Abstract.
We investigated serum C-peptide immunoreactivity (CPR) levels in registered data from a multi-center collaborative nationwide type 1 diabetes study. The CPR levels were obtained from 576 and 409 children during the early registration (2013/2014) and late observation (2016/2017) periods, respectively. The percentages of children with a CPR < 0.1 or < 0.3 ng/mL increased according to the duration since diagnosis. Among patients with 5 or more years since diagnosis, 69% had a CPR < 0.1 and 95% had a CPR < 0.3 in the early registration period. A significant negative correlation was observed between the HbA1c and the CPR levels, and the HbA1c levels were significantly higher among children with a CPR < 0.1 or < 0.3 than among those with a CPR ≥ 0.6 ng/mL. During the late observation period, the prevalence of a CPR < 0.1 ng/mL was 88% among long-standing patients and 77% among patients aged 18–20 yr. Regarding the characteristics of “Responders” with a sustained CPR ≥ 0.6 ng/mL at 5 or more years since diagnosis, six of the seven were adolescent females; five of the seven had an HLA DR4-DQ4 haplotype. When type 1A diabetes mellitus (T1AD) children transit to adult care centers, most of them may have some difficulty in glycemic control because of the depleted endogenous insulin.
Keywords: type 1A diabetes mellitus (T1AD), serum C-peptide immunoreactivity (CPR), Japanese children, human leukocyte antigen (HLA)
Introduction
Type 1A diabetes (T1AD) is mediated by the autoimmune destruction of pancreatic islet β-cells (1,2,3). In patients with T1AD, C-peptide immunoreactivity (CPR), which measures the amount of connecting peptide (C-peptide) co-secreted with insulin from the islets of Langerhans, permits an estimation of the remaining β-cell secretion of insulin. Endogenous insulin secretion in the T1AD patients gradually decreases and becomes depleted within several years after diagnosis (1,2,3).
The Diabetes Control and Complications Trial (DCCT) established that a stimulated CPR concentration of more than 0.6 ng/mL at study entry among subjects with a diabetes duration of up to 5 yr was associated with favorable metabolic and clinical outcomes over the subsequent 7 yr of follow-up (4,5,6). In the intensive treatment group, for patients with a 50% higher stimulated CPR level (such as from 0.30–0.45 ng/mL) upon study entry, the HbA1c level decreased by 0.07%, the insulin dose decreased by 0.0276 U/kg/d, the hypoglycemia risk decreased by 8.2%, and the risk of sustained retinopathy reduced by 25% at the end of the follow-up period (6).
Dramatic technological advances have been made in insulin therapy, and a multi-center collaborative nationwide cohort study (The Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes: JSGIT) has reported an improvement in the control of diabetes in Japan (7, 8). Recently, intensive care with multiple daily injections of insulin and continuous subcutaneous insulin infusion (CSII) soon after diagnosis have been suggested to be beneficial for protecting against the depletion of endogenous insulin in T1AD children.
We analyzed the recent clinical status of endogenous insulin levels in Japanese children with T1AD using the serum CPR levels extracted from the data registered as part of the JSGIT. We also tried to elucidate the clinical and genetic characteristics of so-called “Responders” with a sustained CPR ≥ 0.6 ng/mL at 5 or more years since diagnosis.
Subjects and Methods
This study analyzed serum CPR levels using data from the T1AD children registered in the fourth cohort of the JSGIT (7, 8). Type 1 diabetes was diagnosed according to the criteria of the Japan Diabetes Society and the American Diabetes Association (1,2,3). T1AD was defined as type 1 diabetes with one or more autoantibodies, including glutamic acid decarboxylase autoantibody (GADAb), protein tyrosine phosphatase IA-2 autoantibody (IA-2Ab), and the cation efflux transporter zinc transporter 8 autoantibody (ZnT8Ab), whose presence were determined using a previously described method (9,10,11).
The CPR levels were obtained from 576 children (233 boys and 343 girls; age at diagnosis, 0–18 yr; mean age at diagnosis, 7.3 yr; age at specimen collection, 2–21 yr; mean age at specimen collection, 12.2 yr) during the early registration period from March 2013 to February 2014 (2013/2014). To obtain more information on long-standing T1AD patients, the CPR levels were obtained from an additional 409 children (170 boys and 239 girls; age at specimen collection, 6–26 yr; mean age at specimen collection, 15.6 yr) during the late observation period of the JSGIT from November 2016 to June 2017 (2016/2017).
The time of blood collection for the measurement of CPR levels was not determined, mainly in non-fasting (random) samples at the outpatient clinics of 57 nationwide medical centers in Japan. The serum CPR levels were measured using mainly the electro-chemiluminescence immunoassay (CLEIA) Lumipulse Presto C-peptide (Fujirebio, Tokyo, Japan) (12), and some specimens were measured using the ECLIA Elecsys C-peptide assay (Roche Diagnostics, Mannheim, Germany), EIA ST E test TOSOH II C-peptide (Tosoh, Tokyo, Japan), or CLIA C-peptide Abbott ARCHITECT G06233R01 (Abbott Japan, Tokyo, Japan).
The HbA1c values were measured at each institution. The differences between the HbA1c values measured at each institution and those measured at a core laboratory (SRL Inc., Tokyo, Japan) were evaluated to determine the absolute relative difference by dividing the absolute difference by the HbA1c value measured at the laboratory. The mean absolute relative difference and SD was 2.11 ± 0.28% for this cohort (7). The differences between the participating institutions were thus judged to be acceptable.
Genomic DNA was extracted from whole blood specimens. HLA class II typing was performed using a Luminex Multi-Analyte Profiling system with a WAKFlow HLA typing kit (Wakunaga, Hiroshima, Japan) (13). Briefly, the highly polymorphic exon 2 of the HLA-DRB1 and -DQB1 genes was amplified using the primer pairs included in the kit. Each polymerase chain reaction product was hybridized using sequence-specific oligonucleotide probes that were complementary to the allele-specific sequences.
Statistical analysis
The results were expressed as the mean ± SD. The Chi-square test was applied to a two-by-two contingency table. The Cochran Armitage test was applied for the trend analysis. The Spearman rank correlation test was applied for the correlation analysis. Clinical data among the four groups were analyzed using the Kruskal-Wallis test. A P-value < 0.05 was considered statistically significant. All the statistical analyses were performed using IBM SPSS version 24.0.
Ethical approval
This study was approved by the review board of Tokyo Women’s Medical University and each of the participating institutions and was conducted in accordance with the ethical guidelines and regulations laid out in the Declaration of Helsinki. Written informed consent was obtained from each of the patients or their parents.
Results
The CPR levels of 576 children (233 boys and 343 girls; 2–21 yr old at registration) were obtained during the early registration period between March 2013 and February 2014 (2013/2014). A significant negative correlation was observed (rs = –0.497, P < 0.001) between the serum CPR levels and the duration since diagnosis in the T1AD children (Fig. 1). The percentages of children with a CPR < 0.1 ng/mL increased according to the duration since diagnosis (P < 0.001): 15% for children within 1 yr of diagnosis, 30% for 1–3 yr since diagnosis, 51% for 3–5 yr, 66% for 5–10 yr, and 78% for 10–16 yr (Fig. 2). The percentages of children with a CPR < 0.3 ng/mL also increased according to the duration since diagnosis: 38% for children within 1 year of diagnosis, 60% for 1–3 yr since diagnosis, 86% for 3–5 yr, 93% for 5–10 yr, and 100% for 10–16 yr (Fig. 2). Among long-standing patients (5 or more years since diagnosis), 69% (167/246) had a CPR < 0.1 ng/mL, and 95% (233/246) had a CPR < 0.3 ng/mL.
Fig. 1.
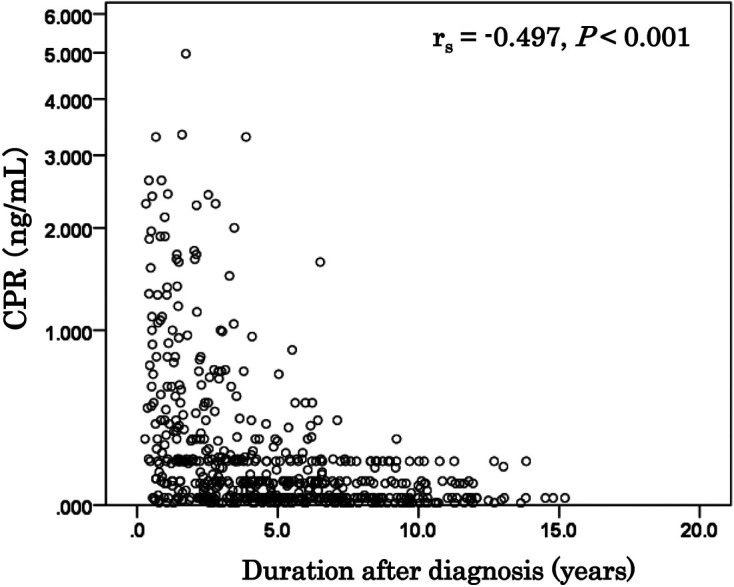
Association between serum C-peptide immunoreactivity (CPR) and duration since diagnosis in children with type 1A diabetes mellitus (T1AD). The CPR levels were obtained for 576 children (233 boys and 343 girls; age at registration, 2–21 yr) during the early registration period from March 2013 to February 2014. A significant negative correlation was observed (rs = –0.497, P < 0.001) between the serum CPR levels and the duration since diagnosis in T1AD children.
Fig. 2.
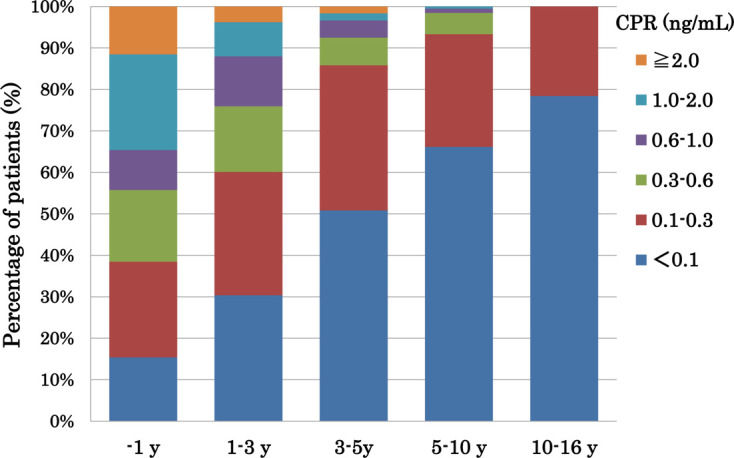
Change in percentages of patients with each C-peptide immunoreactivity (CPR) level according to the duration (yr) since diagnosis in type 1A diabetes mellitus (T1AD) children. Fifty-two subjects were diagnosed with T1AD for 1 yr since diagnosis, 158 for 1–3 yr since diagnosis, 120 for 3–5 yr since diagnosis, 195 for 5–10 yr since diagnosis, and 51 for 10–16 yr since diagnosis. The percentage of children with a CPR < 0.1 ng/mL increased according to the duration since diagnosis (P < 0.001): 15% for within 1 yr, 30% for 1–3 yr, 51% for 3–5 yr, 66% for 5–10 yr, and 78% for 10–16 yr. At 5 or more years since diagnosis, 69% (167/246) had a CPR < 0.1 and 95% (233/246) had a CPR < 0.3 ng/mL.
There was a significant negative correlation (rs = –0.155, P < 0.01) between the HbA1c levels and the CPR levels during the early registration period (2013/2014) (Fig. 3). The mean HbA1c levels of the three records in 2013/2014 were significantly higher (P < 0.001) among children with a CPR < 0.1 ng/mL (Gr. 1) and a CPR of 0.1–0.3 ng/mL (Gr. 2) than among those with a CPR of 0.6 ng/mL or more (Gr. 4) (Fig. 4).
Fig. 3.
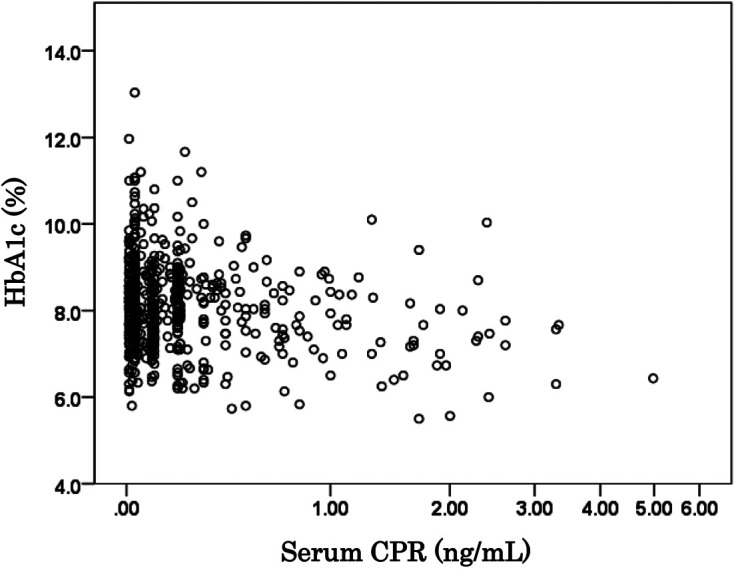
The relation between HbA1c levels and serum C-peptide immunoreactivity (CPR) levels in type 1A diabetes mellitus (T1AD) children. A significant negative correlation (rs = –0.155, P < 0.01) was seen between the HbA1c levels and the CPR levels.
Fig. 4.
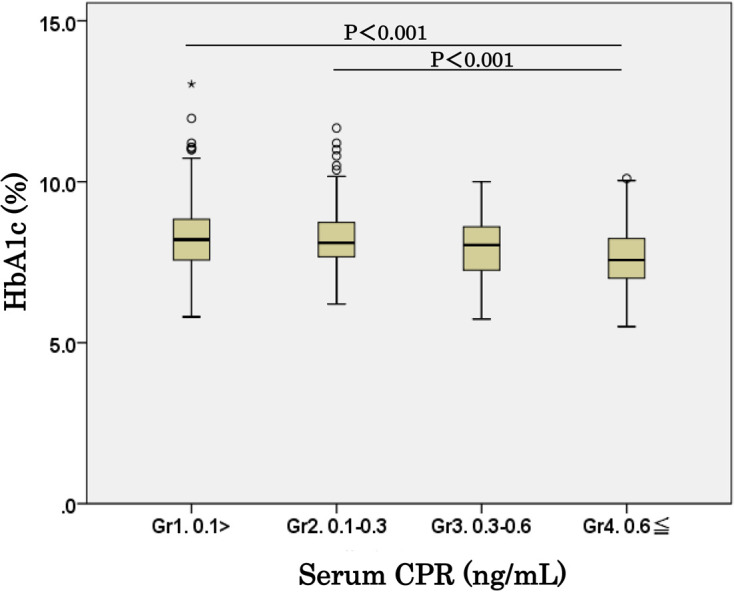
Comparison of HbA1c levels among four groups with different C-peptide immunoreactivity (CPR) levels. The mean HbA1c level of three records at three-time points in 2013/2014 was significantly higher (P < 0.001) in children with a CPR < 0.1 ng/mL (Gr. 1) and those with a CPR level of 0.1–0.3 ng/mL (Gr. 2) than in those with a CPR of ≥ 0.6 ng/mL (Gr. 4).
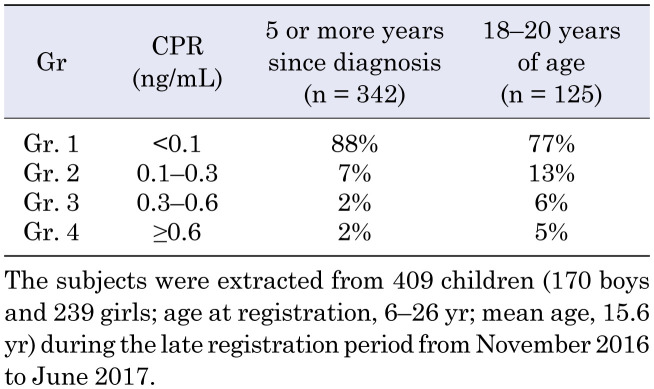
To obtain more information on long-standing T1AD patients, we analyzed data from 409 children (170 boys and 239 girls; age at registration, 6–26 yr; mean age at registration, 15.6 yr) during the late observation period from November 2016 to June 2017 (2016/2017). Table 1 shows the percentages of long-standing T1AD patients according to four groups of CPR levels. The percentages of patients with a CPR < 0.1 ng/mL and < 0.3 ng/mL were 88 and 95%, respectively, among patients at 5 or more years since diagnosis. The percentages of patients with a CPR < 0.1 ng/mL and < 0.3 ng/mL were 77 and 90%, respectively, among patients aged 18–20 yr (Table 1).
Table 1. CPR levels in four groups of long-standing type 1A diabetes mellitus (T1AD) patients.
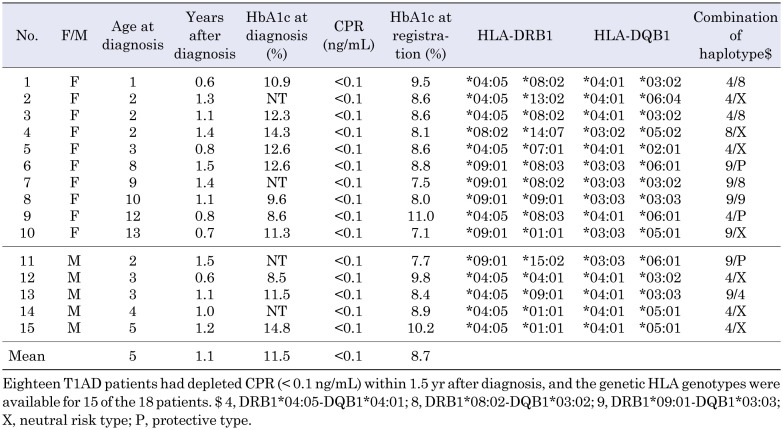
Table 2 shows the clinical and genetic characteristics of 15 T1AD newly diagnosed “Nonresponder” children who had a depleted CPR < 0.1 ng/mL within 1.5 yr after diagnosis. Eighteen T1AD patients had a depleted CPR < 0.1 ng/mL within 1.5 yr after diagnosis, and the genetic HLA genotypes were available for 15 of the 18 Nonresponder patients. Ten of the fifteen Nonresponders were females, and the mean age at diagnosis was relatively low (5 yr). The HLA-DRB1*04:05-DQB1*04:01 (DR4-DQ4) haplotype was found in 9 of the 15 Nonresponders and 9 of the 30 haplotypes. The DRB1*09:01-DQB1*03:03 (DR9-DQ9) haplotype was found in 7 of the 15 patients and 7 of the 30 haplotypes. The DRB1*08:02-DQB1*03:02 (DR8-DQ3) haplotype was found in 4 of the 15 patients and 4 of the 30 haplotypes.
Table 2. Clinical and genetic characteristics of 15 type 1A diabetes mellitus (T1AD) early Nonresponder children with depleted C-peptide immunoreactivity (CPR) levels (< 0.1 ng/mL) within 1.5 yr after diagnosis.
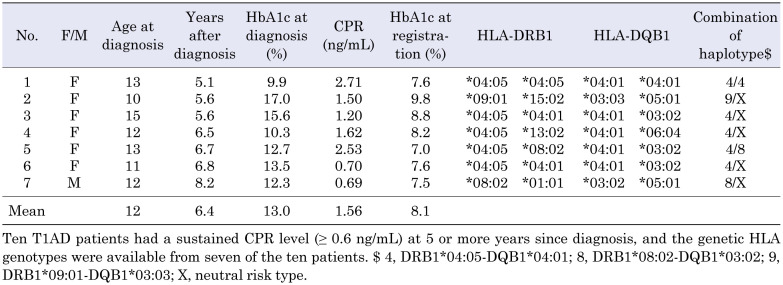
In the JSGIT records, only 10 long-standing Responders had a CPR ≥ 0.6 ng/mL at 5 or more years since diagnosis. Genetic HLA data were available for 7 of the 10 long-standing Responders (Table 3). Of note, the age at diagnosis of the Responders was relatively high (mean age, 12 yr) and was significantly older (P < 0.05) than that of the Nonresponders (mean, 5 yr) who had a depleted CPR < 0.1 ng/mL within 1.5 yr after diagnosis. Furthermore, six of the seven long-standing Responders were females. The mean HbA1c at diagnosis was relatively high (13.0%), but the mean HbA1c at registration improved to 8.1% at 5 or more years since diagnosis. The DR4-DQ4 haplotype was found in five of the seven Responders and six (42.8%) of the fourteen haplotypes. In contrast, only one Responder patient (7.1%) had a DR9-DQ9 haplotype. We previously reported that the DR4-DQ4 haplotype was found in 222 (25.8%) of 860 haplotypes, while the DR9-DQ9 haplotype was found in 275 (32.0%) of 860 haplotypes in 430 T1AD Japanese children (13).
Table 3. Clinical and genetic characteristics of 7 type 1A diabetes mellitus (T1AD) Responder children who had sustained C-peptide immunoreactivity (CPR) levels (≥ 0.6 ng/mL) at 5 or more years since diagnosis.
Discussion
In this study, we identified patients with autoimmune T1AD based on the presence of autoantibodies, including GADAb, IA-2Ab, and ZnT8Ab, and excluded patients with type 1B diabetes and monogenic diabetes. To the best of our knowledge, this is the first multi-center collaborative nationwide study examining residual endogenous insulin levels (using CPR as a marker) in Japanese children with T1AD.
A significant negative correlation was observed (rs = –0.497, P < 0.001) between the serum CPR levels and the duration since diagnosis in T1AD children (Fig. 1). The percentages of children with CPR levels < 0.1 and < 0.3 ng/mL increased according to the duration since diagnosis (P < 0.001) (Fig. 2). The percentage of patients with a depleted CPR < 0.1 ng/mL was 88% among T1AD patients at 5 or more years since diagnosis and 77% among T1AD patients between the ages of 18–20 yr (Table 1).
Steffes et al. reported that in the intensively treated cohort of the DCCT, patients with an undetectable stimulated CPR level (90 min following the ingestion of a mixed meal) of ≤ 0.03 nmol/L(≤ 0.1 ng/mL)at the time of enrollment in the DCCT had a significantly higher incidence of retinopathy and nephropathy than other groups with higher CPR levels. Even a modest CPR level at the time of enrollment in the DCCT was associated with lower incidences of retinopathy and nephropathy. In addition, the continuation of insulin (CPR) secretion was important to avoid hypoglycemia (the major complication of intensive diabetic therapy) (5).
The DCCT also established that “Responders” with a stimulated CPR concentration of ≥ 0.2 nmol/L (≥ 0.6 ng/dL) at study entry among subjects with a diabetes duration of up to 5 yr were associated with favorable metabolic and clinical outcomes over the subsequent 7 yr of follow-up (6). The mean HbA1c during 7 yr of follow-up was 6.9% among Responders and 7.5% among Nonresponders (stimulated CPR < 0.6 ng/dL). In addition, the HbA1c level also decreased by 0.07% per 50% higher CPR level (6).
In our study, blood collection to measure the CPR levels was performed during visits to our outpatient clinics and was suggested to have been performed after a meal, but the detail was not confirmed in the JSGIT records. Unlike the DCCT, we did not examine the stimulated CPR level (90 min after the ingestion of a mixed meal). This is a potential limitation of our study. However, the subjects with a CPR < 0.1 ng/mL in our study may resemble the Nonresponders with an undetectable CPR <0.1 ng/mL in the DCCT.
Our data suggest that childhood-onset T1AD patients who reach the age of 18–20 yr are likely to be confronted with the difficulty of controlling their blood glucose levels because of the depletion of endogenous insulin.
In previous studies conducted in Japan regarding the genetic effects of residual pancreatic β-cell function in type 1 diabetes after diagnosis, Sugihara et al. reported a remarkable difference between HLA DR9-DQ9 and DR4-DQ4 patients in a small longitudinal study (14). The residual pancreatic β-cell function was retained to a larger degree in the patients with DR4-DQ4 than in those with DR9-DQ9 at diagnosis within 12–18 mo after diagnosis. These results suggest that the DR9-DQ9 genotype may induce a stronger immunological attack against the target β-cells than the DR4-DQ4 genotype (14). In a study of mainly adult-onset patients with a mean age of 34 yr at the time of diabetes onset, Nakanishi et al. reported that a complete loss of β-cell function was observed at an earlier stage among patients who possessed both HLA-A24 and HLA-DQA1*03 and among patients with HLA-DR9, compared with patients without these HLA alleles (P = 0.0057 and 0.0093, respectively) (15).
Our current study was a cross-sectional study, not a longitudinal study, of JSGIT data. We next attempted to characterize newly diagnosed Nonresponder children with a depleted CPR < 0.1 ng/mL within 1.5 yr after diagnosis (Table 2) and long-standing Responders with a sustained CPR ≥ 0.6 ng/mL at 5 or more years since diagnosis (Table 3). Of note, the mean age at diagnosis in the Responder group was 12 yr, and this was significantly older than the age at diagnosis in the Nonresponder children (P < 0.05) (Tables 2 and 3). Furthermore, six of the seven Responders were females.
We did not observe statistically significant differences in HLA genotypes between the newly diagnosed Nonresponders and the long-standing Responders mainly because of the small number of patients (Tables 2 and 3). However, among the long-standing Responders, the DR9-DQ9 haplotype was only found in one patient (14.3%); in contrast, the DR4-DQ4 haplotype was found in five (71.4%) of the seven patients.
Kobayashi et al. divided Japanese IDDM patients into groups based on the period between clinical onset and the initiation of insulin therapy to characterize slowly progressive IDDM (period > 13 mo); most of these patients had an adult onset (age of onset >16 yr) (16). The clinical subtype with the slowest progressive course (slowly progressive IDDM) had distinct findings, including a late age of onset, a high prevalence of islet cell antibodies, preserved β-cell function, and a high family history of NIDDM. Kobayashi et al. also demonstrated that HLA-DR4 and DQA1*03:01-DQB1*04:01 were more common in this group than in the control subjects (16). Urakami et al. reported a slowly progressive form of type 1 diabetes detected using urine glucose screening at schools in the Tokyo Metropolitan Area (17). The long-standing Responders who were identified in this study to have a sustained CPR ≥ 0.6 ng/mL at 5 or more years since diagnosis may be a part of the slowly progressive type 1 diabetes.
In conclusion, despite the recent advancement of insulin treatment in Japan, the percentages of children with CPR levels < 0.1 and < 0.3 ng/mL continue to increase as the duration since diagnosis lengthens. The prevalence of a CPR < 0.1 ng/mL was 88% among patients 5 or more years since diagnosis and 77% among patients aged 18–20 yr. Patients with childhood-onset T1AD who reached 18–20 yr of age may have difficulty controlling their blood glucose levels because of the complete depletion of endogenous insulin.
The Specified Pediatric Chronic Diseases Treatment Research Projects (SPCDTRP) were launched in 1974 in Japan to allow all children diagnosed with any of the government-specified chronic diseases, of which type 1 diabetes is one, to be registered. Childhood-onset T1AD patients are registered to receive healthcare subsidies until they come of age (20 yr old at the longest). Additional financial support and care should be offered to childhood-onset T1AD patients during the transition from pediatric to adult care centers, especially after the age of 20 yr.
Conflict of interests
The authors have no relevant conflicts of interest to disclose.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (KAKENHI) (C) from the Japan Society for the Promotion of Science, a research grant from the Ministry of Health, Labor and Welfare, and by the Japan Diabetes Foundation.
We thank all the patients, their families, and members of the JSGIT. The members of the Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT) are as follows: Akemi Koike, Koike Child Clinic; Aki Nishii, JR Sendai Hospital; Yusuke Tanahashi, Asahikawa Medical College; Akira Endo, Iwata City Hospital; Eishin Ogawa, Teikyo University; Emiko Tachikawa, Tokyo Women’s Medical University School of Medicine; Hanako Tajima, Nippon Medical School; Tomohiro Hori, Gifu University; Makoto Anzo, Kawasaki Municipal Hospital; Hiroki Matsuura, Shinshu University; Hiroko Kadowaki, Sanno Hospital; Katsuya Aizu, Saitama Children’s Medical Center; Hisakazu Nakajima, Kyoto Prefectural University of Medicine; Yumiko Kotani, Tokushima University School of Medicine; Ichiro Yokota, Shikoku Medical Center for Children and Adults; Ikuko Takahashi, Akita University Graduate School of Medicine; Ikuma Fujiwara, Tohoku University Hospital; Jiro Iwamoto, Aso-Izuka Hospital; Junichi Nagaishi, Tottori Municipal Hospital; Junko Ito, Toranomon Hospital; Jyunichi Arai, Hosogi Hospital; Kanako Ishii and Kenji Ihara, Kyushu University School of Medicine; Kanshi Minamitani, Teikyo University Chiba Medical Center; Kaori Sasaki, Tokyo Women’s Medical University Yachiyo Medical Center; Kazuhiko Jinno, Hiroshima Prefectural Hospital; Keiichi Hanaki, Tottori Prefectural Kousei Hospital; Yohei Ogawa, Niigata University Graduate School of Medical and Dental Sciences; Hiroki Abe, Niigata City General Hospital; Kenichi Miyako, Fukuoka Children’s Hospital; Kentaro Shiga, Yokohama City University Medical Center; Kimitoshi Nakamura, Kumamoto University School of Medicine; Kisho Kobayashi, University of Yamanashi Faculty of Medicine; Kohei Sato, Sapporo Factory Kids Clinic; Koji Takemoto, Ehime University Graduate School of Medicine; Kosei Hasegawa, Okayama University School of Medicine; Mahoko Hurujyo, Okayama Medical Center; Masanori Adachi, Kanagawa Children’s Medical Center; Masaru Inoue, Okayama Red Cross General Hospital; Michiko Okajima, Kanazawa University School of Medicine; Hitomi Koyama, Dokkyo Medical University; Nobuyuki Kikuchi, Department of Pediatrics, Yokohama City Minato Red Cross Hospital; Kazuteru Kitsuda, Noriyuki Takubo and Shigeyuki Ohtsu, Kitasato University School of Medicine; Reiko Horikawa, National Center for Child Health and Development; Rika Kizu, Yokosuka Kyosai Hospital; Ryuzo Takaya, Osaka Medical College; Sachiko Kitanaka, The University of Tokyo School of Medicine; Shinichiro Miyagawa; National Hospital Organization Kure Medical Center; Shinji Kadoya, Nishinomiya Municipal Central Hospital; Haruo Mizuno, Nagoya City University; Shoji Nakayama, Mominoki Hospital; Shun Soneda, St. Marianna University School of Medicine; Susumu Kanzaki, Tottori University Faculty of Medicine; Susumu Konda, Konda Children’s Clinic; Tadayuki Ayabe, Dokkyo Medical University Koshigaya Hospital; Takahiro Mochizuki, Osaka Police Hospital; Takao Fujisawa, National Mie Hospital; Tokuo Mukai, Asahikawa-Kosei General Hospital; Tomoyuki Hotubo, Sapporo Pediatric Endoclinology Clinic; Kohji Tsubouchi: Department of Pediatrics, Chuno Kosei Hospital; Toshi Tatematsu, Chubu Rosai Hospital; Toshihisa Okada, Kumamoto Hatsuiku Clinic; Toshikazu Takahashi, Takahashi Clinic; Tsutomu Ogata, Hamamatsu University School of Medicine; Utako Sato, Tokyo Hitachi Hospital; Yasusada Kawata, Kyushyu Rosai Hospital; Yoshiya Ito, Kitami Red Cross Hospital; Goro Sasaki: Department of Pediatrics, Tokyo Dental College Ichikawa General Hospital; Yukiyo Yamamoto: Department of Pediatrics, University of Occupational and Environmental Health; Tomoyuki Kawamura, Osaka City University Graduate School of Medicine; Tatsuhiko Urakami: Department of Pediatrics, Nihon University School of Medicine; Toru Kikuchi and Shin Amemiya: Department of Pediatrics, Saitama Medical University; Shigetaka Sugihara: Department of Pediatrics, Tokyo Women’s Medical University Medical Center East.
References
- 1.Couper JJ, Haller MJ, Greenbaum CJ, Ziegler AG, Wherrett DK, Knip M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Stages of type 1 diabetes in children and adolescents. Pediatr Diabetes 2018;19(Suppl 27): 20–7. doi: 10.1111/pedi.12734 [DOI] [PubMed] [Google Scholar]
- 2.Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, Araki E, et al. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010;1: 212–28. doi: 10.1111/j.2040-1124.2010.00074.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1): S62–9. doi: 10.2337/dc10-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The DCCT Research Group. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab 1987;65: 30–6. doi: 10.1210/jcem-65-1-30 [DOI] [PubMed] [Google Scholar]
- 5.Steffes MW, Sibley S, Jackson M, Thomas W. β-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26: 832–6. doi: 10.2337/diacare.26.3.832 [DOI] [PubMed] [Google Scholar]
- 6.Lachin JM, McGee P, Palmer JP, DCCT/EDIC Research Group. Impact of C-peptide preservation on metabolic and clinical outcomes in the Diabetes Control and Complications Trial. Diabetes 2014;63: 739–48. doi: 10.2337/db13-0881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mochizuki M, Kikuchi T, Urakami T, Kikuchi N, Kawamura T, Yokomichi H, et al. Japanese Study Group of Insulin Therapy for Childhood Adolescent Diabetes (JSGIT). Improvement in glycemic control through changes in insulin regimens: findings from a Japanese cohort of children and adolescents with type 1 diabetes. Pediatr Diabetes 2017;18: 435–42. doi: 10.1111/pedi.12409 [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y, Kikuchi T, Urakami T, Goto M, Tsubouchi K, Sasaki G, et al. Status and trends in the use of insulin analogs, insulin delivery systems and their association with glycemic control: comparison of the two consecutive recent cohorts of Japanese children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2019;32: 1–9. doi: 10.1515/jpem-2018-0329 [DOI] [PubMed] [Google Scholar]
- 9.Sugihara S, Yokota I, Mukai T, Mochizuki T, Nakayama M, Tachikawa E, et al. Japanese Study Group of Insulin Therapy for Childhood Adolescent Diabetes (JSGIT). Increased diagnosis of autoimmune childhood-onset Japanese type 1 diabetes using a new glutamic acid decarboxylase antibody enzyme-linked immunosorbent assay kit, compared with a previously used glutamic acid decarboxylase antibody radioimmunoassay kit. J Diabetes Investig 2020;11(3): 594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura N, Uchigata Y, Urakami T, Kawasaki E, Kikuchi N, Kobayashi T, et al. The measurement of IA-2Ab and the combination analysis busing IA-2Ab and GADAb in type1 diabetes mellitus. Practice 1999;16: 567–72. [Google Scholar]
- 11.Kawasaki E, Nakamura K, Kuriya G, Satoh T, Kobayashi M, Kuwahara H, et al. Differences in the humoral autoreactivity to zinc transporter 8 between childhood- and adult-onset type 1 diabetes in Japanese patients. Clin Immunol 2011;138: 146–53. doi: 10.1016/j.clim.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 12.Karasawa M, Tsurue M, Takenami K, Kikuchi M, Wakabayashi T, Suzuki Y, et al. Analysis of basal ability and standard value of “Lumipulse Presto C-peptide” by using Lumipulse Presto II. The Journal of Clinical Laboratory Instruments and Reagents 2006;29: 485–91(in Japanese). [Google Scholar]
- 13.Sugihara S, Ogata T, Kawamura T, Urakami T, Takemoto K, Kikuchi N, et al. Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes (JSGIT). HLA-class II and class I genotypes among Japanese children with Type 1A diabetes and their families. Pediatr Diabetes 2012;13: 33–44. doi: 10.1111/j.1399-5448.2011.00833.x [DOI] [PubMed] [Google Scholar]
- 14.Sugihara S, Sakamaki T, Konda S, Murata A, Wataki K, Kobayashi Y, et al. Association of HLA-DR, DQ genotype with different β-cell functions at IDDM diagnosis in Japanese children. Diabetes 1997;46: 1893–7. doi: 10.2337/diab.46.11.1893 [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi K, Inoko H. Combination of HLA-A24, -DQA1*03, and -DR9 contributes to acute-onset and early complete β-cell destruction in type 1 diabetes: longitudinal study of residual β-cell function. Diabetes 2006;55: 1862–8. doi: 10.2337/db05-1049 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Tamemoto K, Nakanishi K, Kato N, Okubo M, Kajio H, et al. Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care 1993;16: 780–8. doi: 10.2337/diacare.16.5.780 [DOI] [PubMed] [Google Scholar]
- 17.Urakami T, Suzuki J, Yoshida A, Saito H, Mugishima H. Incidence of children with slowly progressive form of type 1 diabetes detected by the urine glucose screening at schools in the Tokyo Metropolitan Area. Diabetes Res Clin Pract 2008;80: 473–6. doi: 10.1016/j.diabres.2008.01.029 [DOI] [PubMed] [Google Scholar]