Abstract
Objectives
This postmarketing study aims to evaluate the safety and effectiveness of oral administration of risedronate at 75 mg once monthly for 36 months in patients with osteoporosis in Japan.
Methods
Participants were ambulatory outpatients with osteoporosis who were ≥ 50 years old and had prevalent vertebral fractures. Outcomes were the incidence rate of adverse drug reaction (ADR), cumulative incidence of vertebral, nonvertebral, and hip fractures, the percent changes of lumbar spinal L2–4 bone mineral density (BMD), and low back pain. In addition, medication compliance was examined.
Results
Safety, vertebral fractures, and other outcomes were analyzed in 542, 328, and 535 patients, respectively. In the safety analysis set, 88.38% of the patients were women and the mean age was 75.9 years. The monthly medication compliance rate ranged from 83.24% to 95.38%. The incidence rate of ADRs, including 4 severe ADRs, was 10.52% (n = 57). The common ADRs were gastrointestinal disorders, musculoskeletal, and connective tissue disorders. No osteonecrosis of the jaw was reported. The cumulative incidences (95% CI) of vertebral, nonvertebral, and hip fractures at 36 months were 12.58% (8.61–18.18), 6.59% (4.31–10.01), and 1.58% (0.64–3.88), respectively. The L2–4 BMD increased by 10.59% compared with baseline value (P < 0.01), and the proportion of patients with low back pain decreased to 30.77%, at 36 months.
Conclusions
Administering 75 mg of risedronate once a month remains a favorable compliance rate and may be useful for the treatment of patients, even the elderly, with osteoporosis in daily practice.
Keywords: Once-monthly risedronate, Postmarketing survey, Osteoporosis, Medication compliance, Vertebral fracture, Low back pain
1. Introduction
Osteoporosis is not merely a bone aging phenomenon but a disease that requires prevention and treatment [1]. In Japan, the number of patients with osteoporosis in 2005 was approximately 13 million [2], which has been increasing annually because of the rapid aging of the population. Therefore, osteoporosis treatment for these patients is important. The primary symptom of a vertebral fracture developing in patients with osteoporosis is lower back or back pain during body movement, resulting in a decrease in activities of daily living. Consequently, bones and muscles weaken, inducing new fractures and pain and further lowering the quality of life (QOL). Considering that mortality increases with the number of vertebral fractures, accumulation of vertebral fractures is regarded as a life-threatening factor [3].
Risedronate is classified as a third-generation bisphosphonate based on its structure with a pyridinyl group in the side chain; it suppresses bone turnover by strongly inhibiting bone resorption [4]. Since the first international launch of risedronate in 1998, it has been used long for patients with osteoporosis in various countries, including Japan. Risedronate has 3 formulations, namely, 2.5-mg once-daily formulation, 17.5-mg once-weekly formulation, and 75-mg once-monthly formulation in Japan. These formulations improve the bone density, bone metabolism markers, and QOL of patients with osteoporosis. With regard to bone fractures, a 96-week clinical trial in Japan reported that its 2.5-mg once-daily formulation of risedronate had a noninferior efficacy in new bone fractures compared with the formulation of etidronate [5]. In a previous study on Japanese patients with osteoporosis, the incidence of hip fractures on the unaffected side at 36 months was significantly lower in the risedronate group than in the control group (calcium, vitamin D3, vitamin K2, and calcitonin preparation or no treatment) [6]. Two overseas large-scale clinical studies also reported that 5-mg once-daily formulation of risedronate decreased the cumulative incidence of new vertebral fractures by 41% [7] and reduced the risk of new vertebral fractures by 49% [8] over 3 years in comparison with a placebo. The manufacturing and marketing of the 75-mg once-monthly formulation of risedronate was approved in Japan in December 2012. In terms of bone mineral density (BMD) and cumulative incidence of vertebral fracture at the end of a 1-year study, this formulation had a comparable efficacy compared with the 2.5-mg once-daily formulation of risedronate [9]. Hence, the fracture-preventing effect of risedronate was confirmed, and the 3 formulations of this drug were found to have similar efficacies. However, the overall compliance rate of daily bisphosphonate was reported to decrease to nearly half 1 year after the initial dose in daily practice in Japan [10] and overseas [11]. Moreover, patient compliance to the treatment regimen is extremely important among individuals with osteoporosis [[12], [13], [14], [15]]. Therefore, the efficacy of long-term treatment with once-monthly risedronate in actual practice remains unclear.
We have completed a 3-year nationwide postmarketing study to investigate its safety and effectiveness, including vertebral fracture risk assessment, in the actual clinical practice. Here, we report the results of the study.
2. Methods
2.1. Study design and subjects
This prospective, longitudinal, 3-year observational study focuses on the effectiveness and safety of 75-mg once-monthly formulation of risedronate in patients with osteoporosis registered through the central registration across Japan. The registration period was from May 2013 to October 2014, and this study was finished in April 2018. This study was performed in accordance with the Japanese Good Postmarketing Study Practice ordinance provided by the Ministry of Health, Labour, and Welfare of the Japanese government. In accordance with these regulations, the need for informed consent from patients was waived. This study was registered in the JAPIC clinical trials registry (JapicCTI-142479).
Eligible subjects were ambulatory outpatients with osteoporosis according to the Diagnostic Criteria for Primary Osteoporosis of the Japanese Society for Bone and Mineral Research [16]. The subjects had 1–4 prevalent baseline (within 3 months prior to the first prescription) fractures in the fourth thoracic spine–fourth lumbar spine (T4–L4) detected radiographically and were aged ≥ 50 years (women had to be in their postmenopausal stage). All subjects were orally administered with 75 mg of risedronate once every month (Actonel® tablet; EA Pharma Co., Ltd., Tokyo, Japan; or Benet® tablet; Takeda Pharmaceutical Company Limited, Osaka, Japan) and observed for 3 years. Patients who had already been treated with such risedronate formulation (75 mg once in a month) at the time of registration, those registered > 15 days after the first prescription date or outside the registration period, or those who had no information on adverse event status were excluded from analysis. During the observation period, the incidence of vertebral fractures was evaluated by spinal radiography at baseline and every 6 months up to 36 months. We also assessed the lumbar spine L2–4 BMD and bone metabolism markers such as serum tartrate-resistant acid phosphatase 5b (TRACP-5b) and type I procollagen-N-propeptide (P1NP), and urinary type I collagen cross-linked N-telopeptide (u-NTX), at baseline and at 3, 6, 12, 18, 24, 30, and 36 months. BMD was measured by dual-energy X-ray absorptiometry. We collected data on baseline characteristics such as sex, age, weight, diagnosis, complications, risk factors for bone fractures, and other current osteoporosis drug use.
2.2. Outcomes
The clinical outcomes were incidence of adverse drug reactions (ADRs); the cumulative incidences (ie, incidence proportion) of vertebral, nonvertebral (including hip), and hip fractures; changes in L2–4 BMD and bone metabolism markers; and the proportion of patients with low back pain. Medication compliance rate was also assessed. Meanwhile, the data of patients who dropped out was evaluated up to the day of dropout.
With regard to safety, we assessed for any presence of ADRs, which we defined as any adverse event for which a causal relationship with risedronate could not be ruled out. Adverse events were coded according to the Medical Dictionary for Regulatory Activities terminology (Japanese, version 21.0).
We defined a vertebral fracture as any new fractures or worsening of a prevalent fracture confirmed on spinal radiography according to the justification criteria for vertebral fractures [17]. A new vertebral fracture occurred if the ratio of the central vertebral height (C) to the anterior vertebral height (A) or C to posterior vertebral height (P) was less than 0.8, the ratio of A to P was less than 0.75, or if A, C, and P all decreased at least 20% from the height of the upper or lower vertebral body. A prevalent vertebral fracture worsened if C/A, C/P, or A/P decreased by 20% or more from the baseline. For the standardization of the evaluations, a summary protocol indicating how to evaluate vertebral fractures was distributed to each institution, and radiographic assessments were performed by attending physicians in accordance with the protocol.
In terms of nonvertebral and hip fractures, we radiographically evaluated patients with suspected fractures and confirmed its diagnosis. Percent changes from baseline in L2–4 BMD and bone metabolism markers were calculated at each evaluation timepoint.
To determine the presence of low back pain and the medication compliance rate during the observation period, we interviewed the patients and asked whether they had low back pain and took risedronate on the scheduled days. We defined the compliance rate as the proportion of the behavior of taking the drug on the scheduled day as per the prescription.
2.3. Statistical analysis
A sample size of 500 patients was determined for the evaluation of the cumulative incidence of vertebral fractures, assuming a dropout rate of 50% because the dropout of the past clinical study on 2.5 mg of risedronate for 2 years resulted in 26.7%. Safety was assessed using a safety analysis set on patients who took 1 or more doses of the drug and was registered as per protocol. Furthermore, an efficacy analysis set included patients whose any efficacy data were available, whereas a vertebral fracture analysis set included patients who had 1 to 4 baseline vertebral fractures.
The incidence proportion of vertebral fractures was estimated using the Kaplan–Meier method, and a two-sided 95% confidence interval was calculated. In this analysis, patients who did not visit or switch to other drugs were considered discontinued and were treated as censored at the time of the last visit. Patients were also censored at the time of the first vertebral fracture. Likewise, in terms of nonvertebral and hip fracture analyses, patients with confirmed fractures were censored at the time of the first fracture. Percent changes from baseline in L2–4 BMD and bone metabolism markers were assessed with a paired t-test. These data were analyzed by SAS 9.2 (SAS Institute Inc., Cary, NC, USA). A value of P < 0.05 indicates statistical significance.
3. Results
3.1. Subject baseline characteristics
A total of 579 patients were registered at 148 sites, and 572 case reports were collected. We excluded 30 patients because of no treatment with the study drug (15 patients), no report on safety data (1 patient), or registration violation (15 patients) such as registration > 15 days after the first prescription date. Ultimately, 542 patients were included in the safety analysis set. Furthermore, 535 patients were included in the efficacy analysis, and 328 of them who had 1 to 4 confirmed baseline vertebral fractures based on the evaluable radiographic data before the start of treatment were included in the vertebral fracture analysis set (Supplemental Fig. 1).
Table 1 summarizes the baseline characteristics of the safety analysis set and the vertebral fracture analysis set. In the safety analysis set, 88.38% of patients were women, the mean age was 75.9 years, and the mean body mass index was 22.81 kg/m2. Primary osteoporosis was present in 88.75% of patients. The common risk factors for fractures were history of steroid use (6.46%) and drinking habit (4.98%). The mean duration of disease was 1.5 years. Osteoporosis drugs were concomitantly used by 46.13% patients, especially active vitamin D3 preparations 39.30%. The vertebral fracture analysis set had similar baseline characteristics.
Table 1.
Baseline characteristics.
| Variable | Safety analysis set (n = 542) | Vertebral fracture analysis set (n = 328) | |||
|---|---|---|---|---|---|
| Characteristic | Number of patients | Percentage or mean ± SD | Number of patients | Percentage or mean ± SD | |
| Sex | |||||
| Female | 479 | 88.38% | 288 | 87.80% | |
| Male | 63 | 11.62% | 40 | 12.20% | |
| Age, yr | 541 | 75.9 ± 8.0 | 328 | 75.6 ± 8.3 | |
| < 65 | 50 | 9.23% | 33 | 10.06% | |
| 65– < 75 | 172 | 31.73% | 113 | 34.45% | |
| ≥ 75 | 319 | 58.86% | 182 | 55.49% | |
| Unknowna | 1 | 0.18% | 0 | 0.00% | |
| Weight, kg | 348 | 50.37 ± 11.79 | 224 | 50.49 ± 11.30 | |
| Height, cm | 304 | 148.65 ± 7.88 | 203 | 149.05 ± 7.12 | |
| Body mass index, kg/m2 | 277 | 22.81 ± 4.81 | 184 | 22.80 ± 4.18 | |
| Diagnosis | |||||
| Primary | 481 | 88.75% | 295 | 89.94% | |
| Secondary | 28 | 5.17% | 17 | 5.18% | |
| Unknown | 33 | 6.09% | 16 | 4.88% | |
| Risk factors for fracture | |||||
| Parent fractured hip | 5 | 0.92% | 5 | 1.52% | |
| History of steroid use | 35 | 6.46% | 23 | 7.01% | |
| Drinking habit | 27 | 4.98% | 21 | 6.40% | |
| Current smoker | 22 | 4.06% | 13 | 3.96% | |
| Duration of disease, yr | 462 | 1.5 ± 2.8 | 274 | 1.5 ± 3.0 | |
| Complications | 453 | 83.58% | 273 | 83.23% | |
| Medical history | 111 | 20.48% | 76 | 23.17% | |
| Prior use of osteoporosis drugs | 201 | 37.08% | 128 | 39.02% | |
| Risedronate | 15 | 2.77% | 10 | 3.05% | |
| Bisphosphonate other than risedronate | 16 | 2.95% | 10 | 3.05% | |
| Calcium preparation | 16 | 2.95% | 12 | 3.66% | |
| Active vitamin D3 | 143 | 26.38% | 87 | 26.52% | |
| Parathyroid hormone | 2 | 0.37% | 1 | 0.30% | |
| Concomitant use of drugs | 452 | 83.39% | 277 | 84.45% | |
| Osteoporosis drugs | 250 | 46.13% | 160 | 48.78% | |
| Calcium preparation | 24 | 4.43% | 19 | 5.79% | |
| Active vitamin D3 | 213 | 39.30% | 131 | 39.94% | |
| Parathyroid hormone | 2 | 0.37% | 2 | 0.61% | |
| Anti-inflammatory analgesics | 233 | 42.99% | 134 | 40.85% | |
| Cardiovascular medicine | 154 | 28.41% | 111 | 33.84% | |
| Central nervous system medicine | 90 | 16.61% | 56 | 17.07% | |
| Antidiabetic drugs | 25 | 4.61% | 21 | 6.40% | |
| Digestive medicine | 204 | 37.64% | 131 | 39.94% | |
| Others | 203 | 37.45% | 127 | 38.72% | |
| Steroid | 33 | 6.09% | 25 | 7.62% | |
| Physiotherapy | 178 | 32.84% | 118 | 35.98% | |
Values are presented as mean ± standard deviation or number (%).
SD, standard deviation.
The first prescription date to define the age was missing.
3.2. Medication compliance
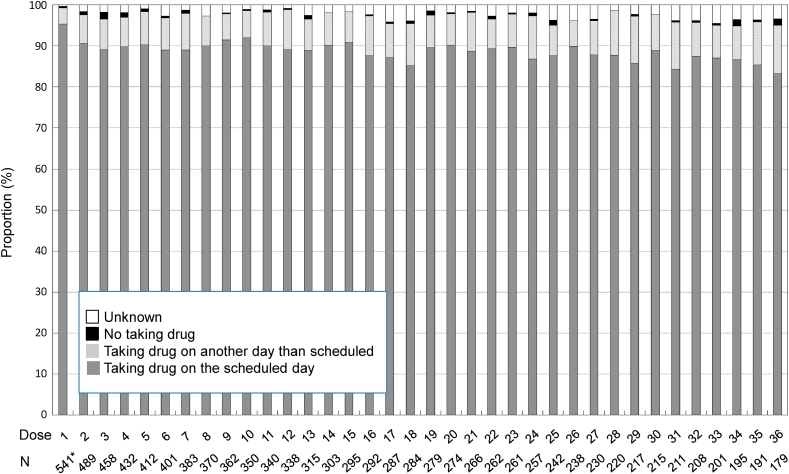
Considering that 1 patient of the safety analysis set had no record of the first administration date of the drug, the 541 remaining patients were analyzed. During the observation period, the proportion of patients who took risedronate on the scheduled day (compliance rate) was 83.24%–95.38% at each month (Fig. 1). The median of dosing interval was 30.0–31.0 days [quartile (Q) 1, 28.0–30.0 days and Q3, 31.0 days]. On the other hand, a very small proportion of patients who did not take risedronate was observed at each month.
Fig. 1.
Change in compliance status of patients to treatment with 75-mg once-monthly formulation of risedronate for 36 months. ∗One patient from the safety analysis set was excluded from analysis because of lack of the first administration date.
3.3. Safety
Among the 542 patients, 57 (10.52%) had 81 ADRs (Table 2). Common ADRs (≥ 3) were gastrointestinal disorders such as nausea, abdominal pain upper, diarrhea, abdominal discomfort, and dyspepsia; musculoskeletal and connective tissue disorders such as back pain and arthralgia; pyrexia; and spinal compression fracture. However, no thigh pain or osteonecrosis of the jaw was reported.
Table 2.
Adverse drug reactions.
| n | (%) | ||||
|---|---|---|---|---|---|
| Number of all the patients | 542 | ||||
| Number of patients with ADRs | 57 | (10.52) | |||
| Number of incidence of ADRs | 81 | ||||
| Number of patients with serious ADRs |
4 | (0.74) | |||
| Number of incidence of serious ADRs | 4 | ||||
| Common ADRs | Serious ADRs | ||||
| n | (%) | n | (%) | ||
| Gastrointestinal disorders | |||||
| Nausea | 7 | (1.29) | |||
| Abdominal pain upper | 5 | (0.92) | |||
| Diarrhea | 4 | (0.74) | |||
| Abdominal discomfort | 3 | (0.55) | |||
| Dyspepsia | 3 | (0.55) | |||
| Tooth ache | 1 | (0.18) | |||
| Musculoskeletal and connective tissue disorders | |||||
| Arthralgia | 3 | (0.55) | |||
| Back pain | 5 | (0.92) | |||
| Osteonecrosis | 1 | (0.18) | |||
| General disorders and administration site conditions | |||||
| Pyrexia | 3 | (0.55) | |||
| Injury, poisoning and procedural complications | |||||
| Spinal compression fracture | 4 | (0.74) | |||
| Femur fracture | 1 | (0.18) | |||
| Surgical and medical procedures | |||||
| Hospitalization | 1 | (0.18) | |||
Values are presented as number (%).
ADRs, adverse drug reactions.
Common ADRs (≥ 3) and serious ADRs are presented.
Four patients (0.74%) had serious ADRs, which included toothache, osteonecrosis, femur fracture, and hospitalization (one each). The toothache occurred in a 68-year-old woman approximately 11 months after the treatment with the drug (12 doses). A causal relationship between the event and the drug could not be assessed because she did not visit the hospital thereafter. The osteonecrosis occurred at the medial condyle of the left femur of a 73-year-old man administered with the drug for approximately 2 years and 3 months. He recovered after discontinuation of the drug. A femur fracture occurred at the neck of femur in an 83-year-old woman approximately 9 months after the treatment with the drug. However, factors other than the drug may also be related to the events because she had a proximal femur with a low mineral density prior to the start of treatment, suffered from comorbid diseases such as diabetes and osteoarthritis, and sustained a fall before the event. Furthermore, hospitalization was reported in an 83-year-old woman who was, however, admitted to another department with unknown reason.
3.4. Bone fracture
In the vertebral fracture analysis set of 328 patients, 196 (59.76%) had one basal fracture, 86 (26.22%) had two, 31 (9.45%) had three, and 15 (4.57%) had four. The mean (SD) number of vertebral fractures at the baseline was 1.6 (0.8).
Four patients in the vertebral fracture analysis set had no radiographic data at the same site at the baseline; consequently, the remaining patients (n = 324) were assessed (Fig. 2A). A total of 29 fractures were reported. The cumulative incidence of vertebral fractures was 7.35% (95% CI: 4.85–11.06) at 12 months, 8.82% (95% CI: 5.97–12.93) at 24 months, and 12.58% (95% CI: 8.61–18.18) at 36 months.
Fig. 2.
Cumulative incidence of vertebral (A), nonvertebral (B), and hip fractures (C).
+, censored; NAR, number at risk.
In total, 535 patients were assessed and 22 nonvertebral fractures (including 5 hip fractures) and 5 hip fractures were recorded within 36 months. The cumulative incidences of nonvertebral and hip fractures were 6.59% (95% CI: 4.31–10.01) and 1.58% (95% CI: 0.64–3.88), respectively (Fig. 2B and C).
3.5. BMD and bone turnover markers
At the baseline, the mean (SD) value of L2–4 BMD in the efficacy analysis set was 0.8149 (0.1668) g/cm2, which gradually and significantly increased at any evaluation timepoint (Fig. 3A). At the baseline, the mean (SD) values of TRACP-5b, P1NP, and u-NTX were 442.19 (180.25) mU/dL, 56.594 (20.611) μg/L, and 70.41 (83.44) nmol BCE/mmol Cr, respectively. The values of these markers subsequently decreased during the observation period (Fig. 3B, C, and D).
Fig. 3.
Time-course changes in L2–4 BMD (A), serum TRACP-5b (B), serum P1NP (C), and urinary NTX (D).
Mean ± SD. ∗P < 0.0001. L2–4 BMD, bone mineral density of the lumbar spine L2–4; LO, last observation; P1NP, type I procollagen-N-propeptide; TRACP-5b, tartrate-resistant acid phosphatase 5b; u-NTX, urinary type I collagen cross-linked N-telopeptide.
3.6. Low back pain
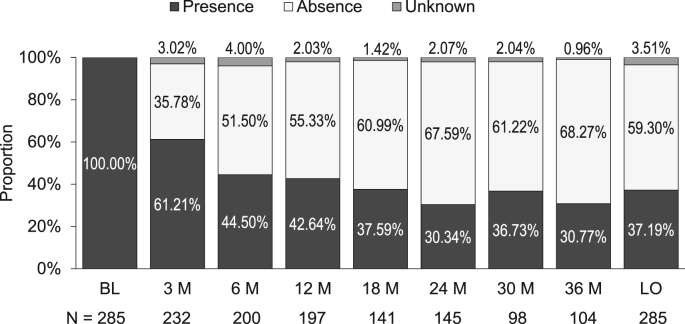
In the efficacy analysis set, 376 patients reported the presence or absence of low back pain at baseline. Among them, 285 complained of low back pain, 83 had no pain, and 8 were unclear. The proportion of patients with low back pain decreased gradually to 30.77% at 36 months (Fig. 4). In contrast, the proportion of patients without pain reached 51.50% at 6 months and increased to approximately 70% at 36 months.
Fig. 4.
Time course of the proportion of patients with low back pain.
BL, baseline; M, month; LO, last observation.
4. Discussion
In this 3-year postmarketing survey among the patients with osteoporosis with prevalent vertebral fractures, half of the patients were aged ≥ 75 years, and most of the patients had complications, indicating that 75-mg once-monthly formulation of risedronate was prescribed to older patients in actual practice. The current study comprised of 63 (11.62%) male patients. The number of male patients included in this study was higher than that included in a phase 3 study [9]. However, the number was still extremely small. Hence, an analysis based on sex could not be performed. The compliance rate for this 75-mg formulation (once-monthly) of risedronate was high in actual clinical practice. Its safety profile was similar to that of the formulation of risedronate used in a previous study [9]. The effectiveness on the bone was demonstrated with an increase in BMD and decrease in bone turnover markers. The incidence proportion of vertebral fractures was 12.58% at 36 months, and the proportion of patients with low back pain gradually decreased to the end of the study.
The monthly compliance rate for 3 years ranged from 83.24% to 95.38%. Approximately 10% of the patients took risedronate on another day than scheduled. The possible causes of this event were forgetting to take medicines on the scheduled days and altered body conditions associated with age. However, only a small proportion of the patients in this study had not taken the drug. An excellent compliance rate has also been observed in the post-marketing studies for the once-daily and once-weekly formulations of risedronate [6,18]. On the other hand, in studies that investigated the adherence and persistence, half of the patients on once-daily formulation of bisphosphonates dropped out [10]. It has been reported that the once-weekly formulation improves the persistence rate when compared with the once-daily formulation [19], and that the once-monthly formulation is even better [20,21]. The persistence rate of monthly bisphosphonate was reported to be approximately 70% in Japanese patients [21]. Similarly, 70% of the patients in the present study continued the treatment for 1 year. A study reported that the main reason for selecting the monthly formulation and not the weekly formulation is the ease of following a treatment regimen for a longer time [22], thus indicating patient preference [23]. This observation should be related to the high compliance rate and persistence rate of the monthly formulation of risedronate. Hence, the compliance rate was favorable and the drug-taking rate was high, indicating that the clinical safety and efficacy profile of risedronate in this study may be compared with those in other clinical studies performed on patients with high adherence to the therapy.
During a 3-year follow-up period, the incidence rate of ADRs associated with once-monthly risedronate was 10.52%, and it was lower than that of a 1-year phase 3 study of this formulation [24]. The types of ADRs in this study were similar to those of previous studies and surveys [9,18]. Four (0.74%) patients presented with serious ADRs, and none of the patients experienced osteonecrosis of the jaw. However, since long-term treatment with bisphosphonate preparations are commonly prescribed, attention must be paid to severe adverse events, including osteonecrosis of the jaw [[25], [26], [27], [28]].
In terms of efficacy, an increase in L2–4 BMD and decrease in bone metabolism markers were observed during the study period. These changes at 12 months, observed in this study, were comparable with those in a phase 3 study of this formulation [9] and a postmarketing study of once-weekly formulation for 3 years [18]. Therefore, the efficacy of 75-mg once-monthly formulation was confirmed in patients with osteoporosis who presented with prevalent vertebral fractures.
Prevention of bone fracture is the most important outcome of the treatment of osteoporosis. The cumulative incidence of new vertebral fractures or exacerbations of prevalent vertebral fractures was 8.82% and 12.58% at months 24 and 36, respectively. A Japanese randomized double-blind study conducted in a similar, but slightly younger population, that is, patients with osteoporosis with 1–4 vertebral fractures, using the 2.5-mg once-daily formulation of risedronate obtained a cumulative incidence of 12.3% at approximately 24 months (week 96) [5]. A postmarketing survey of the 17.5-mg once-weekly formulation of risedronate demonstrated that the cumulative incidences at weeks 96 and 156 were 18.25% and 24.92%, respectively [18]. The cumulative incidence of hip fractures at 36 months was 1.58%. A previous study investigated the incidence of recurrent hip fracture among elderly Japanese women. The results showed that the cumulative incidence of this type of fracture at 36 months was lower in women taking the 2.5-mg once-daily formulation of risedronate (4.3%) than in controls (13.1%) [6]. Thus, the values in this study were similar to or lower than those in the previous study. However, a simple comparison of the bone fracture incidence rate cannot be conducted due to differences in target population and assessment methods.
Chronic low back pain associated with vertebral fractures is a common and serious consequence of osteoporosis that limits body function and negatively affects one’s QOL [29]. In the present study, approximately 80% of the patients had low back pain at baseline among those who had records about pain. The proportion of patients with such pain decreased gradually over the observation period; approximately 70% of patients no longer had low back pain at 36 months. Similar results were reported by previous studies using other anti-osteoporotic drugs [[30], [31], [32]]. The 2.5-mg once-daily formulation [33] and 17.5-mg once-weekly formulation [34] of risedronate demonstrated improvement in QOL using EQ-5D [35] in patients with osteoporosis. In general, osteoporosis pain is directly caused by a spinal compression fracture. Ohtori et al. reported that bone resorption due to osteoporosis may cause low back pain in menopausal women with osteoporosis despite the absence of vertebral fractures, and that the pain was lowered after treatment with bisphosphonate (risedronate) with reducing NTX levels [36]. Based on the results, they proposed another mechanism for pain, which involved neuropeptides such as substance P produced through osteoclast-generated tumor necrosis factor-α. Risedronate would have reduced pain by suppressing osteoclast activation. The mitigation of low back pain by the administration of 75 mg of risedronate may contribute to the improvement of patients’ QOL.
However, this study has some limitations. This was an open-label surveillance with both investigators and the participants being aware of receiving risedronate, thereby possibly affecting the evaluation of safety and efficacy of the drugs being studied. This study is not a placebo-controlled randomized study; hence, the exact extent of risedronate contributing to the prevention of bone fractures in patients with osteoporosis remains unclear.
5. Conclusions
This postmarketing survey revealed that administering 75 mg of risedronate once in a month remained a favorable compliance rate and may be useful for the treatment of patients, even the elderly, with osteoporosis in daily practice.
CRediT author statement
Satoshi Soen: Supervision, Writing - original draft. Yuki Arai: Investigation, Data curation, Writing - original draft, Project administration. Saori Matsuda: Investigation, Data curation, Writing - original draft, Project administration. Kento Emori: Formal analysis, Writing - original draft. Toshimi Ikezaki: Investigation, Writing - original draft. Mitsuharu Osawa: Investigation, Writing - original draft.
Conflicts of interest
Satoshi Soen received a honorarium as a medical professional from EA Pharma Co., Ltd. for this study. Yuki Arai, Saori Matsuda, and Kento Emori are employees of EA Pharma Co., Ltd. Toshimi Ikezaki and Mitsuharu Osawa are employees of Eisai Co., Ltd.
Acknowledgments
This work was supported by EA Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, and Eisai Co., Ltd., which were involved in the study design, implementation, data collection, and data analysis.
The authors would like to express our deepest gratitude to the physicians who provided valuable data and for their cooperation in conducting this study. We also thank EPS Corporation (formerly AC Medical Inc.) for analyzing all data and WysiWyg Co., Ltd. for helping the manuscript preparation. ORCID Satoshi Soen: 0000-0002-0955-2747. Yuki Arai: 0000-0002-3574-7783. Saori Matsuda: 0000-0002-9804-7020. Kento Emori: 0000-0001-5877-7937. Toshimi Ikezaki: 0000-0002-2817-3347. Mitsuharu Osawa: 0000-0002-9572-2422.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.afos.2020.11.002.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Orimo H., Nakamura T., Hosoi T., Iki M., Uenishi K., Endo N. Japanese 2011 guidelines for prevention and treatment of osteoporosis-executive summary. Arch Osteoporos. 2012;7:3–20. doi: 10.1007/s11657-012-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimura N., Nakamura K. Epidemiology of locomotive organ disorders and symptoms: an estimation using the population-based cohorts in Japan. Clin Rev Bone Miner Metabol. 2016;14:68–73. doi: 10.1007/s12018-016-9211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ensrud K.E., Thompson D.E., Cauley J.A., Nevitt M.C., Kado D.M., Hochberg M.C. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 4.Dunn C.J., Goa K.L. Risedronate: a review of its pharmacological properties and clinical use in resorptive bone disease. Drugs. 2001;61:685–712. doi: 10.2165/00003495-200161050-00013. [DOI] [PubMed] [Google Scholar]
- 5.Kushida K., Fukunaga M., Kishimoto H., Shiraki M., Itabashi A., Inoue T. A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metabol. 2004;22:469–478. doi: 10.1007/s00774-004-0509-z. [DOI] [PubMed] [Google Scholar]
- 6.Osaki M., Tatsuki K., Hashikawa T., Norimatsu T., Chiba K., Motokawa S. Beneficial effect of risedronate for preventing recurrent hip fracture in the elderly Japanese women. Osteoporos Int. 2012;23:695–703. doi: 10.1007/s00198-011-1556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris S.T., Watts N.B., Genant H.K., McKeever C.D., Hangartner T., Keller M. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. J Am Med Assoc. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 8.Reginster J., Minne H.W., Sorensen O.H., Hooper M., Roux C., Brandi M.L. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 9.Hagino H., Kishimoto H., Ohishi H., Horii S., Nakamura T. Efficacy, tolerability and safety of once-monthly administration of 75mg risedronate in Japanese patients with involutional osteoporosis: a comparison with a 2.5mg once-daily dosage regimen. Bone. 2014;59:44–52. doi: 10.1016/j.bone.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Kamatari M., Koto S., Ozawa N., Urao C., Suzuki Y., Akasaka E. Factors affecting long-term compliance of osteoporotic patients with bisphosphonate treatment and QOL assessment in actual practice: alendronate and risedronate. J Bone Miner Metabol. 2007;25:302–309. doi: 10.1007/s00774-007-0768-6. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger M.P., Gallagher R., MacCosbe P.E. Medication persistence with weekly versus daily doses of orally administered bisphosphonates. Endocr Pract. 2006;12:522–528. doi: 10.4158/EP.12.5.522. [DOI] [PubMed] [Google Scholar]
- 12.Yood R.A., Emani S., Reed J.I., Lewis B.E., Charpentier M., Lydick E. Compliance with pharmacologic therapy for osteoporosis. Osteoporos Int. 2003;14:965–968. doi: 10.1007/s00198-003-1502-4. [DOI] [PubMed] [Google Scholar]
- 13.Blouin J., Dragomir A., Moride Y., Ste-Marie L.G., Fernandes J.C., Perreault S. Impact of noncompliance with alendronate and risedronate on the incidence of nonvertebral osteoporotic fractures in elderly women. Br J Clin Pharmacol. 2008;66:117–127. doi: 10.1111/j.1365-2125.2008.03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adachi J., Lynch N., Middelhoven H., Hunjan M., Cowell W. The association between compliance and persistence with bisphosphonate therapy and fracture risk: a review. BMC Muscoskel Disord. 2007;8:97. doi: 10.1186/1471-2474-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cramer J.A., Gold D.T., Silverman S.L., Lewiecki E.M. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007;18:1023–1031. doi: 10.1007/s00198-006-0322-8. [DOI] [PubMed] [Google Scholar]
- 16.Orimo H., Hayashi Y., Fukunaga M., Sone T., Fujiwara S., Shiraki M. Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metabol. 2001;19:331–337. doi: 10.1007/s007740170001. [DOI] [PubMed] [Google Scholar]
- 17.Mori S., Soen S., Hagino H., Nakano T., Ito M., Fujiwara S. Justification criteria for vertebral fractures: year 2012 revision. J Bone Miner Metabol. 2013;31:258–261. doi: 10.1007/s00774-013-0441-1. [DOI] [PubMed] [Google Scholar]
- 18.Soen S., Umemura T., Ando T., Kamisaki T., Nishikawa M., Muraoka R. Efficacy on the risk of vertebral fracture with administration of once-weekly 17.5 mg risedronate in Japanese patients of established osteoporosis with prevalent vertebral fractures: a 156-week longitudinal observational study in daily practice. J Bone Miner Metabol. 2017;35:419–427. doi: 10.1007/s00774-016-0771-x. [DOI] [PubMed] [Google Scholar]
- 19.Cramer J.A., Amonkar M.M., Hebborn A., Altman R. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005;21:1453–1460. doi: 10.1185/030079905X61875. [DOI] [PubMed] [Google Scholar]
- 20.Cotté F.E., Fardellone P., Mercier F., Gaudin A.F., Roux C. Adherence to monthly and weekly oral bisphosphonates in women with osteoporosis. Osteoporos Int. 2010;21:145–155. doi: 10.1007/s00198-009-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishimoto H., Maehara M. Compliance and persistence with daily, weekly, and monthly bisphosphonates for osteoporosis in Japan: analysis of data from the CISA. Arch Osteoporos. 2015;10:231. doi: 10.1007/s11657-015-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emkey R., Koltun W., Beusterien K., Seidman L., Kivitz A., Devas V. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO) Curr Med Res Opin. 2005;21:1895–1903. doi: 10.1185/030079905X74862. [DOI] [PubMed] [Google Scholar]
- 23.Silverman S.L., Schousboe J.T., Gold D.T. Oral bisphosphonate compliance and persistence: a matter of choice? Osteoporos Int. 2011;22:21–26. doi: 10.1007/s00198-010-1274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Review report: Actonel Tablets 75 mg/Benet Tablets 75 mg Japan: pharmaceuticals and medical devices agency. https://www.pmda.go.jp/drugs/2012/P201200168/11189000_22400AMX01500_A100_2.pdf [Japanese] [Internet] [cited 2020 12 October]. Available from:
- 25.Yoneda T., Hagino H., Sugimoto T., Ohta H., Takahashi S., Soen S. Bisphosphonate-related osteonecrosis of the jaw: position paper from the allied task force committee of Japanese society for bone and mineral Research, Japan osteoporosis society, Japanese society of periodontology, Japanese society for oral and maxillofacial radiology, and Japanese society of oral and maxillofacial surgeons. J Bone Miner Metabol. 2010;28:365–383. doi: 10.1007/s00774-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 26.Urade M., Tanaka N., Furusawa K., Shimada J., Shibata T., Kirita T. Nationwide survey for bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2011;69:e364–e371. doi: 10.1016/j.joms.2011.03.051. [DOI] [PubMed] [Google Scholar]
- 27.Shibahara T., Morikawa T., Yago K., Kishimoto H., Imai Y., Kurita K. National survey on bisphosphonate-related osteonecrosis of the jaws in Japan. J Oral Maxillofac Surg. 2018;76:2105–2112. doi: 10.1016/j.joms.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Iba K., Takada J., Sonoda T., Yamashita T. Effect of continuous long-term treatment for 10 years with bisphosphonate on Japanese osteoporosis patients. J Bone Miner Metabol. 2020;38:240–247. doi: 10.1007/s00774-019-01049-1. [DOI] [PubMed] [Google Scholar]
- 29.Silverman S.L., Piziak V.K., Chen P., Misurski D.A., Wagman R.B. Relationship of health related quality of life to prevalent and new or worsening back pain in postmenopausal women with osteoporosis. J Rheumatol. 2005;32:2405–2409. [PubMed] [Google Scholar]
- 30.Iwamoto J., Makita K., Sato Y., Takeda T., Matsumoto H. Alendronate is more effective than elcatonin in improving pain and quality of life in postmenopausal women with osteoporosis. Osteoporos Int. 2011;22:2735–2742. doi: 10.1007/s00198-010-1495-8. [DOI] [PubMed] [Google Scholar]
- 31.Hadji P., Zanchetta J.R., Russo L., Recknor C.P., Saag K.G., McKiernan F.E. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int. 2012;23:2141–2150. doi: 10.1007/s00198-011-1856-y. [DOI] [PubMed] [Google Scholar]
- 32.Hongo M., Miyakoshi N., Kasukawa Y., Ishikawa Y., Shimada Y. Additive effect of elcatonin to risedronate for chronic back pain and quality of life in postmenopausal women with osteoporosis: a randomized controlled trial. J Bone Miner Metabol. 2015;33:432–439. doi: 10.1007/s00774-014-0603-9. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T., Umemura T., Kamisaki T., Nishikawa M., Uchida S. QOL-change and examination of factors by treatment of risedronate 2.5mg for patients with osteoporosis [Japanese] Osteoporosis Jpn. 2012;20:551–563. [Google Scholar]
- 34.Nakamura T., Osawa M., Itoh M., Yamaguchi H., Iinuma N., Hayakawa Y. The effect of risedronate (17.5 mg/week) treatment on quality of life in Japanese women with osteoporosis: a prospective observational study. J Bone Miner Metabol. 2012;30:715–721. doi: 10.1007/s00774-012-0372-2. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchiya A., Ikeda S., Ikegami N., Nishimura S., Sakai I., Fukuda T. Estimating an EQ-5D population value set: the case of Japan. Health Econ. 2002;11:341–353. doi: 10.1002/hec.673. [DOI] [PubMed] [Google Scholar]
- 36.Ohtori S., Akazawa T., Murata Y., Kinoshita T., Yamashita M., Nakagawa K. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J Clin Neurosci. 2010;17:209–213. doi: 10.1016/j.jocn.2009.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.