Abstract
Insulin, as a peptide hormone drug, is susceptible to changes in stability when exposed to environmental factors under storage. Proper storage according to the manufacturer’s recommendations is important to maintain its potency and enable precise dosing for people with diabetes (PwD). While it is reasonable to assume that transport conditions and temperature are well controlled during the supply chain, little is known about insulin storage after dispensing and insulin potency at the moment of administration. Insulin is exposed to various environmental factors when carried by PwD and storage recommendations are often not met when it is stored in household refrigerators. It is difficult to assess changes in insulin potency in clinical practice, and there is a gap in the current scientific literature on insulin stability. Package leaflet recommendations only give limited information on the impact of improper storage conditions on insulin stability and guidelines by health organizations are inconsistent. Given the importance of precise dosing in diabetes care, there is a need for more transparency on insulin stability, awareness for proper storage among health care professionals and PwD as well as clear guidelines and practical storage recommendations from manufacturers and health organizations.
Keywords: diabetes therapy, insulin therapy, insulin, insulin stability, insulin concentration, HPLC
Introduction
Tens of millions of vials, pens, and reservoirs of insulin are currently stored or in transit in refrigerated or unrefrigerated distribution centers, pharmacies, hospitals, delivery vehicles, homes, bathroom counters, pockets, purses, backpacks, belt clips, lockers, glove compartments, and planes. It is astonishing that very little published research seems to have been conducted that explores the potency of insulin doses at various steps of the cold chain up to the point they are administered.
Insulin, like many other peptide hormone based drugs, is temperature sensitive and its stability is affected by storage conditions. This is reflected in the resources invested in cold chain maintenance in the insulin supply chain and the specific storage recommendations that apply for insulin when in the hands of people with diabetes (PwD). Insulin, among other temperature-sensitive medications, presents a particularly unique case because of the exposure to environmental factors when in-use. Precise dosing is a key in intensive insulin therapy.
People with diabetes around the world carry insulin with them daily, in vials, pens, or pumps close to their body and store their insulin supply in household refrigerators. Insulin is regularly subjected to risk factors that can affect its potency when in-use, such as high and low ambient temperatures, sun light, and agitation through movement. In insulin pumps worn close to the body, not only is the temperature in the reservoir increased the constant movements also accelerate fibril formation.1-3 Many PwD live in places without access to refrigeration, and recent data indicate that even insulin storage conditions in household refrigerators often do not meet recommendations by manufacturers.4
Exact dosing is essential for PwD to maintain glycemic control. People with diabetes perceive changes in insulin sensitivity often and need to adjust their dose accordingly. Changes in insulin potency can contribute to this observed variability in glucose levels, however, this factor is currently not sufficiently considered. Laboratory analysis is needed to verify if insulin potency has been affected, making it difficult to assess in practice. Furthermore, there is little publicly available data on insulin stability. Limited access to insulin, and recently, the increasing insulin prices in the United States force many PwD to ration their insulin and to obtain it from unconventional sources.5 Insulin might be used beyond expiration date, the recommended in-use timeframe, or after it has been exposed to suboptimal storage conditions. It is complicated for PwD to evaluate which risks they face when using insulin that has or might have been stored outside of recommendations.
This review summarizes what is known about the storage of insulin, how it affects insulin stability, current practice in the distribution chain and when stored by PwD and discusses how the factor of insulin potency could be taken into account to improve diabetes management.
Insulin Stability: Impact of Heat and Cold
Temperature is known to be an important factor that impacts the pharmaceutical quality of drugs based on peptide hormones. This is reflected in insulin storage recommendations (Table 1) and the importance of temperature control throughout distribution (Table 2). Pharmaceutical quality entails many different aspects, such as potency, purity, bioavailability, physicochemical properties, pH, appearance, and sterility. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) defines stability as those quality factors of a specific pharmaceutical product that are susceptible to change during its storage and likely influence its quality, efficacy, purity, and safety.6
Table 1.
Storage Recommendations for Different Insulin Products in the United States and European Union With the Shelf Life In-Use and the Maximum Temperature In-Use.
| Type | Active substance | Manufacturer | Brand name | Region | Year first approval | Packaging | Shelf life in-use | Max. temperature in-use |
|---|---|---|---|---|---|---|---|---|
| Intermediate acting (NPH) | Isophane insulin | Eli Lilly | Humulin N | United States | 1982 | Prefilled pen | 14 days | 30°C |
| Vial | 31 days | 30°C | ||||||
| Novo Nordisk | Novolin N | United States | 1991 | Prefilled pen | 28 days | 30°C | ||
| Vial | 42 days | 25°C | ||||||
| Sanofi | Insuman Basal | EU | 1997 | Pen cartridge | 4 weeks | 25°C | ||
| Prefilled pen | 4 weeks | 25°C | ||||||
| Vial | 4 weeks | 25°C | ||||||
| Long acting | Insulin degludec | Novo Nordisk | Tresiba | EU | 2013 | Pen cartridge | 8 weeks | 30°C |
| Prefilled pen | 8 weeks | 30°C | ||||||
| United States | 2015 | Prefilled pen | 8 weeks | 30°C | ||||
| Vial | 8 weeks | 30°C | ||||||
| Insulin detemir | Novo Nordisk | Levemir | EU | 2004 | Pen cartridge | 6 weeks | 30°C | |
| Prefilled pen | 6 weeks | 30°C | ||||||
| United States | 2005 | Prefilled pen | 42 days | 30°C | ||||
| Vial | 42 days | 30°C | ||||||
| Insulin glargine | Eli Lilly | Abasaglar | EU | 2015 | Pen cartridge | 28 days | 30°C | |
| Prefilled pen | 28 days | 30°C | ||||||
| Basaglar | United States | 2015 | Prefilled pen | 28 days | 30°C | |||
| Mylan | Semglee | EU | 2018 | Prefilled pen | 4 weeks | 30°C | ||
| Sanofi | Lantus | EU | 2000 | Prefilled pen | 28 days | 30°C | ||
| Vial 10 mL | 28 days | 30°C | ||||||
| United States | 2000 | Prefilled pen | 28 days | 30°C | ||||
| Vial 10 mL | 28 days | 30°C | ||||||
| Toujeo | EU | 2015 | Prefilled pen | 6 weeks | 30°C | |||
| United States | 2015 | Prefilled pen | 56 days | 30°C | ||||
| Long acting + glp-1 agonist | Insulin degludec + liraglutide | Novo Nordisk | Xultophy | EU | 2014 | Prefilled pen | 21 days | 30°C |
| United States | 2016 | Prefilled pen | 21 days | 30°C | ||||
| Insulin glargine + lixisenatide | Sanofi | Soliqua | United States | 2016 | Prefilled pen | 28 days | 25°C | |
| Suliqua | EU | 2017 | Prefilled pen | 28 days | 25°C | |||
| Premixed | Human insulin + human insulin isophane | Eli Lilly | Humulin 70/30 | United States | 1989 | Prefilled pen | 10 days | 30°C |
| Vial | 31 days | 30°C | ||||||
| Novo Nordisk | Actraphane | EU | 2002 | Pen cartridge | 6 weeks | 30°C | ||
| Prefilled pen | 6 weeks | 30°C | ||||||
| Vial | 6 weeks | 25°C | ||||||
| Insulatard | EU | 2002 | Pen cartridge | 6 weeks | 30°C | |||
| Prefilled pen | 6 weeks | 30°C | ||||||
| Vial | 4 weeks | 25°C | ||||||
| Mixtard | EU | 2002 | Pen cartridge | 6 weeks | 30°C | |||
| Prefilled pen | 6 weeks | 30°C | ||||||
| Vial | 6 weeks | 25°C | ||||||
| Novolin 70/30 | United States | 1991 | Prefilled pen | 28 days | 30°C | |||
| Vial | 42 days | 25°C | ||||||
| Protaphane | EU | 2002 | Pen cartridge | 6 weeks | 30°C | |||
| Prefilled pen | 6 weeks | 30°C | ||||||
| Vial | 4 weeks | 25°C | ||||||
| Sanofi | Insuman Comb | EU | 1997 | Pen cartridge | 4 weeks | 25°C | ||
| Prefilled pen | 4 weeks | 25°C | ||||||
| Vial | 4 weeks | 25°C | ||||||
| Insulin aspart + protamine-crystallized insulin aspart | Novo Nordisk | NovoLog Mix 70/30 | United States | 2001 | Prefilled pen | 14 days | 30°C | |
| Vial | 28 days | 30°C | ||||||
| NovoMix | EU | 2000 | Pen cartridge | 4 weeks | 30°C | |||
| Prefilled pen | 4 weeks | 30°C | ||||||
| Insulin degludec/insulin aspart | Novo Nordisk | Ryzodeg | EU | 2013 | Pen cartridge | 4 weeks | 30°C | |
| Prefilled pen | 4 weeks | 30°C | ||||||
| Ryzodeg 70/30 | United States | 2015 | Prefilled pen | 28 days | 30°C | |||
| Insulin lispro + insulin lispro protamine | Eli Lilly | Humalog Mix | United States | 1999 | Prefilled pen | 10 days | 30°C | |
| Vial | 28 days | 30°C | ||||||
| Rapid acting | Human insulin | MannKind | Afrezza | United States | 2014 | Inhaler cartridge | 10 days | 30°C |
| Insulin aspart | Novo Nordisk | Fiasp | EU | 2017 | Pen cartridge | 4 weeks | 30°C | |
| Prefilled pen | 4 weeks | 30°C | ||||||
| Vial | 4 weeks | 30°C | ||||||
| United States | 2017 | Prefilled pen | 28 days | 30°C | ||||
| Vial | 28 days | 30°C | ||||||
| NovoLog | United States | 2000 | Pen cartridge | 28 days | 30°C | |||
| Prefilled pen | 28 days | 30°C | ||||||
| Vial | 28 days | 30°C | ||||||
| NovoRapid | EU | 1999 | Pen cartridge | 4 weeks | 30°C | |||
| Prefilled pen | 4 weeks | 30°C | ||||||
| Pump cartridge | in use: 7 days | 30°C | ||||||
| Vial | 4 weeks | 30°C | ||||||
| Insulin glulisine | Sanofi | Apidra | EU | 2004 | Pen cartridge | 4 weeks | 25°C | |
| Prefilled pen | 4 weeks | 25°C | ||||||
| Vial | 4 weeks | 25°C | ||||||
| United States | 2004 | Prefilled pen | 28 days | 25°C | ||||
| Vial | 28 days | 25°C | ||||||
| Vial (use in pump) | 2 days | 37°C | ||||||
| Insulin lispro | Eli Lilly | Humalog | EU | 1996 | Pen cartridge | 28 days | 30°C | |
| Prefilled pen | 28 days | 30°C | ||||||
| Vial | 28 days | 30°C | ||||||
| United States | 1996 | Pen cartridge | 28 days | 30°C | ||||
| Prefilled pen | 28 days | 30°C | ||||||
| Vial | 28 days | 30°C | ||||||
| Liprolog | EU | 2001 | Pen cartridge | 28 days | 30°C | |||
| Prefilled pen | 28 days | 30°C | ||||||
| Vial | 28 days | 30°C | ||||||
| Sanofi | Admelog | United States | 2017 | Prefilled pen | 28 days | 30°C | ||
| Vial | 28 days | 30°C | ||||||
| Insulin lispro Sanofi | EU | 2017 | Pen cartridge | 4 weeks | 30°C | |||
| Prefilled pen | 4 weeks | 30°C | ||||||
| Vial | 4 weeks | 30°C | ||||||
| Short acting/regular | Human insulin | Eli Lilly | Humulin R | United States | 1982 | Vial | 31 days | 30°C |
| Humulin R U-500 | United States | 1994 | Prefilled pen | 28 days | 30°C | |||
| Vial | 40 days | 30°C | ||||||
| Novo Nordisk | Actrapid | EU | 2002 | Pen cartridge | 6 weeks | 30°C | ||
| Prefilled pen | 6 weeks | 30°C | ||||||
| Vial (40 U/mL) | 4 weeks | 25°C | ||||||
| Vial (100 U/mL) | 6 weeks | 25°C | ||||||
| Novolin R | United States | 1991 | Prefilled pen | 28 days | 30°C | |||
| Vial | 42 days | 25°C | ||||||
| Sanofi | Insuman Implantable | EU | 1997 | Vial | 45 days | 37°C | ||
| Insuman Infusat | EU | 1997 | Pump cartridge | 2 weeks | na | |||
| Vial | 2 weeks | 25°C | ||||||
| Insuman Rapid | EU | 1997 | Prefilled pen | 4 weeks | 25°C | |||
| Vial | 4 weeks | 25°C |
Abbreviation: NPH, neutral protein Hagedorn.
Source: Information taken from the Summary of Product Characteristics (SmPC) from FDA and European Assessment Reports (EPAR) from EMA.19
Table 2.
Stakeholders and Quality Assurance Throughout the Cold Chain.
| ||||
|---|---|---|---|---|
| Manufacturer | Distributor/wholesaler | Pharmacy/hospital | People with diabetes | |
| Guidelines | Good Distribution Guidelines by WHO, European Commission, US Pharmacopeia, etc. |
Limited, inconsistent information in guidelines by health organizations | ||
| National law for pharmacies/hospitals | ||||
| Other quality systems | Quality system by manufacturer Contracts with distributors |
Quality system by institution | ||
| Available information | Knowledge of stability data | Package leaflet, might know stability data | Package leaflet, professional training | Package leaflet, education from HCPs, product website |
| Quality assurance measures | - Liability of manufacturer, responsibility to recall products - Risk assessment based on recorded temperature data - Audits, quality control - Training of staff - Use of validated materials (medical refrigerators, tested shipping boxes, refrigerated transporters, etc.) - Temperature monitoring - Documentation |
- No quality control - No risk assessment possible - Suboptimal storage in home refrigerator - Use of cool bags |
||
Abbreviation: HCPs, health care professionals.
Insulin in pharmaceutical formulations undergoes chemical and physical degradation pathways over time, leading to reduced potency. These are accelerated by exposure to high temperatures, direct sunlight, shear stress through agitation and by an increased air-liquid surface, which occurs as the volume in a vial decreases.7 Since PwD take insulin injections several times a day or carry an insulin pump close to the body, insulin is regularly exposed to these stress factors. The chemical degradation pathways of insulin are deamidation and formation of high-molecular weight polymers (HMWPs). Both deamidated insulin molecules and HMWPs are still partially potent.7 As a physical degradation pathway, the aggregation of insulin molecules to fibrils has higher impact on the potency of an insulin formulation.7 Due to the stable bonds of insulin molecules within these fibrils, subcutaneous injections of insulin formulations with fibrils induce a greatly reduced biological response due to the reduced number of monomers that are available for absorption.3 Insulin fibrillation is verified using microscopy in a laboratory, but fibrils can also be visible in insulin vials. However, PwD might not regularly check their insulin for the formation of fibrils which is especially difficult in pens or pump reservoirs.
In addition to high temperatures, freezing must be avoided as this can both compromise the integrity of a formulation and the vial. As an example, freezing might cause fine cracks in the glass of cartridges and therefore impairs its hygiene.7
Even though there are a variety of human insulins and insulin analogs in different formulations and delivery devices on the market today, most of the research on stability and degradation pathways of insulin formulations has been published decades ago and refers to bovine, porcine, and human insulin only. Experimental data presented by Jens Brange from Novo Nordisk in a booklet from 1987 show how storage temperatures induce loss of biological potency of insulin formulations. This publication still remains the main public source of data on insulin stability3,7 among other data from the early 1970s.8-10
It is widely assumed that different insulin products are comparable with respect to their stability, although they vary in peptide structure, formulation, and manufacturing methods. Changes in the primary structure of the insulin molecule or additional substances to enhance or prolong its absorption might influence its stability, eg, differences in sensitivity to structural changes due to temperature, or added excipients like in protein-bound insulin (neutral protein Hagedorn (NPH)-insulins) in suspension, that are more sensitive to physical degradation than insulin solutions.7 Excipients added to the insulin formulations can also have an impact on insulin stability. For example, phenolic preservatives play a role in the stabilization of insulin hexamers. If insulin is stored in such a way as to allow evaporation of such high vapor pressure additives, this will impact insulin stability and solubility.11 This can be a particular problem in insulin pumps, depending on the plastic material used for the insulin infusion set, infusion rates, temperature, etc.
Stability Evaluation of Insulin Formulations
The potency of insulin formulations in units per milliliter (U/mL) indicates the quantified blood glucose lowering activity of an insulin formulation per volume. Originally, insulin potency was determined in bioassays by administering insulin to rabbits or mice and monitoring their blood glucose over time. These bioassays were replaced by high-performance liquid chromatography (HPLC) in the 1980s, measuring the concentration of insulin in a formulation. The correlation between insulin quantity (measured in mass by physicochemical assays) and biological activity (measured in units by bioassays) has been demonstrated in studies at the time.8-10 Today, the United States Pharmacopeia (USP) and many other pharmacopoeias such as Europe and Japan recognize HPLC as the standard method to assess insulin potency.12-14 The USP defines a variability margin of ±5% U/mL for insulin formulations, meaning an insulin vial labeled “100 U/mL” has to contain 95 to 105 U/mL of insulin as measured by HPLC to fulfill quality standards.12
Bioassays have subsequently lost their significance as a quantitative assay but are still used to verify the bioidentity of insulin and are, for example, required as a part of quality control before releasing batches in powder form as an active pharmaceutical ingredient (API) for the US market, in contrast to European regulations.15,16 The USP argues for the validity of this test because it “accurately reflects the effect on the diabetic patient” and bioidentity “cannot be assessed” by HPLC.17 Recently, the insulin manufacturer Sanofi replaced rabbit bioassays with a cell-based assay to verify bioidentity.18
One concern regarding the HPLC method is the pretreatment of insulin by a strong acid for the duration of one hour, which may alternate structural changes, eg, higher molecular weight proteins or fibrils that may be formed previously during storage. The potency of insulin measured by HPLC and the insulin’s actual biological potency therefore might differ; however, no data have been published to substantiate this concern.
A recent independent evaluation of insulin concentration in vials purchased at pharmacies in the Midwest of the United States showed considerably lower insulin concentrations when this was measured with a highly sensitive different method for assessing insulin concentration using Quadrapole Time-of-Flight Mass Spectrometry.20 It remains to be shown if these findings can be confirmed by others using different analytical methods parallel to the USP-approved HPLC method.21 Another research group has conducted a similar study with NPH insulin purchased from pharmacies in the United States. Insulin concentration was assessed using Ultra Performance Liquid Chromatography coupled with UV detection (UPLC-UV) and with Mass Spectrometry (UPLC-MS). The results demonstrate that mass spectrometry yields a lower concentration than liquid chromatography, however, the HPLC method according to USP was not used.22 More recently, NMR methods have been proposed for the measurement of insulin concentrations.23,24 For future studies on insulin stability, it would be helpful to evaluate correlations between established bioassay methods (ie, rabbit hypoglycemia) and modern chemical analytic methods. Such studies would help to better understand the validity of the chemical methods as predictors of biopotency. Other methods to assess insulin pharmacokinetics are immunoassays, which are used to measure small insulin concentrations circulating in blood after subcutaneous insulin administration. Glucose clamp studies allow the estimation of insulin pharmacodynamics. In such studies, insulin is administered to PwD under highly controlled conditions and the glucose infusion rates needed to keep blood glucose constant thereafter is measured. Such studies are a requirement for each new insulin as an essential part of their approval process, but are expensive and cumbersome to perform regularly.
Insulin Storage in the Distribution Cold Chain
Insulin storage temperature is carefully managed and controlled in all steps of the supply chain by manufacturers, distributors, health care professionals (HCPs), and health authorities. Table 2 shows a simplified overview of quality assurance for the main stakeholders in the pharmaceutical cold chain. In distribution, insulin needs to be kept refrigerated between 2°C and 8°C (36°F-46°F) to ensure optimal quality.
The World Health Organization (WHO), the European Commission, and USP issue Good Distribution Practice (GDP) Guidelines, which regulate and advise on how to maintain the pharmaceutical cold chain.25-27 These stipulate that pharmaceutical manufacturers are liable and responsible to recall insulin if possibly damaged. Manufacturers therefore make contracts with logistics companies and wholesalers, defining the conditions under which products are stored and transported, and often work closely together on risk assessment. Pharmacies and hospitals also report to manufacturers when temperature deviations occur, which are then assessed case by case and might result in recalling the packages in question or clearing them safe to dispense.
In 2009, Eli Lilly received a $10-million settlement from their distribution contractor when an insulin shipment was exposed to freezing temperatures. Temperature loggers in the air freight containers had recorded a minimum of −0.1°C. In the case files, it is argued by the manufacturer that “insulin that has been exposed to sub-freezing temperatures may undergo subtle changes that are not detectable by physical inspection.” The shipment was deemed unsalable for human consumption and destroyed.28
Regulatory bodies across regions have different policies on temperature deviations during the supply chain. The USP argues that deviations from the temperature range of 2°C to 8°C (36°F-46°F) are unavoidable in the distribution chain. They are acceptable if the manufacturer can show stability test data that demonstrate the product’s quality is not affected by a certain temperature deviation. The USP GDP also advise to use the metric of mean kinetic temperature that weighs severity and duration of temperature excursion.27 The European GDP however explicitly does not accept any deviations from a temperature range as labeled on a medication package.26 The Australian Health Authority requires manufacturers to share stability data from a real-time temperature simulation of the supply chain, including anticipated temperature deviations. Most biotechnologically derived products such as insulin are manufactured in the United States or Europe and shipping to Australia is lengthy and crosses the equatorial region.29
Once insulin arrives at a pharmacy or hospital, it is supposed to be stored in temperature controlled and monitored refrigerators. The use of a min-max thermometer and daily documentation of the temperature is recommended by USP.27 Furthermore, various national guidelines/legislations are in place, and proper temperature management can be part of a pharmacy/hospital audit.30 In the United States, mail ordering insulin has recently become increasingly popular. Depending on where parcels are delivered to and how long it takes to reach the destination, insulin might be exposed to extended temperature deviations, eg, in the freezing cold during the winter or very hot temperatures or direct sunlight during summer.
Storage Recommendations by Manufacturers
Toward the end of the pharmaceutical supply chain, the people who handle insulin are HCPs and PwD, and the package leaflet becomes the main source of information on insulin stability. Insulin manufacturers need to provide stability data to health authorities as a part of the market authorization process. These tests contain short-term stress tests and long-term tests under different temperature and humidity conditions following general predefined testing protocols of the ICH.6 These data justify shelf life and storage recommendations.
All insulin formulations should be stored in a refrigerator at 2° to 8°C (36°F-46°F) to keep their quality until the expiration date. Insulin can alternatively be stored at room temperature below 25°C or 30°C (77°F or 86°F), provided it is then used within a certain time period that ranges between 10 days and 8 weeks (Table 1). For insulin intended to be used in pumps, recommendations also define a certain shelf life for use at 37°C (98.6°F) as a maximum in-use temperature for a pump reservoir worn close to the body or a patch pump. Table 1 lists all insulin formulations authorized by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) by insulin type, with the respective in-use shelf life and maximum temperature.
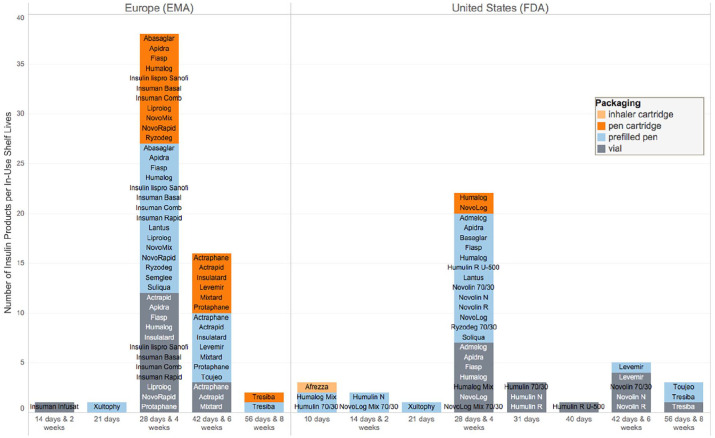
Once removed from the refrigerator, manufacturers recommend to discard insulin after a specified period of time, either if it was in-use or still unopened. For a majority of insulin formulations, this is four weeks: “discard after 28 days even if some of the solution remains.”31 In a Diabetes Care commentary from 2003, the reasons behind this were questioned, since for some PwD, especially for children, this means discarding insulin regularly.32,33 Although three insulin manufacturers and the American Diabetes Association (ADA) provided statements in response, there is little clarity on why these recommendations are in place, whether for sterility, potency, or other quality concerns.32,34 One insulin manufacturer (Eli Lilly) referenced general recommendations by the Committee for Proprietary Medicinal Products for sterile products for human use as a source for the four-week timeframe.32 However, some newer insulin formulations allow a longer shelf life in-use, such as Tresiba® (insulin degludec, Novo Nordisk) with eight weeks (Table 1). Other insulin products should be used no longer than 10 or 14 days. Figure 1 summarizes different in-use shelf lives for insulin products marketed in the United States and EU. A recent study surveyed 269 PwD in the United States on their use of 10 mL Lantus vials (insulin glargine, Sanofi) and 81% of them used their insulin beyond the 28-day recommended shelf life, on average for 43 days. The authors call for a need to increase awareness and education among PwD and recognize that the impact of using insulin past the recommended shelf life is unknown.35
Figure 1.
Number of insulin products per in-use shelf life recommendation and packaging for insulin products authorized by European Medicines Agency and Food and Drug Administration, respectively (information taken from the Summary of Product Characteristics (SmPC) from FDA or EMA, resp. Accessed May 4, 2019).36
Manufacturers have stated that differences in stability occur depending on the type of insulin, volume, and packaging.32 As an example, for Actrapid (human insulin, Novo Nordisk), vials with different insulin concentrations and pens have different storage parameters (Table 1). However, this does not apply in general for all vials and cartridges (Figure 1).
Leaflet storage recommendations are influenced by regulatory framework and give limited information about a product’s stability. Sanofi shared information on the stability of Lantus (insulin glargine, Sanofi), claiming that the long-acting insulin analog has been proven to remain stable during temperature cycles between −15°C and +25°C (5°F and 77°F) for a month, as expected conditions in the supply chain.32 However, the label advises that Lantus should not be frozen. Novo Nordisk recently published stability test data on Actrapid and Insulatard, which are labeled with an in-use shelf life of six weeks, demonstrating that these two insulins are still within FDA specifications after a stress test of six weeks at 30°C and an additional four weeks at 30°C (86°F), to account for potential improper handling during distribution.21 Even though pharmaceutical products remain stable in freeze-thaw studies or can endure single temperature excursions to heat, leaflets recommend either “store in a refrigerator” or “at room temperature”.37 “Room temperature” is not defined exactly the same way across regulatory regions in the world. “Controlled room temperature” according to USP is “20°C to 25°C with excursions allowed to 15°C to 30°C,” while the same product might be labeled as “store below 30°C” in the EU.37 As an example, Xultophy (insulin degludec/liraglutide, Novo Nordisk) should be stored at “room temperature 15°C to 30°C (59°F to 86°F)” in the United States and “below 30°C” in the EU (Table 1). These regulatory differences apply to all temperature-sensitive drugs and have been critically discussed in the past. Hunt et al from USP proposed to label products in a way that more accurately reflects stability data, including excursion times and avoiding narrow temperature ranges of room temperature to remove uncertainty.37 For some rapid acting insulins, like NovoRapid (insulin aspart, Novo Nordisk) or Apidra (insulin glulisine, Sanofi), label recommendations give some idea of a stability budget. When used in a pen, NovoRapid is stable for four weeks below 30°C. When used in a pump, however, insulin manufacturers propose a maximum in-use temperature of 37°C (98.6°F), and insulin should be discarded after one week and can only be stored unrefrigerated (<30°C [86°F]) for two weeks prior (Table 1).
Another regulatory implication is that the same insulin product can have different storage recommendations across different regions, which has been criticized in the past as this is considered to be confusing for HCPs and PwD.38 Today, storage recommendations are harmonized at least across the United States and Europe for a majority of insulins, but there are still some exceptions. As an example, Toujeo (insulin glargine, Sanofi) is currently labeled as stable at room temperature for eight weeks in the United States, but only six weeks in the EU (Table 1).
Insulin Storage at PwD’s Homes
Once insulin is handed over to PwD, they keep it cooled until they start using a vial, cartridge, or prefilled pen. The instructions in the package leaflet are to keep insulin “refrigerated/in a refrigerator between 2°C and 8°C (36°F-46°F)” and “do not freeze”.31 However, for PwD in many parts of the world refrigeration is not available or unreliable.39 What’s more, recent studies demonstrated that real-world conditions often do not successfully meet storage recommendations, even when refrigerators are used. An evaluation of data collected from 230 PwD showed that insulin in 25% of household refrigerators was exposed to temperature fluctuations below freezing point (0°C [32°F]).4 These findings are in line with other observational studies of temperature-sensitive medications stored in patients’ homes,40-42 eg, for antirheumatic biologic drugs, where the recommended temperature range was only maintained for 54.8% of storage time and the proportion of the patients that stored their medication for more than two hours consecutive time below 0°C (32°F) was 24.3%.40 It is unclear what impact these storage conditions have on in insulin stability. However, most PwD store considerable quantities of insulin to cover their needs for many months in their refrigerators, therefore, this represents a risk to insulin quality that would be unacceptable by professional cold chain standards.
Household refrigerators are primarily designed for storing food and optimized for food safety (0°C-4°C [32°F-41°F]) with temperatures a few degrees below range for medication (2°C-8°C [36°F-46°F]). Temperatures have shown to often drop below freezing point. Unlike pharmaceutical refrigerators that have ventilators for air circulation, the air inside household refrigerators is not circulated, which leads to a temperature gradient and the creation of warm and cold zones within the same refrigerator. Many modern refrigerators can adjust their target temperature; however, this regulation is not precise and relies on one measurement only. Compressor cycles introduce fluctuations of high amplitudes. Even if the average temperature is within 2°C to 8°C (36°F-46°F), there may be great deviations outside this range.43-45
The general awareness level of PwD about these technical implications of properly storing insulin in a refrigerator is assumed to be low, as it is not common practice for PwD to use thermometers to monitor their storage temperatures and there is little publicly available information and little training on insulin storage as part of their educational programs. Leaflets do not address the practical aspects of storing insulin properly for extended periods of time in household refrigerators.
Storage and Transport of Insulin When In Use
For PwD, the insulin in-use is a daily companion, and therefore, is exposed to numerous environmental factors. The average ambient temperature in many regions can be much higher than the recommended 30°C (86°F) in summer or can drop below 2°C (36°F) in winter. Package leaflets, in addition to a temperature range, recommend keeping insulin “away from sunlight” and “do not freeze.”
There is little research on how insulin is transported when in-use and its quality at the moment of administration. Observational data from users of the insulin pen cap “Insulclock” showed that during a study period in Spain in summer, injections were performed at an average temperature of 27°C (81°F), with 11.7% above 30°C (86°F) up to 41°C (106°F).46 There are also a few documented cases of diabetic ketoacidosis (DKA) of pump users, whose insulin had stopped working because of exposure to heat or freezing.47,48
The average level of awareness of consequences as a result of exposure to heat seems to be higher than for exposure to low temperatures, as many PwD who live in or travel to warmer climates use cooling bags. In a survey conducted in the south west of the United States, 64% of PwD reported to “carry items in a cooler/ice chest” or “carry items in a commercially produced case with ice/gel packs” to protect their medications and supplies from heat.49 There are several products on the market for protecting insulin pens and vials from heat. However, no systematic analysis was performed to see how stable insulin remains when it is stored within these bags or cases and if recommended temperatures can be maintained. Some products refrigerate by water evaporation (www.frio.co.uk), others by using phase changing material and vacuum insulation (www.tempramed.com). The maximum cooling effect that can be gained from evaporation is strongly dependent on the ambient humidity. People with diabetes also use thermos flasks, regular coolers with ice, or portable refrigerators. When using ice/ice packs, insulin pens or vials should never be kept directly on ice to avoid freezing.50
A number of new insulin delivery systems have an integrated temperature sensor. These include insulin pumps, smart pens, or pen caps https://www.tandemdiabetes.com (t:slimX2), https://www.companionmedical.com (InPen), https://www.insulclock.com (Insulclock), Bigfoot Biomedical pen caps (https://www.bigfootbiomedical.com/), and Common Sensing (https://gocap.me/), a startup supported by Sanofi. There are two products (to the best of the authors’ knowledge at the moment of publishing) that are tailored for PwD and dedicated to monitoring insulin temperature: MedAngel (https://medangel.co/) is a temperature sensor and a mobile app that can be kept with any type of insulin and warns when temperature limits are exceeded, and Safe Med (https://medicool.com/products/safe-med-sensor), a lower-cost alternative. Evaluating data generated by such delivery and monitoring devices will provide further insights into insulin storage conditions.
Quality Assurance and Risk Assessment for PwD
According to the plethora of reports in online forums and social media, PwD experience issues with their insulin often. A decrease in biological potency of insulin over time due to improper storage conditions (while using, eg, the same insulin pen) would potentially result in an increase in insulin doses needed. This does not remain unnoticed by people sensitive to such changes, especially when larger adjustments of insulin doses are required or when people track insulin dosages and glucose levels. However, PwD, caregivers, diabetes educators, and physicians might not consider variations in insulin potency as a possible cause for observed increase in blood glucose fluctuations due to the many other factors influencing insulin requirements. When therapeutic goals are not achieved, insufficient biological potency of the insulin used should be considered as a possible cause as well, by PwD as well as by the diabetes team.
It is unclear how potential issues with insulin quality are handled in practice, as there is currently no way to assess insulin quality in a feasible way for these cases. There is no research on the frequency of replacing insulin or issuing new insulin prescriptions for PwD who perceive changes in insulin sensitivity. Insulin potency is not routinely assessed in a case of a DKA or severe hypoglycemia that has no other apparent cause. In the few documented cases of a DKA caused by spoiled insulin, the reason was only identified because doctors talked extensively to patients and caregivers and because the temperature exposure had been memorable and obvious: a detached insulin pump left in the sun while swimming and an insulin vial that had frozen in a hotel refrigerator.47,48
When PwD or their diabetes team doubt potency of an insulin product, they can return it to the manufacturer. In a recent publication, Novo Nordisk showed quality assessment results of 233 insulin vials that had been sent back to the company because PwD or HPCs in the United States had voiced concerns about their quality.21 The insulin content in over 95% of the vials, that had been collected over three years, was within USP requirements recognized by FDA (±5%) as assessed by HPLC.21 People with diabetes received a reply stating that the insulin in question has been examined and meets release specifications. Only in five of the vials returned to the manufacturer by the consumer, potency and/or appearance was found to be out-of-specification. This is of interest to note, as the returned insulin was subjected to unknown storage and shipping conditions in the hands of the PwD, at least some of the insulin will have been past the recommended discard time period and the vials and pens were already opened and used.
An emerging opportunity to detect changes in insulin potency is to correlate data derived from insulin delivery devices with integrated temperature sensors or systems for automated insulin delivery (AID) with continuous glucose monitoring (CGM). Insulin dosage over time in relation to glycemic outcomes and insulin storage temperature can be analyzed. Such analysis might indicate if insulin used by PwD changes in stability over time, ie, if the biological potency declines while the insulin is in use in the AID system or when stored under insufficient conditions in PwD’s homes. Evaluation of parameters like time in range, insulin sensitivity, and total daily dose are relevant in this respect and information about insulin lots, duration of insulin usage of a given vial, etc., could be taken into consideration. The access to such technologies is an opportunity to enable PwD to manage their insulin’s quality. The insulin delivery device company Bigfoot Biomedical has recently submitted a patent for temperature monitoring that could potentially implement anticipated changes in insulin potency into insulin dosing decisions.51 Recently, methods were proposed that would allow a nondestructive evaluation of insulin concentration.24 This might in the future enable people to assess insulin before injection.
Awareness and Education
After being diagnosed, PwD have to cope with an entirely new life situation and have to learn many things in a short period of time. In diabetes education programs for newly diagnosed PwD, a good portion of time is focused on insulin treatment and all practicalities associated with this. No standardized recommendations for diabetes education programs exist for insulin storage and the priority of the topic is unclear. An increase in awareness about the importance of optimal insulin storage for maintenance of biological potency of insulin by adequate education is important and might lead to a better and higher frequency of reports of reduced insulin potency. This does not only hold true for PwD but also for other stakeholders involved in diabetes care, including pharmacists, diabetes educators, and physicians.
Few health authorities or organizations publicly share recommendations about insulin storage. The current Standards of Medical Care in Diabetes issued by ADA do not cover insulin storage practice or insulin stability, but there is some information on the ADA website.52,53 Table 3 shows recommendations for insulin storage by four different organizations: the ADA, the International Society for Pediatric and Adolescent Diabetes (ISPAD), the East Africa Diabetes Study Group (EADSG), and the Sri Lanka Medical Association (SLMA) published by WHO. All of these guidelines acknowledge that insulin is temperature sensitive and should be protected from heat and freezing to ensure quality. They give practical recommendations to a different level of detail. It is of note that these sources differ in the proposed in-use shelf lives for insulin. The online patient information of the ADA states that “insulin kept at room temperature will last approximately 1 month”, while EADSG recommends six weeks and the SLMA two to three weeks (in bold, Table 3). International Society for Pediatric and Adolescent Diabetes cites degradation kinetics of insulin formulations as a guideline on how to expect insulin to lose potency, however, these data are derived from one comment by one manufacturer (Lilly) from 2003 and it was not specified which type of insulin they apply to.32,54 The EADSG furthermore defines the minimum and maximum temperature limits of 0°C (32°F) and 32°C (90°F) as harmful for insulin quality.50
Table 3.
General Recommendations on Insulin Storage by Independent Organizations.
|
American Diabetes Association (ADA)
“Insulin Storage and Syringe Safety” diabetes.org53 |
International Society for Pediatric and Adolescent Diabetes (ISPAD)
“ISPAD Clinical Practice Consensus Guidelines 2018”54 |
East Africa Diabetes Study Group
(EADSG) “EADSG Guidelines: Insulin Storage and Optimisation of Injection Technique in Diabetes Management”50 |
Sri Lanka Medical Association
“Best Practice in Insulin Injection Technique. A Simplified Guideline”55 |
|---|---|---|---|
| “Although manufacturers recommend storing your insulin in the refrigerator, injecting cold insulin can sometimes make the injection more painful. To avoid this, many providers suggest storing the bottle of insulin you are using at room temperature. Insulin kept at room temperature will last approximately 1 month.
Remember though, if you buy more than one bottle at a time to save money, store the extra bottles in the refrigerator. Then, take out the bottle ahead of time so it is ready for your next injection. Here are some other tips for storing insulin: • Do not store your insulin near extreme heat or extreme cold. • Never store insulin in the freezer, direct sunlight, or in the glove compartment of a car. • Check the expiration date before using, and don’t use any insulin beyond its expiration date. • Examine the bottle closely to make sure the insulin looks normal before you draw the insulin into the syringe. If you use regular, check for particles or discoloration of the insulin. If you use NPH or lente, check for “frosting” or crystals in the insulin on the inside of the bottle or for small particles or clumps in the insulin. If you find any of these in your insulin, do not use it, and return the unopened bottle to the pharmacy for an exchange and/or refund.” |
“Regulatory requirements state that the labelled insulin product must retain at least 95% of its potency at expiry date. At room temperature (25°C, 77°F), insulin will lose <1.0% of its potency over 30 days. In contrast, insulin stored in a refrigerator will lose <0.1% of its potency over 30 days. Storage recommendations are more often based on regulatory requirements regarding sterility than loss of potency. The individual manufacturer’s storage recommendation and expiry dates must be adhered to. These usually recommend that: • Insulin must never be frozen. • Direct sunlight or warming (in hot climates or inside a car on a sunny day) damages insulin. • Patients should not use insulin that has changed in appearance (clumping, frosting, precipitation, or discoloration). • Unused insulin should be stored in a refrigerator (4°C-8°C). • After first usage, an insulin vial should be discarded after 3 months if kept at 2°C to 8°C or 4 weeks if kept at room temperature. However, for some insulin preparations, manufacturers recommend only 10 to 14 days of use in room temperature. • In hot climates where refrigeration is not available, cooling jars, earthenware pitcher or a cool wet cloth around the insulin will help to preserve insulin activity.” |
“To transport insulin from distributor to health care facility, suitable rigid containers should be used to maintain the temperature between 2 and 8°C and to reduce damage to medicines during transit” (. . .) “Store insulin in current use (pen, cartridge or vial) at room temperature (for a maximum of 6 weeks after initial use, and within the expiry date). Store unopened insulin in an area of the refrigerator where freezing is unlikely to occur. If no refrigeration facilities are available in the home, it is recommended to liaise with the nearest health facility for the storage of the unopened insulin.” |
“Insulin vials should ideally be stored between 2-8°C in the middle compartment of a refrigerator. In settings, where a refrigerator is not available, at higher room temperatures, the vial in use should be stored wrapped in a plastic bag in a cool place <25°C, in a clay pot or a flask and discarded in 30 days. If stored above 25°C it may be used for a few weeks and should be discarded in 2-3 weeks. Do not use insulin past the expiry date. Extreme temperatures destroy insulin. Do not freeze insulin. If frozen by mistake, it should be discarded. Vials should not be exposed to direct sunlight or heat. Avoid storing in the kitchen, on top of electrical appliances or heaters. Do not keep vials in the glove compartment (cubby) of a car and leave in a stationary car with closed windows. Prior to use, insulin vials should be inspected thoroughly. Soluble insulin should appear clear, colourless and have no floating particles.” |
There is a paucity of recent stability studies in publicly available literature that could be utilized to give practical recommendations for insulin stability. The last published studies that research degradation kinetics of insulin are a few decades old and do not cover modern insulins.3,56,57 These data show that insulin is far more stable than storage recommendations suggest, but only limited studies have been published about the stability of modern insulins.21 Newer studies do not provide more clarity on insulin degradation kinetics because of different study setups, analytic methods, and research questions. For example, a study from India from 2009 finds that regular insulin formulations start losing potency from three weeks stored at 32°C and 37°C (89.6°F and 98.6°F), as assessed in rabbits and per HPLC.58 Another study from the United States simulated transit conditions for insulin shipped in winter and summer. After exposure to extreme temperatures like −10°C and 45°C (14°F and 113°F) for a cycle of three days, all samples of regular, NPH, and premixed insulin were still within specifications per HPLC analysis and without any visible changes.59 In a viewpoint from the year 2000, it was stated that insulin storage in tropical countries “is not an important practical issue,” referencing studies published in 1987 and 1996.60 To our understanding, this is an important practical issue in many countries—for families, HCPs, and international organizations. In their publication from 2016, comparing and testing clay pot cooling systems for when refrigeration is not available, Ogle et al also mention how scarce public data on insulin stability is. They stress that more studies on thermal stability of insulin are needed to know when the use of cooling systems is warranted so that PwD can reassure whether the storage is adequate.39
The lack of consensus in guidelines and of recent stability studies might be reflected by a varying level of knowledge, awareness, and standard practice between HCPs and PwD. This would be of interest to investigate further with respective survey-based studies. From our point of view, there is also a need for systematic evaluation of insulin stability at different levels of the cold chain, with a clear focus on pharmacies and storage in patients’ homes.
Summary
While insulin storage is well controlled and monitored during the supply chain, optimal insulin storage is not and cannot be guaranteed after dispensing, when used by PwD and when stored at home, since it was shown that household refrigerators bear a risk of storage at temperatures below freezing point in a number of cases. Further investigations are needed to examine possible links of insulin stability impairment and clinical outcomes in real-world use of PwD.
There is a lack of recent studies, scientific literature, and public information on insulin stability under different storage conditions. More transparency would help PwD manage risks better and assess what deviations from the recommended storage conditions mean for their therapy decisions. From a global perspective, better understanding about the impact of storage conditions on insulin stability would take away anxiety from PwD and their families, and health professionals about this. There is a need for a better understanding of PwD’s and HCP’s knowledge and awareness on insulin storage and for harmonized guidelines. This is in line with recent declarations that ask for developing a consensus statement and identification of needs for further research on insulin thermostability.61 Manufacturers should consider releasing more detailed information on insulin stability at varying temperatures. Evaluations of data from CGM sensors, dosage of insulin, and temperature sensors will provide further insight into potential patterns of insulin sensitivity changes under real-world conditions. Development of thermostable insulins is an interesting option to avoid many of the aspects discussed.62
Acknowledgments
We would like to thank several colleagues for their helpful suggestions and comments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LH is a consultant for a number of companies that are developing novel diagnostic and therapeutic options for diabetes treatment. He is a shareholder of Profil Institut für Stoffwechselforschung GmbH, Neuss, Germany and ProSciento, San Diego, CA. KB reports grants from the Berlin Institute of Health (BIH) Clinician Scientist program, the BIH Health Data Platform, fees for medical consulting from Medtronic Diabetes as a member of the Advisory Board “Impact,” medical consulting fees and paid talks from Roche Diabetes Care, Dexcom, Medtronic Diabetes, Diabeloop and Bertelsmann Stiftung, outside the submitted work. AC has no conflict of interest. AZ and LK are employees of MedAngel. This company is involved with product development that is directly related to the topics discussed in this manuscript. AZ reports paid and unpaid engagement activities with the patient committees and boards of Novo Nordisk, Eli Lilly, and Astra Zeneca.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
References
- 1. Herr JK, Keith S, Klug R, Pettis RJ. Characterizing normal-use temperature conditions of pumped insulin. J Diabetes Sci Technol. 2014;8(4):850-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woods RJ, Alarcon J, McVey E, Pettis RJ. Intrinsic fibrillation of fast-acting insulin analogs. J Diabetes Sci Technol. 2012;6(2):265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brange J. Galenics of insulin. Berlin, Heidelberg, New York, London, Paris, Tokyo: Springer-Verlag; 1987. [Google Scholar]
- 4. Braune K, Kraemer LA, Weinstein J, Zayani A, Heinemann L. Storage conditions of insulin in domestic refrigerators and when carried by patients: often outside recommended temperature range. Diabetes Technol Ther. 2019;21(5):238-244. [DOI] [PubMed] [Google Scholar]
- 5. Carter AW, Heinemann L. If PBMs guard access to drugs, then Quis Custodiet Ipsos Custodies? (Who Will Guard the Guardians?). J Diabetes Sci Technol. 2016; 10(6):1406-1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ICH. Stability testing of new drug substances and products Q1A(R2) ICH Quality Guidelines. Geneva: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); 2003. [Google Scholar]
- 7. Beals JM, DeFelippis MR, Kovach PM, Jackson JA. Insulin. In: Crommelin DJA, Sindelar RD, Meibohm B, eds. Pharmaceutical Biotechnology. Vol 4 New York: Springer; 2013. [Google Scholar]
- 8. Farid NA, Atkins LM, Becker GW, et al. Liquid chromatographic control of the identity, purity and “potency” of biomolecules used as drugs. J Pharm Biomed Anal. 1989;7(2):185-188. [DOI] [PubMed] [Google Scholar]
- 9. Fisher BV, Smith D. HPLC as a replacement for the animal response assays for insulin. J Pharm Biomed Anal. 1986;4(3):377-387. [DOI] [PubMed] [Google Scholar]
- 10. ICH. Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products Q6B ICH Quality Guidelines. Geneva: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); 1999. [Google Scholar]
- 11. Teska BM, Alarcon J, Pettis RJ, Randolph TW, Carpenter JF. Effects of phenol and meta-cresol depletion on insulin analog stability at physiological temperature. J Pharm Sci. 2014;103(8):2255-2267. [DOI] [PubMed] [Google Scholar]
- 12. US Pharmacopeia. Insulin Human Drug Products 100 IU/ml. Rockville, MD: United States Pharmacopeial Convention; 2016. [Google Scholar]
- 13. Insulin Human Drug Products 100 IU/ml. European Pharmacopoeia (Ph Eur). 9th ed. Strasbourg: European Pharmacopoeia Commission, Council of European Directorate for the Quality of Medicines; (EDQM); 2017. [Google Scholar]
- 14. Insulin Human Drug Products 100 IU/ml. Japanese Pharmacopoeia. 17th ed. Tokyo: Pharmaceuticals and Medical Devices Agency; 2016. [Google Scholar]
- 15. US Pharmacopeia. Insulin Human. Rockville, MD: United States Pharmacopeial Convention; 2016. [Google Scholar]
- 16. Insulin Human European Pharmacopoeia (Ph Eur). 9th ed. Strasbourg: European Pharmacopoeia Commission, Council of European Directorate for the Quality of Medicines (EDQM); 2017. [Google Scholar]
- 17. US Pharmacopeia. Chapter <121> Insulin Assays. United States Pharmacopeia and National Formulary. Rockville, MD: United States Pharmacopeial Convention; 2015. [Google Scholar]
- 18. Hack R, Rueggeberg S, Schneider L, et al. Progress towards the replacement of the rabbit blood sugar bioidentity assay by an in vitro test for batch release of insulins in quality control. ALTEX. 2017;34(4):565-566. [DOI] [PubMed] [Google Scholar]
- 19. European Medicines Agency: Medicines. EPARs, European Public Assessment Reports [article online]. Available from https://www.ema.europa.eu/en/medicines. Accessed May 4, 2019.
- 20. Carter AW, Heinemann L. Insulin Concentration in vials randomly purchased in pharmacies in the United States: considerable loss in the cold supply chain. J Diabetes Sci Technol. 2018;12(4):839-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moses A, Bjerrum J, Hach M, Waehrens LH, Toft AD. Concentrations of intact insulin concurs with FDA and EMA standards when measured by HPLC in different parts of the distribution cold chain. J Diabetes Sci Technol. 2019;13(1):55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neumiller JJ, Chen G, Newsome C, Hughes S, Lazarus P, White JR., Jr Assessment of regular and NPH insulin concentration via two methods of quantification: the Washington State Insulin Concentration Study (WICS). J Diabetes Sci Technol. (October 2019). doi: 10.1177/1932296819883291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malmodin D, Pedersen A, Karlsson BG, Forsander G. NMR spectroscopic analysis to evaluate the quality of insulin: concentration, variability, and excipient content. J Diabetes Sci Technol. 2020;14(1):180-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Briggs KT, Taraban MB, Wang W, Yu YB. Nondestructive quantitative inspection of drug products using Benchtop NMR Relaxometry-the Case of NovoMix(R) 30. AAPS PharmSciTech. 2019;20(5):189. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organisation Expert Committee on Specifications for Pharmaceutical Preparations. Model Guidance for the Storage and Transport of Time- and Temperature–Sensitive Pharmaceutical Products. WHO Technical Report Series. No.961, Annex 9. Geneva: World Health Organisation; 2011. [Google Scholar]
- 26. European Commission. Guidelines of 5 November 2013 on Good Distribution Practice of Medicinal Products for Human Use. Official Journal of the European Union Commission guideline 2013/C343/012013. [Google Scholar]
- 27. US Pharmacopeia. General Information <1079> Good Storage and Shipping Practices. United States Pharmacopeia and National Formulary. Rockville, MD: United States Pharmacopeial Convention. [Google Scholar]
- 28. 06-23048 - Eli Lilly and Company v. Air Express International USA, Inc. et al. United States District Court Southern District of Florida; 2009. [Google Scholar]
- 29. 14.4 Biological medicines: specific requirements. Australian Regulatory Guidelines for Prescription Medicines (ARGPM). Symonston, Australia: Australian Government Department of Health Therapeutic Goods Administration; 2017. [Google Scholar]
- 30. Quality Improvement and Change Management Unit. WA Health Safe Use of Medication Refrigerators Policy. Perth: Department of Health Western Australia; 2015. [Google Scholar]
- 31. Golay A, Felber JP. Evolution from obesity to diabetes. Diabete Metab. 1994;20:3-14. [PubMed] [Google Scholar]
- 32. Grajower MM, Fraser CG, Holcombe JH, et al. How long should insulin be used once a vial is started? Diabetes Care. 2003;26(9):2665-2666; discussion 266-9. [DOI] [PubMed] [Google Scholar]
- 33. Molitch ME. How long should insulin be used once a vial is started? Diabetes Care. 2004;27(5):1240-1241; author reply 1-2. [DOI] [PubMed] [Google Scholar]
- 34. Grajower MM. How long can a vial of insulin be used after it is started: where are we 10 years later? Endocr Pract. 2014;20(2):188-190. [DOI] [PubMed] [Google Scholar]
- 35. Turner JM, Unni EJ, Strohecker J, Henrichs J. Prevalence of insulin glargine vial use beyond 28 days in a Medicaid population. J Am Pharm Assoc (2003). 2018;58(4S):S37-S40. [DOI] [PubMed] [Google Scholar]
- 36. U.S. Food and Drug Administration: Drugs@FDA (SmPCs, Summary of Product Characteristics) [article online]. Available from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 04 May 2019
- 37. Hunt DG, Chandler C, Ulrich DA, Poska R, Montero A. USP Controlled Room Temperature Range Expansion - Stimuli to Revision Process. International Pharmacopoeia Guidelines 2014. 2014(USP 33–NF 28 Reissue R-11). [Google Scholar]
- 38. Gallo M, Comoglio M, De Micheli A, Monge L, Vespasiani G. Insulin storage in Europe: a comment to Grajower et al., Eli Lilly, and Novo Nordisk. Diabetes Care. 2004;27(5):1225-1226. [DOI] [PubMed] [Google Scholar]
- 39. Ogle GD, Abdullah M, Mason D, Januszewski AS, Besancon S. Insulin storage in hot climates without refrigeration: temperature reduction efficacy of clay pots and other techniques. Diabet Med. 2016;33(11):1544-1553. [DOI] [PubMed] [Google Scholar]
- 40. Vlieland ND, Gardarsdottir H, Bouvy ML, Egberts TC, van den Bemt BJ. The majority of patients do not store their biologic disease-modifying antirheumatic drugs within the recommended temperature range. Rheumatology (Oxford). 2016;55(4):704-709. [DOI] [PubMed] [Google Scholar]
- 41. de Jong MJ, Pierik MJ, Peters A, Roemers M, Hilhorst V, van Tubergen A. Exploring conditions for redistribution of anti-tumor necrosis factors to reduce spillage: a study on the quality of anti-tumor necrosis factor home storage. J Gastroenterol Hepatol. 2018;33(2):426-430. [DOI] [PubMed] [Google Scholar]
- 42. Cuellar MJ, Marco JL, Perez-Castello I, Castello Escriva A. [Quality of storage of thermolabile drugs in patients’ homes]. Rev Calid Asist. 2010;25(2):64-69. [DOI] [PubMed] [Google Scholar]
- 43. Chojcnacky MMW, Ripple D, Strouse G. Thermal Analysis of Refrigeration Systems Used for Vaccine Storage. NISTIR 7656. US Department of Commerce: National Institute of Standards and Technology; 2009. [Google Scholar]
- 44. Laguerre O, Derens E, Palagos B. Study of domestic refrigerator temperature and analysis of factors affecting temperature: a French survey. International Journal of Refrigeration. 2002;25:653-659. [Google Scholar]
- 45. James S, Evans J, James C. A review of the performance of domestic refrigerators. J Food Eng. 2008;87(1):2-10. [Google Scholar]
- 46. Gomez-Peralta F, Abreu C, Gomez-Rodriguez S, Ruiz L. Insulclock: a novel insulin delivery optimization and tracking system. Diabetes Technol Ther. 2019;21(4):209-214. [DOI] [PubMed] [Google Scholar]
- 47. Pryce R. Diabetic ketoacidosis caused by exposure of insulin pump to heat and sunlight. BMJ. 2009;338:a2218. doi: 10.1136/bmj.a2218. [DOI] [PubMed] [Google Scholar]
- 48. Minuto N, Tambroni B, Vannati M, et al. Diabetic ketoacidosis caused by exposure of insulin to low temperature. Diabetes Technol Ther. 2010;12(9):745-746. [DOI] [PubMed] [Google Scholar]
- 49. Nassar AA, Childs RD, Boyle ME, et al. Diabetes in the desert: what do patients know about the heat? J Diabetes Sci Technol. 2010;4(5):1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bahendeka S, Kaushik R, Swai AB, et al. EADSG guidelines: insulin storage and optimisation of injection technique in diabetes management. Diabetes Ther. 2019;10(2):341-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sjolund J. Pen cap for medication injection pen having temperature sensor, Pub. No.: US20190184111A1, Appl. No. 16/218, 251. United States Patent Application Publication; 2019. [Google Scholar]
- 52. American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care; 2019. https://care.diabetesjournals.org/content/43/Supplement_1
- 53. American Diabetes Association. Insulin Storage and Syringe Safety. https://www.diabetes.org/diabetes/medication-management/insulin-other-injectables/insulin-storage-and-syringe-safety. Accessed August 25, 2019.
- 54. Danne T, Phillip M, Buckingham BA, et al. ISPAD clinical practice consensus guidelines 2018: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(suppl 27):115-135. [DOI] [PubMed] [Google Scholar]
- 55. Sri Lanka Medical Association. Best Practice in Insulin Injection Technique. A Simplified Guideline. (Adapted from the Fit-india Guidelines 2012 and 2015) - Sinhalese Version. Essential Medicines and Health Products Information Portal World Health Organization 2017. http://apps.who.int/medicinedocs/en/m/abstract/Js23549sh/.
- 56. Pingel M, Volund A. Stability of insulin preparations. Diabetes. 1972;21(7):805-813. [DOI] [PubMed] [Google Scholar]
- 57. Storvick WO, Henry HJ. Effect of storage temperature on stability of commercial insulin preparations. Diabetes. 1968;17(8):499-502. [DOI] [PubMed] [Google Scholar]
- 58. Vimalavathini R, Gitanjali B. Effect of temperature on the potency & pharmacological action of insulin. Indian J Med Res. 2009;130(2):166-169. [PubMed] [Google Scholar]
- 59. Chandler C, Gryniewicz CM, Pringle T, Cunningham F. Insulin temperature and stability under simulated transit conditions. Am J Health Syst Pharm. 2008;65(10):953-963. [DOI] [PubMed] [Google Scholar]
- 60. Gill GV. Viewpoint: stability of insulin in tropical countries. Trop Med Int Health. 2000;5(9):666-667. [DOI] [PubMed] [Google Scholar]
- 61. Kehlenbrink S, Jaacks LM. Boston Declaration signatories. Diabetes in humanitarian crises: the Boston Declaration. Lancet Diabetes Endocrinol. 2019;7(8):590-592. [DOI] [PubMed] [Google Scholar]
- 62. Glidden MD, Aldabbagh K, Phillips NB, et al. An ultra-stable single-chain insulin analog resists thermal inactivation and exhibits biological signaling duration equivalent to the native protein. J Biol Chem. 2018;293(1):47-68. [DOI] [PMC free article] [PubMed] [Google Scholar]