Abstract
Renal insufficiency is common among patients with various types of malignant tumors. However, the occurrence of anti-glomerular basement membrane (GBM) nephritis in a patient with a malignant tumor is relatively rare. Here, we describe a patient with bronchial carcinoma who exhibited acute kidney injury, hematuria, and non-nephrotic-range proteinuria. The patient had positive serum anti-GBM antibody findings and biopsy-proven anti-GBM nephritis. This is a rare instance of anti-GBM nephritis in a patient with a malignant solid tumor. Neoplasia was presumed to contribute to the development of anti-GBM nephritis through secretion of tumor-related antigens or unusual exposure to GBM.
Keywords: Acute kidney injury, anti-glomerular basement membrane (anti-GBM) nephritis, bronchial carcinoma, cyclophosphamide, methylprednisolone, plasmapheresis
Introduction
Anti-glomerular basement membrane (anti-GBM) nephritis is characterized by focal necrotizing glomerulonephritis with crescents and linear deposition of immunoglobulin G (IgG) and C3 along the GBM.1 Patients develop autoantibodies against the noncollagenous domain 1 of the α3-chain of type IV collagen, which is expressed in specialized basement membranes (e.g., GBM).2 Notably, anti-GBM nephritis has a low incidence of <1 per million population per year in European countries; however, its incidence in China is unclear because of the rarity of the disease.3 Despite this rarity, anti-GBM nephritis is an important disease because it is rapidly progressive in most patients. This report describes a patient with acute kidney injury and bronchial carcinoma who was diagnosed with comorbid anti-GBM nephritis.
Case report
Initial presentation
A 61-year-old Han Chinese man was admitted to the hospital for painless bilateral cervical lymph node enlargement. He denied any medical or family history of diabetes mellitus, hypertension, or renal disease. He had stopped heavy smoking (40 packs per year) 1 year prior to presentation. He denied cocaine inhalation, as well as exposure to other toxic substances. The results of left cervical lymph node biopsy were suggestive of squamous cell carcinoma. Moreover, thoracic computed tomography revealed a mass in the inferior lobe of the left lung. The patient was hospitalized for mild cough, persistent fever between 37.5°C and 38°C, and left thoracic pain. Physical examination revealed that his height was 176 cm, weight was 68 kg (body mass index, 21.95 kg/m2), blood pressure was 126/69 mmHg, heart rate was 87 bpm, and temperature was 37.2°C. This case report received ethical approval from Ruijin Hospital. Written informed consent was obtained from the patient and his next of kin for publication of this case report and any accompanying images.
Laboratory and histopathology findings
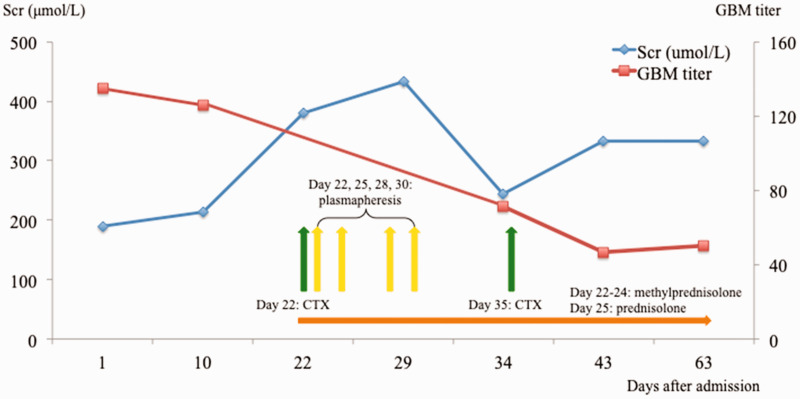
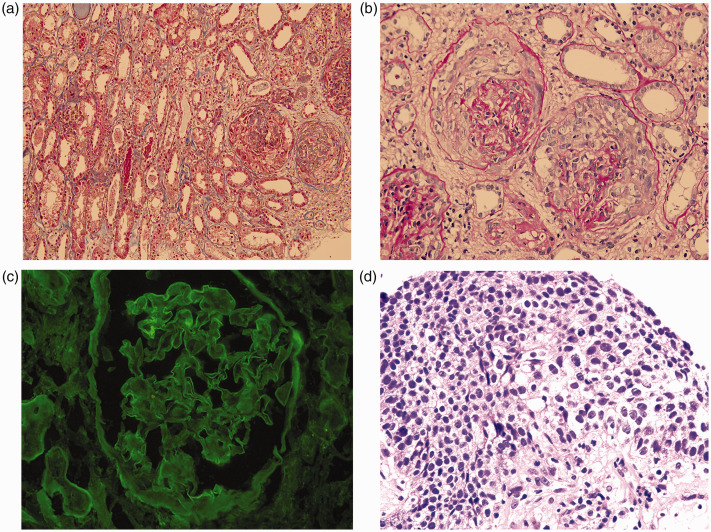
Laboratory examinations showed microscopic hematuria (26–30 red blood cells per high-power field) and proteinuria (1.2 g/24 hours). Complete blood count analysis showed that the patient’s hemoglobin was 86 g/L; no leukopenia or thrombocytopenia was present. The patient’s serum creatinine increased rapidly from 180 μmol/L to 433 μmol/L within 30 days (Figure 1); however, urological ultrasound findings and urine output volume remained normal. His anti-GBM antibody findings were positive, with a titer of 134.88 (normal value, <20). Findings were negative for anti-neutrophil cytoplasmic antibodies, antinuclear antibody, serum and urine immunofixation electrophoresis, and hepatitis B and C serology. The levels of several tumor markers were elevated: cytokeratin-19-fragment, 70.47 ng/mL (normal value, <3.3); squamous cell carcinoma antigen, 11.8 ng/mL (normal value, <1.5); and n-specific enolase, 23.05 ng/mL (normal value, <1.7). Carcinoembryonic antigen, carbohydrate antigen 125, and carbohydrate antigen 153 levels were normal. Renal biopsy of 32–38 glomeruli revealed that 13 of 32 to 18 of 38 exhibited crescent formation and neutrophil infiltration (Figure 2). Immunofluorescence analysis showed linear IgG deposition along the glomerular capillary basement membrane (Figure 2), indicative of anti-GBM nephritis. Pulmonary mass puncture examination confirmed a diagnosis of comorbid bronchial carcinoma T1N3M1 (Figure 2).
Figure 1.
Laboratory examinations revealed changes in serum creatinine and anti-GBM antibody titer during the course of treatment. Intravenous methylprednisolone was administered for 3 days, then switched to oral prednisolone for days 25-63. Abbreviations: Scr, serum creatinine; CTX, cyclophosphamide; GBM, glomerular basement membrane.
Figure 2.
Histopathology analysis revealed findings indicative of anti-GBM nephritis. (a and b) Renal biopsy showing diffuse cellular capsular crescent formation with focal necrosis (A: Periodic acid–Schiff stain, 200×, B: Masson's trichrome stain, 400×). (c) Diffuse linear deposition of IgG along the glomerular capillary basement membrane (immunofluorescence, 400×). (d) Squamous epithelial cells with severe dysplasia, enlarged and intensely stained nuclei, and obvious mitoses (Hematoxylin and eosin stain, 400×).
Abbreviation: GBM, glomerular basement membrane.
Treatment and outcome
A rapid increase in the patient’s creatinine level prompted intense treatment. Intravenous methylprednisolone (80 mg/day) was administered for 3 days (i.e., days 22–24), then switched to oral prednisolone (50 mg/day) for days 25–63; this was followed by four sessions of plasmapheresis (exchange volume, 2.5 L; days 23, 25, 28, and 30 after admission), and two boluses of intravenous cyclophosphamide (10 mg/kg, total dose of 1.2 g; days 22 and 35 after admission). A higher dose of methylprednisolone was not used because the patient exhibited pulmonary infection with sputum cultures positive for Klebsiella pneumoniae and Candida albicans. In addition, he developed steroid-induced diabetes after the administration of three boluses of intravenous methylprednisolone. The patient achieved partial remission, as indicated by a reduced serum creatinine level (245 μmol/L) and reduced GBM titer (46.79) (Figure 1). However, he refused further treatment, including potential cancer treatment, because of financial constraints. The patient was discharged on day 36 after admission. At that time, he had an increased serum creatinine level of 406 μmol/L and an increased GBM titer of 50.34. One month later, he was urgently readmitted with severe edema and died (day 63 after initial admission).
Discussion
Anti-GBM nephritis with a malignant tumor is relatively rare and has been reported only in a few cases. The first case was reported in 1989;4 two separate and distinct bronchial and pancreatic endocrine tumors were found in a 64-year-old woman with Goodpasture’s syndrome. Sugiyama et al.5 described an association between anti-GBM glomerulonephritis and radiotherapy in a patient with prostate cancer. Maes et al.6 reported the presence of IgA anti-GBM disease associated with bronchial carcinoma and monoclonal gammopathy. In these patients, the comorbid conditions were more likely to be coincidental. It remains unclear whether any substance or factor can induce anti-GBM disease. However, invasive tumor growth may cause the degradation of basement membrane matrix structures in early squamous cell lung carcinomas; thus, collagenase IV expression increases in these early tumors,7 leading to the development of anti-GBM nephritis.
In our patient, the original malignant lesion developed in the lung; however, the patient only exhibited a mild cough without clinically evident alveolar hemorrhage. Increased GBM titer and kidney involvement (proteinuria, hematuria, and acute kidney injury) were the main clinical manifestations. Thus, we speculate that the development of comorbid anti-GBM nephritis and bronchial carcinoma was not coincidental. Although the mechanism remains unclear, increased collagenase IV expression in pulmonary basement membrane is presumably involved because of the invasive tumor growth and pulmonary infection secondary to carcinoma; these characteristics may trigger disease development, as indicated by the findings in a recent cohort study of 140 Chinese patients with anti-GBM disease.8 Anti-GBM nephritis can be a life-threatening disease if untreated. However, use of the standard therapy (i.e., a combination of glucocorticoids, cyclophosphamide, and plasmapheresis) dramatically improves prognosis; a retrospective review of 43 patients with anti-GBM disease revealed overall 1-year patient survival and renal survival rates of 88% and 16%, respectively.9 Nevertheless, the outcomes of patients with anti-GBM nephritis combined with malignant tumor seem rather poor. In the case reports by Sugiyama et al.5 and Maes et al.,6 one patient was dialysis-dependent and the other died within 1 month after disease onset. However, our patient was not dialysis-dependent and his urine output remained normal during treatment. This differed from the classic characteristics of patients with anti-GBM nephritis, in whom oliguria, anuria, and end-stage renal disease were more frequent;10 this difference may have been partly related to the inadequate follow-up time of only 2 months.
In conclusion, this report described a patient with bronchial carcinoma and acute kidney injury caused by biopsy-proven anti-GBM nephritis. Although the link between pulmonary and renal manifestations remains unclear, the findings in this case provided new evidence regarding the relationships among neoplasia, tumor-related antigens, GBM exposure, and anti-GBM nephritis origin.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Chenni Gao https://orcid.org/0000-0002-8643-615X
References
- 1.Chen YX, Chen N. Pathogenesis of rapidly progressive glomerulonephritis: what do we learn? Contrib Nephrol 2013; 181: 207–215. [DOI] [PubMed] [Google Scholar]
- 2.Hellmark T, Segelmark M. Diagnosis and classification of Goodpasture's disease (anti-GBM). J Autoimmun 2014; 48–49: 108–112. [DOI] [PubMed] [Google Scholar]
- 3.McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol 2017; 12: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon RF, Lawler W, O'Donoghue DJ, et al. Goodpasture's syndrome in a patient with two endocrine tumours. Postgrad Med J 1989; 65: 582–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiyama M, Yamada Y, Nozaki Y, et al. Anti-glomerular basement membrane glomerulonephritis after radiotherapy for early prostate cancer. Clin Kidney J 2014; 7: 90–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maes B, Vanwalleghem J, Kuypers D, et al. IgA antiglomerular basement membrane disease associated with bronchial carcinoma and monoclonal gammopathy. Am J Kidney Dis 1999; 33: E3. [DOI] [PubMed] [Google Scholar]
- 7.Fisseler-Eckhoff A, Muller KM. [Anti-human collagenase type IV expression in preneoplastic lesions and early squamous cell lung carcinoma]. Verh Dtsch Ges Pathol 1993; 77: 287–291. [PubMed] [Google Scholar]
- 8.Gu QH, Xie LJ, Jia XY, et al. Fever and prodromal infections in anti-glomerular basement membrane disease. Nephrology (Carlton) 2018; 23: 476–482. [DOI] [PubMed] [Google Scholar]
- 9.Alchi B, Griffiths M, Sivalingam M, et al. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol Dial Transplant 2015; 30: 814–821. [DOI] [PubMed] [Google Scholar]
- 10.Cui Z, Zhao MH. Advances in human antiglomerular basement membrane disease. Nat Rev Nephrol 2011; 7: 697–705. [DOI] [PubMed] [Google Scholar]