TLR4 signal via ROS regulation. Our data indicate that SOCS1 and SOCS3 attenuate LPS/TLR4 signals leading to the induction of pro-inflammatory cytokines via ROS downregulation. Specifically, the inhibition of inflammasome activation and induction of thioredoxin were suggested as mechanisms of SOCS action involving ROS suppression to inhibit IL-1β and IL-6, respectively. We further demonstrate that the SOCS1-mediated upregulation of thioredoxin expression involves NRF-2 transcription factor and its binding to the thioredoxin promoter. Taken together, the results of the present study strongly suggest ROS downregulation as a mechanism of anti-inflammatory action of SOCS during macrophage activation.
RESULTS
SOCS1 down-regulates intracellular ROS to suppress LPS/TLR4 signaling for inflammatory cytokine production
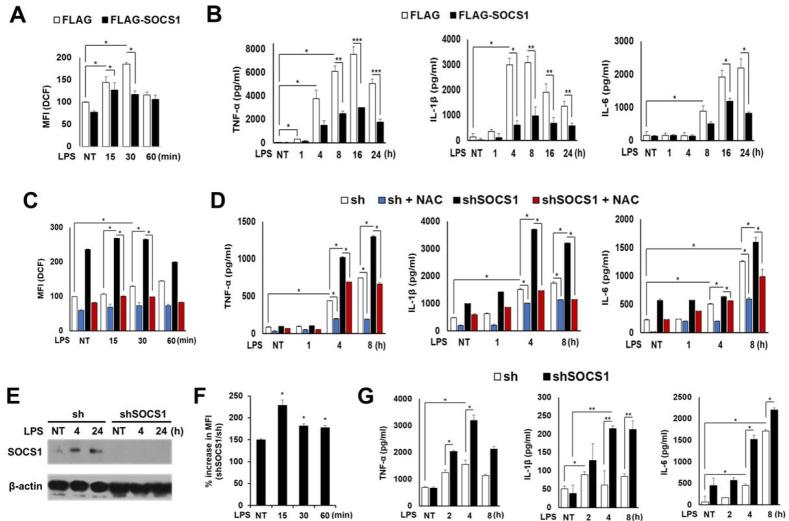
In order to investigate the regulation mechanism of SOCS action in inflammatory signaling, human monocytic cell lines (THP1) expressing inflammatory receptors (e.g., TLRs) were used to establish SOCS1 over-expression or knockdown systems. Initially THP1 cells were subjected to viral transduction of Flag (mock) or Flag-SOCS1 construct using retroviral vectors. Cells were then treated with PMA to induce differentiation prior to stimulation with LPS, a TLR4 agonist. While both mock and SOCS1-transduced cells responded to PMA by up-regulating TLR4 expression, they exhibited similar levels of surface TLR4 regardless of SOCS1 transduction (Supplementary Fig. 1). As LPS-induced ROS generation is thought to play a key role in the initiation of inflammatory signaling, we have determined the intracellular ROS levels upon LPS stimulation. The kinetics revealed that ROS levels induced by LPS peaked in 15 to 30 min, followed by eventual decline in 60 min. Notably, SOCS1-transduced cells showed a substantial decrease in ROS levels when compared with mock cells (Fig. 1A). LPS then induced production of inflammatory cytokines such as TNF-α, IL-1β and IL-6 by 24 h. Upon SOCS1 over-expression, the production of these cytokines was prominently reduced by 40% to 70% (Fig. 1B).
Fig. 1.
Inhibitory effects of SOCS1 on pro-inflammatory cytokine production and ROS generation induced by LPS in THP1 human monocytic cells and mouse BMDMs. Flag or Flag-SOCS1 THP1 cells were differentiated by PMA. Cells were then either not treated (NT) or treated with LPS (1 µg/ml) for the indicated duration. ROS levels were determined (A). The culture supernatants were analyzed to measure inflammatory cytokines by ELISA (B). The sh control and shSOCS1-transduced THP1 cells were stimulated with LPS with or without NAC pretreatment, after which ROS (C) and cytokine levels (D) were determined. BMDMs from C57BL/6 mice were transduced with mouse shSOCS1. The SOCS1 levels were analyzed by western blot (E). ROS levels in shSOCS1 cultures were analyzed as % increase over mock (sh) cultures (F). LPS-induced cytokine production was determined by ELISA (G). Results represent means ± SD obtained from three independent experiments performed in triplicate (*P < 0.05; +n.s.).
In contrast to cells over-expressing SOCS1, the SOCS1-ablated cells exhibited significantly elevated ROS levels. Both the basal and LPS-induced ROS levels were upregulated by shSOCS1 transduction, which was abrogated by treatment with an anti-oxidant NAC (Fig. 1C), indicating that SOCS1 negatively regulates cellular ROS levels. The shSOCS1 cells induced a 2- to 3-fold increase in TNF-α, IL-1β and IL-6 levels compared with mock (sh) cells. NAC not only impaired LPS-induced pro-inflammatory cytokine production in mock cells, but also almost completely blocked the excessive cytokine production in shSOCS1 cells by 4 to 8 h (Fig. 1D). The data indicate that enhanced ROS levels observed in SOCS1-ablated cells may be responsible for the increased production of inflammatory cytokines. The inhibitory effect of SOCS1 on LPS-induced ROS generation and cytokine production in primary cells was also analyzed in bone marrow-derived macrophages (BMDMs) isolated from C57BL/6 mice. The shSOCS1-transduced BMDM cultures exhibited increased ROS levels compared with mock cultures. As seen in THP1 cells, the LPS-induced inflammatory cytokine levels were generally enhanced in SOCS1-ablated BMDMs (Fig. 1E-G).
The role of MAPKs and NF-κB has been widely suggested in the principal signaling pathways evoked by LPS/TLR4 for pro-inflammatory cytokine production (16, 17). We found that the early induction of p-Erk, p-Jnk, and p-p38 by LPS in THP1 cells was reduced in SOCS1-expressing cells (Supplementary Fig. 2A). The NF-κB activation, indicated by the peak nuclear p65 levels after 30 min, was also significantly down-regulated by SOCS1 transduction. In addition, phosphoserine (pS)-STAT1 and phosphotyrosine (pY)-STATs 1, 3 and 5 induced by LPS from 30 to 240 min, were significantly suppressed in SOCS1-over-expressing cells (Supplementary Fig. 2A). In contrast, the LPS-induced activation of MAPKs, NF-κB, and STATs was promoted by SOCS1 ablation and completely inhibited by NAC, which again suggests ROS as a SOCS1 target during LPS-induced inflammation signaling (Supplementary Fig. 2B).
The anti-oxidant NRF-2 and thioredoxin are selectively upregulated by SOCS1
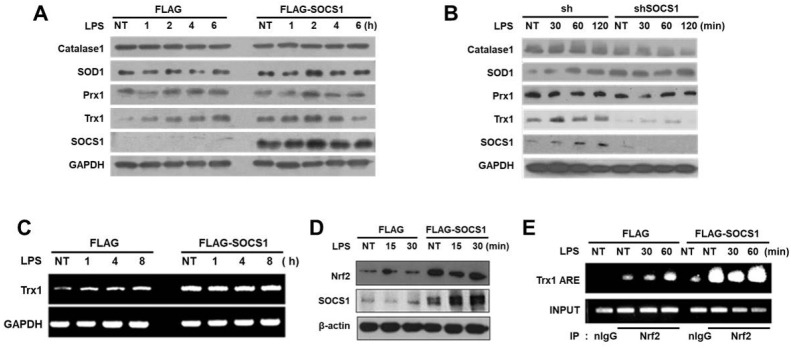
The above data indicate that the downregulation of intracellular ROS by SOCS1 contributes to the anti-inflammatory effects of SOCS1 in both monocytic/macrophagic cell lines and primary macrophages, suggesting that ROS-scavenging proteins mediate the action of SOCS1. Analysis of various anti-oxidant enzymes revealed that thioredoxin is the primary target regulated by SOCS1 and shSOCS1 (Fig. 2A and 2B). While LPS stimulation of mock cells induced a gradual increment in thioredoxin mRNA and protein levels, the basal levels of thioredoxin in SOCS1-transdued cells were up-regulated and maintained at increased levels upon LPS stimulation (Fig. 2A and 2C). In contrast, the levels of other anti-oxidant enzymes such as catalase, SOD1, and peroxidase were not significantly affected by SOCS1 or shSOCS1.
Fig. 2.
Thioredoxin expression is upregulated by SOCS1 via increased NRF-2 binding to the ARE of the thioredoxin promoter. THP1 cells transduced with SOCS1 or shSOCS1 were stimulated with LPS and the expression levels of antioxidant proteins were analyzed by immunoblotting (A, B). Thioredoxin levels were confirmed in SOCS1-transduced cells by RT-PCR (C). NRF-2 levels and NRF-2 binding to the ARE of the thioredoxin promoter were assessed by immu-noblotting and CHIP assays as described in Supplementary Materials (D, E).
Next, to investigate the mechanism of thioredoxin upregulation by SOCS1, the activation of NRF-2 transcription factor implicated in the induction of anti-oxidant response genes was analyzed (18). We have noted that NRF-2 levels were elevated by SOCS1 over-expression, similar to increased thioredoxin levels (Fig. 2D). Furthermore, the CHIP assay revealed that while NRF-2 binding to the anti-oxidant response element (ARE) of thioredoxin promoter was gradually increased by 60 min of LPS treatment in mock cells, its binding was notably enhanced in Flag-SOCS1 cells and maintained at high levels (Fig. 2E). The results suggest that SOCS1 exhibits ROS-scavenging effects by inducing thioredoxin mediated by the action of NRF-2 anti-oxidant transcription factor.
SOCS3 exhibits similar inhibitory effects on LPS-induced ROS and inflammatory cytokine production
We have also analyzed the effect of SOCS3 on LPS-induced ROS signaling leading to inflammation response. Although SOCS1 and SOCS3 share common structural features, they exhibit cell type-dependent expression patterns with distinct roles in inflammation (19).
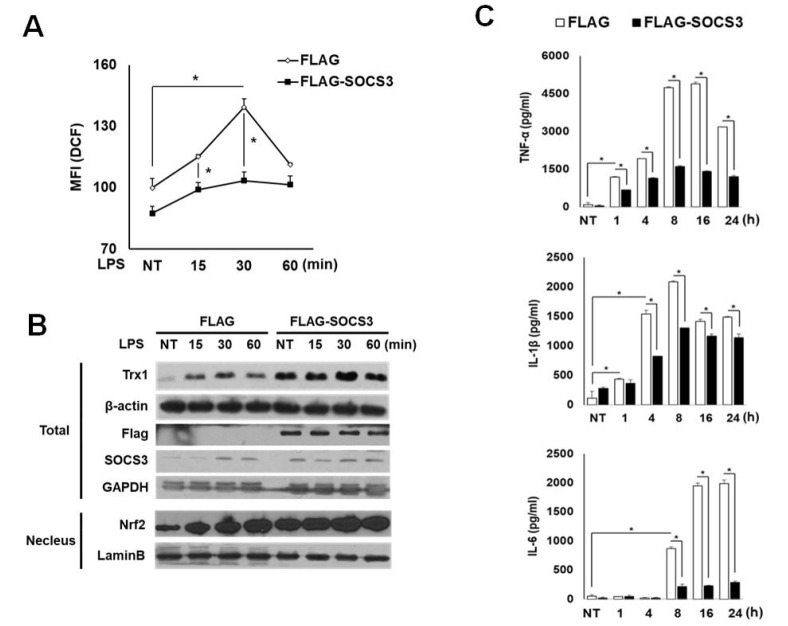
The expression level of SOCS3 is found low in monocytes and macrophages both in vitro and in vivo, unless exposed to inflammatory stimuli (20, 21). While SOCS3 is induced by LPS stimulation within 1 h in mock THP1 cells, the SOCS3-tranduced cells show increased basal SOCS3 levels, which were maintained during LPS treatment. In these cells, a significant down-regulation of LPS-induced ROS levels was correlated with increased expression of NRF2 and TRX1 within 1 h (Fig. 3A and 3B). As in the case for SOCS1, the production of inflammatory cytokines such as TNF-α, IL-1β and IL-6 was strongly suppressed in SOCS3-over-expressing cells (Fig. 3C). Therefore, similar to SOCS1, SOCS3 exhibits prominent inhibitory effects on inflammatory signaling during ROS regulation in response to LPS.
Fig. 3.
Suppressive effects of SOCS3 on LPS-induced ROS and inflammatory cytokine production accompanied by increased NRF-2 and thioredoxin expression. THP1 cells transduced with Flag or Flag-SOCS3 were analyzed for LPS-induced intracellular ROS (A), NRF-2, thioredoxin and SOCS3 levels by Western blot (B), and inflammatory cytokine production by ELISA (C).
Role of thioredoxin in SOCS1-mediated ROS regulation and the suppression of LPS/ROS-induced inflammasome activation by SOCS1
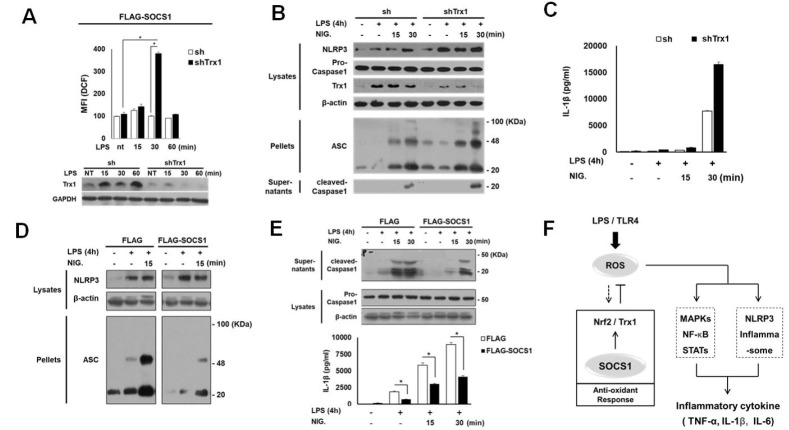
To evaluate whether the increase in thioredoxin levels is responsible for ROS reduction in SOCS over-expressing cells, we investigated the effect of thioredoxin transduction. First, it was observed that thioredoxin gene transduction per se resulted in the suppression of LPS-induced early ROS generation (Supplementary Fig. 3A). Next, to determine the role of thioredoxin in mediating SOCS1 action, the effect of thioredoxin knockdown on LPS-induced ROS generation and cytokine production was analyzed. While Flag-SOCS1 cells exhibited increased thioredoxin and reduced ROS levels, thioredoxin ablation with shTrx introduction into these cells triggered a robust increase in ROS generation by LPS at 30 min (Fig. 4A). The synthesis of inflammatory cytokine IL-6 in both Flag and Flag-SOCS1 cells was upregulated by thioredoxin depletion. Notably, the IL-6 level was re-stored completely in Flag-SOCS1 cells upon thioredoxin ablation both at 4 and 8 h after LPS stimulation (Supplementary Fig. 3B). The result suggests a critical role of thioredoxin in SOCS1-mediated suppression of LPS-induced ROS and cytokine production.
Fig. 4.
Role of thioredoxin in SOCS1-induced suppression of LPS response for ROS generation and inflammasome activation associated with IL-1β production. Flag-SOCS1 THP1 cells were further transfected with sh/shTrx1 and the intracellular ROS levels were analyzed following LPS stimulation (A). The role of Trx1 and SOCS1 in LPS-induced, ROS-promoted inflammasome activation was analyzed. Cells were stimulated with LPS for 4 h and treated with nigericin (NIG) as indicated. Activation of ASC, NLRP3 and caspase-1 was then analyzed by immunoblotting and the secreted IL-1β protein was measured by ELISA (B-E). The proposed model represents the inhibitory mechanism of SOCS1 in TLR4 signaling leading to inflammatory cytokine production via downregulation of ROS (F). The details are described in Supplementary Materials.
The production of inflammatory cytokines in activated macrophages by pathogen sensing occurs via inflammasome assembly, a multi-molecular complex required for caspase 1 activation (22). As inflammasome activation is triggered by ROS (23), we examined the effect of thioredoxin on LPS-induced inflammasome activation leading to caspase1 cleavage for IL-1β maturation. NLRP3 inflammasome was first analyzed in LPS-primed cells by ROS induction with nigericin. The shTrx-transduced cells exhibited a substantial increase in LPS-induced NLRP3 levels, which were further promoted by nigericin. The ASC oligomer formation was induced by LPS and enhanced by nigericin treatment. Subsequently, the shTRX1 transduction promoted the inflammasome assembly leading to caspase-1 cleavage and IL-1β production, indicating that thioredoxin controls LPS/ROS-induced NLRP3 inflammasome activation (Fig. 4B and 4C).
Next, we investigated the possibility that inflammasome activation downstream of TLR4 signaling is regulated by SOCS1. Flag-SOCS1 cells exhibited a significant attenuation of ASC protein synthesis and the ASC oligomer formation induced by the NLRP3 stimulant nigericin (Fig. 4D). This inhibition of assembly correlated strongly with a lack of caspase-1 maturation into its processed 20-kDa form and suppression of IL-1β production (Fig. 4E). The data indicate that both SOCS1 and thioredoxin counteract the LPS-induced inflammasome activation likely via ROS regulation.
DISCUSSION
The present work demonstrates the anti-oxidant function of SOCS in the regulation of inflammatory signaling. Both SOCS1 and SOCS3 increased thioredoxin expression and down-regulated the basal and LPS-induced early ROS generation to attenuate the expression of pro-inflammatory cytokines. The results are consistent with our earlier findings of thioredoxin-promoting effect of SOCS1 resulting in the suppression of the ROS signal-induced apoptosis of Jurkat T cells (15). In the present study, we have further investigated the molecular mechanism of thioredoxin upregulation by SOCS1. As an anti-oxidant defense gene, thioredoxin is considered a target of anti-oxidant transcription factor NRF-2 which binds to the ARE sequence of diverse genes induced under oxidative stress (18, 24). We have in fact identified an ARE-related sequence in the thioredoxin promoter and performed CHIP assays using primers designed to dectect NRF-2 binding. The increases in the basal NRF-2 level and its binding to the thioredoxin promoter correlate with the upregulation of the basal thioredoxin expression in Flag-SOCS1 cells (Fig. 2A and 2C). Currently, the mechanism of NRF-2 upregulation by SOCS1 and SOCS3 is not clear. As NRF-2 level is subject to downregulation by iNRF-2 (Keap1)-mediated degradation, SOCS1 and SOCS3 may competitively inhibit Keap1 action via SOCS-box to bind with Culin/Rbx/E2 ligase required for ubiqutination of NRF-2 (25). The increased basal thioredoxin via NRF-2 upregulation in SOCS1- or SOCS3-transduced cells likey sustains the ROS downregulation in the initial phase of LPS signaling. Such ROS regulation represents an upstream signaling event, which is critical for the downstream response including the production of inflammatory cytokines.
SOCS3 shares common properties with SOCS1 including the structural domains such as KIR, SH2 and SOCS boxes, although their reported functions are often distinct. For example, in contrast to SOCS1, which inhibits the T cell apoptosis induced by hydrogen peroxide and TNF-α via ROS downregulation, SOCS3 promoted the oxidant-induced T cell apoptosis (15). However, in this work, both SOCS1 and SOCS3 strongly inhibited the inflammatory cytokine production involving ROS suppression within 30 min of LPS stimulation. Compared with cells transduced with either SOCS1 or SOCS3, cells transduced with both SOCS1 and SOCS3 exhibited a further inhibition of IL-1 and IL-6 production (Supplementary Fig. 4A), suggesting that SOCS1 and SOCS3 co-operate to down-regulate LPS-induced ROS signaling in inflammatory response. It may also indicate that the two SOCS isoforms utilize distinct additional pathways to synergistically suppress inflammatory signaling. Although both SOCS1 and SOCS3 have been reported to modulate apoptosis induced by ROS and Fas ligation in Jurkat T cells (15, 26), cell death was not affected by SOCS1 or SOCS3 in THP1 cells during LPS treatment (Supplementary Fig. 4B). This finding excludes the possibility that SOCS-mediated suppression of cytokine production occurs due to increased cell death.
The current study also revealed that SOCS1 acts as a potent inhibitor of ROS-mediated NLRP3 inflammasome activation required for caspase 1 activation (Fig. 4). Thus, the anti-inflammatory action of SOCS1 encompasses ROS signal-dependent cytokine induction and processing, which leads to the regulated production of cytokine cascade. The suppressive effect of SOCS1 on inflammasome activation contrasts with the effect of thioredoxin ablation, which promoted the LPS-induced ROS-mediated NLRP3 inflammasome. The data again support the anti-inflammatory mechanism of SOCS1 via thioredoxin induction.
In summary, SOCS1 effectively suppresses LPS-induced signaling and NLRP3 inflammasome by down-regulating intracellular ROS levels via NRF-2/thioredovxin induction (Fig. 4F). During inflammation, SOCS1 is directly induced by ROS upon LPS stimulation (15, 27). SOCS may also be induced by the cytokines produced downstream of LPS signaling as these cells express receptors for inflammatory cytokines (Supplementary Fig. 5). However, the kinetics of ROS-dependent STAT activation observed upstream of cytokine production (Supplementary Fig. 2B) suggest the induction of SOCS in a proximal negative feedback of LPS signaling. The SOCS then likely induces anti-oxidant response for removal of excess ROS triggered by the inflammation. Up-regulation of thioredoxin reduces ROS levels (24) and apparently contributes to the anti-inflammatory action of SOCS in macrophages. Since elevated thioredoxin expression is correlated with increased NRF-2 levels in SOCS-transduced cells, the molecular mechanism by which SOCS upregulates anti-oxidant transcription factor NRF-2 in inflammatory cells warrants further investigation. Such studies are needed to establish the novel function of SOCS as a member of anti-oxidant defense system and its potential therapeutic value in inflammatory diseases caused by ROS deregulation.
MATERIALS AND METHODS
Cell culture and generation of SOCS over-expressing or knockdown cell systems
Human acute monocytic leukemia cell lines (THP1) were maintained in RPMI media containing 10% FBS (Invitrogen, Carsbed) and cultured in a humidified 5% CO2 incubator. The preparation of bone marrow-derived macrophages (BMDMs) from C57BL/6 mice (28) and the viral transduction of SOCS1, SOCS3 and Trx1 genes or sh constructs were performed as detailed in the Supplementary Materials. PMA (100 ng/ml, 16 h)-treated monocytic cells and BMDMs were stimulated by 1 µg/ml LPS (E.Coli 0111:B4, Sigma-Aldrich). Treatment with anti-oxidant NAC (1 mM) was carried out 1 h prior to LPS stimulation.
Determination of intracellular ROS by FACS
Cells were stimulated with LPS for indicated durations and a redox-sensitive cell permeable dye H2DCF-DA (1 µM) was added to cultures 20 min before the end of the incubation. Cells were simultaneously harvested and DCF fluorescence was measured at 530 nm to assess intracellular ROS levels, using FACS Caliber (BD Bioscience, Mountainview, CA).
Cell fractionation and western blot analysis
LPS-primed cells were lysed to prepare total, cytosolic and nuclear extracts (26). The proteins were resolved by SDS-PAGE and transferred to PVDF membranes for immunoblotting using the antibodies described in Supplementary Materials.
Measurement of cytokines by ELISA
TNF-α, IL-1β and IL-6 levels were assessed using human and mouse cytokine ELISA kits (e-Bioscience and R&D Systems).
RT-PCR and qRT-PCR
Cells were harvested for total RNA extraction using TRIzol. RT-PCR of thioredoxin was performed as described (15). The qRT-PCR amplification with POWER SYBRⓇ Green (Applied Biosystems, Warrington, UK) was performed using a Mastercycler realplex thermalcylcer (Eppendorf AG, Hamburg, Germany).
Determination of inflammasome activity
Inflammasome assays were performed in two stages: LPS priming for 4 h and inflammasome activation within 1 h to avoid any chemical induction at the LPS priming stage as described in the Supplementary Materials.
Statistical analysis
Experiments were conducted using at least 3 independent sets in duplicate or triplicate cultures. The values are represented as means ± SD. Staistical significance was determined via Student’s t test. A value of P < 0.05 was considered statisti-cally significant.
Supplemental Materials
ACKNOWLEDGEMENTS
This study is supported by National Research Foundation of Korea (NRF) Grants #2012R1A2A2A01015258, #2015M2B2A9029226, and #2018R1A2B6002201. Hana Jeong is supported in part by NRF-2017-Global Ph.D. Fellowship Program.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 2.Kohchi CI, Inagawa H, Nishizawa T, Soma G. ROS and innate immunity. Anticancer Res. 2009;29:817–821. [PubMed] [Google Scholar]
- 3.Filippin LI, Vercelino R, Marroni NP, Xavier RM. Redoxsignalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 2008;152:415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilgun-Sherki Y, Melamed E, Offen D. The role of oxidativestress in the pathogenesis of multiple sclerosis: the need for effective antioxidanttherapy. J Neurol. 2004;251:261–268. doi: 10.1007/s00415-004-0348-9. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–778. doi: 10.1172/JCI200318174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNF alpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 7.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov R, Preston-Hurlbrt P, Kopp E, et al. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/S1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 9.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander WS, Starr R, Fenner JE, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/S0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa R, Naka T, Tsutsui H, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/S1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- 12.Kimura A, Naka T, Muta T, et al. Suppressor of cytokine signaling-1 selectively inhibits LPS-induced IL-6 production by regulating JAK-STAT. Proc Natl Acad Sci U S A. 2005;102:17089–17094. doi: 10.1073/pnas.0508517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mansell A, Smith R, Doyle SL, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 14.Baetz A, Frey M, Heeg K, Dalpke AH. Suppressor of cytokine signaling (SOCS) proteins indirectly regulate toll-like receptor signaling in innate immune cells. J Biol Chem. 2004;279:54708–54715. doi: 10.1074/jbc.M410992200. [DOI] [PubMed] [Google Scholar]
- 15.Oh J, Hur MW, Lee CE. SOCS1 protects protein tyrosine phosphatases by thioredoxinupregulation and attenuates Jaks to suppress ROS-mediated apoptosis. Oncogene. 2009;28:3145–3156. doi: 10.1038/onc.2009.169. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Yamazaki S, Uematsu S, et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature. 2004;430:218–222. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- 17.Ryan KA, Smith MF, Jr, Sanders MK, Emst PB. Reactive oxygen and nitrogen species differntially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun. 2004;72:2123–2130. doi: 10.1128/IAI.72.4.2123-2130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anuranjani, Bala M. Concerted action of Nrf2-ARE pathway, MRN complex, HMGB1 and inflammatory cytokines: Implication in modification of radiation damage. Redox Biol. 2014;2:832–846. doi: 10.1016/j.redox.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan SA, Baganizi DR, Sahu R, Singh SR, Dennis VA. SOCS proteins as regulators of inflammatory responses induced by bacterial infections: A review. Front Microbiol. 2017;8:2431. doi: 10.3389/fmicb.2017.02431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lui Y, Stewart KN, Bishop E, et al. Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol. 2008;180:6270–6278. doi: 10.4049/jimmunol.180.9.6270. [DOI] [PubMed] [Google Scholar]
- 21.Qin H, Holdbrooks T, Li Y, et al. SOCS3 Deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189:3439–3448. doi: 10.4049/jimmunol.1201168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder K, Tschopp J. Theinflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radical Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh J, Kim SH, Ahn S, Lee CE. Suppressors of cytokine signaling promote Fas-induced apoptosis by down-regulation of NF-κB and mitochondrial Bfl-1 in leukemic T cells. J Immunol. 2012;189:5561–5571. doi: 10.4049/jimmunol.1103415. [DOI] [PubMed] [Google Scholar]
- 27.Kim SY, Jeong J-M, Kim SJ, et al. Pro-inflammatory hepatic macrophages generate ROS through NADPH oxidase 2 via endocytosis of monomeric TLR4-MD2 complex. Nat Commun. 2017;8:2247. doi: 10.1038/s41467-017-02325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Goncalves R, Mosser DM. The isolation and characterizationof murine macrophages. Curr Proc Immunol. 2008;83:14.1.1–14.1.14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.