Abstract
Background:
Glucose control during consecutive days of aerobic exercise has not been well studied. We assessed glycemia, insulin requirements, and carbohydrate (CHO) needs during two consecutive days of prolonged cycling in type 1 diabetes (T1D) adults using sensor-augmented insulin pump therapy.
Methods:
Twenty adults with well-controlled T1D and six healthy adults (for comparison) were enrolled. Assessments were made during two consecutive days of cycling activities (30 miles per day). On day 1 (D1), basal rates were reduced 50% and CHO intake was guided by real-time continuous glucose monitoring (rtCGM) data to maintain a target range (70-180 mg/dL). On day 2 (D2), basal insulin infusion was stopped for the first hour of biking and resumed at a minimal rate during biking. Carbohydrate intake one hour before, during, and ten minutes after biking was recorded. Times within/below target range, glycemic variability, and mean glucose were calculated from rtCGM data.
Results:
Among 17 T1D participants who completed the study, mean glucose levels at the start of cycling were slightly lower on D2 vs D1: 138 ± 16 mg/dL and 122 ± 16, respectively, P = NS. Type 1 diabetes participants achieved near-normal glucose levels at the end of both cycling events; however, the reduction in glucose was most notable at one hour into the event on D2 vs D1. Carbohydrate intake was notably lower during D2 vs D1 with no difference in time <54 mg/dL (both P = NS).
Conclusions:
Type 1 diabetes individuals using rtCGM-augmented insulin pump therapy can safely engage in consecutive days of prolonged aerobic exercise by significantly reducing insulin dosages with no increase in CHO intake.
Keywords: type 1 diabetes, exercise, real-time continuous glucose monitoring, rtCGM, basal rate, carbohydrate, insulin pump
Introduction
Managing diabetes during exercise requires individuals with type 1 diabetes (T1D) to balance their insulin dosing and food intake to maintain target glucose levels.1-3 Although maintaining optimal glycemic control and avoiding hypoglycemia during and after exercise remain primary goals, achieving these goals is challenging for many individuals.
Several factors can influence glycemic responses to physical activity, including current glucose level; timing/dose of last bolus; timing/content of last meal; rate of changing glucose at start of exercise/activity; rate of insulin infusion (insulin pump basal rate); current insulin on board; fitness level; and type, intensity, and duration of exercise/activity.4
Researchers now know that aerobic activities of various intensities and durations increase in insulin sensitivity and a high risk of hypoglycemia during5,6 activity with a risk for late-onset hypoglycemia which may last up to 24 hours.7,8 Individuals may also experience increased postexercise hyperglycemia, resulting from insulin dose reductions and increased carbohydrate (CHO) intake prior to and during the exercise event.9-11
To address the challenges and complexities of safely engaging in exercise and physical activity, many T1D individuals have adopted the use of technologies such as insulin pumps and continuous glucose monitoring systems, which allow for greater flexibility in insulin dosing and more comprehensive real-time monitoring of glucose levels.
Because aerobic exercise increases glucose uptake, insulin sensitivity,12 and counter-regulatory responses,13 individuals with T1D are generally advised to reduce insulin (basal and bolus or both) prior to aerobic activities.4 Carbohydrate intake of 30 to 60 g/h may be needed if the activity is intensive and/or prolonged.4,14 However, although insulin adjustments may be useful in managing glycemia during exercise, it may result in increased postexercise hyperglycemia unless CHO is also carefully managed.15
Although the current evidence describes the glucose response during and postexercise,4,10 glucose metabolism during consecutive days of aerobic exercise has not been well studied. We hypothesized that the “second day” effect would reduce an individual’s basal and correction insulin dose requirements and possibly increase the need for additional CHO intake during exercise.
Methods
Study Design
In this exploratory analysis, we assessed glucose responses, insulin requirements, and CHO needs during prolonged cycling on two consecutive days in adults with T1D using sensor-augmented insulin pump therapy. The cycling took place along the river Danube from October 13-14, 2018. Ethics review was not required because the analysis was not considered a clinical study under the Austrian Medical Devices Act according to the Ethics Committee for the testing of drugs or medical devices or the application of new medical methods to humans (Vienna, Austria).
Participants
Inclusion criteria for participation in the analysis were diagnosed T1D; treatment with insulin pump therapy in conjunction with the Dexcom G6 real-time continuous glucose monitoring (rtCGM) system (Dexcom, Inc., San Diego, CA) for at least two months with 95% use of their rtCGM device; willingness to use their rtCGM exclusively for insulin dosing adjustments; availability of laboratory values (HbA1c, creatinine, sodium, potassium, liver enzymes, TSH, blood count, and blood pressure) obtained within previous two months; and physical ability to safely participate in the cycling activity. Exclusion criteria were T2D, treatment with multiple daily insulin injections therapy, pregnant, incomplete laboratory data, or inability to participate in the cycling activity. Six healthy adults wearing the rtCGM device were also recruited to assess normal glucose response in the tissue during exercise. All participants provided written-informed consent.
Procedures
Two weeks prior to the cycling events, investigators met with participants to upload their insulin pump and rtCGM data to confirm eligibility, review laboratory values, check the functionality of T1D participants’ insulin pump and rtCGM device, and optimize the insulin pump settings for both normal daily use and during the activity. Real-time continuous glucose monitoring was initiated in healthy participants. Participants were instructed to consume a standardized breakfast (bread and cereal) two hours prior to the start of each cycling event and refrain from exercising one day prior to the cycling events. Blood ketone testing was performed prior, during, and at the end of the cycling on each day.
Participants used the Dexom G6 rtCGM) with the OmniPod insulin pump (Insulet, Inc., Billerica, MA, United States) OmniPod, or Medtronic Minimed 640G (Medtronic, Northridge, CA, United States). The GlucoMen aero system (A. Menarini, Firenza, Italy) was used for ketone testing. Participants used either insulin Lispro (Eli Lilly and Company, Indianapolis, Indiana), insulin Aspart, or faster insulin Aspart (Novo Nordisk, Bagsvaerd, Denmark).
Day 1 (D1): After adjusting insulin/CHO to achieve a target range of 150-180 mg/dL, basal rates were reduced 50%, starting 30 minutes prior to cycling, according to a commonly used regimen (eg, 50% basal insulin reduction with increased CHO intake).4,14 Carbohydrate consumed one hour prior to cycling, during cycling, and ten minutes postcycling were entered into the rtCGM device. The supplemental CHO taken were fast-acting CHO: dextrose and fluid maltodextrin with fructose. Participants then completed 30-mile (49 km) cycling course over a time period of around 3.5 hours, consuming CHO as needed to maintain a glucose target range of 70-180 mg/dL. Following the cycling event, investigators uploaded the insulin pump and rtCGM data for analysis, using the Diasend and Clarity System.
On Day 2 (D2): Participants repeated the same cycling course as D1. As a safety measure, we initiated a modified insulin regimen to offset any potential latent hypoglycemia from D1. After adjusting insulin/CHO to achieve a target range of 150 to 180 mg/dL, basal insulin delivery was stopped immediately prior to cycling and then restarted 60 minutes into the cycling event, with a minimum dose of 0.05 to 0.3 IU/h (a reduction of approximately 78% of the normal basal insulin rate) and a 50% reduction in meal/correction insulin doses. Carbohydrate consumed one hour prior to cycling, during cycling, and ten minutes postcycling was entered into the rtCGM device. The cycling period had a duration of about 3.5 hours. Participants were given an individualized algorithm for correction insulin adjustments based on trend arrows and instructions for additive CHO intake to maintain a target glucose range of 70-180 mg/dL (Figure 1).
Figure 1.
Individualized insulin/carbohydrate adjustment based on real-time continuous glucose monitoring.
Measures
The primary outcomes of interest were differences in glycemic control, basal and correction insulin dosages, and CHO intake during the cycling events, comparing D1 vs D2. Glycemic control was assessed by mean glucose, glycemic variability (SD), percentage of time below range (<54 to <70 mg/dL), and time in range (70-180 mg/dL) assessed by downloaded rtCGM data. Secondary outcomes were comparisons glycemic control between T1D vs healthy participants during the cycling activity and during the full cycling days. Safety assessments included the incidence of diabetic ketoacidosis and the number of severe hypoglycemic events, defined as requiring third-party assistance.
Analysis
Data from participants’ rtCGM devices and insulin pumps were uploaded via the Clarity and Diasend software for analysis. All additive CHOs needed to maintain glucose in the target range (70-180 mg/dL) during the cycling events were entered into participants’ rtCGM devices. All CHOs consumed at the start, during, and at the end of the cycling events were included in this analysis. Metabolic status of the healthy participants was similarly obtained and calculated; however, CHO intake was not measured. Significance of differences (0.1) between D1 and D2 in mean glucose and glycemic variability was tested using Student’s t-test.
Results
Participants
Twenty T1D adults (9 females/11 males, age 42 ± 8.8 years, 24 ± 10 years diabetes duration, 6.6 ± 0.8% HbA1c, 26 ± 6 kg/m2 BMI) and six healthy adults (3 females/3 males, age 37 ± 10 years, 5.1 ± 0.1% HbA1c, 24 ± 26 kg/m2 BMI) were enrolled in the analysis. Two T1D participants discontinued prior to the biking activity due to acute illness and one T1D participant did not follow the biking route and was excluded. Seventeen T1D participants and six healthy participants are included in this analysis. Real-time continuous glucose monitoring data from seven days prior to the cycling events showed marked differences between T1D and healthy patients in mean glucose 134 ± 17 vs 95 ± 4 mg/dL) and glycemic variability (44 ± 13 vs 18 ± 3 mg/dL) but not in time spent <54 mg/dL (1.2% ± 1.6% vs 1.8% ± 2.6%) or 70 mg/dL (6.6% ± 3.4% vs 6.8% ± 5.1%).
Outcomes
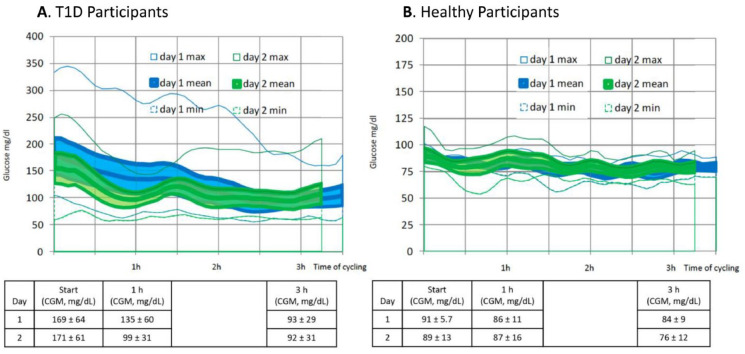
Among T1D participants, mean glucose levels on D2 were slightly lower than D1 at the start of cycling (Figure 2(a)). Participants achieved near-normal glucose levels at the end of both cycling events; however, the reduction in glucose was most notable at one hour into the event on D2 vs D1. Glycemic variability during four hours of cycling on D2 decreased (~28%) compared with D1 (34 ± 7 mg/dL vs 47 ± 13 mg/dL, respectively, P < .01). Glycemic levels remained stable throughout the cycling event among healthy participants (Figure 2(b)).
Figure 2.
Glycemic levels throughout the cycling events.
Analysis of all rtCGM values obtained on cycling days showed that participants in both study groups (T1D and healthy) showed comparable increases in percentage of time spent <70 mg/dL (Level 1), with no significant changes in percentage of time spent <54 mg/dL (Level 2) and a slight increase in percentage of time within target (70-180 mg/dL) (Table 1).
Table 1.
Comparison of Differences in Percentage of Time Spent Below and Within Target Range During Cycling Days: T1D vs Healthy Participants.
| Mean glucose, mg/dL |
Glucose variability (SD), mg/dL |
<54 mg/dL |
<70 mg/dL |
70-180 mg/dL |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1D | Healthy | T1D | Healthy | T1D | Healthy | T1D | Healthy | T1D | Healthy | |
| D1 | 138 ± 16 | 91 ± 3.3 | 48 ± 16 | 13 ± 2.6 | 0.3% ± 0.7% | 0.0% ± 0.0% | 7.6% ± 5.2% | 7.2% ± 7.2% | 66.0% ± 15.0% | 93.0% ± 7.2% |
| D2 | 122 ± 16 | 91 ± 5.4 | 44 ± 12 | 16 ± 5.3 | 0.3% ± 0.7% | 0.2% ± 0.4% | 10.0 ± 8.3% | 12.7% ± 17.5% | 74.0% ± 15.0% | 86.0% ± 18.0% |
| Change | −12% | 0% | −8% | 23% | 0% | 0% | 52% | 76% | 12% | −8% |
| P Value | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Abbreviation: T1D, type 1 diabetes.
The average total basal insulin administrated during each of cycling event was notably lower on D2 (0.69 U) vs D1 (1.5 U). Basal rates during D1 and D2 vs noncycling days are presented in Table 2. None of the patients required correction insulin bolusing during the cycling event.
Table 2.
Pump Settings Prior to and During Exercise in All Type 1 Diabetes Participants.
| Time | Mean basal IU/h ± SD Normal basal program |
Day 1 Basal program during exercise |
Day 2 Basal program during exercise |
|---|---|---|---|
| 0-1 am | 0.68 ± 0.28 | 50% Reduction start: 30 min before |
78% Reduction start : at the beginning |
| 1-2 am | 0.78 ± 0.31 | ||
| 2-3 am | 0.83 ± 0.31 | ||
| 3-4 am | 0.91 ± 0.31 | ||
| 4-5 am | 0.89 ± 0.35 | ||
| 5-6 am | 0.93 ± 0.30 | ||
| 6-7 am | 0.93 ± 0.35 | ||
| 7-8 am | 0.93 ± 0.27 | ||
| 8-9 am | 0.95 ± 0.33 | ||
| 9-10 am | 0.85 ± 0.33 | ||
| 10-11 am | 0.77 ± 0.36 | 0.38 ± 0.18 | 0 |
| 11 am to 12 pm | 0.75 ± 0.33 | 0.37 ± 0.13 | 0.23 ± 0.10 |
| 0-1 pm | 0.73 ± 0.29 | 0.36 ± 0.15 | 0.23 ± 0.10 |
| 1-2 pm | 0.82 ± 0.31 | 0.41 ± 0.20 | 0.23 ± 0.10 |
| 2-3 pm | 0.81 ± 0.34 | ||
| 3-4 pm | 0.82 ± 0.41 | ||
| 4-5 pm | 0.85 ± 0.40 | ||
| 5-6 pm | 0.86 ± 0.38 | ||
| 6-7 pm | 0.90 ± 0.43 | ||
| 7-8 pm | 0.86 ± 0.34 | ||
| 8-9 pm | 0.80 ± 0.31 | ||
| 9-10 pm | 0.82 ± 0.33 | ||
| 10-11 pm | 0.81 ± 0.33 | ||
| 11 pm to 12 am | 0.73 ± 0.26 |
Note: Time of erxercise printed in bold.
Carbohydrate intake among T1D participants were slightly lower during cycling on D2 vs D1: 36 ± 32 g vs 40 ± 27 g and mean glucose on the whole D2 vs D1, respectively, lower 122 ± 16 vs 138 ± 29 mg/dL (both P = NS), Table 1. The healthy participants did not consume CHO during the exercise periods.
Postevent ketone levels were slightly higher among healthy participants on D1 and D2 (0.63 and 0.68 mmol/mol, respectively) compared with T1D participants (0.55 and 0.61 mmol/mol, respectively). No incidents of diabetic ketoacidosis or severe hypoglycemia occurred.
Discussion
Routine physical activity is a key component of effective blood glucose management and critical for maintaining overall health in individuals with diabetes.4 However, for many individuals with T1D, developing strategies for safely engaging in prolonged aerobic exercise is challenging due to the increased risk for hypoglycemia.7
Results from our cycling assessments suggest that individuals with T1D who are using rtCGM in conjunction with insulin pump therapy can significantly reduce their CHO and insulin requirements during prolonged aerobic exercise by stopping their basal insulin delivery during the first hour of activity and then restarting at a significantly reduced dose and, at the same time, reduce their normal prandial and correction insulin doses by approximately 50%. As reported here, reductions in CHO and insulin doses were achieved safely with negligible increases in mean glucose, glycemic variability, and percentage of time spent <54 mg/dL.
For our evaluation, it was assumed that decreased insulin dosages in conjunction with increased CHO intake on D2 would be needed as a safety measure to avoid latent hypoglycemia. As expected, our results showed an increase in insulin sensitivity on D2, characterized by the notable lower gluocse at the start of cycling and the decrease in mean glucose at one hour. However, it was surprising to observe no change in percentage of time spent <54 mg/dL despite a slight decrease in CHO intake. One would have expected a need for increased CHO intake based on the current treatment recommendations.
Importantly, the stark reductions in insulin dosages on D2 are similar to regimens used in other exercise studies. Zaharieva et al16 utilized an 80% reduction of the basal rate 90 minutes before aerobic exercise. In their assessment, the reduced basal rate was used safely and successfully in regard to glucose control during and after the exercise session. In a study by Breton et al,17 investigators managed glucose control in the sensor-augmented pump group by reducing the basal insulin doses by approximately 66% to maintain a time in target range of 70 to 180 mg/dL. As reported, our basal insulin reduction of 78% on D2 yielded better results, but, again, this may be due to the “second day” exercise effect from D1. Although Breton’s sensor-augmented pump group achieved 71% of time in target range, with less time <70 mg/dL (1.8%), the supplemental CHO given in both study groups was substantially greater (50 g/day) then administered in our assessment. In a more recent study, Forlenza et al18 assessed the use of a hybrid closed-loop insulin with moderate exercise (approximately 30 minutes). They found that very similar CHO intake (up to 40 g) was needed to achieve <2.1% of time <70 mg/dL. The low levels of ketones before and during the exercise period demonstrated the safety of the new exercise dosing strategy. We assume that the ketone levels were higher in the group healthy participants because they did not eat anything during the exercise session.
A key limitation of our exploratory analysis was the small number of study participants. Therefore, it is not possible to generalize our findings to the larger T1D patient population. Nevertheless, our results do raise important questions that may warrant further investigation. For example, it has been long understood that exercise-related hypoglycemia can occur in T1D several hours after a single exercise event.19,20 Could latent hypoglycemia be mitigated by more stark reductions in insulin dosages during the first day of exercise? Moreover, it would be valuable to assess the impact of CHO reduction during aerobic exercise on weight loss.
Although individuals with T1D have traditionally been thought to have normal body weight, this is no longer the case. A 2010 study by Conway et al21 followed adult patients with T1D for an average of 18 years and found that the prevalence of overweight increased from 29% to 42%, whereas the prevalence of obesity increased sevenfold from 3% to 23%. Importantly, weight gain was not related to aging but, instead, was associated with clinical factors such as insulin therapy. As reported by Szadkowska et al, individuals with T1D tend to develop overweight and obesity in early adulthood more frequently than the general population and are characterized by higher body fat mass.22
A key contributor to overweight/obesity in T1D is exogenous insulin. As an anabolic hormone, insulin plays a role in inhibiting protein catabolism, stimulating lipogenesis, and slowing basal metabolism,22,23 which results in increased fat accumulation.18,24 The weight-gaining effectives are exacerbated by the administration of exogenous insulin administration,22 since exogenous insulin can only closely mimic endogenous secretion.23 Therefore, many individuals, even those who exercise regularly and/or participate in other physical activities, would like be interested in an insulin dosing algorithm that minimizes CHO intake while optimizing glucose control.
Conclusion
Although reductions in insulin doses and/or additional CHO intake prior to and during aerobic exercise are commonly recommended to maintain safe glucose levels during and after physical activity,6 the treatment regimen utilized on D2 may be valuable for many individuals with T1D who regularly engage in prolonged aerobic excerise, particularly for those who are working toward weight reduction/management. Although our exploratory analysis was not designed to address many of the questions and issues associated with exercise in T1D, our findings may prompt further investigation by others to more fully explore the impact the “second day” exercise effect and perhaps validate the utility of our approach to addressing increased insulin sensitivity and mitigating latent hypoglycemia on consecutive days of prolonged aerobic exercise.
Acknowledgments
The authors wish to thank Christopher G. Parkin, MS, CGParkin Communications, Inc., for editorial support in the preparation of this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Michael Müller-Korbsch, Lisa Frühwald, Michael Heer, Maria Fangmeyer-Binder, and David Reinhart-Mikocki report no disclosures. Peter Fasching has received fees for lectures and consultant activities for Eli Lilly and Company and Novo Nordisk.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by research grants from Eli Lilly and Company, Novo Nordisk, Dexcom, Insulet, Menarini.
References
- 1. Younk LM, Mikeladze M, Tate D, Davis SN. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev Endocrinol Metab. 2011;6(1):93-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallen IW, Hume C, Lumb A. Fueling the athlete with type 1 diabetes. Diabetes Obes Metab. 2011;13(2):130-136. [DOI] [PubMed] [Google Scholar]
- 3. Chu L, Hamilton J, Riddell MC. Clinical management of the physically active patient with type 1 diabetes. Phys Sportsmed. 2011;39(2):64-77. [DOI] [PubMed] [Google Scholar]
- 4. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;5(5):377-390. [DOI] [PubMed] [Google Scholar]
- 5. Zinman B, Murray FT, Vranic M, et al. Glucoregulation during moderate exercise in insulin treated diabetics. J Clin Endocrinol Metab. 1977;45(4):641-652. [DOI] [PubMed] [Google Scholar]
- 6. Moser O, Tschakert G, Mueller A, et al. Effects of high-intensity interval exercise versus moderate continuous exercise on glucose homeostasis and hormone response in patients with type 1 diabetes mellitus using novel ultra-long-acting insulin. PLoS One. 2015;10(8):e0136489 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4552855/. Accessed December 28, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Briscoe VJ, Tate DB, Davis SN. Type 1 diabetes: exercise and hypoglycemia. Appl Physiol Nutr Metab. 2007;32(3):576-582. [DOI] [PubMed] [Google Scholar]
- 8. Teich T, Riddell MC. The enhancement of muscle insulin sensitivity after exercise: a Rac1-Independent handoff to some other player? Endocrinology. 2016;157(8):2999-3001. [DOI] [PubMed] [Google Scholar]
- 9. Yardley JE, Iscoe KE, Sigal RJ, Kenny GP, Perkins BA, Riddell MC. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: an observational study of physically active type 1 diabetes patients. Diabetes Technol Ther. 2013;15(1):84-88. [DOI] [PubMed] [Google Scholar]
- 10. Iscoe KE, Riddell MC. Continuous moderate-intensity exercise with or without intermittent high-intensity work: effects on acute and late glycaemia in athletes with type 1 diabetes mellitus. Diabet Med. 2011;28(7):824-832. [DOI] [PubMed] [Google Scholar]
- 11. Campbell MD, Walker M, Trenell MI, et al. A low-glycemic index meal and bedtime snack prevents postprandial hyperglycemia and associated rises in inflammatory markers, providing protection from early but not late nocturnal hypoglycemia following evening exercise in type 1 diabetes. Diabetes Care. 2014;37(7):1845-1853. [DOI] [PubMed] [Google Scholar]
- 12. McMahon SK, Ferreira LD, Ratnam N, et al. Glucose requirements to maintain euglycemia after moderate-intensity afternoon exercise in adolescents with type 1 diabetes are increased in a biphasic manner. J Clin Endocrinol Metab. 2007;92(3):963-968. [DOI] [PubMed] [Google Scholar]
- 13. Milman S, Leu J, Shamoon H, Vele S, Gabriely I. Opioid receptor blockade prevents exercise-associated autonomic failure in humans. Diabetes. 2012;61(6):1609-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Houlder SK, Yardley JE. Continuous glucose monitoring and exercise in type 1 diabetes: past, present and future. Biosensors (Basel). 2018;8(3):E73. doi: 10.3390/bios8030073 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6165159/. Accessed December 29, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaharieva DP, McGaugh S, Pooni R, Vienneau T, Ly T, Riddell MC. Improved open-loop glucose control with basal insulin reduction 90 minutes before aerobic exercicise in patients with type1 diabetes on continues subcutaneous insulin infusion. Diabetes Care. 2019;42(5):824-831. [DOI] [PubMed] [Google Scholar]
- 17. Breton M, Chernavvsky D, Forlenza G, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artifical pankreas ski study. Diabetes Care. 2017;40(12):1644-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Forlenza GP, Buckingham BA, Christiansen MP, et al. Performance of Omnipod personalized model predictive control algorithm with moderate intensity exercise in adults with type 1 diabetes. Diabertes Technol Ther. 2019;21(5):265-272. doi: 10.1089/dia.2019.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson J-L. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care. 2001;24(4):625-630. [DOI] [PubMed] [Google Scholar]
- 20. Sonnenberg GE, Kemmer FW, Berger M. Exercise in Type 1 (insulin-dependent) diabetic patients treated with continuous subcutaneous insulin infusion: prevention of exercise induced hypoglycaemia. Diabetologia. 1990;33(11):696-703. [DOI] [PubMed] [Google Scholar]
- 21. Conway B, Miller RG, Costacou T, et al. Temporal patterns in overweight and obesity in type 1 diabetes. Diabet Med. 2010;27(4):398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Szadkowska A, Madej A, Ziolkowska K, et al. Gender and age-dependent effect of type 1 diabetes on obesity and altered body composition in young adults. Ann Agric Environ Med. 2015;22(1):124-128. [DOI] [PubMed] [Google Scholar]
- 23. Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799-812. [DOI] [PubMed] [Google Scholar]
- 24. Valla V. Therapeutics of diabetes mellitus: focus on insulin analogues and insulin pumps. Exp Diabetes Res. 2010;2010:178372. doi: 10.1155/2010/178372 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2877202/. Accessed January 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]