Abstract
The atmosphere is host to a complex electric environment, ranging from a global electric circuit generating fluctuating atmospheric electric fields to local lightning strikes and ions. While research on interactions of organisms with their electrical environment is deeply rooted in the aquatic environment, it has hitherto been confined to interactions with local electrical phenomena and organismal perception of electric fields. However, there is emerging evidence of coupling between large- and small-scale atmospheric electrical phenomena and various biological processes in terrestrial environments that even appear to be tied to continental waters. Here, we synthesize our current understanding of this connectivity, discussing how atmospheric electricity can affect various levels of biological organization across multiple ecosystems. We identify opportunities for research, highlighting its complexity and interdisciplinary nature and draw attention to both conceptual and technical challenges lying ahead of our future understanding of the relationship between atmospheric electricity and the organization and functioning of biological systems.
Keywords: Aerosols, Biometeorology, Ecosystem connectivity, Electromagnetics, Electroreception, Electrostatics, Ions, Lightning, Potential gradient, Radionuclides, Thunderstorm
Introduction
The Earth’s atmosphere is a complex physical environment that makes up an intrinsic component of our living environment. For decades, interactions between organisms (animals, plants, bacteria, fungi, archaea, and human beings) and their geophysical and geochemical environment have been a central avenue of empirical research (Halberg 1963). Despite these efforts, biophysical mechanisms underpinning interactions between many atmospheric variables and biological systems remain poorly understood. Thus far, the complexity and diversity of the physical processes operating simultaneously over wide spatio-temporal scales have hampered our understanding whether and how atmospheric physical processes—and their dynamics—can be related to multiple levels of biological organization ranging from molecular dynamics to the functioning of ecosystems.
The atmosphere is host to various sources of electrical variations, spanning spatial dimensions, and electric currents that range from the production of single electrons and ions to the ~ 1000 A global electric circuit of planetary scale (Rycroft et al. 2008). While interactions between organisms and their electrical environment have been mostly studied in the aquatic, electrically rather conductive, environment (Bullock et al. 2006; Crampton 2019), comparatively very little is known about how atmospheric electrical phenomena are tied to biology. However, emerging evidence points to atmospheric electricity interacting with various organisms over various levels of biological organization (e.g., ions, molecules, cells, and organisms; e.g., Morley and Robert 2018; Hunting et al. 2019). As evidence is beginning to highlight the responses of biological systems to known drivers of variations in atmospheric electricity, here we aim to offer several vantage points, synthesizing current understanding of atmospheric electrical phenomena and their interplay with various levels of biological organization. By briefly highlighting some of the prominent historical and contemporary examples, we hope to inspire further forays by other researchers in this fascinating field of interdisciplinary research. To this end, conceptual and technical challenges are identified, providing a platform for further discussions, collaborations, and opportunities for progress and innovation at the interface between meteorology, atmospheric physics, and chemistry, as well as biological and medical sciences.
The atmospheric electrical environment
Various sources of electricity are present in the atmosphere, ranging from global electromagnetic fields and electrostatic fields to more local phenomena such as lightning and ions. Each of these electric phenomena have different degrees of pervasiveness and variability, and potential interactions with biology.
Electromagnetic fields are a ubiquitous physical aspect of the Earth’s atmosphere that historically received scientific attention, especially with respect to its relevance for biology (e.g., Palmer et al. 2006). Electromagnetic fields are composed of electric and magnetic fields of force, generated by natural phenomena or by humans with the use of electrical appliances (e.g., mobile phones, power lines and computers). Electromagnetic fields existing in nature and produced artificially exhibit a wide spectrum of frequencies, ranging from static and quasi-static range (< 3 Hz) to extremely high frequencies (300 GHz) in the microwave range of wavelengths (Mikolajczyk 1990; Saliev et al. 2019). The most well-known natural phenomenon is the static magnetic field of the Earth, putatively generated by electric currents in the melted iron core of the Earth’s core (Kuang and Bloxham 1997). The shape of the Earth’s magnetic field can be approximated by a magnetic dipole, but there may be notable local deviations in which the strength and the actual shape fluctuate on time scales of milliseconds and hours (Hayakawa et al. 2004) to millions of years (McElhinny and McFadden 1998). These natural atmospheric (and cosmic) electromagnetic fields are also an important driver of Earth currents (or telluric currents), and their dynamics, in both soil and water (for review see: Lanzerotti and Gregori 1986; Helman 2013).
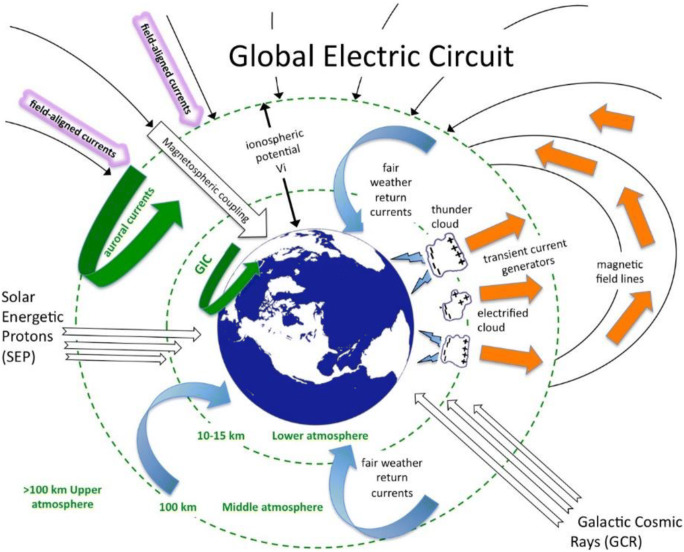
Static electric fields are also pervasive throughout the Earth’s atmosphere as part of the global electric circuit that extends from lower ionospheric layers to the surface of the Earth (see Fig. 1 for an overview of the global electric circuit). In the lower atmosphere, a vertical potential difference exists, the potential gradient (PG), which is fuelled by a positively charged atmosphere and mobile electrical charges within the Earth system. This charge separation generates an electric field between the atmosphere and the Earth during fair weather conditions ranging between 100 and 300 V/m generating a direct current (DC) with a density around 2 pA/m2 (Israël 1971, 1973). These fields exist due to global thunderstorm activity hotspots that push positive charges towards fair weather regions and do so at a global scale (Haldoupis et al. 2017). Near local thunderstorms or in the presence of low clouds carrying or generating local charges (Harrison et al. 2017), however, this electric field becomes erratic with alternating positive and negative potential gradients that can exceed 10 kV/m (Williams and Mareev 2014). The PG is further influenced by local and regional factors, including vertically extending conducting objects (e.g., buildings and vegetation), natural ionizing radiation (e.g., radon decay), solar and auroral activity, the synoptic weather situation, desert dust storms or volcanic ash, and human-induced air pollution (e.g., Leblanc et al. 2008; Matthews et al. 2019; Kourtidis et al. 2020). The PG can show distinct daily variations that depend on the regular fluctuations of the global electric circuit, a variation commonly known as the Carnegie curve (see Harrison et al. 2013). The PG is also known to be altered by variations caused by local influences, e.g., aerosol particle pollution and radioactivity of the air (Reiter 1985). Seasonal variations have also been reported (Adlerman and Williams 1996), whereby the PG typically decreases during summer months.
Fig. 1.
Cosmic and atmospheric phenomena that collectively drive the global electric circuit. Courtesy: National Science Foundation
Both electromagnetic and electrostatic fields are mostly, but not exclusively confined within the vertical atmospheric boundaries formed by the Earth’s surface and ionosphere (Volland 1995a, b). The Earth’s surface material is regarded to be a reasonably good conductor, and so is the lower ionosphere (60–130 km). Arguably arbitrary, these boundaries are considered to play a significant role in the presence and dynamics of the global circuit by providing a waveguide for the electromagnetic (EM) radiation (Rycroft et al. 2008). Although outside the scope of this review, it is important to mention that different EM frequencies across the spectrum will exhibit and experience different behaviours in the atmospheric medium and its complex and heterogeneous chemical composition within the equally diverse and changing boundaries (Volland, 1995a). Practically and measurably, this configuration is held responsible for generating a resonance cavity particularly suitable for the waveguide transmission of radio waves in the extremely low frequency (ELF, 3 Hz–3 kHz) and very low frequency (VLF, 3–30 kHz) range, as well as all forms of electromagnetic radiation (Volland 1995b). Remarkably, some distinct radiofrequency bands are naturally produced by lightning discharges across the globe (Volland 1995b; Price 2016). Natural waves of ultra-low frequency (ULF, 300 Hz – 3 kHz) can also enter the Earth’s atmosphere from the magnetosphere and ionosphere, from where they propagate along geomagnetic field lines as so-called geomagnetic pulsations or ionospheric Alfven resonances (Guglielmi and Pokhotelov 1996). Throughout the atmosphere, ELF electromagnetic waves called Schumann resonances (SR) (e.g., Price 2016) can be measured that result from global lightning discharges in the ground-ionosphere bounded waveguide. These weak waves have peaks at around 7.8, 14.3, and 20 Hz and can show some variations in frequency (± 0.2 Hz) and amplitude depending on the time of the day, the season, and, for instance, the modulation of the height of ionospheric layers due to solar activity.
The Earth’s atmosphere also has a number of local sources of electric variations that, in addition to contributing to local alterations of global patterns in atmospheric electricity, directly govern the local electric landscape and potentially the organisms living therein. These include profound impacts of local thunderstorm activity and in particular lightning strikes (e.g., Schaller et al. 2013), the production of ions through corona discharge (e.g., Matthews et al. 2010), radionuclides (e.g., Krivolutsky and Pokarzhevsky 1992), and the increasing use of electrical technology and devices (e.g., radio’s, portable communication devices) that contribute to shaping the local electric landscape.
Atmospheric electricity and biological systems
Electromagnetic fields
The extent to which geomagnetic and electromagnetic fields and waves affect biological organism has grown into an increasingly important field of study over the last century. The natural electrical, magnetic, and electromagnetic environment created by the existence of a conductive medium, current sources, charge separations, and ducts for wave propagation is admittedly complex. Beginning during the industrial revolution, humans increasingly generated artificial electric fields resulting from developments in industry and especially telecommunication technology, power lines from the electrical grid, transport, and a plethora of consumer electronics. This substantially enhanced the scale and complexity of the electromagnetic environment. In effect, whether naturally or technically generated, variable electrical currents are a substantial source of electromagnetic radiation in the atmosphere. For example, all manners of radio-communication, ranging from older radiolocation, radio-navigation, and portable telephones to the upcoming 5G wireless communication network work in the higher range of frequencies and are widely used in domestic, medical, and industrial appliances (Agiwal et al. 2016).
In biological systems, alternating currents (AC) range from a fraction of hertz to approximately 1000 Hz. Early studies focussed on biological effects of electromagnetic fields in the ELF range in relation to possible effects since ELF is measurable—albeit weak in comparison—in the activity of the human central nervous system (e.g., König et al. 1981). An increasing interest in higher radiofrequencies and microwaves subsequently developed due to the growing application in radio-communication and industry (Repacholi 1998). This interest persists as ELF is now nearly ubiquitous in both industrial and domestic environments (e.g., Bortkiewicz et al. 2006). ELF has also been considered to present potential health or therapeutic applications (König et al. 1981). While biological organisms have been naturally subjected to geomagnetic and electromagnetic fields over the course of the evolution of life on earth, scientific knowledge on the possible beneficial or deleterious effects of such fields remains sparse. Yet, evidence points to responses of biological systems, albeit inconsistent, to the action of electromagnetic fields and waves, including the current expansion of 5G wireless communication with potential adverse effects on DNA and membrane integrity, sperm function, and viability as well as immune and neuronal functioning (Marron et al. 1975; König et al. 1981; Liboff et al. 1984; Mikolajczyk 1990; Bortkiewicz et al. 2006; Valberg et al. 2006; Huss et al. 2007; Engels et al. 2014; Panagopoulos et al. 2015; Kocaman et al. 2018; Russell 2018; Saliev et al. 2019).
While mechanisms underlying the effects of both natural and artificial electromagnetic (EM) fields on biological systems can be expected to be the same, they are not necessarily easy to detect or describe over various levels of biological organization. For instance, effects at the molecular level can already be described with atomistic details, but at the level of cells or tissue require rather profound physical approximations and simplification (Cifra et al. 2020). To date, the cell membrane has been considered a major target of the electric field component of EM field (e.g., Azan et al. 2017), and much less attention has been paid to the direct effects of electric fields on proteins. However, intense electric fields at the nanosecond timescale have been shown to alter protein folding and structures (Marracino et al. 2019; Chafai et al. 2019). This may prove relevant given that proteins are biological nanomachines that execute the vast majority of life processes, so any direct action of EM fields on proteins might have substantial downstream effects.
The fact that variations in atmospheric electric fields have been observed to be biologically relevant to organisms and processes in the natural environment has also encouraged research aimed at disentangling the links between large natural and anthropogenic fluctuations in atmospheric electricity and human well-being. Interactions of atmospheric electricity with human health can be by characterizing anomalous electric environments where unusual biophysical responses in humans become visible (Cannon 1929), although it is difficult to define the personal limits of exposure to natural electric variations. Various atmospheric physical properties have been proposed to be potentially relevant. Although natural electromagnetic fields are generally weak, large-scale variations in various atmospheric phenomena (e.g., radiation, electro-magnetic fields, lunisolar gravitational forces) have been observed to affect cardiovascular systems and biological rhythms (Sollberget 1963; Halberg 1963; Palmer et al. 2006), suggesting local and planetary electrical phenomena have the potential to influence—at least part of—the human population.
Electromagnetic resonances
Lightning discharges generate electromagnetic resonances excited within the Earth-ionosphere waveguide across the globe, the so-called Schumann resonances (SR; Schumann 1952; Price 2016). Lightning events produce signals that are very weak (~ 300 μV m−1 and below 100 Hz) and typically have a low spatial attenuation rate (0.5 dB/Mm), allowing electromagnetic waves from an individual discharge to propagate several times around the globe before it eventually decays (Bliokh et al. 1980). In this physical context, the Earth-ionosphere waveguide behaves like a resonator at extremely low frequencies. This waveguide behaviour results in the amplification of spectral signals from lightning at resonance frequencies due to interference of EM waves propagating in opposite directions around the globe. It is believed that SR has existed throughout the course of Earth history after the formation of the atmosphere (Kasting and Siefert 2002), suggesting SR could be a physical quantity that, much like light, sound and gravity, could have a constituted part of the adaptive landscape in the early evolution of life (e.g., Price et al. 2020). SR occur in the ELF range, with resonant frequencies around 8 Hz, 14 Hz, 20 Hz, 26 Hz, etc. Many living organisms in nature also show electrical activity in the ELF range. From zooplankton to sharks in oceans to the human brain, all show spectral activity between 4 and 40 Hz (Bullock 2002; Freund et al. 2002). For example, the normal brain activity in humans at rest is around 10 Hz (Nunez et al. 1978), between the first two resonant frequencies of the SR. The question remains whether there is a connection between naturally produced SR and organisms and whether organisms have evolved the ability to sense and process the information that is hidden in its weak electric fields (Cherry 2003). Research has shown that entire organisms can be influenced by weak SR fields around 10 Hz (Wever 1973). As early as the 1960s, studies on circadian rhythms have shown that weak 10 Hz SR fields can influence the daily activity cycle of humans, birds, and fruit flies (Wever 1973; Engelmann et al. 1996). Recently, cardiac muscle cells were observed to be influenced by weak magnetic fields in the SR frequency range (Elhalel et al. 2019), which appeared dependent on the frequency (most pronounced at 7.8 Hz) rather than amplitude of the induced field. These studies collectively suggest that very weak alternating magnetic fields can indeed potentially influence biological processes and human health, yet a physical understanding of these findings is still absent (Price et al. 2020; Fdez-Arroyabe et al. 2020).
Static atmospheric electric fields
In the fair-weather regions across the globe, a static atmospheric electric field of the order of amplitudes ca. + 100 to + 300 V/m occurs as a consequence of the global atmospheric electrical circuit. Directed downwards if considered as a vector electric field, this atmospheric potential gradient (PG) undergoes various variations that can be regular (e.g., daily and seasonal) or irregular (locally driven) (Rycroft et al. 2008). The relevance of static atmospheric electric fields for biology has only recently been considered, with a particular focus on the relationship between insect pollinators and plants (e.g., Clarke et al. 2013). It has been found that flowers are surrounded by an electric field that results from a combination of a plants’ placement in the atmospheric PG and electrochemical fluxes through their vascular system and the ground (Maw 1962; Volkov and Shtessel 2018). Hence, a relative negative potential can be observed between flowers and the atmosphere. Several lines of research have assessed this electrostatic linkage and demonstrated empirically that electrostatic forces play a role in the transfer of pollen from flower to pollinator (Armbruster 2001; Corbet and Huang 2014; Clarke et al. 2017). Furthermore, evidence has emerged that bees can detect and use the floral electric fields to associate reward (nectar or pollen) with flowers (Clarke et al. 2013), providing the first documentation of electroreception in air as a resistive medium (Clarke et al. 2013; Greggers et al. 2013). The atmospheric PG also bears direct importance for other arthropods. It was recently shown that spiders can use the electric field in fair weather to balloon upwards in attempts to disperse over longer distances, in which the electric force acts on casted thin strands of silk allowing them to take flight (Morley and Robert 2018). The atmospheric PG has also been observed to extend below the Earth’s surface layers, in which a charge separation between relatively negative soils and sediments and the relatively positive overlying atmosphere results in the movement of respiratory ions and altered bacterial metabolism in subsurface environments (Hunting et al. 2019). The resulting alteration in microbial communities and their metabolic activities likely has wider implications as they serve as a food source for higher trophic levels (Zhai et al. 2018) and are essential for ecosystem processes like decomposition (Hunting et al. 2017). Altogether, these studies indicate that static atmospheric electric fields and their variability are tied to various biological processes, warranting further investigations assessing its significance within an ever-changing and often elusive electrostatic landscape. This encourages efforts to better understand the structure and dynamics of static electric fields at the spatial and temporal scales that are relevant for a potentially wide array of organisms that may use the dynamic electric landscape above, near, and directly below the surface of the Earth.
Lightning
Lightning is a ubiquitous phenomenon on Earth with around 50 lightning strikes per second (Christian et al. 2003). When lightning hits the Earth’s surface, electric current flows through paths of higher conductivity or moisture content (e.g., plants and soil). The energy contained within lightning strikes causes rapid heating of Earth’s surface environment, whereby temperatures may exceed 2500 K (Pasek and Block 2009). Aside from direct effects on biology, it is thus conceivable that these electric currents and associated energy inputs can bear relevance for biology.
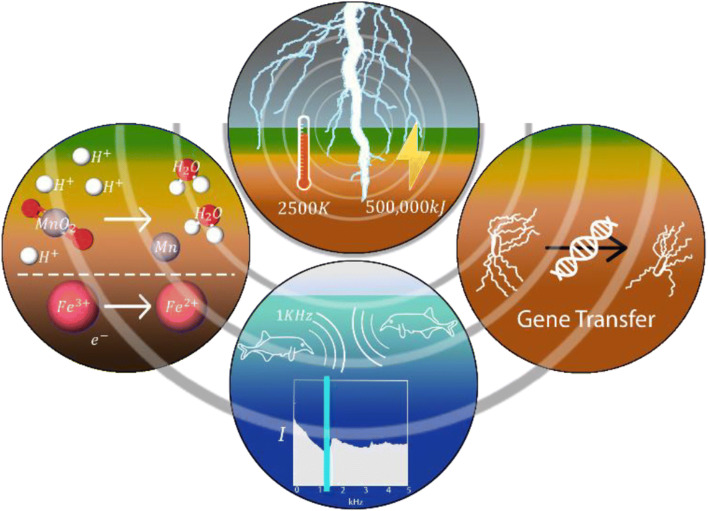
Lightning strikes have long been known to affect biological systems by directly causing injury or death, notably in cattle, humans, and trees (Bernstein 1973; Kautz et al. 2011). While direct effects of lightning on biology are generally obvious, less obvious indirect effects have also been observed (Fig. 2). Lightning can generate electrical noise influencing electrical communication in freshwater electric fish (Hopkins 1973, 1980). Indeed, Gabon mormyrid fish appear to use electrical organ discharge frequencies around 1000 Hz; the low noise bandwidth window, where there is no transmission, allows enhanced propagation of EM energy (Hopkins 1980, Arnason et al. 2002). In addition, lightning and resulting soil currents have been identified as a driver of the transfer of genetic material between different bacterial species (Demanèche et al. 2001). More recently, lightning has also been associated with chemical alterations in the Earth’s surface. This is relevant for organisms living in this environment; subsurface electrochemistry and in particular microorganisms are known to be strongly interdependent (Newman and Banfield 2002; Naudet and Revil 2005; Hunting et al. 2012, 2015; Hunting and Kampfraath 2013). Specifically, lightning has been observed to reduce phosphorus, an important nutrient for microorganisms and phototrophic organisms (algae, plants) in terrestrial and aquatic environments (Pasek and Block 2009). Lightning has also been observed to enhance mobilization of metals in both soils and aquatic sediments, potentially enhancing availability of essential metals or their toxicity to organisms (Schaller et al. 2013). These studies provide important clues on the significant and persisting effects of lightning on the abiotic and biotic environment, upending the steadfast view that lightning is a local and transient phenomenon.
Fig. 2.
Overview of geochemical and biological phenomena that are directly or indirectly affected by lightning. Top: lightning strikes generate 500,000 kJ of energy and heat the ground to 2500 K. Left: lightning reduces soil elements including manganese and iron, increasing their mobility (Schaller et al. 2013). Bottom: Mormyrid fish communicate using frequencies (vertical band on graph) where noise from lightning is lowest (Hopkins 1980). Right: current from lightning mediates gene transfer in soil (Demanèche et al. 2001)
Ions and aerosols
The atmosphere contains a wide variety of ions that can differ significantly in size and charge. These electro-active ions have a tendency to attach to aerosols and originate from both natural (cosmic rays, radioactivity, splashing water, dust storms) and anthropogenic sources (high voltage infrastructure and exhaust fumes from traffic). For instance, a substantial number of corona ions are produced by high-voltage power lines when the voltage is high enough to cause corona breakdown around the cable (Matthews et al. 2012; Jayaratne et al. 2015). If there is a predominance of one polarity of ion, such as near DC power lines and some AC power lines, this can lead to the enhancement of aerosol charge. For AC power lines, the amount of corona has been shown to be affected by local meteorology and time of day (Matthews et al. 2012).
Anthropogenic infrastructures (e.g., high-voltage transmission lines) are generally considered as the main source of this kind of ions (e.g., Matthews et al. 2010). The vast majority of studies hitherto focused on how ions influence microorganisms and human health using model organisms (e.g., mice; e.g., Krueger et al. 1963; Berger et al. 1976; Brun et al. 2018) and effects of increased air ion concentrations on biological systems have been noted (Harrison and Carslaw 2003). For instance, natural ionization of the air has long been known to be bactericidal and to disrupt levels of the neurohormone serotonin (Krueger and Smith 1958) and reduce lifespan in mice (Krueger and Reed 1976; Kellogg III and Yost 1983). The charging of aerosol has also been speculated to lead to an enhanced deposition due to electrostatic effects, potentially increasing deposition of harmful material on the skin (Fews et al. 1999a) or lung tissue through inhalation (Fews et al. 1999b). This has been offered as an explanation for increased rates of childhood leukaemia near high-voltage power lines in some studies (Tynes and Haldorsen 1997; Draper et al. 2005). Enhanced deposition within the lung has been demonstrated within mechanical models of the lung (Cohen et al. 1996) and with multiple charged particles larger than 300 nm in adult human volunteers (Melandri et al. 1983). Yet, air-borne particles measured near to HV power lines represent a relatively low charge enhancement compared with those which have so far demonstrated an effect (Buckley et al. 2008; Matthews et al. 2015; Usmani et al. 2020). It is important to note, however, that effects of ions on biological systems can be caused by electrodynamic, electrostatic, or electrochemical (e.g., ozone production) mechanisms (Fletcher et al. 2007), suggesting a need to control for confounding variables.
Radionuclides
Unstable atoms in the atmosphere, radionuclides, also contribute to the complexity of variations in local atmospheric electricity through ionizing radiation. Most atmospheric radionuclide species originate from the transfer of radioactive material from the Earth surface (e.g., radon) or from extra-planetary ionizing radiation (e.g., cosmogenic beryllium). Among the natural radionuclides, radon and its decay products are considered major contributors to health risk to living organisms, with radon being the second leading cause of lung cancer after tobacco smoke (Sethi et al. 2012). It has been demonstrated in many studies that radionuclides derived from nuclear weapons testing and nuclear accidents can influence the electrical properties of the atmosphere (Israelsson and Knudsen 1986; Tuomi 1988; Yamauchi et al. 2012). Radionuclides can therewith have further direct and indirect effects on organisms: exposure can cause direct effects such as increases in illness or death and result in genotoxic effects such as single- and double-strand deoxyribonucleic acid (DNA) breaks or DNA alterations (Ward 1995), chromosomal aberrations (Geraskin et al. 2003), or morphological abnormalities (Hiyama et al. 2012). Indirect effects of exposure can include suppression of radiosensitive species, disruption of trophic relations, a loss of immunity, and occurrence of novel diseases (Geraskin 2016). For instance, changes in community composition of plants (Suvorova et al. 1993) and soil fauna (Krivolutsky and Pokarzhevsky 1992) have been observed in areas affected by the 1986 Chernobyl nuclear power plant accident.
Implications and future challenges
Methodological challenges
Progress in our understanding of the electric landscape and its biotic constituents is hindered by technical challenges and limitations. The electrical environment is described by interdependent physical parameters (e.g., current, conductivity, electric field, charge location, number, and mobility). Measurement techniques exist for these electrical parameters (Harrison and Ingram 2005; Harrison 1997; Aplin and Harrison 2000; Chubb 2014), but they vary across large spatial and temporal scales, and range across many orders of magnitude (e.g., 10−15 to 103 A currents) for which logarithmic high dynamic range sensors can be required (Marlton et al. 2013). Therefore, sensors are used in arrays, which impose practical constraints such as size and ease of deployment. Likewise, sensors are designed to have the appropriate bandwidth and range to meet the specific scientific questions considered. It is often not feasible to meet all these requirements and, as a result, a variety of different sensors are often needed. Another challenge is to partition the significance of all atmospheric (electric) phenomena that operate simultaneously to directly and indirectly affect the living environment. Therefore, simultaneous measurements of several parameters are often needed to be able to disentangle and partition multiple confounding factors. Miniaturization and integration of several different sensors in a robust and easily deployable measurement package would offer the opportunity to gather more complete and continuous data of these drivers simultaneously and identify key parameters in the interaction between atmospheric electricity and biological systems.
In practise, methodological challenges can be met when experimental protocols ideally necessitate strict controlling and manipulating of the electric fields involved. Substantial difficulties are recognized to arise when a wide range of frequencies have to be shielded from the experimental subject in laboratory situations. Experimental manipulations, including important sham controls, set-up symmetry, stimulus isolation, and other conventional quantification of dose-responses, are not trivial and often onerous. The exploration of the entire parameter space, from DC to GHz frequencies, is desirable yet challenging logistically and financially. One additional challenge stems from the need to document the wave forms and incident magnitudes of exposures and reproduce them in controlled laboratory conditions, in the presence of other physical and biogenic variables. Hence, to date, difficulties remain in designing meaningful and interpretable empirical investigations involving biological systems and their responses to EM fields, which in turn, can be expressed at multiple levels of biological complexity, e.g., behaviour, physiological, molecular, and atomic. It must be recognized that the reproducibility of methodologies, and hence repeatability of experiments, has been an issue in the vast majority of studies published to date, casting uncertainty on our capacity to formulate a solid phenomenology on the effects of atmospheric electricity on biological organisms, including humans.
In studies focussing on human health, the role of atmospheric electricity remains uncertain due to the many external factors, which partially or entirely control exogenous and endogenous biological rhythms. Appropriate control and manipulation of circadian rhythms is thus key for successful experiments (Halberg and Panofsky 1961). The large number of confounding variables in atmospheric parameters, geographic distributions, and lifestyle variability makes this field notoriously challenging. To facilitate progress, a key challenge is the development of Biometeorological Data Infrastructures (Fdez-Arroyabe et al. 2018). These infrastructures can be based on monitoring people and animals in order to collect data and define the vulnerability of individual organisms as well as populations to acclimatize and adapt to normal variability and extreme changes of specific atmospheric parameters. The development of biometeorological data infrastructures based on empirical measurements would be the first step in advancing our understanding on human well-being in relation to its atmospheric electrical environment and ultimately allow for developing tailored early warning systems that could mitigate risks for individuals and populations.
From electrons to ecosystems
The scales at which atmospheric electric phenomena act range from particles to global circuits. How these phenomena interact with different levels of biological organization, which themselves vary spatially and temporally, constitutes a daunting challenge. The electrical landscape of any biome will be a product of the dynamic interplay between abiotic sources (e.g., atmospheric potential gradient) and perturbations by living organisms. Ultimately, for nearly all environments on Earth, abiotic and biotic components will be both sources and sinks, as well as modifiers, of electric fields and ions that interact in intrinsically linked and reciprocal ways. However, the vast ranges in spatial scale and magnitude over which these interactions occur make accurate measurements and comprehensive modelling of the dynamics of this system and its constitutive components a challenging and worthwhile task.
Despite the various interdependent electric and electromagnetic phenomena, not all are expected to be sufficiently strong enough to exert an observable effect on biology, and effects can be expected to differ across various levels of biological organization (e.g., molecules, cells, and organisms: see Cifra et al. 2020 for review). Molecular dynamic simulations (Průša and Cifra 2019; Valle et al. 2019) and further modelling are currently used to identify under what conditions atmospheric electric and electromagnetic fields can modify functions of molecules, an approach that also enables a prediction of the effects on other molecules and organelles (e.g., Tuszyński et al. 2005). While we thereby begin to understand the effects of atmospheric electricity on molecular level processes, a major challenge remains to upscale this analysis to cell and tissue levels, or beyond. Disentangling molecular dynamics at atomic precision could provide a valuable bottom up approach that can inform higher scales of application and complexity in modelling (Apollonio et al. 2013).
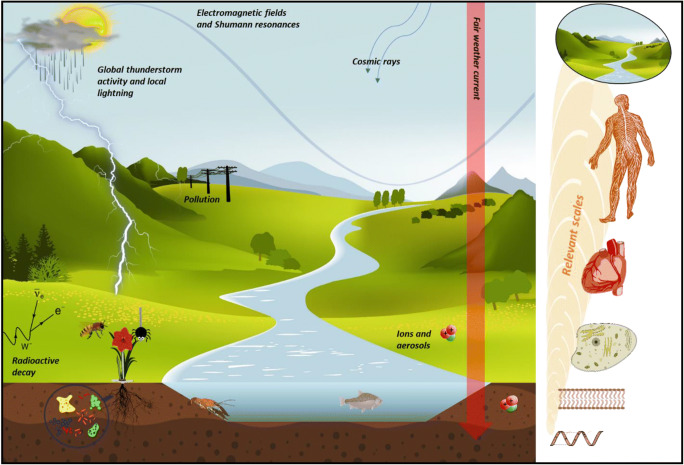
Although challenging, consideration of a wide range of spatial scales in atmospheric electricity is required to identify links across all levels of biological organization (see Fig. 3 for an overview of electrical phenomena tied to different levels of biological organization). For example, the exchange of a relatively small number of electrons on the surface of an insect’s mechanosensory hair could potentially lead to drastic differences in its sensitivity to electric field in a behavioural context stimuli (Sutton et al. 2016). Conversely, on a larger scale, the shielding and distortion effects imparted by trees on the atmospheric potential gradient can effectively nullify or transform the local electric field strength experienced by organisms in their immediate vicinity (Arnold et al. 1965; Williams et al. 2005; Clarke et al. 2017). Likewise, relationships between plants and the atmospheric PG are likely to be species-dependent, owing to species-specific morphology and electrophysiological characteristics. Furthermore, at an even greater scale, the burning of organic matter, as naturally occurs in forest fires, has been suggested as a significant source of negative ions, resulting in anomalous lightning strikes over large areas (Vonnegut et al. 1995). Adding further complexity, many of these interactions transcend multiple tiers of scale, with the largest scale atmospheric electric fields having a marked influence on some of the smallest levels of biological organization. For instance, it has been noted that both local and universal periodic variations in atmospheric electricity can influence the subsurface electrochemistry of soils and water-bodies (Hunting et al. 2019). These changes in electrochemical gradients alter the metabolic activity of microorganisms (Hunting et al. 2019) and could potentially influence the movement of electrotactic organisms (Bespalov et al. 1996; Chrisman et al. 2016). This could further extend to large-scale variations in space weather that are known to influence surface atmospheric electricity (Harrison et al. 2013).
Fig. 3.
Conceptual diagram illustrating various atmospheric electric phenomena that have demonstrated links with various levels of biological organization. While a plethora of studies examined effects on a molecular level and we begin to increase our understanding of higher levels of molecular and cellular organization, a consideration of a wide range of spatial and scales in atmospheric electricity is required to identify links across all levels of biological organization and how they propagate across ecosystems in time and space commensurate with the life cycles of terrestrial organisms
While the basics of electrostatics (Faraday 1839) and electrodynamics (Maxwell 1865) have long been described, the complexity of the biotic environment constitutes a challenge by itself. Through both its physical structural and material diversity, and the possibly countless electrical interactions within, biological material renders the application of said fundamental principles extremely difficult in a biologically relevant setting. In effect, a better identification of the suite of interactions between abiotic and biotic electric fields is much needed to warrant progress in this field. This endeavour, in tandem with measurements and modelling of the electric fields present in complex organic environments, should begin to allow for characterization of the living electrical landscape, and the dynamics therein. Identifying the aspects of the natural electric atmospheric landscape in conjunction with the anthropogenic electric landscape will ultimately allow for establishing a complete picture amenable to experimentation and the gathering of empirical evidence.
Human activities and atmospheric electricity
An increased recognition of a coupling between the electric landscape and biological systems also calls for investigating to what extent this coupling is vulnerable to anthropogenic influences. Various sources of anthropogenic pollution have been identified, ranging from smoke to power lines, which vary in their degree in which they affect the local electric landscape. For instance, smoke and aerosols are known to affect atmospheric electricity (Sheftel and Chernyshev 1994; Kamra and Deshpande 1995; Maricq 2006), and although the number of particles from traffic decays quickly (~ 10 m) (Lee et al. 2012), they can exceed particle numbers near power lines (Maricq 2006; Jayaratne et al. 2015). More pervasive are the effect of electrical wires and power lines. The 50 or 60 Hz “mains hum” can even be detected in aquatic habitats (Peters and Bretschneider 1972), and electrical pollution by high-voltage power lines is a wide spread factor affecting local variations in AE (Maruvada 2011) that can be measured hundreds of meters away from power lines (Matthews et al. 2010, 2012).
Whether sources of anthropogenic pollution affect the electric landscape sufficiently enough to influence biology remains a central issue and studies are often ambiguous. Power lines have been observed to trigger behavioural responses in insects and planarians (Jackson et al. 2011; Petri et al. 2017; Schmiedchen et al. 2018), but no physiological mechanism underlying these observations has been detected so far. It has also been proposed that resulting fluctuations in E-fields can be disruptive to circadian rhythms (Henshaw et al. 2008), and power frequency fields have resulted in melatonin disruption in rats (e.g., Wilson et al. 1981; Wilson et al. 1986; Reiter et al. 1988; Grota et al. 1994). In addition to AC and DC fields, power lines can shed ions, thereby providing a secondary and indirect source of electrical pollution that potentially alters local direct current and ion transport, adding further complexity. The myriad potential perturbations caused by human activities may therefore—in concert—interfere with linkages between atmospheric electricity and biological systems in ways that remain largely unexplored.
Concluding statement
Collectively, the research reviewed in this article serves to document and highlight the links between atmospheric electricity and biological systems. The evidence presented illustrates the multiple facets of current research while shedding light on gaps that warrant investigation. One key emerging perspective is the expectation that variations in atmospheric electricity affect various biological systems across multiple ecosystem boundaries. It is also becoming apparent that in addition to directly influencing biology, atmospheric electricity can have various indirect links to organisms and biological processes. Technical and methodological challenges create a number of pitfalls that prevent the gathering of conclusive evidence and warrant the development of interdisciplinary research that seeks the integration and harmonization of research disciplines such as atmospheric physics, biometeorology, behavioural and sensory biology, ecology and ecophysiology, and medical and environmental sciences. While many examples show the interactions of atmospheric electrical phenomena at multiple organizational scales (e.g., effects on molecules, cells, and organisms), it becomes progressively more important to consider wider spatial and temporal scales. At stake is a deeper understanding of how and why diverse interactions can propagate across ecosystems in time and space commensurate with the life cycles of terrestrial organisms.
Acknowledgements
This paper is based upon work from COST Action “Atmospheric Electricity Network: coupling with the Earth System, climate and biological systems (ELECTRONET),” supported by COST (European Cooperation in Science and Technology).
Funding information
EH received financial support from the Swiss National Science Foundation, SNF (CRSK-2 190855). SD received financial support from the Ministry of Education, Science and Technological Development of the Republic of Serbia (project III43009). AO received funding from Poland Ministry of Science and Higher Education for statutory research of the Institute of Geophysics, Polish Academy of Sciences (Grant No 3841/E-41/S/2019). DR received financial support from the European Research Commission (ERC-ADG 743093), supporting EH, SJE, and KuK. KM is supported by the Natural Environment Research Council, DFT FRESH. MC received financial support from the Czech Science Foundation, GAČR (GA20-06873X).
Contributor Information
Ellard R. Hunting, Email: e.r.hunting@bristol.ac.uk
Daniel Robert, Email: d.robert@bristol.ac.uk.
References
- Adlerman EJ, Williams ER. Seasonal variation of the global electrical circuit. J Geophys Res Atmos. 1996;101:29679–29688. doi: 10.1029/96JD01547@10.1002/(ISSN)2169-8996.ATMELECT1. [DOI] [Google Scholar]
- Agiwal M, Roy A, Saxena N. Next generation 5G wireless networks: a comprehensive survey. IEEE Commun Surveys Tuts. 2016;18(3):1617–1655. [Google Scholar]
- Aplin KL, Harrison RG. A computer-controlled Gerdien atmospheric ion counter. Rev Sci Instrum. 2000;71(8):3037–3041. doi: 10.1063/1.1305511. [DOI] [Google Scholar]
- Apollonio F, Liberti M, Paffi A, Merla C, Marracino P, Denzi A, Marino C, d’Inzeo G (2013) Feasibility for microwaves energy to affect biological Systems via nonthermal mechanisms: a systematic approach. IEEE T Microw Theory 61(5):2031–2045
- Armbruster WS. Evolution of floral form: electrostatic forces, pollination and adaptive compromise. New Phytol. 2001;152:181–183. [Google Scholar]
- Arnason BT, Hart LA, O'Connell-Rodwell CE. The properties of geophysical fields and their effects on elephants and other animals. J Comp Psychol. 2002;116(2):123–132. doi: 10.1037/0735-7036.116.2.123. [DOI] [PubMed] [Google Scholar]
- Arnold HR, Pierce ET, Whitson AL. The effect of a living tree upon the fair weather potential gradient. J Atmos Terr Phys. 1965;27:429–430. doi: 10.1016/0021-9169(65)90045-0. [DOI] [Google Scholar]
- Azan A, Gailliègue F, Mir LM, Breton M. Cell membrane electropulsation: chemical analysis of cell membrane modifications and associated transport mechanisms. In: Kulbacka J, Satkauskas S, editors. Transport across natural and modified biological membranes and its implications in physiology and therapy. Cham: Springer International Publishing; 2017. pp. 59–71. [Google Scholar]
- Berger TJ, Spadaro JA, Chapin SE, Becker RO. Electrically generated silver ions: quantitative effects on bacterial and mammalian cells. Antimicrob Agents Chemother. 1976;9:357–358. doi: 10.1128/aac.9.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein T. Effects of electricity and lightning on man and animals. J Forensic Sci. 1973;18:10002J. doi: 10.1520/jfs10002j. [DOI] [PubMed] [Google Scholar]
- Bespalov VA, Zhulin IB, Taylor BL. Behavioral responses of Escherichia coli to changes in redox potential. Proc Natl Acad Sci U S A. 1996;93:10084–10089. doi: 10.1073/pnas.93.19.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliokh PV, Nikolaenko AP, Fillippov Yu. F (1980) Schumann resonances in the earth-ionosphere cavity, Peter Peregrinus, 166 p
- Bortkiewicz A, Gadzicka E, Zmyślony M, Szymczak W. Neurovegetative disturbances in workers exposed to 50 Hz electromagnetic fields. Int J Occup Med Environ Health. 2006;19(1):53–60. doi: 10.2478/v10001-006-0001-1. [DOI] [PubMed] [Google Scholar]
- Brun NR, Koch BEV, Varela M, Peijnenburg WJGM, Spaink HP, Vijver MG. Nanoparticles induce dermal and intestinal innate immune system responses in zebrafish embryos. Environ Sci Nano. 2018;5:904–916. doi: 10.1039/c8en00002f. [DOI] [Google Scholar]
- Buckley AJ, Wright MD, Henshaw DL. A technique for rapid estimation of the charge distribution of submicron aerosols under atmospheric conditions. Aerosol Sci Technol. 2008;42:1042–1051. doi: 10.1080/02786820802400645. [DOI] [Google Scholar]
- Bullock TH. Biology of brain waves: natural history and evolution of an informationrich sign of activity. In: Arikan K, Moore N, editors. Advances in electrophysiology in clinical practice and research. Wheaton: Kjellberg; 2002. [Google Scholar]
- Bullock TH, Hopkins CD, Fay RR, editors. Electroreception. Berlin: Springer Science & Business Media; 2006. [Google Scholar]
- Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9(3):399–431. [Google Scholar]
- Chafai DE, Sulimenko V, Havelka D, Kubínová L, Dráber P, Cifra M. Reversible and irreversible modulation of tubulin self-assembly by intense nanosecond pulsed electric fields. Adv Mater. 2019;31:1903636. doi: 10.1002/adma.201903636. [DOI] [PubMed] [Google Scholar]
- Cherry NJ. Human intelligence: the brain, an electromagnetic system synchronised by the Schumann resonance signal. Med Hypotheses. 2003;60(6):843–844. doi: 10.1016/s0306-9877(03)00027-6. [DOI] [PubMed] [Google Scholar]
- Chrisman SD, Waite CB, Scoville AG, Carnell L (2016) C elegans demonstrates distinct behaviors within a fixed and uniform electric field. PLoS One 11. 10.1371/journal.pone.0151320 [DOI] [PMC free article] [PubMed]
- Christian HJ, et al. Global frequency and distribution of lightning as observed from space by the optical transient detector. J Geophys Res. 2003;108:4005. [Google Scholar]
- Chubb J. The measurement of atmospheric electric fields using pole mounted electrostatic fieldmeters. J Electrost. 2014;72(4):295–300. doi: 10.1016/j.elstat.2014.05.002. [DOI] [Google Scholar]
- Cifra M, Apollonio F, Liberti M, García-Sánchez T, Mir LM (2020) Possible molecular and cellular mechanisms at the basis of atmospheric electromagnetic field bioeffects. Int J Biometeorol, in press. 10.1007/s00484-020-01885-1 [DOI] [PMC free article] [PubMed]
- Clarke D, Whitney H, Sutton G, Robert D. Detection and learning of floral electric fields by bumblebees. Science. 2013;340:66–69. doi: 10.1126/science.1230883. [DOI] [PubMed] [Google Scholar]
- Clarke D, Morley E, Robert D. The bee, the flower, and the electric field: electric ecology and aerial electroreception. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2017;203:737–748. doi: 10.1007/s00359-017-1176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BS, Xiong JQ, Li W. Aerosol inhalation: recent research frontiers. Dordrecht: Springer; 1996. The influence of charge on the deposition behavior of aerosol particles with emphasis on singly charged nanometer sized particles; pp. 153–164. [Google Scholar]
- Corbet S a, Huang S-Q. Buzz pollination in eight bumblebee-pollinated Pedicularis species: does it involve vibration-induced triboelectric charging of pollen grains? Ann Bot. 2014;114:1665–1674. doi: 10.1093/aob/mcu195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampton W. Electroreception, electrogenesis, and electric signal evolution. J Fish Biol. 2019;95(1):92–134. doi: 10.1111/jfb.13922. [DOI] [PubMed] [Google Scholar]
- Demanèche S, Bertolla F, Buret F, et al. Laboratory-scale evidence for lightning-mediated gene transfer in soil. Appl Environ Microbiol. 2001;67:3440–3444. doi: 10.1128/AEM.67.8.3440-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper G, Vincent T, Kroll ME, Swanson J. Childhood cancer in relation to distance from high voltage power lines in England and Wales: a case-control study. BMJ. 2005;330(7503):1290. doi: 10.1136/bmj.330.7503.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhalel G, Price C, Fixler D, Shainberg A. Cardioprotection from stress conditions by weak magnetic fields in the Schumann resonance band. Sci Rep. 2019;9:1645. doi: 10.1038/s41598-018-36341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann W, Hellrung W, Johnsson A (1996) Circadian locomotor activity of Musca flies: Recording method and effects of 10 Hz square‐wave electric fields. Bio Electro Magnetics 17(2):100–110 [DOI] [PubMed]
- Engels S, Schneider N-L, Lefeldt N, Hein CM, Zapka M, Michalik A, Elbers D, Kittel A, Hore PJ, Mouritsen H. Anthropogenic electromagnetic noise disrupts magnetic compass orientation in a migratory bird. Nature. 2014;509:353–356. doi: 10.1038/nature13290. [DOI] [PubMed] [Google Scholar]
- Faraday M. Experimental researches in electricity. London: Bernard Quaritch; 1839. [Google Scholar]
- Fdez-Arroyabe P, Lecha Estela L, Schimt F. Digital divide, biometeorological data infrastructures and human vulnerability definition. Int J Biometeorol. 2018;62:733–740. doi: 10.1007/s00484-017-1398-x. [DOI] [PubMed] [Google Scholar]
- Fdez-Arroyabe P, Fornieles-Callejón J, Santurtún A, Szangolies L, Donner RV. Schumann resonance and cardiovascular hospital admission in the area of Granada, Spain: an event coincidence analysis approach. Sci Total Environ. 2020;705:135813. doi: 10.1016/j.scitotenv.2019.135813. [DOI] [PubMed] [Google Scholar]
- Fews AP, Henshaw DL, Keitch PA, Close JJ, Wilding RJ. Increased exposure to pollutant aerosols under high voltage power lines. Int J Radiat Biol. 1999;75:1505–1521. doi: 10.1080/095530099139115. [DOI] [PubMed] [Google Scholar]
- Fews AP, Henshaw DL, Wilding RJ, Keitch PA. Corona ions from powerlines and increased exposure to pollutant aerosols. Int J Radiat Biol. 1999;75:1523–1531. doi: 10.1080/095530099139124. [DOI] [PubMed] [Google Scholar]
- Fletcher LA, Gaunt LF, Beggs CB, Shepherd SJ, Sleigh PA, Noakes CJ, Kerr KG. Bactericidal action of positive and negative ions in air. BMC Microbiol. 2007;7(1):32. doi: 10.1186/1471-2180-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund JA, Schimansky-Geier L, Beisner B, Nieman A, Russell DF, Yakusheva T, Moss F. Behavioral stochastic resonance: how the noise from a Daphnia swarm enhances prey capture by juvenile paddlefish. J Theor Biol. 2002;214:71–83. doi: 10.1006/jtbi.2001.2445. [DOI] [PubMed] [Google Scholar]
- Geraskin SA. Ecological effects of exposure to enhanced levels of ionizing radiation. J Environ Radioact. 2016;162-163:347–357. doi: 10.1016/j.jenvrad.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Geraskin SA, Zimina LM, Dikarev VG, Dikareva NS, Zimin VL, Vasiliyev DV, Oudalova AA, Blinova LD, Alexakhin RM. Bioindication of the anthropogenic effects on micropopulations of Pinus sylvestris, L in the vicinity of a plant for the storage and processing of radioactive waste and in the Chernobyl NPP zone. J Environ Radioact. 2003;66:171–180. doi: 10.1016/S0265-931X(02)00122-4. [DOI] [PubMed] [Google Scholar]
- Greggers U, Koch G, Schmidt V, Dürr A, Floriou-Servou A, Piepenbrock D, Göpfert MC, Menzel R. Reception and learning of electric fields in bees. Proc R Soc B Biol Sci. 2013;280(1759):20130528. doi: 10.1098/rspb.2013.0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grota LJ, Reiter RJ, Keng P, Michaelson S. Electric field exposure alters serum melatonin but not pineal melatonin synthesis in male rats. Bioelectromagnetics. 1994;15:427–437. doi: 10.1002/bem.2250150506. [DOI] [PubMed] [Google Scholar]
- Guglielmi AV, Pokhotelov OA. Geoelectromagnetic waves. Bristol: Institute of Physics Publishing; 1996. p. 382. [Google Scholar]
- Halberg F. Periodicity analysis a potential tool for biometeorologists. Int J Biometeorol. 1963;7(2):167–191. [Google Scholar]
- Halberg F, Panofsky H. Thermo-variance spectra; method and clinical illustrations. I. Exp Med Surg. 1961;19:284. [PubMed] [Google Scholar]
- Haldoupis C, Rycroft M, Williams E, Price C. Is the “Earth-ionosphere capacitor” a valid component in the atmospheric global electric circuit? J Atmos Sol Terr Phys. 2017;164:127–131. [Google Scholar]
- Harrison RG. An antenna electrometer system for atmospheric electrical measurements. Rev Sci Instrum. 1997;68(3):1599–1603. doi: 10.1063/1.1147932. [DOI] [Google Scholar]
- Harrison RG, Carslaw KS. Ion-aerosol-cloud processes in the lower atmosphere. Rev Geophys. 2003;41:3. [Google Scholar]
- Harrison RG, Ingram WJ. Air–earth current measurements at Kew, London, 1909–1979. Atmos Res. 2005;76:49–64. doi: 10.1016/j.atmosres.2004.11.022. [DOI] [Google Scholar]
- Harrison RG, Nicoll KA, McWilliams KA (2013) Space weather driven changes in lower atmosphere phenomena. J Atmos Sol Terr Phys 98:22–30
- Harrison RG, Nicoll KA, Aplin KL. Evaluating stratiform cloud base charge remotely. Geophys Res Lett. 2017;44:6407–6412. doi: 10.1002/2017GL073128. [DOI] [Google Scholar]
- Hayakawa M, Hattori K, Ando Y. Natural electromagnetic phenomena and electromagnetic theory: a review. IEEJ Trans Fundam Mater. 2004;124:72–79. [Google Scholar]
- Helman DS. Earth electricity: a review of mechanisms which cause telluric currents in the lithosphere. Ann Geophys. 2013;56(5):0564. [Google Scholar]
- Henshaw DL, Ward JP, Matthews JC. Can disturbances in the atmospheric electric field created by powerline corona ions disrupt melatonin production in the pineal gland? J Pineal Res. 2008;45:341–350. doi: 10.1111/j.1600-079X.2008.00594.x. [DOI] [PubMed] [Google Scholar]
- Hiyama A, Nohara C, Kinjo S, Wataru T, Gima S, Tanahara A, Otaki JM. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci Rep. 2012;2:570. doi: 10.1038/srep00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins CD. Lightning as background noise for communication among electric fish. Nature. 1973;242(5395):268–270. [Google Scholar]
- Hopkins CD. Evolution of electric communication channels of mormyrids. Behav Ecol Sociobiol. 1980;7(1):1–3. [Google Scholar]
- Hunting ER, Kampfraath AA. Contribution of bacteria to redox potential (Eh) measurements in sediments. Int J Environ Sci Technol. 2013;10:55–62. doi: 10.1007/s13762-012-0080-4. [DOI] [Google Scholar]
- Hunting ER, Whatley MH, van der Geest HG, Mulder C, Kraak MH, Breure AM, Admiraal W. Invertebrate footprints on detritus processing, bacterial community structure, and spatiotemporal redox profiles. Freshw Sci. 2012;3:724–732. [Google Scholar]
- Hunting ER, Vijver MG, van der Geest HG, Mulder C, Kraak MHS, Breure AM, Admiraal W. Resource niche overlap promotes stability of bacterial community metabolism in experimental microcosms. Front Microbiol. 2015;6:105. doi: 10.3389/fmicb.2015.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunting ER, Barmentlo SH, Schrama M, van Bodegom PM, Zhai Y, Vijver MG. Agricultural constraints on microbial resource use and niche breadth in drainage ditches. PeerJ. 2017;5:e4175. doi: 10.7717/peerj.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunting ER, Harrison RG, Bruder A, van Bodegom PM, van der Geest HG, Kampfraath AA, Vorenhout M, Admiraal W, Cusell C, Gessner MO (2019) Atmospheric electricity influencing biogeochemical processes in soils and sediments. Front Physiol 10. 10.3389/fphys.2019.00378 [DOI] [PMC free article] [PubMed]
- Huss A, Egger M, Hug K, Huwiler-Müntener K, Röösli M. Source of funding and results of studies of health effects of mobile phone use: systematic review of experimental studies. Environ Health Perspect. 2007;115(1):1–4. doi: 10.1289/ehp.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël H. Fundamentals, conductivity, ions. Jerusalem: Israel Program for Scientifc Translations; 1971. Atmospheric electricity; p. 318. [Google Scholar]
- Israël H. Fields, charges, currents. Jerusalem: Israel Program for Scientific Translations; 1973. Atmospheric electricity; p. 479. [Google Scholar]
- Israelsson S, Knudsen E. Effect of radioactive fallout from a nuclear power plant accident on electrical parameters. J Geophys Res. 1986;91:11909–11910. doi: 10.1029/JD091iD11p11909. [DOI] [Google Scholar]
- Jackson CW, Hunt E, Sharkh S, Newland PL. Static electric fields modify the locomotory behaviour of cockroaches. J Exp Biol. 2011;214(12):2020–2026. doi: 10.1242/jeb.053470. [DOI] [PubMed] [Google Scholar]
- Jayaratne ER, Ling X, Morawska L. Comparison of charged nanoparticle concentrations near busy roads and overhead high-voltage power lines. Sci Total Environ. 2015;526:14–18. doi: 10.1016/j.scitotenv.2015.04.074. [DOI] [PubMed] [Google Scholar]
- Kamra AK, Deshpande CG. Possible secular change and land-to-ocean extension of air pollution from measurements of atmospheric electrical conductivity over the Bay of Bengal. J Geophys Res. 1995;100:7105–7110. doi: 10.1029/94JD03246. [DOI] [Google Scholar]
- Kasting JF, Siefert JL. Life and the evolution of the Earth's atmosphere. Science. 2002;296:1066–1068. doi: 10.1126/science.1071184. [DOI] [PubMed] [Google Scholar]
- Kautz M, Berger U, Stoyan D, Vogt J, Khan NI, Diele K, Saint-Paul U, Triet T, Nam VN. Desynchronizing effects of lightning strike disturbances on cyclic forest dynamics in mangrove plantations. Aquat Bot. 2011;95:173–181. doi: 10.1016/j.aquabot.2011.05.005. [DOI] [Google Scholar]
- Kellogg EW, III, Yost MG. The effects of long-term air ion and DC electric field exposures on survival characteristics in female NAMRU mice. J Gerontol. 1983;41(2):147–153. doi: 10.1093/geronj/41.2.147. [DOI] [PubMed] [Google Scholar]
- Kocaman A, Altun G, Kaplan AA, Deniz ÖG, Yurt KK, Kaplan S. Genotoxic and carcinogenic effects of non-ionizing electromagnetic fields. Environ Res. 2018;163:71–79. doi: 10.1016/j.envres.2018.01.034. [DOI] [PubMed] [Google Scholar]
- König HL, Krueger AP, Lang S, Sönning W, editors. Biologic effects of environmental electromagnetism, topics in environmental physiology and medicine. New York: Springer-Verlag; 1981. p. 332. [Google Scholar]
- Kourtidis K, Szabóné André K, Karagioras A, Nita IA, Sátori G, Bór J, Kastelis N (2020) The influence of circulation weather types on the exposure of the biosphere to atmospheric electric fields. Int J Biometeorol:1–3. 10.1007/s00484-020-01923-y [DOI] [PMC free article] [PubMed]
- Krivolutsky DA, Pokarzhevsky AD. Effects of radioactive fallout on soil animal populations in the 30 km zone of the Chernobyl atomic power plant. Sci Total Environ. 1992;112:69–77. doi: 10.1016/0048-9697(92)90239-o. [DOI] [PubMed] [Google Scholar]
- Krueger AP, Reed EJ. Biological impact of small air ions. Science. 1976;193:1209–1213. doi: 10.1126/science.959834. [DOI] [PubMed] [Google Scholar]
- Krueger AP, Smith RF. Effects of air-ions on the living mammalian trachea. J Gen Physiol. 1958;42:69–82. doi: 10.1085/jgp.42.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger AP, Andries PC, Kotaka S. The biological mechanism of air ion action. The effect of C02+ in inhaled air on the blood level of 5-hydroxytryptamine in mice. Int J Biometeorol. 1963;7:3–16. [Google Scholar]
- Kuang W, Bloxham J. An earth-like numerical dynamo model. Nature. 1997;389:371–374. doi: 10.1038/38712. [DOI] [Google Scholar]
- Lanzerotti LJ, Gregori GP. The Earth's Electrical Environment. Washington, D.C.: The National Academies Press; 1986. Telluric currents: the natural environment and interactions with man-made systems; pp. 232–257. [Google Scholar]
- Leblanc F, Aplin KL, Yair Y, Harrison RG, Blanc M, editors. Planetary atmospheric electricity, Space Science Series of ISSI. Berlin: Springer; 2008. p. 532. [Google Scholar]
- Lee ES, Xu B, Zhu Y. Measurements of ultrafine particles carrying different number of charges in on- and near-freeway environments. Atmos Environ. 2012;60:564–572. doi: 10.1016/j.atmosenv.2012.06.085. [DOI] [Google Scholar]
- Liboff AR, Williams T, Jr, Strong DM, Wistar R., Jr Time-varying magnetic fields: effect on DNA synthesis. Science. 1984;223:818–820. doi: 10.1126/science.6695183. [DOI] [PubMed] [Google Scholar]
- Maricq MM. On the electrical charge of motor vehicle exhaust particles. J Aerosol Sci. 2006;37:858–874. doi: 10.1016/j.jaerosci.2005.08.003. [DOI] [Google Scholar]
- Marlton GJ, Harrison RG, Nicoll KA. Atmospheric point discharge current measurements using a temperature-compensated logarithmic current amplifier. Rev Sci Instrum. 2013;84:066103. doi: 10.1063/1.4810849. [DOI] [PubMed] [Google Scholar]
- Marracino P, Havelka D, Průša J, Liberti M, Tuszynski J, Ayoub AT, Apollonio F, Cifra M. Tubulin response to intense nanosecond-scale electric field in molecular dynamics simulation. Sci Rep. 2019;9:10477. doi: 10.1038/s41598-019-46636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marron MT, Goodman EM, Greenebaum B. Mitotic delay in the slime mould Physarum polycephalum induced by low intensity 60 and 75 Hz electromagnetic fields. Nature. 1975;254(5495):66–67. doi: 10.1038/254066a0. [DOI] [PubMed] [Google Scholar]
- Maruvada PS (2011) Electric field and ion current environment of HVdc transmission lines: comparison of calculations and measurements. IEEE Trans Power Deliv 27(1):401–410
- Matthews JC, Ward JP, Keitch PA, Henshaw DL. Corona ion induced atmospheric potential gradient perturbations near high voltage power lines. Atmos Environ. 2010;44:5093–5100. doi: 10.1016/j.atmosenv.2010.09.007. [DOI] [Google Scholar]
- Matthews JC, Buckley AJ, Wright MD, Henshaw DL. Comparisons of ground level measurements of ion concentration and potential gradient upwind and downwind of HV power lines in corona. J Electrost. 2012;70:407–417. doi: 10.1016/j.elstat.2012.05.005. [DOI] [Google Scholar]
- Matthews JC, Wright MD, Biddiscombe MF, et al (2015) Re-creation of aerosol charge state found near HV power lines using a high voltage corona charger. In: Journal of Physics: Conference Series. Institute of Physics Publishing
- Matthews J, Wright M, Clarke D, Morley E, Silva HG, Bennett A, et al. Urban and rural measurements of atmospheric potential gradient. J Electrost. 2019;97:42–50. [Google Scholar]
- Maw MG (1962) Some biological effects of atmospheric electricity. In Proceedings of the Entomological Society of Ontario 92: 33–37
- Maxwell C. VIII. A dynamical theory of the electromagnetic field. Philos Trans R Soc Lond. 1865;155:459–512. doi: 10.1098/rstl.1865.0008. [DOI] [Google Scholar]
- McElhinny M, McFadden PL. The magnetic field of the earth: paleomagnetism, the core, and the deep mantle. Cambridge: Academic Press; 1998. [Google Scholar]
- Melandri C, Tarroni G, Prodi V, de Zaiacomo T, Formignani M, Lombardi CC. Deposition of charged particles in the human airways. J Aerosol Sci. 1983;14:657–669. doi: 10.1016/0021-8502(83)90070-8. [DOI] [Google Scholar]
- Mikolajczyk H. Działanie pól i promieniowania elektromagnetycznego na obiekty biologiczne (influence of electromagnetic field and radiation on biological objects) In: Twardowski J, editor. Biospektroskopia. Warsaw: PWN; 1990. pp. 153–234. [Google Scholar]
- Morley EL, Robert D. Electric fields elicit ballooning in spiders. Curr Biol. 2018;28:2324–2330.e2. doi: 10.1016/j.cub.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudet V, Revil A. A sandbox experiment to investigate bacteria-mediated redox processes on self-potential signals. Geophys Res Lett. 2005;32:1–4. doi: 10.1029/2005GL022735. [DOI] [Google Scholar]
- Newman DK, Banfield JF. Geomicrobiology: how molecular-scale interactions underpin biogeochemical systems. Science. 2002;296:1071–1077. doi: 10.1126/science.1010716. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Reid L, Bickford RG. The relationship of head size to alpha frequency with implications to a brain wave model. Electroencephalogr Clin Neurophysiol. 1978;44:344–352. doi: 10.1016/0013-4694(78)90309-7. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Rycroft MJ, Cermack M. Solar and geomagnetic activity, extremely low frequency magnetic and electric fields and human health at the Earth’s surface. Surv Geophys. 2006;27(5):557–595. [Google Scholar]
- Panagopoulos DJ, Johansson O, Carlo GL. Polarization: a key difference between man-made and natural electromagnetic fields, in regard to biological activity. Sci Rep. 2015;5:14914. doi: 10.1038/srep14914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M, Block K. Lightning-induced reduction of phosphorus oxidation state. Nat Geosci. 2009;2:553–556. doi: 10.1038/ngeo580. [DOI] [Google Scholar]
- Peters RC, Bretschneider F. Electric phenomena in the habitat of the catfishIctalurus nebulosus LeS. J Comp Physiol. 1972;81(4):345–362. [Google Scholar]
- Petri AK, Schmiedchen K, Stunder D et al (2017) Biological effects of exposure to static electric fields in humans and vertebrates: a systematic review. Environ Health 16(1):41 [DOI] [PMC free article] [PubMed]
- Price C (2016) ELF electromagnetic waves from lightning: the schumann resonances. Atmosphere (Basel) 7. 10.3390/atmos7090116
- Price C, Williams E, Elhalel G, Sentman D. Natural ELF fields in the atmosphere and in living organisms. Int J Biometeorol. 2020;8:1–8. doi: 10.1007/s00484-020-01864-6. [DOI] [PubMed] [Google Scholar]
- Průša J, Cifra M. Molecular dynamics simulation of the nanosecond pulsed electric field effect on kinesin nanomotor. Sci Rep. 2019;9(1):19721. doi: 10.1038/s41598-019-56052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ (1985) Fields, currents and aerosols in the lower atmosphere, Steinkopff Verlag, NSF Translation TT 76–52030, 714 pp
- Reiter RJ, Anderson LE, Buschbom RL, Wilson BW. Reduction of the nocturnal rise in pineal melatonin levels in rats exposed to 60-Hz electric fields in utero and for 23 days after birth. Life Sci. 1988;42:2203–2206. doi: 10.1016/0024-3205(88)90371-2. [DOI] [PubMed] [Google Scholar]
- Repacholi MH. Low-level exposure to radiofrequency electromagnetic fields: health effects and research needs. Bioelectromagnetics. 1998;19:1–19. [PubMed] [Google Scholar]
- Russell CL. 5 G wireless telecommunications expansion: public health and environmental implications. Environ Res. 2018;165:484–495. doi: 10.1016/j.envres.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Rycroft MJ, Harrison RG, Nicoll KA, Mareev EA (2008) An overview of Earth’s global electric circuit and atmospheric conductivity. In: Planetary atmospheric electricity. Springer, New York, pp 83–105
- Saliev T, Begimbetova D, Masoud A-D, Matkarimov B. Biological effects of non-ionizing electromagnetic fields: two sides of a coin. Prog Biophys Mol Biol. 2019;141(25–36):2019–2036. doi: 10.1016/j.pbiomolbio.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Schaller J, Weiske A, Berger F (2013) Thunderbolt in biogeochemistry: galvanic effects of lightning as another source for metal remobilization. Sci Rep 3. 10.1038/srep03122 [DOI] [PMC free article] [PubMed]
- Schmiedchen K, Petri A, Driessen S, et al (2018) Systematic review of biological effects of exposure to static electric fields. Part II: invertebrates and plants. Elsevier [DOI] [PubMed]
- Schumann WO. Über die strahlungslosen Eigenschwingungen einer leitenden Kugel, die von einer Luftschicht und einer Ionosphärenhülle umgeben ist. Z Nat Forsch A J Phys Sci. 1952;7:149–154. doi: 10.1515/zna-1952-0202. [DOI] [Google Scholar]
- Sethi TK, El-Ghamry MN, Kloecker GH. Radon and lung cancer. Clin Adv Hematol Oncol. 2012;10:157–164. [PubMed] [Google Scholar]
- Sheftel V, Chernyshev A. Air conductivity and atmospheric electric field as an indicator of anthropogenic atmospheric pollution. J Geophys Res. 1994;99(D5):10793–10795. [Google Scholar]
- Sollberget A. Significance of biological rhythm study for human biometeorology. Int J Biometeorol. 1963;7(2):193–220. [Google Scholar]
- Sutton GP, Clarke D, Morley EL, Robert D. Mechanosensory hairs in bumblebees (Bombus terrestris) detect weak electric fields. Proc Natl Acad Sci U S A. 2016;113:7261–7265. doi: 10.1073/pnas.1601624113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvorova LI, Spirin DA, Martyushov VZ, Smirnov EG, Tarasov OV, Shein GP (1993) Assessment of biological and ecological consequences of radioactive contamination of biogeocenoses (in Russian). In: Izrael Yu A (ed) Radiation aspects of the Chernobyl accident, vol. 2, St. Stavroulakis P (ed) (2003), Biological effects of electromagnetic fields mechanisms, modeling, biological effects, therapeutic effects, international standards, exposure criteria Springer-Verlag, Berlin Heidelberg, 793 pp 10.1007/978-3-662-06079-7.
- Tuomi TJ (1988) Observations of atmospheric electricity 1986. Geophys Publ 7, 551.506.1, Finish Meteorological Institute, Helsinki, pp 61
- Tuszyński JA, Brown JA, Crawford E, Carpenter EJ, Nip MLA, Dixon JM, Satarić MV. Molecular dynamics simulations of tubulin structure and calculations of electrostatic properties of microtubules. Math Comput Model. 2005;41(10):1055–1070. [Google Scholar]
- Tynes T, Haldorsen T. Electromagnetic fields and cancer in children residing near Norwegian high-voltage power lines. Am J Epidemiol. 1997;145(3):219–226. doi: 10.1093/oxfordjournals.aje.a009094. [DOI] [PubMed] [Google Scholar]
- Usmani OS, Matthews JC, Wright MD, Meah S, Underwood SR, Barnes PJ, Shallcross DE, Biddiscombe MF. No evidence electric charge increases inhaled ultrafine particle deposition in human lungs. Am J Respir Crit Care Med. 2020;201:1301–1303. doi: 10.1164/rccm.201912-2502LE. [DOI] [PubMed] [Google Scholar]
- Valberg PA, van Deventer TE, Repacholi MH. Workgroup report: base stations and wireless networks—radiofrequency (RF) exposures and health consequences. Environ Health Perspect. 2006;115(3):416–424. doi: 10.1289/ehp.9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle E d, Marracino P, Pakhomova O, Liberti M, Apollonio F. Nanosecond pulsed electric signals can affect electrostatic environment of proteins below the threshold of conformational effects: the case study of SOD1 with a molecular simulation study. PLoS One. 2019;14(8):e0221685. doi: 10.1371/journal.pone.0221685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov AG, Shtessel YB. Electrical signal propagation within and between tomato plants. Bioelectrochemistry. 2018;1:195–205. doi: 10.1016/j.bioelechem.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Volland H, editor. Handbook of atmospheric electrodynamics. Boca Raton: CRC Press; 1995. p. 432. [Google Scholar]
- Volland H, editor. Handbook of atmospheric electrodynamics. Boca Raton: CRC Press; 1995. p. 526. [Google Scholar]
- Vonnegut B, Latham DJ, Moore CB, Hunyady SJ. An explanation for anomalous lightning from forest fire clouds. J Geophys Res. 1995;100:5037–5050. doi: 10.1029/94JD02956. [DOI] [Google Scholar]
- Ward JF. Radiation mutagenesis: the initial DNA lesions responsible. Radiat Res. 1995;142:362–368. doi: 10.2307/3579145. [DOI] [PubMed] [Google Scholar]
- Wever R. Human circadian rhythms under the influence of weak electric fields and the different aspects of these studies. Int J Biometeorol. 1973;17:227–232. doi: 10.1007/BF01804614. [DOI] [PubMed] [Google Scholar]
- Williams E, Mareev E. Recent progress on the global electrical circuit. Atmos Res. 2014;135–136:208–227. [Google Scholar]
- Williams E, Markson R, Heckman S. Shielding effects of trees on the measurement of the Earth’s electric field: implications for secular variations of the global electrical circuit. Geophys Res Lett. 2005;32:1–4. doi: 10.1029/2005GL023717. [DOI] [Google Scholar]
- Wilson BW, Anderson LE, Ian Hilton D, Phillips RD. Chronic exposure to 60-Hz electric fields: effects on pineal function in the rat. Bioelectromagnetics. 1981;2:371–380. doi: 10.1002/bem.2250020408. [DOI] [PubMed] [Google Scholar]
- Wilson BW, Chess EK, Anderson LE. 60 Hz electric field effects on pineal melatonin rhythms: time course for onset and recovery. Bioelectromagnetics. 1986;7:239–242. doi: 10.1002/bem.2250070213. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Takeda M, Makino M, Owada T, Miyagi I. Settlement process of radioactive dust to the ground inferred by the atmospheric electric field measurement. Ann Geophys. 2012;30:49–56. doi: 10.5194/angeo-30-49-2012. [DOI] [Google Scholar]
- Zhai Y, Brun NR, Bundschuh M, Schrama M, Hin E, Vijver MG, Hunting ER. Microbially-mediated indirect effects of silver nanoparticles on aquatic invertebrates. Aquat Sci. 2018;80(4):44. [Google Scholar]