Abstract
Background
Evidence comparing fibrin sealants (FSs) in surgery are limited. This study evaluated the efficacy and safety of FSs, and manual compression in peripheral vascular surgery.
Methods
A systematic review of randomized trials was conducted in Medline, Embase, and Cochrane databases within the last 15 years. Data were available to conduct a network meta-analysis (NMA) in peripheral vascular surgery. Fibrin sealant treatment arms were further broken-down and assessed by clotting time (i.e., 2-min [2C] or 1-min [1C]). The primary efficacy outcome was the proportion of patients achieving hemostasis by 4 min (T4). Treatment-related serious and non-serious adverse events (AEs) were qualitatively assessed.
Results
Five studies (n = 693), were included in the NMA. Results predicted VISTASEAL 2C, followed by EVICEL 1C, had the highest probability of achieving T4. Compared with manual compression, significant improvements in T4 were found with VISTASEAL 2C (relative risk [RR] = 2.67, 95% CrI: 2.13–3.34), EVICEL 1C (RR = 2.58, 95% CrI: 2.04–3.23), VISTASEAL 1C (RR = 2.00, 95% CrI: 1.45–2.65), and TISSEEL 2C (RR = 1.99, 95% CrI: 1.48–2.60). TISSEEL 1C was not significantly different than manual compression (RR = 1.40, 95% CrI: 0.70–2.33). Among FSs, VISTASEAL 2C was associated with a significant improvements in T4 compared with VISTASEAL 1C (RR = 1.33, 95% CrI: 1.02–1.82), TISSEEL 2C (RR = 1.34, 95% CrI: 1.05–1.77), and TISSEEL 1C (RR = 1.90, 95% CrI: 1.18–3.74). Treatment-related serious and non-serious AE rates were typically lower than 2%.
Conclusions
In peripheral vascular surgeries, VISTASEAL 2C and EVICEL 1C were shown to have the highest probabilities for achieving rapid hemostasis among the treatments compared. Future studies should expand networks across surgery types as data become available.
Keywords: Fibrin tissue adhesive, Hemostasis, Network meta-analysis, Systematic review, Vascular surgical procedures
Highlights
-
•
Fibrin sealants can control perioperative bleeding, yet comparative evidence is limited.
-
•
This network meta-analysis compared fibrin sealants and compression in peripheral vascular surgery.
-
•
VISTASEAL and EVICEL had the highest probability of achieving hemostasis by 4 min.
-
•
These results show differences exist between fibrin sealants in peripheral vascular surgery.
1. Introduction
Perioperative bleeding is a common complication of surgery associated with substantial clinical and economic burden [[1], [2], [3]]. Uncontrolled bleeding is associated with increased operative time, length of stay, infections, and ventilator use [1,4]. Furthermore, changing patient demographics creates further challenges in bleeding management. For instance, the rising incidence of cardiovascular disease increases use of anticoagulants and antiplatelet medications which can increase perioperative bleeding risk [[5], [6], [7], [8]]. Red blood cell transfusions are often required as a result of perioperative bleeding; however, issues with their use include limited availability, costs, and complications that can impact patient safety and quality of care [[9], [10], [11]].
In an effort to manage perioperative bleeding and limit the use of transfusions, several adjunctive hemostatic agents have been developed [12]. These hemostatic agents can include one or a combination of gelatin, collagen, oxidized cellulose, thrombin and/or fibrinogen [12,13]. Fibrin sealants (FSs), one type of active hemostat, typically contain two major components - fibrinogen and thrombin - which work together to create a cross-linked fibrin clot that mimics the final steps of the coagulation cascade. Over the past several years, FSs have emerged as one of the leading adjunctive hemostatic agents for controlling perioperative bleeding across a range of surgery types, including cardiovascular, orthopedic, thoracic, gynecologic, and urologic surgeries [11,14]. Fibrin sealants can have beneficial characteristics including their safety (i.e., no tissue toxicity), rapid action, reabsorbability (broken down through endogenous fibrinolytic mechanisms), ability to mimic natural coagulation mechanisms regardless of the patient's coagulation profile, and promotion of local tissue growth and repair [15,16]. Further, several randomized trials and systematic reviews have reported favorable outcomes with the use of FSs compared with standard techniques for achieving hemostasis and reduction of blood loss during surgical procedures [11,13,17,18].
Despite the large and growing evidence-base for FSs, their comparative efficacy and safety remains unclear as the design of randomized trials has so far precluded such comparisons. Moreover, formulations, dosages, applicators, usage instructions, and manufacturing processes can differ amongst these products, despite similarity in core components. A network meta-analysis (NMA) enables the indirect comparison of treatments that have not been directly compared in clinical trials but have a direct comparator in common. Therefore, the objective of this study was to conduct a systematic literature review and NMA to evaluate the efficacy and safety of FSs as adjuvant to hemostasis in patients undergoing peripheral vascular surgery.
2. Materials and Methods
2.1. Data source and search
This systematic review and NMA followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19,20]. A systematic search of MEDLINE, Embase, and the Cochrane Library databases was conducted for systematic reviews and randomized controlled trials (RCTs) between January 2005 to January 18th, 2019 using a search strategy developed by a library scientist that included vocabulary and key words related to our research question (e.g., “fibrin sealants”, “fibrin tissue adhesive”, “fibrin glue”, “hemostatic agent”). Only English language articles were reviewed. Reference lists of retrieved articles were hand-searched. Specific details regarding the search strategy appear in Appendix A.
2.2. Eligibility criteria
Studies were considered for inclusion if they were RCTs, included adult (>18 years old) surgical patients, compared a FS to another hemostatic method for open surgery, and reported outcomes related to hemostatic efficacy at specific time points after product application. Originally, most general, gynecological, urological, and vascular surgery types were considered for inclusion; however, this was later restricted to only peripheral vascular surgeries given the dearth of data in other surgery types and to minimize population heterogeneity. Based on this inclusion, the eligibility of each publication was evaluated in the title and abstract review. Full-text screening was conducted if the abstract suggested potential eligibility. Systematic reviews and meta-analyses were reviewed for insights and reference searching. Records were evaluated by two independent reviewers and discrepancies resolved either through consensus or by adjudication from a third reviewer.
2.3. Data extraction and quality assessment
Data from included studies were extracted independently by the same reviewers and included: study authors, publication year, study time frame, study design, country, sample size, key patient characteristics (e.g., mean age, surgery type, anticoagulant medication use), intervention and comparator details (e.g., FS type and clotting time), and detailed outcomes. In some instances, the number of patients experiencing events was calculated from percentage values reported in the studies. Potential risks of bias were assessed independently using the Cochrane Collaboration Risk of Bias Tool (version 5.2).
2.4. Study outcomes
Outcomes of interest included hemostatic efficacy and safety. The primary efficacy outcome evaluated across included trials and this NMA was the proportion of patients achieving hemostasis by 4 min after treatment application (T4). The secondary outcome was the proportion of patients achieving hemostasis by 10 min (T10). Insufficient data were available for other hemostasis related outcomes (e.g., transfusion) or healthcare resource use (eg, length of hospital stay) and therefore were not included in the analysis. For safety outcomes, the analysis focused on treatment-related adverse events (AEs) (i.e., definitely, probably, or possibly related). Due to inconsistent reporting and data insufficiency, an NMA for treatment-related AEs could not be constructed. Instead, outcomes were assessed qualitatively.
2.5. Data synthesis and Bayesian network meta-analysis
Upon completion of the data extraction it was found that the amount of time between FS application and re-establishment of blood flow by the release of vessel clamps (clotting/vessel clamping time) differed amongst studies. Therefore, FS treatment arms were further broken-down and assessed by clotting time (i.e., 2-min [2C] or 1-min [1C]).
All identified treatments were compared pairwise using Bayesian NMA based on the binomial likelihood model. Each was considered as an individual node in the network, with analyses performed using R Software (version 5.3.1) and JAGS (version 4.3.0) based on code from NICE TSD2 [21]. Results of pairwise comparisons were reported as relative risk (RR) and 95% credible intervals (CrI). For hemostasis efficacy, RR can be interpreted as the ratio of the proportion of patients with hemostasis success in the two treatment arms at any one particular time point.
Study and patient characteristics were assessed to ensure similarity and investigate the potential effect of clinical heterogeneity on results. A fixed-effects model was chosen for the primary analysis, as the evidence network was largely comprised of single study connections, and random-effect models may not provide realistic intervals when fit on sparse networks. Analysis of the agreement between direct and indirect evidence using unrelated mean effect models as described in NICE TSD4 [22] was planned, however, the network did not include any independent loops, making statistical analysis of consistency between direct/indirect evidence impossible. A sensitivity analysis acknowledging potential population heterogeneity was performed using a random-effects NMA with informative priors based on one of the settings described by Turner et al. which comprises a semi-objective outcome and a non-pharmacological intervention comparison, was conducted [23].
3. Results
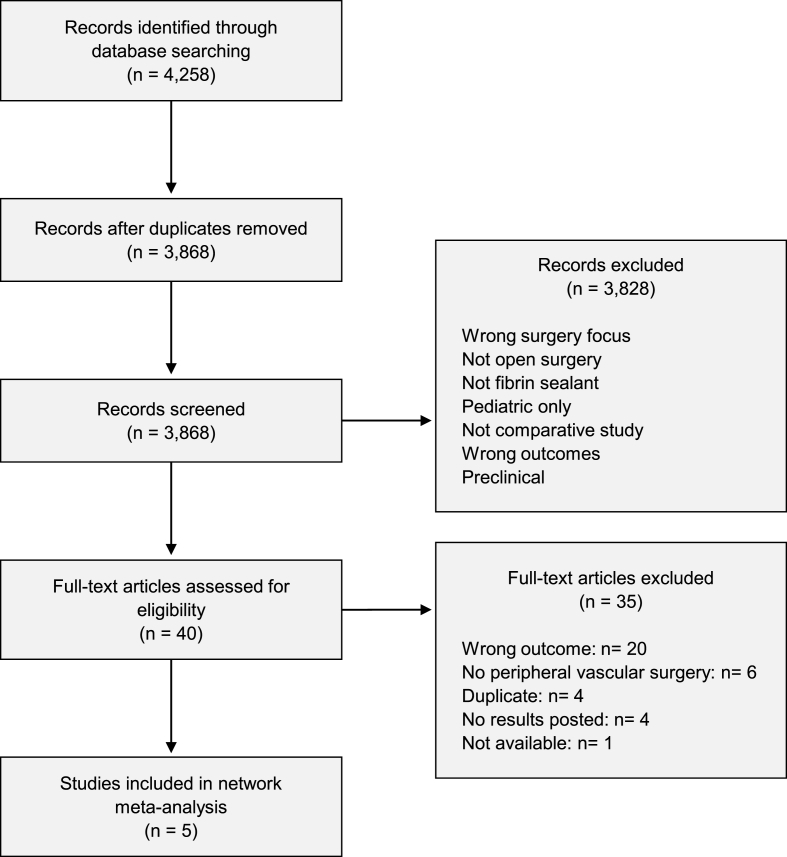
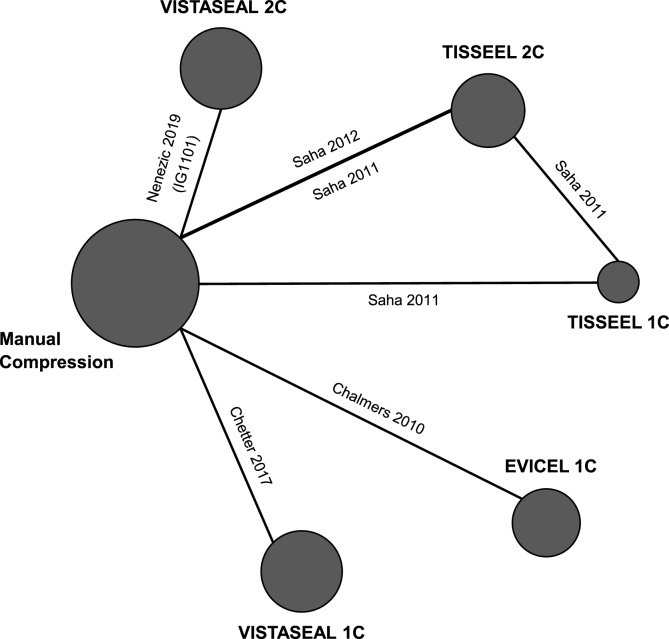
A total of 3868 records were identified, of which 3828 records were excluded during title and abstract review. Of the 40 full-text articles assessed for inclusion, five publications were included in the NMA (Fig. 1) [[24], [25], [26], [27], [28]]. During the development of this study, the official publication for trial IG1101 was released, and was included for completeness [24,29]. All studies compared the FS of interest to manual compression. The FSs captured included VISTASEAL 1C, VISTASEAL 2C, TISSEEL 1C, TISSEEL 2C, and EVICEL 1C (Fig. 2) [[24], [25], [26], [27], [28]]. Sample sizes ranged from 73 to 167 participants, totaling 693 patients. Mean/median age across trials ranged from 61 to 68 years; the proportion of male participants ranged from 48% to 86%. In all trials, patients were heparinized during the surgical procedure [[25], [26], [27], [28], [29]]. More than half of the total 693 patients underwent bypass grafting as the peripheral vascular surgery. Key demographic information and efficacy outcomes for T4/T10 by trial are summarized in Table 1 and Table 2, respectively.
Fig. 1.
Prisma flowchart.
Fig. 2.
Evidence network for hemostasis success at both 4 and 10 min.
Note: In the evidence networks, the width of the lines for each connection is proportional to the number of randomized controlled trials comparing each pair of treatments. The size of each treatment node is proportional to the number of randomized participants (sample size).
Abbreviations: 1C = 1-min clotting time; 2C = 2-min clotting time.
Table 1.
Study characteristics and patient demographics.
| Study | Comparator (n) | Age, YearsMean (SD) or Median (Range) | Male n (%) | Surgery type | Surgery number (%) | Intraoperative Coagulation Medications |
|---|---|---|---|---|---|---|
| Nenezic 2019 [20,28] | VISTASEAL 2C (109) | 64(44–84) | 76 (70) | Bypass Grafting | 95 (87.2%) | Heparin 100IU/kg before clamping for bypass and 50IU/kg for AVG |
| Arteriovenous Graft Formation | 14 (12.8%) | |||||
| Manual Compression (57) | 61(22–82) | 31 (54) | Bypass Grafting | 47 (82.5%) | ||
| Arteriovenous Graft Formation | 10 (17.5%) | |||||
| Chetter 2017 [19] | VISTASEAL 1C (110) | 68(8.9) | 95 (86) | Bypass grafting | 53 (48.2%) | According to the respective institution's standards (included systemic heparin regime/dose, reversal with protamine, and timing of reversal) |
| Endarterectomy requiring patch angioplasty | 27 (24.5%) | |||||
| Aneurysm resection and graft replacement | 18 (16.4%) | |||||
| Iliofemoral bypass | 4 (3.63%) | |||||
| Other type of surgery (≤1 each) | 8 (7.3%) | |||||
| Manual Compression (57) | 67(10.3) | 39 (68) | Bypass grafting | 27 (47.4%) | ||
| Endarterectomy requiring patch angioplasty | 11 (19.3%) | |||||
| Aneurysm resection and graft replacement | 13 (22.8%) | |||||
| Iliofemoral bypass | 2 (3.5%) | |||||
| Other type of surgery (≤1 each) | 1 (1.8%) | |||||
| Saha 2012 [31] | TISSEEL 2C (70) | 63(12.6) | 30 (43) | Bypass grafting | 39 (55.7%) | Heparin 100IU/kg before clamping for bypass and 50IU/kg for AV shunts |
| Arteriovenous shunt | 31 (44.3%) | |||||
| Manual Compression (70) | 66(11.5) | 37 (53) | Bypass grafting | 28 (40%) | ||
| Arteriovenous shunt | 42 (60%) | |||||
| Saha 2011 [30] | TISSEEL 1C (26) | 63(12.7) | 21 (81) | Bypass grafting | NR | Mean heparin units (IU) = 4621.2 |
| Arteriovenous shunt with graft | ||||||
| TISSEEL 2C (24) | 64(14.6) | 12 (50) | Bypass grafting | NR | Mean heparin units (IU) = 5804.2 | |
| Arteriovenous shunt with graft | ||||||
| Manual Compression (23) | 63(14.4) | 11 (48) | Bypass grafting | NR | Mean heparin units (IU) = 5731.8 | |
| Arteriovenous shunt with graft | ||||||
| Chalmers 2010 [29] | EVICEL 1C (75) | 66(11) | 41 (55) | Femoral procedures | Heparin: 70IU/kg for bypass and 35IU/kg for AVG. Protamine in 5–7% | |
| Bypass grafting | 44 (58.7%) | |||||
| Arteriovenous access graft | 1 (1.3%) | |||||
| Aneurysm graft | 3 (4%) | |||||
| Upper extremity graft procedures | 27 (36%) | |||||
| Manual Compression (72) | 66(14) | 36 (50) | Femoral procedures | |||
| Bypass grafting | 48 (66.7%) | |||||
| Arteriovenous access graft | 3 (4.2%) | |||||
| Aneurysm graft | 0 | |||||
| Upper extremity graft procedures | 21 (29.2%) | |||||
Abbreviations: 1C = 1-min clotting time; 2C = 2-min clotting time; AVG = arteriovenous graft; IU = international unit, NR = not reported; n = number of patients; SD = standard deviation. characteristics.
Table 2.
Hemostasis success at 4 and 10 min for studies included in the network meta-analysis.
| Study | Comparator (n) | Percentage of patients achieving hemostasis by T4 (%) | Percentage of patients achieving hemostasis by T10 (%) |
|---|---|---|---|
| Nenezic 2019 [20,28] | VISTASEAL 2C (109) | 76 | 88 |
| Manual Compression (57) | 23 | 46 | |
| Chetter 2017 [19] | VISTASEAL 1C (110) | 63 | 88 |
| Manual Compression (57) | 32 | 72 | |
| Saha 2012 [31] | TISSEEL 2C (70) | 63 | 76 |
| Manual Compression (70) | 31 | 56 | |
| Saha 2011 [30] | TISSEEL 1C (26) | 46 | 65 |
| TISSEEL 2C (24) | 63 | 75 | |
| Manual Compression (23) | 35 | 43 | |
| Chalmers 2010 [29] | EVICEL 1C (75) | 85 | 96 |
| Manual Compression (72) | 39 | 69 |
Abbreviations: 1C = 1-min clotting time; 2C = 2-min clotting time; T4 = 4 min; T10 = 10 min; n = number of patients.
3.1. Risk of bias assessment
The risk of bias was largely consistent across studies, with only slight differences in risk of selection bias (sequence generation, allocation concealment) due to unclear reporting. There was a low risk of performance bias (blinding of participants and personnel), attrition bias (incomplete outcome data), and reporting bias (selective outcome reporting). Since the outcome assessors (surgeons) could not be blinded, the risk of detection bias was considered unclear across the studies. Two of the five studies reported use of computers to generate the random sequence, and two studies reported use of sealed opaque envelopes for allocation concealment, although only one clarified that the envelopes were sequentially numbered [25,26,29]. A summary of the bias risk is reported in Table 3.
Table 3.
Risk of bias assessment.
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| Nenezic2019 [20,28] | Unclear | Low | Low | Unclear | Low | Low |
| Chetter2017 [19] | Low | Unclear | Low | Unclear | Low | Low |
| Saha2012 [31] | Unclear | Unclear | Low | Unclear | Low | Low |
| Saha2011 [30] | Unclear | Unclear | Low | Unclear | Low | Low |
| Chalmers2010 [29] | Low | Unclear | Low | Unclear | Low | Low |
3.2. Hemostasis by 4 min
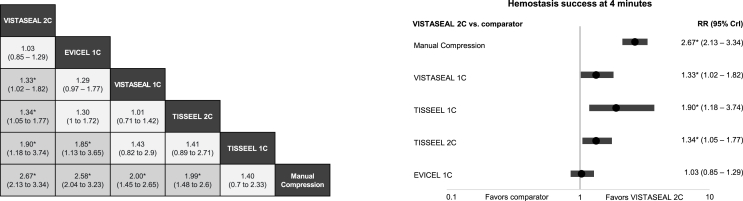
For the primary efficacy outcome, results predicted the treatments with the highest probability of achieving T4 to be VISTASEAL 2C and EVICEL 1C. Compared with manual compression, significant improvements in T4 were found with VISTASEAL 2C (relative risk [RR] = 2.67, 95% CrI: 2.13–3.34), EVICEL 1C (RR = 2.58, 95% CrI: 2.04–3.23), VISTASEAL 1C (RR = 2.00, 95% CrI: 1.45–2.65), and TISSEEL 2C (RR = 1.99, 95% CrI: 1.48–2.60). TISSEEL 1C was the only FS found to not be significantly different from manual compression for the probability of achieving T4 (RR = 1.40, 95% CrI: 0.70–2.33). When comparing FSs, VISTASEAL 2C was associated with a significantly higher probability of achieving T4 compared with VISTASEAL 1C (RR = 1.33, 95% CrI: 1.02–1.82), TISSEEL 2C (RR = 1.34, 95% CrI: 1.05–1.77), and TISSEEL 1C (RR = 1.90, 95% CrI: 1.18–3.74). The only other significant difference observed among FSs was EVICEL 1C having a significantly higher probability of achieving T4 compared to TISSEEL 1C. Similar hemostasis rates were observed for VISTASEAL 2C and EVICEL 1C (RR = 1.03, 95% CrI: 0.85–1.29). Results of pairwise comparisons for T4 are reported in Fig. 3a Fig. 3b presents a forest plot for VISTASEAL 2C vs. other comparators.
Fig. 3.
a) Fixed-effects network meta-analysis for hemostasis success by 4 min: a) for all comparators (RR, 95% CrI), b) for VISTASEAL 2C versus comparators (RR, 95% CrI).
Abbreviations: 1C = 1-min clotting time; 2C = 2-min clotting time; CrI = credible interval; RR = relative risk.
*Indicates statistically significant differences vs. comparator to the far right.
3.3. Hemostasis by 10 min
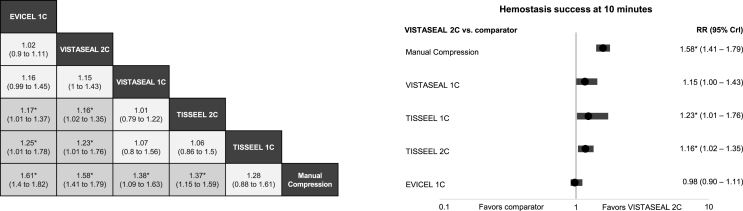
For the secondary efficacy outcome, treatments with the highest probabilities of achieving T10 were EVICEL 1C and VISTASEAL 2C. Similar to the primary analysis, all FSs, except TISSEEL 1C, significantly improved the probability of achieving T10 compared with manual compression (Fig. 4a). Both EVICEL 1C and VISTASEAL 2C were associated with a significantly higher probability of achieving T10 compared to TISSEEL 2C and with TISSEEL 1C (Fig. 4a). Fig. 4b presents a forest plot of VISTASEAL 2C vs. other comparators.
Fig. 4.
a) Fixed-effects network meta-analysis for hemostasis success by 10 min: a) for all comparators (RR, 95% CrI), b) for VISTASEAL 2C versus comparators (RR, 95% CrI).
Abbreviations: 1C = 1-min clotting time; 2C = 2-min clotting time; CrI = credible interval; RR = relative risk.
*Indicates statistically significant differences vs. comparator to the far right.
3.4. Sensitivity analysis
Results of the sensitivity analysis using a random-effects model with informative priors found similar findings to the primary fixed-effects analysis (Appendix B). Compared with manual compression, a significantly higher probability of achieving T4 was observed for all FSs except TISSEEL 1C. Comparisons between FS resulted in the same trend; however, the only products showing significant improvements in probability of achieving T4 included VISTASEAL 2C and EVICEL 1C, both compared with TISSEEL 1C. All other differences remained non-significant.
3.5. Adverse events
Data was available on the overall rate of serious adverse events (SAE) and non-SAE from four of the five included trials [24,[26], [27], [28]]. Overall rates of SAE and non-SAE ranged from 15% –29%, and 15%–83%, respectively. None of the trials reported an AE categorized as definitely or probably related to a FS (Table 4). When comparing AEs related to FSs across studies, it was observed that the number of events considered possibly related to FSs was much lower than the overall rate; that is, 0%–1.2% for SAE and 0.5%–2% for non-SAE (Table 4). Chalmers et al. did not classify AEs as whether they were serious or not; however, they reported that 12% of patients experienced AEs that were considered possibly related to treatment overall (EVICEL 1C) [25].
Table 4.
Treatment-relateda adverse events data reported in included studies.
| Study | Treatment | Treatment-Related SAE | Treatment-Related Non-SAE |
|---|---|---|---|
| Nenezic 2019 [20,28] | VISTASEAL 2C | 2/168 (1.2%) | 2/168 (1.2%) |
| Possibly Related | Possibly Related | ||
| Cellulitis, B19V test positiveb | Vascular graft complication, B19V test positivec | ||
| Manual Compression | 0 | 0 | |
| Chetter 2017 [19] | VISTASEAL 1C | 1/187 (0.5%) | 1/187 (0.5%) |
| Possibly Related | Possibly Related | ||
| Post-Procedure Hemorrhage | Wound Infection | ||
| Manual Compression | 0 | 0 | |
| Saha 2012 [31] | TISSEEL 2C | 0 | 1/70 (1.4%) |
| Possibly Related | |||
| Intraoperative bleeding | |||
| Manual Compression | 0 | 1/70 (1.4%) | |
| Possibly Related | |||
| Postoperative hematoma | |||
| Saha 2011 [30] | TISSEEL 1C | 0 | 1/50 (2%) |
| TISSEEL 2C | 0 | Possibly Related | |
| Venous stenosis in the area of the anastomosis | |||
| Manual Compression | 0 | 1/23 (4.3%) | |
| Probably Related | |||
| Intraoperative rebleeding | |||
| Chalmers 2010 [29] | EVICEL 1C | NR | NR |
| Manual Compression | NR | NR |
Abbreviations: 1C = 1-min clotting time; 2C = 2-min clotting time; NR = not reported; SAE = serious adverse event.
Treatment-related defined as an adverse event definitely, probably, or possibly related to study treatment.
The data suggest that there was no treatment emergent viral infection.
The data suggest passive transmission of B19V IgG antibody with the transfusion of blood products.
4. Discussion
This NMA demonstrates that currently studied FS have a significantly higher probability of achieving hemostasis compared with manual hemostasis methods in peripheral vascular surgery. Among FSs, the newest generation product, VISTASEAL, is associated with a similar or improved hemostatic effect, while providing a similar safety profile compared with other FSs. Patients treated with VISTASEAL 2C and EVICEL 1C had the highest probability of achieving hemostasis by 4 min, with VISTASEAL 2C demonstrating significantly improved results compared with TISSEEL 1C and 2C. Findings were similar with hemostasis at 10 min. EVICEL 1C and VISTASEAL 2C were the only treatments associated with a significantly higher probability of achieving hemostasis, at either 4 or 10 min, compared to another FS. No significant differences were observed between EVICEL 1C and VISTASEAL 1C or 2C. A random-effects sensitivity analysis that acknowledges the potential for larger heterogeneity across trials similarly ranked VISTASEAL 2C and EVICEL 1C as the treatments with the highest probability of success for hemostasis at 4 min.
The present study is the first NMA comparing the hemostatic efficacy and safety between different FSs. Literature on FS efficacy and safety has proliferated rapidly; however, published systematic reviews and meta-analyses are limited with respect to comparison of performance between FS products [13]. Several systematic reviews and meta-analyses have assessed the group-based efficacy of FSs (i.e., with no comparison between individual products) or the efficacy of a broader group of hemostatic agents including FSs and other surgical sealants, such as those consisting of polyethylene glycol, gelatin and thrombin, or protein-based adhesives [[30], [31], [32], [33]]. Common comparators included in these systematic reviews are manual compression, standard of care, gelatine sponge, argon beam coagulation, PlasmaJet, polyglycolic acid felt, and oxidized cellulose [[30], [31], [32], [33], [34]].
Results of this NMA are important as the burden of bleeding in surgery can be considerable and the comparative efficacy between hemostats/fibrin sealants is largely unknown. A study using data from the Premier Perspectives Database reported that, despite the use of hemostatic agents, uncontrolled bleeding events occurred in 56%–68% of cardiovascular surgery patients [1]. This analysis included 13%–16% of patients receiving FSs, with others receiving active, mechanical, or multiple agents. Despite hemostat use, 25%–71% of patients still required transfusions [1]. Comparative effectiveness data between product types were not available from this database analysis. The persistence of surgical bleeding, despite currently available hemostasis methods, represents a continued risk of unfavorable clinical outcomes, an unmet need requiring advanced hemostats, and a more critical, substantiated understanding of comparative performance [1].
Several individual clinical factors have been linked to a higher risk of surgical bleeding including coagulopathies, renal and liver diseases, and various medications such as chronic antiplatelet therapies, non-steroidal anti-inflammatory drugs, and anticoagulants which have been recently associated with an increased number of users [6,35]. These factors can complicate surgeries as well as increase the likelihood of transfusion, reoperation, and associated complications. Mortality rates can increase to 20% in cases of severe bleeding [1]. Fibrin sealants represent an important option in patients at high risk of bleeding as they typically remain effective in the presence of congenital or acquired coagulation disorders, including heparinized patients; as their use are not dictated by the functionality of the patient coagulation system [36]. However, a FS should not be used as a substitute for the primary hemostatic techniques or to control life-threatening severe bleeds, typically in arteries or arterioles of significant diameter [29].
Although the FSs studied in this analysis consist of similar concentrations and activities of human plasma-derived fibrinogen and thrombin, respectively, some differences in their manufacturing processes, biochemical properties, formulations, and application may account for the variation in efficacy observed. A study assessing the biochemical properties of fibrin clot formation found a superior clot strength and resilience with EVICEL compared with TISSEEL, and concluded that it may be largely attributed to higher factor XIII levels with EVICEL [37]. Dickneite et al. compared the properties of several commercially available FSs and noted that TISSEEL showed a significantly lower adhesive strength to tissue when compared with six products [38]. Another difference between TISSEEL and VISTASEAL involves the viral inactivation process. VISTASEAL components undergo dual nanofiltration, while TISSEEL undergoes a high temperature two-step vapor heating process, which can potentially have detrimental effects on the proteins; however, the clinical relevance of this is unclear [39]. Other varying factors amongst FSs include the presence of aprotinin in TISSEEL, the concentrations of polysorbate 80, viscosity, and the range of working temperature for clinical use, all of which may contribute to differential efficacy; however, the impacts are not known.
The following study has some important limitations. The network has a small number of studies in each node and limited direct comparative evidence. These factors impacted the ability to evaluate the consistency of the NMA assumptions and conduct subgroup analyses. The network was further restricted due to the separation of interventions by clotting time. These methods were, however, deemed necessary since some data have shown that the hemostatic efficacy of FSs may be influenced by length of clotting time [27]. Some inevitable heterogeneity is still to be expected because of the nature of FS utilization, which may include differences in surgical techniques and application methods. In addition to the outcomes of T4 and T10, the included trials assessed the achievement of hemostasis by other time points, such as 3, 5, and 7 min. As these time points were not consistently measured by all the trials, these results were not analyzed in the NMA. This may potentially hamper the identification of unknown differences between the treatments and any patterns occurring over the duration of the hemostasis process. Additionally, there were some inconsistencies, overlap, or absences of information involving the definitions of bleeding severity used by the FS studies. As such, the conduct of an analysis stratified by bleeding severity, was not possible. Finally, there are limitations associated with the outcomes evaluated across included studies. The primary efficacy measure was based on the surrogate endpoint of hemostasis at 4 min, which not reflect effectiveness in a real-world setting, and limited data was available on direct hemostasis-related outcomes (i.e., transfusions) or healthcare resource use which precluded analysis for these types of outcomes.
5. Conclusions
This systematic review and NMA provides new indirect evidence on the comparative efficacy and safety of FSs for peripheral vascular surgery. Results demonstrate that differences are observed between FSs and that this can possibly be explained by biochemical, formulation, and processing. VISTASEAL 2C and EVICEL 1C were shown to have the highest probability of achieving hemostasis at 4 min, and all FSs had low rates of treatment-related adverse events. Future research should compare FSs in other surgical specialties, as well as consider other type of analyses, such as matched adjusted indirect comparisons, which could better address some limitations that arise when using aggregate data.
Provenance and peer review
Not commissioned, externally peer reviewed.
Ethical approval
Ethical approval not required for the systematic review and network meta-analysis.
Sources of funding
Funding provided by Ethicon Inc.
Author contribution
W. Danker III, N. Ferko, D. Garcia, and A. Hogan designed the research.
N. Ferko, D. Garcia, and A. Hogan collected the data.
W. Danker III, A. DeAnglis, N. Ferko, D. Garcia, and A. Hogan analyzed/interpreted the data.
N. Ferko, D. Garcia, and A. Hogan drafted the manuscript.
W. Danker III, A. DeAnglis, N. Ferko, D. Garcia, and A. Hogan revised the manuscript for important intellectual content and approved the final version for publication.
Registration of research studies
1. Name of the registry: Research Registry.
2. Unique Identifying number or registration ID: reviewregistry992.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
The Guarantor is the one or more people who accept full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Nicole Ferko.
Walter Danker III.
Consent
No data on patients or volunteers were captured with this systematic review and network meta-analysis, as such, consent was not required.
Declaration of competing interest
CRG-EVERSANA (N. Ferko, D. Garcia, and A. Hogan) was provided consulting fees from Ethicon Inc., for analysis and development of the manuscript. W. Danker III and A. DeAnglis are employees of Ethicon Inc., the manufacturer for VISTASEAL and EVICEL.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.12.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Corral M., Ferko N., Hollmann S., Broder M.S., Chang E. Health and economic outcomes associated with uncontrolled surgical bleeding: a retrospective analysis of the Premier Perspectives Database. Clinicoecon Outcomes Res. 2015;7:409–421. doi: 10.2147/CEOR.S86369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corral M., Ferko N., Hogan A., Hollmann S.S., Gangoli G., Jamous N., Batiller J., Kocharian R. A hospital cost analysis of a fibrin sealant patch in soft tissue and hepatic surgical bleeding. Clinicoecon Outcomes Res. 2016;8:507–519. doi: 10.2147/CEOR.S112762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghadimi K., Levy J.H., Welsby I.J. Perioperative management of the bleeding patient. Br. J. Anaesth. 2016;117:iii18–iii30. doi: 10.1093/bja/aew358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokes M.E., Ye X., Shah M., Mercaldi K., Reynolds M.W., Rupnow M.F., Hammond J. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv. Res. 2011;11:135. doi: 10.1186/1472-6963-11-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyasaka Y., Barnes M.E., Gersh B.J., Cha S.S., Bailey K.R., Abhayaratna W.P., Seward J.B., Tsang T.S. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 6.Barnes G.D., Lucas E., Alexander G.C., Goldberger Z.D. National trends in ambulatory oral anticoagulant use. Am. J. Med. 2015;128:1300–1305 e2. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavallari I., Patti G. Efficacy and safety of oral anticoagulation in elderly patients with atrial fibrillation. Anatol. J. Cardiol. 2018;19:67–71. doi: 10.14744/AnatolJCardiol.2017.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoeb M., Fang M.C. Assessing bleeding risk in patients taking anticoagulants. J. Thromb. Thrombolysis. 2013;35:312–319. doi: 10.1007/s11239-013-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soril L.J.J., Noseworthy T.W., Stelfox H.T., Zygun D.A., Clement F.M. A retrospective observational analysis of red blood cell transfusion practices in stable, non-bleeding adult patients admitted to nine medical-surgical intensive care units. J. Intensive Care. 2019;7:19. doi: 10.1186/s40560-019-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker L., Park L., Gilbert R., Martel A., Ahn H., Davies A., McIsaac D.I., Saidenberg E., Tinmouth A., Fergusson D.A., Martel G. Guidelines on the intraoperative transfusion of red blood cells: a protocol for systematic review. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards S.J., Crawford F., van Velthoven M.H., Berardi A., Osei-Assibey G., Bacelar M., Salih F., Wakefield V. The use of fibrin sealant during non-emergency surgery: a systematic review of evidence of benefits and harms. Health Technol. Assess. 2016;20:1–224. doi: 10.3310/hta20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spotnitz W.D., Burks S. Hemostats, sealants, and adhesives III: a new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion. 2012;52:2243–2255. doi: 10.1111/j.1537-2995.2012.03707.x. [DOI] [PubMed] [Google Scholar]
- 13.Spotnitz W.D. Fibrin sealant: the only approved hemostat, sealant, and adhesive-a laboratory and clinical perspective. ISRN Surg. 2014;2014:203943. doi: 10.1155/2014/203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carless P.A., Henry D.A., Anthony D.M. Fibrin sealant use for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst. Rev. 2003:CD004171. doi: 10.1002/14651858.CD004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tawes R.L., Jr., Sydorak G.R., DuVall T.B. Autologous fibrin glue: the last step in operative hemostasis. Am. J. Surg. 1994;168:120–122. doi: 10.1016/s0002-9610(94)80049-9. [DOI] [PubMed] [Google Scholar]
- 16.Bjelovic M., Ayguasanosa J., Kim R.D., Stojanovic M., Vereczkei A., Nikolic S., Winslow E., Emre S., Xiao G., Navarro-Puerto J., Courtney K., Barrera G. Investigators of the fibrin sealant grifols in hepatic resection clinical investigation study G. A prospective, randomized, phase III study to evaluate the efficacy and safety of fibrin sealant grifols as an adjunct to hemostasis as compared to cellulose sheets in hepatic surgery resections. J. Gastrointest. Surg. 2018;22:1939–1949. doi: 10.1007/s11605-018-3852-4. [DOI] [PubMed] [Google Scholar]
- 17.Carless P.A., Anthony D.M., Henry D.A. Systematic review of the use of fibrin sealant to minimize perioperative allogeneic blood transfusion. Br. J. Surg. 2002;89:695–703. doi: 10.1046/j.1365-2168.2002.02098.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z., Xiao L., Guo H., Zhao G., Ma J. The efficiency and safety of fibrin sealant for reducing blood loss in primary total hip arthroplasty: a systematic review and meta-analysis. Int. J. Surg. 2017;37:50–57. doi: 10.1016/j.ijsu.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P., Straus S., Thorlund K., Jansen J.P., Mulrow C., Catala-Lopez F., Gotzsche P.C., Dickersin K., Boutron I., Altman D.G., Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 20.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Dias S., Welton N.J., Sutton A.J., Ades A.E. 2014. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. London. [PubMed] [Google Scholar]
- 22.Dias S., Welton N.J., Sutton A.J., Caldwell D.M., Lu G., Ades A.E. 2014. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. London. [PubMed] [Google Scholar]
- 23.Turner R.M., Davey J., Clarke M.J., Thompson S.G., Higgins J.P. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int. J. Epidemiol. 2012;41:818–827. doi: 10.1093/ije/dys041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NCT01662856 Cg. 2017. Safety and Efficacy Study of Fibrin Sealant Grifols as an Adjunct to Hemostasis during Peripheral Vascular Surgery. [Google Scholar]
- 25.Chalmers R.T., Darling R.C., Iii, Wingard J.T., Chetter I., Cutler B., Kern J.A., Hart J.C. Randomized clinical trial of tranexamic acid-free fibrin sealant during vascular surgical procedures. Br. J. Surg. 2010;97:1784–1789. doi: 10.1002/bjs.7235. [DOI] [PubMed] [Google Scholar]
- 26.Chetter I., Stansby G., Sarralde J.A., Riambau V., Gimenez-Gaibar A., MacKenzie K., Acin F. Navarro-puerto J, investigators of the fibrin sealant grifols study G. A prospective, randomized, multicenter clinical trial on the safety and efficacy of a ready-to-use fibrin sealant as an adjunct to hemostasis during vascular surgery. Ann. Vasc. Surg. 2017;45:127–137. doi: 10.1016/j.avsg.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 27.Saha S.P., Muluk S., Schenk W., 3rd, Burks S.G., Grigorian A., Ploder B., Presch I., Pavlova B.G., Hantak E. Use of fibrin sealant as a hemostatic agent in expanded polytetrafluoroethylene graft placement surgery. Ann. Vasc. Surg. 2011;25:813–822. doi: 10.1016/j.avsg.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Saha S.P., Muluk S., Schenk W., 3rd, Dennis J.W., Ploder B., Grigorian A., Presch I., Goppelt A. A prospective randomized study comparing fibrin sealant to manual compression for the treatment of anastomotic suture-hole bleeding in expanded polytetrafluoroethylene grafts. J. Vasc. Surg. 2012;56:134–141. doi: 10.1016/j.jvs.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Nenezic D., Ayguasanosa J., Menyhei G., Tamas H., Matyas L., Muluk S., Courtney K., Ibanez J., Chen J. Investigators of the Fibrin Sealant Grifols in Vascular Surgery Clinical Investigation Study G. A prospective, single-blind, randomized, phase III study to evaluate the safety and efficacy of Fibrin Sealant Grifols as an adjunct to hemostasis compared with manual compression in vascular surgery. J. Vasc. Surg. 2019;70:1642–1651. doi: 10.1016/j.jvs.2018.12.051. [DOI] [PubMed] [Google Scholar]
- 30.Ding H., Yuan J.Q., Zhou J.H., Zheng X.Y., Ye P., Mao C., Chen Q. Systematic review and meta-analysis of application of fibrin sealant after liver resection. Curr. Med. Res. Opin. 2013;29:387–394. doi: 10.1185/03007995.2013.768216. [DOI] [PubMed] [Google Scholar]
- 31.Moggia E., Rouse B., Simillis C., Li T., Vaughan J., Davidson B.R., Gurusamy K.S. Methods to decrease blood loss during liver resection: a network meta-analysis. Cochrane Database Syst. Rev. 2016;10:CD010683. doi: 10.1002/14651858.CD010683.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanjay P., Watt D.G., Wigmore S.J. Systematic review and meta-analysis of haemostatic and biliostatic efficacy of fibrin sealants in elective liver surgery. J. Gastrointest. Surg. 2013;17:829–836. doi: 10.1007/s11605-012-2055-7. [DOI] [PubMed] [Google Scholar]
- 33.Rogers A.C., Turley L.P., Cross K.S., McMonagle M.P. Meta-analysis of the use of surgical sealants for suture-hole bleeding in arterial anastomoses. Br. J. Surg. 2016;103:1758–1767. doi: 10.1002/bjs.10308. [DOI] [PubMed] [Google Scholar]
- 34.Brustia R., Granger B., Scatton O. An update on topical haemostatic agents in liver surgery: systematic review and meta analysis. J. Hepatobiliary Pancreat Sci. 2016;23:609–621. doi: 10.1002/jhbp.389. [DOI] [PubMed] [Google Scholar]
- 35.Koh M.B., Hunt B.J. The management of perioperative bleeding. Blood Rev. 2003;17:179–185. doi: 10.1016/s0268-960x(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 36.Chiara O., Cimbanassi S., Bellanova G., Chiarugi M., Mingoli A., Olivero G., Ribaldi S., Tugnoli G., Basilico S., Bindi F., Briani L., Renzi F., Chirletti P., Di Grezia G., Martino A., Marzaioli R., Noschese G., Portolani N., Ruscelli P., Zago M., Sgardello S., Stagnitti F., Miniello S. A systematic review on the use of topical hemostats in trauma and emergency surgery. BMC Surg. 2018;18:68. doi: 10.1186/s12893-018-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickerson W.L., Nur I., Meidler R. A comparison of the mechanical, kinetic, and biochemical properties of fibrin clots formed with two different fibrin sealants. Blood Coagul. Fibrinolysis. 2011;22:19–23. doi: 10.1097/MBC.0b013e32833fcbfb. [DOI] [PubMed] [Google Scholar]
- 38.Dickneite G., Metzner H., Pfeifer T., Kroez M., Witzke G. A comparison of fibrin sealants in relation to their in vitro and in vivo properties. Thromb. Res. 2003;112:73–82. doi: 10.1016/j.thromres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Montana M., Tabele C., Curti C., Terme T., Rathelot P., Gensollen S., Vanelle P. Organic glues or fibrin glues from pooled plasma: efficacy, safety and potential as scaffold delivery systems. J. Pharm. Pharmaceut. Sci. 2012;15:124–140. doi: 10.18433/j39k5h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.