Abstract
Globally, hepatitis B virus (HBV) infection and its related liver diseases account for 780,000 deaths every year. Outcomes of HBV infection depend on the interaction between the virus and host immune system. It is becoming increasingly apparent that Kupffer cells (KCs), the largest population of resident and monocyte-derived macrophages in the liver, contribute to HBV infection in various aspects. These cells play an important role not only in the anti-HBV immunity including virus recognition, cytokine production to directly inhibit viral replication and recruitment and activation of other immune cells involved in virus clearance but also in HBV outcome and progression, such as persistent infection and development of end-stage liver diseases. Since liver macrophages play multiple roles in HBV infection, they are directly targeted by HBV to benefit its life cycle. In the present review, we briefly outline the current advances of research of macrophages, especially the studies of their phenotypes, in chronic HBV infection.
Keywords: Hepatitis B virus, Chronic HBV infection, Macrophage, Phenotype
Introduction
As a major world health problem, hepatitis B virus (HBV) infects 257 million people, representing about 3% of the world’s population (https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b). While approximately 95% of HBV infections acquired during adulthood are resolved, the virus cannot be cleared in most individuals infected in perinatal period or early childhood.1 It is widely accepted that the virus-host interaction, which is affected by age, transmission route, immune status and other factors, determines the outcome of infection.2,3
Both adaptive and innate immunity are involved in anti-HBV immune response. On one hand, antigen-presenting cells (APCs), including macrophages and dendritic cells (DCs), initialize the virus-specific adaptive immunity characterized by activation of T helper lymphocytes and secretion of various cytokines, which then mobilize the cytotoxic T lymphocyte (CTL) to kill the HBV-infected cells. Additionally, HBV-specific antibodies are developed by the humoral immune system to neutralize the virus and facilitate its clearance.4 On the other hand, the essential role of non-specific defense, especially the function of the liver macrophages (i.e. KCs), has gained growing attention (reviewed in Faure-Dupuy et al.5), albeit the precise mechanism remains incompletely elucidated because of the difficulty in identifying asymptomatic early infections in human studies.6 Unlike hepatitis C virus (HCV), HBV was once considered as a “stealth virus”, due to the fact that HBV could not induce significant innate immune response in an acute HBV-infected chimpanzee model.7 Limited evidence from clinical study8 also showed that no intense cytokine storm, such as type I interferon (IFN) and type III IFN production, occurs in patients with acute HBV infection. Nevertheless, one typical characteristic of HBV infection is macrophage hyperplasia in the liver,9,10 suggesting an important role of macrophages in HBV pathogenesis. It has been demonstrated that some effecter molecules, such as interleukin (IL)-6,11 were produced by KCs to replace IFNs to control HBV infection.11 Another interesting study12 showed that HBV DNA in the liver and blood were cleared before the adaptive immune response was elicited, indicating that innate immune response is much more than a simple branch to control virus invasion until onset of the adaptive response.
Herein, we will review the effects of liver macrophages on HBV infection, focusing on macrophage phenotypes in HBV persistent infections.
Macrophages: Functions and phenotypes
In the healthy liver, the compartment of liver macrophages is dichotomic, involving tissue-resident macrophages (i.e. KCs) and monocyte-derived macrophages (MDMs). KCs, as well as the liver DCs and sinusoidal endothelial cells (LSECs), are mainly localized in the sinusoids of the liver and they form the first line of defense to diverse antigens and toxic components contained in portal venous blood.13 MDMs are mainly localized near the portal triad. When the KCs are depleted experimentally or pathologically, the MDMs can be infiltrated from the peritoneal cavity, replacing KCs by acquiring virtually the same phenotype (reviewed in14). In fact, the liver macrophages (i.e. KCs and MDMs) are very plastic and no specific marker is used to discriminate KCs from MDMs in human.5
As an important component of innate immunity, liver macrophages can function as: 1) phagocytes to remove dead cells, debris and pathogens;15 2) effective APCs, with their expression of major histocompatibility complex (MHC) and co-stimulatory molecules; 3) immune mediators involved in immune suppression and allograft tolerance of liver; and, 4) key players in rapid erythrocyte removal and iron recycling (mainly the MDMs).16
Polarization, which is equally vague as activation, is indispensable before the macrophages achieve their different functions. Generally speaking, macrophages can be polarized into two major subsets with different combinations of stimuli:17 M1 macrophages of classical activation, which induce inflammation and cause tissue damage by facilitating Th1 response, and M2 macrophages of alternative activation, which maintain tissue integrity by promoting the Type 2 T helper cell response (Fig. 1). M1 and M2 type cytokines or surface markers are referred to, to differentiate different macrophage activation phenotypes. There are other subsets, such as M2a, M2b, M2c, etc. What has been overlooked, however, is that polarization is a process which changes continuously18 and various mixtures of M1 and M2 type macrophages may result in confusion. As a matter of fact, much more effort is required to define the criteria for assessing phenotypes. However, for the rest of this review, we will discuss the association between macrophages and HBV infection on the basis of the current understanding of M1/M2 type macrophages.
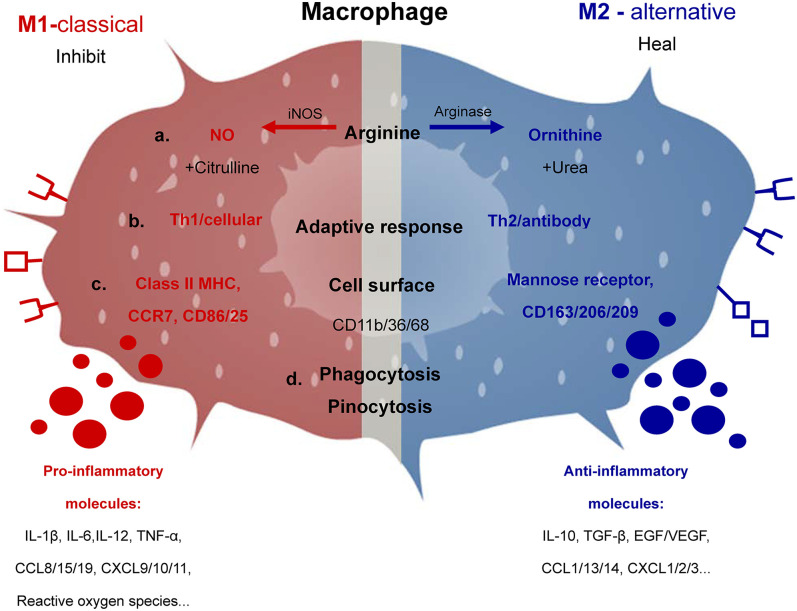
Fig. 1. Characteristic products and functions of M1 and M2 macrophages. Macrophages can metabolize arginine with the inducible nitric oxide synthase enzyme into nitric oxide and citrulline or with arginase into ornithine and urea which is the biochemical basis of the M1 or M2 macrophage responses, respectively.
(a). M1- or M2-dominant macrophages stimulate the Type 1 T helper cell or Type 2 T helper cell responses (b). Also shown are the major molecules involved, including cell surface molecules, cytokines, chemokines and so on, which are closely associated with the M1 or M2 phenotypes (c). Phagocytosis and pinocytosis are general properties of macrophages, which are not dependent on M1 or M2 type responses (d).
Do liver macrophages sense HBV infection?
First of all, although the exact interaction between liver macrophages and HBV is still unclear, nonhepatic cell surface presentations of molecules interacting with PreS or hepatitis B core antigen have been documented. Peripheral blood mononuclear cells (PBMCs), the monocytic cell line THP-1 and U93719–23 were reported to express the PreS-binding receptor of HBV. Lipoprotein lipase (LPL), which can be produced by THP-1 macrophages,24,25 has an linear motif for PreS binding and may interact with HBV particles during infection.23 Additional candidate Pre-S receptors, which can be expressed by KCs,26 are lipopolysaccharide (LPS) binding protein (LBP), the LPS receptor CD14,22 and mannose receptor (MR).27,28 These receptors are involved in the binding of hepatitis B surface antigen to macrophages, monocytes or DCs. Hepatitis B core antigen was also reported to bind to PBMCs and trigger the release of IL-18.29 Consistently, Cooper et al.30 demonstrated that hepatitis B core antigen could bind to receptor(s), like the Toll-like receptor (TLR)2 and heparan sulfate proteoglycan (HSPG), on THP-1 macrophages by its arginine-rich domain at the C-terminal and effectively induce expression of pro-inflammatory molecules. Given the fact that hepatitis B core antigen mainly exists within the hepatocytes and viral particles, whether liver macrophages interact with hepatitis B core antigen during HBV infection in patients is still not clear. Accordingly, although there is a probable involvement of HBV antigen receptors in initializing viral infection, it is more likely that these receptors only mediate cellular recognition or internalization of HBV/HBV antigens. Little work has addressed the expression of the recently identified HBV functional receptor sodium taurocholate cotransporting polypeptide (NTCP)31,32 in liver macrophages. In an interesting study, Neurath et al.20 reported that HUT-78 and MOLT3 cells (both T cell lines) could covalently attach to PreS-cellulose or hepatitis B surface antigen-cellulose after treatment with concanavalin A linked with a peptide of HBV PreS1. This result suggests a similar possibility that HBV receptors could be induced by appropriate stimulations in liver macrophages or monocytes. Moreover, HBV antigens and nucleic acid have been detected in macrophages and monocytes,21,33–35 raising the possibility that HBV might be “taken into” the macrophages or monocytes.
Secondly, the ability of macrophages to produce cytokines upon exposure to HBV potentially renders them as indispensable immune cells sensing and discriminating invading HBV. Hösel’s group11 observed an early-time, nuclear factor kappa-B (NF-κB)-dependent induction of inflammatory mediators in primary human KCs stimulated with HBV inoculum generated from the HepG2.2.15 cell line. This cluster of soluble inflammatory cytokines, including IL-6, IL-8, IL-1β and tumor necrosis factor (TNF)-α but no type I IFN, inhibited HBV replication significantly. In a more recent study,21 KCs isolated from patients with persistent HBV infection showed a higher activation status (characterized by elevated expressions of CD40, HLA-ABC and HLA-DR) than those of healthy control. And, in accordance with previous report, their experiments21 also revealed obvious inductions of IL-6, IL-15, TNF, chemokine (C-C motif) ligand 4 (CCL4), C-X-C motif chemokine ligand 8 (CXCL8), as well as IL-10 in human primary KCs and PBMC-generated macrophages cultured with patient plasma-derived hepatitis B surface antigen. Most recently, Cheng et al.36 reported that human macrophages showed an inflammatory cytokine storm when stimulated with high level HBV, while the hepatocytes sensed HBV DNA poorly. Other in vitro studies demonstrated that HBV antigens (e.g., HBV envelop protein, PreS and HBV core antigen) were able to induce cytokine secretion in monocytes and MDMs after binding to the receptors (see below).
M1 and M2 macrophages involved in HBV infection
As a major source of cytokines and immune regulators, macrophages are involved in HBV infection in at least two aspects: (1) antiviral effects, mainly mediated by M1 type molecules (Fig. 2a); and, (2) immunotolerance, mediated by M2 type molecules (Fig. 2b).
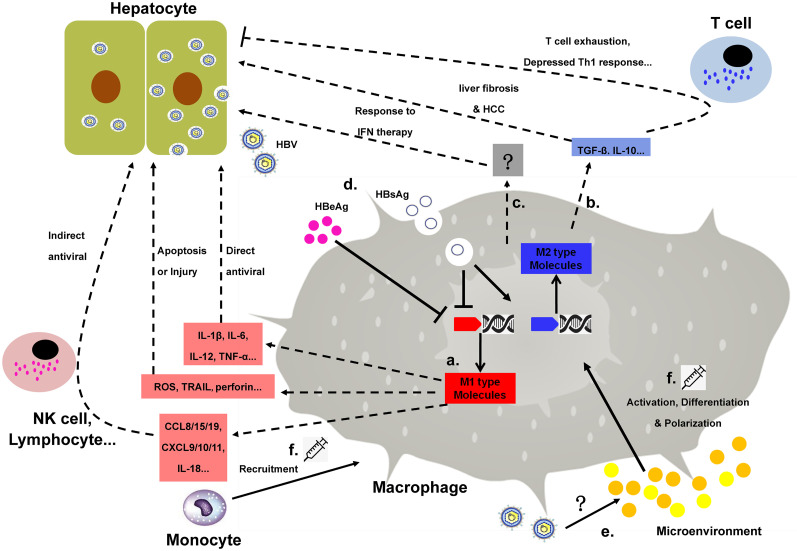
Fig. 2. Macrophage involvement in HBV infection. The anti-HBV effect of macrophages is mediated mainly by pro-inflammatory cytokines inducing a direct antiviral response or molecules recruiting or activating other immune cells. Meanwhile, another group of M1 KCs produces molecules that may result in injury or apoptosis of the hepatocytes.
(a). Immunomodulatory mediators, such as IL-10 and TGF-β, are closely associated with suppressed antiviral T cell responses and/or end-stage HBV liver disease (b).Macrophages may also contribute to the inflammatory or anti-inflammatory liver microenvironment and, consequently, alter hepatic response to IFN treatment (c). The phenotype and function of macrophages can be modified by either HBV itself (d) or the microenvironment (e). Thus, the therapeutic strategies targeting macrophages in an HBV infection may aim at modulating macrophage polarization/phenotype, monocyte recruitment/activation and so on (f).
Antiviral effects
Activation of M1-type macrophages and production of pro-inflammatory cytokines usually indicate a robust immune response to HBV infection. CD16+ is one of the M1-like phenotype markers. Zhang’s group37 investigated 110 hepatitis B e antigen-negative chronic hepatitis B (CHB) patients and found that the immune-activated group was characterized by lower HBV DNA and that high alanine aminotransferase (ALT) is associated with more CD16+ monocytes and/or macrophages in the peripheral blood and liver, when compared with the immune tolerant group. High level of M1-like CD16+ macrophages was an indicator for immune activation that helped patients to defend against the virus.
Direct antiviral effects: Agonists of TLRs38–41 and HBV antigens (as discussed above) can induce macrophages to express soluble inflammatory mediators and other effective molecules, which are the major effectors to assume direct antiviral activity of macrophages. With diverse mechanisms, these effective molecules can either control HBV without obvious cytotoxicity or result in injury or apoptosis of the infected hepatocytes.
Type I IFN, for example, one of the key cytokines potently inhibiting HBV replication in hepatocytes, is routinely used in the clinic to treat HBV patients. Despite plenty of work having addressed the anti-HBV mechanisms of type I IFN, there is limited clinical or in vivo evidence for the idea that after the lag phase of HBV replication with negligible release of type I IFN, liver macrophage-synthesized IFN α/β may act as essential controller for HBV. Fortunately, circumstantial evidence is available. First of all, type I IFNs induced by in vitro activated KCs effectively suppress HBV production. Injections of diverse TLR (TLR3/4/5/7/9) agonists could control HBV replication, which is IFN α/β-dependent in transgenic mice.40 Wu’s group38 confirmed and extended these findings by collecting the supernatants of primary C57BL/6 mouse KCs after stimulation with ligands specific for TLR1 to TLR9 and evaluating their effects on HBV-Met cells. Their study showed a significant TLR3- or TLR4-mediated suppression of HBV replication, which can be abolished, or at least partially abolished, by IFN-β antibodies.
Another study42 using KCs activated by the agonist of stimulator of IFN genes (STING) revealed a predominant type I IFN production and subsequent inhibition of HBV replication in the AML12HBV10 cell line. The anti-HBV effect of STING agonist was further confirmed in the HBV DNA hydrodynamic NOD/SCID mouse model.42 Secondly, unrelated viral infection may activate KCs and noncytopathically inhibit HBV production via IFN α/β. Guidotti et al.43 used lymphocytic choriomeningitis virus (LCMV) to infect HBV transgenic mice and assayed the production of HBV. They found that 3.5- and 2.1-kb HBV mRNAs were decreased or even absent, as well as HBV DNA replication forms. Similar results were found in HBV transgenic mouse model with malaria infection.44 Recruitment of macrophages and subsequently elevated expressions of IFN α/β/γ suppressed HBV gene expression and replication in vivo. Thirdly, HBV has evolved some strategies specifically targeting IFN production in KCs, indicating the potential anti-HBV effects of macrophage-derived IFNs. Using the murine nonparenchymal liver cells, Wu’s group45 demonstrated that hepatitis b surface antigen, hepatitis B e antigen, as well as HBV virion, could suppress TLR3-mediated IFN-β, IFN-γ and IFN-stimulated gene (ISG) production by interfering with the activation of interferon regulatory factor 3 and NF-κB.
Activated macrophages are the major source of TNF-α,46 which has been identified as a potent anti-HBV molecule. It has been well established that TNF-α production increases in the primary KCs47 or PBMCs48 isolated from CHB patients. In vitro studies also demonstrated that the expression levels of TNF-α in primary KCs,11,21 MDMs21 and THP-130 cells were up-regulated in response to HBV challenge. In addition, HBV replication in primary tupaia hepatocytes (PTHs) was partially inhibited by recombinant tupaia TNF-α.49 In transgenic mice, TNF-α produced by macrophages during LCMV,43 adenovirus or cytomegalovirus50 infection inhibited HBV gene expression and DNA replication noncytopathically. Furthermore, substantial clinical data also raised the importance of TNF-α in HBV infection.
Elimination of hepatitis B e antigen and suppression of HBV replication in patients receiving IFNα treatment was accompanied by spontaneously induced TNF-α in PBMCs.51 Anti-TNF-α therapy in patients with chronic inflammatory diseases was associated with higher risk of HBV activation, reactivation and hepatotoxicity,52–55 which may be attributed to the setting of immune suppression. Accumulating evidence also suggests an important role of TNF-α gene polymorphisms in HBV infection (reviewed in56). These clinical studies, together with the data from basic research and animal models, mirror the fact that TNF-α, as well as the macrophages, is one of the prerequisites for virus clearance and permanent control of HBV.
Other macrophage-derived anti-HBV cytokines include IL-1β,11,51 IL-6,11,21,30 IL-12,57 IL-15,21 and macrophage migration inhibitory factor (MIF),58 some of which may perform synergistic actions with each other (reviewed in59). Meanwhile, another group of KCs produced molecules, such as reactive oxygen species (ROS),10 Fas-ligand,60 TNF-related apoptosis-inducing ligand (TRAIL),61 granzyme B and perforin,62 may result in injury or apoptosis of the hepatocytes.
Indirect antiviral effects through recruiting or activating other immune cells: Liver macrophages synthesize several cytokines and chemokines to activate or recruit inflammatory cells involved in the anti-HBV roles. IL-18, an inflammatory cytokine belonging to the IL-1 family, is mainly expressed by liver macrophages (reviewed in63). Previous studies have demonstrated that IL-18 plays a powerful anti-HBV role by inducing cytokine production (e.g. IFN-γ, IFN α/β, TNF-α)64 in some immune cells. Kakimi et al.65 showed that IL-18 was a type I- and type II-IFN inducing factor, acting on both intrahepatic natural killer (NK) and natural killer T (NKT) cells in a transgenic mouse model, resulting in suppressed HBV replication. Interestingly, this inhibitory effect of IL-18 on HBV replication is dependent on IL-12, which is able to be released by activated macrophages. Boltjes et al.21 analyzed the function of human primary KCs and in vitro-generated MDMs. They found both could be activated by exposure to patient-derived hepatitis B surface antigen, resulting in activation of NK cells characterized by up-regulation of CD69 and IFN-γ.
It has been well established that Type 1 T helper cells, B cells and DCs can also produce IFN-γ in response to IL-18 stimulation.66–68 In addition, activated liver macrophages also produce CXCL, CXCL-9 and CXCL-10, which assist in trafficking of lymphocytes and monocyte/macrophages into the tissue.69,70 Another study from Kakimi group71 demonstrated that CXCL-9 and CXCL-10 derived from nonparenchymal cells (including KCs) chemoattracted lymphomononuclear inflammatory cells into the liver in a transgenic murine model.
Immunotolerance/immunosuppressive activity
Constantly exposed to diverse antigens derived from food or microbial products, the immune cells, in addition to other cells72 in the liver, develop some mechanisms to prevent excessive activation and continuous pathology, known as inherent tolerogenicity of the liver. KCs are involved in the well-known tolerogenic milieu by secreting soluble immunoregulators (e.g., IL-10, TGF-β, and amphiregulin) or expressing inhibitory molecules on the membrane, both of which could be exploited by HBV for their favorable immunosuppressive microenvironment.
For instance, IL-10 could depress inflammation response by inhibiting Type 1 T helper cell cytokine expression. An HBV-carrier mouse model showed no significant immune response to hepatitis B surface antigen vaccination, which could be reversed by KC depletion or IL-10 deficiency.73 Clinical data74 also revealed an association between CHB and elevated plasma IL-10 level, though it was uncertain whether the increased IL-10 was derived mainly from macrophages or not. Consistently, Li’s group75 also reported that the increased production of IL-10 by KCs, which was stimulated by HBV core antigen, resulted in inhibition of the antiviral function of CD8+ T cells in mice.
Interestingly, IL-10 gene promoter polymorphisms were reported to be associated with HBV progression.76 It was reported that murine KCs preferred to produce TGF-β, which is able to restrain immune response and to develop tolerance towards self-antigens,77 rather than functioning as a pro-inflammatory cytokine in response to HBV infection.78 Although the precise mechanism remains unclear, the tolerogenic role of HBV by modulating liver macrophage polarization should not be ignored, since IL-10 and TGF-β are typical cytokines of M2 macrophages. Previous studies also suggested that KCs were primarily immunosuppressive, mediated by prostaglandin E2 (PGE2) (reviewed in13); accordingly, HBV may maintain or even promote this immunosuppressive status by regulating macrophage polarization and/or PGE2 production to benefit its replication.
Bility and colleagues79 found that CHB patients with fibrosis and/or hepatocellular carcinoma (HCC) and patients with acute HBV-associated liver failure experienced a M2 phenotype, including increased M2 macrophages in the liver infiltration and predominate M2-type gene expression profile in the liver. The authors developed a humanized mouse model, supporting HBV replication to investigate the HBV-associated immunopathogenesis. They found that impaired immune response in parallel with robust M2-type macrophage activation in the liver contributed to the development of persistent HBV infection, indicating the M2 macrophages might act as immune suppressors. Nowadays, accumulating evidence indicates that macrophages play an important role in HBV-induced immune suppression, not only in persistent infection establishment but also in the development of the end-stage liver diseases, such as liver fibrosis and HCC.80
Potential role of macrophages in response to IFNα treatment in CHB patients
Type I IFN is still one of the most important therapies for CHB infection or chronic hepatitis C virus (CHC). However, only a subset of the patients respond. Our previous studies showed that cell-type specific ISGs’ expression in the liver predicts whether a given patient will respond to IFN treatment among CHC81 or CHB82 patients. We analyzed the pre-treatment gene expression in 38 CHB livers by immunohistochemical staining and found that in the treatment responders, increased ISG15 and myxovirus resistance gene (MxA) protein expression was more pronounced in macrophages than that in hepatocytes. In contrast, in the non-responders, elevated expression of ISG15 and MxA was more pronounced in hepatocytes compared with that in macrophages. A similar result was found in CHC patients before receiving pegylated-IFN/ribavirin treatment, indicating that the liver macrophages might be involved in mediating patients’ response to IFN and other anti-viral therapy.
Many studies correlated IFN and a subset of typical ISGs (e.g., ISG15, USP18) with macrophage phenotypes and functions. Fleetwood et al.83 reported that the type I IFN signaling pathway played an essential role in regulating phenotype and function of macrophage-colony stimulating factor (M-CSF)- or granulocyte-macrophage colony-stimulating factor (GM-CSF)-treated bone marrow-derived macrophages in mice. ISG15, a typical ISG, may play an important role in macrophage polarization and function. Macrophage polarization is characterized by mitochondrial functions regulated by different metabolic patterns, and the lack of ISG15 was responsible for mitochondrial dysfunction, including diminished oxidative phosphorylation (OXPHOS), depressed oxygen consumption rate, as well as reduced adenosine triphosphate (ATP) and ROS production in bone marrow-derived macrophages in mice.84 Macrophages from the ISG15-deficient mice have been shown to have depressed phagocytic capacity, which is dependent on protein kinase AKT.85 Moreover, we previously described USP18, another typical ISG, as a modulator of macrophage activity in mice. Compared with wild type control, both primary KCs or peritoneal exudative macrophages (PEMs) from USP18-/- preferred to polarize to the M2-like phenotype, producing more anti-inflammatory cytokines (e.g., IL-10 and IL-4) and less inflammatory cytokines (e.g., TNF-α and IL-12) in response to murine hepatitis virus (MHV)-3 infection (unpublished data).
The inflammation microenvironment may be changed by macrophages, influencing the response of hepatocytes to IFN treatment. Our previous study86 found that pre-treatment with TNF-α or LPS led to an IFNα refractory state in human hepatoma cells and primary murine hepatocytes. We have also investigated the response of primary murine hepatocytes to IFNα after co-culturing with the primary murine hepatocytes and the primary murine USP18-/- (M2 like) or wide type (M1-like) PEMs, using the Transwell co-culture system. We found that hepatocytes co-cultured with USP18-/- PEMs experienced much higher expression of ISGs (including of ISG15, USP18 and MxA) with IFNα stimulation (unpublished data).
We therefore hypothesize that liver macrophages regulate inflammatory and anti- inflammatory responses, contributing to the liver microenvironment and, consequently, alter hepatic response to IFN treatment. However, more in-depth investigations are needed to uncover the underlying molecular mechanism (Fig. 2c).
Effect of HBV on macrophage phenotype
The distinct roles of M1 and M2 macrophages involved in HBV infection raise the possibility that HBV may promote M2 polarization of macrophages to impair the Type 1 T helper cell immune response, resulting in persistent infection and disease progression. A most recent study87 supported the hypothesis that HBV suppresses M1 macrophage cytokine (IL-6 and IL-1β) expression and promotes M2 macrophage cytokine (IL-10) expression to favor HBV infection. Although the precise mechanism remains unclear, several in vitro studies have revealed that either hepatitis B surface antigen or hepatitis B e antigen may make a contribution. Expression of M1-type cytokines, such as TNF-α, IL-1b and IL-8, was inhibited by hepatitis B surface antigen in PBMCs,19 while the expression of IL-10 was not affected or even promoted.19,88 Similar results were observed in THP-1-derived macrophages: hepatitis B surface antigen acted as a potent suppressor of M1-type cytokines, including IL-12, TNF-α, IL-1β and IL-6.88–90 Moreover, Yu et al.91 reported a decreased IL-1β secretion in liver macrophages induced by hepatitis B e antigen. However, it has been reported that pro-inflammatory macrophages and monocytes expressing TNF-α and GM-CSF accumulated in chronic HBV or HCV-related liver disease.92 Moreover, high level of IL-23 (as well as IL-1β, IL-6 and IL-17) expression in liver inflammatory macrophages was demonstrated to be associated with HCC development.93 These contradictory lines of evidence have indicated the complicated association between different macrophage phenotypes and disease progression of hepatitis B virus infection (Fig. 2d). In addition, the microenvironment altered by HBV infection may also contribute to the activation, differentiation and polarization of macrophages (Fig. 2e).
Conclusions
Although our knowledge about the exact immunological pathogenesis during chronic HBV infection is limited, the remarkable heterogeneity of liver macrophages concerning not only the defense but also the homeostasis and metabolism make it a promising option for treating HBV infection (reviewed in14, Fig. 2f). Binding and/or up-taking virus/ viral antigens, as well as the signaling from infected hepatocytes, may result in the activation of liver macrophages. The activated liver macrophages, on one hand, enforce virus clearance by producing pro-inflammatory cytokines targeting hepatocytes to suppress HBV directly or recruiting, interacting or activating other immune cells to get rid of the virus, and on the other hand, modulating immunotolerance as a negative feedback to avoid unchecked inflammation. This functional diversity or contrary action makes it possible that liver macrophages may be exploited by HBV.
HBV is a stealth virus which “hides” itself in the early stage of life cycle to escape macrophage defenses, and may then manipulate the polarization/phenotype of macrophages to benefit its persistent infection. It is important to note that the response of liver macrophages to HBV infection is not only limited to innate defense but also links the innate immunity with acquired immunity. However, whether macrophages resolve virus, worsen liver immunopathogenesis, promote persistent infection or modulate the response to IFNα therapy depends on a combination of various factors and is finely tuned. This is indeed a complicated process and the interaction between macrophages and HBV deserves further study.
Abbreviations
- ALT
alanine aminotransferase
- APC
antigen-presenting cell
- ATP
adenosine triphosphate
- CHB
chronic HBV infection
- CHC
chronic hepatitis C virus
- CTL
cytotoxic T lymphocyte
- CXCL
chemokine (C-C motif) ligand, CCL, C-X-C motif chemokine ligand
- DC
dendritic cell
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HSPG
heparan sulfate proteoglycan
- IFN
interferon
- IL
interleukin
- IRF3
interferon regulatory factor
- ISGs
IFN-stimulated genes
- KC
Kupffer cell
- LBP
LPS binding protein
- LCMV
lymphocytic choriomeningitis virus
- LPL
lipoprotein lipase
- LSEC
liver sinusoidal endothelial cell
- M-CSF
macrophage-colony stimulating factor
- MDM
monocyte-derived macrophage
- MHC
major histocompatibility complex
- MHV
murine hepatitis virus
- MIF
macrophage migration inhibitory factor
- MR
mannose receptor
- MxA
myxovirus resistance gene
- NF-κB
nuclear factor kappa-B
- NK
natural killer
- NKT
natural killer T
- NTCP
sodium taurocholate cotransporting polypeptide
- OXPHOS
oxidative phosphorylation
- PBMC
peripheral blood mononuclear cell
- PEM
peritoneal exudative macrophage
- PGE2
prostaglandin E2
- PTH
primary tupaia hepatocytes
- ROS
reactive oxygen species
- STING
stimulator of IFN genes
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
References
- 1.Aspinall EJ, Hawkins G, Fraser A, Hutchinson SJ, Goldberg D. Hepatitis B prevention, diagnosis, treatment and care: a review. Occup Med (Lond) 2011;61:531–540. doi: 10.1093/occmed/kqr136. [DOI] [PubMed] [Google Scholar]
- 2.Publicover J, Jespersen JM, Johnson AJ, Nishimura SL, Goodsell A, Wakil AE, et al. Liver capsule: Age-influenced hepatic immune priming determines HBV infection fate: Implications from mouse to man. Hepatology. 2016;63:260. doi: 10.1002/hep.28284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevens CE, Beasley RP, Tsui J, Lee WC. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975;292:771–774. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- 4.Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439–1449. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- 5.Faure-Dupuy S, Durantel D, Lucifora J. Liver macrophages: Friend or foe during hepatitis B infection? Liver Int. 2018;38:1718–1729. doi: 10.1111/liv.13884. [DOI] [PubMed] [Google Scholar]
- 6.Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117–1124. doi: 10.1053/jhep.2000.19324. [DOI] [PubMed] [Google Scholar]
- 7.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 10.Hagen TM, Huang S, Curnutte J, Fowler P, Martinez V, Wehr CM, et al. Extensive oxidative DNA damage in hepatocytes of transgenic mice with chronic active hepatitis destined to develop hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1994;91:12808–12812. doi: 10.1073/pnas.91.26.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 13.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 14.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Naito M, Hasegawa G, Ebe Y, Yamamoto T. Differentiation and function of Kupffer cells. Med Electron Microsc. 2004;37:16–28. doi: 10.1007/s00795-003-0228-x. [DOI] [PubMed] [Google Scholar]
- 16.Theurl I, Hilgendorf I, Nairz M, Tymoszuk P, Haschka D, Asshoff M, et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945–951. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 19.Vanlandschoot P, Van Houtte F, Roobrouck A, Farhoudi A, Leroux-Roels G. Hepatitis B virus surface antigen suppresses the activation of monocytes through interaction with a serum protein and a monocyte-specific receptor. J Gen Virol. 2002;83:1281–1289. doi: 10.1099/0022-1317-83-6-1281. [DOI] [PubMed] [Google Scholar]
- 20.Neurath AR, Strick N, Sproul P. Search for hepatitis B virus cell receptors reveals binding sites for interleukin 6 on the virus envelope protein. J Exp Med. 1992;175:461–469. doi: 10.1084/jem.175.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boltjes A, van Montfoort N, Biesta PJ, Op den Brouw ML, Kwekkeboom J, van der Laan LJ, et al. Kupffer cells interact with hepatitis B surface antigen in vivo and in vitro, leading to proinflammatory cytokine production and natural killer cell function. J Infect Dis. 2015;211:1268–1278. doi: 10.1093/infdis/jiu599. [DOI] [PubMed] [Google Scholar]
- 22.Vanlandschoot P, Van Houtte F, Roobrouck A, Farhoudi A, Stelter F, Peterson DL, et al. LPS-binding protein and CD14-dependent attachment of hepatitis B surface antigen to monocytes is determined by the phospholipid moiety of the particles. J Gen Virol. 2002;83:2279–2289. doi: 10.1099/0022-1317-83-9-2279. [DOI] [PubMed] [Google Scholar]
- 23.Deng Q, Zhai JW, Michel ML, Zhang J, Qin J, Kong YY, et al. Identification and characterization of peptides that interact with hepatitis B virus via the putative receptor binding site. J Virol. 2007;81:4244–4254. doi: 10.1128/JVI.01270-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Beauchamp MC, Renier G. Peroxisome proliferator-activated receptor alpha and gamma agonists upregulate human macrophage lipoprotein lipase expression. Atherosclerosis. 2002;165:101–110. doi: 10.1016/s0021-9150(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 25.Makoveichuk E, Castel S, Vilaró S, Olivecrona G. Lipoprotein lipase-dependent binding and uptake of low density lipoproteins by THP-1 monocytes and macrophages: possible involvement of lipid rafts. Biochim Biophys Acta. 2004;1686:37–49. doi: 10.1016/j.bbalip.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Ono K, Nishitani C, Mitsuzawa H, Shimizu T, Sano H, Suzuki H, et al. Mannose-binding lectin augments the uptake of lipid A, Staphylococcus aureus, and Escherichia coli by Kupffer cells through increased cell surface expression of scavenger receptor A. J Immunol. 2006;177:5517–5523. doi: 10.4049/jimmunol.177.8.5517. [DOI] [PubMed] [Google Scholar]
- 27.Op den Brouw ML, Binda RS, Geijtenbeek TB, Janssen HL, Woltman AM. The mannose receptor acts as hepatitis B virus surface antigen receptor mediating interaction with intrahepatic dendritic cells. Virology. 2009;393:84–90. doi: 10.1016/j.virol.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Zhou J, Zhang B, Tian Z, Tang J, Zheng Y, et al. Hepatitis B virus induces IL-23 production in antigen presenting cells and causes liver damage via the IL-23/IL-17 axis. PLoS Pathog. 2013;9:e1003410. doi: 10.1371/journal.ppat.1003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manigold T, Böcker U, Chen J, Gundt J, Traber P, Singer MV, et al. Hepatitis B core antigen is a potent inductor of interleukin-18 in peripheral blood mononuclear cells of healthy controls and patients with hepatitis B infection. J Med Virol. 2003;71:31–40. doi: 10.1002/jmv.10445. [DOI] [PubMed] [Google Scholar]
- 30.Cooper A, Tal G, Lider O, Shaul Y. Cytokine induction by the hepatitis B virus capsid in macrophages is facilitated by membrane heparan sulfate and involves TLR2. J Immunol. 2005;175:3165–3176. doi: 10.4049/jimmunol.175.5.3165. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto M, Watashi K, Tsukuda S, Aly HH, Fukasawa M, Fujimoto A, et al. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem Biophys Res Commun. 2014;443:808–813. doi: 10.1016/j.bbrc.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 32.Watashi K, Urban S, Li W, Wakita T. NTCP and beyond: opening the door to unveil hepatitis B virus entry. Int J Mol Sci. 2014;15:2892–2905. doi: 10.3390/ijms15022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oquendo J, Karray S, Galanaud P, Petit MA. Effect of hepatitis B virus on tumour necrosis factor (TNF alpha) gene expression in human THP-1 monocytic and Namalwa B-cell lines. Res Immunol. 1997;148:399–409. doi: 10.1016/s0923-2494(97)82873-8. [DOI] [PubMed] [Google Scholar]
- 34.Oquendo J, Dubanchet S, Capel F, Mabit H, Petit MA. Suppressive effect of hepatitis B virus on the induction of interleukin-1 beta and interleukin-6 gene expression in the THP-1 human monocytic cell line. Eur Cytokine Netw. 1996;7:793–800. [PubMed] [Google Scholar]
- 35.Bouffard P, Lamelin JP, Zoulim F, Pichoud C, Trepo C. Different forms of hepatitis B virus DNA and expression of HBV antigens in peripheral blood mononuclear cells in chronic hepatitis B. J Med Virol. 1990;31:312–317. doi: 10.1002/jmv.1890310413. [DOI] [PubMed] [Google Scholar]
- 36.Cheng X, Xia Y, Serti E, Block PD, Chung M, Chayama K, et al. Hepatitis B virus evades innate immunity of hepatocytes but activates cytokine production by macrophages. Hepatology. 2017;66:1779–1793. doi: 10.1002/hep.29348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang JY, Zou ZS, Huang A, Zhang Z, Fu JL, Xu XS, et al. Hyper-activated pro-inflammatory CD16 monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B. PLoS One. 2011;6:e17484. doi: 10.1371/journal.pone.0017484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A, et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46:1769–1778. doi: 10.1002/hep.21897. [DOI] [PubMed] [Google Scholar]
- 39.Zhang E, Lu M. Toll-like receptor (TLR)-mediated innate immune responses in the control of hepatitis B virus (HBV) infection. Med Microbiol Immunol. 2015;204:11–20. doi: 10.1007/s00430-014-0370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Z, Liao W, Ren J. Physicochemical characterization of a polysaccharide fraction from platycladus orientalis (L.) franco and its macrophage immunomodulatory and anti-hepatitis B virus activities. J Agric Food Chem. 2016;64:5813–5823. doi: 10.1021/acs.jafc.6b01387. [DOI] [PubMed] [Google Scholar]
- 42.Guo F, Han Y, Zhao X, Wang J, Liu F, Xu C, et al. STING agonists induce an innate antiviral immune response against hepatitis B virus. Antimicrob Agents Chemother. 2015;59:1273–1281. doi: 10.1128/AAC.04321-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guidotti LG, Borrow P, Hobbs MV, Matzke B, Gresser I, Oldstone MB, et al. Viral cross talk: intracellular inactivation of the hepatitis B virus during an unrelated viral infection of the liver. Proc Natl Acad Sci U S A. 1996;93:4589–4594. doi: 10.1073/pnas.93.10.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasquetto V, Guidotti LG, Kakimi K, Tsuji M, Chisari FV. Host-virus interactions during malaria infection in hepatitis B virus transgenic mice. J Exp Med. 2000;192:529–536. doi: 10.1084/jem.192.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 46.Bradham CA, Plümpe J, Manns MP, Brenner DA, Trautwein C. I. TNF-induced liver injury. Am J Physiol Gastrointest Liver Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- 47.González-Amaro R, García-Monzón C, García-Buey L, Moreno-Otero R, Alonso JL, Yagüe E, et al. Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J Exp Med. 1994;179:841–848. doi: 10.1084/jem.179.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheron N, Lau J, Daniels H, Goka J, Eddleston A, Alexander GJ, et al. Increased production of tumour necrosis factor alpha in chronic hepatitis B virus infection. J Hepatol. 1991;12:241–245. doi: 10.1016/0168-8278(91)90945-8. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Köck J, Lu Y, Yang D, Lu M, Zhao X. Suppression of hepatitis B virus replication in Tupaia hepatocytes by tumor necrosis factor alpha of Tupaia belangeri. Comp Immunol Microbiol Infect Dis. 2011;34:361–368. doi: 10.1016/j.cimid.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Cavanaugh VJ, Guidotti LG, Chisari FV. Inhibition of hepatitis B virus replication during adenovirus and cytomegalovirus infections in transgenic mice. J Virol. 1998;72:2630–2637. doi: 10.1128/JVI.72.4.2630-2637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniels HM, Meager A, Eddleston AL, Alexander GJ, Williams R. Spontaneous production of tumour necrosis factor alpha and interleukin-1 beta during interferon-alpha treatment of chronic HBV infection. Lancet. 1990;335:875–877. doi: 10.1016/0140-6736(90)90475-k. [DOI] [PubMed] [Google Scholar]
- 52.Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, Lopez-Roses L, Brito-Zerón P, Pérez-de-Lis M, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore) 2011;90:359–371. doi: 10.1097/MD.0b013e3182380a76. [DOI] [PubMed] [Google Scholar]
- 53.Murdaca G, Spanò F, Contatore M, Guastalla A, Penza E, Magnani O, et al. Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf. 2015;14:571–582. doi: 10.1517/14740338.2015.1009036. [DOI] [PubMed] [Google Scholar]
- 54.French JB, Bonacini M, Ghabril M, Foureau D, Bonkovsky HL. Hepatotoxicity associated with the use of anti-TNF-α agents. Drug Saf. 2016;39:199–208. doi: 10.1007/s40264-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Temel T, Cansu DÜ, Korkmaz C, Kaşifoğlu T, Özakyol A. The long-term effects of anti-TNF-α agents on patients with chronic viral hepatitis C and B infections. Int J Rheum Dis. 2015;18:40–45. doi: 10.1111/1756-185X.12467. [DOI] [PubMed] [Google Scholar]
- 56.Sawhney R, Visvanathan K. Polymorphisms of toll-like receptors and their pathways in viral hepatitis. Antivir Ther. 2011;16:443–458. doi: 10.3851/IMP1820. [DOI] [PubMed] [Google Scholar]
- 57.Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71:3236–3243. doi: 10.1128/JVI.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moudi B, Heidari Z, Mahmoudzadeh-Sagheb H, Hashemi M. Gene polymorphisms of macrophage migration inhibitory factor affect susceptibility to chronic hepatitis B virus infection in an Iranian cohort. Microbiol Immunol. 2016;60:390–396. doi: 10.1111/1348-0421.12382. [DOI] [PubMed] [Google Scholar]
- 59.Ebrahim M, Bagheri K, Arababadi MK. Potential roles played by IL-6 in hepatitis B infection. Future Virology. 2014;9:431–438. doi: 10.2217/fvl.14.21. [DOI] [Google Scholar]
- 60.Tang TJ, Kwekkeboom J, Laman JD, Niesters HG, Zondervan PE, de Man RA, et al. The role of intrahepatic immune effector cells in inflammatory liver injury and viral control during chronic hepatitis B infection. J Viral Hepat. 2003;10:159–167. doi: 10.1046/j.1365-2893.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- 61.Boltjes A, Movita D, Boonstra A, Woltman AM. The role of Kupffer cells in hepatitis B and hepatitis C virus infections. J Hepatol. 2014;61:660–671. doi: 10.1016/j.jhep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 62.Tordjmann T, Soulie A, Guettier C, Schmidt M, Berthou C, Beaugrand M, et al. Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver. 1998;18:391–397. doi: 10.1111/j.1600-0676.1998.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 63.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/s1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 64.Revill P, Yuan Z. New insights into how HBV manipulates the innate immune response to establish acute and persistent infection. Antivir Ther. 2013;18:1–15. doi: 10.3851/IMP2542. [DOI] [PubMed] [Google Scholar]
- 65.Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J Virol. 2002;76:10702–10707. doi: 10.1128/jvi.76.21.10702-10707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 67.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 68.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 69.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 70.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 71.Kakimi K, Lane TE, Chisari FV, Guidotti LG. Cutting edge: Inhibition of hepatitis B virus replication by activated NK T cells does not require inflammatory cell recruitment to the liver. J Immunol. 2001;167:6701–6705. doi: 10.4049/jimmunol.167.12.6701. [DOI] [PubMed] [Google Scholar]
- 72.Buonaguro L, Tagliamonte M, Petrizzo A, Damiano E, Tornesello ML, Buonaguro FM. Cellular prognostic markers in hepatocellular carcinoma. Future Oncol. 2015;11:1591–1598. doi: 10.2217/fon.15.39. [DOI] [PubMed] [Google Scholar]
- 73.Xu L, Yin W, Sun R, Wei H, Tian Z. Kupffer cell-derived IL-10 plays a key role in maintaining humoral immune tolerance in hepatitis B virus-persistent mice. Hepatology. 2014;59:443–452. doi: 10.1002/hep.26668. [DOI] [PubMed] [Google Scholar]
- 74.Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230, 1230. e1-3. doi: 10.1053/j.gastro.2011.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li M, Sun R, Xu L, Yin W, Chen Y, Zheng X, et al. Kupffer cells support hepatitis B virus-mediated CD8+ T cell exhaustion via hepatitis B core antigen-TLR2 interactions in mice. J Immunol. 2015;195:3100–3109. doi: 10.4049/jimmunol.1500839. [DOI] [PubMed] [Google Scholar]
- 76.Miyazoe S, Hamasaki K, Nakata K, Kajiya Y, Kitajima K, Nakao K, et al. Influence of interleukin-10 gene promoter polymorphisms on disease progression in patients chronically infected with hepatitis B virus. Am J Gastroenterol. 2002;97:2086–2092. doi: 10.1111/j.1572-0241.2002.05926.x. [DOI] [PubMed] [Google Scholar]
- 77.Taylor AW. Review of the activation of TGF-beta in immunity. J Leukoc Biol. 2009;85:29–33. doi: 10.1189/jlb.0708415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H, Zheng HW, Chen H, Xing ZZ, You H, Cong M, et al. Hepatitis B virus particles preferably induce Kupffer cells to produce TGF-β1 over pro-inflammatory cytokines. Dig Liver Dis. 2012;44:328–333. doi: 10.1016/j.dld.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. doi: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol. 2019;25:3527–3537. doi: 10.3748/wjg.v25.i27.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Borozan I, Sun J, Guindi M, Fischer S, Feld J, et al. Cell-type specific gene expression signature in liver underlies response to interferon therapy in chronic hepatitis C infection. Gastroenterology. 2010;138:1123–1133.e1-3. doi: 10.1053/j.gastro.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 82.Zhu Y, Qin B, Xiao C, Lu X, Chen L. Cell-type specific interferon stimulated gene staining in liver underlies response to interferon therapy in chronic HBV infected patients. Dig Dis Sci. 2012;57:2355–2361. doi: 10.1007/s10620-012-2169-5. [DOI] [PubMed] [Google Scholar]
- 83.Fleetwood AJ, Dinh H, Cook AD, Hertzog PJ, Hamilton JA. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J Leukoc Biol. 2009;86:411–421. doi: 10.1189/jlb.1108702. [DOI] [PubMed] [Google Scholar]
- 84.Baldanta S, Fernández-Escobar M, Acín-Perez R, Albert M, Camafeita E, Jorge I, et al. ISG15 governs mitochondrial function in macrophages following vaccinia virus infection. PLoS Pathog. 2017;13:e1006651. doi: 10.1371/journal.ppat.1006651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yángüez E, García-Culebras A, Frau A, Llompart C, Knobeloch KP, Gutierrez-Erlandsson S, et al. ISG15 regulates peritoneal macrophages functionality against viral infection. PLoS Pathog. 2013;9:e1003632. doi: 10.1371/journal.ppat.1003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacParland SA, Ma XZ, Chen L, Khattar R, Cherepanov V, Selzner M, et al. Lipopolysaccharide and tumor necrosis factor alpha inhibit interferon signaling in hepatocytes by increasing ubiquitin-like protease 18 (USP18) expression. J Virol. 2016;90:5549–5560. doi: 10.1128/JVI.02557-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Faure-Dupuy S, Delphin M, Aillot L, Dimier L, Lebossé F, Fresquet J, et al. Hepatitis B virus-induced modulation of liver macrophage function promotes hepatocyte infection. J Hepatol. 2019;71:1086–1098. doi: 10.1016/j.jhep.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 88.Vanlandschoot P, Roobrouck A, Van Houtte F, Leroux-Roels G. Recombinant HBsAg, an apoptotic-like lipoprotein, interferes with the LPS-induced activation of ERK-1/2 and JNK-1/2 in monocytes. Biochem Biophys Res Commun. 2002;297:486–491. doi: 10.1016/s0006-291x(02)02243-x. [DOI] [PubMed] [Google Scholar]
- 89.Cheng J, Imanishi H, Morisaki H, Liu W, Nakamura H, Morisaki T, et al. Recombinant HBsAg inhibits LPS-induced COX-2 expression and IL-18 production by interfering with the NFkappaB pathway in a human monocytic cell line, THP-1. J Hepatol. 2005;43:465–471. doi: 10.1016/j.jhep.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 90.Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu M, et al. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J Immunol. 2013;190:5142–5151. doi: 10.4049/jimmunol.1201625. [DOI] [PubMed] [Google Scholar]
- 91.Yu X, Lan P, Hou X, Han Q, Lu N, Li T, et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. J Hepatol. 2017;66:693–702. doi: 10.1016/j.jhep.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 92.Tan-Garcia A, Wai LE, Zheng D, Ceccarello E, Jo J, Banu N, et al. Intrahepatic CD206+ macrophages contribute to inflammation in advanced viral-related liver disease. J Hepatol. 2017;67:490–500. doi: 10.1016/j.jhep.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 93.Zang M, Li Y, He H, Ding H, Chen K, Du J, et al. IL-23 production of liver inflammatory macrophages to damaged hepatocytes promotes hepatocellular carcinoma development after chronic hepatitis B virus infection. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3759–3770. doi: 10.1016/j.bbadis.2018.10.004. [DOI] [PubMed] [Google Scholar]