Abstract
During Camellia sinensis tea processing, manufacturers usually remove the internodes, which are classified as waste. This study presents the first determination of plant part contribution, particularly internodes, to green tea quality, in order to find the best blend to maximize impact on human health. Catechins, caffeine and free amino acid (FAA) profiles were determined by RP-HPLC/DAD, total phenolics (TPC) and total flavonoids (TFC) by Folin-Ciocalteu and colorimetric methodologies, respectively, and antioxidant activities by free radical-scavenging activity (FRSA), ferric-reducing antioxidant power (FRAP) and ferrous ion-chelating (FIC) methods. Individual esterified catechins content decreased as follows: epicatechin-3-gallate > epigallocatechin-3-gallate ≫ gallocatecin-3-gallate, and epicatechin derivatives content ranged from 63.91 to 91.22% of total catechins. Caffeine content was higher in internodes. L-theanine, histidine, asparagine, phenylalanine, glutamic acid and methionine were the major FAAs, and internodes contained the highest amounts of L-theanine and histidine (17 and 13.73 mg/g of sample, respectively). TPC ranged from 201.51 to 265.48 mg gallic acid equivalents/g dry extract (DE) and TFC ranged from 23.84 to 72.02 mg rutin equivalents/g DE. Internodes presented the lowest FRSA (EC50 = 6.10–13.50 μg/mL), FRAP (EC50 = 5.70–11.40 μg/mL) and FIC activity (36.96–79.21%). Bud presented the highest FRSA and FRAP, and bud+1st+2ndleaves + internodes the highest FIC activity. The results revealed the potential contribution of the internodes to green tea quality and, consequently, to human health.
Keywords: Internode waste valorization, Tea antioxidants, Total phenolics, Total flavonoids, Catechin profiles, Free amino acid profiles, L-theanine/caffeine content, RP-HPLC analysis
Graphical abstract
Highlights
-
•
Metabolites variability is observed in different parts of Azorean Camellia sinensis.
-
•
Camellia sinensis plant parts are good sources of polyphenols (catechins).
-
•
Internodes are not useless tea plant waste but a valuable tea quality component.
-
•
Theanine is the major amino acid in Camellia sinensis particularly in internodes.
-
•
Addition of internodes to the tea leaves have significative impact on human health.
1. Introduction
The drink made from tea (Camellia sinensis L.) leaves is one of the most ancient and most widely consumed non-alcoholic beverage worldwide following water. This popularity is attributed to its sensory properties, stimulant effects, health benefits, and relatively low retail price. The tea plant, originally from Southeast China was gradually introduced into many tropical and subtropical countries. Since the last decade of the 19th century, tea is also produced in one unique place in Europe, the volcanic São Miguel Island of the Azores Archipelago (Portugal) (Fig. 1) that is characterized by an oceanic climate, with mild temperatures all year around (Baptista et al., 2012).
Fig. 1.
Azorean tea plantation.
In recent years, C. sinensis tea, particularly green tea, has received considerable attention in Western countries due to its several health benefits, including antioxidant, antimutagenic, anticarcinogenic, cardioprotective, antimicrobial, antidiabetic, and neuroprotective effects on cognitive function which is particularly relevant to senior adults (Bolling et al., 2009; Chacko et al., 2010; Khan & Mukhtar, 2013; Pastoriza et al., 2017; Rubab et al., 2020). Tea polyphenols (TPs) represent about 30% of the leaf dry weight (DW) and exist principally as catechins. These powerful natural antioxidants (particularly epigallocatechin-3-gallate and epicatechin-3-gallate) are the main contributors to the health-promoting effects of green tea (Chacko et al., 2010; Khan & Mukhtar, 2018). Tea plants are also rich in amino acids (AAs) that are the main contributors to its taste and, together with TPs, caffeine (CAF) and aromatic compounds, are considered fundamental to tea's nutraceutical and quality effects (Yu & Yang, 2019). The predominant AA in C. sinensis is L-theanine (5-N-ethylglutamine; THEA), a unique non-protein AA that constitutes between 1.0 and 3.0% of leaf DW, accounting for up to 50% of all free AAs (FAAs) (Baptista et al., 2012). Currently, there is great interest in THEA due to its health benefits. These concern mainly nervous effects, which include its calming effects by stimulating the release of dopamine (a brain's neurotransmitter responsible for confidence and sense of well-being), stimulating the production of alfa brain waves creating a state of deep relaxation, improving immune function, promoting neuroprotection, and enhancing anti-tumor activity. Furthermore, THEA, in a specific ratio to CAF, promotes a positive effect on cognitive performance and mental alertness (Baptista et al., 2012; Türközü; Şanlier, 2017; Williams et al., 2016). On the other hand, THEA is a major tea flavour compound contributing to the “umami” taste of green tea infusions by counteracting the astringency and/or bitterness associated with catechins, particularly the gallate type, and CAF (Yu & Yang, 2019).
It is well established that tea chemical composition, and therefore its quality and price, can be significantly affected by the region of tea production and plant variety, as well as by pre-harvest agronomics and post-harvest manufacturing processes (Too et al., 2015; Tounekti et al., 2013). Furthermore, bioactive metabolites have different distributions in different tea plant tissues. Previous studies by the authors on Azorean C. sinensis revealed the potential use of the flowers (Paiva et al., 2019) as well as off-season fresh leaves (Baptista et al., 2014) as valuable sources of catechins and THEA.
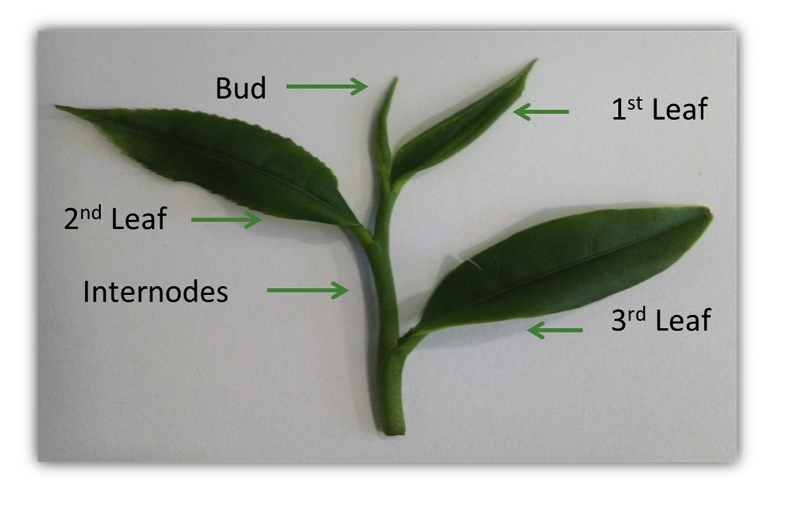
Tea manufacturers usually remove stems (the internode) during tea processing as their size can lead to a non-uniform and unpleasant appearance (Zeng et al., 2017). Knowing the strong impact of TPs, FAAs, and CAF on human health and tea taste, one major question remains to be answered: What is the potential contribution of the internodes to the quality of commercial Azorean green tea? To answer this question, we performed a comparative study on the antioxidant activities (scavenging effects, reducing ability, and chelating capacity) and total phenolics, total flavonoids, catechins, FAAs (including THEA), and CAF contents in different parts of Azorean C. sinensis (bud, 1st leaf, 2nd leaf, and internodes) and in combinations of these plant parts (Fig. 2). The results will aim to find the best combination of tea plant parts to maximize its flavour and impact on human health.
Fig. 2.
Different tea leaves and internodes.
2. Materials and methods
2.1. Chemicals and reagents
THEA (lot 606,121) was generously donated by Taiyo Kagaku International Inc. (Boise, ID). AA standard mixture, catechins, namely, catechin (C), epicatechin (EC), gallocatechin (GC), epigallocatechin (EGC), gallocatechin-3-gallate (GCG), epigallocatechin-3-gallate (EGCG) and epicatechin-3-gallate (ECG), CAF, gallic acid, rutin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), butylated hydroxytoluene (BHT), ethylenediaminetetraacetic disodium salt (EDTA), Folin–Ciocalteu reagent, potassium ferricyanide, iron (II) chloride, iron (III) chloride, aluminum chloride, ferrozine and o-phthaldialdehyde (OPA) were obtained from Sigma–Aldrich (St. Louis, MO, USA). β-mercaptoethanol, potassium monobasic phosphate, sodium acetate, sodium phosphate (Na2HPO4) and orthophosphoric acid were purchased from E. Merck (Darmstad, Hessen, Germany). Acetonitrile, tetrahydrofuran (THF), methanol, chloroform and ethyl acetate, HPLC-grade, were obtained from Riedel-de Häen (Aktiengesellschaft, Seelze, Germany) and other chemicals and solvents were analytical grade. Deionised water was obtained from an in-house Milli-Q water purification system (Millipore, Bedford, MA, USA).
2.2. Sample collection and water extract preparation for antioxidant assays
Samples of different parts of Azorean C. sinensis var. sinensis (internodes, bud, and 1st, 2nd and 3rd leaves) were provided by Gorreana Tea Plantation (São Miguel Island, Azores, Portugal). The freshly plucked samples were indoor-withered at 25–30 °C to achieve a relative humidity of 70%. The aqueous extracts of C. sinensis samples (different parts and combinations of plant parts) were prepared using 1 g of dried material in 20 mL of distilled water using a water bath for 30 min at 70 °C to avoid loss of their antioxidant properties at temperatures higher than 70 °C. The extraction process was repeated three times and the combined extract was filtered through a paper filter, then through 0.45 μm porosity cellulose acetate membranes, and then dried on a rotary evaporator and lyophilized.
2.3. Antioxidant activity assays on water extracts
2.3.1. DPPH free radical scavenging activity (FRSA) determination
The free radical scavenging activity (FRSA) of C. sinensis samples on DPPH radicals was determined according to the method of Molyneux (2004) with some modifications (Paiva et al., 2019). Butylated hydroxytoluene (BHT) was used as the reference sample. The results were expressed as the EC50 value (μg/mL), which is defined as the sample concentration that can quench 50% of the DPPH free radicals. A lower EC50 value means a higher antioxidant activity.
2.3.2. Ferric reducing antioxidant power (FRAP) determination
The ferric reducing antioxidant power (FRAP) of C. sinensis samples was determined according to the method of Oyaizu (1986) with some modifications (Paiva et al., 2019), and evaluated on the basis of their ability to reduce the Fe3+ complex to Fe2+. BHT was used for comparison. The results were expressed as the EC50 value (μg/mL). The extract concentration providing 0.5 of absorbance (EC50) was calculated from the graph of absorbance at 700 nm against extract concentration. A lower EC50 value means a higher antioxidant activity.
2.3.3. Ferrous ion-chelating (FIC) activity determination
The chelating ability of C. sinensis samples was determined according to the modified method of Wang et al. (2009) with some modifications (Paiva et al., 2019). The chelating ability is the percentage inhibition of the Fe2+– ferrozine complex formation. EDTA was used as the reference standard.
2.4. Total phenolic content (TPC) determination in water extracts
Total phenolic content (TPC) was determined using Folin–Ciocalteu colorimetric methodology as described by Waterhouse (2001) with some modifications (Paiva et al., 2019). Gallic acid was used as the standard and the results were expressed as mg of gallic acid equivalents per gram of dried extract (mg GAE/g DE).
2.5. Total flavonoid content (TFC) determination in water extracts
Total flavonoid content (TFC) was determined by following the colorimetric method of Chang et al. (2002) with some modifications (Paiva et al., 2019). TFC was expressed as mg of rutin equivalents per gram of dried extract (mg RE/g DE).
2.6. Extraction of crude catechins, CAF and FAAs (including THEA)
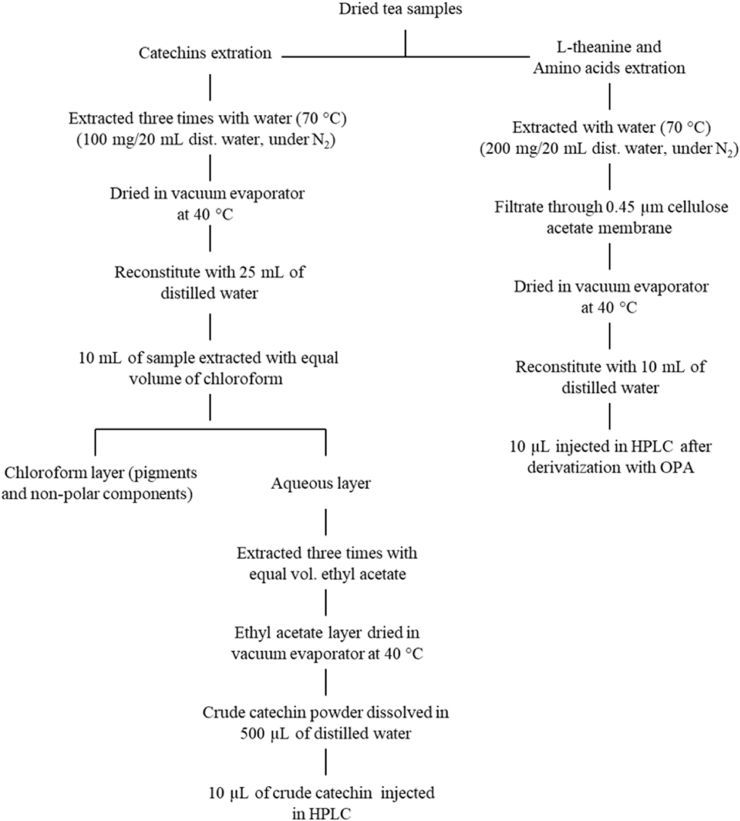
The protocol for crude catechins and caffeine (CAF) extraction from the samples under study was a slightly modified methodology described by Baptista et al. (2014). The extraction of FAAs, including THEA, was performed according to the protocol of Baptista et al. (2012) with some modifications. The dried ground samples were steeped in distilled water under nitrogen (to prevent oxidation by atmospheric oxygen) using a thermal flask following the steps in Fig. 3. For THEA determination, a stock solution was prepared at a final concentration of 40 μg/mL.
Fig. 3.
Extraction of crude catechins, caffeine, and free amino acids (including L-theanine) from Azorean Camellia sinensis samples (different plant parts or combinations of plant parts).
2.7. RP-HPLC analysis of catechins and CAF
2.7.1. HPLC analysis conditions
The HPLC system consisted of an Agilent Technologies (Palo Alto, CA, USA) series 1200 Liquid Chromatograph equipped with a diode array detector (DAD) fixed at 280 nm. Before HPLC analysis, samples were filtered through a polytetrafuoroethylene (PTFE) membrane cartridge. The analysis was carried out on an Ultropac Spherisorb ODS2 column (100 × 4.6 mm i. d.) from LKB (Bromma, Sweden) maintained at 35 °C. Baseline separation was achieved with binary elution as follows: 100% A (acetonitrile:ethyl acetate:0.1% orthophophoric acid:water, 4.25:1:44.75:50, v/v/v/v) for 10 min, followed by a linear gradient between phase A and phase B (acetonitrile:water, 1:1, v/v), at an increasing rate of 2% per min of phase B and held at this composition until the end of the run, at a flow rate of 0.8 mL/min. The chromatograms were recorded according to the retention time (RT), and peak identity was performed by RT based on comparison with the authentic standards and/or by spiking the sample with the same standards. Individual catechins were also confirmed by the absorbance spectra (DAD) and by superimposing the spectrum of the peak with the corresponding standard spectrum. The average of three measurements was used to calculate the tea catechins content and results were expressed as a percentage by mass of the total catechins on a DW basis. The CAF content was expressed in mg/g DW.
2.7.2. HPLC method validation
The catechins and CAF concentrations were expressed as the mean of three independent replicates and the relative standard deviation (RSD). The multiple-level calibration curves at five different concentrations were constructed at the peak-area vs concentration. Linear regression analysis provided the equations with y as the peak area and x as the concentration of tea component in mg/g of sample DW. Re-calibration was performed regularly. The detection limit (LOD) and quantification limit (LOQ) were defined as the amount of injected sample that gave a signal to noise ratio of 3 and 10, respectively. The accuracy of the methodology was evaluated by determining the recovery of CAF (medium RT) and ECG (longer RT) in a sample with known levels of 22.65 μg and 34.73 μg, respectively, per 10 μL of injection volume. Three different amounts of standards were added to each sample which was subjected to HPLC chromatography. Recovery was calculated based on the difference between the total amount determined in the spiked sample and the amount observed in non-spiked samples. All analyses were carried out in triplicate.
2.8. RP-HPLC analysis of FAAs (including THEA) after sample derivatisation
The determination of FAAs (protein-FAAs and THEA) in the study samples was performed by HPLC analysis after derivatisation with OPA as previously described in Baptista et al. (2012). The HPLC system consisted of an Agilent Technologies (Palo Alto, CA, USA) series 1200 Liquid Chromatograph with a DAD fixed at 338 nm. Before HPLC analysis, the samples were filtered through a PTFE membrane cartridge. A RP-HPLC column Spherisorb ODS-2 – 5 μm (150 × 4.6 mm i. d.) from Varian Inc. (Palo Alto, CA, USA) was used with the following eluent system: phase (A) 50 mM sodium acetate plus 50 mM Na2HPO4 (pH 7.5):methanol:THF (96:2:2, v/v/v) and phase (B) methanol:water (70:30, v/v), using the following linear gradient elution: t = 0 min–25% B, t = 10 min–25% B, t = 30 min–40% B and t = 40 min–100% B, with a flow rate of 0.7 mL/min at a column temperature of 35 °C. The chromatograms were recorded according to RT, and AAs were identified by RT based on comparison with the authentic standard and/or by spiking the sample with standard. The average of three measurements was used to calculate FAA content and results were expressed in mg of AA per g of DW sample.
2.9. Statistical analysis
All determinations were performed in triplicate and results were expressed as means ± standard deviations (SD). One-way analysis of variance (ANOVA) was carried out to assess for any significant differences between the means. Differences between means at the 95% confidence level (p < 0.05) were considered statistically significant.
3. Results and discussion
3.1. Antioxidant activities, total phenolic content (TPC), and total flavonoid content (TFC) of water extract samples
The antioxidant properties of natural compounds are very important due to their applications in medicine, food and cosmetics. The studied samples were evaluated by three different tests as reported in Table 1. The values were also compared to the synthetic antioxidants BHT and EDTA under the same assay conditions, which is a good approach to compare results to available literature data.
Table 1.
Free radical scavenging activity (FRSA), ferric reducing antioxidant power (FRAP), ferrous ion-chelating (FIC) activity, total phenolics content (TPC) and total flavonoids content (TFC) in water dry extracts (DE) of Azorean Camellia sinensis samples (different plant parts and combinations of plant parts).a
| C. sinensis samples and control | FRSA (EC50b, μg/mL) | FRAP (EC50c, μg/mL) | FIC (%) | TPC (mg GAE/g DE) | TFC (mg RE/g DE) |
|---|---|---|---|---|---|
| B | 6.10 ± 0.33 | 5.70 ± 0.10 | 74.88 ± 1.53 | 253.23 ± 3.08 | 29.52 ± 0.92 |
| 1st L | 10.50 ± 0.70 | 9.40 ± 0.15 | 55.94 ± 2.33 | 254.25 ± 3.33 | 53.61 ± 0.47 |
| 2nd L | 10.70 ± 0.64 | 8.50 ± 0.09 | 49.34 ± 0.59 | 265.48 ± 2.06 | 72.02 ± 1.33 |
| I | 13.50 ± 0.57 | 11.40 ± 0.10 | 36.96 ± 3.23 | 201.51 ± 3.36 | 23.84 ± 1.25 |
| B+1st+2nd L | 9.00 ± 0.89 | 9.60 ± 0.08 | 78.35 ± 1.88 | 243.44 ± 4.14 | 53.76 ± 1.99 |
| B+1st+2nd L + I | 8.60 ± 0.35 | 9.40 ± 0.30 | 79.21 ± 1.33 | 244.57 ± 2.32 | 54.06 ± 0.27 |
| B+1st+2nd+3rd L + I | 11.50 ± 0.37 | 10.00 ± 0.43 | 68.75 ± 0.41 | 220.22 ± 2.10 | 46.79 ± 1.73 |
| BHT | 20.80 ± 0.60 | 5.60 ± 0.09 | – | – | – |
| EDTA | – | – | 97.64 ± 0.58 | – | – |
Values are mean ± SD (n = 3). GAE, gallic acid equivalents. RE, rutin equivalents. B, bud. I, internodes. L, leaves. BHT, butylated hydroxytoluene. EDTA, ethylenediaminetetraacetic disodium salt.
Half-maximal effective concentration.
Effective concentration at which the absorbance is 0.5.
For the free radical scavenging assay (FRSA) assessment of tea extracts, the DPPH assay was chosen since it is considered to be one of the standard colorimetric methods for evaluating the antioxidant power of bioactive natural products. The range of FRSA EC50 values of the samples (6.10–13.50 μg/mL) was better than that of BHT (20.80 μg/mL), and antioxidant capacity of plant part mixtures decreased as follows: B > B+1st+2nd L + I ~ B+1st+2nd L > 1st L~2nd L > B+1st+2nd+3rd L + I > internodes. Chan, Lim, and Chew (2007) reported a FRSA EC50 value of 30 μg/mL for young leaves of C. sinensis var. assamica from Malaysia, and therefore, lower antioxidant capacity than those observed in our study. Concerning the reducing capacity of the samples, which may serve as a significant indicator of its potential antioxidant activity, the range of the ferric reducing antioxidant power (FRAP) EC50 values was narrower (5.70–11.40 μg/mL), and the antioxidant capacity decreased in the following order: B > 2nd L > 1st L = B+1st+2nd L + I ~ B+1st+2nd L ~ B+1st+2nd+3rd L + I > internodes. The results revealed that, in general, a similar order was observed in the FRAP and FRSA for the samples, being consistent with our previous research on the antioxidant activity of tea extracts (Baptista et al., 2014; Paiva et al., 2019). These results may be explained by the fact that both assays rely on an electron/hydrogen donation mechanism. It should also be highlighted that the FRSA of bud (5.70 μg/mL) was like that of BHT (5.60 μg/mL), which is known to be a strong reducing agent. Since metal chelating capacity is claimed as one of the important mechanisms of antioxidant activity (Wang et al., 2009), ferrous ion chelating (FIC) assay was chosen to better characterize the antioxidant activity of the samples. Similarly, as observed for FRSA and FRAP assays, the internodes showed the lowest FIC activity (36.96%). On the other hand, the most active plant parts sample was B+1st+2nd L + I (79.21%), followed by B+1st+2nd L (78.35%) and bud (74.88%). Furthermore, they had a significant FIC activity compared to that of EDTA (97.64%), a potent metal-ion chelator.
Table 1 summarizes the total phenolic content (TPC) and total flavonoid content (TFC) for each sample. The TPC levels of the samples can also be considered an indirect measure of their antioxidant activity because the basic redox mechanism of the Folin–Ciocalteu method was chosen to screen phenolic content. The highest TPC was found in 2nd leaf (265.48 mg GAE/g DE) and the lowest in internodes (201.51 mg GAE/g DE). The other samples presented very similar and significant TPC values that ranged from 220.22 to 254.25 mg GAE/g DE. Our results were higher than those reported by Rana et al. (2016) for acetone:water (70:30, v/v) extracts of tea plant material from the experimental tea farm CSIR-IHBT, Palampur. However, both results showed good correlation since the authors also reported higher TPC levels in bud, 1st leaf, and 2nd leaf (74.93, 102.24 and 91.02 mg GAE/g DE, respectively) in comparison to stem (internode) extract (36.63 mg GAE/g DE).
Many in vitro and in vivo studies have shown that green tea flavonoids have strong antioxidant and metal chelating properties and may therefore protect cells and tissues against free radicals (Chacko et al., 2010; Khan & Mukhtar, 2018). Similarly, as observed for TPC, the 2nd leaf presented the highest TFC value (72.02 mg RE/g DE) and the internodes had the lowest value (23.84 mg RE/g DE). The bud also presented a low TFC value (29.52 mg RE/g DE), that can be explained by the lower TFC content as compared to the 2nd leaf. The other four samples presented very similar but significant TFC values that ranged between 46.79 mg RE/g DE (B+1st+2nd+3rd L + I) and 54.06 mg RE/g DE (B+1st+2nd L + I).
Overall, the results indicate that the bud and the 1st and 2nd leaves present significantly better antioxidant activities and higher TPC values compared to the internodes, which may be due to the superior concentration of polyphenols (catechins) in these tea plant parts. Similar results were found by Rusaczonek et al. (2007) who reported that the antioxidant capacity and TPC of several herbal tea leaves were higher than that of stems and leafstalks. Furthermore, the results also indicate that the combination B+1st+2nd L + I presented slightly better values than B+1st+2nd L in all the parameters evaluated, revealing the potential contribution of the internodes to green tea quality.
3.2. Catechin profiles and caffeine (CAF) content
In several studies, the antioxidant activity of tea samples has only been correlated with TPC. However, it is well known that antioxidant activity not only depends on the levels of antioxidants, but also on their chemical structure, as well as the synergetic effects among active compounds that share a close natural botanical association, such as for example, green tea polyphenols and caffeine (CAF) (Jagdeo & Brody, 2011). The variability of catechin profiles and CAF content in the samples was determined by a gradient reverse-phase high Performance Liquid Chromatography-Diode Array (RP-HPLC/DAD) procedure that provides a fast, precise, and accurate method for the simultaneous separation of seven major tea catechins and CAF. The results of individual catechin contents, as a percentage of total catechins, and CAF are summarized in Table 2. In all samples, epicatechin-3-gallate (ECG) was the major catechin, ranging between 34.82% (internodes) and 60.87% (B+1st+2nd leaves) followed by epigallocatechin-3-gallate (EGCG), except for bud and internodes that had gallocatechin (GC) as the second major catechin. The catechins that presented lower values in all samples were catechin (C), epigallocatechun (EGC) and gallocatechin-3-gallate (GCG) (0.24–1.68%, 0.67–1.88%, and 0.67–4.81%, respectively). On the other hand, internodes presented the highest CAF content and 2nd leaf the lowest content (51.50 and 22.89 mg/g of DW, respectively). Rana et al. (2016) reported higher total catechins content in 2nd leaf than in buds and the lowest value in stems. Lin, Tsai, Tsay, and Lin (2003), who analyzed several fresh tea leaf samples from the Tea Experimental Station in Wen-Shan or Taitung (Taiwan), observed that old leaves contained less CAF but more EGCG than young ones, showing a good agreement with the results in the present study. As also shown in Table 2, the content of various catechin groups as a percentage of the total catechins, was calculated, including esterified catechins (sum of EGCG, ECG and GCG), non-esterified catechins (sum of C, EC, EGC and GC), epicatechin derivatives (ECDs), or cis-catechins (sum of EC, EGC, EGCG and ECG) and trans-catechins (sum of C, GC and GCG). The cis/trans catechins ratio was also reported. In all samples, the individual esterified catechins decreased as follows: ECG > EGCG ≫ GCG. The 2nd leaf showed the highest esterified catechins value (85.69%), followed by the B+1st+2nd L (85.29%), and the internodes had the lowest value (55.35%). Relative to ECDs (cis-catechins), the values ranged from 63.91 to 91.22% and the trans-catechins group ranged from 8.77 to 36.10% of the total catechins. As a result, the cis/trans catechins ratio ranged from 1.77 to 10.40, decreasing in the following order: B+1st+2nd L > 2nd L > B+1st+2nd+3rd L + I > B+1st+2nd L + I > 1st L > internodes ~ bud. Similar findings were reported by Lee et al. (2014) who showed that total ECD contents, particularly the higher values of EGCG and ECG, were the key factor affecting the antioxidant activity of tea extracts. In fact, many studies report that the antioxidant potential of esterified catechins was stronger than that of non-esterified catechins. Lee et al. (2014) also reported that the antioxidant potential of EGC and GC, which have ortho-trihydroxyl groups in the B ring, are stronger than those of EC and C, which have ortho-dihydroxyl groups in the B ring.
Table 2.
Comparison of catechins (percentage of total catechins) and caffeine (mg/g) in different Azorean Camellia sinensis samples.a
| Catechins and CAF |
C. sinensis samples (different plant parts and combinations of plant parts) |
||||||
|---|---|---|---|---|---|---|---|
| B | 1st L | 2nd L | I | B+1st+2nd L | B+1st+2nd L + I | B+1st+2nd+3rd L + I | |
| Individual catechins and CAF contentsb | |||||||
| C | 0.24 ± 0.02 | 0.42 ± 0.03 | 0.78 ± 0.02 | 0.63 ± 0.02 | 1.10 ± 0.05 | 0.69 ± 0.02 | 1.68 ± 0.05 |
| EC | 1.92 ± 0.03 | 4.64 ± 0.10 | 7.18 ± 0.12 | 11.61 ± 0.15 | 6.65 ± 0.08 | 8.48 ± 0.14 | 10.14 ± 0.14 |
| EGC | 1.14 ± 0.08 | 1.82 ± 0.08 | 1.30 ± 0.07 | 1.21 ± 0.05 | 1.88 ± 0.06 | 0.67 ± 0.04 | 0.83 ± 0.05 |
| EGCG | 25.32 ± 0.15 | 22.72 ± 0.11 | 29.54 ± 0.23 | 16.61 ± 0.08 | 21.83 ± 0.14 | 23.56 ± 0.16 | 30.96 ± 0.15 |
| ECG | 35.53 ± 1.04 | 46.57 ± 1.12 | 52.42 ± 1.21 | 34.82 ± 0.34 | 60.87 ± 1.32 | 48.59 ± 1.17 | 42.40 ± 1.06 |
| GC | 35.19 ± 0.31 | 21.24 ± 0.20 | 5.05 ± 0.09 | 31.19 ± 0.17 | 5.08 ± 0.06 | 15.33 ± 0.15 | 9.17 ± 0.05 |
| GCG | 0.67 ± 0.04 | 2.60 ± 0.09 | 3.73 ± 0.11 | 3.92 ± 0.13 | 2.59 ± 0.09 | 2.67 ± 0.08 | 4.81 ± 0.11 |
| CAF | 29.01 ± 1.19 | 34.45 ± 1.31 | 22.89 ± 0.98 | 51.50 ± 2.37 | 37.75 ± 1.86 | 41.76 ± 2.03 | 36.41 ± 1.67 |
| Catechin groups contentb | |||||||
| Est. CAT | 61.52 | 71.89 | 85.69 | 55.35 | 85.29 | 74.82 | 78.17 |
| Non-est. CAT | 38.49 | 28.12 | 14.31 | 44.64 | 14.71 | 25.17 | 21.82 |
| cis-CAT (ECDs) | 63.91 | 75.75 | 90.44 | 64.25 | 91.22 | 81.30 | 84.33 |
| trans-CAT | 36.10 | 24.26 | 9.56 | 35.74 | 8.77 | 18.69 | 15.66 |
| cis/trans ratio | 1.77 | 3.12 | 9.46 | 1.80 | 10.40 | 4.35 | 5.39 |
Values are mean ± SD (n = 3). B, bud. I, internodes. L, leaves. C, (+)-catechin. EC, (−)-epicatechin. EGC, (−)-epigallocatechin. EGCG, (−)-epigallocatechin-3-gallate. ECG, (−)-epicatechin-3-gallate. GC, (+)-gallocatechin. GCG, (+)-gallocatechin-3-gallate. CAF, caffeine. CAT, catechins. Est. CAT (esterified catechins), sum of EGCG, ECG and GCG. Non-est. CAT (non-esterified catechins), sum of C, EC, EGC and GC. cis-CAT or ECDs (epicatechin derivatives), sum of EC, EGC, EGCG and ECG. trans-CAT, sum of C, GC and GCG.
Dry weight basis.
Overall, the results indicate that the studied C. sinensis samples presented a total ECD content above 60% of the total catechins, thus revealing its high antioxidant value. Furthermore, the results also indicate that, among the combinations of plant parts, B+1st+2nd+3rd L + I and B+1st+2nd L + I presented only a slightly lower content of total ECDs as compared to B+1st+2nd L, thus the presence of internodes in green tea had no significant impact on the levels of these powerful antioxidants. Recent studies suggest that the consumption of both CAF and catechin affected body weight management in humans (Hursel and Westerterp-Plantenga, 2013). Thus, catechin- and CAF-rich teas may be useful agents that can help in preventing a positive energy balance and obesity, although we should pay attention to the acceptable daily intake.
3.3. HPLC method validation
The repeatability of HPLC analysis was evaluated intraday using a known level of CAF and ECG, tea components that have medium and higher coefficients of diffusion in the HPLC column, respectively. The detection limit (LOD) ranged from 0.7 to 1.10 μmol/L and the quantification limit (LOQ) from 2.31 to 3.4 μmol/L for CAF (component with medium retention time (RT)) and ECG (component with longer RT), respectively, under the experimental conditions used. The results presented in Table 3 show that the relative standard deviation (RSD) for the intraday repeatability was 1.44% and 1.82% for CAF and ECG, respectively, whereas interday precision (data acquired over a period of 5 days) was better than 4.27% (data not shown), indicating a high degree of repeatability for the determination of tea catechin content under the analytical conditions used. The accuracy of the CAF and ECG determination was evaluated by determining the recovery of CAF and ECG in a sample with a known level of these components. Results with the RSD are shown in Table 4. The RSD was better than 3.25% for CAF and the mean recovery ranged from 96.0% to 97.7%, and better than 2.70% for ECG and the mean recovery ranged from 98.99% to 99.98% indicating a high degree of accuracy of the method.
Table 3.
Intraday precision data for retention time (RT), standard deviation (SD) and relative standard deviation (RSD) for CAF and ECG.a
| Analytes | Intraday precision (n = 9) |
||
|---|---|---|---|
| RT mean (min) | SD (min) | RSD (%) | |
| CAF | 16.40 | 0.318 | 1.44 |
| ECG | 33.70 | 0.614 | 1.82 |
Chromatography conditions referred in section 2.7. CAF, caffeine. ECG, (−)-epicatechin-3-gallate.
Table 4.
Recovery of CAF (medium RT) and ECG (longer RT) from studied samples (n = 3).a
| Analytes | Concentration (inj. vol. 10 μL) |
Recovery (%) | RSD (%) | ||
|---|---|---|---|---|---|
| Catechin (μg) | Spiked (μg) | Measured (μg) | |||
| CAF | 22.65 | 10 | 31.91 | 97.70 | 2.62 |
| 20 | 40.94 | 96.00 | 2.86 | ||
| 30 | 51.08 | 97.00 | 3.25 | ||
| ECG | 34.73 | 20 | 54.18 | 98.99 | 2.10 |
| 30 | 64.51 | 99.66 | 2.70 | ||
| 50 | 84.71 | 99.98 | 2.12 | ||
Chromatography conditions as in section 2.7. CAF, caffeine. ECG, (−)-epicatechin-3-gallate. RT, retention time.
3.4. Free amino acids (FAAs) and THEA contents
It is known that free amino acids (FAAs) in green tea are essential contributors to the overall quality in terms of freshness and mellowness. According to Yu and Yang (2019), THEA, Glu, Asp, Gln and Asn are “umami”-like taste compounds, while Gly, Ala, Thr, Ser, Met, Cys and Pro are sweet-tasting compounds, and the other nine common amino acids (AAs) are bitter-tasting compounds. Furthermore, several AAs, including THEA, Glu, Gln and Arg are conducive to the formation of volatile compounds in tea, contributing to its aromatic quality (Yu & Yang, 2019).
This study determines, for the first time, the FAA profile, including THEA, of Azorean C. sinensis samples using a RP-HPLC/DAD system after derivatisation with o-phthaldialdehyde (OPA) with the data are presented in Table 5 and Fig. 4. The total FAA contents ranged from 22.44 to 50.49 mg/g of dry weight (DW) sample, being THEA, His, Asn, Phe, Glu and Met the major AAs. THEA was present at higher levels in internodes (17 mg/g) followed by B+1st+2nd L + I (10.59 mg/g) and B+1st+2nd+3rd L + I (8.78 mg/g), and lower values in the other studied samples (2.30–2.74 mg/g). His content was also higher in internodes (13.73 mg/g) followed by B+1st+2nd+3rd L + I (10.75 mg/g) and B+1st+2nd L + I (10.18 mg/g), and lower in the other studied samples (2.68–3.98 mg/g). Asn content ranged from 3.01 to 7.70 mg/g, presenting higher level in bud followed by 1st leaf (6.40 mg/g), internodes (5 mg/g) and B+1st+2nd L + I (4.76 mg/g). Phe (1.43–4.13 mg/g) and Glu (2.10–3.13 mg/g) were also higher in internodes followed by B+1st+2nd L + I that presented values of 3.39 mg/g and 2.50 mg/g, respectively. Concerning Met content (1.55–2.93 mg/g), higher level was found in bud followed by 1st leaf (2.68 mg/g) and B+1st+2nd L + I (2.50 mg/g). Horanni and Engelhardt (2013) analyzed the FAA profile of 23 commercial green tea samples and found an average value of 20.15 mg/g of DW for total FAA content, and 9.75 mg/g for THEA, which was similar or lower than the results in our study.
Table 5.
Free amino acid profiles in different Azorean Camellia sinensis samples (mg amino acid/g of dry weight sample).a
| Amino acids (AA) |
C. sinensis samples (different plant parts and combinations of plant parts) |
||||||
|---|---|---|---|---|---|---|---|
| B | 1st L | 2nd L | I | B+1st+2nd L | B+1st+2nd L + I | B+1st+2nd+3rd L + I | |
| Asp | 0.98 ± 0.02 | 0.88 ± 0.05 | 1.78 ± 0.05 | 0.55 ± 0.08 | 1.63 ± 0.04 | 0.78 ± 0.02 | 0.78 ± 0.04 |
| Glu | 2.10 ± 0.15 | 2.40 ± 0.07 | 2.45 ± 0.05 | 3.13 ± 0.10 | 2.28 ± 0.07 | 2.50 ± 0.03 | 2.20 ± 0.02 |
| Asn | 7.70 ± 0.23 | 6.40 ± 0.10 | 4.06 ± 0.13 | 5.00 ± 0.010 | 3.56 ± 0.10 | 4.76 ± 0.11 | 3.01 ± 0.04 |
| Ser | 1.88 ± 0.11 | 2.50 ± 0.02 | 1.88 ± 0.09 | 1.20 ± 0.04 | 1.73 ± 0.03 | 2.01 ± 0.08 | 1.28 ± 0.02 |
| His | 2.68 ± 0.12 | 3.48 ± 0.14 | 3.98 ± 0.09 | 13.73 ± 0.06 | 3.93 ± 0.08 | 10.18 ± 0.11 | 10.75 ± 0.08 |
| Gly | tr | 0.38 ± 0.03 | 0.45 ± 0.07 | 1.28 ± 0.02 | 0.28 ± 0.02 | 0.21 ± 0.02 | 0.93 ± 0.05 |
| Thr | 0.73 ± 0.08 | 0.80 ± 0.04 | 0.65 ± 0.09 | 1.28 ± 0.01 | 0.60 ± 0.07 | 1.07 ± 0.05 | 1.73 ± 0.03 |
| Arg | 0.95 ± 0.06 | 1.23 ± 0.09 | tr | tr | 0.75 ± 0.09 | 1.68 ± 0.03 | tr |
| Thea | 2.35 ± 0.07 | 2.30 ± 0.07 | 2.73 ± 0.06 | 17.00 ± 0.07 | 2.74 ± 0.02 | 10.59 ± 0.09 | 8.78 ± 0.04 |
| Ala | tr | 0.58 ± 0.08 | 0.60 ± 0.07 | tr | tr | 0.25 ± 0.01 | 0.45 ± 0.02 |
| Tyr | 0.45 ± 0.06 | 0.53 ± 0.08 | 0.55 ± 0.07 | tr | 0.50 ± 0.03 | 1.20 ± 0.04 | 0.83 ± 0.02 |
| Met | 2.93 ± 0.01 | 2.68 ± 0.08 | 1.75 ± 0.09 | 1.58 ± 0.04 | 1.65 ± 0.07 | 2.50 ± 0.03 | 1.55 ± 0.05 |
| Trp | nd | nd | 2.30 ± 0.03 | nd | nd | nd | nd |
| Phe | 2.50 ± 0.08 | 2.33 ± 0.02 | 1.60 ± 0.04 | 4.13 ± 0.07 | 1.43 ± 0.05 | 3.39 ± 0.09 | 1.95 ± 0.06 |
| Ile | 2.30 ± 0.08 | 1.90 ± 0.03 | 1.15 ± 0.04 | 0.83 ± 0.02 | 1.18 ± 0.02 | 1.27 ± 0.07 | 1.00 ± 0.07 |
| Leu | 1.95 ± 0.06 | 1.93 ± 0.02 | 1.17 ± 0.02 | 0.78 ± 0.03 | 1.18 ± 0.04 | 1.39 ± 0.07 | 1.03 ± 0.06 |
| Total AA | 29.50 | 30.32 | 27.10 | 50.49 | 22.44 | 43.78 | 36.27 |
Values are mean ± SD (n = 3). B, bud. I, internodes. L, leaves. Thea, theanine. nd, not detected. tr, traces.
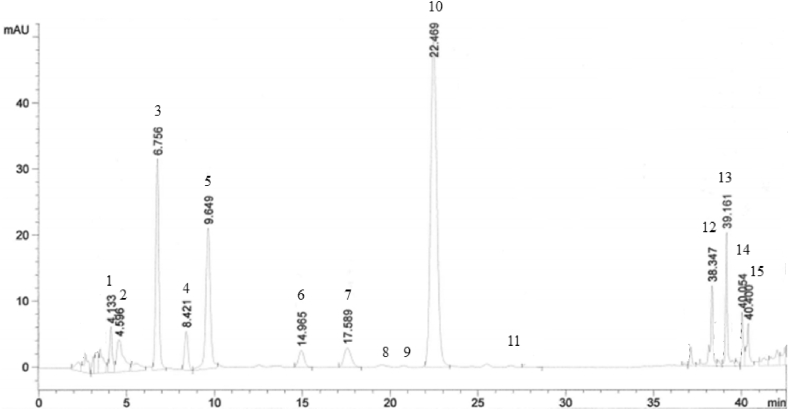
Fig. 4.
Amino acids determination by RP-HPLC/DAD from internodes of Azorean Camellia sinensis sample. Legend: 1, Aspartic acid (Asp). 2, Glutamic acid (Glu). 3, Asparagine (Asn). 4, Serine (Ser). 5, Histidine (His). 6, Glycine (Gly). 7, Threonine (Thr). 8, Arginine (Arg). 9, Alanine (Ala). 10, theanine (Thea). 11, Tyrosine (Tyr). 12, Methionine (Met). 13, Phenylalanine (Phe). 14, Isoleucine (Ile). 15, Leucine (Leu).
Overall, results indicate that internodes had the highest amounts of THEA, His, Phe and Glu, and are also a good source of Asn. A similar trend was found in B+1st+2nd L + I that, in addition, showed a relatively high Met content, thus revealing the potential contribution of internodes to green tea valorization since it is known that high amounts of FAAs in green tea is essential to good quality. Namely, the presence in internodes of Glu, Asn and, particularly, its high THEA content can provide a strong “umami” flavour that confers the special taste of green tea. In fact, THEA generates a pronounced caramel aroma, and Glu and Phe generate similar floral aromas (Yu & Yang, 2019). Concerning the contribution of internode AAs to the health benefit functions of tea, THEA has many biological effects beneficial to human health, particularly nervous system-related effects, and Glu is a major neurotransmitter in the mammalian brain. Phe improves memory, eliminates depression, reduces hunger, and promotes the synthesis of thyroxine and adrenaline. Asn reduces blood pressure, anti-peptic ulcer and relieves gastric dysfunction (Yu & Yang, 2019).
4. Conclusions
Limited published data is available on metabolite composition and biological activities of tea internodes. To the best of our knowledge, this is the first study that reports the variability of major functional metabolites and antioxidant properties in different parts of Azorean C. sinensis and its plant part combinations.
All samples presented high TPC values, and the total ECD content was above 60% of the total catechins, consistent with their high antioxidant activities. All samples were also good sources of FAAs, particularly, THEA, histidine, asparagine, phenylalanine, glutamic acid, and methionine, mainly in the internodes followed by the combination of bud +1st leaf +2nd leaf + internodes. These results may reflect the influence of relevant factors (e.g. genetic strain, geographic origin, climate conditions, and volcanic soil) on the chemical composition of the Azorean tea plant and consequently, its tea quality.
The combination of bud +1st + 2nd leaves + internodes presented slightly better FRSA, FRAP, and FIC activity, as well as TPC, TFC, and EGCG content. In addition, there was significantly higher amounts of total FAA (including THEA) as compared to samples without internodes. These results revealed the potential contribution of internodes to green tea quality and subsequently, human health. Furthermore, a large amount of time and expense is required to manually remove the internodes. Therefore, the presence of internodes in green tea may be economically beneficial.
Funding
This study was supported by PO Açores 2020 and by the Community Structural Funds FEDER and FSE for the 2014–2020 programming period, with implementation in the Azores Region, under the project “ACORES-01-0247-FEDER-000014”.
Declaration of competing interest
The authors declare no conflict of interest.
Abbreviations
- AAs
Amino acids
- BHT
Butylated hydroxytoluene
- DW
Dry weight
- CAF
Caffeine
- C
Catechin
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- EDTA
Ethylenediaminetetraacetic acid
- EC
Epicatechin
- EGC
Epigallocatechin
- EGCG
Epigallocatechin-3-gallate
- ECG
epicatechin-3-gallate
- ECD
epicatechin derivatives
- FAA
Free amino acids
- FRSA
Free radical-scavenging activity
- FRAP
Ferric-reducing antioxidante power
- FIC
Ferrous ion-chelating
- GC
Gallocatechin
- GCG
Gallocatechin-3-gallate
- TPs
Tea polyphenols
- THEA
L-theanine
- TFC
Total flanonoids content
- TPC
Total phenolic content
References
- Baptista J., Lima E., Paiva L., Andrade A.L., Alves M.G. Comparison of Azorean tea theanine to teas from other origins by HPLC/DAD/FD. Effect of fermentation, drying temperature, drying time and shoot maturity. Food Chem. 2012;132:2181–2187. [Google Scholar]
- Baptista J., Lima E., Paiva L., Castro A.R. Value of off-season fresh Camellia sinensis leaves. Antiradical activity, total phenolics content and catechin profiles. LWT – Food Science and Technology. 2014;59:1152–1158. [Google Scholar]
- Bolling B.W., Chen C.-Y., Blumberg J.B. Tea and health: preventive and therapeutic usefulness in the elderly? Curr. Opin. Clin. Nutr. Metab. Care. 2009;12:42–48. doi: 10.1097/MCO.0b013e32831b9c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko S.M., Thambi P.T., Kuttan R., Nishigaki I. Beneficial effects of green tea: a literature review. Chin. Med. 2010;5:13–14. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E.W.C., Lim Y.Y., Chew Y.L. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007;102:1214–1222. [Google Scholar]
- Chang C.-C., Yang M.-H., Wen H.-M., Chern J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Hursel R., Westerterp-Plantenga M.S. Catechin- and caffeine-rich teas for control of body weight in humans. Am. J. Clin. Nutr. 2013;98:1682S–1693S. doi: 10.3945/ajcn.113.058396. [DOI] [PubMed] [Google Scholar]
- Horanni R., Engelhardt U.H. Determination of amino acids in white, green, black, oolong, pu-erh teas and tea products. J. Food Compos. Anal. 2013;31:94–100. [Google Scholar]
- Jagdeo J., Brody N. Complementary antioxidant function of caffeine and green tea polyphenols in normal human skin fibroblasts. J. Drugs Dermatol. JDD. 2011;10:753–761. [PubMed] [Google Scholar]
- Khan N., Mukhtar H. Tea and health: studies in humans. Curr. Pharmaceut. Des. 2013;19:6141–6147. doi: 10.2174/1381612811319340008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N., Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2018;11:39. doi: 10.3390/nu11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.-S., Kim S.-H., Kim Y.-B., Kim Y.-C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules. 2014;19:9173–9186. doi: 10.3390/molecules19079173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.S., Tsai Y.J., Tsay J.S., Lin J.K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003;51:1864–1873. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004;26:211–219. [Google Scholar]
- Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese Journal of Nutrition and Dietetics. 1986;44:307–315. [Google Scholar]
- Paiva L., Lima E., Motta M., Baptista J. The surplus value of Azorean Camellia sinensis flowers as an important contributor affecting the nutraceutical benefits of green tea quality. Pharmacy & Pharmacology International Journal. 2019;7:327–332. [Google Scholar]
- Pastoriza S., Mesías M., Cabrera C.J., Rufián-Henares A. Healthy properties of green and white teas: an update. Food & Function. 2017;8:2650–2662. doi: 10.1039/c7fo00611j. [DOI] [PubMed] [Google Scholar]
- Rana A., Sharma E., Rawat K., Sharma R., Verma S., Padward Y., Gulati A. Screening and purification of catechins from underutilized tea plant parts and their bioactivity studies. J. Food Sci. Technol. 2016;53:4023–4032. doi: 10.1007/s13197-016-2406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubab S., Rizwani G.H., Bahadur S., Shah M., Alsamadany H., Alzahrani Y., Alghamdi S.A., Anwar Y., Shuaib M., Shah A.A., Muhammad I., Zaman W. Neuropharmacological potential of various morphological parts of Camellia sinensis L. Saudi J. Biol. Sci. 2020;27:567–573. doi: 10.1016/j.sjbs.2019.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusaczonek A., Żebrowska M., Waszkiewicz-Robak B., Ślusarczyk E. Evaluation of phenolic compounds content and antioxidant capacity of herbs. Pol. J. Food Nutr. Sci. 2007;57:483–488. [Google Scholar]
- Too J.C., Kinyanjui T., Wanyoko J.K., Wachira F.N. Effect of sunlight exposure and different withering durations on theanine levels in tea (Camellia sinensis) Food Nutr. Sci. 2015;6:1014–1021. [Google Scholar]
- Tounekti T., Joubert E., Hernández I., Munné-Bosch S. Improving the polyphenol content of tea. Crit. Rev. Plant Sci. 2013;32:192–215. [Google Scholar]
- Türközü D., Şanlier N. L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit. Rev. Food Sci. Nutr. 2017;57:1681–1687. doi: 10.1080/10408398.2015.1016141. [DOI] [PubMed] [Google Scholar]
- Wang T., Jónsdóttir R., Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009;116:240–248. [Google Scholar]
- Waterhouse A.L. Current Protocols in Food Analytical Chemistry (Units I.1.1.1–I1.1.8) John Wiley & Sons; New York: 2001. Determination of total phenolics. R E. [Google Scholar]
- Williams J., Kellett J., Roach P.D., McKune A., Mellor D., Thomas J., Naumovski N. L-theanine as a functional food additive: its role in disease prevention and health promotion. Beverages. 2016;2:13. [Google Scholar]
- Yu Z., Yang Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Crit. Rev. Food Sci. Nutr. 2019:1–15. doi: 10.1080/10408398.2018.1552245. [DOI] [PubMed] [Google Scholar]
- Zeng L., Zhou Y., Fu X., Mei X., Cheng S., Gui J., Dong F., Tang J., Ma S., Yang Z. Does oolong tea (Camellia sinensis) made from a combination of leaf and stem smell more aromatic than leaf-only tea? Contribution of the stem to oolong tea aroma. Food Chem. 2017;237:488–498. doi: 10.1016/j.foodchem.2017.05.137. [DOI] [PubMed] [Google Scholar]