Graphical abstract
Keywords: Drosophila, Insecticide, Cuticle, Barrier, Oxidative stress, Apoptosis, Cncc/Keap1
Highlights
-
•
Contact to Dithiothreitol (DTT), a widely used antioxidant, is lethal to the fruit fly Drosophila melanogaster.
-
•
At low concentrations, DTT contact induces the expression of some apoptosis genes.
-
•
In parallel, DTT triggers the expression of a cuticle barrier gene.
-
•
Expression of some genes involved in detoxification is intensified with increasing DTT concentrations.
-
•
Toxicity of DTT to the exnvironment should be tested.
Abstract
The thiol-containing compound Dithiothreitol (DTT) has been shown to be toxic to cultured cells by inducing the generation of reactive oxygen species that ultimately cause cell death. However, its effects on multicellular organisms and the environment have not been investigated yet in detail. In this work, we tested the toxicity of DTT to the model insect Drosophila melanogaster. We show that DTT is lethal to D. melanogaster by topical application but not through feeding. DTT treatment triggers the transcription of the canonical apoptosis regulators grim, hid and rpr at low amounts. The amplitude of this induction declines with elevating DTT amounts. By live microscopy, we observe apoptotic cells especially in the gut of DTT treated flies. In parallel, low DTT amounts also activate the expression of the cuticle barrier component gene snsl. This indicates that a physical defence response is launched upon DTT contact. This combined measure is seemingly successful in preventing fly death. The expression of a number of known detoxification genes including cyp6a2, cyp6a8, cyp12d1 and GstD2 is also enhanced through DTT contact. The degree of upregulation of these genes is proportional to the applied DTT amounts. Despite this effort, flies exposed to high amounts of DTT eventually die. Together, D. melanogaster is able to sense DTT toxicity and adjust the defence response successfully at least at low concentrations. This is the first time to analyse the molecular consequences of DTT exposure in a multicellular organism. Our work provides a new model to discuss the physiological response of animals against thiol toxins and to resurvey the effect of redox agents on the environment.
1. Introduction
Dithiothreitol (DTT or 1,4-Dimercapto-2,3-butandiol) has two SH groups with strong reducibility and is therefore commonly used as antioxidant in diverse biomedical practices. For instance, DTT prevents interference of the anti-CD38 antibody daratumumab, that is used in a therapy against malignant myeloma, with CD38 exposed on red blood cells during transfusion [1]. Furthermore, DTT has been used in an assay to assess the oxidation property of ambient fine particulate matter 2.5 (PM2.5) in polluted air [2]. Besides these special applications, DTT is a routinely used as a thiol molecule in molecular-biological laboratories. It is applied for protein denaturation.
The biocompatibility of DTT has not been analysed systematically in multicellular organisms and is therefore unclear. In different cell cultures, like other thiols, DTT was reported to induce programmed cell death [3]. There are two proposed mechanisms to explain the effects of DTT in these cases. According to the first possible explanation, thiol oxidation produces hydrogen peroxide following the formula:
| 2RSH + O2 → RSSR +H2O2 |
Increased H2O2 levels, in turn, and other reactive oxygen species (ROS) formed in the Fenton reaction ultimately cause lethality [4,5]. A report that catalase, an enzyme that converses H2O2 to H2O and O2, attenuates DTT toxicity to hamster V79 cells supports this hypothesis [4,6]. However, another report suggests that DTT does not promote cell death in HL-60 cells via H2O2 production, but by the activation of caspase 3 [7]. Catalases do not rescue cell death in this case. The second possible explanation is based on the ability of DTT to break disulphide bonds thereby causing protein misfolding [8,9,3]. The accumulation of misfolded proteins, in turn, induces ER stress and ER stress mediated apoptosis. The amplitude of this response depends on the concentration of DTT. At a low concentration (3.2 mM), the c-Jun N-terminal kinase (JNK) and p38 are activated upon DTT application. At a high concentration (6.4 mM), in addition, ERK activity is reduced. Treatment of the unicellular parasite Trypanosoma cruzi with DTT interferes with mitochondrial homeostasis, ultimately killing the organism [10]. As T. cruzi does not display a canonical ER stress response, the underlying mechanisms of DTT exposure remain to be studied in detail in this case.
Despite the work on cell cultures, we lack evaluations of DTT toxicity in animals. Here we present our assay of DTT toxicity on the fruit fly Drosophila melanogaster that in a number of cases has served as a model organism in toxicology [[11], [12], [13], [14]].
2. Methods
2.1. Fly husbandry
The D. melanogaster stocks w1118 and Dijon 2000 were used in this work. Stocks were kept in vials with standard yeast-cornmeal-molasses food at 25 °C. In all experiments, five to six days old females and males were used. The apoptosis sensor line UAS-GC3Ai was obtained from Magali Suzanne [15]. It was crossed to da-Gal4 for ubiquitous expression in flies of the next generation.
2.2. DTT toxicity assay
DTT (1,4-dithiolthreitol, Sigma-Aldrich) stock solution had a concentration of 2% (w/v) in acetone. Dilution of 0.2 %, 0.1 %, 0.02 % and 0.002 % (w/v) were prepared using this stock solution. For each assay, 1 mL DTT solution was applied to a glass vial with a diameter of 1 cm. The concentrations and amounts used were hence 0%-0 mg (acetone alone, negative control), 2 %–20 mg, 0.2 %–2 mg, 0.1 %–1 mg, 0.02 %–0.2 mg, 0.002 %–0.02 mg. Glass vials were placed in a fume hood overnight to allow complete evaporation of acetone. 10 flies were put into the vial that was closed with a cotton plug. The paralysis or knockdown rate as a standard read-out for toxicity in insects [16] was recorded every hour. Knockdown was defined as the inability of coordinated movement and climbing to the top of the vial; these flies eventually died. For analysis of the DTT penetration route, we cut the proboscis with fine scissors one day before the DTT contact assay (Fig. S1). The survived flies were used the next day to evaluate DTT toxicity. To analyse the effect of oral DTT delivery, a feeding assay based on the CAFÉ assay was designed [17]. 2 % DTT was diluted 1:10 in water and filled into a glass capillary that was inserted into the glass vial to allow the flies to take up DTT water. 0.1 mg/mL chlorantraniliprole and water were used as positive and negative controls, respectively. In this assay, consumption of liquid is visible in the shifting liquid meniscus.
2.3. Quantitative PCR
For quantitative PCR, gene expression in five female flies of each DTT treatment (2 mg, 1 mg, 0.2 mg, 0.02 mg and 0 mg) was analysed. Total RNA was isolated with the RNEasy Kit (Qiagen, Hilden Germany). RNA was used to generate cDNA using oligo(dT) primers (Enhanced Avian First Strand Synthesis Kit, Sigma-Aldrich). qPCR experiments were performed with Roche SybrGreen kit (Roche, Basel, Switzerland) on a Roche LightCycler Nano and evaluated by the respective software. The expression of Rps20 was used as the reference. Four independent samples were prepared in experiments with two technical repeats. Primers for qPCR reaction are listed in Table S1. Student’s T-test was applied for statistical analyses of the expression data.
3. Results and discussion
3.1. DTT is toxic to fruit flies
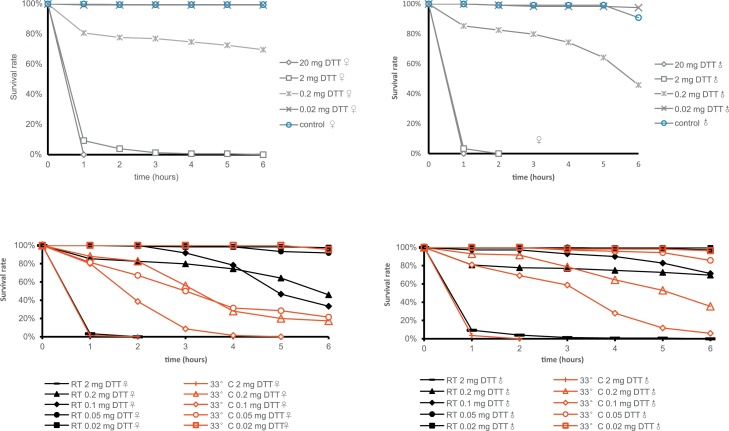
To characterise DTT toxicity, we coated the bottom of glass vials with four different doses of DTT (20 mg, 2 mg, 0.2 mg, 0.02 mg). At room temperature (25 °C), both male and female flies (w1118), which were exposed to the highest DTT amount (20 mg) were all dead after one hour of incubation (Fig. 1). Exposed to 2 mg DTT, most flies survived for several hours, but they lost their ability of coordinated movement and of climbing up the walls of the vial. This effect of paralysis has been reported for a number of insecticides targeting the nervous system, such as DDT and chlorantraniliprole and was called “knockdown” [18]. In vials with 0.2 mg DTT, female flies showed to be more tolerant to DTT than males. With 6 h continuous exposure, only less than 30 % of females displayed the knockdown phenotype, while over 50 % of males were paralysed at the same time. This sexual dimorphic toxin resistance might be due to the different body mass of the genders, females being generally 50 % bigger than males [19]. Flies exposed to 0.02 mg DTT did not show any difference to flies without DTT contact regarding behaviour and mobility. To nevertheless assess any effect of this non-lethal DTT dose on flies, experiments such as larval crawling and climbing assays as described for the evaluation of the toxicity of graphene oxide and zinc oxide nanoparticles on flies shall be performed in the next future [20].
Fig. 1.
DTT is toxic to fruit flies.
Exposure of females (A) and males (B) to DTT is lethal at high amounts of DTT (20 mg and 2 mg). 0.2 mg of DTT is lethal to about 20 % and 50 % of female and male flies, respectively. Treatment with ten times less DTT does not affect fly viability. These experiments were performed at room temperature (RT).
We compared the effects of DTT exposure in dependence of the incubation temperature at 25 °C (RT) and 33 °C in females (C) and males (D). There is an obvious tendency that at 33 °C, the toxicity of DTT increases.
Since the uptake concentration of DTT is unknown, a LC50 value cannot specified. For the same reason, it is difficult to evaluate the potency of DTT as a toxin. By approximation, compared with DDT, another contact insecticide, the toxic amounts of DTT are ten times higher than the toxic amounts of DDT (0.1 mg) [18]. In an early work, three rats weighing 260 g, 300 g and 300 g, injected with 100 mg DTT died immediately [21]. The molecular consequences of DTT injections were not studied in this case. The companies PanReac Applichem and Affimetrix (Germany) indicate an LD50 value of 400 mg/kg for rats (oral) and an LC50 (48 h) value of 27.000 μg/l freshwater for Daphnia magna, respectively. Again, no molecular consequences of these tests were reported.
3.2. DTT is more toxic at high temperatures
The efficacy of a toxic substance may be temperature-dependent. This relationship is called temperature coefficient (TC) of the substance toxicity. TCs vary between toxins and insect species and populations. For example, diazinon, pirimicarb, fenazaquin, indoxacarb (oxadiazine), teflubenzuron, acetamiprid and beta-cyfluthrin were reported to have positive TCs against the forest beetle Anoplotrupes stercorosus [22]. Likewise, sensitivity of the brown planthopper Nilaparvata lugens to cycloxaprid, nitenpyram, triflumezopyrim and chlorpyrifos increased with higher temperatures [23]. By contrast, deltamethrin, DDT and pyrethroid were reported to have negative TCs in a number of insect species including the malaria vector Anopheles arabiensis and Anopheles funestus [24,25]. Similarly, nymphs of the bug winchuka (Triatoma infestans) were more resistant to pyrethroids at higher temperatures than at lower temperatures [26].
To further characterize the toxic effect of DTT, we analysed the impact of temperature on DTT sensitivity. In particular, using five doses (Fig. 1), we compared DTT sensitivity at a mild temperature (25 °C) and at a high temperature (33 °C). Without DTT, flies showed normal behaviour and activity at least during six hours of the assay. At the lowest dose (0.02 mg), the flies from both 25 °C and 33 °C cohorts survived during the whole assay. At the highest dose (2 mg), all flies died during the first hour of the assay regardless of the temperature. At the other three doses on average both males and females were more sensitive to DTT at the higher temperature. For example, exposed to 0.1 mg DTT at 33 °C, all females died within 4 h and almost all males died after 6 h. By contrast, at 25 °C, 33 % of the females and 71 % of the males exposed to the same dose of DTT survived after 6 h.
Temperature may affect the efficacy of a toxic substance in different ways. It may have an impact on the stability of the substance, it may influence the interaction between the substance and its target, or it may modulate the metabolic rate of the organism. A cellular mechanism that attenuates TCs is a heat shock response induced by high temperature. A. arabiensis display higher pyrethoid resistance even after a short-term exposure to high temperature. This induced resistance was demonstrated to be mediated by increased levels of heat shock proteins [25]. In contrast to this linear relationship, some insects developed a trade-off between thermal tolerance and resistance against insecticides. For example, the chlorpyrifos susceptible strain of Plutella xylostella (Diamondback moth) shows higher fitness in terms of longevity and fecundity than resistance strains at high temperatures [27].
In our case, DTT toxicity on D. melanogaster has a positive TC. Trivially, moreover, longer incubation at the higher temperature enhanced lethality of DTT indicating that a possible induction of a heat shock response was unable to attenuate DTT toxicity. One conceivable reason for a positive TC may be the positive correlation between temperature and cuticle permeability to xenobiotics [28,29]. In our experiments, increased DTT penetration through the cuticle at higher temperatures may explain increased toxicity. Quantification of DTT in the insect body at different temperatures may shed light on this issue. Alternatively, higher metabolic rate at higher temperatures may promote the toxic effect of DTT.
3.3. DTT penetrates the insect body through the cuticle
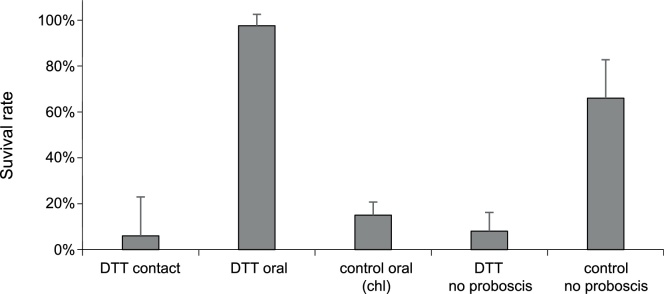
We next addressed the question as to whether DTT penetrates the insect directly through the cuticle or by ingestion. Two assays were performed to answer this question. First, we removed the proboscis of wild-type flies to make sure that DTT was not taken up by ingestion (Fig. S1). Most of these flies survived to the next day with the wound covered by a melanized scab. They were sensitive to DTT as flies with a functional proboscis (Fig. 2). This suggests that DTT directly penetrates the insect through the cuticle causing the full toxic effect. Second, flies in a vial were watered with DTT resuspended in water via a glass capillary. Uptake of DTT by ingestion did not affect fly activity (Fig. 2). For this assay, as a positive control, the insecticide chlorantraniliprole (0.1 mg/mL) was supplied to fruit flies by the same method. Almost all the flies were knocked down within one hour after insecticide uptake. We conclude that either flies did not ingest a toxic DTT amount in our assay, or that ingestion of DTT is harmless. Together, these experiments indicate that DTT is toxic to D. melanogaster after penetration through the cuticle. Hence, this DTT mode of action corresponds to the mode of action of contact insecticides like DDT [18]. The amounts of DDT that are toxic to flies are, however, ten times lower (0.1 mg). While DDT is a hydrophobic molecule, DTT has two hydroxy groups that may attenuate its penetration through the hydrophobic cuticle. We reckon that the efficient concentration of DTT inside the fly body is, therefore, probably, rather low.
Fig. 2.
Not feeding but contact with DTT is lethal to D. melanogaster.
Topical application of 2 mg DTT is lethal to D. melanogaster (DTT contact). Feeding flies with 20 mg/mL DTT (DTT oral) is not lethal, while feeding flies with 0.1 mg/mL chlorantraniliprole (control oral chl) to demonstrate that the feeding device is functional is lethal. Removal of the proboscis (DTT no proboscis) does not have any effect on DTT-toxicity. Flies with a removed proboscis (control no proboscis) survive the time of experiment under identical conditions without DTT.
3.4. DTT exposure induced apoptosis
DTT is known to induce apoptosis in cell cultures [3]. We tested whether exposure to DTT may induce apoptosis also in the fruit fly. Commonly, apoptosis in both mammals and insects are controlled by the stability of caspase inhibitors named the IAPs (inhibitor of apoptosis proteins) [30]. The half-life of IAPs is regulated by IAP-binding-motif (IBM) proteins, which bind to IAPs, elicit IAP degradation and ultimately trigger apoptosis. To some extent, in D. melanogaster, apoptosis is induced by transcriptional upregulation of the genes grim, head-involution defective (hid) and reaper (rpr) that code for Drosophila IAP (DIAP) binding proteins with IBMs [31,32].
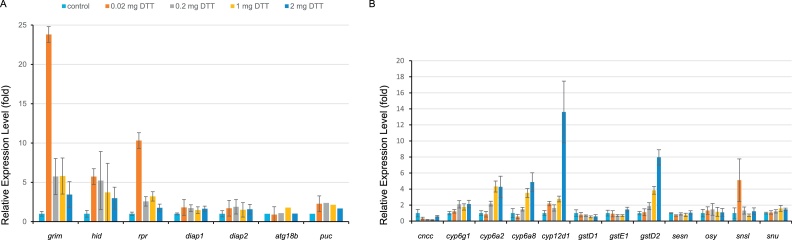
Here, we analysed the expression levels of the three IBM protein coding genes in fruit flies after a one-hour exposure to different doses of DTT (Fig. 3A). Although expression levels vary in biological replicates, generally, we observe a dosage-dependent induction of the expression levels of grim, hid and rpr after treatment with different amounts of DTT. Interestingly, the highest induction occurred at the lowest, non-lethal DTT dose (0.02 mg). At this dose, the expression levels of grim increased in the four biological replicates in a range between 5–58 folds compared to the average value of control flies. For hid and rpr, we recorded 1.5–9 folds and 2–30 folds increased expression levels compared to the expression levels in control flies, respectively. At 0.2 mg DTT dose, the grim and rpr expression were also induced by 2–11 folds and 1.5–4 folds compared to control levels, respectively. The hid expression levels of the four biological repeats varied between 0.8 and 16.2 folds of the control average. At 1 mg DTT dose, the expression levels of rpr in the four replicates was higher than the control average levels (1.6–5.9 folds). The expression levels of grim and hid in three replicates were higher and in one replicate lower than control average expression values. At the highest dose (2 mg), the expression levels of the three genes was normal. Based on these data, we assume that at lethal (2 mg) or sublethal (1 mg) doses, flies were unable to mount an apoptosis response because of uncontrolled lethal damages, whereas at lower doses DTT induced a normal apoptosis response. In a next step, we plan to study the cellular survival response in these flies.
Fig. 3.
Topical DTT induces the expression of apoptosis- and detoxification-related genes.
Topical application of low amounts of DTT (0.02 mg) activates the expression of the apoptosis-related genes rpr, grim and hid (A). Diap1 and Diap2 expression, by contrast, is not induced by 0.02 mg DTT. The apoptosis response is less pronounced at higher DTT amounts (0.2 mg–2 mg). A detoxification-response increases with increasing amounts of DTT (B). The expression of the genes osy and snu involved in inward barrier formation is unchanged upon DTT application. Low amounts of DTT (0.02 mg), however, activate the expression of snsl that codes for a barrier forming extracellular protein. Bars indicate standard errors (SE). In cases of seemingly missing bars, the SE values are extremely small.
We also monitored the expression levels of two IAP coding genes of D. melanogaster, Diap1 and Diap2 (Fig. 3A). Diap1 was identified as the major apoptosis inhibitor [33], while Diap2 was found not to be involved in apoptosis regulation, but in immune response to bacterial infection [34,35]. Usually, the expression of diaps is constitutive and not regulated by an apoptotic signal. Consistently, the expression levels of Diap1 and Diap2 transcripts were relatively stable in DTT treated flies.
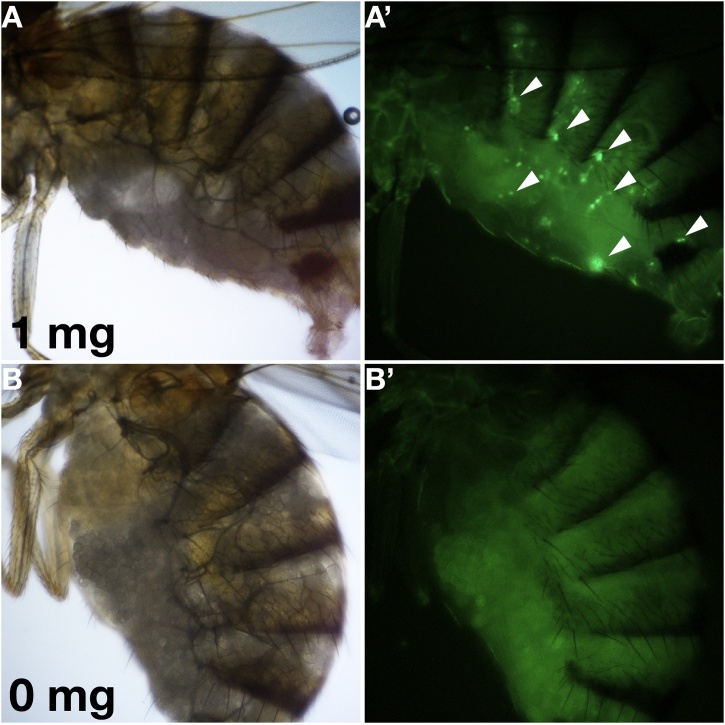
To visualise apoptosis, we expressed the fluorescence apoptosis sensor GC3Ai in the whole fly [15]. Contact with DTT induced fluorescence in the gut tissue, while in control flies no such signal was detected (Fig. 4).
Fig. 4.
DTT induces GC3Ai fluorescence in the gut of adult flies.
GC3Ai does not fluoresce in flies that are not exposed to DTT (A). When exposed to DTT (1 mg) in our contact assay, some gut cells show a fluorescence signal indicating that apoptosis has been triggered in them (B). GC3Ai is a GFP version that needs cleavage of DEVD linker in order to emit green light upon excitation with blue light. Images were taken on a Nikon AZ100 microscope.
Together, these results suggest that DTT contact induces apoptosis in the fruit fly at the transcriptional level. However, it remains unclear whether apoptosis is the cause of DTT lethality.
3.5. DTT activates the detoxification system at the transcriptional level
Cytochrome P450 monooxygenases (CYPs) and Glutathione S-transferases (GSTs) are two enzyme families that are involved in xenobiotic detoxification in multicellular organisms. CYPs catalyse the reduction of molecules including toxins and insecticides thereby potentially inactivating them [36]. GSTs ligate electrophilic xenobiotic substrates with thiol groups, thereby increasing their solubility and, by consequence, their metabolic processing [37]. A number of CYPs and GSTs were found to be implicated in insecticide resistance in different insect species including among many others Spodoptera exigua [38], Anopheles gambiae [39], Anopheles arabiensis [40], Bombyx mori [41] and Musca domestica [42,43]. In D. melanogaster, several CYP and GST coding genes were expressed at higher levels in a DDT-resistant than in a DDT-susceptible strain [44]. Transgenic overexpression of cyp6g1, cyp6g2 and cyp12d1 in fruit flies conferred selective tolerance against the insecticides DDT (cyp6g1, cyp12d1), nitenpyram (cyp6g1, cyp6g2), dicyclanil (cyp6g1, cyp12d1) and diazinon (cyp6g2) [45]. Additionally, gstD1 was identified as DDTase, which converses DDT to the harmless DDE [46].
CYP and GST expression is also activated upon contact with non-insecticide, but nevertheless toxic xenobiotics. For example, phenobarbital, caffeine or chlorpromazine treatment entails the expression of cyp6a2, cyp6a21, cyp6a8, cyp12d1, cyp6a12, gstD2 and gstD7 in D. melanogaster [47]. Presence of H2O2 was reported to increase the transcript levels of GstD1, GstD2, GstD3, GstD9 and GstE1 in the fruit fly [44].
The detoxification response pathway in insects follows the Cncc/Keap1 signalling pathway, which is also present in mammals [48,49]. In absence of toxic molecules, Cncc (named Nrf2 in mammals) is present in the cell in a complex with Keap1 and is rapidly degraded by proteasomes. Under oxidative stress, Cncc dissociates from Keap1, enters the nucleus where it associates with Maf and binds to promoters of a number of genes, including CYPs and GSTs to relieve the oxidative stress. To deepen our understanding on the impact of DTT on oxidative stress in D. melanogaster, we plan to analyse the effects of antioxidants on DTT toxicity. A promising antioxidant is curcumin that has been shown to attenuate the adverse effects of copper ions (Cu2+) that induce oxidative stress, for instance, in D. melanogaster and rat renal cells [50,51].
Here, we tested the transcript levels of cncc and six of its target genes including cyp6g1, cyp6a8, cyp12d1, GstD1, GstE1 and GstD2 [47] after DTT exposure (Fig. 3B). Transcript levels of cncc, cyp6g1, GstD1 and GstE1 were unchanged after application of any DTT dose, whereas the transcript levels of cyp6a2, cyp6a8 and GstD2 were enhanced in a dose-dependent manner. These results suggest that DTT induces a detoxification response in the fruit fly body. Regarding the model that DTT produces ROS [3], we speculate that the detoxification response to DTT runs through the Cncc/Keap1 signalling pathway. We note, however, that the signal is selective, activating some of the Cncc targets while some others remain silent. This suggests that despite of being Cncc targets, some genes at the same time are targets of a negative regulator that suppresses their expression in presence of Cncc and DTT. Indeed, a context-dependent response to the Cncc/Keap1 signal has been observed in another example. 70 % of genes, which are upregulated by phenobarbital, are also upregulated in response to Cncc overexpression [47]. On the other hand, only 15 % of Cncc response genes also respond to phenobarbital application. The situation is probably even more complex as there are two additional pathways reported to activate a xenobiotic response in D. melanogaster, i.e. the JNK pathway and the DHR96 pathway [52,53]. As the JNK pathway was shown to be responsive to DTT in HeLa cells [3], we tentatively assessed the possibility that it was induced by DTT also in flies by analysing the transcript levels of two JNK targets, namely atg18b and puc (Fig. 3A). Transcript levels of atg18b were unchanged when flies were exposed to DTT; by contrast, puc transcripts were highly enriched in DTT-exposed flies. Although the determination of the DTT-induced transcriptome would be necessary for a comprehensive conclusion, we dare to state that only a partial JNK response might be elicited by DTT. We did not test the impact of DTT on the DHR96 pathway as DHR96 is sensitive to starvation to which flies in our assay are exposed to [54]. What we can state, nevertheless, is that transcript levels of cyp6a8 and GstD2 were elevated when flies contacted DTT (Fig. 3B), whereas their expression was shown to be independent of DHR96 function [53].
Another genetic program that is activated during general external stress infliction involves the evolutionary conserved Sestrin protein [55]. Sestrin intervenes with the adenosine monophosphate-dependent protein kinase (AMPK)-Target of rapamycin (Tor) signalling pathway, thereby regulating cellular homeostasis. Among others, Sesn function leads to a reduction of ROS amounts. Interestingly, in human cell lines, it has been shown that Sesn is able to promote Keap1 degradation, thereby stabilising Nrf2 (the Cncc ortholog) and enhancing its function as a transcriptional regulator [56]. In order to verify whether a Sesn-dependent stress response is mounted upon DTT application, we detected its transcripts in DTT treated flies. Indeed, sens transcription has been shown to be sensitive to stress [57]. Overall, sesn expression was normal in flies exposed to DTT. Thus, Sens is not involved in the DTT-control program in flies.
3.6. Barrier fortification is only induced by non-lethal DTT dosage
The cuticle surface that consists of the envelope and free hydrocarbons (CHCs) is the primary inward barrier to prevent the penetration of xenobiotics [28,29]. In D. melanogaster, the ATP-binding cassette (ABC) transporters Snustorr (Snu) and Oskyddad (Osy) and the extracellular protein Snustorr-snarlik (Snsl) are key proteins involved in the establishment of the surface barrier [58,59]. Snu and Osy belong to the Arthropod-unique ABC transporter subfamily ABCH. Dysfunction of Snu or Osy causes reduction of CHC amounts. Here we monitored the levels of transcripts coding for these three proteins by qPCR following exposure to different amounts of DTT. No change in transcript levels was detected for either of these factors after DTT treatment except for snsl transcripts at very low DTT amounts compared to the control situation (Fig. 3B). One may speculate that this defence measure is the cause for survival at very low DTT dosage. In summary, we assume that when exposed to very low DTT amounts, flies react with an immediate change in barrier efficiency via alteration of snsl transcript levels; when exposed to higher DTT amounts, however, rather a detoxification program is launched while barrier fortification is neglected.
4. Conclusion
This is the first report on the molecular consequences of DTT toxicity to an animal. Our results indicate that DTT has a toxic effect on D. melanogaster in a time and dose depended manner. We speculate that DTT may also be toxic to other, especially small animals including vertebrates. Further toxicological investigations are needed to assess the full impact of DTT on ecosystems.
CRediT authorship contribution statement
Yiwen Wang: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft. Maïlys Misto: Data curation, Formal analysis, Investigation, Methodology. Jing Yang: Data curation, Formal analysis, Investigation, Methodology. Nicole Gehring: Formal analysis, Investigation, Methodology. Xiaoyu Yu: . Bernard Moussian: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This work was supported by a grant from the German Research Foundation (DFG, MO1714/10-1). We thank Magali Suzanne (Ubiversitévery much for the apoptosis reporter flies).
Edited by Dr. A.M Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.12.014.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Chapuy C.I., Aguad M.D., Nicholson R.T., AuBuchon J.P., Cohn C.S., Delaney M., Fung M.K., Unger M., Doshi P., Murphy M.F., Dumont L.J., Kaufman R.M., Collaborative, D.-D.S.G.f.t.B International validation of a dithiothreitol (DTT)-based method to resolve the daratumumab interference with blood compatibility testing. Transfusion. 2016;56:2964–2972. doi: 10.1111/trf.13789. [DOI] [PubMed] [Google Scholar]
- 2.Wang J., Lin X., Lu L., Wu Y., Zhang H., Lv Q., Liu W., Zhang Y., Zhuang S. Temporal variation of oxidative potential of water soluble components of ambient PM2.5 measured by dithiothreitol (DTT) assay. Sci. Total Environ. 2019;649:969–978. doi: 10.1016/j.scitotenv.2018.08.375. [DOI] [PubMed] [Google Scholar]
- 3.Xiang X.Y., Yang X.C., Su J., Kang J.S., Wu Y., Xue Y.N., Dong Y.T., Sun L.K. Inhibition of autophagic flux by ROS promotes apoptosis during DTT-induced ER/oxidative stress in HeLa cells. Oncol. Rep. 2016;35:3471–3479. doi: 10.3892/or.2016.4725. [DOI] [PubMed] [Google Scholar]
- 4.Held K.D., Biaglow J.E. Mechanisms for the oxygen radical-mediated toxicity of various thiol-containing compounds in cultured mammalian cells. Radiat. Res. 1994;139:15–23. [PubMed] [Google Scholar]
- 5.Held K.D., Tuttle S.W., Biaglow J.E. Role of the pentose cycle in oxygen radical-mediated toxicity of the thiol-containing radioprotector dithiothreitol in mammalian cells. Radiat. Res. 1993;134:383–389. [PubMed] [Google Scholar]
- 6.Held K.D., Melder D.C. Toxicity of the sulfhydryl-containing radioprotector dithiothreitol. Radiat. Res. 1987;112:544–554. [PubMed] [Google Scholar]
- 7.Tartier L., McCarey Y.L., Biaglow J.E., Kochevar I.E., Held K.D. Apoptosis induced by dithiothreitol in HL-60 cells shows early activation of caspase 3 and is independent of mitochondria. Cell Death Differ. 2000;7:1002–1010. doi: 10.1038/sj.cdd.4400726. [DOI] [PubMed] [Google Scholar]
- 8.Merksamer P.I., Trusina A., Papa F.R. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavender T.J., Bulleid N.J. Peroxiredoxin IV protects cells from oxidative stress by removing H2O2 produced during disulphide formation. J. Cell. Sci. 2010;123:2672–2679. doi: 10.1242/jcs.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messias Sandes J., Nascimento Moura D.M., Divina da Silva Santiago M., Barbosa de Lima G., Cabral Filho P.E., da Cunha Goncalves de Albuquerque S., de Paiva Cavalcanti M., Fontes A., Bressan Queiroz Figueiredo R.C. The effects of endoplasmic reticulum stressors, tunicamycin and dithiothreitol on Trypanosoma cruzi. Exp. Cell Res. 2019;383 doi: 10.1016/j.yexcr.2019.111560. [DOI] [PubMed] [Google Scholar]
- 11.Affleck J.G., Walker V.K. Drosophila as a model for developmental toxicology: using and extending the drosophotoxicology model. Methods Mol. Biol. 2019;1965:139–153. doi: 10.1007/978-1-4939-9182-2_10. [DOI] [PubMed] [Google Scholar]
- 12.Chifiriuc M.C., Ratiu A.C., Popa M., Ecovoiu A.A. Drosophotoxicology: an emerging research area for assessing nanoparticles interaction with living organisms. Int. J. Mol. Sci. 2016;17:36. doi: 10.3390/ijms17020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rand M.D. Drosophotoxicology: the growing potential for Drosophila in neurotoxicology. Neurotoxicol. Teratol. 2010;32:74–83. doi: 10.1016/j.ntt.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rand M.D., Vorojeikina D., Peppriell A., Gunderson J., Prince L.M. Drosophotoxicology: elucidating kinetic and dynamic pathways of methylmercury toxicity in a Drosophila model. Front. Genet. 2019;10:666. doi: 10.3389/fgene.2019.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schott S., Ambrosini A., Barbaste A., Benassayag C., Gracia M., Proag A., Rayer M., Monier B., Suzanne M. A fluorescent toolkit for spatiotemporal tracking of apoptotic cells in living Drosophila tissues. Development. 2017;144:3840–3846. doi: 10.1242/dev.149807. [DOI] [PubMed] [Google Scholar]
- 16.Knipple D.C., Doyle K.E., Marsella-Herrick P.A., Soderlund D.M. Tight genetic linkage between the kdr insecticide resistance trait and a voltage-sensitive sodium channel gene in the house fly. Proc. Natl. Acad. Sci. U. S. A. 1994;91:2483–2487. doi: 10.1073/pnas.91.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ja W.W., Carvalho G.B., Mak E.M., de la Rosa N.N., Fang A.Y., Liong J.C., Brummel T., Benzer S. Prandiology of Drosophila and the CAFE assay. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt J.M., Battlay P., Gledhill-Smith R.S., Good R.T., Lumb C., Fournier-Level A., Robin C. Insights into DDT resistance from the Drosophila melanogaster genetic reference panel. Genetics. 2017;207:1181–1193. doi: 10.1534/genetics.117.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Farine J.P., Yang Y., Yang J., Tang W., Gehring N., Ferveur J.F., Moussian B. Transcriptional control of quality differences in the lipid-based cuticle barrier in Drosophila suzukii and Drosophila melanogaster. Front. Genet. 2020;11:887. doi: 10.3389/fgene.2020.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sood K., Kaur J., Singh H., Kumar Arya S., Khatri M. Comparative toxicity evaluation of graphene oxide (GO) and zinc oxide (ZnO) nanoparticles on Drosophila melanogaster. Toxicol. Rep. 2019;6:768–781. doi: 10.1016/j.toxrep.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walshe J.M. Toxicity of dithiothreitol. Lancet. 1970;2:263. doi: 10.1016/s0140-6736(70)92611-5. [DOI] [PubMed] [Google Scholar]
- 22.Poechowicz B., Grodzicki P. Effect of temperature on toxicity of selected insecticides to forest beetle Anoplotrupes stercorosus. Chem. Didact. Ecol. Metrol. 2013;18:103–108. [Google Scholar]
- 23.Mao K., Jin R., Li W., Ren Z., Qin X., He S., Li J., Wan H. The influence of temperature on the toxicity of insecticides to Nilaparvata lugens (Stal) Pestic. Biochem. Physiol. 2019;156:80–86. doi: 10.1016/j.pestbp.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Glunt K.D., Oliver S.V., Hunt R.H., Paaijmans K.P. The impact of temperature on insecticide toxicity against the malaria vectors Anopheles arabiensis and Anopheles funestus. Malar. J. 2018;17:131. doi: 10.1186/s12936-018-2250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver S.V., Brooke B.D. The effect of elevated temperatures on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae) Malar. J. 2017;16:73. doi: 10.1186/s12936-017-1720-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alzogaray R.A., Zerba E.N. Temperature effect on the insecticidal activity of pyrethroids on Triatoma infestans. Comp. Biochem. Physiol. C. 1993;104:485–488. doi: 10.1016/0742-8413(93)90022-d. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L.J., Jing Y.P., Li X.H., Li C.W., Bourguet D., Wu G. Temperature-sensitive fitness cost of insecticide resistance in Chinese populations of the diamondback moth Plutella xylostella. Mol. Ecol. 2015;24:1611–1627. doi: 10.1111/mec.13133. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Carballo R.G., Moussian B. Double cuticle barrier in two global pests, the whitefly Trialeurodes vaporariorum and the bedbug Cimex lectularius. J. Exp. Biol. 2017;220:1396–1399. doi: 10.1242/jeb.156679. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., Yu Z., Zhang J., Moussian B. Regionalization of surface lipids in insects. Proc. Biol. Sci. 2016:283. doi: 10.1098/rspb.2015.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lalaoui N., Vaux D.L. Recent advances in understanding inhibitor of apoptosis proteins. F1000Res. 2018:7. doi: 10.12688/f1000research.16439.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steller H. Regulation of apoptosis in Drosophila. Cell Death Differ. 2008;15:1132–1138. doi: 10.1038/cdd.2008.50. [DOI] [PubMed] [Google Scholar]
- 32.Yoo S.J., Huh J.R., Muro I., Yu H., Wang L., Wang S.L., Feldman R.M., Clem R.J., Muller H.A., Hay B.A. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat. Cell Biol. 2002;4:416–424. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
- 33.Wilson R., Goyal L., Ditzel M., Zachariou A., Baker D.A., Agapite J., Steller H., Meier P. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat. Cell Biol. 2002;4:445–450. doi: 10.1038/ncb799. [DOI] [PubMed] [Google Scholar]
- 34.Huh J.R., Foe I., Muro I., Chen C.H., Seol J.H., Yoo S.J., Guo M., Park J.M., Hay B.A. The Drosophila inhibitor of apoptosis (IAP) DIAP2 is dispensable for cell survival, required for the innate immune response to gram-negative bacterial infection, and can be negatively regulated by the reaper/hid/grim family of IAP-binding apoptosis inducers. J. Biol. Chem. 2007;282:2056–2068. doi: 10.1074/jbc.M608051200. [DOI] [PubMed] [Google Scholar]
- 35.Leulier F., Lhocine N., Lemaitre B., Meier P. The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol. Cell. Biol. 2006;26:7821–7831. doi: 10.1128/MCB.00548-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair P.C., McKinnon R.A., Miners J.O. Cytochrome P450 structure-function: insights from molecular dynamics simulations. Drug Metab. Rev. 2016;48:434–452. doi: 10.1080/03602532.2016.1178771. [DOI] [PubMed] [Google Scholar]
- 37.Tew K.D., Townsend D.M. Glutathione-s-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012;17:1728–1737. doi: 10.1089/ars.2012.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu B., Hu S., Huang H., Wei Q., Ren M., Huang S., Tian X., Su J. Insecticides induce the co-expression of glutathione S-transferases through ROS/CncC pathway in Spodoptera exigua. Pestic. Biochem. Physiol. 2019;155:58–71. doi: 10.1016/j.pestbp.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Nikou D., Ranson H., Hemingway J. An adult-specific CYP6 P450 gene is overexpressed in a pyrethroid-resistant strain of the malaria vector, Anopheles gambiae. Gene. 2003;318:91–102. doi: 10.1016/s0378-1119(03)00763-7. [DOI] [PubMed] [Google Scholar]
- 40.Simma E.A., Dermauw W., Balabanidou V., Snoeck S., Bryon A., Clark R.M., Yewhalaw D., Vontas J., Duchateau L., Van Leeuwen T. Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with deltamethrin and DDT resistance in Anopheles arabiensis populations from Ethiopia. Pest Manag. Sci. 2019;75:1808–1818. doi: 10.1002/ps.5374. [DOI] [PubMed] [Google Scholar]
- 41.Hu J., Chen J., Wang H., Mao T., Li J., Cheng X., Hu J., Xue B., Li B. Cloning and functional analysis of CncC and Keap1 genes in silkworm. J. Agric. Food Chem. 2018;66:2630–2636. doi: 10.1021/acs.jafc.7b05820. [DOI] [PubMed] [Google Scholar]
- 42.Kasai S., Scott J.G. Overexpression of cytochrome P450 CYP6D1 is associated with monooxygenase-mediated pyrethroid resistance in house flies from Georgia. Pest Biochem. Physiol. 2000;68:34–41. [Google Scholar]
- 43.Zhu F., Li T., Zhang L., Liu N. Co-up-regulation of three P450 genes in response to permethrin exposure in permethrin resistant house flies, Musca domestica. BMC Physiol. 2008;8:18. doi: 10.1186/1472-6793-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L., Schemerhorn B., Jannasch A., Walters K.R., Jr., Adamec J., Muir W.M., Pittendrigh B.R. Differential transcription of cytochrome P450s and glutathione S transferases in DDT-susceptible and -resistant Drosophila melanogaster strains in response to DDT and oxidative stress. Pest Biochem. Physiol. 2011;100:7–15. [Google Scholar]
- 45.Daborn P.J., Lumb C., Boey A., Wong W., Ffrench-Constant R.H., Batterham P. Evaluating the insecticide resistance potential of eight Drosophila melanogaster cytochrome P450 genes by transgenic over-expression. Insect Biochem. Mol. Biol. 2007;37:512–519. doi: 10.1016/j.ibmb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Low W.Y., Feil S.C., Ng H.L., Gorman M.A., Morton C.J., Pyke J., McConville M.J., Bieri M., Mok Y.F., Robin C., Gooley P.R., Parker M.W., Batterham P. Recognition and detoxification of the insecticide DDT by Drosophila melanogaster glutathione S-transferase D1. J. Mol. Biol. 2010;399:358–366. doi: 10.1016/j.jmb.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Misra J.R., Horner M.A., Lam G., Thummel C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011;25:1796–1806. doi: 10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilding C.S. Regulating resistance: CncC:maf, antioxidant response elements and the overexpression of detoxification genes in insecticide resistance. Curr. Opin. Insect Sci. 2018;27:89–96. doi: 10.1016/j.cois.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Abolaji A.O., Fasae K.D., Iwezor C.E., Aschner M., Farombi E.O. Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Toxicol. Rep. 2020;7:261–268. doi: 10.1016/j.toxrep.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashemzaei M., Tabrizian K., Alizadeh Z., Pasandideh S., Rezaee R., Mamoulakis C., Tsatsakis A., Skaperda Z., Kouretas D., Shahraki J. Resveratrol, curcumin and gallic acid attenuate glyoxal-induced damage to rat renal cells. Toxicol. Rep. 2020;7:1571–1577. doi: 10.1016/j.toxrep.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batista J.E., Sousa L.R., Martins I.K., Rodrigues N.R., Posser T., Franco J.L. Data on the phosphorylation of p38MAPK and JNK induced by chlorpyrifos in Drosophila melanogaster. Data Brief. 2016;9:32–34. doi: 10.1016/j.dib.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.King-Jones K., Horner M.A., Lam G., Thummel C.S. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006;4:37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Sieber M.H., Thummel C.S. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab. 2009;10:481–490. doi: 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee J.H., Budanov A.V., Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bae S.H., Sung S.H., Oh S.Y., Lim J.M., Lee S.K., Park Y.N., Lee H.E., Kang D., Rhee S.G. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Lee J.H., Budanov A.V., Park E.J., Birse R., Kim T.E., Perkins G.A., Ocorr K., Ellisman M.H., Bodmer R., Bier E., Karin M. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y., Norum M., Oehl K., Yang Y., Zuber R., Yang J., Farine J.P., Gehring N., Flotenmeyer M., Ferveur J.F., Moussian B. Dysfunction of Oskyddad causes Harlequin-type ichthyosis-like defects in Drosophila melanogaster. PLoS Genet. 2020 doi: 10.1371/journal.pgen.1008363. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuber R., Norum M., Wang Y., Oehl K., Gehring N., Accardi D., Bartozsewski S., Berger J., Flotenmeyer M., Moussian B. The ABC transporter Snu and the extracellular protein Snsl cooperate in the formation of the lipid-based inward and outward barrier in the skin of Drosophila. Eur. J. Cell Biol. 2018;97:90–101. doi: 10.1016/j.ejcb.2017.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.