Abstract
Patients affected by neurological pathologies with motor disorders when they are of working age have to cope with problems related to employability, difficulties in working, and premature work interruption. It has been demonstrated that suitable job accommodation plans play a beneficial role in the overall quality of life of pathological subjects. A well-designed return-to-work program should consider several recent innovations in the clinical and ergonomic fields. One of the instrument-based methods used to monitor the effectiveness of ergonomic interventions is surface electromyography (sEMG), a multi-channel, non-invasive, wireless, wearable tool, which allows in-depth analysis of motor coordination mechanisms. Although the scientific literature in this field is extensive, its use remains significantly underexploited and the state-of-the-art technology lags expectations. This is mainly attributable to technical and methodological (electrode-skin impedance, noise, electrode location, size, configuration and distance, presence of crosstalk signals, comfort issues, selection of appropriate sensor setup, sEMG amplitude normalization, definition of correct sEMG-related outcomes and normative data) and cultural limitations. The technical and methodological problems are being resolved or minimized also thanks to the possibility of using reference books and tutorials. Cultural limitations are identified in the traditional use of qualitative approaches at the expense of quantitative measurement-based monitoring methods to design and assess ergonomic interventions and train operators. To bridge the gap between the return-to-work rehabilitation and other disciplines, several teaching courses, accompanied by further electrodes and instrumentations development, should be designed at all Bachelor, Master and PhD of Science levels to enhance the best skills available among physiotherapists, occupational health and safety technicians and ergonomists.
Keywords: return-to-work rehabilitation, surface electromyography, instrumental-based biomechanical risk assessment, exoskeletons control, manual material handling monitoring
Introduction
Bipolar and multi-channel (high-density) surface electromyography (sEMG and HDsEMG) represent non-invasive physiological approaches, which enable greater comprehension of the upper limb, lower limb, and trunk muscle behaviors during the execution of movement (1–4). Differential bipolar sEMG signals, currently acquired using miniaturized wireless sensors attached to the skin, represent the spatio-temporal summation of all the motor unit action potentials, which propagate from the innervation zones to the tendon regions along the muscle fibers closest to the skin. In view of the complexity of these interference signals, simple indices facilitate an appropriate and complete analysis of the electrical activity of muscles. Simple indices provide information on “when” and “how much” the muscles are electrically active during the execution of both isometric and dynamic activities, and include the following parameters:
- muscle activation timings (7).
Furthermore, sEMG-based algorithms enable greater in-depth comprehension of the muscle coordination mechanisms adopted by the central nervous system (CNS) by estimating the following:
- simultaneous activation of several muscles or muscle groups (co-activation), a mechanism adopted by the CNS to stabilize joints, upper and lower limbs, and the spine (8–11),
- myoelectric manifestation of muscle fatigue, estimated by measuring the decrease in fiber conduction velocity (12, 13), which is reflected in an amplitude increase and spectral compression over time (14–16), and
- locomotor coordination (17–26), analyzed to comprehend how CNS lesions of neurological subjects with motor deficits influence plasticity and modular control of muscle patterns (27). It has been demonstrated that the CNS can linearly combine, with different weights, a limited number of basic functions called primitives or muscle synergies, to implement several motor tasks. During steady-state walking and running, five and four primitives, respectively, account for muscle activity (28–30).
High-density sEMG recordings are performed using high-density surface grids placed on the skin to evaluate the online spatial distribution of the sEMG activity and estimate the discharge times of several motor units by using decomposition algorithms (2, 31).
Despite the many advantages of sEMG and HDsEMG, these instrumental tools are largely underexploited and their application lags expectations in the fields of ergonomics and occupational medicine. These tools have begun to be applied in the prevention of work-related musculoskeletal disorders, a set of painful inflammatory and degenerative conditions affecting the joints, spinal dizcs, cartilage, muscles, tendons, ligaments and peripheral nerves, caused by manual lifting, pushing and pulling, repetitive movements, and patients handling activities (32–39). However, they remain underused in return-to-work rehabilitation plans for people with neurological pathologies with motor disorders.
People affected by neurological pathologies need to be integrated/reintegrated into their workplaces because their motor disease symptoms appeared when they were of working age, reducing their working capacity (40, 41) and employability (42). Recent studies have proven that avoiding early exit from employment plays a beneficial and key role in the overall quality of life of people affected by neurological disorders (41, 42). Clinicians manage their patients' premature work interruption (43, 44) by designing appropriate traditional and innovative pharmacological, surgical, and rehabilitation treatments, such as robotic rehabilitation, virtual reality, and neuromodulation (45–49). Furthermore, job accommodation plans are being enriched with new ergonomic options, such as work task rehabilitation and workplace interventions (50–52). In fact, the fourth industrial revolution has recently opened new occupational scenarios within which key human-robot collaborative (HRC) technologies, such as exoskeletons (Figure 1) and cobots, assist workers in their workplaces. Cobots can be defined as reconfigurable collaborative robots able to interact with workers within a shared space and to respond to the worker intentions and task variations in a timely manner while simultaneously offloading them from external loadings, and keep them in task-optimum and ergonomic working conditions. Small-medium enterprises can currently use collaborative technologies allowing flexible and ergonomic workplaces, which can adapt to the characteristics of workers with neurological disorders (53). Quite recently, the European Union's Horizon 2020 research and innovation program funded the SOPHIA (Socio-physical Interaction Skills for Cooperative Human-Robot Systems in Agile Production) project to establish and achieve, among others, the goal of validating the HRC technologies developed under the aegis of the project in the healthcare sector and in return-to-work rehabilitation of patients affected by neurological disorders. In particular, the European consortium is developing myoelectric HRC interfaces to study how new hybrid work environments can flexibly adapt to the human physical states and needs, thereby contributing to improvements in ergonomic interventions (53). Furthermore, the project has the aims, for prevention, to design training plans of professionals specialized in the workers motor performance measurement by using sEMG-based approaches, develop miniaturized wearable devices to monitor human-motor variables and render haptic stimuli to specific areas of the worker's body and develop new standards for adaptation of work environments and biomechanical risk assessment in collaborative manufacturing scenarios. Within this context, it is quite evident that sEMG can play a crucial role in complex vocational reintegration programs in classifying residual motor functions, assessing pre-post-rehabilitation and ergonomic interventions, and controlling wearable robotics. The professionals trained for the sEMG in return-to-work programs should be physiotherapists, occupational health and safety technicians and ergonomists which should operate in a multidisciplinary team also constituted by neurologists, occupational physicians, physiatrists, biomedical engineers and movement scientists. We believe that no new professions should be created.
Figure 1.
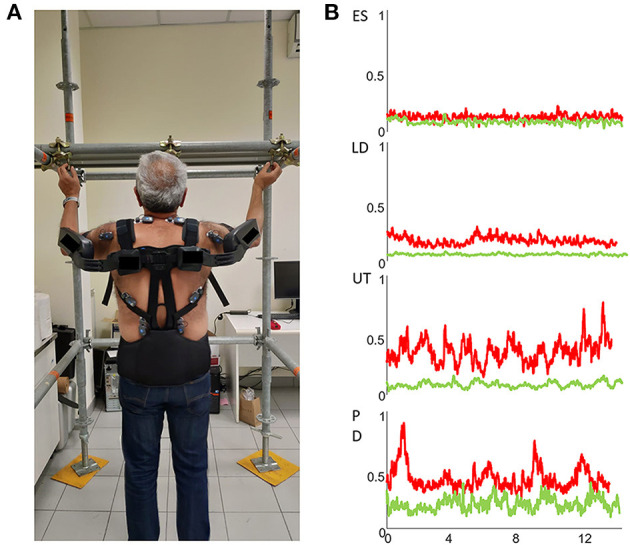
(A) Wearable wireless sEMG sensors placed bilaterally over the erector spinae (ES), latissimus dorsi (LD), upper trapezius (UT) and posterior deltoids (PD) muscles to assess the efficacy of a passive exoskeleton during the execution of an overhead screwing activity. (B) sEMG envelopes of right ES, LD, UT and PD muscles of a representative subject without (red traces) and with (green traces) the use of a passive exoskeleton.
Occupational health and safety technicians have an academic degree offered at Bachelor of Science level in the same field of physiotherapists (health professions sciences). Instead, professionals from different disciplines, for example occupational physicians, who aspire to practice the profession of ergonomist, need a degree that is not conferred by universities. In Europe, the Center for the Registration of the European Ergonomists (CREE) is the professional certification supported by European ergonomics associations and recognized by the International Ergonomics Association (IEA) which provides the title of “Eur.Erg.” and allows to practice the profession of ergonomist in 47 countries.
The above premise serves as a rationale for identifying and discussing the barriers to the coherent and widespread use of sEMG in work integration/reintegration.
This article represents the perspective, under the aegis of the SOPHIA project, of the Laboratory of Ergonomics and Physiology of the Italian Institute for Insurance against Accidents at Work (INAIL), a public non-profit entity aimed at facilitating the return-to-work of people with motor disorders.
An Overview of sEMG Use in Return-To-Work of Patients With Neurological Disorders
Ethical review and approval and written informed consent were not required for the study because no human participants were recruited in the study and in accordance with the local legislation and institutional requirements.
Degenerative and acquired neurological diseases, including neuropathies, multiple sclerosis, stroke, spastic paraplegia, cerebellar ataxia, dystonia, traumatic spine and brain lesions, and encephalitis, are disorders, which can affect the motor function during working age and severely limit the autonomy and efficiency of workers (42, 54–58). The motor impairment of workers affected by neurological diseases may encompass several motor domains, including hand function, balance, and locomotion, resulting in considerable onus on the society in terms of reduced work productivity and cost. The main purpose of pharmacological, surgical, and rehabilitation treatments must be to improve the motor performance, autonomy, and daily lives of patients, thereby offering them the possibility of returning to work and optimizing their work capability.
Combined with kinematic and kinetic measurements, sEMG is currently widely used in research laboratories by movement scientists and still little in clinical routine by health operators to classify quantitatively the nature and degree of motor dysfunction, analyze the complex relationship between the primary deficit and the adaptive and compensatory mechanisms, categorize patients based on their specific neurological disease, and finally monitor pre-post-treatment. Importantly, as sEMG essentially investigates the final output of motor commands, it can quantify the residual motor function, which can theoretically be monitored continuously in workplace adaptation and integrated into HRC technologies.
Application of sEMG in Monitoring
When a worker affected by a neurological pathology with motor disorders is reintegrated at work, an exhaustive assessment of his/her residual motor function is of primary importance to design and/or adapt his/her workplace well. Additionally, it is necessary to verify and monitor the efficacy of these ergonomic interventions over time.
Although no consistent studies have specifically investigated the sEMG of patients with neurological disorders in the workplace context, there are several reports on the residual muscle function assessment of patients with neurological disorders [(10), for review see (59, 60)]. Many muscle activation measures and indices exploring several aspects of motor control have been proposed for patients with neurological diseases: co-contraction/co-activation of single-joint or multi-joint muscles [(9, 23, 61), for review see (62)], spatio-temporal modular muscle activation (19–22, 27), muscle activation asymmetry (63), time-frequency coherences between joint muscle signals during dynamic contractions to detect the spasticity in the upper limbs (64), and muscle fatigue (65, 66). Furthermore, it is possible to obtain from these studies a set of functional measures to consider for work reintegration while simultaneously taking into account the uniqueness of the motor deficit specific to each disease.
Some long-lasting degenerative neurological diseases, such as cerebellar ataxia and spastic paraplegia, often begin at a young age and can persist for the entire duration of a subject's working life. This is of interest in the context of work-related rehabilitation because the majority of patients consider themselves capable of working, and approximately 78% of non-working patients seek employment (42). Furthermore, workers with cerebellar ataxia show low or average-to-low job stress-related risk (42).
The use of sEMG analysis in patients with degenerative cerebellar ataxia (for instance, spinocerebellar ataxia) reveals a series of muscle activation abnormalities (67). Specifically, patients with spinocerebellar ataxia show increased amplitude and duration of sEMG bursts in both the upper and lower (21, 22) limbs, with significant differences in muscle activation timing (21, 22). Further evidence reveals increases in both single-joint antagonist muscle co-activation (61) and multi-joint multi-muscle co-activation (68). All these abnormalities are related to the severity of the disease and balance features, suggesting they could be exploited as potential biomarkers for the work-related biomechanical risk evaluation and workplace adaptation monitoring of these patients. One possible approach is the planning of assistive devices for workplace adaptation, such as the use of supportive elastic suits (69). These soft wearable devices can improve movement stability and reduce the need to co-activate muscles. Consequently, they can lower the associated energy costs and tissue-overuse injuries owing to excessive compression and shear forces at, for instance, the L5-S1 joint of the spine.
It has been reported that patients with degenerative spastic paraplegia (for instance, hereditary paraplegia) show increased global (23) and segmental lower limb muscle co-activation (9), which correlate positively with disease severity, degree of spasticity, and energetic costs (9). In addition, when mapping the simultaneous activities of a large number of muscles during walking onto the anatomical rostrocaudal location of the motor neuron pools (70), patients with spastic paraplegia show an abnormal spread of muscle activation during gait, initially involving the sacral segments and, at more severe stages, the lumbar segments (20).
Application of sEMG in Controlling HRC Technologies
Human-robot collaborative technologies, particularly exoskeletons, have proven significantly useful in the rehabilitation programs of patients affected by neurological diseases. Physiological parameters with sEMG play a key role in monitoring muscle activation amplitude and fatigue in the design of innovative active exoskeleton controller systems and assessment of their effectiveness on motor performance (71).
Recent improvements in the measurement and real-time classification of myoelectric signals have already facilitated the use of sEMG in man-machine interfaces for controlling prostheses and orthoses among other devices (72–76).
Very recently, human-exoskeleton interfaces were developed for the purpose of rehabilitation to support physically weak and disabled people in performing several motor activities of daily living, such as walking. These interfaces were developed based on new technical and mathematical approaches, including new sEMG signal-processing procedures (77–82).
The performance of these man-machine interfaces is quickly improving owing to neuro-musculoskeletal models driven by neural information obtained from the decomposition of HDsEMG (72).
Barriers to sEMG Use in Return-To-Work of Patients With Neurological Diseases
The critical issues hindering the widespread adoption of sEMG in return-to-work programs are mainly attributable to technical, methodological and cultural limitations. The technical issues are attributable to both monitoring and control functions. With regard to monitoring, the most critical technical aspects are strongly associated with the sEMG technique:
- electrode-skin impedance, noise, and electrode contact stability;
- while the methodological aspects are associated to:
- problems linked to electrode location, size, configuration, and distance;
- presence of crosstalk signals (16).
- placement of sEMG electrodes for long hours;
- selection of the right sensor setup on the base of the neurological pathology and manual handling activity to be investigated;
- management the sEMG amplitude normalization;
- definition of appropriate sEMG-related outcomes and normative data.
Fortunately, the effect of these critical issues on the sEMG signal quality can be reduced with the aid of authoritative reference books and tutorials. In particular, the European Recommendations for Surface Electromyography (83), which needs to be updated, and the Atlas of Muscle Innervation Zones (84) are recommended as guides for the use of sEMG together with recent tutorials and consensus papers (85–87). A knowledge of the contents of these texts and tutorials makes users aware of the current limitations of the sEMG approach given its ability to monitor only a limited number of superficial muscles. Despite this, the technical and methodological limitations of the sEMG approach can be minimized and do not justify its non-usage in return-to-work rehabilitation plans. In addition, new wearable sensors and electronic smart devices such as smartphones and tablets allow simple monitoring of the worker at the workplace. Wearable sensors do not interfere with the typical movements performed by workers owing to their miniaturization and wireless communication protocols. In addition, multi-channel sEMG systems are available in wireless versions despite their high number of channels and high data rate. The combination of sensor networks and intelligent work environments provides real-time estimation of physiological parameters, enabling direct feedback to workers who are monitored directly and constantly at the workplace. Real-time monitoring is additionally useful for providing acoustic, visual, and vibro-tactile stimuli to workers (53, 88) executing manual handling tasks in awkward postures or requiring significant physical effort, or when muscle fatigue sets in.
As regard the sEMG use in work environment, each manual handling activity represent a risk of onset of well-classified diseases affecting the musculoskeletal system. For instance, lifting activities imply high compression and shear forces at L5-S1 joints (strongly correlated with muscle co-activation) with a significant involvement of erector spinae and rectus abdominus muscles, while handling low loads at high frequency imply neck and upper limbs muscles fatigue. For this reason the choice of channels and the rationale for selecting them should be task-guided.
The criticisms related to the use of sEMG to control collaborative wearable trunk and upper limb devices designed to assist people with neurological disabilities are attributable to the algorithms used in human-robot interfaces. These algorithms are used for pattern recognition and classification of patients' movement intentions. Only a few years ago, the performance of sEMG-based interfaces had not reached accuracies acceptable for widespread commercial use (74). Accuracy was limited by the high inter-subject variability, which required subjective calibrations and training. Fortunately, these interfaces, including machine-learning algorithms, have since been significantly improved, enabling the acceptable optimization of HRC control mostly for people with severe upper and lower limb disabilities (89).
Another technical limitation of sEMG-based interfaces for use in HRC technology control is the fact that most wearable assistive devices use traditional control tools, such as bipolar sEMG, to record antagonist muscle activities. This low spatial sampling implies that a maximum of one degree of freedom (DoF) can be controlled. Furthermore, managing up to two DoFs requires slow, sequential, and unintuitive control. The related limited functionality in conjunction with the extensive training required of neurological subjects led to the high rejection rates of these technologies (72). Currently, classification and regression approaches outperform traditional control tools in controlling complex motor activities in terms of speed and accuracy, providing a promising method for advanced myoelectric control (72).
Without doubt, in job integration/reintegration, the sEMG approach has yet to be adopted and the critical issues associated with it are managed with difficulty. In fact, while reasonable workplace accommodation and disability employment issues are being historically and widely addressed by the governments of the industrialized world, the adoption of instrument-based quantitative assessments of ergonomic interventions has so far been disregarded, owing to cultural barriers, which lead to a preference for qualitative approaches.
A clear testimony of the presence of this educational gap is the worldwide social policies for persons with disabilities (90, 91), such as the European Disability Strategy (2010–2020) and the directives 89/654/EEC, 2000/78/EC, and 2000/78/EC are the corresponding policies. Another example is that of the Job Accommodation Network, a facility of the United States Department of Labor's Office of Disability Employment Policy, which provides a valuable strategy for the inclusion of people with neurological disabilities (92). Nevertheless, only half of the population with disabilities has been accommodated well in terms of workplace design and there is evidence of poor knowledge about adaptation of workers with neurological pathologies.
The lack of quantitative approaches suggests that, except in rare cases, sEMG is not taught in the Ph.D. programs of universities and occupational medicine specialization schools. Furthermore, the domestic and international chapters of the Human Factors and Ergonomics Society do not actively promote meetings, conferences, and events to address the challenges of sEMG use. Finally, the reference society for sEMG, the International Society of Electrophysiology and Kinesiology, has thus far not dedicated specific training programs regarding job accommodation. All the above mentioned training activities would typically be successful to address methodological issues, as increased knowledge alone cannot overcome technical limitations.
The enormous potential of the sEMG and HDsEMG approaches and their very limited use in return-to-work programs represent a real paradox. It is difficult to determine why sEMG is underused. Perhaps, the most likely reason is that the training provided to professionals is based on more qualitative rather than quantitative approaches, and the transition from one approach to the other is evidently difficult. Professionals in the field have to be trained to understand the extremely variable abnormalities of workers suffering from neurological pathologies and to associate an appropriate return-to-work plan with them.
Discussion
Although sEMG is considered the most informative instrument for muscle monitoring when wearable robots are used, its use poses a number of challenges.
One such challenge, which we believe is on the verge of being addressed, pertains to the use of exoskeletons for active rehabilitation therapies. It pertains to the optimization of appropriate smart algorithms to detect patients' intentions and allow exoskeletons to act in synergy with them (93, 94). The challenge is to enable symbiotic physical HRC by incorporating accurate subject-specific computer models of each individual's neuro-musculoskeletal system to enable appropriate anticipation mechanisms. This is crucial to estimate muscle and joint stiffness accurately to determine the onset of excessive rigidity, which may be related to fatigue or negative compensatory strategies (95).
Myocontrol should be increasingly based on HDsEMG to increase spatial resolution with respect to low-density sEMG and to improve the accuracy of motor workers' intentions recognition. Moreover, machine learning approaches such as artificial neural networks should be used to evaluate the capacity of workers with neurological diseases for myocontrol.
In a recent study (96), HDsEMG was used to test the ability of participants with Duchenne muscular dystrophy (DMD) to produce repeatable HDsEMG patterns, which were unexpectedly comparable with those of healthy participants, suggesting a clear potential for the myocontrol of wearable exoskeletons. High-density sEMG can be applied to analyze the altered motor control of people with DMD and potentially interface them with assistive wearable robots. In addition, non-invasive decoding of individual α-motor neuron activation may represent a new option for the design of real-time closed-loop control applications, such as transcutaneous and epidural electrical stimulations.
To bridge the gap between the return-to-work concept and other disciplines, several educational activities should be developed to enhance and apply the best skills available in rehabilitation engineering, physiotherapy, occupational therapy, and ergonomics. For a few years, INAIL has been promoting a Ph.D. program together with the Sapienza University of Roma titled “Kinematic, Kinetic, and Electromyographic Characterization of Motor Disabilities and Biomechanical Overload Risk Management for Job Reintegration,” a Masters' course titled “New Methodologies for the Evaluation and Management of Biomechanical Risk and Criteria and Methods for the Adaptation of Workplaces,” and several training courses regarding the role of sEMG in occupational medicine and ergonomics. The participation, although the events were not free and not recognized as continuing education, has always been conspicuous with a number of participants (occupational health and safety technicians, physiotherapists, ergonomists, occupational physicians, rehabilitation engineers and movement scientists) that has always reached the maximum allowed limit (30 in the case of training courses). The authors of this article begin to observe a first positive impact of these initiatives on the need that operators in the field have in using the sEMG approach.
These mandatory educational opportunities must capitalize on the skills of the leaders and innovators of sEMG to serve physiotherapists, health and safety technicians, and ergonomists by providing them with qualified training focused on the management of the monitoring and HRC technologies and instrumental recordings. In particular, these professionals should know the physiology of sEMG signals, electrodes placement, software and hardware for acquisition. Furthermore, they are expected to know basic and some more complex concepts of signal processing, do a general visual interpretation and be able to generate reports that can be interpreted by the other team members. The multidisciplinary team should plan, implement and evaluate the return-to-work program in both the clinical and occupational environment. It should be considered that when patients with neurological disorders are involved, the key professionals leading the team should be neurologists in the health sector and occupational physicians at workplace. INAIL is organized throughout the national territory in order to fully manage the job integration/reintegration process but this service is also offered by consultant companies. Rehabilitation engineers should be given greater options to work with patients and physiotherapists, health and safety technicians end ergonomists. We believe that no other hybrid professions should be created. The contents must be based first on elementary concepts [which are illustrated in free online materials, such as those available at [99]] and, second, on integrated approaches promoting the culture and acceptance of instrument-based quantitative methodologies. All these actions should be taken as early as possible by guiding workers with chronic neurological disorders to return to work and stay in work with well-managed occupational safety and health interventions. The abovementioned activities may additionally yield tangible savings for businesses and national health and welfare systems.
Obviously, the teaching activities directed to strategic professions alone cannot solve the problem of under-use of sEMG in return-to-work plans. In fact, they must be accompanied by further electrodes and instrumentations development especially from the perspective of emerging artificial intelligence that may be encapsulate sEMG knowledge. Such tools may provide the professional with information to act on, thereby reducing the current “art” aspect of using EMG to something more in everyone's hands to use and exploit.
In addition to the motor impairments, cognitive and speech impairments are major contributors that strongly impact on the returning to work. Important aspects to consider are to understand how patients with cognitive and speech problems can adapt to the sEMG monitoring and how they can be assisted by using specific sEMG technology (96).
There are other challenges that are specific to application of sEMG in return-to-work environment according to the specific motor impairment characterizing the different neurological diseases. For instance, muscle fatigue, which is a common feature of several neurological diseases (i.e., multiple sclerosis, stroke, muscle dystrophy) results in altered motor unit recruitment and decreased maximal voluntary motor unit firing rate that can be detected by sEMG monitoring. A specific scenario could be to adapt the workplace and to modify the work-task according to the subjects' abnormal fatiguing performance, by assisting the workers with devices and/or reducing the amount and the duration of the work-related activity. Furthermore, an individualized rehabilitative program could be planned to improve the impact of the work-task on the fatigability in a long-term period.
The abnormal muscle co-activation is another example of common problem identifiable by sEMG in several neurological disorders (e.g., cerebellar ataxia, Parkinson disease, multiple sclerosis, stroke) which can be detected by measuring the simultaneous time-varying sEMG signal in many muscles. It is known that patients with balance disorders increase the muscle co-activation to control their walking instability in the attempt to stiffen the body segments. Unfortunately, this compensatory mechanism has some negative effects, such as increased metabolic cost and risk of cartilage degeneration. A specific scenario for these workers is to plan an appropriate workplace rehabilitation to improve their balance, to stabilize the body segments and to reduce the need to increase the muscle co-activation.
In conclusion, sEMG should be used in job integration plans to:
- classify the nature and degree of residual motor function in order to design/adapt the workplace;
- assess the efficacy of work-task rehabilitation and ergonomic interventions;
- control new assistive technologies such as collaborative robots;
- evaluate the biomechanical risk during the execution of manual handling activities;
- plan a preventive rehabilitation program to prevent injury.
Including the sEMG approach in work integration/reintegration offers the possibility of designing programs based on the residual motor abilities of the worker and adapting his/her workplace. This allows to consider a wide range of workers with several neurological pathologies and different levels of severity but without a complete inability to perform activities of daily life.
To make this possible, Bachelor, Master and PhD programs should be promoted, or at least, supervised and monitored, by scientific societies in the fields of physiotherapy, ergonomics, occupational medicine, biomechanics, electrophysiology and kinesiology, and should include continuing education courses on the use of sEMG specifically oriented to teachers in these fields.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval and written informed consent were not required for the study as per local legislation and institutional requirements.
Author Contributions
AR, MS, and FD planned the manuscript, performed the literature searches, wrote the text and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The research presented in this article was carried out as part of the SOPHIA project, which has received funding from the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 871237.
References
- 1.Merletti R, Botter A, Troiano A, Merlo E, Minetto MA. Technology and instrumentation for detection and conditioning of the surface electromyographic signal: state of the art. Clin Biomech. (2009) 24:122–34. 10.1016/j.clinbiomech.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 2.Farina D, Negro F, Muceli S, Enoka RM. Principles of Motor Unit Physiology Evolve With Advances in Technology. Physiology (Bethesda). (2016) 31:83–94. 10.1152/physiol.00040.2015 [DOI] [PubMed] [Google Scholar]
- 3.Merletti R, Farina D, Gazzoni M. The linear electrode array: a useful tool with many applications. J Electromyogr Kinesiol. (2003) 13:37–47. 10.1016/s1050-6411(02)00082-2 [DOI] [PubMed] [Google Scholar]
- 4.Zwarts MJ, Stegeman DF. Multichannel surface EMG: basic aspects and clinical utility. Muscle Nerve. (2003) 28:1–17. 10.1002/mus.10358 [DOI] [PubMed] [Google Scholar]
- 5.Hägg GM, Luttmann A, Jäger M. Methodologies for evaluating electromyographic field data in ergonomics. J Electromyogr Kinesiol. (2000) 10:301–12. 10.1016/s1050-6411(00)00022-5 [DOI] [PubMed] [Google Scholar]
- 6.Merletti R, Knaflitz M, De Luca CJ. Myoelectric manifestations of fatigue in voluntary and electrically elicited contractions. J Appl Physiol. (1990) 69:1810–20. 10.1152/jappl.1990.69.5.1810 [DOI] [PubMed] [Google Scholar]
- 7.Torres-Oviedo G, Ting LH. Muscle synergies characterizing human postural responses. J Neurophysiol. (2007) 98:2144–56. 10.1152/jn.01360.2006 [DOI] [PubMed] [Google Scholar]
- 8.Varrecchia T, Rinaldi M, Serrao M, Draicchio F, Conte C, Conforto S, et al. Global lower limb muscle coactivation during walking at different speeds: Relationship between spatio-temporal, kinematic, kinetic, and energetic parameters. J Electromyogr Kinesiol. (2018);43:148–57. 10.1016/j.jelekin.2018.09.012 [DOI] [PubMed] [Google Scholar]
- 9.Rinaldi M, Ranavolo A, Conforto S, Martino G, Draicchio F, Conte C, et al. Increased lower limb muscle coactivation reduces gait performance and increases metabolic cost in patients with hereditary spastic paraparesis. Clin Biomech (Bristol, Avon). (2017) 48:63–72. 10.1016/j.clinbiomech.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 10.Rosa MC, Marques A, Demain S, Metcalf CD, Rodrigues J. Methodologies to assess muscle co-contraction during gait in people with neurological impairment—a systematic literature review. J Electromyogr Kinesiol. (2014) 24:179–91. 10.1016/j.jelekin.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 11.Tatarelli A, Serrao M, Varrecchia T, Fiori L, Draicchio F, Silvetti A, et al. Global muscle coactivation of the sound limb in gait of people with transfemoral and transtibial amputation. Sensors. (2020) 20:E2543. 10.3390/s20092543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farina D, Merletti R. A novel approach for estimating muscle fiber conduction velocity by spatial and temporal filtering of surface EMG signals. IEEE Trans Biomed Eng. (2003) 50:1340–51. 10.1109/TBME.2003.819847 [DOI] [PubMed] [Google Scholar]
- 13.Farina D, Merletti R. Methods for estimating muscle fibre conduction velocity from surface electromyographic signals. Med Biol Eng Comput. (2004) 42:432–45. 10.1007/BF02350984 [DOI] [PubMed] [Google Scholar]
- 14.Cifrek M, Medved V, Tonković S, Ostojić S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin Biomech. (2009) 24:327–40. 10.1016/j.clinbiomech.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 15.Merletti R, Farina D. Surface Electromyography: Physiology, Engineering, and Applications. John Wiley & Sons; (2016). [Google Scholar]
- 16.Merletti R, Parker P. Electromyography—Physiology, Engineering and Noninvasive Applications. 1st ed Hoboken, New Jersey: John Wiley & Sons, Inc. (2004). [Google Scholar]
- 17.Janshen L, Santuz A, Ekizos A, Arampatzis A. Fuzziness of muscle synergies in patients with multiple sclerosis indicates increased robustness of motor control during walking. Sci Rep. (2020) 10:7249. 10.1038/s41598-020-63788-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Marchis C, Ranaldi S, Serrao M, Ranavolo A, Draicchio F, Lacquaniti F, et al. Modular motor control of the sound limb in gait of people with trans-femoral amputation. J Neuroeng Rehabil. (2019) 16:132 10.1186/s12984-019-0616-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martino G, Ivanenko Y, Serrao M, Ranavolo A, Draicchio F, Casali C, et al. Locomotor coordination in patients with Hereditary Spastic Paraplegia. J Electromyogr Kinesiol. (2019) 45:61–9. 10.1016/j.jelekin.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Martino G, Ivanenko Y, Serrao M, Ranavolo A, Draicchio F, Rinaldi M, et al. Differential changes in the spinal segmental locomotor output in Hereditary Spastic Paraplegia. Clin Neurophysiol. (2018) 129:516–25. 10.1016/j.clinph.2017.11.028 [DOI] [PubMed] [Google Scholar]
- 21.Martino G, Ivanenko YP, d'Avella A, Serrao M, Ranavolo A, Draicchio F, et al. Neuromuscular adjustments of gait associated with unstable conditions. J Neurophysiol. (2015) 114:2867–82. 10.1152/jn.00029.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martino G, Ivanenko YP, Serrao M, A Ranavolo A, d'Avella A, Draicchio F, et al. Locomotor patterns in cerebellar ataxia. J Neurophysiol. (2014) 112:2810–21. 10.1152/jn.00275.2014 [DOI] [PubMed] [Google Scholar]
- 23.Serrao M, Rinaldi M, Ranavolo A, Lacquaniti F, Martino G, Leonardi L, et al. Gait patterns in patients with hereditary spastic paraparesis. PLoS ONE. (2016) 11:e0164623. 10.1371/journal.pone.0164623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacquaniti F, Ivanenko YP, d'Avella A, Zelik KE, Zago M. Evolutionary and developmental modules. Front Comput Neurosci. (2013) 7:61. 10.3389/fncom.2013.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.d'Avella A, Lacquaniti F. Control of reaching movements by muscle synergy combinations. Front Comput Neurosci. (2013) 7:42 10.3389/fncom.2013.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ivanenko YP, Cappellini G, Dominici N, Poppele RE, Lacquaniti F. Coordination of locomotion with voluntary movements in humans. J Neurosci. (2005) 25:7238–53. 10.1523/JNEUROSCI.1327-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanenko YP, Cappellini G, Solopova IA, Grishin AA, Maclellan MJ, Poppele RE, et al. Plasticity and modular control of locomotor patterns in neurological disorders with motor deficits. Front Comput Neurosci. (2013) 7:123. 10.3389/fncom.2013.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janshen L, Santuz A, Ekizos A, Arampatzis A. Modular control during incline and level walking in humans. J Exp Biol. (2017) 220:807–13. 10.1242/jeb.148957 [DOI] [PubMed] [Google Scholar]
- 29.Santuz A, Ekizos A, Janshen L, Mersmann F, Bohm S, Baltzopoulos V, et al. Modular control of human movement during running: an open access data set. Front Physiol. (2018) 9:1509. 10.3389/fphys.2018.01509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol. (2004) 556:267–82. 10.1113/jphysiol.2003.057174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Negro F, Muceli S, Castronovo AM, Holobar A, Farina D. Multi-channel intramuscular and surface EMG decomposition by convolutive blind source separation. J Neural Eng. (2016) 13:026027. 10.1088/1741-2560/13/2/026027 [DOI] [PubMed] [Google Scholar]
- 32.Ranavolo A, Mari S, Conte C, Serrao M, Silvetti A, Iavicoli S, et al. A new muscle co-activation index for biomechanical load evaluation in work activities. Ergonomics. (2015) 58:966–79. 10.1080/00140139.2014.991764 [DOI] [PubMed] [Google Scholar]
- 33.Ranavolo A, Varrecchia T, Rinaldi M, Silvetti A, Serrao M, Conforto S, et al. Mechanical lifting energy consumption in work activities designed by means of the “revised NIOSH lifting equation.” Ind Health. (2017) 55:444–54. 10.2486/indhealth.2017-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ranavolo A, Draicchio F, Varrecchia T, Silvetti A, Iavicoli S. Erratum, Alberto R. et al. Wearable monitoring devices for biomechanical risk assessment at work: current status and future challenges—a systematic review. Int J Environ Res Public Health. (2018) 15:2569. 10.3390/ijerph15112569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranavolo A, Varrecchia T, Iavicoli S, Marchesi A, Rinaldi M, Serrao M, et al. “Surface electromyography for risk assessment in work activities designed using the “revised NIOSH lifting equation””. Int J Ind Ergon. (2018) 68:34–45. 10.1016/j.ergon.2018.06.003 [DOI] [Google Scholar]
- 36.Ranavolo A, Chini G, Silvetti A, Mari S, Serrao M, Draicchio F. Myoelectric manifestation of muscle fatigue in repetitive work detected by means of miniaturized sEMG sensors. Int J Occup Saf Ergon. (2018) 24:464–74. 10.1080/10803548.2017.1357867 [DOI] [PubMed] [Google Scholar]
- 37.Alberto R, Draicchio F, Varrecchia T, Silvetti A, Iavicoli S. Wearable monitoring devices for biomechanical risk assessment at work: current status and future challenges–a systematic review. Int J Environ Res Public Health. (2018) 15:2001. 10.3390/ijerph15092001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varrecchia T, De Marchis C, Draicchio F, Schmid M, Conforto S, Ranavolo A. Lifting activity assessment using kinematic features and neural networks. Appl Sci. (2020) 10:1989 10.3390/app10061989 [DOI] [Google Scholar]
- 39.Varrecchia T, De Marchis C, Rinaldi M, Draicchio F, Serrao M, Schmid M, et al. Lifting activity assessment using surface electromyographic features and neural networks. Int J Ind Ergon. (2018) 66:1–9. 10.1016/j.ergon.2018.02.003 [DOI] [Google Scholar]
- 40.Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol. (2010) 9:94–104. 10.1016/S1474-4422(09)70305-9 [DOI] [PubMed] [Google Scholar]
- 41.Miller A, Dishon S. Health-related quality of life in multiple sclerosis: The impact of disability, gender and employment status. Qual Life Res. (2006) 15:259–71. 10.1007/s11136-005-0891-6 [DOI] [PubMed] [Google Scholar]
- 42.Ranavolo A, Serrao M, Varrecchia T, Casali C, Filla A, Roca A, Silvetti A, et al. The Working Life of People with Degenerative Cerebellar Ataxia. Cerebellum. (2019) 18:910–21. 10.1007/s12311-019-01065-x [DOI] [PubMed] [Google Scholar]
- 43.Toldrá RC, Santos MC. People with disabilities in the labor market: facilitators and barriers. Work. (2013) 45:553–63. 10.3233/WOR-131641 [DOI] [PubMed] [Google Scholar]
- 44.Honan CA, Brown RF, Hine DW, Vowels L, Wollin JA, Simmons RD, et al. The multiple sclerosis work difficulties questionnaire. Mult Scler. (2012) 18:871–80. 10.1177/1352458511431724 [DOI] [PubMed] [Google Scholar]
- 45.Karamians R, Proffitt R, Kline D, Gauthier LV. Effectiveness of virtual reality- and gaming-based interventions for upper extremity rehabilitation poststroke: a meta-analysis. Arch Phys Med Rehabil. (2020) 101:885–96. 10.1016/j.apmr.2019.10.195 [DOI] [PubMed] [Google Scholar]
- 46.Triegaardt J, Han TS, Sada C, Sharma S, Sharma P. The role of virtual reality on outcomes in rehabilitation of Parkinson's disease: meta-analysis and systematic review in 1031 participants. Neurol Sci. (2020) 41:529–36. 10.1007/s10072-019-04144-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naro A, Leo A, Russo M, Casella C, Buda A, Crespantini A, et al. Breakthroughs in the spasticity management: Are non-pharmacological treatments the future? J Clin Neurosci. (2017) 39:16–27. 10.1016/j.jocn.2017.02.044 [DOI] [PubMed] [Google Scholar]
- 48.Iosa M, Morone G, Cherubini A, Paolucci S. The three laws of neurorobotics: a review on what neurorehabilitation robots should do for patients and clinicians. J Med Biol Eng. (2016) 36:1–11. 10.1007/s40846-016-0115-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis PM, Thomson RH, Rosenfeld JV, Fitzgerald PB. Brain neuromodulation techniques: a review. Neuroscientist. (2016) 22:406–21. 10.1177/1073858416646707 [DOI] [PubMed] [Google Scholar]
- 50.Ashley KD, Lee LT, Heaton K. Return to work among stroke survivors. Workplace Health Saf . (2019) 67:87–94. 10.1177/2165079918812483 [DOI] [PubMed] [Google Scholar]
- 51.Skamagki G, King A, Duncan M, Wåhlin C. A systematic review on workplace interventions to manage chronic musculoskeletal conditions. Physiother Res Int. (2018) 23:e1738. 10.1002/pri.1738 [DOI] [PubMed] [Google Scholar]
- 52.Hoosain M, de Klerk S, Burger M. Workplace-based rehabilitation of upper limb conditions: a systematic review. J Occup Rehabil. (2019) 29:175–93. 10.1007/s10926-018-9777-7 [DOI] [PubMed] [Google Scholar]
- 53.Ajoudani A, Albrecht P, Bianchi M, Cherubini A, Del Ferraro S, Fraisse P, et al. Smart collaborative systems for enabling flexible and ergonomic work practices. IEEE Robot Autom Mag. (2020) 27:169–76. 10.1109/MRA.2020.2985344 [DOI] [Google Scholar]
- 54.Vinci P, Serrao M, Millul A, Deidda A, De Santis F, Capici S, et al. Quality of life in patients with charcot-marie-tooth disease. Neurology. (2005) 65:922–4. 10.1212/01.wnl.0000176062.44360.49 [DOI] [PubMed] [Google Scholar]
- 55.Doogan C, Playford ED. Supporting work for people with multiple sclerosis. Mult Scler. (2014) 20:646–50. 10.1177/1352458514523499 [DOI] [PubMed] [Google Scholar]
- 56.Raggi A, Covelli V, Schiavolin S, Scaratti C, Leonardi M, Willems M. Work-related problems in multiple sclerosis: a literature review on its associates and determinants. Disabil Rehabil. (2016) 38:936–44. 10.3109/09638288.2015.1070295 [DOI] [PubMed] [Google Scholar]
- 57.Hendricks DJ, Sampson E. Accommodating individuals with traumatic brain injury: an analysis of employer-initiated cases handled by the Job Accommodation Network. Work. (2017) 58:29–4. 10.3233/WOR-172594 [DOI] [PubMed] [Google Scholar]
- 58.van der Kemp J, Kruithof WJ, Nijboer TCW, van Bennekom CAM, van Heugten C, Visser-Meily JMA. Return to work after mild-to-moderate stroke: work satisfaction and predictive factors. Neuropsychol Rehabil. (2019) 29:638–53. 10.1080/09602011.2017.1313746 [DOI] [PubMed] [Google Scholar]
- 59.Wimalaratna HS, Tooley MA, Churchill E, Preece AW, Morgan HM. Quantitative surface EMG in the diagnosis of neuromuscular disorders. Electromyogr Clin Neurophysiol. (2002) 42:167–74. [PubMed] [Google Scholar]
- 60.Labarre-Vila A. Electromyographie de surface et fonction musculaire en pathologie [Assessment of muscle function in pathology with surface electrode EMG]. Rev Neurol. (2006) 162:459–65. 10.1016/s0035-3787(06)75037-8 [DOI] [PubMed] [Google Scholar]
- 61.Mari S, Serrao M, Casali C, Conte C, Martino G, Ranavolo A, et al. Lower limb antagonist muscle co-activation and its relationship with gait parameters in cerebellar ataxia. Cerebellum. (2014) 13:226–36. 10.1007/s12311-013-0533-4 [DOI] [PubMed] [Google Scholar]
- 62.Latash ML. Muscle coactivation: definitions, mechanisms, and functions. J Neurophysiol. (2018) 120:88–104. 10.1152/jn.00084.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castagneri C, Agostini V, Rosati S, Balestra G, Knaflitz M. Asymmetry index in muscle activations. IEEE Trans Neural Syst Rehabil Eng. (2019) 27:772–9. 10.1109/TNSRE.2019.2903687 [DOI] [PubMed] [Google Scholar]
- 64.Becker S, von Werder SCFA, Lassek AK, Disselhorst-Klug C. Time-frequency coherence of categorized sEMG data during dynamic contractions of biceps, triceps, and brachioradialis as an approach for spasticity detection. Med Biol Eng Comput. (2019) 57:703–13. 10.1007/s11517-018-1911-3 [DOI] [PubMed] [Google Scholar]
- 65.Morrison S, Kavanagh J, Obst SJ, Irwin J, Haseler LJ. The effects of unilateral muscle fatigue on bilateral physiological tremor. Exp Brain Res. (2005) 167:609–21. 10.1007/s00221-005-0050-x [DOI] [PubMed] [Google Scholar]
- 66.Menotti F, Bazzucchi I, Felici F, Damiani A, Gori MC, Macaluso A. Neuromuscular function after muscle fatigue in Charcot-Marie-Tooth type 1A patients. Muscle Nerve. (2012) 46:434–9. 10.1002/mus.23366 [DOI] [PubMed] [Google Scholar]
- 67.Bodranghien F, Bastian A, Casali C, Hallett M, Louis ED, Manto M, et al. Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum. (2016) 15:369–91. 10.1007/s12311-015-0687-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiori L, Ranavolo A, Varrecchia T, Tatarelli A, Conte C, Draicchio F, et al. Impairment of global lower limb muscle coactivation during walking in cerebellar ataxias. Cerebellum. (2020) 19:583–96. 10.1007/s12311-020-01142-6 [DOI] [PubMed] [Google Scholar]
- 69.Serrao M, Casali C, Ranavolo A, Mari S, Conte C, Chini G, et al. Use of dynamic movement orthoses to improve gait stability and trunk control in ataxic patients. Eur J Phys Rehabil Med. (2017) 53:735–43. 10.23736/S1973-9087.17.04480-X [DOI] [PubMed] [Google Scholar]
- 70.Ivanenko YP, Poppele RE, Lacquaniti F. Spinal cord maps of spatiotemporal alpha-motoneuron activation in humans walking at different speeds. J Neurophysiol. (2006) 95:602–18. 10.1152/jn.00767.2005 [DOI] [PubMed] [Google Scholar]
- 71.de Looze MP, Bosch T, Krause F, Stadler KS, O'Sullivan LW. Exoskeletons for industrial application and their potential effects on physical work load. Ergonomics. (2016) 59:671–81. 10.1080/00140139.2015.1081988 [DOI] [PubMed] [Google Scholar]
- 72.Kapelner T, Sartori M, Negro F, Farina D. Neuro-musculoskeletal mapping for man-machine interfacing. Sci Rep. (2020) 10:5834. 10.1038/s41598-020-62773-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kapelner T, Vujaklija I, Jiang N, Negro F, Aszmann OC, Principe J, et al. Predicting wrist kinematics from motor unit discharge timings for the control of active prostheses. J Neuroeng Rehabil. (2019) 16:47. 10.1186/s12984-019-0516-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lobov S, Krilova N, Kastalskiy I, Kazantsev V, Makarov VA. Latent factors limiting the performance of sEMG-interfaces. Sensors. (2018) 18:1122. 10.3390/s18041122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roche AD, Rehbaum H, Farina D, Aszmann OC. Prosthetic myoelectric control strategies: a clinical perspective. Curr Surg Rep. (2014) 2:44 10.1007/s40137-013-0044-8 [DOI] [Google Scholar]
- 76.Hahne JM, Biessmann F, Jiang N, Rehbaum H, Farina D, Meinecke FC, et al. Linear and nonlinear regression techniques for simultaneous and proportional myoelectric control. IEEE Trans Neural Syst Rehabil Eng. (2014) 22:269–79. 10.1109/TNSRE.2014.2305520 [DOI] [PubMed] [Google Scholar]
- 77.Gordon KE, Kinnaird CR, Ferris DP. Locomotor adaptation to a soleus EMG-controlled antagonistic exoskeleton. J Neurophysiol. (2013) 109:1804–14. 10.1152/jn.01128.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiguchi K, Hayashi Y. An EMG-based control for an upper-limb power-assist exoskeleton robot. IEEE Trans Syst Man Cybern B Cybern. (2012) 42:1064–71. 10.1109/TSMCB.2012.2185843 [DOI] [PubMed] [Google Scholar]
- 79.Yagi E, Harada D, Kobayashi M. Upper-limb power assist control for agriculture load lifting. Int J Autom Technol. (2009) 3:716–22. 10.20965/ijat.2009p0716 [DOI] [Google Scholar]
- 80.Su H, Li Z, Li G, Yang C. EMG-based neural network control of an upper-limb power-assist exoskeleton robot. In: Guo C, Hou ZG, Zeng Z, editors. Advances in Neural Networks—ISNN 2013. Berlin, Heidelberg: Springer; (2013). [Google Scholar]
- 81.Khokhar ZO, Xiao ZG, Menon C. Surface EMG pattern recognition for real-time control of a wrist exoskeleton. Biomed Eng Online. (2010) 9:41. 10.1186/1475-925X-9-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gopura RARC, Kiguchi K, Li Y. SUEFUL-7: a 7DOF upper-limb exoskeleton robot with muscle-model-oriented EMG-based control. In: IEEE/RSJ International Conference on Intelligent Robots and Systems. St. Louis, MO: (2009). [Google Scholar]
- 83.Hermens HJ, Freriks B. European Recommendations for Surface Electromyography: Results of the SENIAM Project. Enschede: Roessingh Research and Development; (1999). [Google Scholar]
- 84.Barbero M, Merletti R, Rainoldi A. Atlas of Muscle Innervation Zones: Understanding Surface Electromyography and its Applications. New York, NY: Springer; (2012). [Google Scholar]
- 85.Besomi M, Hodges PW, Van Dieën J, Carson RG, Clancy EA, Disselhorst-Klug, et al. Consensus for experimental design in electromyography (CEDE) project: electrode selection matrix. J Electromyogr Kinesiol. (2019) 48:128–44. 10.1016/j.jelekin.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 86.Merletti R, Muceli S. Tutorial. Surface EMG detection in space and time: best practices. J Electromyogr Kinesiol. (2019) 49:102363. 10.1016/j.jelekin.2019.102363 [DOI] [PubMed] [Google Scholar]
- 87.Merletti R, Cerone GL. Tutorial. Surface EMG detection, conditioning and pre-processing: best practices. J Electromyogr Kinesiol. (2020) 54:102440. 10.1016/j.jelekin.2020.102440 [DOI] [PubMed] [Google Scholar]
- 88.Ajoudani A, Godfrey SB, Bianchi M, Catalano MG, Grioli G, Tsagarakis N, et al. Exploring teleimpedance and tactile feedback for intuitive control of the pisa/iit softhand. IEEE Trans Haptics. (2014) 7:203–15. 10.1109/TOH.2014.2309142 [DOI] [PubMed] [Google Scholar]
- 89.Gao B, Wei C, Ma H, Yang S, Ma X, Zhang S. Real-time evaluation of the signal processing of sEMG used in limb exoskeleton rehabilitation system. Appl Bionics Biomech. (2018) 2018:1391032. 10.1155/2018/1391032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krahn GL. WHO World report on disability: a review. Disabil Health J. (2011) 4:141–2. 10.1016/j.dhjo.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 91.de Jong PR. Sickness, disability and work: breaking the barriers—a synthesis of findings across OECD countries—OECD. Int Rev für Soz Sicherheit. (2011) 64:115–7. 10.1111/j.1752-1726.2011.01406.x| [DOI] [Google Scholar]
- 92.Copaci D, Serrano D, Moreno L, Blanco D. A high-level control algorithm based on sEMG signalling for an elbow joint SMA exoskeleton. Sensors. (2018) 18:2522. 10.3390/s18082522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suplino LO, Sommer LF, Forner-Cordero A. EMG-based control in a test platform for exoskeleton with one degree of freedom. Conf Proc IEEE Eng Med Biol Soc. (2019) 5366–9. 10.1109/EMBC.2019.8856836 [DOI] [PubMed] [Google Scholar]
- 94.Sartori M, Maculan M, Pizzolato C, Reggiani M, Farina D. Modeling and simulating the neuromuscular mechanisms regulating ankle and knee joint stiffness during human locomotion. J Neurophysiol. (2015) 114:2509–27. 10.1152/jn.00989.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nizamis K, Rijken NHM, van Middelaar R, Neto J, Koopman BFJM, Sartori M. Characterization of forearm muscle activation in duchenne muscular dystrophy via high-density electromyography: a case study on the implications for myoelectric control. Front Neurol. (2020) 11:231. 10.3389/fneur.2020.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janke M, Diener L. EMG-to-speech: direct generation of speech from facial electromyographic signals. IEEE/ACM Trans Audio Speech Lang Proc. (2017) 12:2375–85. 10.1109/TASLP.2017.2738568 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


