Abstract
The basis of pharmacotherapy requires knowledge of two properties of a drug: pharmacokinetics (PK) and pharmacodynamics (PD). In the era of precision medicine, there is growing interest in determining between-individual variations in PK and PD. While these two dimensions of pharmacotherapy are key foci of investigation, a third property is also emerging as a critical factor in understanding how a drug affects an individual. This third property of a drug is known as phamacoadherence (PA). There can be wide variation in PA among people with diabetes, whether they are using oral or injectable medications. The use of new digital health interventions and telehealth communication tools, such as smart insulin pens, is now creating opportunities for health care professionals to have a more complete understanding of the PA of drugs, which allows for more personalized prescribing practices.
Keywords: diabetes, digital health, missed insulin, pharmacoadherence
Pharmacokinetics and Pharmacodynamics
Pharmacotherapy is the treatment of a disease with medications. This field traditionally examines two properties of a medication: its pharmacokinetics (PK) and pharmacodynamics (PD). A drug’s PK is the link between a dose and the concentration of the drug in various body fluids over time, including the drug’s absorption, distribution, metabolism, and excretion. A drug’s PD is the effect of a drug on the body, including its molecular, biochemical, and physiological actions.1 Traditionally, data on a drug’s PK and PD provide a rational basis for selecting the choice and dose of a medication as a therapeutic intervention. Application of these two principles of pharmacotherapy to support timely, effective, and safe prescribing assumes that the patient is actually going to take the drug in the prescribed dose and the agreed time.
The Third Dimension of Pharmacotherapy: Pharmacoadherence
In 2008, Chisholm-Burns and Spivey coined the term “pharmacoadherence” (PA), which they defined as the “extent to which a patient follows a given therapeutic medication regimen as agreed on in partnership with a healthcare professional.”2 The three dimensions of pharmacotherapy—PK, PD, and PA—are necessary to plan and understand the effect of prescribing for any disease, including diabetes. Combining PA data from sensors measuring drug adherence in an individual with established laboratory PK and PD data can provide researchers and clinicians with valuable information for personalized drug development and prescribing in clinical practice. The combination of PK, PD, and PA has been referred to as the Internet of Pharmaceutical Things.3
Failure to initiate or change therapy when needed can be attributed to the health care professional (HCP). The concept of PA is attributed to a person with diabetes—that is, when they do not use an agreed upon dose of a medication or do not use any medication at all. The concept of adherence (to prescribed therapies) contrasts with the term compliance, which is a pejorative term suggesting obedience. Failure of a clinician to initiate or intensify therapy is known as therapeutic inertia, and failure of a person with diabetes to use prescribed therapy, which has been agreed with their HCP, is known as nonadherence.4
The Importance of Adherence to Diabetes Pharmacotherapy
According to a report by the World Health Organization, “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.”5 Improving adherence to oral medications in type 2 diabetes is associated with a significant reduction in diabetes-related complications, improved quality of life, lower risk of premature death, fewer emergency department visits, fewer hospitalizations, and reduced medical costs.6
Failure to acknowledge PA as a clinical challenge can negatively influence the approach used by an HCP to prescribing. For example, when a person with diabetes using insulin reports high blood glucose levels or above target hemoglobin A1C (HbA1c) levels, it is common for the HCP to suggest altering the dose, timing, or frequency of insulin injections/boluses or the addition of other therapies based on the potentially erroneous assumption that prior doses have been taken. Similarly, in approaching the challenge of preventing hypoglycemia, consideration of the PK and PD of the type of insulin being prescribed is included in therapeutic planning (eg, thinking about insulin on board). For people with diabetes using insulin injections, until recently, these approaches have taken place in the absence of accurate information of the timing and dose of administered insulin. Without this information as surrogate measures of adherence, a recommendation to increase the usual dose of insulin could lead to an increase in the frequency, duration, and/or severity of hypoglycemia. Similarly, failure to appreciate adherence to a prescribed insulin regimen can also put an individual at increased risk of severe complications in the future.
Barriers to Pharmacoadherence
Taking a medication at the right time and at the right dose can be challenging. A person with diabetes is asked to consider seven properties of any drug they are prescribed (Table 1).7 This type of routine can cause a significant cognitive burden. Reasons for poor adherence are often multifactorial, including the frequency of medications being suggested, their mode of delivery (eg, oral medicines may be preferred over injections, alternative modes of delivery such as injection versus infusion), cost, and the personal burden. Concerns about anticipated outcomes may also influence adherence; for example, a fear of side effects from insulin, such as injection site reactions, hypoglycemia, or weight gain, can lead to insulin omission.8 Other factors influencing adherence relate to psychosocial influences, such as peer influences, culture, myths, and stigma.9
Table 1.
Seven Properties of a Drug That Must Be Remembered to Successfully Take a Single Pill.7
| 1. The name of the drug |
| 2. The disease for which it is used |
| 3. How to take the medication |
| 4. The number of daily doses |
| 5. When to take the drug relative to a meal |
| 6. The dose |
| 7. The duration |
A dose of insulin may be missed for many reasons. These reasons can be categorized as either intentional or unintentional nonadherence.10 With the former, there is a conscious decision to not take the medication. In contrast, when omission is unintentional, the medication is omitted because of forgetfulness, misunderstanding of the instructions, or lack of access to the medication.11 Unintentional adherence is more easily remedied because it is not dependent on a conscious decision to omit the dose. For unintentional nonadherence, factors such as lifestyle or workload might contribute to forgetfulness.12 Challenges to health literacy and numeracy may also be influential.13 To reduce intentional nonadherence, there must be an understanding of the personal gain from taking a medication. Trust in physicians and constancy of habits are important modifiable factors associated with adherence that can be reinforced through education and a collaborative physician-patient relationship.14 Fifteen behavioral strategies that can be applied to digital health (DH) tools to promote PA are listed in Table 2.15
Table 2.
Fifteen Process Motivators That Can Lead to Increased PA.15
| Challenge Choice/control |
| Community |
| Competence |
| Competition |
| Context |
| Curiosity |
| Growth mind-set |
| Identity |
| Personalization |
| Piggybacking |
| Pride |
| Reframing |
| Taste |
| Teamwork |
Abbreviation: PA, phamacoadherence.
Consequences of Poor Pharmacoadherence to Insulin Therapy
Nonadherence to drugs prescribed for diabetes may range from 53% to 65%4 and may account for up to 75% of the gap between the HbA1c lowering demonstrated in randomized controlled trials compared to real-world evidence.16 It is now estimated that mealtime insulin dosing is late or missed with 25%-27% of meals.17,18 Modeling studies suggest that insulin omission can lead to worse glycemic control. For example, omitting 2.1 meal-related injections per week can lead to an increase in HbA1c of at least 0.3%-0.4%, omitting 2.1 bolus injections per week would lead to an increase in HbA1c of 0.2%-0.3%, and omitting 39% of all injections would lead to an increase in HbA1c of 1.8%.19 Similarly, in a survey of Type 1 Diabetes (T1D) Exchange members under the age of 26 years, those who reported missing ≥1 insulin dose per week (compared to those rarely missing an injection) had higher HbA1c concentrations (9.8% vs 8.3%, P < .001) and were more likely to experience at least one episode of diabetic ketoacidosis (9% vs 5%, adjusted P = .001).20 In a study in children, the rise in postprandial glycemia following a snack either with or without pre-snack insulin was, respectively, 52 mg/dL compared to 114 mg/dL (P < .001).21 Among children with T1D, as few as two missed insulin bolus doses each week can result in an increased HbA1c concentration of 0.5%.22 In a study of children using insulin pump therapy, in any given day, 38% of the patients missed at least 15% of their insulin doses, and these children also had HbA1c levels 0.8% higher than the cohort that missed no more than 15% of their bolus insulin doses.23 In a series of Austrian children with T1D, intentional overdose of insulin was almost as common as insulin omission.24 Among a cohort of children that included both insulin dose manipulators and insulin users, psychiatric comorbidity was found in 46.3% compared to 17.5% of patients, respectively, and the former group compared to the latter group had higher HbA1c levels by an average of 0.89%.25
For people with diabetes prescribed oral agents, the traditional measures of success are HbA1c, blood pressure, and/or levels of cholesterol and other lipids. These variables are rarely associated with symptoms and are often dependent on a third-party professional for providing the numbers. Side effects are more common than positive symptoms or alleviation of negative ones. Therefore, a positive feedback loop to maintain PA is lacking. It is already known that people with diabetes have very limited opportunities to interact with HCPs26 and are therefore left on their own to consider PA. Although real-time continuous glucose monitoring (CGM) has the potential to provide immediate feedback from changes in therapy, only a minority of people with diabetes have access to this technology. Similarly, self-monitoring of blood glucose (SMBG) can be helpful, but there are additional limitations to this form of self-monitoring.27 Increased use of structured SMBG may be more useful.28
Both nonelectronic and electronic tools can be used to promote PA. An advantage of nonelectronic tools is that they are simple to use and require no electronic components, but any documentation of adherence must be carried out manually. An advantage of electronic tools is that they contain sensors that make them part of the Internet of Things, which is a network of objects that automatically communicates with other systems by way of the Internet, and they can send information automatically to a cloud-based electronic patient record.
Nonelectronic Tools to Promote Adherence
Drug reminder packages, such as pill organizers and blister packs with a calendar, are widely used as a means to promote medication adherence.29 These approaches have been shown to work well in the geriatric population, which includes people with diabetes who must deal with polypharmacy. Direct supervision of drug therapy can improve medication adherence in residential care settings. This approach requires that a caregiver either observe or dispense therapy directly to the patient.30
Electronic Communication Tools to Promote Pharmacoadherence
DH and telehealth are modern electronic communication tools applied to healthcare. Telehealth is the exchange of medical information using platforms, such as email, texting, phones, and video.31 DH is the convergence of wearable devices, information technology, and electronic communication tools32 (ie, sensors, software, and mobile communication platforms) to support the practice of medicine. DH converts sensor information into software that provides either information, treatment recommendations, or controlled drug delivery. The three functions of DH mobile software applications or apps are to: (1) provide enhanced access to health information to people with diabetes, HCPs, and researchers; (2) facilitate remote monitoring and diagnosis; and (3) deliver timely treatment recommendations or facilitate remotely controlled actions for treatment.33 This third function is an opportunity to improve PA. When an intervention that treats a disease or improves adherence to a prescribed medication is driven by software, then this treatment reflects an application of digital therapeutics.34
Seven types of electronic telehealth communication tools without sensor input have been used to provide reminder messages intended to improve adherence to diabetes medications. These include:
nudge reminders that subtly encourage rather than mandate change,37
mobile apps sending messages,38
voice messaging by phone,41
serious video games to educate or motivate about medications,42 and
multimodal interventions, eg, “Smartphone Medication Adherence Saves Kidneys” for kidney transplantation recipients, consisting of automated reminders and text messages along with automated summary reports for HCPs.43
These seven forms of communication platforms are examples of telehealth communication rather than true DH, which uses sensor input to determine the type and intensity of the intervention. DH tools may help improve PA by generating sensor data to (1) provide feedback on the benefits of adherence, (2) overcome fear of the consequences of being adherent to medication (eg, risk of hypoglycemia), and (3) support patient-doctor conversations.7
Digital Health Interventions to Promote Pharmacoadherence
Three types of DH interventions for drugs used in diabetes have been developed, which integrate sensor information to inform a person with diabetes and/or their HCP. These can be used with (1) specially formulated oral agents, (2) all oral agents, or (3) insulin. Furthermore, an association between the frequency of using DH activity trackers and adherence to diabetes medications has been observed, leading to a hypothesis that incentivizing health tracking might result in better adherence to diabetes medications.44
For promoting adherence to oral medications, a digital medicine offering has been tested that measures adherence to ingestion of medications, including oral agents for diabetes. With the use of a wearable sensor patch, a mobile device app can inform the person with diabetes or HCP wirelessly through a mobile app if or when a specially formulated pill (containing a medication co-encapsulated with a sensor) has been ingested.45,46 Further development of this product has recently been discontinued.47
An electronic pillbox with a sensor that records when a lid opens could be used to improve adherence through (1) audible alarms, (2) visible alarms, and (3) messages sent to cellphones and computers. However, these types of products can be challenging to use or are expensive.48 Also, because of electronic malfunction or incorrect medication self-administration, even if the pillbox sensor signals that the lid was opened, there is no assurance that the medication was actually removed from the box and taken.49
Smart Insulin Pens
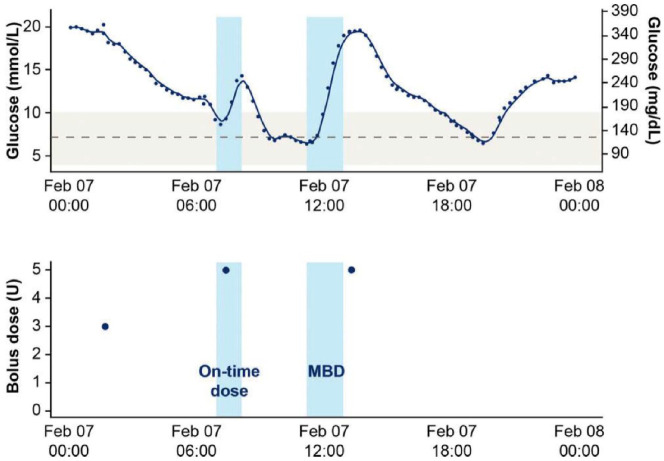
Smart insulin pens are by far the most promising DH tool for improving PA to insulin. These devices use a sensor, which is part of an insulin pen, to collect and store data on the date and time of injections and the number of units administered. This information can be wirelessly downloaded to a cloud-based database and visualized on a mobile platform or computer. Smart insulin pens provide accurate information to the HCP about (1) missed doses, (2) injection times relative to meals, and (3) dose sizes relative to meals. Insulin dosing data can be superimposed on CGM data to provide an accurate picture of the relationship between actually administered doses of insulin and levels of glycemia.50 See Figure 1 for an example of superimposed glucose data from a CGM and insulin dosing data from a smart insulin pen.51
Figure 1.
(Top): CGM data from a day with two meals detected. The solid dark blue line represents the CGM signal. The light blue shaded areas each represent a meal detected with a glycemia rate-of-change algorithm. The gray, dashed line represents a glucose level of 130 mg/dL and the light gray shaded area represents a target glycemic range of 70-180 mg/dL.
(Bottom): Insulin dosing data from a smart insulin pen on the same day. The blue circles in the lower figure indicate bolus doses. A bolus dose within 15 minutes before to 60 minutes after the start of a meal is considered to be an on-time bolus dose. A dose outside of this 75-minute time window is considered a missed bolus dose (MBD). Note. CGM, continuous glucose monitoring.
Reproduced from Adolfsson et al51 with permission from Diabetes Technology and Therapeutics.
The first study of clinical outcomes using a smart insulin pen was reported in 2020.51 This study reported real-world, individual patient-controlled results from 94 participants with T1D using CGM, who administered bolus and basal insulin using the Novo Nordisk NovoPen6 (Novo Nordisk, Bagsværd, Denmark) for a mean duration of 223 days. Blinded baseline data were used so that each participant served as their own control. From baseline to follow-up, there was a significant increase in time in range (70-180 mg/dL) of 1.9 hours/day (P < .001), which was an absolute improvement of 8.5% of the day. There was also a significant reduction in time above 180 mg/dL of 1.8 hours per day, a significant reduction in time spent in Level 2 hypoglycemia (<54 mg/dL) of 0.3 hours (P = .005), and a nonsignificant reduction in time spent in Level 1 hypoglycemia (54-69 mg/dL) of 0.2 hours (P = .181). The absolute incidence of missed bolus doses decreased from 25% to 14%, which was a relative improvement of 43%. The authors concluded that a smart insulin pen can contribute to insulin management and improve glycemic control and dosing behavior.51
Digital Health Interventions for Pharmacoadherence Without Reminders
Other types of DH and telehealth interventions (ie, educational, behavioral, and physiological) could indirectly promote PA without necessarily sending reminders to take medications. First, PA interventions may be based on mobile apps or software that offer diabetes education; for example, by improving access to Diabetes Self-Management Education and Support and upgrading the content and delivery of the education to avoid boredom and loss of engagement with participants.15 Second, PA interventions may be based on behavior-modifying software; for example, by facilitating greater use of glucose monitoring devices that automatically upload data to the cloud and from there to mobile apps and similar software. Finally, PA interventions may be based on new physiological monitoring sensors that can allow new short-term outcomes to be measured; for example, by promoting specific exercises through exercise tracking sensors rather than only focusing on long-term glycemic outcomes.
The Future of DH and Telehealth for Diabetes
The current COVID-19 pandemic has expedited the adoption of DH in clinical practices. The widespread use of telehealth is expected to continue even after the pandemic.52 For diabetes care, telehealth services now can range from downloading and interpreting BG data, insulin delivery data, and other sensor data, to providing both synchronous and asynchronous diabetes education, to the facilitation of clinician patient interaction over an online platform. Ceriello have described a six-step cycle for personalized diabetes management, which portrays telehealth and DH as iterative processes.53 DH and telehealth can be thought of as enablers of PA, and optimal PA can be thought of as a digitally enabled essential component of therapy. The use of DH to promote PA should now be considered for all routine interactions with patients, and especially those with chronic diseases like diabetes. The increasing availability of automated and electronic tools for PA will support these interactions.
Conclusion
The most effective therapy for diabetes is safe, effective, and, most importantly, followed. A collaborative agreement between a person with diabetes and their healthcare professional can be described as PA when it relates to drug treatment. PA requires that the person with diabetes must have (1) motivation, (2) knowledge, (3) skills, and (4) access to any agreed-upon treatment. In all these four domains, there are opportunities for DH. Using PA as an outcome for DH brings in the key components of value in the delivery of care, namely clinical outcomes and the experience of care with lower costs, with cost being defined as personal burden, as well as the health-economic cost. With DH tools becoming increasingly integrated into diabetes care and telehealth now becoming mainstream amidst the COVID-19 pandemic, software and hardware that enhances PA could become critically important for facilitating personalized medication treatment plans.
Acknowledgments
We acknowledge Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DCK is a consultant for Dexcom, Eoflow, Fractyl, Lifecare, Novo Nordisk, Roche Diagnostics, Samsung, and Thirdwayv. JYZ, TS, and CM have nothing to disclose. DK has participated in paid advisory boards for NovoNordisk, Sanofi and Abbott Diabetes Care and is in receipt of research support from Lilly. DK is also a medical advisor to Glooko for which he is in receipt of share options.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
Jennifer Y. Zhang  https://orcid.org/0000-0002-3374-9777
https://orcid.org/0000-0002-3374-9777
Trisha Shang  https://orcid.org/0000-0001-9687-9336
https://orcid.org/0000-0001-9687-9336
Chhavi Mehta  https://orcid.org/0000-0003-0223-5009
https://orcid.org/0000-0003-0223-5009
David Kerr  https://orcid.org/0000-0003-1335-1857
https://orcid.org/0000-0003-1335-1857
References
- 1. Derendorf H, Meibohm B. Modeling of pharmacokinetic/pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharm Res. 1999;16(2):176-185. [DOI] [PubMed] [Google Scholar]
- 2. Chisholm-Burns MA, Spivey CA. Pharmacoadherence: a new term for a significant problem. Am J Health Syst Pharm. 2008;65(7):661-667. [DOI] [PubMed] [Google Scholar]
- 3. Özdemir V, Endrenyi L. A new approach to measure adherence to medicines using biomarkers and sensors. OMICS. 2019;23(7):334-337. [DOI] [PubMed] [Google Scholar]
- 4. Giugliano D, Maiorino MI, Bellastella G, Esposito K. Clinical inertia, reverse clinical inertia, and medication non-adherence in type 2 diabetes. J Endocrinol Invest. 2019;42(5):495-503. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Adherence to long-term therapies: evidence for action. 2003. https://www.who.int/chp/knowledge/publications/adherence_introduction.pdf?ua=1. Accessed September 7, 2020.
- 6. Pednekar P, Heller DA, Peterson AM. Association of medication adherence with hospital utilization and costs among elderly with diabetes enrolled in a state pharmaceutical assistance program. J Manag Care Spec Pharm. 2020;26(9):1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reach G. Can technology improve adherence to long-term therapies? J Diabetes Sci Technol. 2009;3(3):492-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frias JP, Dex T, Roberts M, Kaplan A. A review of the safety and adverse event profile of the fixed-ratio combination of insulin glargine and lixisenatide. Diabetes Ther. 2019;10(1):21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar K, Greenfield S, Raza K, Gill P, Stack R. Understanding adherence-related beliefs about medicine amongst patients of South Asian origin with diabetes and cardiovascular disease patients: a qualitative synthesis. BMC Endocr Disord. 2016;16(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chan AHY, Horne R, Hankins M, Chisari C. The Medication Adherence Report Scale: a measurement tool for eliciting patients’ reports of nonadherence. Br J Clin Pharmacol. 2020;86(7):1281-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reach G. Decisions in the psychology of glucose monitoring. J Diabetes Sci Technol. 2019;13(6):1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harashima SI, Nishimura A, Inagaki N. Attitudes of patients and physicians to insulin therapy in Japan: an analysis of the Global Attitude of Patients and Physicians in Insulin Therapy study. Expert Opin Pharmacother. 2017;18(1):5-11. [DOI] [PubMed] [Google Scholar]
- 13. Marden S, Thomas PW, Sheppard ZA, Knott J, Lueddeke J, Kerr D. Poor numeracy skills are associated with glycaemic control in Type 1 diabetes. Diabet Med. 2012;29(5):662-669. [DOI] [PubMed] [Google Scholar]
- 14. Reach G, Pellan M, Crine A, Touboul C, Ciocca A, Djoudi Y. Holistic psychosocial determinants of adherence to medication in people with type 2 diabetes. Diabetes Metab. 2018;44(6):500-507. [DOI] [PubMed] [Google Scholar]
- 15. Klonoff DC. Behavioral theory: the missing ingredient for digital health tools to change behavior and increase adherence. J Diabetes Sci Technol. 2019;13(2):276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425-1432. [DOI] [PubMed] [Google Scholar]
- 17. Adolfsson P, Hartvig NV, Kaas A, Møller JB, Hellman J. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther. 2020;22:709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norlander LM, Anderson SA, Levy CJ, Ekhlaspour L. Late and missed meal boluses with multiple daily insulin injections. Diabetes. 2018;68(suppl 1):A259. [Google Scholar]
- 19. Randløv J, Poulsen JU. How much do forgotten insulin injections matter to hemoglobin a1c in people with diabetes? A simulation study. J Diabetes Sci Technol. 2008;2(2):229-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Datye KA, Boyle CT, Simmons J, Moor DJ, Jaser SS, Sheanon N. Timing of meal insulin and its relation to adherence to therapy in type 1 diabetes. J Diabetes Sci Technol. 2018;12(2):349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vanderwel BW, Messer LH, Horton LA, et al. Missed insulin boluses for snacks in youth with type 1 diabetes. Diabetes Care. 2010;33(3):507-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burdick J, Chase HP, Slover RH, et al. Missed insulin meal boluses and elevated hemoglobin A1c levels in children receiving insulin pump therapy. Pediatrics. 2004;113(3 pt 1):e221-e224. [DOI] [PubMed] [Google Scholar]
- 23. Olinder AL, Kernell A, Smide B. Missed bolus doses: devastating for metabolic control in CSII-treated adolescents with type 1 diabetes. Pediatr Diabetes. 2009;10(2):142-148. [DOI] [PubMed] [Google Scholar]
- 24. Schober E, Wagner G, Berger G, et al. Prevalence of intentional under- and overdosing of insulin in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12(7):627-631. [DOI] [PubMed] [Google Scholar]
- 25. Berger G, Waldhoer T, Barrientos I, et al. Association of insulin-manipulation and psychiatric disorders: a systematic epidemiological evaluation of adolescents with type 1 diabetes in Austria. Pediatr Diabetes. 2019;20(1):127-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schatz D. 2016 presidential address: diabetes at 212°-confronting the invisible disease [published correction appears in Diabetes Care 2017 May;40(5):726]. Diabetes Care. 2016;39(10):1657-1663.27660119 [Google Scholar]
- 27. Weatherly J, Kishnani S, Aye T. Challenges with patient adoption of automated integration of blood glucose meter data in the electronic health record. Diabetes Technol Ther. 2019;21(11):671-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zedler BK, Kakad P, Colilla S, Murrelle L, Shah NR. Does packaging with a calendar feature improve adherence to self-administered medication for long-term use? A systematic review. Clin Ther. 2011;33(1):62-73. [DOI] [PubMed] [Google Scholar]
- 30. Boeni F, Spinatsch E, Suter K, Hersberger KE, Arnet I. Effect of drug reminder packaging on medication adherence: a systematic review revealing research gaps. Syst Rev. 2014;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klonoff DC. Telemedicine for diabetes: current and future trends. J Diabetes Sci Technol. 2015;10(1):3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klonoff DC, Kerr D. Overcoming barriers to adoption of digital health tools for diabetes. J Diabetes Sci Technol. 2018;12(1):3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klonoff DC, Kerr D. Digital diabetes communication: there’s an app for that. J Diabetes Sci Technol. 2016;10(5):1003-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dang A, Arora D, Rane P. Role of digital therapeutics and the changing future of healthcare. J Family Med Prim Care. 2020;9(5):2207-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graetz I, Huang J, Muelly ER, Fireman B, Hsu J, Reed ME. Association of mobile patient portal access with diabetes medication adherence and glycemic levels among adults with diabetes. JAMA Netw Open. 2020;3(2):e1921429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quinn CC, Butler EC, Swasey KK, et al. Mobile diabetes intervention study of patient engagement and impact on blood glucose: mixed methods analysis. JMIR Mhealth Uhealth. 2018;6(2):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kwan YH, Cheng TY, Yoon S, et al. A systematic review of nudge theories and strategies used to influence adult health behaviour and outcome in diabetes management. Diabetes Metab 2020;46:450-460. [DOI] [PubMed] [Google Scholar]
- 38. Wang Y, Min J, Khuri J, et al. Effectiveness of mobile health interventions on diabetes and obesity treatment and management: systematic review of systematic reviews. JMIR Mhealth Uhealth. 2020;8(4):e15400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kassavou A, Mirzaei V, Brimicombe J, et al. A highly tailored text and voice messaging intervention to improve medication adherence in patients with either or both hypertension and type 2 diabetes in a UK primary care setting: feasibility randomized controlled trial of clinical effectiveness. J Med Internet Res. 2020;22(5):e16629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prinjha S, Ricci-Cabello I, Newhouse N, Farmer A. British South Asian patients’ perspectives on the relevance and acceptability of mobile health text messaging to support medication adherence for type 2 diabetes: qualitative study. JMIR Mhealth Uhealth. 2020;8(4):e15789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Som A, Patel K, Sink E, et al. A novel patient engagement platform using accessible text messages and calls (Epharmix): feasibility study. JMIR Form Res. 2017;1(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abraham O, LeMay S, Bittner S, Thakur T, Stafford H, Brown R. Investigating serious games that incorporate medication use for patients: systematic literature review. JMIR Serious Games. 2020;8(2):e16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McGillicuddy J, Chandler J, Sox L, et al. “Smartphone medication adherence saves kidneys” for kidney transplantation recipients: protocol for a randomized controlled trial. JMIR Res Protoc. 2019;8(6):e13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quisel T, Foschini L, Zbikowski SM, Juusola JL. The association between medication adherence for chronic conditions and digital health activity tracking: retrospective analysis [published correction appears in J Med Internet Res 2019 Dec 10;21(12):e17375]. J Med Internet Res. 2019;21(3):e11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moorhead P, Zavala A, Kim Y, Virdi NS. Efficacy and safety of a medication dose reminder feature in a digital health offering with the use of sensor-enabled medicines. J Am Pharm Assoc (2003). 2017;57(2):155-161.e1. [DOI] [PubMed] [Google Scholar]
- 46. Frias J, Virdi N, Raja P, Kim Y, Savage G, Osterberg L. Effectiveness of digital medicines to improve clinical outcomes in patients with uncontrolled hypertension and type 2 diabetes: prospective, open-label, cluster-randomized pilot clinical trial. J Med Internet Res. 2017;19(7):e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Farr C. Proteus digital health, once valued at $1.5 billion, files for chapter 11 bankruptcy. CNBC [Internet]; 2020. June https://www.cnbc.com/2020/06/15/proteus-digital-health-once-worth-1point5-billion-files-for-chapter-11.html. Accessed September 7, 2020.
- 48. Miguel-Cruz A, Felipe Bohórquez A, Aya Parra PA. What does the literature say about using electronic pillboxes for older adults? A systematic literature review. Disabil Rehabil Assist Technol. 2019;14(8):776-787. [DOI] [PubMed] [Google Scholar]
- 49. Lieb M, Hepp T, Schiffer M, Opgenoorth M, Erim Y. Accuracy and concordance of measurement methods to assess non-adherence after renal transplantation—a prospective study. BMC Nephrol. 2020;21(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Klonoff DC, Kerr D. Smart pens will improve insulin therapy. J Diabetes Sci Technol. 2018;12(3):551-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adolfsson P, Hartvig NV, Kaas A, Møller JB, Hellman J. Increased time in range and fewer missed bolus injections after introduction of a smart connected insulin pen. Diabetes Technol Ther. 2020;22:709-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Klonoff DC. Telemedicine for diabetes after the COVID-19 pandemic: we can’t put the toothpaste back in the tube or turn back the clock. J Diabetes Sci Technol. 2020;14(4):741-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ceriello A. “Diabetes as a case study of chronic disease management”: eight years later. The opportunity learned from the COVID-19 pandemic. Diabetes Res Clin Pract. 2020;167:108384. [DOI] [PMC free article] [PubMed] [Google Scholar]