Abstract
Osteoarthritis (OA) is a disease characterized by degeneration of the joint complex due to cartilage destruction. Fraxetin, a widely used and studied coumarin compound extracted from a traditional Chinese herb (Qin Pi), has shown anti-inflammatory and antioxidant properties, but its effects on OA have not been studied. In the present study, western blotting, immunofluorescence, and terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) were used to evaluate the effects of fraxetin on IL-1β-induced apoptotic activity, inflammatory responses, and catabolism in rat chondrocytes. The results showed that fraxetin prevented IL-1β-induced apoptosis of chondrocytes and inhibited inflammatory mediator release by regulating the Toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MyD88)/nuclear factor (NF)-κB pathway in chondrocytes. Additionally, fraxetin suppressed the upregulation of matrix metalloproteinase 13 (MMP13) and degradation of collagen II in the extracellular matrix (ECM). Moreover, the effects of fraxetin in vivo were assessed in a monosodium iodoacetate (MIA)-induced rat model of OA using hematoxylin and eosin (H&E) and Safranin O-fast green staining and magnetic resonance imaging (MRI). The results showed that fraxetin protected the cartilage against destruction. In conclusion, fraxetin could be a potential therapeutic for OA.
Keywords: Fraxetin, Osteoarthritis, IL-1β, Inflammation, Catabolism, TLR4/MyD88/NF-κB signaling
Abbreviations: BSA, bovine albumin serum; CCK-8, cell counting kit-8; DAMP, damage-associated molecular pattern; DAPI, 4,6-diamidino-2-phenylindole; ECL, enhanced chemiluminescence; ECM, extracellular matrix; H&E, hematoxylin and eosin; HRP, horseradish peroxidase; IgG, immunoglobulin G; IL, interleukin; IκBα, inhibitor of NF-κB-α; MIA, monosodium iodoacetate; MMP, matrix metalloproteinase; MRI, magnetic resonance imaging; MyD88, myeloid differentiation primary response 88; NF, nuclear factor; OA, osteoarthritis; PBS, phosphate buffered saline, PMSF, phenylmethanesulphonyl fluoride; PRR, pattern recognition receptor; RIPA, radio-immunoprecipitation assay; SD, Sprague-Dawley; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SPF, specific pathogen free; TdT, terminal deoxynucleotidyl transferase; TLR, Toll-like receptor; TNF-α, tumour necrosis factor; TUNEL, TdT dUTP nick-end labeling
1. Introduction
Osteoarthritis (OA) is a complex, degenerative form of arthritis with a high incidence that causes pain, stiffness, and functional limitation. OA results in considerable medical costs and debilitating effects on patients, and the present lack of disease-modifying drugs needs to be addressed urgently (Callhoff et al., 2019). OA is characterized by the loss of articular cartilage with ongoing structural, functional, and metabolic changes (Kovács et al., 2019). There is abundant evidence that chondrocyte apoptosis, inflammation, and extracellular matrix (ECM) degradation lead to cartilage degeneration and histological manifestations.
Apoptosis, which is strongly correlated with OA, has been found in human OA chondrocytes, but not in normal chondrocytes (Musumeci et al., 2015b). Apoptosis causes matrix degradation and cartilage destruction (Musumeci et al., 2015a), which makes it an important target in the regulation of cartilage degeneration (Hwang and Kim, 2015). The caspase family of proteins has been identified as a core effector of apoptosis. Caspase-8 initiates extrinsic apoptosis by activating caspase-3 (Van Opdenbosch and Lamkanfi, 2019) and pro-inflammatory cytokines, such as interleukin (IL)-1β, activate caspase family members.
Inflammation occurs during the early process of OA and throughout its progression (Felson, 2006). Potent pro-inflammatory cytokines such as IL-1β, tumor necrosis factor (TNF)-α, and IL-6 are key cytokines that induce apoptosis and contribute to the destruction of cartilage (Liao et al., 2019, Woodell‐May and Sommerfeld, 2020Woodell‐May and Sommerfeld, 2020Woodell‐May and Sommerfeld, 2020). Nuclear factor (NF)-κB is a key transcription factor during this process, and p65/p50 is the most common of its five subunits. Intrinsic signals that are dependent on Toll-like receptor (TLR) activation have been found in OA cartilage lesions (Kim et al., 2018). TLRs, as a family of surface expression pattern recognition receptors (PRR), activate inflammatory cytokines, especially TLR2 and TLR4. In particular, TLR4 binds to its ligand myeloid differentiation primary response 88 (MyD88), and then activates NF-κB to upregulate IL-1β and TNF-α (Huang et al., 2019, Kim et al., 2018).
Increased catabolism in the articular cartilage ECM contributes to the occurrence and aggravation of OA (Rahmati et al., 2017). The ECM consists of gelatinous substances and collagen fibers including type II collagen, which is the key component and a biomarker of cartilage degradation. Matrix metalloproteinases (MMPs) such as MMP-1, MMP-9, and MMP-13 are key catabolic enzymes in collagen degradation and are secreted by chondrocytes. MMP-13 especially is a key regulator of cartilage destruction (Aspden and Saunders, 2019, Chen et al., 2019, Kovács et al., 2019, Liao et al., 2019).
Fraxetin (7,8-dihydroxy-6-methoxycoumarin), an active ingredient that is extracted from the herb Cortex Fraxini (also known as Qin Pi in Chinese), was previously reported to alleviate tissue inflammation and edema, and inhibit the production of lipoxygenase and cyclooxygenase enzymes (Fylaktakidou et al., 2004). These actions indicate its effects against inflammation and oxidation (Fylaktakidou et al., 2004). There is currently no report on the effects of fraxetin on OA. Therefore, in this study, we investigated this phenomenon in rat chondrocytes and cartilage using a monosodium iodoacetate (MIA)-induced rat model of OA for the first time, focusing on three aspects: 1) inflammatory response, 2) ECM degradation; and 3) chondrocyte apoptosis.
2. Materials and methods
2.1. Chemicals
Fraxetin (CAS No. 574–84-5; purity ≥ 98%; Fig. 1A) was obtained from Aladdin Biochemical Technology (Shanghai, China). Antibodies against TLR4, MyD88, NF-κB p65, inhibitor of NF-κB-α (IκBα), IL-6, TNF-α, caspase-3, caspase-8, and β-actin, as well as horseradish peroxidase (HRP) goat anti-rabbit and anti-mouse immunoglobulin G (IgG) were obtained from ABclonal (Wuhan, China). Antibodies against type II collagen and MMP-13 were from Abcam (Cambridge, UK). Other reagents were from Beyotime Biotechnology (Shanghai, China) unless otherwise specified. The material used for the immunopharmacological experiments were endotoxin-free, including fraxetin, all biologics, and synthetics.
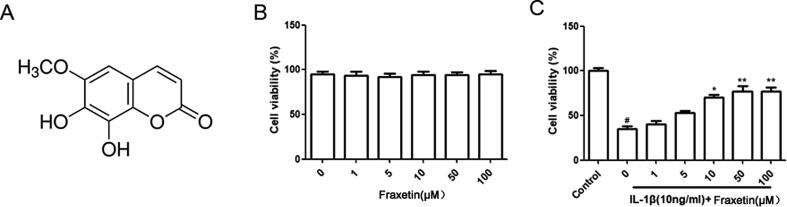
Fig. 1.
Effects of fraxetin on chondrocyte viability. (A) Chemical structure of fraxetin. (B) Chondrocytes were incubated with fraxetin alone for 24 h and analyzed using cell counting kit (CCK)-8 assay and no significant difference was found. (C) Chondrocytes were incubated with fraxetin and exposed to interleukin (IL)-1β for 24 h and analyzed using the CCK-8 assay. #P < 0.05 vs. Control group. *P < 0.05 and **P < 0.01 vs. IL-1β group.
2.2. MIA-induced OA rat model
Animal protocols used in this study were approved by the Laboratory Animal Ethical and Welfare Committee of Xin Hua Hospital, which is affiliated with Shanghai Jiao Tong University School of Medicine (Approval No. XHEC-F-2019-048). We used specific pathogen free (SPF), Sprague-Dawley (SD) rats (Shanghai Institute of Planned Parenthood Research [SIPPR]-BK Laboratory Animals Co., Ltd., Shanghai, China). Every effort was made to minimize the pain and suffering of the animals. Male SD rats (3-month-old, 180–220 g, n = 18, SPF) were purchased from SIPPR BK Laboratory Animal Co., Ltd. The rats were housed for 1 week under controlled conditions with temperature of 22 ± 2 °C, 70% humidity, and a 12 h dark/light cycle for environmental acclimation and provided standard laboratory chow and water. The rats were weighed and intraperitoneally injected with 3% sodium pentobarbital (0.1 mL/100 g), the right knee joints of those used to establish the model were disinfected with Iodophor, and then 50 μL of MIA (60 mg/mL, Aladdin Biochemical Technology Co. China) was injected into the joint space. The control unmodeled and MIA plus fraxetin-treated rats were similarly injected intra-articularly with 50 μL saline and fraxetin (5 mg·kg−1·day -1), whereas the other rats were intra-articularly injected with saline at an equal dose. Then, 4 weeks later, the rats were euthanized and the knee joints were collected as described below.
2.3. Chondrocyte isolation and culture
Cartilage was separated from 4-week-old SPF, SD rats (Shanghai Institute of Planned Parenthood Research [SIPPR]-BK Laboratory Animals Co., Ltd., Shanghai, China). Primary chondrocytes were isolated from twelve newborn SD rats that were sacrificed. To isolate the cartilage from the muscle and connective tissue, the articular cartilage around the knee joint was removed and dissected in aseptic conditions. The cartilage was cut into pieces and digested with 0.25% trypsin for 0.5 h, and then incubated overnight with 0.04% collagenase II at 37 °C. Chondrocytes were obtained after centrifuging (2000 rpm, 3 min). Next, the cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and penicillin–streptomycin (100 U/mL) at 37 °C. Only primary chondrocytes were used for the subsequent studies.
2.4. Cell viability
The cell counting kit-8 (CCK-8) assay was used to determine cell viability. Chondrocytes were plated into a 96-well plate (8 × 103 cells/well) and cultured for 24–48 h until 80% cell confluency was reached. Then, cells were treated with fraxetin (0, 1, 10, 50, and 100 μM) alone or incubated with IL-1β (10 ng/mL) for 24 h. After processing the treated cells with the kit reagents, the absorbance of the resultant solution was measured at a wavelength of 450 nm using a microplate reader (BioTek, Winooski, VT, USA).
2.5. Western blotting
Total proteins were extracted using radioimmunoprecipitation assay (RIPA) buffer containing phenylmethanesulfonyl fluoride (PMSF), separated by centrifugation, and the supernatant was aspirated. The protein content was measured using a bicinchoninic acid (BCA) assay kit. The total protein content (30 μg) of each sample was separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a PVDF membrane, which was then blocked using a fast-blocking solution. The membrane was then incubated with primary antibodies overnight at 4 °C, followed by the corresponding secondary antibody at 37 °C for 1 h. The immunoreactive bands were detected using enhanced chemiluminescence (ECL) and the blots were analyzed using the ChemiDoc MP imaging system (Bio-Rad, Santa Rosa, CA, USA).
2.6. Immunofluorescence staining
Chondrocytes were seeded in 96-well plates, incubated with IL-1β (10 ng/mL) in the presence or absence of fraxetin (50 μM), and fixed with 4% paraformaldehyde (PFA) for 0.5 h, followed by permeabilization with 0.2% Triton X-100 for 5 min. The cells were then incubated with 5% bovine serum albumin (BSA) for 0.5 h, followed by a primary antibody against type II collagen (1:1000) at 4 °C overnight. After that, they were washed by phosphate-buffered saline (PBS). This was followed by incubation with a secondary antibody at 37 °C in the dark for 1 h and then 4,6-diamidino-2-phenylindole (DAPI). The chondrocytes were observed using a fluorescence microscope (Olympus, Tokyo, Japan).
2.7. Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) staining
The apoptosis of chondrocytes in each group was measured using TUNEL staining. The chondrocytes were incubated with or without fraxetin (50 μM) in combination with IL-1β for 24 h. Next, the chondrocytes were fixed by 4% PFA in PBS for 30 min, incubated with hydrogen peroxide for 10 min, and then with PBS containing Triton X-100 for 2 min, followed by the TUNEL reaction system at 37 °C for 1 h in the dark. Finally, the chondrocytes were stained with DAPI for 5 min and an in situ cell death detection kit was used to detect apoptosis. Images were captured using a fluorescence microscope.
2.8. MRI scanning
MRI images were captured using 11.7 t Bruker Ultra High Field at the Zhangjiang International Brain Imaging Center, Fudan. The rat knee joints were imaged in the same position in sagittal slice orientation ensuring that the femoral and tibial articular cartilage were in direct contact in the central slice.
2.9. Histological staining
The degree of cartilage degeneration was evaluated using H&E and Safranin O-fast green staining. The rat knee joints were harvested, fixed, decalcified, embedded in paraffin, and cut into 5 μm continuous histological sections. Two serial sections from the middle were analyzed using H&E and Safranin O-fast green staining. The Osteoarthritis Research Society International (OARSI) and Mankin's scores were used to evaluate the degeneration of cartilage.
2.10. Statistical analysis
Data are presented as means ± standard error of the mean (SEM). A one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test was used to calculate the statistical differences. Statistical significance was accepted when P < 0.05.
3. Results
3.1. Effects of fraxetin on chondrocyte viability
The CCK-8 analysis was used to test the effect of fraxetin on chondrocyte viability and 0, 1, 10, 50, and 100 μM showed no obvious toxicity at 24 h (Fig. 1B). Moreover, fraxetin partially inhibited the IL-1β-induced chondrocyte apoptosis (Fig. 1C). As the response to IL-1β plateaus at concentrations above 50 μM, we used 10 μM and 50 μM fraxetin for subsequent experiments. Therefore, fraxetin may exhibit cytoprotective effects on chondrocytes stimulated by IL-1β.
3.2. Effects of fraxetin on chondrocyte apoptosis
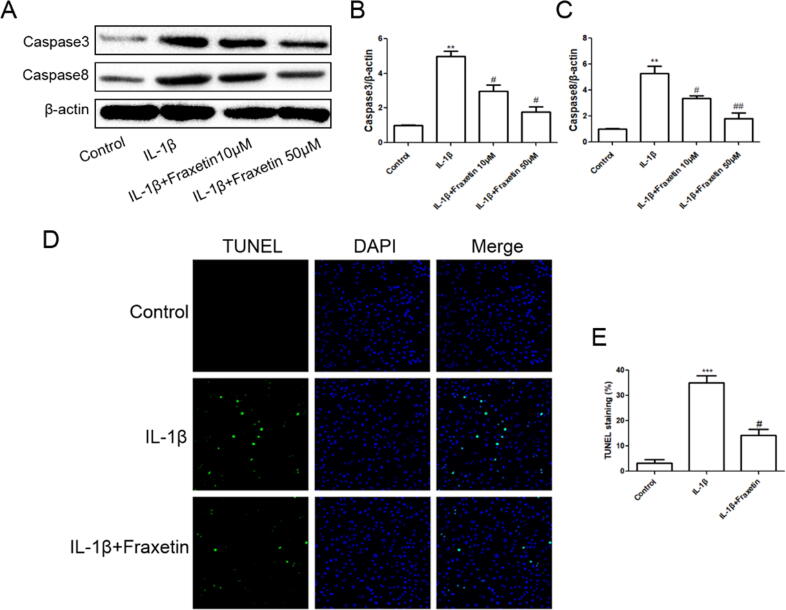
The effects of fraxetin on apoptosis of rat chondrocytes were evaluated. The TUNEL assay showed that fraxetin attenuated IL-1β-induced apoptotic activity (Fig. 2D and 2E) and suppressed the expression of caspase-3 and caspase-8 (Fig. 2A–C).
Fig. 2.
Effects of fraxetin on chondrocyte apoptosis. (A) Western blot analysis. (B, C) Quantification of caspase-3 and caspase-8 protein expression. (D) Terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL). (E) Quantification of detected apoptotic activity. ***P < 0.001 (vs. control group); #P < 0.05 and ##P < 0.01 vs. control and IL-1β groups, respectively, n = 3.
3.3. Anti-inflammatory action of fraxetin on chondrocytes
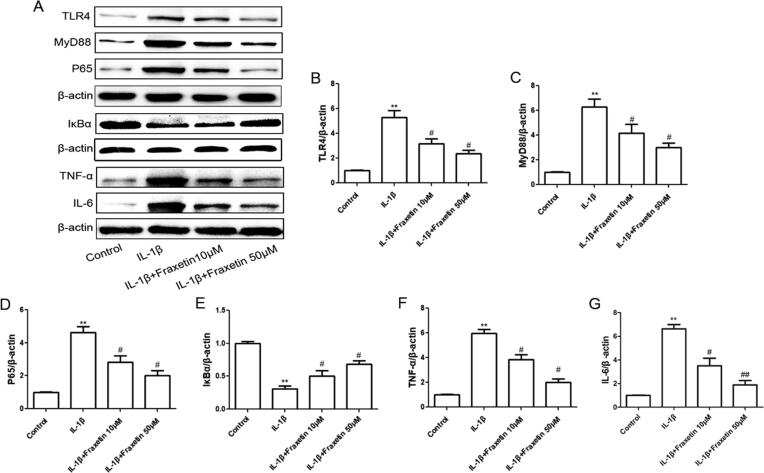
The effects of the different interventions on the expression of OA-relative inflammatory proteins and genes were detected using western blotting. The IL-1β-induced increase in protein expression levels of TLR4, MyD88, NF-κB p65, TNF-α, and IL-6 was decreased when treated with fraxetin. Moreover, the protein expression of IκBα was decreased by IL-1β and increased using fraxetin treatment (Fig. 3). These results indicate that fraxetin suppressed inflammatory activity via the TLR4/MyD88/NF-κB pathway.
Fig. 3.
Effects of fraxetin on inflammatory factor expression in chondrocytes. (A) Western blot analysis. (B–G) Quantification of Toll-like receptor 4 (TLR4), myeloid differentiation primary response 88 (MyD88), nuclear factor (NF)-κB p65, inhibitor of NF-κB-α (IκBα), tumor necrosis factor (TNF)-α, and IL-6 expression. **P < 0.01 vs. control group; #P < 0.05 and ##P < 0.01 vs. IL-1β group, n = 3.
3.4. Protective effect of fraxetin against ECM degradation in chondrocytes
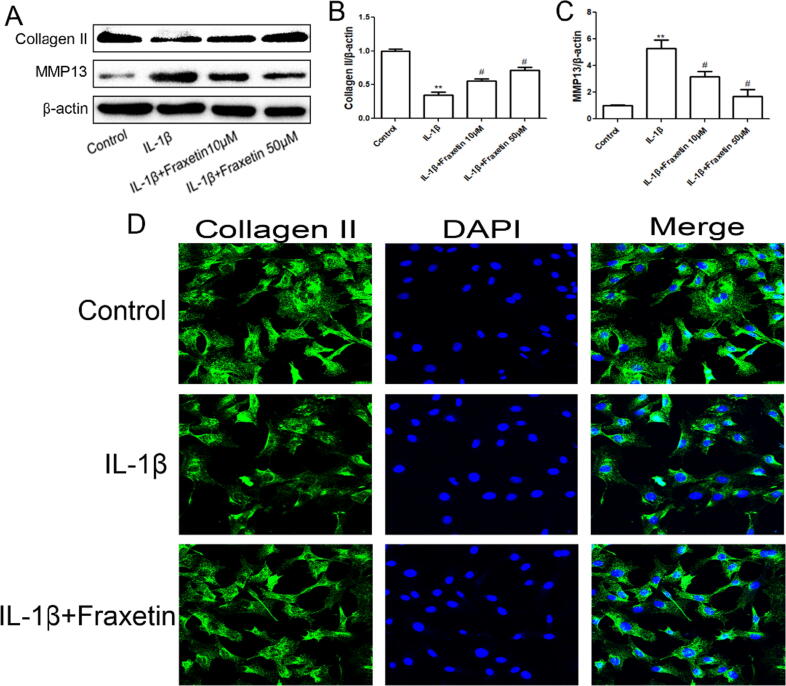
The western blot results showed that the IL-1β-induced increase in the degradation of type II collagen and secretion of MMP-13 was inhibited by fraxetin treatment (Fig. 4A–C). In addition, the immunofluorescence results revealed that the IL-1β-induced increase in type II collagen content was effectively reversed by fraxetin (Fig. 4D).
Fig. 4.
Protective effect of fraxetin against extracellular matrix (ECM) degradation in chondrocytes. (A) Western blot analysis. (B, C) Quantification of collagen II and matrix metalloproteinase (MMP)-13 expression levels. **P < 0.01 and #P < 0.05 vs. control and IL-1β groups, respectively, n = 3. (D) Immunofluorescence detection of collagen II.
3.5. Protective effect of fraxetin on cartilage in MIA-induced OA rats
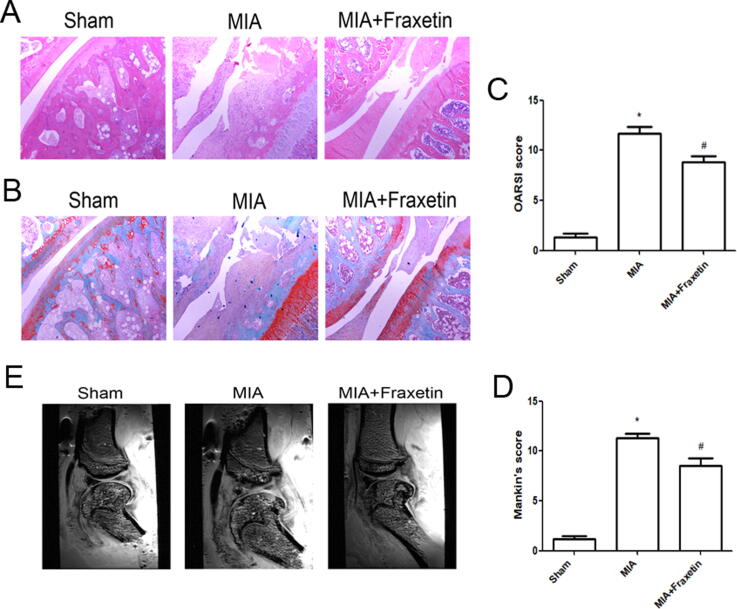
Both the Mankin’s and OARSI scores of MIA-induced OA rats were significantly higher than those of rats in the sham group, whereas fraxetin reduce the scores (Fig. 5C and 5D). Severe cartilage destruction was found in the MIA group, whereas fraxetin reduced this effect. The MRI showed considerable cartilage damage, erosions, defects, and focal thickening in the MIA group, whereas fraxetin treatment alleviated these OA symptoms (Fig. 5E). These results show that fraxetin attenuated cartilage degeneration in the rat OA model.
Fig. 5.
Effects of fraxetin on histomorphology of monosodium iodoacetate (MIA)-induced osteoarthritis (OA) rat model. (A) Hematoxylin and eosin (H&E). (B) Safranin O-fast staining of articular cartilage sections (40 × magnification). (C) Assessment of cartilage destruction using Osteoarthritis Research Society International (OARSI). (D) Mankin’s scores. **P < 0.01 and #P < 0.05 vs. sham and MIA groups, respectively, n = 6. (E) Magnetic resonance imaging (MRI) of rat knee joints of each group.
4. Discussion
OA has been shown to be a major contributor to global disability (Cross et al., 2014). The aging world population and increasing incidence of obesity has substantially increased the demand for OA-related medical services (Cross et al., 2014). Presently, more effective therapeutic drugs for OA are being explored. In the pathological process of OA, chondrocyte apoptosis, inflammation, and matrix catabolism play an important role and may create a vicious cycle of events.
Traditional medicinal plants are promising therapies for OA and many have been shown to be effective in vitro and in vivo; however, their mechanisms of action are often uncertain and there is a lack of human clinical evidence (Dragos et al., 2017). Fraxetin has shown antioxidant and anti-inflammatory properties in hepatic fibrosis (Chen et al., 2018, Wu et al., 2019), neuroblastoma cells (Molina-Jiménez et al., 2004, MOLINAJIMENEZ et al., 2005), intestinal inflammatory disease (Witaicenis et al., 2014), and atherosclerosis (Thuong et al., 2009). Fraxetin has also been reported to inhibit osteoclast differentiation and formation by suppressing p38 signaling in vitro and in vivo (Liao et al., 2018).
In our study, the effects of fraxetin on rat chondrocytes stimulated by IL-1β were studied for the first time, to the best of our knowledge. Furthermore, the potential underlying mechanisms were preliminarily explored and phenotypic validation was performed in rats.
Apoptosis of OA chondrocytes was detected, indicating its importance in the progression of OA (Musumeci et al., 2015). Fraxetin is not directly cytotoxic but it scavenges superoxide anion free radicals, which may have protective effects at leukocyte activation sites in the presence of inflammation (Paya et al., 1994). This notion is supported by our findings that chondrocyte viability did not decrease following incubation with fraxetin. Moreover, the cell viability was decreased significantly by stimulation with IL-1β, and this negative effect was inhibited by fraxetin. These results showed that fraxetin was non-toxic to chondrocytes and effectively inhibited IL-1β-induced apoptosis.
The TUNEL method was used to further explore the effect of fraxetin on chondrocyte apoptosis, and showed that fraxetin inhibited apoptosis induced by IL-1β. Caspases, the key apoptotic molecules, are activated in both the extrinsic and intrinsic pathways of apoptosis (Musumeci et al., 2015). Furthermore, caspase inhibitors are the most studied apoptotic inhibitors of OA (Hwang and Kim, 2015). Caspases possess different structures and functions, which determine whether they trigger inflammation or apoptosis. Caspase-3 and caspase-8 are apoptotic, but caspase-3 is the key effector caspase. Caspase-8, as an initiator caspase, activates caspase-3 to trigger apoptosis (Van Opdenbosch and Lamkanfi, 2019). Fraxetin has also been reported to inhibit caspase-8 and caspase-3 activation, which is mediated by Fas signaling in MG-63 cells (Kuo et al., 2006). In our study, fraxetin inhibited the expression of caspase-8 and caspase-3 induced by IL-1β, suggesting that fraxetin suppressed apoptosis, which was consistent with the TUNEL results.
Chronic inflammation is an inducer and aggravating factor of OA. Pro-inflammatory cytokines are key mediators in the pathological progression of OA. Common inflammatory cytokines such as TNF, IL-1 β, and IL-6 accelerate the pathological progress of OA (Liu-Bryan and Terkeltaub, 2015, Urban and Little, 2018). Additionally, TNF-α and IL-1β induce ECM denaturation, thereby making the ECM a major therapeutic target (Kapoor et al., 2011). In our study, the expression of TNF-α and IL-6 induced by IL-1β in chondrocytes was decreased by fraxetin, indicating that it regulated inflammation.
NF-κB is recognized as a key transcription factor that induces chondrocyte apoptosis, cartilage metabolism, and inflammation (Choi et al., 2019, Lepetsos et al., 2019). The inhibition of NF-κB has been demonstrated to reduce catabolic gene expression (Choi et al., 2018), whereas its knockdown attenuates early inflammation (Yan et al., 2016). Stimulation by pro-inflammatory cytokines such as TNF-α, IL-6, and ECM degradation products activates the NF-κB pathway in chondrocytes, leading to aggravation caused by inflammation and cartilage destruction (Kapoor et al., 2011, Rigoglou and Papavassiliou, 2013). This study showed that fraxetin reduced the nuclear translocation of NF-κB p65. IκBα is an important factor in the NF-κB pathway and degradation of IκB enables NF-κB protein to translocate to the nucleus (Li and Verma, 2002). The results of this study showed that fraxetin inhibited IL-1β-induced degradation of IκBα. Therefore, we speculated that the anti-inflammatory mechanism of fraxetin may involve the NF-κB pathway.
The TLR4 signaling pathway is involved in inflammation and catabolism of chondrocytes, initiating the destruction of articular cartilage (Lei et al., 2019). TLR4 is an effective target for blocking damage-associated molecular pattern (DAMP)-mediated OA lesions. TLR4 activates its ligand MyD88 via classical pathways, which further phosphorylate NF-κB, promoting innate immune responses (Gómez et al., 2015). This study showed that fraxetin decreased the expression of TLR4, MyD88, and NF-κB p65 in IL-1β-induced chondrocytes. Therefore, fraxetin may regulate inflammation in OA through the TLR4/MyD88/NF-κB pathway.
NF-κB enters the nucleus, where it is phosphorylated following stimulation; thus, NF-κB promotes the expression of MMPs and leads to cartilage matrix degradation (Zhang et al., 2019). MMPs and collagenase produced by IL-1β-stimulated chondrocytes are important proteolytic enzymes for ECM degradation. The expression of MMPs enhances the inflammatory and oxidative state. Collagen accounts for more than 50% of the dry weight of cartilage. Type II collagen, which is unique to cartilage, is the main macromolecule of the ECM, accounting for more than 90% of the total tissue collagen (Martel-Pelletier et al., 2008). In this study, fraxetin inhibited the degradation of collagen II and secretion of MMP-13, which indicates that fraxetin may protect the ECM against degradation.
In addition, these effects were further confirmed in vivo. MRI of OA joint samples can be used to clearly evaluate changes in biochemical components and tissues around the joint (Hayashi et al., 2018). The 11.7 T MR images of the rat knee joints showed the degree of cartilage destruction. The focal thickening in cartilage appeared to be edema, which may have been caused by inflammation and matrix degradation products. The progress of cartilage erosions, defects, and destruction in rats was alleviated by fraxetin treatment, indicating that fraxetin slowed the progress of cartilage destruction in OA rats. The degree of subchondral bone injury was evaluated using OARSI and Mankin’s scores and the values of the MIA plus fraxetin group were lower than those of the MIA group alone. Therefore, fraxetin showed protective effects against cartilage destruction in vivo.Conclusion
Collectively, our findings demonstrate that fraxetin suppressed IL-1β-induced inflammation via the TLR4/MyD88/NF-κB pathway in rat chondrocytes. Moreover, fraxetin inhibited apoptosis and matrix degradation in chondrocytes. The in vivo experiments confirmed that fraxetin delayed the destruction of cartilage in MIA-induced OA rats. Finally, to the best of our knowledge, this study is the first to reveal the effects of fraxetin on OA and OA chondrocytes isolated from a (MIA)-induced rat model and its possible mechanism of action, thereby providing preliminary data for a potential new drug candidate for OA. However, while the mechanism of its effects on OA was preliminarily explored in our study, a more in-depth, effective mechanism needs to be explored to better understand its pharmacological effects to truly benefit mankind.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
MRI Fudan University, China support was provided by the Zhangjiang International Brain Imaging Center, Fudan. The authors thank Ye Wang for providing care to the rats.
Funding
The study was supported by the Shanghai Municipal Science and Technology Commission, China fund (Nos. 15401970500 and 17401931400).
Footnotes
Peer review under responsibility of King Saud University.
References
- Aspden, R.M., Saunders, F.R., 2019. Osteoarthritis as an organ disease: from the cradle to the grave. Eur. Cell Mater. 37, 74–87. https://doi.org/10.22203/eCM.v037a06. [DOI] [PubMed]
- Callhoff J., Albrecht K., Redekere I., Lange T., Goronzy J., Günther K.P., Zink A., Schmitt J., Saam J., Postier A. Disease burden of persons with osteoarthritis: results of a cross-sectional survey linked to claims data. Arthritis Care Res (Hoboken) 2019 doi: 10.1002/acr.24058. [DOI] [PubMed] [Google Scholar]
- Chen X., Ying X., Sun W., Zhu H., Jiang X., Chen B. The therapeutic effect of fraxetin on ethanol-induced hepatic fibrosis by enhancing ethanol metabolism, inhibiting oxidative stress and modulating inflammatory mediators in rats. Int. Immunopharmacol. 2018;56:98–104. doi: 10.1016/j.intimp.2018.01.027. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang C., Wang X., Huo S. Juglanin inhibits IL-1beta-induced inflammation in human chondrocytes. Artif. Cells Nanomed. Biotechnol. 2019;47:3614–3620. doi: 10.1080/21691401.2019.1657877. [DOI] [PubMed] [Google Scholar]
- Choi M.C., Jo J., Park J., Kang H.K., Park Y. NF-kappaB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8 doi: 10.3390/cells8070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.C., MaruYama T., Chun C.H., Park Y. Alleviation of murine osteoarthritis by cartilage-specific deletion of ikappabzeta. Arthritis Rheumatol. 2018;70:1440–1449. doi: 10.1002/art.40514. [DOI] [PubMed] [Google Scholar]
- Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., Bridgett L., Williams S., Guillemin F., Hill C.L., Laslett L.L., Jones G., Cicuttini F., Osborne R., Vos T., Buchbinder R., Woolf A., March L. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014;73(7):1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- Dragos D., Gilca M., Gaman L., Vlad A., Iosif L., Stoian I., Lupescu O. Phytomedicine in joint disorders. Nutrients. 2017;9(1):70. doi: 10.3390/nu9010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felson D.T. Osteoarthritis of the Knee. N. Engl. J. Med. 2006;354(8):841–848. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- Fylaktakidou K.C., Hadjipavlou-Litina D.J., Litinas K.E., Nicolaides D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/ antioxidant activities. Curr. Pharm. Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- Gómez R., Villalvilla A., Largo R., Gualillo O., Herrero-Beaumont G. TLR4 signalling in osteoarthritis—finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2015;11(3):159–170. doi: 10.1038/nrrheum.2014.209. [DOI] [PubMed] [Google Scholar]
- Hayashi D., Roemer F.W., Guermazi A. Recent advances in research imaging of osteoarthritis with focus on MRI, ultrasound and hybrid imaging. Clin. Exp. Rheumatol. 2018;36(Suppl 114):43–52. [PubMed] [Google Scholar]
- Huang X., Qiao F., Xue P. The protective role of microRNA-140-5p in synovial injury of rats with knee osteoarthritis via inactivating the TLR4/Myd88/NF-kappaB signaling pathway. Cell Cycle. 2019;18:2344–2358. doi: 10.1080/15384101.2019.1647025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hwang H.S., Kim H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015;16(11):26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.-P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Bang J., Son C.N., Baek W.K., Kim J.M. Grape seed proanthocyanidin extract ameliorates murine autoimmune arthritis through regulation of TLR4/MyD88/NF-kappaB signaling pathway. Korean J. Intern. Med. 2018;33:612–621. doi: 10.3904/kjim.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács B., Vajda E., Nagy E.E. Regulatory effects and interactions of the Wnt and OPG-RANKL-RANK signaling at the bone-cartilage interface in osteoarthritis. IJMS. 2019;20(18):4653. doi: 10.3390/ijms20184653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo P.L., Huang Y.T., Chang C.H., Chang J.K. Fraxetin inhibits the induction of anti-Fas IgM, tumor necrosis factor-alpha and interleukin-1beta-mediated apoptosis by Fas pathway inhibition in human osteoblastic cell line MG-63. Int. Immunopharmacol. 2006;6:1167–1175. doi: 10.1016/j.intimp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Lei J., Fu Y., Zhuang Y., Zhang K. miR-382-3p suppressed IL-1beta induced inflammatory response of chondrocytes via the TLR4/MyD88/NF-kappaB signaling pathway by directly targeting CX43. J. Cell. Physiol. 2019;234:23160–23168. doi: 10.1002/jcp.28882. [DOI] [PubMed] [Google Scholar]
- Lepetsos P., Papavassiliou K.A., Papavassiliou A.G. Redox and NF-kappaB signaling in osteoarthritis. Free. Radic. Biol. Med. 2019;132:90–100. doi: 10.1016/j.freeradbiomed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- Li Q., Verma I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Liao C.R., Wang S.N., Zhu S.Y., Wang Y.Q., Li Z.Z., Liu Z.Y., Jiang W.S., Chen J.T., Wu Q. Advanced oxidation protein products increase TNF-alpha and IL-1beta expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2019;28:101306. doi: 10.1016/j.redox.2019.101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J.C., Wei Z.X., Zhao C., Ma Z.P., Cai D.Z. Inhibition of osteoclastogenesis for periprosthetic osteolysis therapy through the suppression of p38 signaling by fraxetin. Int. J. Mol. Med. 2018;42:1257–1264. doi: 10.3892/ijmm.2018.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Bryan R.u., Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015;11(1):35–44. doi: 10.1038/nrrheum.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J., Boileau C., Pelletier J.-P., Roughley P.J. Cartilage in normal and osteoarthritis conditions. Best Practice Res. Clin. Rheumatol. 2008;22(2):351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Molina-Jiménez M.F., Sánchez-Reus M.I., Andres D., Cascales María, Benedi J. Neuroprotective effect of fraxetin and myricetin against rotenone-induced apoptosis in neuroblastoma cells. Brain Res. 2004;1009(1-2):9–16. doi: 10.1016/j.brainres.2004.02.065. [DOI] [PubMed] [Google Scholar]
- Molinajimenez M., Sanchezreus M., Cascales M., Andres D., Benedi J. Effect of fraxetin on antioxidant defense and stress proteins in human neuroblastoma cell model of rotenone neurotoxicity. Comparative study with myricetin and N-acetylcysteine. Toxicol. Appl. Pharmacol. 2005;209(3):214–225. doi: 10.1016/j.taap.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Musumeci G., Aiello F., Szychlinska M., Di Rosa M., Castrogiovanni P., Mobasheri A. Osteoarthritis in the XXIst century: risk factors and behaviours that influence disease onset and progression. IJMS. 2015;16(12):6093–6112. doi: 10.3390/ijms16036093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musumeci G., Castrogiovanni P., Trovato F., Weinberg A., Al-Wasiyah M., Alqahtani M., Mobasheri A. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. IJMS. 2015;16(9):20560–20575. doi: 10.3390/ijms160920560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paya M., Goodwin P.A., De Las Heras B., Hoult J.R.S. Superoxide scavenging activity in leukocytes and absence of cellular toxicity of a series of coumarins. Biochem. Pharmacol. 1994;48(3):445–451. doi: 10.1016/0006-2952(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Rahmati M., Nalesso G., Mobasheri A., Mozafari M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Research Reviews. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- Rigoglou S., Papavassiliou A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013;45:2580–2584. doi: 10.1016/j.biocel.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Thuong P.T., Pokharel Y.R., Lee M.Y., Kim S.K., Bae KiHwan, Su N.D., Oh W.K., Kang K.W. Dual anti-oxidative effects of fraxetin isolated from fraxinus rhinchophylla. Biol. Pharm. Bull. 2009;32(9):1527–1532. doi: 10.1248/bpb.32.1527. [DOI] [PubMed] [Google Scholar]
- Urban, H., Little, C. B., 2018. The role of fat and inflammation in the pathogenesis and management of osteoarthritis. Rheumatology (Oxford). 57, iv10–iv21. https://doi.org/10.1093/rheumatology/kex399. [DOI] [PubMed]
- Van Opdenbosch N., Lamkanfi M. Caspases in cell death, inflammation, and disease. Immunity. 2019;50(6):1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witaicenis A., Seito L.N., da Silveira Chagas A., de Almeida L.D., Junior, Luchini A.C., Rodrigues-Orsi P., Cestari S.H., Di Stasi L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine. 2014;21(3):240–246. doi: 10.1016/j.phymed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Woodell‐May J.E., Sommerfeld S.D. Role of inflammation and the immune system in the progression of osteoarthritis. J. Orthop. Res. 2020;38(2):253–257. doi: 10.1002/jor.24457. [DOI] [PubMed] [Google Scholar]
- Wu B., Wang R., Li S., Wang Y., Song F., Gu Y., Yuan Y. Antifibrotic effects of Fraxetin on carbon tetrachloride-induced liver fibrosis by targeting NF-kappaB/IkappaBalpha, MAPKs and Bcl-2/Bax pathways. Pharmacol. Rep. 2019;71:409–416. doi: 10.1016/j.pharep.2019.01.008. [DOI] [PubMed] [Google Scholar]
- Yan H., Duan X., Pan H., Holguin N., Rai M.F., Akk A., Springer L.E., Wickline S.A., Sandell L.J., Pham C.T.N. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc. Natl. Acad. Sci. USA. 2016;113:E6199–E6208. doi: 10.1073/pnas.1608245113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Ji, L., Yang, Y., Wei, Y., Zhang, X., Gang, Y., Lu, J., Bai, L., 2019. The Therapeutic Effects of Treadmill Exercise on Osteoarthritis in Rats by Inhibiting the HDAC3/NF-KappaB Pathway in vivo and in vitro. Front Physiol. 10, 1060. https://doi.org/10.3389/fphys.2019.01060. [DOI] [PMC free article] [PubMed]