Graphical abstract
Keywords: Withametelin, Withanolides, Anticancer, Analgesic, Anti-inflammatory, Antidepressant
Abstract
Withanolides are natural medicinal agents whose safety and therapeutic profiles make them valuable to mankind. Among multiple withanolides, withametelin is underexplored. The present study was aimed to create a general biological profile of isolated withametelin from Datura innoxia Mill. targeting different biological models. In-silico studies include drug-likeliness, pharmacokinetics, toxicity, molecular targets and cytotoxicity to cancer cell lines predictions. In silico directed preliminary in-vitro evaluation comprised of cancer/normal cell cytotoxicity, DPPH and protein kinase inhibition assays while in-vivo bioactivities include antiinflammatory, analgesic, antidepressant and anticoagulant assays. Pharmacological findings were strengthened by molecular docking studies to check interactions with various proteins and to propose the future path of studies. Results indicated compliance with Lipinski drug-likeliness rule (score −0.55). ADMET prediction showed strong plasma protein binding, GI absorption (Caco-2 cells permeability = 46.74 nm/s), blood brain barrier penetration (Cbrain/Cblood = 0.31), efflux by P-glycoprotein, metabolism by CYP1A2, CYP2C19 and CYP3A4, medium hERG inhibition and non-carcinogenicity in rodents. Predicted molecular targets included mainly receptors (glucocorticoid, kappa opioid, delta opioid, adrenergic and dopamine), oxidoreductase (arachidonate 5-lipoxygenase and cyclooxygenase-2), enzymes (HMG-CoA reductase) and kinase (NFκb). Withametelin was more cytotoxic to cancer cells (DU145 IC50 7.67 ± 0.54 µM) than normal lymphocytes (IC50 33.55 ± 1.31 µM). It also showed good antioxidant and protein kinase inhibition potentials. Furthermore, withametelin (20 mg/kg) significantly reduced inflammatory paw edema (68.94 ± 5.55%), heat-induced pain (78.94 ± 6.87%) and immobility time (50%) in animals. Molecular docking showed hydrogen bonding interactions (binding energies: −11.3 to −7.8 kcal/mol) with arachidonate 5 lipoxygenase, NFκb and glucocorticoid receptor. Withametelin has potential for advance investigations for its cytotoxic, anti-inflammatory, analgesic and antidepressant activities.
List of Abbreviations
- ADMET
Absorption Distribution Metabolism Excretion and Toxicity
- BBB
Blood brain barrier
- CYP
CytochromeP
- DMEM
Dulbecco Modified Eagle’s Medium
- DPPH
2-2 diphenyl 1 picryl hydrazyl
- DMSO
Dimethylsulfoxide
- HIA
Human Intestinal Absorption
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- NIH
National Institute of Health
- PK
Protein Kinase
1. Introduction
Investigating the extraordinary chemical diversity possessed by plants is still a mainstay in drug discovery process. High chemical diversity, biochemical specificity and other molecular properties of natural compounds offer added advantages over the pool of available synthetic and combinatorial compounds (Koehn and Carter, 2005). There has been a constant increase in the number of reported natural compounds from plants since 1940s and 1950s, from few to approximately more than 1500 chemical entities being discovered per year. Some approved therapeutic breakthroughs of plant origin in the last thirty years include artemisinin for malaria, colchicine for gout, cannabidol for chronic neuropathic pain and paclitaxel for cancer. Many compounds are in the last stages of clinical trials; for example, curcumin, resveratrol, quercetin and genistein for inflammation, diabetes, chronic obstructive pulmonary disease and Alzheimer’s disease, respectively (Atanasov et al., 2015). In this context, it can be inferred that the natural sources still has the potential to yield novel bioactive compounds. Discovering chemical compounds from natural sources sound scientifically interesting but unearthing their biological roles is the actual challenge (Pye et al., 2017).
Drug discovery from natural origin received a backlash due to “more investment-less outcome” paradigm (Sliwoski et al., 2014). In-silico directed real time screening has helped to resolve this issue. Literature shows that traditional high throughput screening and virtual high throughput screening provides almost an equal number of bioactive hits. It provides alternative to the time consuming and expensive in-vitro procedures and prevents waste of resources on compounds with negative predictions in in-silico analysis. This approach has been used on various drugs approved by US Food and Drug Administration (FDA). Drugs that owe their success mostly to computer-aided drug discovery include dorzolamide (carbonic anhydrase inhibitor), captopril (angiotensin-converting enzyme inhibitor), tirofiban (fibrinogen antagonist) and HIV protease inhibitors namely saquinavir, ritonavir and indinavir (Sliwoski et al., 2014).
Among multiple plant products, withanolides are under scrutiny for their therapeutic potentials in various disease models. Withanolides, mostly 1-oxosteroids, have steroidal nucleus in the side chain that provides opportunity to synthesize diverse derivatives of these compounds. These possess antitumor, antifeedant, antistress, cytotoxic, immunosuppressive, antimicrobial, and antiinflammatory activities (Chen et al., 2011). Withametelins is a sub class of withanolides possessing a C21–O–C24 ether bridge and a bicyclic lactone side chain with a six membered homocycle and an exocyclic double bond at C25–C27 (Oshima et al., 1987). These compounds including withametelin is mainly found in Datura genus (Chen et al., 2011). The current work is on isolation of withametelin from D. innoxia leaves. It is reported to possess antifungal (Singh et al., 2001) and cytotoxicity against various cancer cell lines (Rao et al., 2016). Here, we have reported the in-silico absorption, distribution, metabolism, elimination and toxicity (ADMET) profile, predicted molecular targets and cancer cell line cytotoxicity of withametelin. We then performed in-vitro cytotoxicity, antioxidant and protein kinase (PK) inhibition assays as well as in-vivo analgesic, antiinflammatory, antidepressant and anticoagulant activities of withametelin. It was followed by an attempt to determine protein ligand interactions via docking analysis to verify the findings of pharmacological investigations and propose the roadmap for future studies.
2. Material and methods
2.1. Chemicals and cell lines
Dimethylsulfoxide (DMSO), vincristine, cabazitaxel, ibuprofen, tramadol, carboxymethyl cellulose (CMC), fluoxetine and aspirin were obtained from Sigma Aldrich (Germany). Unless otherwise stated, all chemicals were obtained from Sigma Aldrich (USA). ISP4 medium was prepared in the laboratory.
For the purpose of this study, hepatic, prostate and breast cancer cell lines including HuH7.0 (CCL-185) and HuH7.5 (PTA-8561), DU145 (HTB-81) and PC3 (CRL-1435) and MCF-7 (HTB-22), respectively were used. Cell were grown in Dulbecco's Modified Eagle Medium (DMEM; 100 μg/mL streptomycin sulfate, 100 IU/mL penicillin G sodium, 0.25 μg/mL amphotericin B; MCF7) and RPMI-1640 (pH 7.4, 2.2 g/l NaHCO3) supplemented with 10% v/v heat inactivated fetal bovine serum in a humidified incubator (37 °C, 5% CO2).
2.2. Isolation of withametelin
D. innoxia leaves were collected in June 2018 from Muzaffargarh Pakistan. Credentials of the field gathered plant was authenticated as Datura innoxia Mill by Prof. Dr. Rizwana Aleem Qureshi, Department of Plant Sciences, Faculty of Biological Sciences, Quaid-i-Azam University Islamabad, Pakistan. Dried voucher specimen was submitted in the Herbarium of medicinal plants, Quaid-i-Azam University Islamabad under herbarium number PHM-525. The isolation and characterization is detailed in supplementary information (Supplementary Fig. 1a–d) (Jahromi et al., 1993, Sinha et al., 1989).
2.3. Animals
Mice (Balb/c) of mean weights (25–30 g) and age (7–8 weeks) of either sex were used in this study. Animals were housed in the Primate facility of Faculty of Biological Sciences, Quaid-i-Azam University Islamabad, Pakistan in compliance with the National Institute of Health, USA guidelines for the care and use of laboratory animals. They were provided with standard light/dark conditions and food and water ad libitum. Study was conducted after ethical approval from the Institutional Animal Ethics Committee (letter number # BEC-FBS-QAU2019-135) and adhered with strict cautions to diminish mice distress.
2.4. In-silico screening
2.4.1. Drug likeliness prediction
PreADMET (Lee et al., 2003), SwissADME (Daina et al., 2017) and Molsoft (Waseem et al., 2017) tools were utilized to determine the drug-likeliness of withametelin and reference drugs. Prediction was done following previously described method (Waseem et al., 2017).
2.4.2. ADMET profile prediction
ADMET profile was predicted using PreADMET (Lee et al., 2003) and SwissADME (Daina et al., 2017) tools. Molfiles or SMILES of withametelin were added into the database and searched for ADMET properties. Plasma protein binding (PPB), blood brain barrier (BBB) permeation (Ajay and Murcko, 1999), human intestinal absorption (HIA) (Yamashita et al., 2000) profiles were calculated. BOILED-Egg method is used to predict BBB permeation and HIA in SwissADME (Daina and Zoete, 2016). Target CYP enzymes and possibility of mutagenicity and carcinogenicity were also predicted as per criteria of the tools. Furthermore, GLORY web tool was used to CYP-mediated metabolites of withametelin based on the site, probabilities and frequency of metabolic reaction (de Bruyn Kops et al., 2019).
2.4.3. Molecular target prediction
Molecular targets were predicted by SwissTargetPrediction online database using PubChem (CID: 10873797) SMILES based on chemical (2D) and structural similarities (3D) with the bioactive molecules available in the database (Waseem et al., 2017, Gfeller et al., 2014).
2.4.4. Cancer cell line cytotoxicity prediction
Next, cytotoxicity to cancer cell lines was predicted using PASS CLC-Pred online tool. This creates structure–activity relationship model based on experimental data from ChEMBL (version 23) and known cytotoxicity of similar compounds against 278 tumor cell lines. Results are shown as probabilities of being active (Pa) and inactive (Pi) (Lagunin et al., 2018).
2.5. In-vitro studies
2.5.1. Cell cytotoxicity evaluation
In-vitro cytotoxicity of withametelin (20 µg/mL) against cancer cell lines (HuH7.0, HuH7.5, DU145, PC3 and MCF7; 72 h) and freshly isolated lymphocytes (24 h) cells was conducted by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay as described previously (Waseem et al., 2017, Ahmed et al., 2017). Lymphocytes were stimulated by phytohaemagglutinin (PHA) and maintained in RPMI-1640. Withametelin (0.25–20 µg/mL), vincristine (20 µg/mL), cabazitaxel (20 µg/mL) and 1% DMSO in PBS served as sample, positive and negative controls, respectively. Assay was performed in triplicate. IC50 values were calculated in micromolar (µM) concentrations using table curve 2D v5.01 software.
2.5.2. Antioxidant assay
Antioxidant activity of withametelin was estimated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay (Majid et al., 2019). Briefly, withametelin (50 µg/ml; DMSO, n = 3) or ascorbic acid (50–1.85 μg/mL; n = 3) was incubated with DPPH solution (9.2 mg/100 mL methanol) for 30 min at 37 °C in 96-well plate. It was followed by absorbance measurement at 517 nm. Percent free radical scavenging (FRS) and IC50 values were calculated in terms of micromolar (µM) concentrations.
2.5.3. Protein kinase inhibition assay
PK inhibition potential of withametelin was determined using disc diffusion method according to previously stated protocol (Zahra et al., 2017). Concisely, spores of refreshed Streptomyces 85E culture were swabbed on sterile ISP4 medium plates. Next, sterile 6 mm filter paper discs loaded with withametelin (20 μg; C20) or Surfactin (positive control; 20 μg) or DMSO (negative control) were placed on the seeded plates and incubated at 30 °C for 96 h. Results were noted as bald and clear zones of inhibition around samples and controls infused discs. The assay was run in triplicate and IC50 values were calculated in micromolar (µM) concentrations.
2.6. In-vivo studies
2.6.1. Treatment groups
Lymphocyte cytotoxicity data in our study showed comparatively safe nature of withametelin than vincristine. In acute toxicity studies, LD50 range of withametelin is >50 mg/kg (unpublished data). Withametelin 20, 10 and 5 mg/kg were used for subsequent in-vivo analysis. Animals (n = 5) were randomly divided into six groups. Groups-I to -III were administered normal saline (0.6 mL; negative control), 10% DMSO in CMC (0.6 mL; vehicle control) and standard drugs (10 mg/kg; positive control), respectively. Groups-IV to -VI were treated with withametelin 20 mg/kg (C20), 10 mg/kg (C10) and 5 mg/kg (C5), respectively.
2.6.2. Carrageenan-induced paw edema test
Antiinflammatory activity of withametelin was evaluated using standard carrageenan-induced paw edema test (Majid et al., 2015, Ismail and Mirza, 2015). Briefly, Balb/c mice were administered withametelin (C20, C10 or C5), ibuprofen (10 mg/kg) or negative/vehicle controls by oral gavage. After one hour, edema was induced by injecting carrageenan (50 μL; 1% saline) into the subplanter region of right hind paw. Paw thickness was measured using microgauge (Mitutoyo, Japan) before, immediately after injection and at regular intervals for 4 h and percentage inhibition of edema was calculated.
2.6.3. Hot-plate algesia test
Analgesia in mice was determined by induction of thermal pain using standard hot-plate method according to previously described protocol (Kayani et al., 2016). Mice were administered withametelin, tramadol (10 mg/kg) or controls. Initial (Ti) and after treatment (Tf) reaction times in terms of paw licking or jumping responses were recorded before, 30 min and 60 min after treatment by placing the mice on hot-plate (Barnstead Thermolyne; 55 ± 2 °C; 30 sec cut-off) and percentage analgesia was calculated.
2.6.4. Tail suspension-induced depression test
Tail suspension test is an effective model to determine the behavioural antidepressant effects of samples (Peng et al., 2007). Here, mice were administered withametelin, Fluoxetine (10 mg/kg) or controls as described above. After 1 h of treatment, mice were individually suspended 7.5 cm above the surface of a table that was 70 cm high. An adhesive tape was placed 1 cm away from tip of the tail. Immobility of animal was considered as the sign of depression and was recorded for 6 min. Mice were considered immobile only when they hung passively and were completely motionless. The reduction in immobility time (sec) reflected the antidepressant effect of the treatment.
2.6.5. Capillary tube coagulation test
Anticoagulant activity of withametelin was investigated by capillary tube method (Ismail and Mirza, 2015). Concisely, withametelin, Aspirin (10 mg/kg) or controls were administered to mice as given in Section 2.6.1. After 1 h, mice tails disinfected by methylated spirit were pricked and sufficient quantity of blood was immediately collected in capillary tubes by gently squeezing the tails of mice. Capillary tubes were sealed and immersed in water bath at 37 °C for 30 s. Afterwards; 4–5 mm portion of the capillary tube was sequentially broken until a fibrin thread was seen between the two broken ends. Time interval between the appearance of a drop of blood and thread formation was recorded as the coagulation time (sec).
2.7. Molecular docking studies
Molecular docking studies were performed to varify the pharmacological findings of in silico, in vitro and in vivo investigations and to also propose the future path of research. SMILES of withametelin were used to draw structure and energy was minimized using Chem 3D Pro and saved as mol2 file for final use. Protein data bank (PDB) database was used to obtain 3D structures of arachidonate 5-lipoxygenase, nuclear factor kappa beta subunit (NFκB), glucocorticoid receptor, apoptosis regulator Bcl-X, CDK8/cyclin C, dopamine D4, Kappa opiod receptor and prostaglandin E synthase with PDB IDs 3V99, 4G3E, 6EL7, 1YSI, 5CEI, 5WIU, 4DJH and 4YK5 respectively. Ligands and heteroatoms were removed and proteins were optimized and minimized using UCSF Chimera software to obtain structurally correct protein. Docking was performed using Pyrx-virtual screening tool, binding energies were saved as CSV file and Discovery studio was used to visualize the final protein–ligand interactions (Ibrahim et al., 2020).
2.8. Statistical analysis
Data was analyzed using One-way Analysis of Variance (ANOVA) followed by suitable post hoc tests. Results are represented as mean ± SD of respective parameters and p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Predicted drug-likeliness of withametelin
In-silico drug-likeliness properties aids in optimizing pharmaceutical and pharmacokinetic properties of new compounds (Vistoli et al., 2008). Withametelin has passed five out of nine rules of drug-likeliness (Table 1), which conforms to the available approved FDA drugs. As all drugs do not comply with all rules of drug-likeliness e.g. deflazacort, an antiinflammatory drug, violates lead-like and WDI-like rules but passes CMC-like and Lipinski rules. Likewise, MDDR-like rule predicted aspirin to be a mid-structure and it does not follow WDI-like rule. Cisplatin has failed all rules of drug-likeliness as assessed by PreADMET. Drug-likeliness score of withametelin computed via Molsoft tool is −0.55, which makes it a moderately drug-like candidate. Its score is either comparable to or better than some marketed drugs such as tetrahydrocannabinol (psychoactive; −0.01), cisplastin (anticancer; −1.12) and valproic acid (antiepileptic; −0.02). Assessment of drug-likeliness as a first step in drug discovery process helps to eliminate the non-drug-like compounds. This benefits the researchers save time and resources and focus on drug-like compounds only in research (Shen et al., 2012). Hence, withametelin being a drug-like compound based on its structural properties is suitable for further investigations into its biological profile.
Table 1.
Drug likeliness, ADMET and cancer cell line cytotoxicity prediction of withametelin.
| Drug likeliness Profile | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rules |
Scores |
|||||||||
| abLipinski | aCMC | aLead | aMDDR | aWDI | bGhose | bVeber | bEgan | bMuegge | bBioavailabilty | cDrug likeliness |
| Qual | Qual | Viol | Viol | Viol | Qual | Qual | Qual | Viol | 0.55 | −0.55 |
| ADME Profile | |||||||
| aPPB (%) |
abBBB permeability |
bLipophilicity consensus LogPo/w | bWater Sol | bGI Abs | aCaco-2 cells (nm/s) | bP-gp substrate | |
| Cbrain/Cblood | Pred | ||||||
| 100 | 0.31 | Moderate | 4.7 | Moderate | High | 46.74 | Yes |
| Toxicity Profile | |||||||
| aAmes Test | ahERG inhibition | aCarcinogenicity in mouse | aCarcinogenicity in rat | ||||
| Pred | TA100 | TA1535 | |||||
| +S9 | −S9 | +S9 | −S9 | ||||
| Mutagen | −ve | −ve | −ve | −ve | Medium risk | Non-carcinogenic | Non-carcinogenic |
| dCytotoxicity against cancer cell lines (Pa > Pi) | |||||
| Glioma | Breast adenocarcinoma | Lung carcinoma | Stomach adenocarcinoma | Hepatoblastoma | Melanoma |
| U-251 | MDA-MB-453 | A-549 | BGC-823 | HepG2 | SK-ML-28 |
| 8.99 > 0.00 | 0.42 > 0.05 | 0.66 > 0.02 | 0.57 > 0.00 | 0.21 > 0.09 | 0.38 > 0.03 |
Data were predicted from PreADMET (a), SwissADME (b) MOLSOFT (c) and PASS CLC-Pred (d) tools. Qual = qualified; Viol; violated; Sol = solubility; abs = absorption; -ve means no toxicity in Ames test; Pred = prediction.+S9 and –S9 means with or without metabolism of compound. Pa > Pi represents probability of compound to be active or inactive in respective model.
3.2. ADMET prediction of withametelin
Next, we estimated the ADMET properties of withametelin, since, pharmacokinetic parameters of a compound direct its availability to the site of action for the desired biological response. ADME analysis predicted withametelin with moderate BBB permeability and as substrate of P-glycoprotein, which is present on the epithelial cells of BBB and causes efflux of molecules from central nervous system thereby preventing CNS side effects (Ramakrishnan, 2003). High HIA and moderate Caco-2 cell permeability prediction presents it as a candidate for oral formulation (Table 1). Caco-2 cell model is a reliable model for the prediction of oral drug absorption where high penetrability across Caco-2 monolayer is anticipated to be absorbed well by the human intestinal mucosa (Yamashita et al., 2000). Withametelin showed moderate water solubility. Furthermore, withametelin can also be formulated as a topical agent or transdermal drug delivery system. Its LogKp skin permeation value is comparable to well absorbed topical antiinflammatory diclofenac (−5.21 cm/s vs −4.96 cm/s, respectively). Higher the LogKp value, higher the skin permeability (Potts and Guy, 1992). PreADMET reported inhibition of CYP2C9 and CYP3A4 by withametelin while SwissADME computed inhibition of the former only (Table 1). Inhibition of cytochrome P450 enzymes CYP2C9 and CYP3A4 results in increased bioavailability (Wang et al., 2015) of the tested specimen. This can be both beneficial as well as possibly induce the risk of toxicity of compounds. In-vivo toxicity studies are in process to determine the safety profile of withametelin and its interaction with CYP enzymes in biosystem. In present study, the compound showed bioactivity in in-vivo animal models indicating its access to the target sites of action. This means that High PPB prediction did not much affect the bioavailability of compound. Withametelin was predicted as a mutagen in Ames test by PreADMET. Database has anticipated mutagenicity both with and without metabolism by S9 liver homogenate. Likewise, many drugs including indomethacin, diphenylhydramine, valproic acid and cisplatin used for comparative analysis were also found mutagenic in Ames test results from the database. In-vivo genotoxicity testing is a prerequisite to prove this claim of PreADMET software However, it was predicted as non-carcinogenic in rat and mouse models when correlated with the available toxicity data of FDA and National Toxicology Program. There was also possible medium risk of hERG inhibition (Table 1). This gene codes for cardiac potassium channels and its inhibition causes an abnormality of cardiac muscle repolarization that is characterized by the prolongation of the QT interval in the electrocardiogram resulting in sudden cardiac arrhythmias and death. Safety concerns such as hERG inhibition or hepatotoxicity of drugs have only been identified in last stages of clinical trials or in post marketing surveillance. Reliable in-silico filters for these untoward effects would greatly improve drug candidate survival and benefit the ultimate goal of making drug therapy safer (Aronov, 2005).
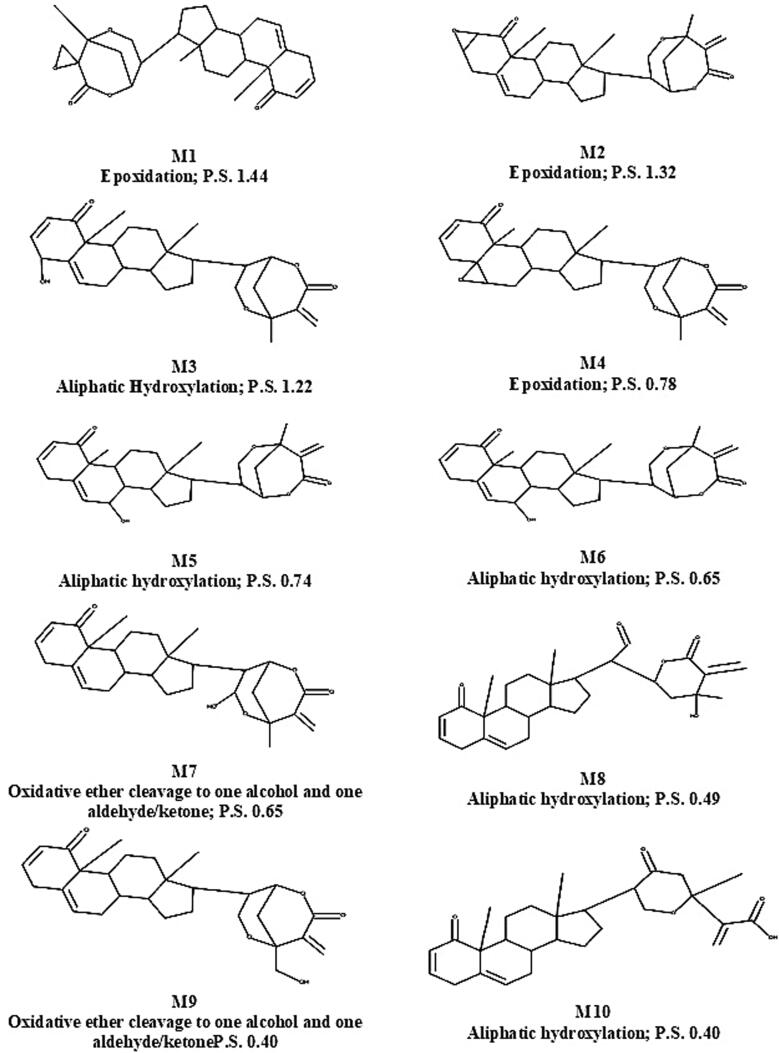
We then predicted the metabolites of withametelin by GLORY software (Fig. 1). GLORY reduces the number of putative false positive predictions while still keeping a high rate of recovery of reported metabolites. It can predict at least one known metabolite within the top three ranked positions for 76% of the molecules (de Bruyn Kops et al., 2019). Major predicted metabolites of withametelin were processed by epoxidation, aliphatic hydroxylation, oxidative ether cleavage to one alcohol/one aldehyde/ketone and alkyl dehydrogenation. These are dominantly Phase-I metabolism reactions that render withametelin more water soluble ensuring clearance from urine. Preclinical drug evaluations require estimation of drug metabolism to ensure active/inactive metabolites and drug transport into and out of target cells (T Issa et al., 2017). In-silico prediction is only based on chemical structures that can take place in any organ of the body dominantly liver. Hence, comprehensive assessment of metabolism of withametelin in in-vitro/in-vivo hepatic and extra-hepatic models is required in future studies.
Fig. 1.
Predicted metabolites of withametelin. CYP450 metabolites of withametelin were predicted by GLORY web predictor. Structures of top ten (M1 to M10) metabolites involved reactions to make respective metabolite as well as priority score (P.S.) are given in the figure. P.S. are ranked on the basis of predicted site of metabolism, probabilities of the atoms involved in the reaction and whether the reaction type is common or not.
3.3. Predicted molecular interactions
Molecular target prediction by SwissTargetPrediction tool provided information on the interactions of withametelin with various biomolecules (Table 2). It was predicted that withametelin can bind with certain oxidoreductase (20%), G-protein coupled receptor (26.7%), Toll-like receptors (20%), enzymes (13.3%), voltage-gated ion channels (6.7%), nuclear receptors (20%) and other cytosolic proteins (6.7%). It was predicted to bind with many inflammatory mediators like arachidonate 5-lipoxygenase, cycloxygenase-2 (COX-2), prostaglandin E synthase and NF-kappa B beta subunit (NFκB). Arachidonate 5-lipoxygenase/COX-2 inhibitors act by blocking the formation of both prostaglandins and leukotrienes. Such drugs have sparing side effects on the gastric linings dominantly due to the inhibition of arachidonate 5-lipoxygenase products. It can, therefore, be expected that dual blockers like withametelin can induce an enhanced antiinflammatory effect without damaging the gastrointestinal mucosa (Martel-Pelletier et al., 2003). Withametelin was predicted to interact with delta and kappa opioid receptors. Scientists are more interested in developing selective kappa and delta opioid receptor agonists in the management of pain with limited effect on Mu receptors. This curbs the possibility of abuse and opioid dependence (Vanderah, 2010). Likewise, overexpression of antiapoptotic Bcl-2 family proteins in various cancers is associated with tumor initiation, progression and resistance to therapy. Compounds, which reduce Bcl-2 protein expression can increase sensitivity to anticancer drugs and enhance in-vivo survival. Development of the inhibitors of antiapoptotic and inducers of apoptotic proteins can be potential anticancer therapies (Oltersdorf et al., 2005). Predicted interaction of withametelin with Bcl-X provides a possible avenue of molecular studies, since withametelin has demonstrated cytotoxicity against cancer cell lines in literature (Rao et al., 2016) and our subsequent in-vitro anticancer screening (3.5). Furthermore, major predicted targets of withametelin were family A G-protein coupled receptors (Table 2) that are associated with numerous physiological processes and pathological conditions especially related to psychiatric illnesses (Catapano and Manji, 2007). The α-2 adrenergic receptor agonists have been used for decades to treat common medical conditions such as hypertension, attention-deficit/hyperactivity disorder, panic disorders, cigarette craving and symptoms of opioid, benzodiazepine and alcohol withdrawal (Giovannitti Jr et al., 2015). Preclinical evidence suggests that targeting α-adrenergic receptors is beneficial for the treatment of both major depressive disorder and schizophrenia (Maletic et al., 2017). Additionally, homology based similarity of withametelin with more than 100 compounds in the SwissTargetPrediction database regarding interaction with HMG-CoA reductase enzyme, signifies the need for further investigation as an anticancer (Bjarnadottir et al., 2013), hypocholesterolemic (Nawrocki et al., 1995) and antiinflammatory (Jain and Ridker, 2005) agent. Likewise, glucocorticoid receptor is an important antiinflammatory and analgesic target via different mechanisms (Lim et al., 2005, Rhen and Cidlowski, 2005). Predicted interaction of withametelin with glucocorticoid receptors opens a research prospect in inflammatory models. In summary, in-silico analysis presents withametelin a possible medicinal agent that is proposed for detailed evaluation in in-vitro and in-vivo cancer, brain disorders, inflammation and pain models.
Table 2.
Predicted molecular targets of withametelin.
| Sr. No. | Target | Number of similar compounds |
Target Class | |
|---|---|---|---|---|
| 3D | 2D | |||
| 1 | Arachidonate 5-lipoxygenase | 100 | 7 | Oxidoreductase |
| 2 | Prostaglandin E synthase | 26 | 8 | Enzyme |
| 3 | Cyclooxygenase-2 | 126 | 14 | Oxidoreductase |
| 4 | Glucocorticoid receptor | 242 | 49 | Nuclear receptor |
| 5 | Delta opioid receptor | 56 | 1 | Family A G-protein coupled receptor |
| 6 | Kappa opioid receptor | 159 | 8 | Family A G-protein coupled receptor |
| 7 | HMG-CoA reductase (by homology) | 43 | 105 | Transporter |
| 8 | Toll-like receptor (TLR7/TLR9) | 9 | 2 | Toll-like and IL-1 receptors |
| 9 | CDK8/Cyclin C | 24 | 0 | Kinase |
| 10 | Dopamine D4 receptor | 55 | 1 | Family A G-protein coupled receptor |
| 11 | Nuclear factor kappa beta subunit (NFκB) | 13 | 1 | Kinase |
| 12 | Apoptosis regulator Bcl-X | 5 | 3 | Other ion channel |
| 13 | Thrombin | 28 | 9 | Protease |
| 14 | Alpha-2a adrenergic receptor | 11 | 0 | Family A G-protein coupled Receptor |
| 15 | Epoxide hydratase | 140 | 0 | Protease |
Results are expressed as interaction of withametelin with the molecular targets and number of structurally similar bioactive compounds in the database on the basis of 3D and 2D structures interacting with the targets.
3.4. Predicted cytotoxicity in cancer cell lines
Since, withametelin showed interaction with NFκB and Bcl-X; therefore, cytotoxicity in cancer cell lines was initially predicted in-silico. This was done as a pre-requisite for further in-vitro studies (3.5). Comparison to number of compounds in database on structural basis provided possible cancer models that can be the target of withametelin. Withametelin was predicted with high probability to be cytotoxic to cancer cell lines of glioma, breast adenocarcinoma, hepatoblastoma, stomach adenocarcinoma, lung carcinoma and melanoma models (Table 1). This is in line with preceding in-silico analysis that has depicted interaction with inflammatory, cell proliferation and apoptosis regulators (3.3). Literature has reported cytotoxicity of withanolides in lung (A549) and breast (MDA-MB-231) cancer cells (Rao et al., 2016). The complex and diverse morphologic, histologic and genetic features of tumors require the discovery and creation of new potent and safe drugs (Lagunin et al., 2018). Predicting cytotoxicity using in-silico tools helps in designing the subsequent in-vitro and/or in-vivo anticancer studies.
3.5. In-vitro cytotoxicity of withametelin in cancer cells
Considering the results of in-silico analysis, in-vitro cytotoxicity of withametelin was assessed in cancer cells and normal isolated lymphocytes. Results showed that withametelin induced maximum (p < 0.05) cytotoxicity in prostate cancer cells, i.e., DU145 and PC3 with IC50 values of 7.67 ± 0.54 and 7.85 ± 0.52 µM, respectively. It was comparable to cabazitaxel that demonstrated IC50 values of 5.68 ± 1.12 and 7.91 ± 2.11 µM, respectively (Table 3). Moreover, withametelin showed good activity against Huh7.0, Huh7.5 and MCF-7 cells with IC50 values of 19.53 ± 1.21, 15.5 ± 1.09 and 11.68 ± 1.37 µM, respectively as compared to vincristine. This also conforms to the in-silico cell line cytotoxicity analysis where hepatic and breast carcinoma models were predicted targets of withametelin (3.3). Our results are in corroboration with previous studies where withametelin was active against A549, MDA-MB-231 and HT-29 cell lines (Rao et al., 2016). We then evaluated the cytotoxicity in normal lymphocytes to assess selectivity of response in normal versus cancer cells. Withametelin was comparatively less cytotoxic to normal lymphocytes at the same concentration with IC50 value (33.55 ± 1.31 µM) that was 4.37–1.71-folds higher than the cancer cells. It was also higher than vincristine (8.31 ± 0.49 µg/mL) (Table 3). This lowers the concern of withametelin being toxic to normal cells of the body where hematological side effects are major concern of cancer chemotherapy (Calvo, 2019). Furthermore, molecular docking studies performed (3.11) also showed interaction with apoptosis and cell proliferation markers (NFkB and Bcl-X) which expands the spectrum of investigations as an anticancer. Literature showed that withametelin induced cell cycle arrest at G2/M phase via down regulation of cyclin B1, cdc2 and cdc25 expression and mitochondria-mediated apoptosis (Rao et al., 2016). Withaferin A (withanolide) caused cell cycle arrest in colon and breast carcinoma via NFkB inhibition. Several reports have also shown synergistic effect of withanolides with standard chemotherapy (Samadi, 2015). Thus, our in-vitro and in-silico analysis pave the path for in-vivo and molecular level anticancer studies.
Table 3.
In-vitro cytotoxicity assessment of withametelin in cancer and normal cells.
|
In-vitro cytotoxicity assessment in cancer and normal cells | |||||||
|---|---|---|---|---|---|---|---|
| Cell lines | C20 |
Vincristine |
Cabazitaxel |
1% DMSO |
|||
| % inhibition | IC50 (µM) | % inhibition | IC50 (µM) | % inhibition | IC50 (µM) | % inhibition | |
| Huh7.0 | 76.48 ± 1.81 | 19.53 ± 1.21b | 86.75 ± 0.56 | 8.18 ± 0.79a | – | ||
| Huh7.5 | 69.14 ± 1.27 | 15.5 ± 1.09b | 84.57 ± 0.49 | 6.8 ± 0.72a | – | ||
| DU145 | 78.70 ± 1.52 | 7.67 ± 0.54a | 94.75 ± 0.15 | 5.68 ± 1.12b | – | ||
| PC3 | 81.06 ± 0.42 | 7.85 ± 0.52a | 92.76 ± 1.76 | 7.91 ± 2.11b | – | ||
| MCF7 | 70.14 ± 0.99 | 11.68 ± 1.48a | 78.55 ± 2.36 | 5.24 ± 1.87a | – | ||
| Lymphocytes | 57.23 ± 1.22 | 33.55 ± 1.31c | 67.73 ± 1.70 | 8.31 ± 0.49a | – | ||
Sample (C20) and positive controls (vincristine and cabazitaxel) were tested at 20 µg/mL and IC50 results calculated in µM. Cytotoxicity was evaluated in cancer cells after 72 h and in normal isolated lymphocytes after 24 h. Values are presented as mean ± standard deviation (n = 3) of cytotoxicity. (–) means no activity. a–c means difference is highly significant, slightly significant, significant at p < 0.05. 1% DMSO did not show any cytotoxicity in cells.
3.6. Antioxidant and PK inhibition properties of withametelin
Free radicals produced endogenously or exogenously cause oxidative stress that is mediator of inflammation, brain damage and cancer. Scavenging free radicals can impede pathogenesis of inflammatory disorders and carcinogenesis (Samadi, 2015). Consequently, antioxidant potential of withametelin was determined by measuring it capacity to scavenge DPPH free radical. Withametelin showed moderate in-vitro antioxidant potential as compared to ascorbic acid with IC50 values of 85.0 ± 1.19 µM and 24.4 ± 1.02 µM, respectively (Table 4). Free radicals can initiate a vicious circle of inflammatory mediators like NFκB that can lead to the expression of other inflammatory cytokines, cell cycle regulatory molecules and neurotransmitter (Reuter et al., 2010). Thus, withametelin as an antioxidant agent has capacity to reduce free radical mediated damage to variety of biological systems. Predicted interaction with inflammatory mediators and in-vitro cytotoxicity can also be partially attributed to antioxidant potential of withametelin. Research has shown the effectiveness of withanolides in suppressing oxidative stress and inflammation in microglial cells (Sun et al., 2016).
Table 4.
Free radical scavenging and protein kinase inhibition by withametelin.
| Sr. No. | Sample | Free radical scavenging |
Protein kinase inhibition |
|||
|---|---|---|---|---|---|---|
| % inhibition | IC50 (µM) | Clear zone (mm) | Bald zone (mm) | MIC (µM/disc) | ||
| 1 | C50 | 62.15 ± 2.37 | 85.0 ± 1.19 | |||
| 2 | C20 | 11.19 ± 2.32 | 14.25 ± 2.11 | 45.8 | ||
| 3 | Ascorbic acid | 95.15 ± 2.46 | 24.4 ± 1.02 | |||
| 4 | Surfactin | 0 | 28.23 ± 2.63 | – | ||
| 5 | DMSO | – | – | – | – | – |
Values are presented as mean ± Standard deviation (n = 3). (--) No activity. Ascorbic acid (50 µg/ml) and Surfactin (20 µg/disc) are positive controls of DPPH scavenging and protein kinase inhibition assays, respectively. MIC is minimum inhibitory concentration. DMSO did not showed any results.
We have also evaluated the potential protein kinase (PK) inhibition activity of withametelin. PKs contribute to maintenance and development of inflammatory and neoplastic phenotypes. Mutations elevate kinase activity at serine/threonine residues, commonly found in human cancers, making those critical factors in carcinogenesis (Zahra et al., 2017). In present study, withametelin significantly (p < 0.05) inhibited hyphae formation of Streptomyces that requires PKs to originate and protrude from surface. The bald phenotype of 14.25 ± 2.11 mm around the withametelin discs depicts PK inhibition when as compared to control surfactin (28.23 ± 2.63 mm) (Table 4). PK inhibitory drugs are currently in phase-II/III of clinical trials that have so far shown good efficacy in cancer models (Fabbro et al., 2002). Herein, in-vitro cytotoxicity of withametelin in cancer cells can be associated with its PK inhibition property. The antioxidant and PK inhibition potentials have added benefit to the medicinal profile of withametelin rendering it a promising drug candidate.
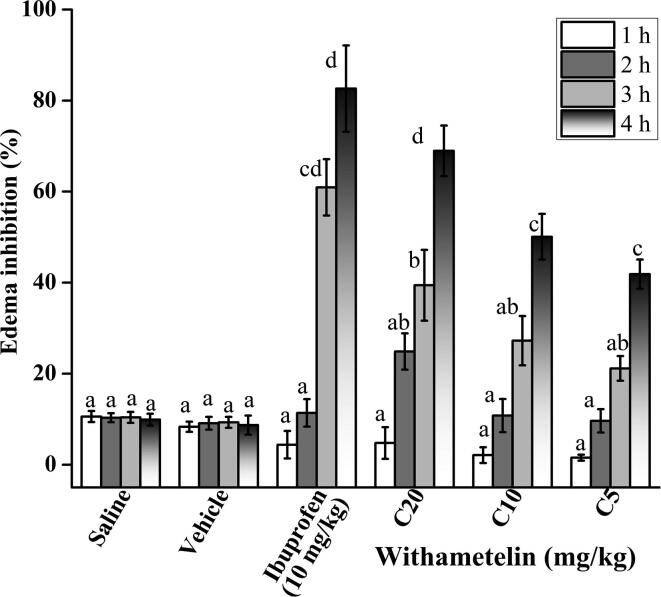
3.7. Withametelin reduced inflammation in mice
Subsequently, we assessed in-vivo antiinflammatory, analgesic, antidepressant and anticoagulation activities on the basis of results obtained from in-silico and in-vitro studies. Inflammation has long been a well-known symptom of many diseases. Molecular and epidemiological research suggests its linkage with broad range of non-infectious diseases, perhaps all of them (Hunter, 2012). Literature suggests that inflammatory responses have important role in pain, depression, cancer, blood coagulation and pathophysiology of brain and blood disorders (Vogelzangs et al., 2012, Witkowski et al., 2016). In the present study, carrageenan-induced inflammation protocol was used that is an acute, reproducible, thoroughly investigated and non-immune procedure. A decrease in paw thickness after sample administration points to the antiinflammatory response (Morris, 2003). In this context, maximum mouse paw edema inhibition was shown by C20 with 68.94 ± 5.55% (p < 0.05 compared to vehicle control) reduction in inflammation after 4 h of injection (Fig. 2). The antiinflammatory response was comparable to positive control, ibuprofen (10 mg/kg) with the edema inhibition of 82.64 ± 9.49%. This indicates that withametelin has aniinflammatory properties that make it valuable as an important remedy for inflammatory conditions. Inhibition of inflammation in carrageenan-induced paw edema model indicates activity of withametelin on second phase inflammatory mediators where prostaglandins and various cytokines such as IL-1β, IL-6, IL-10, and TNF-α are implicated (Posadas et al., 2004). Withanolides are acknowledged for antiinflammatory properties via selective inhibition of COX-2, prostaglandins, NFκB and nitric oxide production (Chen et al., 2011). Physalin type withanolides exert antiinflammatory response by triggering glucocorticoid receptors (Vieira et al., 2005). Withametelin was predicted in in-silico analysis to interact with arachidonate 5-lipoxygenase, COX-2, NFκB and glucocorticoid receptors. Moreover, it was found to inhibit free radicals in in-vitro analysis. This interaction with inflammatory and oxidative stress mediators in our study could be the mechanism of antiinflammatory response observed in our in-vivo experiment. Additionally, this also supports in-vitro cytotoxicity results where inhibition of cancer cell proliferation by withametelin was in part due to its potent antiinflammatory activity.
Fig. 2.
Antiinflammatory activity of withametelin. Antiinflammatory effect was assessed by carrageenan-induced method in terms of percentage reduction in paw edema of mice at different time intervals. Values are expressed as mean ± SD (n = 6) of % edema inhibition. C5, C10 and C20 represents 5, 10 and 20 mg/kg dose of withametelin. Ibuprofen was used as positive control. Means with different letters (a–d) are significantly (p < 0.05) different from one another.
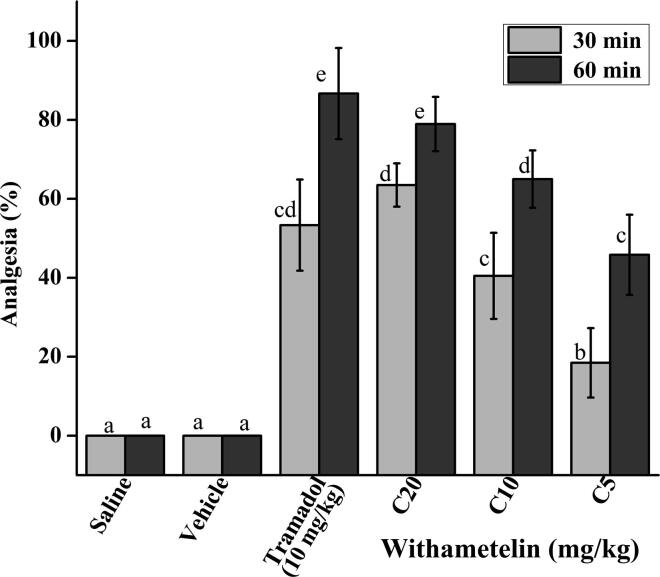
3.8. Withametelin diminished in-vivo thermal pain
Thermal pain is mediated from the periphery to central nervous system, which directs the withdrawal response in animals in terms of paw licking or withdrawal of hind paw from the thermal surface. An increase in the latency time to lick one of the hind paw is interpreted as an analgesic response (Bannon and Malmberg, 2007). It was observed that tramadol (10 mg/kg) showed significant reduction in pain with 86.6 ± 11.5% (p < 0.05) analgesia (Fig. 3). Withametelin exhibited dose dependent response where maximum activity was depicted by C20 with 63.4 ± 5.4% and 78.9 ± 6.8% (p < 0.05 compared to vehicle control) analgesia after 30 and 60 min, respectively. The C10 and C5 showed 65.0 ± 7.2% and 45.8 ± 10.1% analgesia, respectively after 60 min of treatment. Generation of free radicals and inflammatory mediators especially prostaglandins at the site of thermal exposure are the source of local nerve sensitivity and pain (Bannon and Malmberg, 2007). Withametelin being a good antioxidant and antiinflammaroty agent, as shown in our study, has capacity to mitigate localized pain and produce analgesia. Additionally, the central component of thermal pain could have been addressed by interaction with opioid receptors as predicted by our in-silico analysis. Likewise, α2 adrenoceptors are widely distributed in the peripheral and central nervous systems. Although, both α2A and α2C are expressed in the spinal cord; however, the α2A adrenoceptor agonists have been related more strongly to pain relief (Sudo et al., 2017). Since, withametelin reduced inflammation and showed predicted interaction with α2A receptors, these along with kappa opioid receptors (Millan, 1989) could be the mechanism for withametelin induced analgesia in in-vivo experiment. Thus, withametelin is a multipurpose compound that has capacity to inhibit thermal pain.
Fig. 3.
Analgesic activity of withametelin. Hot-plate method was used to measure the analgesic activity in mice. Data values represent mean ± SD of percent analgesia (n = 6). C5, C10 and C20 represents 5, 10 and 20 mg/kg dose of withametelin. Tramadol was used as positive control. Means with different letters (a–e) are significantly (p < 0.05) different from one another.
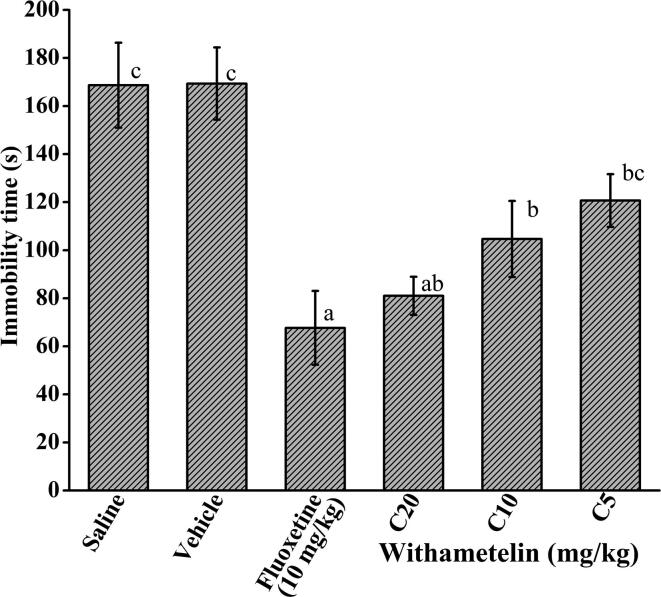
3.9. Withametelin can improve depression in in-vivo animal model
Animals models like tail suspension test, mimicking the depressive behavior, are helpful to evaluate the potential antidepressant drugs and to assess the determinants affecting symptoms of depression (Can et al., 2012). Withametelin (C20) reduced the immobility time of mice from 169.33 ± 15.04 s in vehicle control group to maximum of 81 ± 7.93 s (p < 0.05) (Fig. 4). This 50% reduction in immobility time indicates the reduction in mice depression after treatment with withametelin. These results were comparable with fluoxetine (10 mg/kg) where immobility time was reduced to 67.66 ± 15.37 s. The C10 and C5 doses of withametelin also showed significant results with 104.66 ± 15.82 and 120.66 ± 10.96 s immobility times, respectively. Determination of antidepressant activity unveils the importance of withametelin in depression. As dysfunction of monoaminergic neurotransmission is implicated in major depressive disorder; therefore, compounds regulating their role may be of clinical importance (Cottingham and Wang, 2012). Since, monoamine receptors are an important target of withametelin; these could be primarily responsible for its antidepressant response. Furthermore, high predicted BBB permeability in our study enabled withametelin to reach the target site in brain. Previous studies affirm curative potential of glycowithanolides in chronic stress-induced depression in in-vivo models (Bhattacharya et al., 2000, Ghosal et al., 1989). However, in-depth studies are required to justify these proposed mechanisms of depression.
Fig. 4.
Antidepressant activity of withametelin. Tail immersion method was conducted in mice and immobility time in seconds was measured after treatment with 5, 10 and 20 mg/kg doses (C5, C10, C20). Fluoxetine was used as positive control. Values are expressed in mean ± SD (n = 6). Means with different superscript (a–c) letters are significantly (p < 0.05) different from one another.
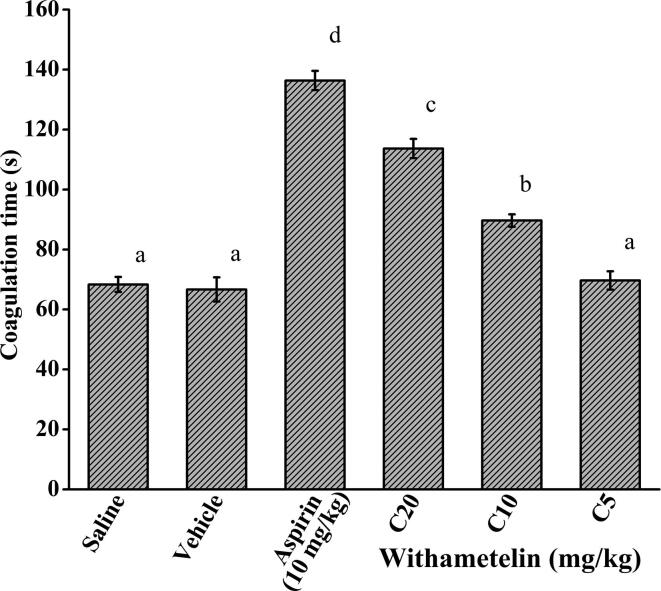
3.10. Withametelin is a moderate anticoagulant agent
One of the least explored areas on withanolides is estimation of their anticoagulant properties. In the present work, we determined the anticoagulation activity of withametelin using the basic and simple capillary tube assay. Results showed that the compound gradually increased coagulation time with increasing doses. Coagulation time of vehicle control group was 66.66 ± 4.04 s. It was increased to 113 ± 3.21 (p < 0.05) and 89 ± 2.08 s at C20 and C10 concentrations, respectively. However, the C5 dose did not demonstrate significant results (Fig. 5). Normal healing process prevents excessive blood loss following injury. Literature shows evidence of cross-talk between inflammation and coagulation, whereby pro-inflammatory cytokines and other mediators activate the coagulation cascade. Contrariwise, triggered coagulation proteases may modulate the inflammatory response (Levi and van der Poll, 2010). Over stimulation or suppression of any one mechanism may lead to hemorrhage or thrombosis (Haines and Bussey, 1995). The anticoagulant activity of withametelin in our study adds to the significance of withanolides in hemostasis. Our results are in accordance with previous report, where withaferin A (withanolide) showed anticoagulant properties by inhibiting fibrin polymerization and platelet aggregation, prolonging activated partial prothrombin time and thrombin time, and diminishing the production of thrombin and factor Xa (Ku and Bae, 2014). The antioxidant, anti-inflammatory profile of withametelin and predicted interaction with thrombin, arachidonate 5-lipoxygenase and COX-2 in our study could be the underlying mechanism of anticoagulant response.
Fig. 5.
Anticoagulant activity of withametelin. Coagulation time in seconds was calculated by capillary method. Aspirin was used as positive control. C5, C10 and C20 represents 5, 10 and 20 mg/kg dose of withametelin. Values are expressed in mean ± SD (n = 6) of coagulation time. Means with different letters (a–c) are significantly (p < 0.05) different from one another.
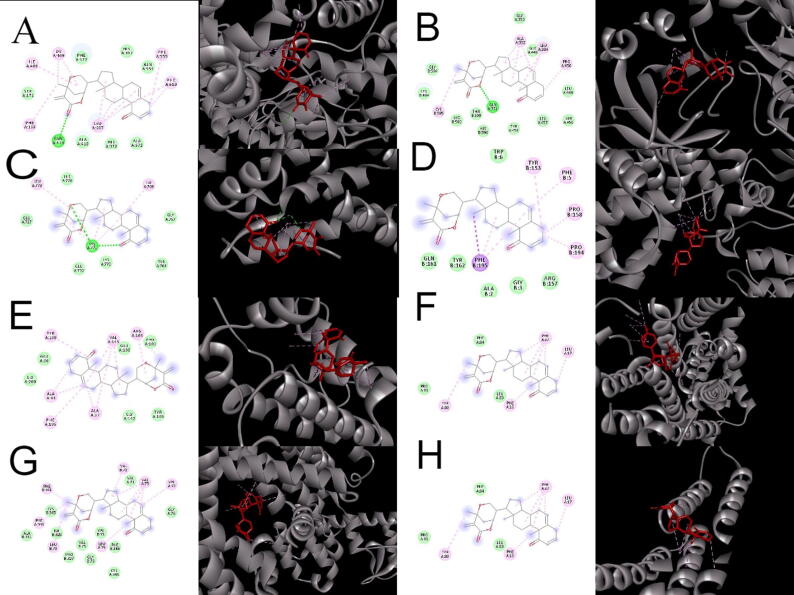
3.11. Molecular docking analysis
Compound behavior and interaction can be predicted at atomic level in the binding site of targeted protein via molecular docking approach. Anticancer, antiinflammatory, analgesic and antidepressant potential of withametelin was also strengthened by docking analysis (Table 5; Fig. 6). Withametelin interacted with arachidonate 5 lipoxygenase by forming hydrogen bond with GLN 413 and binding energy was estimated to be −11.3 kcal/mol. It also showed hydrogen bonding interaction with NFκB (GLN 351) with binding energy of −9.5 kcal/mol. Moreover, withametelin formed two hydrogen bonds with LYS 771 of glucocorticoid receptor and binding energy was calculated to be −7.8 kcal/mol. Withametelin exhibited hydrophobic and vander vaal interactions with CDK8/Cyclin C, Bcl-X, dopamine D4 receptor, K opiod receptor and prostaglandin E synthase. Proteins which did not show hydrogen bonding interaction with withametelin are recommended to be checked by other docking approaches. Ligands which exhibited H-bond interaction with withametelin have important implications in cancer, inflammation, hyperalgesia and depression.
Table 5.
Binding energies of withametelin interaction with proteins in kCal/mol using molecular docking analysis.
| Protein | Binding energy (kCal/mol) |
|---|---|
| Arachidonate 5 lipoxygenase | −11.3 |
| nfκb | −9.5 |
| Glucocorticoid receptor | −7.8 |
| CDK8/Cyclin C | −9.8 |
| Bcl-X | −10.7 |
| Dopamine D4 | −9.6 |
| K opiod receptor | −10.8 |
| Prostaglandin E synthase | −7.8 |
Fig. 6.
Graphical representation of withametelin binding modes in proteins. (A) Arachidonate 5 Lipoxygenase, (B) NFκB, (C) Glucocorticoid receptor, (D) CDK8/cyclin C, (E) BCL-X, (F) Dopamine D4, (G) Kappa opiod receptor and (H) Prostaglandin E synthase.
Depression and pain, the two most common and health relevant symptoms among cancer patients, are also linked to inflammation. People with cancer associated depression have elevated proinflammatory cytokines like IL6 which also disrupts neural encoding of pain stimulus resulting in greater pain severity (Hughes et al., 2014). Arachidonate 5 lipoxygenase inhibitors significantly reduce IL6 (Lin et al., 2014) thus targeting multiple associated symptoms at the same time. Similarly, NFκB is a target for cancer therapeutics and is also a pivotal link between cancer and inflammation. Its inhibition down regulates the expression of tumor promoting cytokines and survival genes (Karin, 2009). NFκB activation in CNS links to depression also, thus providing a possible avenue for antidepressant drugs (Caviedes et al., 2017). Moreover, glucocorticoid receptor abnormalities and hyperactivity of hypothalamus pituitary adrenal axis (HPA) contribute to pathophysiology of major depressive illness. Antidepressants restore glucocorticoid receptor function by regulating receptor expression and post translational modifications (Anacker et al., 2011). Withametelin might inhibit arachidonate 5 lipoxygenase, NFκB and restore glucocorticoid receptor function, which could be the mechanisms underlying pharmacological responses. However, in depth real time evaluation is required to justify with more certainty.
4. Conclusions
In the present study, withametelin was predicted as drug-like with good ADME properties and partial risk of toxicity. Multiple possible molecular targets were perceived that have important roles in inflammation, analgesia, brain disorders, cancer and hemostasis. Withametelin showed significant in-vitro cytotoxic, antioxidant and PK inhibition activities. Furthermore, potent to moderate in-vivo antiinflammatory, analgesic, antidepressant and anticoagulant activities were also observed. On the basis of related in-silico, in-vitro and in-vivo studies, we propose withametelin as a drug candidate for tested bioactivities and suggest detailed mechanistic, toxicity and clinical studies of this natural compound.
Contributions
IH conceptualized and designed the study, supervised execution of experiments, critically revised the manuscript and approved the final version of this manuscript. MWB performed experiments, analyzed and interpreted the data, wrote and revised the manuscript. BN contributed in compound isolation, in vitro and in vivo experiments, acquisition of the data and critical review of the manuscript. DW contributed in the conduction of in silico experiments, acquisition of the data and critical review of the manuscript. MM contributed in study design, experimentation and acquisition of the data. MZIK assisted in experiments, data analysis, interpretation and made critical revisions. All authors have read and approved the final manuscript.
Availability of data
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors except mentioned in acknowledgement section.
Ethics approval and consent to participate
Ethical approval for animal studies was obtained from the Institutional Animal Ethics Committee (letter number # BEC-FBS-QAU2019-135). Blood sampling from healthy volunteer was also approved by Institutional Review board of Quaid-i-Azam University (letter number # BEC-FBS-QAU2019-135). A written informed consent was obtained from the participant in this regard.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Professor Dr. Rizwana Aleem Qureshi is acknowledged for identification of plant sample. HEC Pakistan is acknowledged for the funding through Indigenous PhD fellowship program for Muhammad Waleed Baig to execute the study.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2020.09.021.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ahmed M., Fatima H., Qasim M., Gul B. Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Complement. Altern. Med. 2017;17(386) doi: 10.1186/s12906-017-1894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajay B.G.W., Murcko M.A. Designing libraries with CNS activity. J. Med. Chem. 1999;42:4942–4951. doi: 10.1021/jm990017w. [DOI] [PubMed] [Google Scholar]
- Anacker C., Zunszain P.A., Carvalho L.A., Pariante C.M. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. 10.1016%2Fj.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov A.M. Predictive in silico modeling for hERG channel blockers. Drug Discov. Today. 2005;10:149–155. doi: 10.1016/S1359-6446(04)03278-7. [DOI] [PubMed] [Google Scholar]
- Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.-M., Linder T., Wawrosch C., Uhrin P. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon A.W., Malmberg A.B. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr. Protoc. Neurosci. 2007;41 doi: 10.1002/0471142301.ns0809s41. 8.9.1–8.9.16. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Bhattacharya A., Sairam K., Ghosal S. Anxiolytic-antidepressant activity of Withania somnifera glycowithanolides: an experimental study. Phytomedicine. 2000;7:463–469. doi: 10.1016/S0944-7113(00)80030-6. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir O., Romero Q., Bendahl P.-O., Jirström K., Rydén L., Loman N. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res. Treat. 2013;138:499–508. doi: 10.1007/s10549-013-2473-6. [DOI] [PubMed] [Google Scholar]
- Calvo R. Hematological side effects of immune checkpoint inhibitors: the example of immune-related thrombocytopenia. Front. Pharmacol. 2019;10:454. doi: 10.3389/fphar.2019.00454. 10.3389%2Ffphar.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A., Dao D.T., Terrillion C.E., Piantadosi S.C., Bhat S., Gould T.D. The tail suspension test. J. Visual. Exp. 2012 doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catapano L.A., Manji H.K. G protein-coupled receptors in major psychiatric disorders. Biochimica et Biophysica Acta (BBA) – Biomembranes. 2007;1768:976–993. doi: 10.1016/j.bbamem.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviedes A., Lafourcade C., Soto C., Wyneken U. BDNF/NF-κB signaling in the neurobiology of depression. Curr. Pharm. Des. 2017;23:3154–3163. doi: 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- Chen L.-X., He H., Qiu F. Natural withanolides: an overview. Nat. Prod. Rep. 2011;28:705–740. doi: 10.1039/c0np00045k. [DOI] [PubMed] [Google Scholar]
- Cottingham C., Wang Q. α2 adrenergic receptor dysregulation in depressive disorders: Implications for the neurobiology of depression and antidepressant therapy. Neurosci. Biobehav. Rev. 2012;36:2214–2225. doi: 10.1039/c0np00045k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daina A., Zoete V. A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11:1117–1121. doi: 10.1002/cmdc.201600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn Kops C., Stork C., Šícho M., Kochev N., Svozil D., Jeliazkova N. GLORY: generator of the structures of likely cytochrome P450 metabolites based on predicted sites of metabolism. Front. Chem. 2019;7:402. doi: 10.3389/fchem.2019.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro D., Ruetz S., Buchdunger E., Cowan-Jacob S.W., Fendrich G., Liebetanz J. Protein kinases as targets for anticancer agents: from inhibitors to useful drugs. Pharmacol. Therap. 2002;93:79–98. doi: 10.1016/s0163-7258(02)00179-1. [DOI] [PubMed] [Google Scholar]
- Gfeller D., Grosdidier A., Wirth M., Daina A., Michielin O., Zoete V. SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucl. Acid Res. 2014;42:W32–W38. doi: 10.1093/nar/gku293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S., Lal J., Srivastava R., Bhattacharya S.K., Upadhyay S.N., Jaiswal A.K. Immunomodulatory and CNS effects of sitoindosides IX and X, two new glycowithanolides from Withania somnifera. Phytotherap. Res. 1989;3:201–206. doi: 10.1002/ptr.2650030510. [DOI] [Google Scholar]
- Giovannitti J.A., Jr, Thoms S.M., Crawford J.J. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth. Prog. 2015;62:31–38. doi: 10.2344/0003-3006-62.1.31. 10.2344%2F0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines S.T., Bussey H.I. Thrombosis and the pharmacology of antithrombotic agents. Annal. Pharmacother. 1995;29:892–905. doi: 10.1177/106002809502900912. 10.1177%2F106002809502900912. [DOI] [PubMed] [Google Scholar]
- Hughes S., Jaremka L.M., Alfano C.M., Glaser R., Povoski S.P., Lipari A.M. Social support predicts inflammation, pain, and depressive symptoms: longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology. 2014;42:38–44. doi: 10.1016/j.psyneuen.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P. The inflammation theory of disease: The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012;13:968–970. doi: 10.1038/embor.2012.142. 10.1038%2Fembor.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M.T., Uzairu A., Shallangwa G.A., Ibrahim A. In-silico studies of some oxadiazoles derivatives as anti-diabetic compounds. J. King. Saud. Univ.-Sci. 2020;32:423–432. doi: 10.1016/j.jksus.2018.06.006. [DOI] [Google Scholar]
- Ismail H., Mirza B. Evaluation of analgesic, anti-inflammatory, anti-depressant and anti-coagulant properties of Lactuca sativa (CV. Grand Rapids) plant tissues and cell suspension in rats. BMC Complement. Altern. Med. 2015;15(199) doi: 10.1186/s12906-015-0742-0. 10.1186%2Fs12906-015-0742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi F., Manickam M., Gupta M., Oshima Y., Hatakeyama S. Withametelins F and G, two new withanolides of Datura metel. J. Chem. Res. Synop. (Print) 1993:234–235. [Google Scholar]
- Jain M.K., Ridker P.M. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug. Discov. 2005;4:977. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayani W.K., Dilshad E., Ahmed T., Ismail H., Mirza B. Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities. BMC Complem. Altern Med. 2016;16:375. doi: 10.1186/s12906-016-1363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn F.E., Carter G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005;4:206. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- Ku S.-K., Bae J.-S. Antiplatelet, anticoagulant, and profibrinolytic activities of withaferin A. Vascul. Pharmacol. 2014;60:120–126. doi: 10.1016/j.vph.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Lagunin A.A., Dubovskaja V.I., Rudik A.V., Pogodin P.V., Druzhilovskiy D.S., Gloriozova T.A. CLC-Pred: a freely available web-service for in silico prediction of human cell line cytotoxicity for drug-like compounds. PloS One. 2018;13 doi: 10.1371/journal.pone.0191838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee I., Kim H., Chang G., Chung J., No K. EuroQSAR 2002 Designing Drugs and Crop Protectants: Processes, Problems and Solutions. 2003. The PreADME Approach: Web-based program for rapid prediction of physico-chemical, drug absorption and drug-like properties; pp. 418–420. [Google Scholar]
- Levi M., van der Poll T. Inflammation and coagulation. Crit. Care. Med. 2010;38:S26–S34. doi: 10.1097/ccm.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- Lim G., Wang S., Mao J. Central glucocorticoid receptors modulate the expression of spinal cannabinoid receptors induced by chronic morphine exposure. Brain Res. 2005;1059:20–27. doi: 10.1016/j.brainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Lin H.-C., Lin T.-H., Wu M.-Y., Chiu Y.-C., Tang C.-H., Hour M.-J. 5-Lipoxygenase inhibitors attenuate TNF-α-induced inflammation in human synovial fibroblasts. PLoS One. 2014;9:e107890. doi: 10.1371/journal.pone.0107890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid M., Ijaz F., Baig M.W., Nasir B., Khan M.R., Haq I.-U. Scientific validation of ethnomedicinal use of Ipomoea batatas L. Lam. as aphrodisiac and gonadoprotective agent against bisphenol A induced testicular toxicity in male Sprague Dawley rats. BioMed Res. Intern. 2019 doi: 10.1155/2019/8939854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid M., Khan M.R., Shah N.A., Haq I.U., Farooq M.A., Ullah S. Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides. BMC Complement. Altern. Med. 2015;15:349. doi: 10.1186/s12906-015-0868-0. 10.1186%2Fs12906-015-0868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletic V., Eramo A., Gwin K., Offord S.J., Duffy R.A. The role of norepinephrine and its α-adrenergic receptors in the pathophysiology and treatment of major depressive disorder and schizophrenia: a systematic review. Front. Psych. 2017;8:42. doi: 10.3389/fpsyt.2017.00042. 10.3389%2Ffpsyt.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J., Lajeunesse D., Reboul P., Pelletier J.-P. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Annal. Rheum. Dis. 2003;62:501–509. doi: 10.1136/ard.62.6.501. 10.1136%2Fard.62.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M. Kappa-opioid receptor-mediated antinociception in the rat. I. Comparative actions of mu-and kappa-opioids against noxious thermal, pressure and electrical stimuli. J. Pharmacol. Exp. Therap. 1989;251:334–341. http://jpet.aspetjournals.org/content/251/1/334 [PubMed] [Google Scholar]
- Morris C.J. Inflamm. Protoc. Springer; 2003. Carrageenan-induced paw edema in the rat and mouse. [DOI] [PubMed] [Google Scholar]
- Nawrocki J.W., Weiss S.R., Davidson M.H., Sprecher D.L., Schwartz S.L., Lupien P.-J. Reduction of LDL cholesterol by 25% to 60% in patients with primary hypercholesterolemia by atorvastatin, a new HMG-CoA reductase inhibitor. Arterioscler. Thromb. Vasc. Biol. 1995;15:678–682. doi: 10.1161/01.ATV.15.5.678. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Elmore S.W., Shoemaker A.R., Armstrong R.C., Augeri D.J., Belli B.A. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Oshima Y., Bagchi A., Hikino H., Sinha S.C., Sahai M., Ray A.B. Withametelin, a hexacyclic withanolide of Datura metel. Tetrahedron. Lett. 1987;28:2025–2027. doi: 10.1016/S0040-4039(00)96036-2. [DOI] [Google Scholar]
- Peng W.-H., Lo K.-L., Lee Y.-H., Hung T.-H., Lin Y.-C. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007;81:933–938. doi: 10.1016/j.lfs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Posadas I., Bucci M., Roviezzo F., Rossi A., Parente L., Sautebin L. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004;142:331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts R.O., Guy R.H. Predicting skin permeability. Pharmaceut. Res. 1992;9:663–669. doi: 10.1023/a:1015810312465. [DOI] [PubMed] [Google Scholar]
- Pye C.R., Bertin M.J., Lokey R.S., Gerwick W.H., Linington R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Nat. Acad. Sci. 2017;114:5601–5606. doi: 10.1073/pnas.1614680114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan P. The role of P-glycoprotein in the blood-brain barrier. Einstein. QJ. Biol. Med. 2003;19:160–165. http://www.einstein.yu.edu/uploadedFiles/EJBM/19Ramakrishnan160.pdf [Google Scholar]
- Rao P.C., Begum S., Jahromi M.A.F., Jahromi Z.H., Sriram S., Sahai M. Cytotoxicity of withasteroids: Withametelin induces cell cycle arrest at G2/M phase and mitochondria-mediated apoptosis in non-small cell lung cancer A549 cells. Tumor. Biol. 2016;37:12579–12587. doi: 10.1007/s13277-016-5128-5. [DOI] [PubMed] [Google Scholar]
- Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: how are they linked? Free Rad. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. 10.1016%2Fj.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhen T., Cidlowski J.A. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. New. Eng. J. Med. 2005;353:1711–1723. doi: 10.1056/nejmra050541. [DOI] [PubMed] [Google Scholar]
- Samadi A.K. Elsevier; 2015. Potential Anticancer Properties and Mechanisms of Action of Withanolides. The Enzymes. https://doi.org/10.1016/bs.enz.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Shen M., Tian S., Li Y., Li Q., Xu X., Wang J. Drug-likeness analysis of traditional Chinese medicines: 1. property distributions of drug-like compounds, non-drug-like compounds and natural compounds from traditional Chinese medicines. J. Cheminf. 2012;4(31) doi: 10.1186/1758-2946-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U., Prakash O., Ray A. Antifungal activity of withametelin, a withanolide isolated from Datura metel. Mycobiology. 2001;29:96–99. doi: 10.1080/12298093.2001.12015768. [DOI] [Google Scholar]
- Sinha S.C., Kundu S., Maurya R., Ray A.B., Oshima Y., Bagchi A. Structures of withametelin and isowithametelin, withanolides of Datura metel leaves. Tetrahedron. 1989;45:2165–2176. doi: 10.1016/S0040-4020(01)80076-4. [DOI] [Google Scholar]
- Sliwoski G., Kothiwale S., Meiler J., Lowe E.W. Computational methods in drug discovery. Pharmacol. Rev. 2014;66:334–395. doi: 10.1124/pr.112.007336. 10.1124%2Fpr.112.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo R.T., do Amaral R.V., da Silva Monteiro C.E., da Rocha Pitta I., do Carmo Lima M., Montes G.C. et al., 2017. Antinociception induced by a novel α2A adrenergic receptor agonist in rodents acute and chronic pain models. Eur. J. Pharmacol. 815, 210–218. 10.1016/j.ejphar.2017.09.018. [DOI] [PubMed]
- Sun G.Y., Li R., Cui J., Hannink M., Gu Z., Fritsche K.L. Withania somnifera and its withanolides attenuate oxidative and inflammatory responses and up-regulate antioxidant responses in BV-2 microglial cells. Neuromol. Med. 2016;18:241–252. doi: 10.1007/s12017-016-8411-0. [DOI] [PubMed] [Google Scholar]
- Issa N.T., Wathieu H., Ojo A., Byers S.W., Dakshanamurthy S. Drug metabolism in preclinical drug development: a survey of the discovery process, toxicology, and computational tools. Curr. Drug. Metab. 2017;18:556–565. doi: 10.2174/1389200218666170316093301. https://dx.doi.org/10.2174%2F1389200218666170316093301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah T.W. Delta and kappa opioid receptors as suitable drug targets for pain. Clin. J. Pain. 2010;26:S10–S15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- Vieira A.T., Pinho V., Lepsch L.B., Scavone C., Ribeiro I.M., Tomassini T. Mechanisms of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. British. J. Pharmacol. 2005;146:244–251. doi: 10.1038/sj.bjp.0706321. 10.1038%2Fsj.bjp.0706321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistoli G., Pedretti A., Testa B. Assessing drug-likeness–what are we missing? Drug. Discov. Today. 2008;13:285–294. doi: 10.1016/j.drudis.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N., Duivis H.E., Beekman A.T., Kluft C., Neuteboom J., Hoogendijk W. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl. Psych. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Sun W., Huang C.-K., Wang L., Ia M.-M., Cui X. Inhibitory effects of curcumin on activity of cytochrome P450 2C9 enzyme in human and 2C11 in rat liver microsomes. Drug. Dev. Indust. Pharm. 2015;41:613–616. doi: 10.3109/03639045.2014.886697. [DOI] [PubMed] [Google Scholar]
- Waseem D., Butt A.F., Haq I.-U., Bhatti M.H., Khan G.M. Carboxylate derivatives of tributyltin (IV) complexes as anticancer and antileishmanial agents. DARU. J. Pharm. Sci. 2017;25:8. doi: 10.1186/s40199-017-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski M., Landmesser U., Rauch U. Tissue factor as a link between inflammation and coagulation. Trend Cardiovasc. Med. 2016;26:297–303. doi: 10.1016/j.tcm.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Furubayashi T., Kataoka M., Sakane T., Sezaki H., Tokuda H. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Euro. J. Pharm. Sci. 2000;10:195–204. doi: 10.1016/s0928-0987(00)00076-2. [DOI] [PubMed] [Google Scholar]
- Zahra S.S., Ahmed M., Qasim M., Gul B., Zia M., Mirza B. Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complement. Altern Med. 2017;17(443) doi: 10.1186/s12906-017-1951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.