Abstract
The article published by Kevin Cowart in this issue of the Journal of Diabetes Science and Technology (JDST) is a detailed overview of the clinical trial data and analysis used to demonstrate the safety and effectiveness of the Eversense continuous glucose monitoring (CGM) System for regulatory approval and clinical acceptance. The article describes the published study results for safety, accuracy, reliability, ease of insertion/removal, adverse events, and ease of diabetes patient-use for controlling their glucose levels short and long term. The author nicely compares Eversense CGM System safety and performance with the short-term subcutaneous tissue CGM systems being commercialized by Dexcom, Medtronic Diabetes, and Abbott Diabetes. This comparison may help the clinician define which type of patient with diabetes might benefit the most from the long-term implantable CGM system. The majority of studied patients describe a positive experience managing their diabetes with the Eversense CGM System and request implantation of a new sensor 90 or 180 days later.
Keywords: continuous glucose monitoring system, glucose sensor, artificial pancreas, automated insulin delivery, Eversense CGM System, Eversense XL CGM System, short-term implantation, long-term implantation, acute inflammation, foreign body response
People with type 1 diabetes are able to safely use real-time continuous glucose monitoring (CGM or glucose sensor) systems to more effectively control their blood glucose levels.1-4 The real-time CGM systems measure, analyze, and display the interstitial fluid glucose concentration, direction of change, and rate of change of the glucose trend data. Commercial CGM systems produce audible, visual, and/or vibration signals to alert the patient when they detect or predict hyperglycemia or hypoglycemia—15 to 30 minutes into the future.5-8
Real-time CGM system has become the standard of care for patients with type 1 diabetes and end-stage type 2 diabetes attempting to minimize diabetic complications using intensive insulin therapy.9-11 Improved long-term glucose control decreases the incidence and severity of microvascular disease (diabetic retinopathy, diabetic kidney disease, peripheral neuropathy, autonomic neuropathy, cardiomyopathy, dementia, etc.).12 Improved long-term glucose control also decreases the incidence, time of onset, and severity of macrovascular disease (ischemic heart disease, myocardial infarction, stroke, and peripheral arterial disease).13-18
Severe and prolonged hypoglycemia is a feared complication, causing many patients to live with a chronically elevated glucose level.19 Almost all patients practicing intensive insulin therapy using finger stick blood samples and a glucose meter/test strips develop one or more serious episodes of hypoglycemia every year, defined as a seizure, loss of consciousness, or rescue by a family member or emergency personnel. Many patients require treatment in the emergency room and hospital for severe hypoglycemia, uncontrolled hyperglycemia, diabetic ketoacidosis (DKA), and hyperglycemic hyperosmolar syndrome. Severe hypoglycemia can cause brain damage, cardiac arrhythmias, and death.19-24
Using real-time CGM system’s data and open-loop control methods, patients with diabetes learn how to titrate insulin doses in relation to meals, exercise, sleep, and illness. Clinical use of the real-time CGM system enables many patients to significantly improve their overall glucose control, defined as increased time in the desired glucose range (eg, 70 to 180 mg/dL); lower % HbA1c; lower glycemic variability; and decreased incidence, magnitude, and duration of hypoglycemia.1-4
The clinical benefits of using real-time CGM system can be achieved using multiple syringe injections of insulin each day (MDI or multidose insulin therapy) and insulin pump therapy using a continuous subcutaneous infusion of insulin.25,26 Clinical trials have clearly demonstrated that patients need to use the real-time CGM system to manage their diabetes a high percentage of the time, and adjust insulin doses in response to changing CGM system’s trend data, in order to achieve effective intensive insulin therapy.27-30
Real-time CGM systems that are coupled with an insulin pump having a “low glucose suspend” software algorithm can significantly decrease the incidence and severity of hypoglycemia, without causing DKA, rebound hyperglycemia, and increased % HbA1c.28
Real-time CGM systems that are coupled with an insulin pump having a “hybrid closed-loop” software algorithm also significantly decrease the incidence and severity of hyperglycemia, hypoglycemia, and glycemic variability. This so-called artificial pancreas automatically adjusts the amount of rapid acting insulin delivered into the subcutaneous tissue every five minutes. The concept of closed-loop insulin delivery is called automated insulin delivery. Patients with diabetes comment that waking up most mornings with their glucose concentration in the desired range (eg, 100 ± 30 mg/dL) greatly improves their quality of life and overall glucose control. Of note, these hybrid systems require the patient to manually program an appropriate bolus of rapid acting insulin prior to each meal, to minimize postprandial hyperglycemia.31-36
Advanced CGM systems that are coupled with an insulin pump having a “fully closed-loop” software algorithm are being developed that automatically deliver a meal insulin bolus based on the CGM system’s trend data, without manual input from the patient.37,38 Some closed-loop AP systems under development monitor the intensity and duration of exercise, and automatically adjust the rate of insulin delivery to avoid postexercise hypoglycemia.39 One closed-loop system utilizes a dual pump that automatically delivers insulin and glucagon into the subcutaneous tissue.40
There are two types of CGM systems that are Food and Drug Administration and CE Mark approved for monitoring the tissue fluid glucose concentration with sufficient accuracy to dose insulin: (1) real-time CGM systems with a subcutaneous tissue electrode inserted short term (≤10 days) and (2) real-time CGM systems that are implanted long term within the subcutaneous tissue (90 or 180 days). A variety of new and novel short-term and long-term implantable CGM systems are currently being developed by academia and industry.
Real-Time CGM Systems With a Subcutaneous Tissue Electrode
Dexcom, Medtronic Diabetes, and Abbott Diabetes manufacture CGM systems with a subcutaneous tissue electrode that uses an enzyme with specificity for the glucose molecule, and electrochemistry to measure the concentration of glucose in the interstitial fluid surrounding the sensor. All of the CGM systems are factory calibrated and do not need any finger stick self-monitored blood glucose (SMBG) measurements to recalibrate a sensor. Food and Drug Administration labeling, however, continues to require that the patient performs an SMBG if the CGM system’s data does not match their clinical situation, for added safety.6-8
The Dexcom and Medtronic CGM systems continuously measure and analyze the glucose sensor’s output signal, then displays the glucose concentration every five minutes on a cell phone or smart watch. Data are reliably transmitted to the phone’s software application using low-energy Bluetooth. Clinical decisions are made by the patient using the displayed current glucose value; the glucose direction of change; the glucose rate of change trend data; and alerts for hypoglycemia, hyperglycemia, and rapid rate of change. The patient’s cell phone can automatically transmit the glucose data and alerts to the cell phone of multiple family members, friends, and caregivers.6,7
A Medtronic CGM system reliably communicates with a Medtronic insulin pump (Enlite sensor with a 530G, 630G, or 670G pump) that contains control software for “low-glucose suspend” or “hybrid closed-loop.” A Dexcom CGM system (G5 and G6) reliably communicates with a variety of commercial insulin pumps (Tandem Diabetes Care t:slimX2, Insulet OmniPod, Roche Accu-Chek Spirit Combo, and Animas Vibe) that contain similar glucose control software algorithms.6,7
The current Abbott CGM system continuously measures and analyzes the glucose sensor’s output signal, but only displays the glucose concentration when the patient manually downloads sensor data to a software application on their cell phone.8 Abbott is currently developing a real-time CGM system with threshold and predictive alerts and alarms, similar to the Dexcom and Medtronic CGM systems.
The real-world performance of commercial subcutaneous tissue glucose sensors has significantly improved over the last 30 years, due to optimization of their electrode’s size, flexibility, porous membranes, enzyme electrochemistry, automated insertion, and data analytics. Despite these technological advancements, the accuracy, stability, reliability, and longevity of an individual CGM sensor can be significantly lower than required for safe and effective intensive insulin therapy. Improved manufacturing and quality control methods have significantly decreased the incidence of an individual CGM sensor that produces an inaccurate glucose measurement or fails prematurely.41 All of the commercial CGM systems routinely measure the concentration of glucose with sufficient accuracy for safely dosing insulin into the subcutaneous tissue (mean absolute relative difference [MARD] approximately 9% to 11%).42-46
An individual glucose sensor’s performance is directly affected by the individual patient’s subcutaneous tissue cellular and humoral immune response to glucose sensor electrode insertion and maintenance. Insertion of a glucose sensor needle and electrode through the skin into the subcutaneous tissue damages cells, connective tissue, and extracellular matrix. Damaged capillaries release plasma, red blood cells, white blood cells, and activated platelets into the tissue surrounding the implanted electrode. Damaged adipose cells release nuclei, organelles, plasma membranes, and triglyceride molecules into the adjacent subcutaneous tissue. Neutrophils and macrophages infiltrate the area of thrombus and adjacent tissue to actively phagocytize bacteria and debris from damaged cells, connective tissue, and extracellular matrix. Fibroblasts, lymphocytes, and mast cells may infiltrate the damaged tissue to modulate the acute inflammatory reaction and produce new fibrous connective tissue. Current CGM systems do not display a glucose reading for two or more hours after implantation due to variable sensor stability. A layer of inflammatory tissue that becomes thick, continuous, and dense with metabolically active cells may decrease a CGM system’s accuracy and stability, and increase lag time. Current commercial CGM systems need to be removed and replaced with a new CGM system at an alternate location every 6 to 14 days, due to this foreign body immune response to electrode insertion.41,47-65
Real-Time CGM Systems That are Implanted Long-Term Within the Subcutaneous Tissue
Senseonics, Inc. manufactures a long-term implantable subcutaneous tissue CGM system (Eversense CGM System) that measures the concentration of interstitial fluid glucose every five minutes and displays the measurements on the patient’s cell phone for 90 days (United States) or 180 days (Europe and South Africa). The Eversense System measures the concentration of glucose over the 40 mg/dL to 400 mg/dL range with accuracy similar to the other commercial systems. However, the implanted sensor requires a finger stick SMBG measurement approximately every 12 hours for recalibration, in order to accurately measure the concentration of interstitial tissue fluid glucose (MARD 8.5% to 11.5%). The CGM system is designed to replace finger stick blood glucose testing for diabetes treatment decisions.9,30,43,66-73
The Eversense CGM System consists of an implantable optical sensor, an external transmitter worn on the skin surface directly above the sensor, and a software application located on the patient’s smartphone, watch, tablet, or PC. The implanted sensor does not have a battery, and is powered on once every five minutes by the external transmitter, and lies dormant the rest of the time. The external transmitter and sensor use an inductive link to communicate across the skin (near-field communication or NFC), consisting of a magnetic-coupled coil pair that wirelessly powers the sensor’s optics and electronics, and transfers data between the two devices. The transmitter analyzes the optical sensor’s output signal to calculate the concentration of glucose and detect an alert condition, then transmits the data via low-energy Bluetooth to the Senseonics application on the patient’s smartphone. The wearable transmitter will vibrate to alert the patient when it detects or predicts the onset of hypoglycemia or hyperglycemia. Data can be easily transmitted to multiple caregivers and the manufacturer’s cloud-based platform for advanced analysis and display.9,66-69
The sterile sensor (3.3 mm diameter × 15 mm length) is implanted within the subcutaneous tissue of the upper arm by a trained physician or a nurse practitioner during an office procedure using local anesthesia and a novel implantation tool. The small skin incision is easily closed with steri-strips and covered with a sterile bandage until healed.
The miniature optical sensor consists of a microfluorometer encased in a translucent polymer capsule. The rigid capsule is coated with a soft hydrogel that contains proprietary chemicals that increase in fluorescence when bound to glucose molecules, with good specificity. This chemical layer is covered with a proprietary porous membrane designed to enhance biocompatibility with the adjacent subcutaneous tissue. In addition, the external surface of the implanted sensor has a silicone collar that slowly releases the glucocorticoid steroid dexamethasone acetate into the adjacent subcutaneous tissue to suppress inflammation and the foreign body immune response.
The fluorometer consists of a light-emitting diode (LED), two photodiode light detectors, microelectronics, and an antennae. The LED is briefly turned on once every five minutes to energize or excite the fluorescent chemistry, causing the hydrogel to fluoresce. The photodiodes measure the degree of fluorescence, which is directly proportional to the concentration of glucose within the hydrogel layer.9,66-69
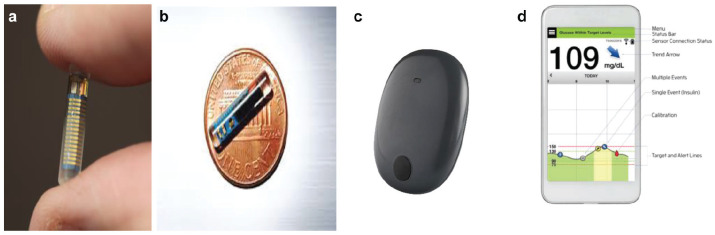
Clinical decisions are made by the patient using the displayed current glucose value; the glucose direction of change; the glucose rate of change trend data; and alerts for hypoglycemia, hyperglycemia, and rapid rate of change. The external transmitter and adhesive tape are removed every 24 to 72 hours for recharging the battery (takes 10 to 20 minutes), then readhered to the skin surface above the sensor (Figure 1).
Figure 1.
Miniature optical sensor for long-term implantation within the subcutaneous tissue of the upper arm (a and b), external transmitter adhered to the skin surface above the implanted sensor with 3M silicone adhesive tape (c), and Apple iPhone with Eversense System software application—displaying the glucose concentration; direction of change; rate of change; target glucose range; and markers for the timing of meals, insulin, and calibration (d) (www.Senseonics.com).
A trained health care worker needs to surgically remove the Eversense sensor after 90 and 180 days of implantation in the United States and outside of the United States, respectively. The incision can be easily closed with steri-strips and a bandage. The manufacturer recommends a new sterile sensor be implanted into the subcutaneous tissue of the opposite upper arm, allowing the prior wound time to heal. There is real-world data that a new sensor can be inserted into the same arm near the prior insertion site, with good clinical performance. However, there is limited data to support implanting a new sensor in the same tissue capsule that formed around the previous sensor. There is also limited long-term data demonstrating the feasibility of using only the upper arm location for implantation, due to the possible formation of avascular fibrous scar throughout the subcutaneous tissue.9,70,71
Removal requires a small skin incision and dissection to fish the sensor out of the tissue and surrounding fibrous capsule. Clinicians typically close the incision with steri-strips and a bandage. Sensor removal, however, may be difficult in a small number of patients, requiring assistance from a surgeon. The overall benefits of the real-time Eversense CGM System exceed the short-term discomfort following implantation and the small risk of infection, hematoma, skin irritation, and premature sensor failure.
The Eversense CGM System does not display glucose measurements for 24 hours after implantation due to the changing physiology of the damaged tissue adjacent to the sensor. The sensor may be less accurate for several days after implantation due to the acute inflammatory response to tissue damage and presence of a foreign body. The CGM system requires a finger stick SMBG measurements approximately every 12 hours to ensure accuracy, due to the changing tissue environment around the sensor’s electrode. Errors in calibration may significantly affect sensor accuracy until the next recalibration.74
A layer of fibrous tissue may form around the implanted sensor over time, depending upon the age and immune status of the individual. Glucose diffusion from adjacent capillaries into the sensor’s hydrogel chemistry may be inhibited by this fibrous layer.47-65 The reasons why some implanted sensors fail to function before the 90 or 180 days of expected wear time may be due to this immune response and possible degradation of the fluorescent chemistry.
Of interest, the company Glysens, Inc. is developing a long-term implantable CGM system with multiple enzyme-based electrochemical glucose electrodes and oxygen electrodes that accurately measure the concentration of interstitial fluid glucose every five minutes, with data transmitted to an external receiver for display.74 Surgeons implant the Glysens CGM system into the subcutaneous adipose tissue of the lower abdomen, using local anesthesia and IV sedation. The implanted CGM system is small, thin, and unobtrusive once implanted under the skin. The sensor’s outer membrane was designed to minimize biofouling and enhance vascular tissue in-growth. The implanted CGM system has functioned reliably with satisfactory accuracy for more than one year during animal and human clinical trials, while requiring infrequent recalibration once the tissue surrounding the sensor has healed. Recent clinical trials demonstrated satisfactory CGM system’s performance when a new sensor was immediately implanted with the fibrous capsule formed by the previously inserted sensor (Figure 2).74-79
Figure 2.
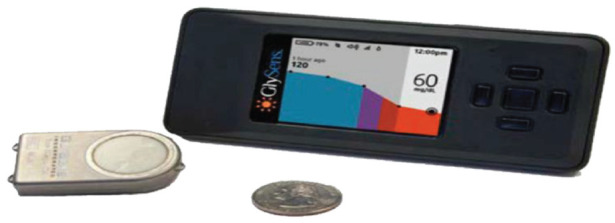
The Eclipse ICGM System was developed by Glysens, Inc. The implantable continuous glucose monitoring system (left) accurately measures the concentration of tissue fluid glucose, remains stable between infrequent recalibration periods, and reliably communicates with an external transmitter-receiver (right) using low-energy Bluetooth for more than one year (www.glysens.com).
The article published by Kevin Cowart, PharmD, MPH, BCACP, CDE in this issue of the Journal of Diabetes Science and Technology (JDST) entitled “A review of the first long-term implantable continuous glucose monitoring system available in the United States” is a detailed overview of the clinical trial data and analysis used to demonstrate the safety and effectiveness of the Eversense CGM System for regulatory approval and clinical acceptance.
The article describes the published study results for safety, accuracy, reliability, ease of insertion/removal, adverse events, and ease of diabetes patient-use for controlling their glucose levels short and long term. The author nicely compares Eversense CGM System safety and performance with the short-term subcutaneous tissue CGM systems being commercialized by Dexcom, Medtronic Diabetes, and Abbott Diabetes. This comparison may help the clinician define which type of patient with diabetes might benefit the most from the long-term implantable CGM system. A thorough review of this article is therefore highly recommended for all clinicians taking care of patients with diabetes using a CGM system.80
The number of patients with diabetes being implanted with the Eversense CGM System is increasing rapidly worldwide due to the safety profile; recent regulatory approvals; insurance coverage for the device and implantation/explantation procedures; and the education of many endocrinologists, diabetes educators, and nurse practitioners. The majority of studied patients describe a positive experience managing their diabetes with the Eversense CGM System and request implantation of a new sensor 90 or 180 days later.70
In conclusion, although many patients with diabetes will choose the long-term implantable Eversense CGM System for their day-to-day glucose control, the current system requires two finger stick blood glucose measurements per day for recalibration to ensure accuracy good enough for intensive insulin therapy. There is also limited clinical data demonstrating the feasibility of implanting the sensor in the upper arm subcutaneous tissue multiple times. Additional clinical trials are also needed to demonstrate the safety and efficacy of combining the Eversense CGM System with a smart insulin pump or insulin/glucagon pump containing a closed-loop or hybrid closed-loop control algorithm providing automated insulin delivery.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Joseph is a co-founder, equity owner, and Chairman of the Scientific and Clinical Advisory Boards of Capillary Biomedical, Inc. He is also the Director of the Jefferson Artificial Pancreas Center.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Joseph’s laboratory at Thomas Jefferson University has received research funding from the diabetes technology companies Capillary Biomedical, Inc.; Dexcom, Inc.; Medtronic Diabetes, Inc.; Glumetrics, Inc.; Flowsion; Echo Therapeutics; Thermalin, Inc.; and Animas Corporation.
ORCID iD: Jeffrey I. Joseph  https://orcid.org/0000-0002-4945-2070
https://orcid.org/0000-0002-4945-2070
References
- 1. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379-387. [DOI] [PubMed] [Google Scholar]
- 2. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self-monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. doi: 10.1136/bmj.d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371-378. [DOI] [PubMed] [Google Scholar]
- 4. Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011;5(3):668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kruger DF, Edelman SV, Hinnen DA, Parkin CG. Reference guide for integrating continuous glucose monitoring into clinical practice. Diabetes Educ. 2019;45(suppl 1):S3-S20. [DOI] [PubMed] [Google Scholar]
- 6. Dexcom. Introducing Dexcom G4 Platinum. http://www.dexcom.com. Accessed February 9, 2020.
- 7. Medtronic Diabetes. Next tech pathway. http://www.medtronicdiabetes.com. Accessed February 9, 2020.
- 8. Abbott Diabetes. https://www.diabetescare.abbott. Accessed February 9, 2020
- 9. Senseonics. Creators of the first and only long-term implantable CGM. http://www.senseonics.com. Accessed February 9, 2020.
- 10. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365-374. [DOI] [PubMed] [Google Scholar]
- 12. Wong JC, Foster NC, Maahs DM, et al. Real time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care. 2014;37(10):2702-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Control and Complications Trial Study Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14): 977-986. [DOI] [PubMed] [Google Scholar]
- 14. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goff DC, Jr, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. 2007;99(12A):i4-i20. [DOI] [PubMed] [Google Scholar]
- 16. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 17. Gerstein HC, Pogue J, Mann JF, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia. 2005;48(9):1749-1755. [DOI] [PubMed] [Google Scholar]
- 18. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of the critically ill? Diabetologia. 2006;49(8):1722-1725. [DOI] [PubMed] [Google Scholar]
- 20. Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care. 1996;19(4):324-327. [DOI] [PubMed] [Google Scholar]
- 21. Stahl M, Berger W. Higher incidence of severe hypoglycaemia leading to hospital admission in Type 2 diabetic patients treated with long-acting versus short-acting sulphonylureas. Diabet Med. 1999;16(7):586-590. [DOI] [PubMed] [Google Scholar]
- 22. Suh SW, Hamby AM, Swanson RA. Hypoglycemia, brain energetics, and hypoglycemic neuronal death. Glia. 2007;55(12):1280-1286. [DOI] [PubMed] [Google Scholar]
- 23. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovatchev BP. Hypoglycemia reduction and accuracy of continuous glucose monitoring. Diabetes Technol Ther. 2015;17(8):530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365-374. [DOI] [PubMed] [Google Scholar]
- 26. Foster N, Miller K, Tamborlane W, Bergenstal R, Beck R. Continuous glucose monitoring in T1D patients using injections of insulin: a report from the T1D Exchange clinic registry. Diabetes Care. 2016;39(6):e81-e82. doi: 10.2337/dc16-0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bergenstal RM, Tamborlane WV, Ahmann A, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311-320. [DOI] [PubMed] [Google Scholar]
- 28. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224-232. [DOI] [PubMed] [Google Scholar]
- 29. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012;35(1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanchez P, Ghosh-Dastidar S, Tweden KS, Kaufman FR. Real-world data from the first U.S. commercial users of an implantable continuous glucose sensor. Diabetes Technol Ther. 2019;21(12):677-681. [DOI] [PubMed] [Google Scholar]
- 31. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407-1408. [DOI] [PubMed] [Google Scholar]
- 32. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stone JY, Haviland N, Bailey TS. Review of a commercially available hybrid closed-loop insulin-delivery system in the treatment of type 1 diabetes. Ther Deliv. 2018;9(2):77-87. [DOI] [PubMed] [Google Scholar]
- 34. Tauschmann M, Allen JM, Wilinska ME, et al. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2016;39(7):1168-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Messer LH, Forlenza GP, Sherr JL, et al. Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care. 2018;41(4):789-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38(7):1205-1211. [DOI] [PubMed] [Google Scholar]
- 37. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934-939. [DOI] [PubMed] [Google Scholar]
- 38. Cameron FM, Ly TT, Buckingham BA, et al. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther. 2017;19(9):527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Breton MD, Cherñavvsky DR, Forlenza GP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40(12):1644-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. El-Khatib FH, Russell SJ, Nathan DM, Sutherlin RG, Damiano ER. A bihormonal closed-loop artificial pancreas for type 1 diabetes. Sci Transl Med. 2010;2(27):27ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joseph JI, Eisler G, Diaz D, Khalf A, Loeum C, Torjman M. Glucose sensing in the subcutaneous tissue-attempting to correlate the immune response with CGM accuracy. Diabetes Technol Ther. 2018;20(5):321-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Therapy. 2017;8(1):55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christiansen MP, Klaff LJ, Brazg R, et al. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol Ther. 2018;20(3):197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a Factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19(8):446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andelin M, Kropff J, Matuleviviene V, et al. Assessing the accuracy of continuous glucose monitoring (CGM) calibrated with capillary values using capillary or venous glucose levels as a reference. J Diabetes Sci Technol. 2016;10(4):876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wisniewski N, Klitzman B, Miller B, Reichert WM. Decreased analyte transport through implanted membranes: differentiation of biofouling from tissue effects. J Biomed Mater Res. 2001;57(4):513-521. [DOI] [PubMed] [Google Scholar]
- 48. Klueh U, Liu Z, Feldman B, et al. Metabolic biofouling of glucose sensors in vivo: role of tissue microhemorrhages. J Diabetes Sci Technol. 2011;5(3):583-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klueh U, Czajkowski C, Ludzinska I, Qiao Y, Frailey J, Kreutzer DL. Impact of CCL2 and CCR2 chemokine/receptor deficiencies on macrophage recruitment and continuous glucose monitoring in vivo. Biosens Bioelectron. 2016;86:262-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klueh U, Frailey JT, Qiao Y, Antar O, Kreutzer DL. Cell based metabolic barriers to glucose diffusion: macrophages and continuous glucose monitoring. Biomaterials. 2014;35(10):3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dungel P, Long N, Yu B, Moussy Y, Moussy F. Study of the effects of tissue reactions on the function of implanted glucose sensors. J Biomed Mater Res. 2007;85(3):699-706. [DOI] [PubMed] [Google Scholar]
- 52. Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor tissue interface-effects of motion, pressure, and design on sensor performance and the foreign body response-part I: theoretical framework. J Diabetes Sci Technol. 2011;5(3):632-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sharkawy AA, Klitzman B, Truskey GA, Reichert WM: Engineering the tissue which encapsulates subcutaneous implants. I. Diffusion properties. J Biomed Mater Res. 1997;37(3):401-412. [DOI] [PubMed] [Google Scholar]
- 54. Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. II. Plasma-tissue exchange properties. J Biomed Mater Res. 1998;40(4):586-597. [DOI] [PubMed] [Google Scholar]
- 55. Sharkawy AA, Klitzman B, Truskey GA, Reichert WM. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J Biomed Mater Res. 1998;40(4):598-605. [DOI] [PubMed] [Google Scholar]
- 56. Kvist PH, Iburg T, Bielecki M, et al. Biocompatibility of electrochemical glucose sensors implanted in the subcutis of pigs. Diabetes Technol Ther. 2006;8(4):463-475. [DOI] [PubMed] [Google Scholar]
- 57. Gerritsen M, Jansen JA, Kros A, et al. Influence of inflammatory cells and serum on the performance of implantable glucose sensors. J Biomed Mater Res. 2001;54(1):69-75. [DOI] [PubMed] [Google Scholar]
- 58. Kamath A, Mahalingam A, Brauker J. Analysis of time lags and other sources of error of the DexCom SEVEN continuous glucose monitor. Diabetes Technol Ther. 2009;11(11):689-695. [DOI] [PubMed] [Google Scholar]
- 59. Rigla M, Pons B, Rebasa P, et al. Human subcutaneous tissue response to glucose sensors: macrophages accumulation impact on sensor accuracy. Diabetes Technol Ther. 2018;20(4):296-302. [DOI] [PubMed] [Google Scholar]
- 60. Woodward SC. How fibroblasts and giant cells encapsulate implants: considerations in design of glucose sensors. Diabetes Care. 1982;5(3):278-281. [DOI] [PubMed] [Google Scholar]
- 61. Hunt TK. The physiology of wound healing. Ann Emerg Med. 1988;17(12):1265-1273. [DOI] [PubMed] [Google Scholar]
- 62. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang Y, Vaddiraju S, Gu B, Papadimitrakopoulos F, Burgess DJ. Foreign body reaction to implantable biosensors: effects of tissue trauma and implant size. J Diabetes Sci Technol. 2015;9(5):966-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Joseph JI, Torjman MC. Implantable glucose sensors. In: Wnek G, Bowlin GL. (eds) Encyclopedia of Biomaterial and Biomedical Engineering. 2nd ed, Vol 2 Boca Raton: CRC Press; 2008:1174-1181. [Google Scholar]
- 65. Ward KW. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J Diabetes Sci Technol. 2008;2(5):768-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kropff J, Choudhary P, Neupane S, et al. Accuracy and longevity of an implantable continuous glucose sensor in the PRECISE study: a 180-day, prospective, multicenter, pivotal trial. Diabetes Care. 2017;40(1):63-68. [DOI] [PubMed] [Google Scholar]
- 67. Christiansen MP, Klaff LJ, Bailey TS, Brazg R, Carlson G, Tweden KS. A prospective multicenter evaluation of the accuracy and safety of an implanted continuous glucose sensor: the PRECISION study. Diabetes Technol Ther. 2019;21(5):231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aronson R, Abitbol A, Tweden KS. First assessment of the performance of an implantable continuous glucose monitoring system through 180 days in a primarily adolescent population with type 1 diabetes. Diabetes Obes Metab. 2019;21(7):1689-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jafri RZ, Balliro CA, El-Khatib F, et al. A three-way accuracy comparison of the Dexcom G5, Abbott Freestyle Libre Pro, and Senseonics Eversense CGM devices in an outpatient study of subjects with type 1 diabetes. Diabetes. 2018;67(suppl 1):14-OR. [DOI] [PubMed] [Google Scholar]
- 70. Deiss D, Irace C, Carlson G, Tweden KS, Kaufman FR. Real-world safety of an implantable continuous glucose sensor over multiple cycles of use: a post-market registry study. Diabetes Technol Ther. 2020;22(1):48-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Deiss D, Szadkowska A, Gordon D, et al. Clinical practice recommendations on the routine use of Eversense, the first long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. 2019;21(5):254-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lorenz C, Sandoval W, Mortellaro M. Interference assessment of various endogenous and exogenous substances on the performance of the Eversense long-term implantable continuous glucose monitoring system. Diabetes Technol Ther. 2018;20(5):344-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. King C, Anderson SM, Breton M, Clarke WL, Kovatchev BP. Modeling of calibration effectiveness and blood-to-interstitial glucose dynamics as potential confounders of the accuracy of continuous glucose sensors during hyperinsulinemic clamp. J Diabetes Sci Technol. 2007;1(3):317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. GlySens, Inc. http://www.glysens.com. Accessed February 9, 2020.
- 75. Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. Function of an implanted tissue glucose sensor for more than 1 year in animals. Sci Transl Med. 2010;2(42):42ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lucisano JY, Routh TL, Lin JT, Gough DA. Glucose monitoring in individuals with diabetes using a long-term implanted sensor/telemetry system and model. IEEE Trans Biomed Eng. 2017;64(9):1982-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kumosa LS, Routh TL, Lin JT, Lucisano JY, Gough DA. Permeability of subcutaneous tissues surrounding long-term implants to oxygen. Biomaterials. 2014;35(29):8287-8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29(12):1517-1524. [DOI] [PubMed] [Google Scholar]
- 79. Klueh U, Antar O, Qiao Y, Kreutzer DL. Role of vascular networks in extending glucose sensor function: impact of angiogenesis and lymphangiogenesis on continuous glucose monitoring in vivo. J Biomed Mater Res A. 2014;102(10):3512-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cowart K. A review of the first long-term implantable continuous glucose monitoring system available in the United States. J Diabetes Sci Technol. 2021;15:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]