Abstract
Rheumatoid arthritis (RA) is considered a debilitating disease that increases the risk of significant morbidity and premature mortality. To circumvent drug-related toxicity and ineffectiveness of anti-inflammatory drugs, there is a significant need for an advanced delivery system that increases bioavailability. The feasibility of in situ gel of methotrexate sodium (MTS) as an effective management for Rheumatoid arthritis was investigated. It was formulated with pluronic F-127 (PLF-127) as primary polymer, hydroxypropyl methylcellulose K4M (HK4M), and polycarbophil (PCL) as a copolymer and characterized by various parameters. The efficacy evaluation by Freund's complete adjuvant (FCA) model, biocompatibility assessment by histopathological studies conducted. The optimized in situ gel (M4) was thermoresponsive, released 93.26 ± 2.39% MTS at 96 hours. In addition, distribution of MTS was even in the optimized sterile and syringeable in situ gel. In vivo studies on wistar rats demonstrated a substantial reduction in paw oedema during the 28-day study period and were biocompatible with the tissues at the injection site. The study was successful in formulating, optimizing MTS in situ gel for effective management of RA.
Keywords: Methotrexate sodium, In situ gel, Drug targeting, Rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is an “immune mediated disease characterized by joint pain and inflammation, with progressive erosion of the cartilage and bone, accompanied by synovial proliferation” (Chopra, 2000, Lee and Kavanaugh, 2003). The cause of RA is unknown, 1% of the population suffers from RA, usually beginning at the age of 30–50 (Pai et al., 2019). An estimated 2.5 million people in US is affected by RA and has economic impact in billions (Lipsky and Kavanaugh, 1999). About 0.5–0.75% Indian population suffer from the disease, with a woman to man ratio 3:1 (Misra et al., 2008). RA also promotes death rate, with continued existence of patients with ruthless ischemic heart disease or lymphoma which has great impact on monetary cost (Pincus, 1998, Feldmann et al., 1996).
Based on the pathogenic criteria, RA is an autoimmune disease against unidentified self-antigens. In RA, the damage to group of cells is caused by synovium inflammation (Magne et al., 2006). As a consequence of extra-articular immunologic defects, other organs are prone to get damaged (Abdel-Nasser et al., 1997, Sokka et al., 2008). RA leads to disability, joint deformation, and mortality as reported by various studies (Leigh and Fries, 1994, Pincus et al., 1984, Wolfe et al., 1994). Multiple studies have reported higher death rates along with RA patients with other comorbidities. The death is caused due to heart related disease, carcinomas and infections (Avina-Zubieta et al., 2008, Meune et al., 2009, Sacks et al., 2004, Larsen et al., 2008).
Despite of advancements in basic science and therapeutics, the present treatment of RA remains partly efficient with several disadvantages which include dose, time of administration or other related toxicities (Butoescu et al., 2009). The ideal system must be administered easily, delivering the drug in a sustained manner and retain optimum drug concentration without disagreeable adverse events. Various therapeutic agents available for the treatment of RA comprises of (a) NSAIDs (non-steroidal anti-inflammatory drugs)/selective cyclooxygenase-2 (COX-2) inhibitors, (b) Disease-modifying anti-rheumatic drugs (DMARDs), (c) Glucocorticoids, (d) Natural origin compounds and (e) Biological agents (Pincus, 1998).
Methotrexate (MTS), a folic acid antagonist that inhibits DNA and RNA synthesis, is the most potent, and commonly prescribed synthetic DMARD, and can retard joint destruction. It acts by reversibly inhibiting dihydrofolate reductase which reduces dihydrofolate to tetrahydrofolate. Inhibition of this enzyme interferes with purine and pyrimidine synthesis. MTX is used in low doses for the treatment of RA and is termed as the “Gold Standard Treatment for Rheumatoid Arthritis”. Also, MTS has been very commonly used as the “anchor” agent for combination therapy with other DMARDs and biological agents for the effective treatment of RA. But the precise mechanism of action of MTX in RA is understood only to a limited extent; however, MTS acts as both an antiproliferative as well as an anti-inflammatory agent against the cells responsible for the joint inflammation in RA. The studies showed that MTS induce immunosuppression by inhibiting both dihydrofolate reductase and other folate-dependent enzymes, leading to adenosine overproduction (Cronstein, 1997).
To avoid various toxic effects of anti-arthritic drugs, the requirement for developing customized formulation which delivers drug to reach effective concentration at the desired site is warranted. Novel injectable systems have gained considerable attention in this arena. Targeted delivery has numerous benefits such as site specific delivery, maximum drug concentration at the site and limiting systemic-drug related toxicities (Albert et al., 2006). The rapid clearance of drug from target site is a significant problem which can be overcome by giving multiple injections. However, multiple injections may cause infection or joint disability (Yang et al., 2011). A depot system which can retain the drug concentration for the desired time interval at injection site may help resolve the issue.
Polymeric in-situ gels have been used as drug delivery vehicles because of their ability to undergo transformation from solution to gels at body temperature. The formulated system can be sterilized easily, deliver the active ingredient at desired location and have prolonged action due to gel formation. The gels are modified to decrease the drug amount required for beneficial effects, thus provides better patient comfort and compliance. Pluronic F-127 (PLF-127) is listed in the FDA's inactive substance database in “GRAS” (generally considered safe) ingredients. These polymers are non-toxic, non-irritant, biocompatible and biodegradable (Vohra, T et al., 2013).
Various DMARDs such as methotrexate sodium (MTS) are used in therapy; however, the treatment is still unsatisfactory due to its undesired pharmacokinetic parameters such as low bioavailability, long half life and narrow therapeutic window limit its therapeutic benefits via conventional delivery. Treatment using MTS is discontinued in 8–19% of patients due to diverse drug-related toxicities, including suppression of the formation of new blood cells leading to a severe form of anaemia, liver damage, pulmonary disturbances, renal disturbances, gastrointestinal disturbances (stomatitis, enteritis, ulcerations, and bleeding of the mucous membranes of the mouth or other portions of the gastrointestinal (GI) tract and abdominal distress) and potential effects on the central nervous system. Therefore, its use in normal routine therapy is limited. In the present scenario, MTS is the preferred drug of choice as it has dual anti-inflammatory and immunosuppressant effects, which can be useful in RA (Wenham et al., 2013, Venkatesh et al., 2013, Schmolka and Artificial skin, 1972).
The ultimate goal in the treatment of RA is to prevent joint damage and restore normal life. To reduce the drug related toxic effects on the body, there is a need of dosage form which can ensure the availability of MTX at right amounts with minimum exposure to body tissues and still be effective in the treatment of RA.
The main objective of the research work was to prepare an injectable MTS in-situ gel for sustained release of MTS for the successful management of RA.
2. Material and methods
2.1. Materials
PLF-127 was sourced from Sigma-Aldrich, St. Louis, United States. MTS was gifted by Samarth Life Sciences, Bangalore. Hydroxy Propyl Methyl Cellulose K4M (HK4M) was sourced from Loba Chemie Pvt. Ltd., Mumbai; Polycarbophil (PCL) procured from Arihant Trading Co., Mumbai, India. All formulations were prepared with analytical grade chemicals and double distilled water.
3. Methods
3.1. Fourier transform infrared (FTIR) spectroscopy
FT-IR studies were carried out to assess the compatibility between drug and excipients using FT-IR spectrophotometer (8400S, Shimadzu, Japan). Compatibility was ascertained by matching the IR spectra of the pure drug, total lipid and its physical mixture to identify any shift, appearance or disappearance of peaks.
3.2. Differential scanning calorimeter (DSC) studies
The thermal behavior of the sample was analyzed using DSC (DSC-60, Shimadzu, Japan). The required amounts of samples (pure drug, total lipid and its physical mixture) were placed in aluminum pans which were accurately weighed and then crimped. The samples were heated at a rate of 20 °C/min from 40 °C to 300 °C under constant nitrogen purging at a rate of 40 ml/min.
3.3. MTS in-situ gels formulation
Traditional “cold method” was employed for MTS in-situ gels preparation (Yang et al., 2009). With reference to formulation chart (Table 1) weighed amounts of PLF-127, other copolymers (HK4M / PCL) were gradually added in varying concentrations and dispersed in cold water (5–10 °C) under magnetic stirring. As per the cold method, the mixture was kept at 4 °C for overnight to aid into the gel formation. Dissolved MTS in 0.1 N NaOH and added to the preformed gel to obtain final concentration. While triethanolamine was added to adjust the pH to neutral, 0.99%w/v benzyl alcohol added to polymeric gel as antibacterial and benzalkonium chloride (0.001%w/v) as preservative.
Table 1.
Composition of MTS in situ gels.
| Formulation Code | MTS | PLF-127 | HK4M | PCL |
|---|---|---|---|---|
| M1 | 0.5 | 20 | – | – |
| M2 | 0.5 | 20 | 0.5 | – |
| M3 | 0.5 | 20 | 1.0 | – |
| M4 | 0.5 | 20 | 1.5 | – |
| M5 | 0.5 | 20 | – | 0.5 |
| M6 | 0.5 | 20 | – | 1.0 |
| M7 | 0.5 | 20 | – | 1.5 |
*Hydroxy Propyl Methyl Cellulose K4M (HK4M).
Polycarbophil (PCL).
Methotrexate Sodium (MTS).
Pluronic F-127 (PLF-127).
3.4. MTS in-situ gels characterization
3.4.1. Gelation temperature and time
Heated, MTS in-situ gelling solution in specially designed thin walled (0.6 mm) borosilicate glass tube (65 mm length & 10 mm internal diameter) by placing in a thermostatically controlled water bath with incremental rise in temperature (every 2 °C for 5 min) till it converted to gel. The absence of flow when the tube was overturned indicated the gel formation and was rendered ‘gelation temperature’ (Schuetz et al., 2008).
Heated 2 ml of MTS in-situ gelling solution at respective gelation temperature (determined earlier) in specially designed thin-walled borosilicate tube (as described above) in a thermostatically controlled water bath. The tube was inspected at periodic intervals, overturned to observe the flow of gelling solution. The time lag, when the gelling solution didn’t flow was considered as ‘gelation time’ (Schmolka and Artificial skin, 1972).
3.4.2. Syringeability test
The syringeability test apparatus consisted of MTS in-situ gelling solution (5 ml, maintained at 5 ± 1 °C) loaded 5 ml glass syringe and 18G needle held by vertical support. The time taken for syringeability of gelling solution (expel completely from syringe) under constant pressure (0.5 kg placed on pan) was recorded (Ravani et al., 2013).
3.4.3. Viscosity study
The viscosity of MTS in-situ gels was measured (n = 3) at 5 ± 1 °C and at 37 ± 1 °C using viscometer (Brookfield DV Pro-II, United States). The samples were equilibrated in thermo stated water jacket for 10 min; viscosity read at 50 rpm with spindle no. 5 (Thakkar et al., 2007).
3.4.4. Drug content
The drug content in MTS in-situ gels equivalent to 1 ml was extracted in phosphate buffer solution (PBS) pH 7.4, diluted upto 100 ml. The drug content was determined spectrophotometrically at 303.5 nm (Florey, 2001).
3.4.5. Sterilization
Gamma irradiation is simple, efficient, and convenient for terminal sterilization of pharmaceuticals and is recommended by European Pharmacopoeia (Dorpema, 1990, General texts on sterility, 2005, Helena Madruga Lima-Ribeiro et al., 2012). The optimized formulation purged with nitrogen, sealed in borosilicate glass vials was gamma irradiated (Microtrol, Bangalore, India -Cobalt-60 source) for 15.76 kGy dose at 25 °C for sterilization. Compendial sterility testing was carried out on irradiated samples to ascertain the effectiveness of sterilization (Indian Pharmacopoeia, 2014).
3.4.6. Sterility testing of formulations
Sterility test was performed as per the compendial method. The effectiveness of sterilization was determined by direct inoculation of MTS in-situ gels in fluid thioglycolate (FT) media and soyabean casein digest (SD) media. 2 ml of the formulation was transferred aseptically (and mixed) to 20 ml of FT media and 20 ml of SD media separately. The inoculated media were incubated for 14 days at 30 °C to 35 °C in the case of FT medium and 20 °C to 25 °C in the case of SD medium. Sterile liquidmedia was used as negative control and unsterilized gel was used as positive control. Clouding of the liquid media implied contamination and ineffective sterilization, while a clear and uncontaminated liquid media indicates efficient sterilization (Snekhalatha et al., 2012). Effect of sterilization on all the characterization parameters was ascertained.
3.4.7. In vitro drug release studies
In vitro release of MTS from in-situ gels was performed using a dialysis bag diffusion method. Dialysis membrane 110 (Hi-media Labs, India) with an average flat width of 32.34 mm (21.5 mm average diameter, approximate capacity of 3.63 ml cm−1) was used for diffusion. 1 ml of in-situ gel was placed in hydrated dialysis membrane (soaked in freshly prepared 7.4 PBS), and tied from both ends. The dialysis tube was positioned (lower end touching the surface of buffer) in a glass beaker with 30 ml of pH 7.4 PBS maintained at 37 ± 0.5 °C. Aliquots of 3 ml were pipetted out at predetermined intervals and the same was replenished with fresh, warm diffusion medium to maintain constant volume. Subsequent to appropriate dilutions, samples were evaluated spectrophotometrically at 303.5 nm for MTS (Florey, 2001). The in vitro drug release profile was plotted and mechanism of drug release from gels was ascertained by fitting release data into various mathematical models.
3.4.8. In vivo study
Approval from Institutional Animal Ethics Committee for the conduct of preclinical study was obtained (IAEC No: JSSCPM/249/2017). Twelve wistar rats of either sex weighing 150–200 g were used for the study. The rats were randomly grouped into two with six rats in each group. The grouping and treatment schedule of experimental animals are shown in Table 2. On day “0”, induction of arthritis was initiated by injecting 0.1 ml of Freund’s complete adjuvant (FCA) into the sub-planter area of the right hind paw for all rats after anaesthesia. Animals were cautiously and carefully examined on daily basis by examining the induced paw and their general well-being. The sub-planter injection of FCA produces local oedema after few hours with a step by step elevation and reaching its peak level by 28th day. On day 14, the animals were randomized into control and test groups. The same day, test group was administered with MTS in-situ gel subcutaneously (SC), with second administration on day 21. The control group was administered saline SC weekly once, till day 28. Paw volume was measured on alternate days till day 28. The paw inflammation was measured (read as paw volume) using Digital Plethysmometer (7141 UGO Basile, Italy) on alternative days till day 14 to assess the development of arthritis in animals and its subsequent reduction till day 28 (Nagai et al., 2012, Miao et al., 2011).
Table 2.
Grouping of animals and treatment schedule for Freund’s complete adjuvant (FCA) induced chronic arthritis in rat model.
| Group | Induction of arthritis | Treatment |
|---|---|---|
| Control | Arthritis was induced by injecting 0.1 ml of FCA into the sub planter region of the right hind paw on day 0. | Received saline solution subcutaneously every week. |
| MTS in situ gel | Arthritis was induced by injecting 0.1 ml of FCA into the sub planter region of the right hind paw on day 0. | Received a single dose of MTS in situ gel subcutaneously per week |
3.4.9. Assessment of biocompatibility
Biocompatibility of optimized MTS in-situ gels was assessed in male wistar rats (150–200 g weight, aged 6–8 weeks). The right and left knee joint received 100 µL of optimized formulation and 100 µL saline solution respectively by IA. Both joints were monitored for swelling till 7th day; both joints were separated by euthanizing the animals on 8th day. The dissected joints were soaked in formalin (10%) for 48 h, dehydrated serially, embedded in paraffin, stained with haematoxylin and eosin. Histopathology of these samples were studied using light microscope (Olympus BX 51: Olympus, Japan) by a qualified pathologist for tissue reactions and cell infiltration in the synovium (Klouda and Mikos, 2008).
3.4.10. Stability studies
During storage conditions, the degradation of formulation may occur due to chemical modification or lack of formulation stability. The objective of stability studies was to ascertain the effect of the temperature and relative humidity (RH) conditions on stability of formulation by increasing rate of degradation. The optimized MTS in-situ gel were stored at 5 ± 3 °C and 25 ± 2 °C, 60 ± 5%RH for 6 months (as per ICH Q1A (R2) guidelines) in amber coloured glass vials sealed secured with rubber bungs (ICH harmonized tripartite guidelines, 2003). Samples were analyzed at 3 and 6 months for changes in physical characteristics and drug content.
4. Results and discussion
The FT-IR spectra of the pure drug (MTS) and formulation M4 & M7 showed that the characteristics peaks of MTS were not altered without any change in their position, thereby indicating no chemical interactions between the drug and polymers used (Fig. 1 and Table 3). FT-IR study demonstrated the compatibility of MTS with PLF-127, HK4M and PCL.
Fig. 1.
FT-IR spectrum (A- Methotrexate, B- Formulation M4, C- Formulation M7).
Table 3.
Interpretation of FT-IR spectra of Methotrexate and physical mixtures (M4 & M7).
| Sl No. | Peak Observed (cm−1) | Interpretation | Formulation M4 | Formulation M7 |
|---|---|---|---|---|
| 1. | 2960.70 | O—H Stretch of COOH | 2891.39 | 2881.75 |
| 2. | 1642.74 | C O Stretch | 1645.33 | 1647.26 |
| 3. | 1546.72 | C—C Stretch (aromatic) | 1545.03 | 1546.96 |
| 4. | 1448.35 | C—H Deformation (CH3) | 1496.81 | 1464.02 |
| 5. | 1404.22 | C C Stretch (aromatic) | 1458.23 | 1350.22 |
| 6. | 1207.72 | C—O Stretch | 1350.00 | 1300.00 |
| 7. | 831.11 | C—H Deformation (aromatic) | 850.04 | 850.06 |
| 8. | 3408.33 | N—H Stretching | 3408.33 | 3446.91 |
MTS showed three endotherms; the first two endotherms that emerge at about 95 °C and 120 °C are correlated with the loss of free and bound water, respectively (Fig. 2). The third endotherm at 178.11 °C represents the melting point of MTS. The DSC analysis of MTS indicated that the obtained sample was a hydrate form (see Fig. 3).
Fig. 2.

Formulation of Methotrexate in-situ gels.
Fig. 3.
DSC thermogram of Methotrexate.
The MTS in-situ gels were prepared by cold method technique in which the concentrations of HK4M and PCL were varied; whereas PLF-127concentration was kept constant to our pre-optimized level [20% (w/v)] (Yang et al., 2009). The preliminary study revealed that PLF-127system alone at 20–22% w/v concentration was not quite satisfactory and exhibited prolonged gelation temperature and time. Therefore, the addition of co-polymer was initiated; HK4M and PCL were experimented in different concentration. The prepared in-situ gels were clear and transparent (Fig. 2). The overnight stay at cold temperature (2–8 °C) ensured the complete solubility of drug and polymers due to surfactant effect of PLF-127. Six formulations of MTS in-situ gels (M2 to M7) were prepared by altering two polymer constituents, the concentrations of HK4M and PCL (Table 1). The results of gelation temperature (°C), gelation time (sec), syringeability test (sec), in vitro drug release studies (% cumulative drug release at 96 h) and viscosity (cps) are presented in Table 4. The final concentration of MTS in the in-situ gels was 0.5% (w/v).Table 5.
Table 4.
Characterization of MTS in situ gels.
| Formulation Code | Gelation temperature (°C) | Gelation time (Sec) | Syringeability (Sec) | Cumulative % drug release (at 96 h) | Viscosity |
|
|---|---|---|---|---|---|---|
| 8 °C | 37 °C | |||||
| M1 | 37.3 ± 0.42 | 65 ± 2 | 5.33 ± 0.58 | – | 544 ± 12 | 26248 ± 223 |
| M2 | 35.6 ± 0.46 | 65 ± 1 | 6.33 ± 0.58 | 98.80 ± 2.39 | 1053 ± 34 | 32021 ± 246 |
| M3 | 35.2 ± 0.62 | 60 ± 2 | 6.66 ± 0.58 | 98.93 ± 3.04 | 1126 ± 42 | 39088 ± 258 |
| M4 | 34.8 ± 0.51 | 58 ± 1 | 7.00 ± 1.00 | 93.26 ± 2.39 | 1432 ± 61 | 43179 ± 269 |
| M5 | 37.2 ± 0.24 | 65 ± 1 | 5.33 ± 0.58 | 99.39 ± 4.93 | 583 ± 18 | 28356 ± 234 |
| M6 | 36.7 ± 0.28 | 60 ± 2 | 6.00 ± 1.00 | 96.18 ± 4.83 | 721 ± 21 | 28924 ± 286 |
| M7 | 36.0 ± 0.57 | 58 ± 2 | 6.66 ± 0.58 | 91.27 ± 4.48 | 915 ± 28 | 32623 ± 169 |
| Post sterilization (M4) | 34.5 ± 0.58 | 57 ± 1 | 6.86 ± 1.00 | 94.28 ± 3.12 | 1394 ± 49 | 42981 ± 198 |
*Mean ± Standard deviation, n = 3.
Table 5.
In-vitro dissolution data and mathematical model fitting.
| Formulation Code | T50% (Hrs) | Order of drug release | Mechanism of drug release | ‘n' value |
|---|---|---|---|---|
| M1 | 03.87 | First-Order | Non-fickian | 0.654 |
| M2 | 44.42 | First-Order | Non-fickian | 0.760 |
| M3 | 52.92 | First-Order | Non-fickian | 0.629 |
| M4 | 55.02 | First-Order | Non-fickian | 0.585 |
| M5 | 39.15 | First-Order | Non-fickian | 0.532 |
| M6 | 43.58 | First-Order | Non-fickian | 0.613 |
| M7 | 48.80 | First-Order | Non-fickian | 0.535 |
*Mean ± Standard deviation, n = 3.
An increase in the concentration of PLF-127 by 2% resulted in noticeable changes in the evaluation parameters of gel. PLF-127 gels with 22%w/w exhibited gelation at 35.1 °C within 59 sec and syringeability time of 6.18 sec. The complete satisfactory in-situ gel system could be achieved by increasing the concentration of PLF-127 or by incorporating co-polymers in the formulation. Hence, co-polymers (HK4M and PCL) were incorporated to investigate the feasibility of formulating thermoresponsive PLF in-situ gels.
Gelation of PLF-127 occurs due to dehydration of the polymer leading to an increased chain friction and entanglement, producing hydrophobic association. Micelles are formed in aqueous pluronic solutions. At higher temperature or at high concentrations, these micelles associate to form various lyotropic liquid crystalline phases. With the increase in temperature, micellar entanglement proceeds, leading to gel formation and an overall increase in bulk viscosity. PLF solutions forms gel at body temperature forming liquid crystalline phase due to increased intermicellar interactions. With increase in length of PEO chain the onset and temperature of gelation and thermal stability of the gel also increases.
The prepared MTS in-situ gels showed sol-to-gel transformation. The gelation temperatures were in the range of 34.8 ± 0.51 °C to 37.3 ± 0.42 °C (close to human body temperature).The formulation M2 and M5 exhibited highest gelation temperature whereas M4 and M7 showed lowest gelation temperature.
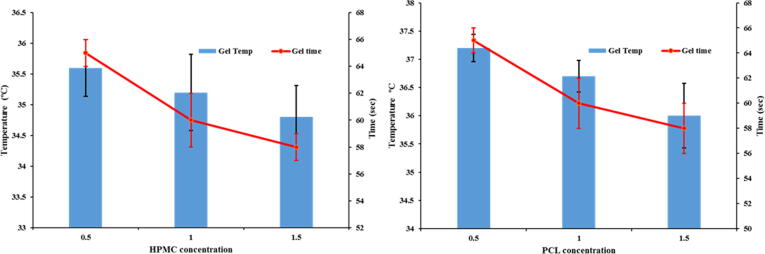
The results suggest concentration dependent gelation of the system; an upsurge in HK4M and PCL concentration, lowered the gelation temperature of the system. Although PLF-127 concentration had significant effects on the sol–gel transformation, HK4M and PCL concentrations also affected the gelation temperature. It was observed that the concentrations of the HK4M and PCL increased the viscosity of the system linearly; which lead to lowered gelation temperature (Fig. 4). Although both in-situ gels showed considerable impacts on the sol–gel transition, HK4M concentration had a greater impact on the gelation temperature. Klouda et al., addressed changes in PLF-127 molecule properties upon changes in temperature and concentration in the environment, which was a main reason for choosing PLF-127 (20%) for the study.
Fig. 4.
Effect of polymer (HK4M and PCL) concentration on gelation time (Sec) and gelation temperature (°C).
The gelation temperature lowering effect of co-polymer could be explained by two possibilities: (a) The gelation temperature lowering effect might be caused by increased viscosity after dissolution of co-polymer or (b) Due to their ability to bind to the polyoxyethylene chains present in the Pluronic molecules, they promote dehydration, causing an increase in entanglement of adjacent molecules and extensively increasing intermolecular hydrogen bonding which will lead to gelation at lower temperature.
The results of the gelation time revealed that the prepared MTS in-situ gels rapidly responded to gelation temperature, exhibiting quick gelation by a maximum of 65 ± 1 sec. The gelation times were in the range of 58 ± 1 to 65 ± 1 sec. The formulation M4 and M7 showed lowest gelation time, whereas M2 and M5 showed highest gelation time.
The gelation time was significantly influenced by polymeric concentrations of HK4M and PCL. The change of gelation temperature with polymer concentration is depicted in the Fig. 4. The rise in the polymer concentration (HK4M and PCL) from zero to 1.5%w/w resulted in a decreased gelation time of about 7 sec, due to enhanced gel viscosity. Din et al. (2015) found similar results in designing novel solid-lipid nanoparticle hydrogels for the rectal administration.
The duration to drive out the syringe contents by use of constant energy is called as syringeability time. The findings of syringeability testing revealed that the prepared MTS in-situ gels were conveniently syringeable via 18-gauge needle. It was observed that the syringeability times reported for gel formulations are within the range of 5.33 ± 0.58 sec to 7 ± 1.00 sec. Fig. 5 revealed a significant linear trend in syringeability time with proportional increase in polymer concentration (M2 to M7). This recognized the fact that, rise in the viscosity of the system resulted in improved opposition to flow (Cabana et al., 1997).
Fig. 5.
Effect of polymers (HK4M and PCL) concentration on syringeability (Sec), in vitro drug release studies (cumulative % drug release).
All the formulations exhibited sol state at refrigerated temperature (5 ± 3 °C) but transformed into clear stiff gels at body temperature (37 °C). The conversion from sol-to-gel is dependent on temperature, which is reversible when refrigerated. The viscosity of the MTS in-situ gels was measured at 5 ± 3 °C (storage temperature) and 37 ± 0.5 °C (human body temperature). A temperature dependent raise in viscosity was exhibited by the gel (Fig. 6). The viscosity of the system was proportionally increased with increment in HK4M and PCL concentrations. Whereas Pluronic polymers, which are polypropylene oxide tri block copolymers and non-ionic in nature, got aggregated into micelles at 37 °C; this relates to dehydration of the polymer blocks with changes (rise) in temperature. It has been confirmed that the protective material and micellar concentration promotes gel formation; at larger PLF-127concentrations, the gel is more tangled. Consequent to these, separation from each other becomes evidently difficult, causing inflexibility and higher gel viscosity at higher PLF concentrations (Jain et al., 1998, Dyondi et al., 2015). The viscosity values for optimized formulation was low at 5 ± 3 °C; although, a significant rise was reported at 37 ± 0.5 °C, owing to sol–gel conversion. The MTS in-situ gel (M4) viscositywas 1432cps (at 5 ± 3 °C), a notable increase was observed at 37 °C (43179cps). Taking into account all the results/findings of characterization, formulation M4 was considered for further studies.
Fig. 6.
Effect of polymers (HK4M and PCL) concentration on viscosity (cps) at two different temperature i.e. 8 °C & 37 °C.
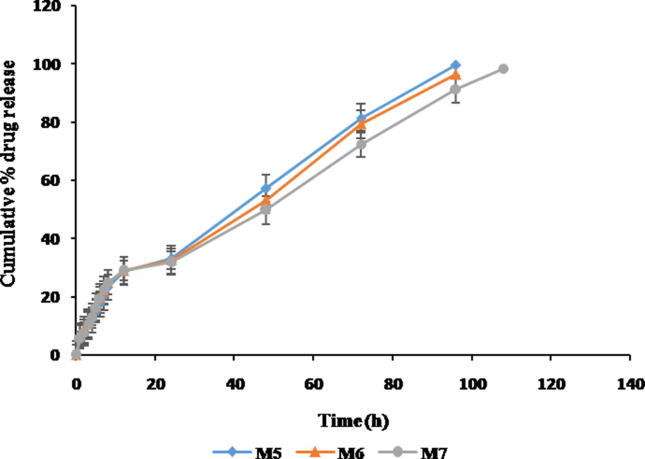
The in vitro drug release studies help in providing important data regarding the performance of a dosage form under in vivo conditions. Sustained MTS release from formulations upto 96 h due to gradual degradation of the polymer matrix and chiefly influenced by the combination of polymers resulted in rapid gelation time and lowered gelation temperature. The formulations M4 and M7 prolonged the release compared to other formulations (Fig. 7, Fig. 8). Preliminary burst discharge followed by a sustained release of MTS was observed with all in-situ gels. The burst release of MTS helps in immediate management of symptoms, prolonged release helps for maintaining the therapeutic concentration in the management of RA (Bozdag et al., 2005).
Fig. 7.
Comparative in vitro release profile of MTS in situ gels (Formulation- M2, M3 & M4).
Fig. 8.
Comparative in vitro release profile of MTS in situ gels (Formulation- M5, M6 & M7).
In-vitro drug release data was fit into various mathematical models to ascertain the best fit model and to determine the release mechanism. From the kinetics study, it was found that drug release from all the formulations followed first-order kinetics (concentration dependant) and the best fit model was peppas model. Drug release data (<70%) was fitted during the peppas model. The value of ‘n’ determined from Korsmeyer-Peppas equation if found to be <0.45 = Fickian diffusion, ‘n’ value is between 0.5 and 0.85 = non-fickian diffusion or anomalous mechanism (relaxation controlled) and if ‘n’ value is >0.89 = super case II transport. The calculated ‘n’ values were between 0.5 and 0.85, indicating that the release of MTS was by non-fickian diffusion. The statistical analysis of cumulative drug release data, duration of drug release (hours) along with T50% with reference to increase in concentration of co-polymers (HK4M and PCL) was found to be highly significant (p < 0.005).
The MTS content in the M4 gel was 98.08 ± 0.12%, which implied significant loading of in-situ gel formulation.
Sterility is very important requisite for a parenteral formulation. MTS in-situ gel is designed for parenteral administration to target site. The optimized formulation (M4) was sterilized by gamma irradiation and accessed for sterility after the exposure. The microbiological evaluation assures the product’s sterility and effectiveness of the sterilization method. Post incubation period, no turbidity was observed both in the negative control as well as test sample; implying absence of microbial growth. Only in the non-irradiated formulation, used as a positive control, saw clouding of broth indicating microbial presence and its proliferation. With consideration of observations from the sterility test, the developed formulations passed the test for sterility.
Sterilization by gamma rays helps in terminal sterilization of the dosage form before its administration into the body. Among other methods for sterilization, gamma irradiation was preferred for its high ability to penetrate the product that permits sterilization of even susceptible material without elevation of temperature. However, gamma irradiation may have affected characterization of in-situ gels (Memisoglu-Bilensoy and Hincal, 2006). Moreover, the effect of gamma sterilization on gelation temperature and time, syringeability time, drug release -in vitroand drug content was evaluated. The results demonstrated that there were no significant changes in the said parameters between non-irradiated and irradiated formulations (Table 3).
FCA is a denatured Mycobacterium butyricum suspended in mineral oil (paraffin oil). FCA induced arthritis model for chronic inflammation in rats is considered critical for the pathophysiological and pharmacological management of inflammatory processes. It helps in the assessment and evaluation of anti-inflammatory and anti-arthritic agents since it mimics the RA progression in humans.
Paw volume reports for the MTS in-situ gel treated rats (test) and control rats were compared to evaluate whether the site targeted injection of MTS in-situ gels may indicate positive course for arthritis. Fig. 9 demonstrates the impact of MTS in-situ gels on swelling of the right hind paw in different groups. The MTS in-situ gels showed a more beneficial result than control group during the study period. In comparison, the paw swelling with MTS in-situ gels treated rats (0.94 ± 0.2%; P < 0.05) was substantially less when compared to the rats in the control group (1.26 ± 0.4%) after 14 days treatment period. This variation remained significantly throughout the duration of the study up to 28th day, where paw volume for the MTS in-situ gel treated rats was 0.48 ± 0.3% (P < 0.05). The effectiveness of MTS in-situ gel in FCA-induced arthritis model was aimed to retain MTS within the joint space. The findings from the study can be supported by the previous literature where lipid nano emulsion of MTS was assessed for anti-inflammatory activity in RA treatment (Maranhao et al., 2013). It was concluded that improvement in the RA inflammatory condition could be ultimately resulted from sustained MTS release and its concentration in the target site.
Fig. 9.
The anti-inflammatory effect of MTS in situ gels as assessed by a reduction in paw volume in rats with adjuvant arthritis model. (One day after treatment).
Drug delivery systems can enhance the drug retention at target site while reducing its toxicity. The findings of the current research verified these hypotheses by demonstrating gradual reduction in paw volume in MTS in-situ gels treated rats which progressed towards normal condition by the end of treatment period. These findings establish that incorporation of MTS into in-situ gels could desirably improve efficiency in suppressing inflammation in RA.
Significant paw swelling was not reported in test group; since macroscopic signs of paw stiffness, swelling or redness was not observed. The histological samples of haematoxylin and eosin stained joints of both test and control group are shown in Fig. 10. Inflammatory infiltration was not found in the joint tissue. The joints treated with MTS in-situ gels did not differ from the control joint tissue. These results inferred that MTS in-situ gel shows favourable biocompatibility with tissues at the target site. Thus, MTS in-situ gels could provide a non-toxic, biocompatible solution for the successful management of RA.
Fig. 10.
Representative H and E (hematoxylin and eosin) stained histological slides of joint tissues from healthy rat knees after injection with (A) 100 µL of saline and (B) 100 µL of MTS in situ gels (magnification: 100×).
The stability studies for optimized formulation were conducted at 5 ± 3 °C (long term storage condition) and at 25 °C/60% RH (accelerated storage condition) for 6 months. There was no remarkable difference in the physical properties; gelation temperature and time, and drug content during the stability period, which indicated that the optimized formulation displayed superior stability during evaluation.
5. Conclusion
MTS in-situ gels were formulated to provide a novel injectable, biodegradable system for managing RA. The MTS in-situ gel remained stable, thermoresponsive and transformed to gel lower than the body temperature. The optimized in-situ gel exhibited rapid and sustained release of MTS enhancing the treatment modality; it was non-toxic and biocompatible with joint tissue. A novel drug delivery system for prolonged release of MTS which can reduce dose, dosing frequency, associated side effects, cost of therapy and there by improve patient compliance was developed. This research demonstrated advantages of MTS in-situ gel and may be suitable as an injectable drug delivery system for management of RA to overcome the limitations of currently available formulations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank JSS Academy of Higher Education and Research and JSS College of Pharmacy, Mysuru for providing necessary facilities to carry out this research work.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2020.10.003.
Contributor Information
Madhugiri Prakash Venkatesh, Email: venkateshmpv@jssuni.edu.in.
Tegginmat Pramod Kumar, Email: pramodkumar@jssuni.edu.in.
Deeksha Ramananda Pai, Email: deeksharpai@jssuni.edu.in.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.

References
- Abdel-Nasser A.M., Rasker J.J., Vaikenburg H.A. Epidemiological and clinical aspects relating to the variability of rheumatoid arthritis. Semin. Arthritis. Rheum. 1997;27:123–140. doi: 10.1016/s0049-0172(97)80012-1. [DOI] [PubMed] [Google Scholar]
- Albert C., Brocq O., Gerard D., Roux C., Euller-Ziegler L. Septic knee arthritis after intra-articular hyaluronate injection. Joint. Bone. Spine. 2006;73:205–207. doi: 10.1016/j.jbspin.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Avina-Zubieta J.A., Choi H.K., Sadatsafavi M., Etminan M., Esdaile J.M., Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis. Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- Bozdag S., Dillen K., Vandervoort J., Ludwig A. The effect of freeze-drying with different cryoprotectants and gamma-irradiation sterilization on the characteristics of ciprofloxacin HCl-loaded poly(D, L-lactide-glycolide) nanoparticles. J. Pharm. Pharmacol. 2005;57:699–707. doi: 10.1211/0022357056145. [DOI] [PubMed] [Google Scholar]
- Butoescu N., Jordan O., Doelker E. Intra-articular drug delivery systems for the treatment of rheumatic diseases: A review of the factors influencing their performance. Eur. J. Pharm. Biopharm. 2009;73:205–218. doi: 10.1016/j.ejpb.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Cabana A., Aït-Kadi A., Juhasz J. Study of the gelation process of polyethylene oxidea-polypropylene oxideb–polyethylene oxideacopolymer (poloxamer 407) aqueous solutions. J. Colloid. Interface. Sci. 1997;190:307–312. doi: 10.1006/jcis.1997.4880. [DOI] [PubMed] [Google Scholar]
- Chopra A. Ayurvedic medicine and arthritis. Rheum. Dis. Clin. North Am. 2000;26:133–144. doi: 10.1016/s0889-857x(05)70127-7. [DOI] [PubMed] [Google Scholar]
- Cronstein B.N. The mechanism of action of methotrexate. Rheum. Dis. Clin. N.Am. 1997;23(4):739–755. doi: 10.1016/s0889-857x(05)70358-6. [DOI] [PubMed] [Google Scholar]
- Din F.U., Mustapha O., Kim D.W., Rashid R., Park J.H., Choi J.Y., Ku S.K., Yong C.S., Kim J.O., Choi H. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur. J. Pharm. Biopharm. 2015;94:64–72. doi: 10.1016/j.ejpb.2015.04.019. [DOI] [PubMed] [Google Scholar]
- Dorpema J.W. Review and state of the art on radiation sterilization of medical devices. Int. J. Radiat. Appl. Instrum. C. Radiat. Phys. Chem. 1990;35:357–360. [Google Scholar]
- Dyondi D., Sarkar A., Banerjee R. Joint surface-active phospholipid-mimetic liposomes for intra-articular delivery of paclitaxel. J. Biomed. Nanotechnol. 2015;11:1225–1235. doi: 10.1166/jbn.2015.2061. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F., Maini R. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Florey K. Elsevier; New Delhi: 2001. Analytical Profile of Drug Substances. [Google Scholar]
- General texts on sterility, 2005. European Pharmacopoeia (EP 5); Council of Europe: Edom, Strasbourg. Vol. 1, pp. 445–449.
- Helena Madruga Lima-Ribeiro, M., Santos-Oliveira, R., Firmino de Santana, M., de Jesus Andreoli Pinto, T., Satiko Kikuchi, I., GonçalvesMothé, C., Cassandra BreitenbachBarroso Coelho, L., Tereza dos Santos Correia, M., Maria dos AnjosCarneiro-Leão, A., 2012. In vitro evaluation of gamma irradiation on a gel formulation of Cratyliamollis: rheological proporties and microbiological control. J. Cosmet. Dermatol. 02, 45–50.
- ICH harmonized tripartite guidelines, 2003: Stability testing of new drug substances and products Q1A(R2).
- Jain N.J., Aswal V.K., Goyal P.S., Bahadur P. Micellar structure of an ethylene oxide−propylene oxide block copolymer: a small-angle neutron scattering study. J. Phys. Chem. B. 1998;102:8452–8458. [Google Scholar]
- Klouda L., Mikos A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C., Ostergaard J., Larsen S.W., Jensen H., Jacobsen S., Lindegaard C., Andersen P.H. Intra-articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders. J. Pharm. Sci. 2008;97:4622–4654. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kavanaugh A. Pharmacological treatment of established rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 2003;17:811–829. doi: 10.1016/s1521-6942(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Leigh J.P., Fries J.F. Arthritis and mortality in the epidemiological follow-up to the National Health and Nutrition Examination Survey I. Bull. N. Y. Acad. Med. 1994;71:69–86. [PMC free article] [PubMed] [Google Scholar]
- Lipsky P., Kavanaugh A. The impact of pharmaco-economic considerations on the utilization of novel anti-rheumatic therapies. Rheumatology (Oxford). 1999;38:41–44. [PubMed] [Google Scholar]
- Magne D., Palmer G., Barton J., Mézin F., Talabot-Ayer D., Bas S., Duffy T., Noger M., Guerne P., Nicklin M., Gabay C. The new IL-1 family member IL-1F8 stimulates production of inflammatory mediators by synovial fibroblasts and articular chondrocytes. Arthritis. Res. Ther. 2006;8:1–11. doi: 10.1186/ar1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranhao, R., Verissimo de Mello, S.B., Tavares, Bulgarelli, Maria, 2013. Intra-articular methotrexate associated to lipid nanoemulsions: anti-inflammatory effect upon antigen-induced arthritis. Int. J. Nanomed. 443. [DOI] [PMC free article] [PubMed]
- Memisoglu-Bilensoy, E., Hincal, A.A., 2006. Sterile, injectable cyclodextrin nanoparticles: Effects of gamma irradiation and autoclaving. Int. J. Pharm. 311, 203–208. [DOI] [PubMed]
- Meune C., Touze E., Trinquart L., Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology(Oxford) 2009;48:1309–1313. doi: 10.1093/rheumatology/kep252. [DOI] [PubMed] [Google Scholar]
- Miao B., Song C., Ma G. Injectable thermosensitive hydrogels for intra-articular delivery of methotrexate. J. Appl. Polym. Sci. 2011;122:2139–2145. [Google Scholar]
- Misra R., Sharma B., Gupta R., Pandya S., Agarwal S., Agarwal P., Grover S., Sarma P., Wangjam K. Indian rheumatology association consensus statement on the management of adults with rheumatoid arthritis. Indian. J. Rheumatol. 2008;3:S1–S16. [Google Scholar]
- Nagai T., Kyo A., Hasui K., Takao S., Matsuyama T. Efficacy of an immunotoxin to folate receptor beta in the intra-articular treatment of antigen-induced arthritis. Arthritis. Res. Ther. 2012;14:R106. doi: 10.1186/ar3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai D.R., Prakash V.M., Kumar T.P. Current developments in therapeutic drug targeting for the management of rheumatoid arthritis: an emerging paradigm. Crit. Rev. Ther. Drug. 2019;36(6) doi: 10.1615/CritRevTherDrugCarrierSyst.2019025729. [DOI] [PubMed] [Google Scholar]
- Pincus T. Aggressive treatment of early rheumatoid arthritis to prevent joint damage. Bull. Rheum. Dis. 1998;47:2–7. [PubMed] [Google Scholar]
- Pincus T., Callahan L.F., Sale W.G., Brooks A.L., Payne L.E., Vaughn W.K. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis. Rheum. 1984;27:864–872. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- Ravani L., Esposito E., Bories C., Moal V.L., Loiseau P.M., Djabourov M., Cortesi R., Bouchemal K. Clotrimazole-loaded nanostructured lipid carrier hydrogels: Thermal analysis and in vitro studies. Int. J. Pharm. 2013;454:695–702. doi: 10.1016/j.ijpharm.2013.06.015. [DOI] [PubMed] [Google Scholar]
- Sacks J.J., Helmick C.G., Langmaid G. Deaths from arthritis and other rheumatic conditions, United States, 1979–1998. J. Rheumatol. 2004;31:1823–1828. [PubMed] [Google Scholar]
- Schmolka I.R., Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972;6:571–582. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- Schuetz Y.B., Gurny R., Jordan O. novel thermoresponsive hydrogel based on chitosan. Eur. J. Pharm. Biopharm. 2008.A;68:19–25. doi: 10.1016/j.ejpb.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Snekhalatha U., Anburajan M., Venkatraman B., Menaka M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Z. Rheumatol. 2012;72:375–382. doi: 10.1007/s00393-012-1083-8. [DOI] [PubMed] [Google Scholar]
- Sokka T., Abelson B., Pincus T. Mortality in rheumatoid arthritis 2008 update. Clin. Exp. Rheumatol. 2008;26:35–61. [PubMed] [Google Scholar]
- Test for sterility, 2014. Indian Pharmacopoeia.; The Indian Pharmacopoeia Commision: Ghaziabad. Vol1, pp. 59–66.
- Thakkar H., Kumar Sharma R., Murthy R.S.R. Enhanced retention of celecoxib-loaded solid lipid nanoparticles after intra-articular administration. Drugs. R. D. 2007;8:275–285. doi: 10.2165/00126839-200708050-00002. [DOI] [PubMed] [Google Scholar]
- Venkatesh M.P., Anis S., Kumar T.P. Design and development of an injectable in-situ forming drug delivery system of methotrexate for the treatment of rheumatoid arthritis. J. Drug. Deliv. Sci. Technol. 2013;23:445–453. [Google Scholar]
- Yang Y., Wang J., Zhang X., Lu W., Zhang Q. A novel mixed micelle gel with thermosensitive property for the local delivery of docetaxel. J. Control. Release. 2009;135(2):175–182. doi: 10.1016/j.jconrel.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Wenham C.Y.J., Grainger A.J., Hensor E.M.A., Caperon A.R., Ash Z.R., Conaghan P.G. Methotrexate for pain relief in knee osteoarthritis: an open-label study. Rheumatology. 2013;52:888–892. doi: 10.1093/rheumatology/kes386. [DOI] [PubMed] [Google Scholar]
- Wolfe F., Mitchell D.M., Siblety J.T., Fries J.F., Bloch D.A., Williams C.A., Spitz P.W., Haga M., Kleinheksel S.M., Cathey M.A. The mortality of rheumatoid arthritis. Arthritis. Rheum. 1994;37:481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- Yang Z., Nie S., Hsiao W., Pam W. ThermoreversiblePluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: in vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011:151–166. doi: 10.2147/IJN.S15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.