Abstract
The evaluation of the seminal plasma plays a relevant role in the definition of male infertility and in assisted reproduction outcomes; for this reason, it would be recommended to find biochemical markers able to characterize sperm pathology. In this study, 53 infertile patients (grouped by the presence leukocytospermia, idiopathic infertility, or varicocele) and 10 fertile men were selected. Spermiogram was performed by light microscopy, and sperm ultrastructure was evaluated by transmission electron microscopy (TEM) mathematically elaborated. Testosterone (TESTO), estradiol (E2), ferritin (FERR), iron (Fe), transferrin (TRSF), triglycerides (TRG), cholesterol (CHOL), and isoprostanes (F2-IsoPs) were detected in seminal plasma. Sperm characteristics and biochemical components were correlated by Spearman’s rank correlation coefficient in the whole population and in each group. The levels of TESTO and E2 were positively correlated with sperm quality in particular, and E2 was correlated with fertility index expressing the number of sperm free of ultrastructural defects evaluated by TEM. On the contrary, the indices of iron metabolism (FERR, Fe, and TRSF) were positively associated with low sperm quality and sperm necrosis, particularly in leukocytospermia and varicocele groups, pathologies in which an inflammatory status and oxidative stress condition are present. The study of the seminal plasma composition deserves attention because the levels of the various components seem to be associated with specific reproductive pathologies.
Keywords: Iron metabolism, Isoprostanes, Leukocytospermia, TEM, Testosterone, Varicocele
Introduction
Seminal plasma is the protein-rich fluid part of the semen and it plays an important role in sperm metabolism as well as sperm function. The analyses of seminal enzyme activities and the concentrations of biochemical elements have been performed in men and animals [1–4] in order to establish the functions of the different components, because some of them are still obscure.
The evaluation of seminal components may be used to characterized sperm quality and pathological conditions related to male infertility [5]. Many molecules are involved in the pathophysiology of spermatozoa. Iron is an essential trace nutrient that plays an important role in general health and fertility; however, it is highly toxic if accumulated in large quantities. The excess or the deficiency may lead to defective spermatogenesis and to fertility impairment [6]. About this topic, Hashemi et al. [7] reported a negative impact of iron on human sperm motility.
Transferrin is reported to be an iron-binding protein that regulates free iron levels in biological fluid in the body [8]. In the past, Barthelemy et al. [9] proposed that the seminal fluid transferrin, that correlated with sperm concentration, could be considered as a direct index of the functional state of Sertoli cells and seminiferous tubules. Recently, it has been found that transferrin could markedly extend sperm longevity in vitro [10].
Ferritin is an intracellular protein that stores iron in the atoxic form, and it is found in the extracellular environment as a result of cellular synthesis and secretion. Seminal ferritin is found in abundance in the seminal plasma, and Sertoli cells produce approximately 70% of its contents [11].
The investigation of other different semen biochemical components may explain their role in seminal pathologies. Recently, Murgia et al. [12] indicated that seminal fluid metabolome may represent a marker for oligozoospermia. Moreover, the identification of the lipids most closely related to the reproductive success may be of great interest to the scientific community. Cholesterol is a component of the mammalian plasma membrane and its redistribution and depletion from the sperm membrane are the key parts of the spermatozoon’s preparation for fertilization [13].
Many hormones are also found among the constituents of seminal plasma. When compared with blood serum levels, concentrations of testosterone in seminal plasma are lower by almost one order of magnitude, while estradiol is in some instances higher [14]. Other authors [15, 16] observed that the seminal balance between estradiol and testosterone provides a valuable biological indicator for predicting the spermatogenic state.
Our previous paper reported that increased albumin, total proteins, ferritin levels, and γ-glutamyl transferase and creatine kinase activities concomitant with decreased amounts of alkaline phosphatase and folic acid were associated with reduced sperm parameters. These results suggested their possible involvement in male infertility [17]. In humans, many pathological conditions as varicocele, urogenital infections, leukocytospermia, and inflammation influence sperm quality as well as seminal compositions. Oxidative stress in seminal fluid may be the common denominator responsible of male infertility associated with these diseases; the increased presence of reactive oxygen species (ROS) affects seminal components and it causes alterations in sperm lipids, proteins, and DNA with consequent sperm impairment [18].
Changes in the concentrations of the trace elements in human seminal plasma may be related to sperm quality since they are involved in the maintenance of the pro-/antioxidative balance in ejaculate [19]. In the presence of oxidative stress, a non-enzymatic oxidative damage in sperm plasma membranes, particularly rich in polyunsaturated fatty acid, may generate prostaglandin-like end-products known as isoprostanes (IsoPs). Seminal F2-isoprostanes (F2-IsoPs), a specific class of IsoPs, have been indicated as a marker of sperm immaturity in semen of infertile patients with varicocele [20].
To clarify the role of specific biochemical semen components in sperm quality, we have investigated the levels of cholesterol (CHOL), estradiol (E2), testosterone (TESTO), iron (Fe), ferritin (FERR), transferrin (TRSF), and triglycerides (TRG) in seminal fluid of fertile and infertile men grouped according to their reproductive pathologies. Isoprostane (F2-IsoP) amount was also dosed. Semen analysis was performed by light and transmission electron microscopy (TEM) mathematically elaborated. As a result, all the variables were correlated and compared.
Materials and Methods
Patients
In this research, from January 2018 through December 2018, we selected 53 Italian infertile patients (aged 28–39) attending AGI Medica, Fertility Center lab for semen analysis. Infertile patients did not obtain pregnancy after 2 years of unprotected sexual intercourses; the female factor was not present.
The infertile patients were grouped as follows: 16 idiopathic infertile patients, 19 patients with varicocele, and 18 patients with abacterial leukocytospermia. Leukocytospermia has been defined as reported in WHO guidelines [21]. The varicocele group was composed by patients who underwent both physical examination and scrotal color Doppler ultrasonography analysis carried out in laboratory other than ours. Patients with subclinical and grade 1 varicocele were not included in the study.
Inclusion selection criteria were non-azoospermic men, normal karyotype, BMI < 25 kg/m2, normal serum hormonal levels (follicle-stimulating hormone, luteinizing hormone, testosterone, prolactin), and absence of genitourinary infections. We did not include carriers of chromosome Y microdeletions and patients showing germ cells in their ejaculates. Patients with chronic diseases and receiving radiotherapy, chemotherapy, and medication were excluded. Selected men did not take an oral antioxidant supplement for at least 4 months before the study. Men with a history of recreational drug use and alcohol consumption were not selected.
Only individuals with moderate and heavy smoking habit (> 10 cigarettes/day) were excluded [22].
The fertile group was composed of 10 individuals (aged 26–40) which fathered at least one child in the last 3 years. The fertile subjects were not affected by infections and/or anatomical problems.
All selected men provided an informed written consent before the inclusion on this research where the aims of the study were accurately described.
Semen Analysis
Semen analysis was performed according to WHO guidelines [21]. Briefly, specimens were collected by masturbation after 3–5 days of sexual abstinence and analyzed after liquefaction for 30 min at 37 °C. Conventional semen parameters were determined: volume, pH, sperm concentration, motility, and vitality. The sperm morphology was assessed by the stain-coated testsimplets slides (Origio, Italy). Peroxidase stain was used to identify the leukocytes in semen samples. A leukocyte concentration ≥ 1 million cell/ml was considered abnormal [21].
After semen evaluation, all the samples were divided into three aliquots to be used for different analyses.
An aliquot was processed for transmission electron microscopy, and the seminal volume was doubled with cold Karnovsky fixative and maintained at 4 °C for 2 h. Then samples were centrifuged at 200g for 15 min, the supernatant was discarded, and sperm cells were washed in 0.1 mol/l cacodylate buffer (pH 7.2) for 12 h, postfixed in 1% buffered osmium tetroxide for 1 h at 4 °C, and it was washed again in 0.1 mol/l cacodylate buffer; the samples were dehydrated in a graded ethanol series and embedded in Epon Araldite. Ultra-thin sections were cut with a Supernova ultramicrotome (Reickert Jung, Vienna, Austria), mounted on copper grids, stained with uranyl acetate and lead citrate, and at least observed and photographed with a Philips CM12 (Philips Scientifics, Eindhoven, The Netherlands) at the CE.M.E, CNR (Via Madonna del Piano, 10, 50019 Sesto Fiorentino, Italy). Three hundred sperm sections were analyzed from each sample. The TEM data was elaborated using a mathematical formula [23] which provides numerical scores such as fertility index (number of sperm free of structural defects in a semen sample) and percentage of sperm pathologies such as immaturity, apoptosis, and necrosis [24], defined by distinctive ultrastructural characteristics. Sperm immaturity include the presence of cytoplasmic droplets, altered acrosomes, roundish or elliptical nuclei with uncondensed chromatin. Marginated chromatin, translucent vacuoles included in cytoplasmic residues, swollen and badly assembled mitochondria are ultrastructural indicators of apoptosis. Sperm with reacted or absent acrosome, misshaped nuclei with disrupted chromatin, broken plasma membrane, and poor axonemal and periaxonemal cytoskeletal structures are affected by necrosis.
Clinical Biochemistry Determinations
A semen aliquot of each ejaculate was centrifuged at 12,000g to obtain a sample free of cells as reported by Feng et al. [1], and the seminal plasma was collected by micropipette and stored in 2-ml cryotubes at − 80 °C until use, then thawed at room temperature. Seminal plasma samples (1 ml at room temperature) were tested using a COBAS 8000 modular analyzer (Roche Diagnostics, Mannheim, GmbH, Germany) by means of two analytical modules: C702, the high-throughput clinical chemistry module, and E602, the immunoassay module.
We measured the following parameters in seminal plasma: iron (Fe, μg/dl), triglycerides (TRG, mg/dl), cholesterol (CHOL, mg/dl), transferrin (TRSF, mg/dl), estradiol (E2, pg/μl), ferritin (FERR, ng/ml), testosterone (TESTO, ng/ml). For the analytes measured in module C702 (Fe, TRG, CHOL, TRSF), COBAS 8000 calibration was done with the human lyophilized serum Calibrator C.f.a.s. (Roche Diagnostics, Mannheim, GmbH, Germany). The C.f.a.s. (calibrator for automated systems) is a universal calibrator for adjusting most photometric methods.
Human lyophilized serum PreciControl ClinChem level 1 was used as normal control and PreciControl ClinChem level 2 was used as pathologic control (Roche Diagnostics, Mannheim, GmbH, Germany).
For the analytes measured in module E602 (FERR, E2, TESTO), we used specific calibrator for each analyte (Roche Diagnostics, Mannheim, GmbH Germany). PreciControl Varia levels 1 and 2 (Roche 470 Diagnostics, Mannheim, GmbH, Germany), PreciControl Universal levels 1 and 2 (Roche Diagnostics, Mannheim, GmbH, Germany), and PreciControl Tumor Marker levels 1 and 2 (Roche 470 Diagnostics, Mannheim, GmbH, Germany) were used as normal and pathologic controls respectively.
F2-Isoprostane Determination
An aliquot of each semen sample was centrifuged at 200 g for 15 min, then the pellet was discarded. Seminal sample, devoid of cells, was supplemented with butylated hydroxytoluene (BHT, final concentration 100 μM) and was stored at − 80 °C until use.
F2-isoprostanes (F2-IsoPs) are originally formed in situ, when the fatty acid precursor is esterified in phospholipids and subsequently released in biological fluids in a free-form through a process likely mediated by a phospholipase A2 (PLA2) activity(ies) [25]. Here, total (free plus esterified) F2-IsoPs were analyzed by gas chromatography/negative-ion chemical ionization-tandem mass spectrometry (GC/NICI-MS/MS). After collection, as previously reported, BHT was added in order to avoid unspecific oxidation processes. For each sample, basic hydrolysis was carried out by incubation at 45 °C for 45 min in the presence of 1 N KOH; at the end of the incubation, HCl 1 N was added to stabilize a value of pH 3.0. At this stage, in each sample, tetradeuterated derivative of prostaglandin F2α (PGF2α-d4) (500 pg) was added as an internal standard, and solid-phase extractions were consecutively carried out on an octadecylsilane (C18) cartridge and an aminopropyl (NH2) cartridge. The NH2 final eluate was derivatized to convert the carboxyl group of F2-IsoPs into pentafluorobenzyl ester and the hydroxyl group into trimethylsilyl ethers [26]. For 8-iso-PGF2α, also referred as 15-F2t-IsoP, one of the most represented isomers of F2-IsoPs, the product ion at m/z 299, derived from the [M−181]− precursor ion (m/z 569), was detected and referred to the internal standard PGF2α-d4 (m/z 573 → m/z 303) [27].
Statistical Analysis
Data were reported as medians and interquartile range (IQR 75°–25° centile). The comparisons between groups (fertile, idiopathic infertility, varicocele, leukocytospermia) were evaluated by Kruskal-Wallis test followed, only for significant cases, by Dunn’s post hoc test for multiple comparisons. Spearman rho (R) coefficient was calculated to measure the correlation between variables for all enrolled patients and separately for each group. A P value < 0.05 (two-tailed) was considered statistically significant. All analyses were performed by IBM SPSS Statistics Software v.25.
Results
Semen characteristics of the infertile patients with leukocytospermia, idiopathic infertility, varicocele, and fertile group are reported in Table 1. Idiopathic infertility group significantly differed in almost all evaluated sperm characteristics from fertile men. Patients with leukocytospermia showed a significant decrease in sperm motility, normal sperm morphology, and vitality with respect to fertile patients, and higher percentage of necrosis (Fig. 1a) compared to that observed in fertile and varicocele groups (P < .001). Finally, in varicocele patients, immaturity percentage was significantly elevated (P < .001) with respect to that detected in fertile, idiopathic infertility, and leukocytospermia groups (Table 1).
Table 1.
Medians and interquartile ranges (IQR 75°–25° centile) of semen parameters evaluated by light microscopy, sperm apoptosis, necrosis, immaturity, and fertility index evaluated by transmission electron microscopy in semen samples of 63 men divided into 4 groups (leukocytospermia, L; idiopathic infertility, I; varicocele, V; fertile men, F) according to their clinical condition. Statistics are also reported: *P < .05, **P < .01, ***P < .001
| Variables | Diagnosis | Statistics | ||||
|---|---|---|---|---|---|---|
| Leukocytospermia (L no. 18) |
Idiopathic infertility (I no. 16) |
Varicocele (V no. 19) |
Fertile men (F no. 10) |
Kruskal-Wallis (P value) |
Multiple comparisons | |
| Volume (ml) | 3.25 (2.13) | 3.75 (1.50) | 4.00 (0.50) | 4.25 (1.13) | 0.068 | |
| Sperm ml × 106 | 40.50 (68.35) | 6.50 (28.56) | 38.50 (36.38) | 118.50 (128.06) | *** | F vs I |
| Progressive motility % | 25.50 (15.50) | 26.50 (19.75) | 25.00 (24.00) | 48.00 (30.25) | * | F vs I; F vs L |
| normal morphology % | 9.00 (3.25) | 7.50 (4.75) | 10.00 (7.00) | 16.00 (7.75) | *** | F vs I; F vs L |
| Vitality % | 65.00 (30.50) | 66.50 (28.75) | 75.00 (21.00) | 85.00 (12.75) | ** | F vs I; F vs L |
| Apoptosis % | 6.48 (3.42) | 9.11 (6.83) | 4.13 (1.65) | 4.13 (0.86) | *** | F vs I; I vs V |
| Necrosis % | 44.00 (19.95) | 37.74 (10.72) | 34.24 (19.65) | 30.05 (11.11) | *** | F vs L; L vs V |
| Immaturity % | 50.65 (23.04) | 53.89 (13.15) | 75.09 (10.89) | 47.20 (8.26) | *** | F vs V; I vs V; L vs V |
| Fertility index | 266,546 (785,647) | 6811 (420,986) | 287,471 (1,497,605) | 4,025,583 (5,444,460) | *** | F vs I; F vs L; F vs V |
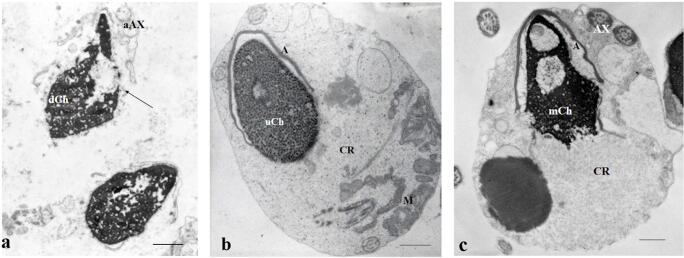
Fig. 1.
TEM micrographs of longitudinal sections of spermatozoa. In a, two necrotic spermatozoa are shown, the chromatin (dCh) appears disrupted, the plasma membranes are broken (arrow), and the axonemal components are absent (aAx). An immature sperm is represented in b, the chromatin is uncondensed (uCh), the acrosome (A) reduced and far from the nucleus, the tail is coiled into a cytoplasmic residue (CR), and mitochondria (M) are disassembled. An apoptotic sperm is shown in c, the chromatin is marginated (mCh), the tail is coiled but the axonemal components are well assembled (Ax); acrosome (A); and cytoplasmic residue (CR). a–c Bar, 1 μm
The medians and interquartile ranges of seminal levels of F2-IsoPs, CHOL, E2, TESTO, Fe, FERR, TRSF, and TRIG are shown in Table 2.
Table 2.
Medians and interquartile ranges (IQR 75°–25° centile) of isoprostanes (F2-IsoPs), cholesterol (CHOL), estradiol (E2), testosterone (TESTO), iron (Fe), ferritin (FERR), transferrin (TRSF), and triglycerides (TRG) assayed in semen samples of 63 men divided into 3 groups according to their clinical condition (leukocytospermia, L; idiopathic infertility, I; varicocele, V; fertile men, F). Statistics are also reported: *P < .05, ***P < .001
| Variables | Diagnosis | Statistics | ||||
|---|---|---|---|---|---|---|
| Leukocytospermia (L no. 18) | Idiopathic infertility (I no. 16) | Varicocele (V no. 19) | Fertile men (F no. 10) | Kruskal-Wallis (P value) | Multi-comparison test | |
| F2-IsoPs (ng/ml) | 27.22 (31.50) | 7.03 (32.63) | 76.16 (55.46) | 8.56 (17.42) | *** | I vs V; F vs V; L vs V |
| CHOL (mg/dl) | 22.00 (14.75) | 19.00 (10.00) | 17.00 (9.00) | 19.00 (13.75) | 0.326 | |
| E2 (pg/μl) | 45.83 (27.77) | 55.63 (16.57) | 58.93 (29.24) | 47.09 (19.36) | 0.154 | |
| TESTO (ng/ml) | 0.94 (0.37) | 1.04 (1.33) | 1.10 (2.03) | 1.26 (1.57) | 0.264 | |
| Fe (μg/dl) | 23.00 (16.50) | 13.00 (8.25) | 15.00 (10.00) | 14.50 (11.00) | * | I vs L |
| FERR (ng/ml) | 337.2 (179.4) | 233.9 (91.3) | 238.2 (77.3) | 158.4 (75.8) | *** | L vs F; L vs V; L vs I |
| TRSF (mg/dl) | 1.50 (7.00) | 0.50 (1.00) | 3.64 (3.00) | 0.50 (3.00) | 0.117 | |
| TRG (mg/dl) | 99.50 (181.75) | 7.00 (59.25) | 2.00 (126.00) | 56.50 (164.50) | 0.053 | |
F2-IsoP level, a reliable indicator of oxidative stress, was significantly increased in varicocele group compared to leukocytospermia, idiopathic infertility, and fertile groups (P < .001). The values of CHOL, E2, TESTO, TRSF, and TRG did not show differences in the considered groups. In leukocytospermia group, the concentration of FERR was significantly increased than that observed in fertile, varicocele, and idiopathic infertility groups (P < .001) and the Fe level was higher than that detected in idiopathic infertility group (P < .05).
In order to understand the possible correlations among the studied variables, we used the Spearman rank correlation coefficient considering the whole population of interest (Table 3). Regarding biochemical components, F2-IsoP levels correlated positively with sperm immaturity (Fig. 1b; P < .01). Positive correlations between the TRSF (P < .01) and TRG (P < .05) levels and sperm concentration were observed. FERR showed negative correlations with sperm morphology, vitality, and fertility index (P < .05) and positive correlations with sperm necrosis (P < .01) and Fe (P < .05); Fe compound also positively correlated with sperm necrosis (P < .05). Interestingly, TESTO displayed positive correlations with the sperm vitality (P < .05) and E2 level (P < .01) and negative correlations with TRG (P < .01) and sperm apoptosis (Fig. 1c; P < .05).
Table 3.
Correlations (rho Spearman’s coefficient) between all considered variables in 63 individuals
| Sperm/ml × 106 | Motility % | Normal morphology % | Vitality% | A % | N % | I % | FI | E2 | FERR | Fe | TESTO | TRG | TRSF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sperm/ml × 106 | 1 | |||||||||||||
| Motility % | .400** | 1 | ||||||||||||
| Normal morphology % | .677** | .753** | 1 | |||||||||||
| Vitality% | .271* | .639** | .645** | 1 | ||||||||||
| A % | NS | NS | NS | − .359** | 1 | |||||||||
| N % | − .299* | − .250* | − .462** | − .454** | .356** | 1 | ||||||||
| I % | NS | − .264* | NS | NS | NS | NS | 1 | |||||||
| FI | .697** | .522** | .642** | .392** | NS | − .457** | − .263* | 1 | ||||||
| E2 (pg/μl) | NS | NS | NS | NS | NS | NS | .303* | NS | 1 | |||||
| FERR (ng/ml) | NS | NS | − .259* | − .285* | NS | .562** | NS | − .253* | NS | 1 | ||||
| Fe (μg/dl) | NS | NS | NS | NS | NS | .253* | NS | NS | NS | .314* | 1 | |||
| TESTO (ng/ml) | NS | NS | NS | .272* | − .254* | NS | NS | NS | .581** | NS | NS | 1 | ||
| TRG (mg/dl) | .248* | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | − .357** | 1 | |
| TRSF (mg/dl) | .326** | NS | NS | NS | NS | NS | NS | NS | NS | NS | .306* | NS | NS | 1 |
| F2-IsoPs (ng/ml) | NS | NS | NS | NS | NS | NS | .640** | NS | NS | NS | NS | NS | NS | NS |
Sperm/ml × 106, number of sperm/ml; motility %, percentage of progressive sperm motility; normal morphology %, percentage of normal sperm morphology assessed with testsimplets; vitality %, percentage of viable sperm; A %, percentage of sperm apoptosis assessed by transmission electron microscopy; N %, percentage of sperm necrosis assessed by transmission electron microscopy; I %, percentage of sperm immaturity assessed by transmission electron microscopy; FI, fertility index, the number of sperm probably devoid of ultrastructural defects; F2-IsoPs, isoprostanes; CHOL, cholesterol; E2, estradiol; TESTO, testosterone; Fe, iron; FERR, ferritin; TRSF, transferrin; TRG, triglycerides, NS, not significant
*P < .05, **P < .01
Different correlations between biochemical indices and sperm parameters were analyzed in each studied group (Table 4, Spearman’s rank correlation coefficient).
Table 4.
Correlations (rho Spearman’s coefficient) between all considered variables in 63 individuals grouped in fertile men (line 1), idiopathic infertile patients (line 2), leukocytospermic men (line 3), and infertile patients with varicocele (line 4)
| Sperm/ml × 106 | Motility % | Normal morphology % | Vitality % | A % | N % | I % | FI | E2 | FERR | Fe | TESTO | TRG | TRSF | CHOL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sperm/ml × 106 | 1 | ||||||||||||||
| Motility % |
NS NS .486* NS |
1 | |||||||||||||
| Normal morphology % |
.671* NS .693** .656** |
.725* .650** .705** .712** |
1 | ||||||||||||
| Vitality% |
NS .635** .618** .534* |
NS .766** NS .519* |
1 | ||||||||||||
| A % |
NS NS − .489* − .500* |
1 | |||||||||||||
| N% |
NS NS − .579* NS |
NS NS .627** NS |
1 | ||||||||||||
| I % |
NS − .570* NS NS |
NS NS NS − .498* |
− .695* NS NS − .476* |
1 | |||||||||||
| FI |
NS .560* .650** .706** |
NS NS .605** .575** |
NS NS .602** .691** |
NS NS NS − .502* |
1 | ||||||||||
| E2 (pg/μl) |
.709* NS NS NS |
1 | |||||||||||||
| FERR (ng/ml) |
NS NS NS − .493* |
NS NS NS .487* |
NS NS − .498* NS |
1 | |||||||||||
| Fe (μg/dl) | 1 | ||||||||||||||
| TESTO (ng/ml) |
NS NS − .518* NS |
.891** NS .752** .598** |
NS NS − .586* NS |
1 | |||||||||||
| TRG (mg/dl) |
NS NS NS NS |
1 | |||||||||||||
| TRSF (mg/dl) |
NS NS NS − .607** |
NS NS NS − .560* |
NS NS .506* NS |
1 | |||||||||||
| CHOL (mg/dl) |
NS NS .524* NS |
.786** NS NS NS |
NS NS .520* NS |
1 | |||||||||||
| F2-IsoPs (ng/ml) |
NS NS .530* .786** |
Sperm/ml × 106, number of sperm/ml; motility %, percentage of progressive sperm motility; normal morphology %, percentage of normal sperm morphology assessed with testsimplets; vitality %, percentage of viable sperm; A %, percentage of sperm apoptosis assessed by transmission electron microscopy; N%, percentage of sperm necrosis assessed by transmission electron microscopy; I %, percentage of sperm immaturity assessed by transmission electron microscopy; FI, fertility index, the number of sperm probably devoid of ultrastructural defects; F2-IsoPs, isoprostanes; CHOL, cholesterol; E2, estradiol; TESTO, testosterone; Fe, iron; FERR, ferritin; TRSF, transferrin; TRG, triglycerides; NS, not significant
*P < .05, **P < .01
In fertile men, E2 positively correlated with fertility index (P < .05) and TESTO (P < .01); CHOL positively correlated with TRG (P < .001).
In idiopathic infertility group, we did not observe significant correlations between seminal plasma components and sperm characteristics.
In leukocytospermia group, E2 positively correlated with TESTO (P < .01) and TESTO negatively correlated with sperm necrosis (P < .05); both TESTO and E2 negatively correlated with FERR (P < .05). TRSF had positive correlations with CHOL and TRG (both P < .01). Sperm immaturity positively correlated with F2-IsoPs (P < .05). Sperm necrosis is positively correlated with CHOL (P < .05).
In infertile patients affected by varicocele, E2 showed positive correlation with TESTO (P < .01), and both E2 and TESTO negatively correlated with TRSF (respectively P < .01; P < .05). FERR displayed a positive correlation with sperm necrosis (P < .05) and negative correlation with sperm motility (P < .05). Finally, F2-IsoPs had a positive relationship with sperm immaturity (P < .01).
Discussion
The evaluation of the seminal plasma composition may play a relevant role in the definition of male infertility and in assisted reproduction outcomes [28]; for this reason, it would be recommended to find biochemical markers able to characterize sperm pathology [29]. Our and other groups have stressed on the importance of the research of seminal indicators in the different male infertility conditions [30–33]. In our recent paper, we suggested that some seminal biochemical components may be associated with pathological conditions of human semen. Patients with sperm vitality ≤ 5th percentile showed increased albumin concentration and creatine kinase activity. The presence of germ cells in semen was associated with high values of FERR; the alkaline phosphatase activity and folic acid were decreased in hyperviscous semen samples [17]. There are several studies focused on the proteomic composition of human seminal plasma, which may be linked to specific pathways of infertility [3, 34]. Mirnamniha et al. [35] reported that the deficiency of trace elements such as calcium copper, manganese, magnesium, zinc, and selenium is associated with impaired spermatogenesis, altered levels of sex hormones, seminal oxidative stress, inflammation, and apoptosis. Recently, Murgia et al. [12] detected altered levels of fructose, myo-inositol, aspartate, and choline in oligozoospermic men.
In this paper, the data regarding the analyzed variables suggested some intriguing hypotheses. The level of TESTO did not differ in the examined groups but it was strongly positively correlated with sperm quality and with E2; in turn, E2 level positively correlated with fertility index expressing the number of sperm free of ultrastructural defects evaluated by TEM. These results confirmed that these hormones have a relevant positive influence in sperm physiology and the seminal balance between estrogenic and androgenic actions is essential for maintaining spermatogenesis and male fertility [15, 16, 36]. In agreement with our data, Thanaboonyawat et al. [37] demonstrated that in vitro supplementation of TESTO determined a significant retardation in the normal reduction of sperm motility over time. Moreover, the effects of E2 on human sperm have been associated with an increased motility, oocyte penetration, longevity, oxygen intake, and metabolization of exogenous substrates [38–40]. The correlation between these hormones is justified by the fact that in adult human and animal testis, TESTO is converted to E2 by aromatase (CYP19A1), the ubiquitous NADPH cytochrome P450 reductase enzyme principally found in spermatids and mature sperm in seminiferous tubules and Leydig cells [41]. A further confirmation that TESTO is a positive marker of semen quality is suggested by its negative correlation with sperm necrosis.
On the contrary, the indices of iron metabolism (FERR, Fe, and TRSF) were positively associated with low sperm quality and negatively with TESTO and E2 levels, particularly in leukocytospermia and varicocele groups, pathologies in which an inflammatory status and oxidative stress condition are generally present [42].
The relationship among TESTO, FERR, and inflammation is well known in the literature [43–46] even if the determinations are performed in blood serum. In our study, they were detected in human seminal plasma and these associations were confirmed suggesting the involvement of cytokines [43] and ROS [47] also in the seminal fluid from patients with varicocele or leukocytospermia. It is known that an increased number of leukocytes are positively associated with altered sperm characteristics, sperm necrosis, intracellular ROS [30, 48, 49], and level of Fe in the seminal plasma of men with different fertility potentials [50]. Overload of Fe may cause disturbances to spermatogenesis as well as to crucial sperm cell structures accompanied by oxidative stress and cell death [6, 50].
FERR has been localized in human Sertoli and Leydig cells [51, 52] and an increased level of FERR was detected in human semen concomitantly to the presence of germ cells [17]. Seminal fluid TRSF values were proposed as a reliable clinical index for determining the functional status of Sertoli cells or seminiferous tubules [9]. In mice, TRSF overexpression had negative effects on testicular function and TRSF level required strict regulation in the testis [53]. In our paper, the negative correlation between TRSF and TESTO and E2 in varicocele patients may suggest a role of TRSF as an index of testis malfunctioning since it is well known that varicocele can alter Sertoli cell function and consequently influence the TSRF level.
This study confirmed the close relationship between increased levels of F2-IsoP varicocele and sperm immaturity observed also in previous studies [20, 54]; since these studies included different patient populations, the semen level of F2-IsoPs can be considered a semen biochemical marker of sperm immaturity and varicocele.
Noteworthy is the behavior of CHOL and TRG that in the first analysis seems to be irrelevant, either as marker of pathologies either in correlations performed in the whole studied population. When the correlations between variables were carried out in each group separately, we observed that in the case of leukocytospermia, CHOL and TRG positively correlated with sperm necrosis. Previous studies suggested that elevated lipid levels in seminal plasma might have adverse effect on sperm quality [55], and seminal plasma TRG and total CHOL levels had a positive correlation with sperm DNA fragmentation [56]. Also increased lipid serum levels play a negative influence on sperm quality [57].
Patients with idiopathic infertility showed an altered sperm quality as the other infertile groups; however, they did not show correlations between semen parameters and seminal plasma markers highlighted for leukocytospermia and varicocele groups. This observation could be explained by the intrinsic meaning of idiopathic infertility since it is difficult to group patients with homogeneous characteristics. Indeed, in semen of many men who are currently diagnosed as having unexplained male infertility, oxidative stress was detected, but other genetic, epigenetic, or environmental unknown causes could be supposed [58, 59].
Conclusions
In conclusion, infertile men with varicocele, leukocytospermia, and idiopathic infertility differed in seminal fluid composition. TESTO and E2 levels appeared to be necessary for a regular spermatogenesis and play a role in sperm quality; FERR, Fe, and TRSF appeared to be linked to inflammatory conditions and sperm necrosis as well as F2-IsoPs to sperm immaturity. We are aware that the number of cases is low and should be extended in a future study; however, these preliminary data indicated that the seminal plasma composition deserves attention because the levels of the various components seem to be associated with specific reproductive pathologies.
The knowledge of the role of seminal molecules in male infertility will help in the development of targeted treatments.
Acknowledgments
Open access funding provided by Università degli Studi di Siena within the CRUI-CARE Agreement. We sincerely acknowledged the assistance given by Dr. Maria Cristina Salvatici, CE.M.E, CNR (Via Madonna del Piano, 10, 50019 Sesto Fiorentino, Italy), in this study.
Authors’ Contributions
All the authors approved the submitted version of this paper and gave substantial contributions to the research design and drafting or critically revising the paper. In particular: Giulia Collodel—paper writing, research design, TEM analysis, data interpretation; Cinzia Signorini—isoprostane determination, data interpretation; Fabiola Nerucci—biochemical component determination, data interpretation; Francesca Iacoponi—statistical analysis; Laura Gambera—patient recruitment, semen analysis, data interpretation; Elena Moretti—patient selection, TEM analysis, data interpretation, research design.
Funding Information
This research was supported by the Plan Support to the Research (PSR) 2019, Department of Molecular and Developmental Medicine, University of Siena, Italy.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Patient Consent
All participants were informed and gave written consent prior to the collection of samples.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feng RX, Lu JC, Zhang HY, Lü NQ. A pilot comparative study of 26 biochemical markers in seminal plasma and serum in infertile men. Biomed Res Int. 2015;2015:805328. doi: 10.1155/2015/805328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang HY, Lu JC, Feng RX. Correlations of 24 biochemical markers in seminal plasma with routine semen parameters. Zhonghua Nan Ke Xue. 2015;21(12):1087–1092. [PubMed] [Google Scholar]
- 3.Camargo M, Intasqui P, Bertolla RP. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin Androl. 2018;28:6. doi: 10.1186/s12610-018-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Recuero S, Fernandez-Fuertes B, Bonet S, Barranco I, Yeste M. Potential of seminal plasma to improve the fertility of frozen-thawed boar spermatozoa. Theriogenology. 2019;137:36–42. doi: 10.1016/j.theriogenology.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 5.Vickram AS, Kamini AR, Das R, Pathy MR, Parameswari R, Sridharan TB. Validation of artificial neural network models for predicting biochemical markers associated with male infertility. Syst Biol Reprod Med. 2016;62(4):258–265. doi: 10.1080/19396368.2016.1185654. [DOI] [PubMed] [Google Scholar]
- 6.Tvrda E, Peer R, Sikka SC, Agarwal A. Iron and copper in male reproduction: a double-edged sword. J Assist Reprod Genet. 2015;32(1):3–16. doi: 10.1007/s10815-014-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemi MM, Behnampour N, Nejabat M, Tabandeh A, Ghazi-Moghaddam B, Joshaghani HR. Impact of seminal plasma trace elements on human sperm motility parameters. Rom J Intern Med. 2018;56(1):15–20. doi: 10.1515/rjim-2017-0034. [DOI] [PubMed] [Google Scholar]
- 8.Kleven MD, Jue S, Enns CA. The transferrin receptors, TfR1 and TfR2 bind transferrin through differing mechanisms. Biochemistry. 2018;57(9):1552–1559. doi: 10.1021/acs.biochem.8b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barthelemy C, Khalfoun B, Guillaumin JM, Lecomte P, Bardos P. Seminal fluid transferrin as an index of gonadal function in men. J Reprod Fertil. 1998;82(1):113–118. doi: 10.1530/jrf.0.0820113. [DOI] [PubMed] [Google Scholar]
- 10.Matsuzaki M, Mizushima S, Dohra H, Sasanami T. Expression of transferrin and albumin in the sperm-storage tubules of Japanese quail and their possible involvement in long-term sperm storage. J Poult Sci. 2020;57(1):88–96. doi: 10.2141/jpsa.0190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva GP, De La Vega Elena CD, Pirani Carneiro F, Russomano Veiga JP. Effect of systemic inflammation on level of ferritin seminal in chronic renal male patient undergoing hemodialysis. Int Arch Med. 2014;7:23. doi: 10.1186/1755-7682-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murgia F, Corda V, Serrenti M, et al. Seminal fluid metabolomic markers of oligozoospermic infertility in humans. Metabolites. 2020;10(2):E64. doi: 10.3390/metabo10020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leahy T, Gadella BM. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J Androl. 2015;17(4):561–567. doi: 10.4103/1008-682X.153309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitku J, Kolatorova L, Hampl R. Occurrence and reproductive roles of hormones in seminal plasma. Basic Clin Androl. 2017;27:19. doi: 10.1186/s12610-017-0062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr Rev. 2001;22(3):289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Bai Q, Yuan Y, Liu P, Qiao J. Assessment of seminal estradiol and testosterone levels as predictors of human spermatogenesis. J Androl. 2010;31(2):215–20. [DOI] [PubMed]
- 17.Collodel G, Nerucci F, Signorini C, Iacoponi F, Moretti E. Associations between biochemical components of human semen with seminal conditions. Syst Biol Reprod Med. 2019;65(2):155–163. doi: 10.1080/19396368.2018.1548668. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, De Stefani S, et al. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod BioMed Online. 2012;25(3):300–306. doi: 10.1016/j.rbmo.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Nenkova G, Petrov L, Alexandrova A. Role of trace elements for oxidative status and quality of human sperm. Balkan Med J. 2017;34(4):343–348. doi: 10.4274/balkanmedj.2016.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collodel G, Moretti E, Longini M, Pascarelli NA, Signorini C. Increased F2-isoprostane levels in semen and immunolocalization of the 8-Iso prostaglandin F2α in spermatozoa from infertile patients with varicocele. Oxidative Med Cell Longev. 2018;2018:7508014. doi: 10.1155/2018/7508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . WHO laboratory manual for the examination and processing of human semen. 5. Geneva: WHO Press; 2010. [Google Scholar]
- 22.Collodel G, Capitani S, Iacoponi F, Federico MG, Pascarelli NA, Moretti E. Retrospective assessment of potential negative synergistic effects of varicocele and tobacco use on ultrastructural sperm morphology. Urology. 2009;74(4):794–799. doi: 10.1016/j.urology.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 23.Baccetti B, Bernieri G, Burrini AG, Collodel G, Crisà N, Mirolli M, Moretti E, Piomboni P. Notulae Seminologicae. 5. Mathematical evaluation of interdependent submicroscopic sperm alterations. J Androl. 1995;16(4):356–371. [PubMed] [Google Scholar]
- 24.Collodel G, Moretti E. Morphology and meiotic segregation in spermatozoa from men of proven fertility. J Androl. 2008;29(1):106–114. doi: 10.2164/jandrol.107.002998. [DOI] [PubMed] [Google Scholar]
- 25.Stafforini DM, Sheller JR, Blackwell TS, Sapirstein A, Yull FE, McIntyre TM, et al. Release of free F2-iso-prostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J Biol Chem. 2006;281(8):4616–4623. doi: 10.1074/jbc.M507340200. [DOI] [PubMed] [Google Scholar]
- 26.Signorini C, De Felice C, Durand T, et al. Relevance of 4-F(4t)-neuroprostane and 10-F(4t)-neuroprostane to neurological diseases. Free Radic Biol Med. 2018;115:278–287. doi: 10.1016/j.freeradbiomed.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Signorini C, Cardile V, Pannuzzo G, Graziano ACE, Durand T, Galano JM, Oger C, Leoncini S, Cortelazzo A, Lee JCY, Hayek J, de Felice C. Increased isoprostanoid levels in brain from murine model of Krabbe disease—relevance of isoprostanes, dihomo-isoprostanes and neuroprostanes to disease severity. Free Radic Biol Med. 2019;139:46–54. doi: 10.1016/j.freeradbiomed.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 29.Lazzarino G, Listorti I, Muzii L, Amorini AM, Longo S, di Stasio E, Caruso G, D’Urso S, Puglia I, Pisani G, Lazzarino G, Tavazzi B, Bilotta P. Low-molecular weight compounds in human seminal plasma as potential biomarkers of male infertility. Hum Reprod. 2018;33(10):1817–1828. doi: 10.1093/humrep/dey279. [DOI] [PubMed] [Google Scholar]
- 30.Collodel G, Moretti E, Micheli L, Menchiari A, Moltoni L, Cerretani D. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology. 2015;3:280–286. doi: 10.1111/andr.297. [DOI] [PubMed] [Google Scholar]
- 31.Micheli L, Cerretani D, Collodel G, Menchiari A, Moltoni L, Fiaschi AI, Moretti E. Evaluation of enzymatic and non-enzymatic antioxidants in seminal plasma of men with genitourinary infections, Varicocele and Idiopathic Infertility. Andrology. 2016;4(3):456–464. doi: 10.1111/andr.12181. [DOI] [PubMed] [Google Scholar]
- 32.Moretti E, Sutera G, Collodel G. The importance of transmission electron microscopy analysis of spermatozoa: diagnostic applications and basic research. Syst Biol Reprod Med. 2016;62(3):171–183. doi: 10.3109/19396368.2016.1155242. [DOI] [PubMed] [Google Scholar]
- 33.Gatimel N, Moreau, Parinaud J, Léandri RD. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology. 2017;5(5):845–886. doi: 10.1111/andr.12389. [DOI] [PubMed] [Google Scholar]
- 34.Behrouzi B, Kenigsberg S, Alladin N, Swanson S, Zicherman J, Hong SH, Moskovtsev SI, Librach CL. Evaluation of potential protein biomarkers in patients with high sperm DNA damage. Syst Biol Reprod Med. 2013;59(3):153–163. doi: 10.3109/19396368.2013.775396. [DOI] [PubMed] [Google Scholar]
- 35.Mirnamniha M, Faroughi F, Tahmasbpour E, Ebrahimi P, Harchegani AB. An overview on role of some trace elements in human reproductive health, sperm function and fertilization process. Rev Environ Health. 2019;34(4):339–348. doi: 10.1515/reveh-2019-0008. [DOI] [PubMed] [Google Scholar]
- 36.Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2–13. doi: 10.1016/j.semcdb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thanaboonyawat I, Chantrapanichkul P, Petyim S, Kaewjunun C, Laokirkkiat P, Choavaratana R. Application of testosterone supplementation in semen to improve sperm motility in asthenozoospermic males. Arch Gynecol Obstet. 2017;296(3):589–596. doi: 10.1007/s00404-017-4451-4. [DOI] [PubMed] [Google Scholar]
- 38.Idaomar M, Guerin JF, Lorange J, Cziba JC. Stimulation of motility and energy metabolism of spermatozoa from astenozoospermic patients by 17β-estradiol. Arch Androl. 1989;22:197–202. doi: 10.3109/01485018908986772. [DOI] [PubMed] [Google Scholar]
- 39.Baldi E, Luconi M, Bonaccorsi L, Muratori M, Forti G. Intracellular events and signaling pathways involved in sperm acquisition of fertilizing capacity and acrosome reaction. Front Biosci. 2000;5:110–123. doi: 10.2741/baldi. [DOI] [PubMed] [Google Scholar]
- 40.Aquila S, Sisci D, Gentile M, Carpino A, Middea E, Catalano S, et al. Towards a physiological role for cytochrome P450 aromatase in ejaculated human sperm. Hum Reprod. 2003;18(8):1650–1659. doi: 10.1093/humrep/deg340. [DOI] [PubMed] [Google Scholar]
- 41.Cooke PS, Nanjappa MK, Ko CM, Gail S, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50(11):e13126. doi: 10.1111/and.13126. [DOI] [PubMed] [Google Scholar]
- 43.Chao KC, Chang CC, Chiou HY, Chang JS. Serum ferritin is inversely correlated with testosterone in boys and young male adolescents: a cross-sectional study in Taiwan. PLoS One. 2015;10(12):e0144238. doi: 10.1371/journal.pone.0144238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremellen K, McPhee N, Pearce K, Benson S, Schedlowski M, Engler H. Endotoxin-initiated inflammation reduces testosterone production in men of reproductive age. Am J Physiol Endocrinol Metab. 2018;314(3):E206–E213. doi: 10.1152/ajpendo.00279.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueda N, Takasawa K. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients. 2018;10(9):1173. doi: 10.3390/nu10091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darbandi M, Darbandi S, Agarwal A, Sengupta P, Durairajanayagam D, Henkel R, Sadeghi MR. Reactive oxygen species and male reproductive hormones. Reprod Biol Endocrinol. 2018;16:87. doi: 10.1186/s12958-018-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zorn B, Ihan A, Kopitar AN, Kolbezen M, Sesek-Briski A, Meden-Vrtovec H. Changes in sperm apoptotic markers as related to seminal leukocytes and elastase. Reprod Biomed Online. 2010;21(1):84–92. doi: 10.1016/j.rbmo.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Moretti E, Collodel G, Mazzi L, Campagna MS, Iacoponi F, Mazzi L, et al. Resistin, interleukin-6, tumor necrosis factor-alpha, and human semen parameters in the presence of leukocytospermia, smoking habit, and varicocele. Fertil Steril. 2014;102(2):354–360. doi: 10.1016/j.fertnstert.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 50.Ammar O, Houas Z, Mehdi M. The association between iron, calcium, and oxidative stress in seminal plasma and sperm quality. Environ Sci Pollut Res Int. 2019;26(14):14097–14105. doi: 10.1007/s11356-019-04575-7. [DOI] [PubMed] [Google Scholar]
- 51.Koeva YA. Immunoreactivity for ferritin in Leydig cells of human testis. Folia Med (Plovdiv) 2002;44(3):24–26. [PubMed] [Google Scholar]
- 52.Mikuz G. The multitasking Sertoli cell. Pathologe. 2019;40(Suppl):318–324. doi: 10.1007/s00292-019-00711-9. [DOI] [PubMed] [Google Scholar]
- 53.Lécureuil C, Staub C, Fouchécourt S, Maurel MC, Fontaine I, Martinat N, Gauthier C, Daudignon A, Delaleu B, Sow A, Jégou B, Guillou F. Transferrin overexpression alters testicular function in aged mice. Mol Reprod Dev. 2007;74(2):197–206. doi: 10.1002/mrd.20523. [DOI] [PubMed] [Google Scholar]
- 54.Longini M, Moretti E, Signorini C, Noto D, Iacoponi F, Collodel G. Relevance of seminal F2-dihomo-IsoPs, F2-IsoPs and F4-NeuroPs in idiopathic infertility and varicocele. Prostaglandins Other Lipid Mediat. 2020;149:106448. doi: 10.1016/j.prostaglandins.2020.106448. [DOI] [PubMed] [Google Scholar]
- 55.Lu JC, Jing J, Yao Q, Fan K, Wang GH, Feng RX, Liang YJ, Chen L, Ge YF, Yao B. Relationship between lipids levels of serum and seminal plasma and semen parameters in 631 Chinese subfertile men. PLoS One. 2016;11(1):e0146304. doi: 10.1371/journal.pone.0146304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu JC, Jing J, Chen L, Ge YF, Feng RX, Liang YJ, Yao B. Analysis of human sperm DNA fragmentation index (DFI) related factors: a report of 1010 subfertile men in China. Reprod Biol Endocrinol. 2018;16(1):23. doi: 10.1186/s12958-018-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saez Lancellotti TE, Boarelli PV, Monclus MA, Cabrillana ME, Clementi MA, Espínola LS, Cid Barría JL, Vincenti AE, Santi AG, Fornés MW. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS One. 2010;5(1):e13457. doi: 10.1371/journal.pone.0013457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tournaye H, Krausz C, Oates RD. Novel concepts in the aetiology of male reproductive impairment. Lancet Diabetes Endocrinol. 2016;8587:1–10. doi: 10.1016/S2213-8587(16)30040-7. [DOI] [PubMed] [Google Scholar]
- 59.Bracke A, Peeters K, Punjabi U, Hoogewijs D, Dewilde S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod Biomed Online. 2018;36(3):327–339. doi: 10.1016/j.rbmo.2017.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.