Abstract.
Selenium, one of the essential trace minerals, is present in vivo in form of selenoproteins. Iodothyronine deiodinase, a selenoprotein, is involved in the activation and inactivation of thyroid hormone. Therefore, patients with selenium deficiency may present changes in thyroid hormone levels due to inhibition of T4 to T3 conversion; however, this assumption is still under debate. In the present study, we retrospectively investigated the thyroid function in 22 patients with selenium deficiency. Thyroid stimulating hormone (TSH) and free T4 (FT4) levels were increased in 3 (14%) and 5 (23%) patients, respectively, and free T3 (FT3) levels were decreased in 6 (27%) patients. The FT4/FT3 ratio was significantly higher in patients with selenium deficiency than that in the control group. There appeared to be a positive correlation between the decreased rate of selenium levels and FT4/FT3 ratio, thereby indicating that patients with severe selenium deficiency also exhibited abnormal thyroid hormone levels. Furthermore, when selenium was supplemented in seven patients with abnormal thyroid hormone levels, the TSH, FT4, and FT4/FT3 ratio were significantly decreased and FT3 levels were increased. Collectively, patients with selenium deficiency could present the characteristics of not only low FT3 but also high FT4 and FT4/FT3 ratio.
Keywords: selenoprotein, selenium deficiency, FT4/FT3 ratio, iodothyronine deiodinase, thyroid function
Introduction
Selenium, an essential trace mineral, is found in soil and water and enters the food chain through plant roots and aquatic organisms (1). Healthy Japanese individuals do not present selenium deficiency as they regularly consume selenium-rich foods like meat, fish, milk, and eggs (2); however, some patients with dietary restrictions due to food allergies or congenital metabolic diseases, those who depend on enteral formula by tube feeding due to difficulty in oral intake, and those who receive intravenous nutrition, are at a high risk of selenium deficiency, because certain food items/formulas lack enough selenium (3, 4). Selenium-deficient patients present various symptoms such as whitened nails, hair loss, dermatitis, muscle weakness, cardiac myopathy, and abnormal laboratory findings suggesting macrocytic anemia and liver dysfunction (5, 6).
Selenium is involved in in vivo mechanisms in the form of selenoproteins. Humans possess 25 types of selenoproteins, essentially glutathione peroxidases (GPx), thioredoxin reductase, and iodothyronine deiodinase (DIO) (7, 8). Of the 8 forms of GPxs present in the human body, 5 (GPx 1–4 and 6) are selenoproteins; all these utilize glutathione to catalyze the reduction of hydrogen peroxide and phospholipid peroxide and act as antioxidants. Thioredoxin reductase is an oxidoreductase that catalyzes the reduction of oxidized thioredoxin and regulates cell signaling. Deiodinase is an important enzyme required to convert T4 into T3. Of the three deiodinases (DIO1, DIO2, and DIO3), DIO1 and DIO2 activate T4 by converting it into T3, whereas DIO3 inactivates T3 (9). Therefore, presumably selenium deficiency results in increased T4 and decreased T3 levels due to the inhibition of T4 to T3 conversion.
Nevertheless, according to some studies that reported the influence of thyroid hormone levels due to selenium administration, the decrease in T4 and/or thyroid stimulating hormone (TSH) levels appeared unlikely to be always common (10); and it has, this assumption remains controversial. The diagnostic criteria for selenium deficiency in Japan established in 2015 suggests low T3 levels as one of the characteristic findings (11); however, the change in thyroid hormone levels may be not limited to only low T3 level. This study aimed to further assess the relationship between selenium deficiency status and thyroid hormone metabolism and to find the characteristics of thyroid function in patients with selenium deficiency.
Materials and Methods
This study was approved by the Medical Research Ethics Committee at Todaiji Ryoiku Hospital for Children and Nara Medical University, Japan.
Patients
This retrospective study included 22 patients (at risk for selenium deficiency owing to causes such as tube feeding, parenteral nutrition, and dietary restrictions) who presented low serum selenium levels at Todaiji Ryoiku Hospital for Children and Nara Medical University Hospital in Nara from January 2014 to December 2017. Patients with abnormal thyroid function due to primary or secondary hypothyroidism or who presented low T3 due to malnutrition by physical status (BMI z-score < –2SD in children or BMI < 18.5 in adult) were excluded from this study. Moreover, the study included 44 patients (25 males and 19 females) as a control group aged 3–28 yr (median age; 13 yr old). The euthyroid controls measured the thyroid hormone levels to assess the cause of diseases or to take regular follow-up for the diseases such as short stature, epilepsy, and mental illness, in which the thyroid hormone levels were within standard values. Eligibility criteria were: i) to be considered fit based on the nutritional and physical status by assessing the BMI or nutrition-related blood examinations, ii) lack diseases associated with selenium intake such as food allergy and anorexia, and iii) lack diseases affecting the thyroid function such as growth hormone deficiency. The standard value of thyroid function in children ≤ 15 yr of age differs from those in adults and also varies by age (12). In addition, the standard value of thyroid function differed between the two participating facilities. Therefore, thyroid hormone levels between the selenium deficiency group and control group at ≤ 15 yr of age were compared at 1:2 ratio of cases with age-matching for each facility. In contrast, the thyroid hormones between two groups at > 15 yr of age were compared at 1:2 ratio of cases without age-matching for each facility. Informed consents were obtained from the patients and their parents.
Assessments
Based on their medical records, patients were retrospectively examined for thyroid hormone levels and for changes in thyroid hormone levels after selenium supplementation. The data extracted from the records were: i) underlying medical problems and complications, ii) dietary contents, iii) symptoms and laboratory findings of selenium deficiency, iv) serum selenium, TSH, free T4 (FT4), and free T3 (FT3) levels before and after selenium supplementation, and v) selenium supplementation. The serum TSH, FT3, and FT4 levels of the healthy controls were also extracted from their records. Based on the collected data, we investigated the following parameters: i) the relationship between the severity of selenium deficiency and thyroid function, ii) the association between thyroid function and other symptoms of selenium deficiency (such as whitened nails, hair loss, dermatitis, and macrocythemia), and iii) the comparison of thyroid hormone levels in the selenium deficiency group with those in the control group, and furthermore, the comparison of thyroid hormone levels in the selenium deficiency group accompanied by abnormal thyroid hormone levels (abnormal thyroid group) with those in the control group, and iv) changes in thyroid hormone levels after selenium supplementation in abnormal thyroid group were analyzed as subgroup analysis. The severity of selenium deficiency was assessed by comparing the decrease rate to the lower limits of standard serum selenium levels for each age group (11). The decrease rate was calculated as follows: (1 − serum selenium level/lower limit of the standard serum selenium level for that age group) ×100 (%).
Serum selenium levels were measured using flameless atomic absorption spectrometry, whereas serum TSH, FT3, and FT4 levels were measured using chemiluminescence immunoassay (ARCHITECT®; ABBOTT JAPAN Co., Ltd., Tokyo, Japan) at Todaiji Ryoiku Hospital for Children and Eclusys® (Roche Diagnostics K.K., Tokyo, Japan) in Nara Medical University. Based on the guidelines for selenium deficiency (11), low levels of serum selenium were defined as follows: ≤ 6.0 μg/dL for patients aged 0–5 yr, ≤ 7.0 μg/dL for 6–14 yr, ≤ 8.0 μg/dL for 15–18 yr, and ≤ 10.0 μg/dL for patients aged >19 yr. An abnormal thyroid level was defined as the deviation from the standard values of each facility.
Statistical analyses
GraphPad Prism 5 (GraphPad Software Inc; San Diego, California, USA) was used for data analysis, and values of p < 0.05 were considered to be statistically significant. Two-tailed Mann-Whitney U-tests were used for between-group comparisons of the median values of continuous variables, and the Wilcoxon rank-sum test was used to compare the thyroid hormone levels before and after selenium supplementation. Spearman’s correlation coefficient was used to assess the correlation between the two groups.
Results
Population characteristics
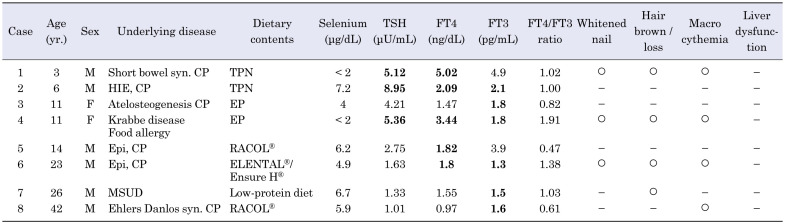
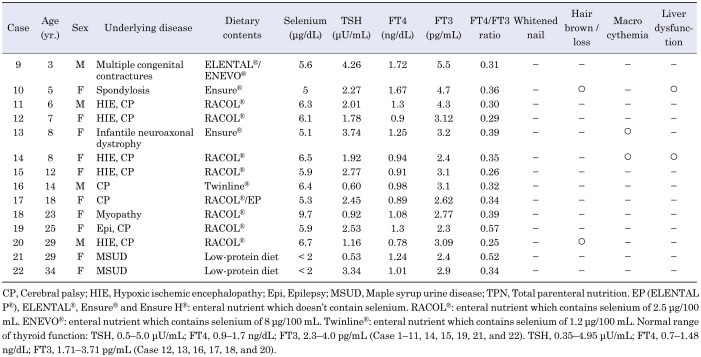
The study included 22 patients (10 males and 12 females) with selenium deficiency, aged 3–42 yr (median age; 13 yr). Abnormal thyroid hormone levels were observed in 8 (36%) patients. Detailed data regarding all the patients is listed in Table 1A and Table 1B. The underlying diseases included cerebral palsy due to central nerve system or muscular disorders (n = 15), amino acid metabolism disorders (n = 3), bowel dysfunction due to short bowel syndrome (n = 1), milk allergy (n = 1), and others (n = 2). Considering the nutritional intake, 17 patients received enteral nutrition formula (containing little or no selenium) via tube feeding due to oral motor dysfunction, 3 patients consumed protein-restricted diets due to amino acid metabolism disorders, and 2 patients received total parenteral nutrition without selenium. One patient (Case 9) was administered enteral nutrition containing selenium (ENEVO®; Abbott Japan LLC, Tokyo, Japan), but due to insufficient intake of 100 mL/d (8 μg/d), he did not meet the recommended daily intake criterion (13).
Table 1A. Characteristics of clinical and laboratory findings in selenium-deficient patients with abnormal thyroid hormone levels.
Table 1B. Characteristics of clinical and laboratory findings in selenium-deficient patients with normal thyroid hormone levels.
Thyroid function in selenium-deficient patients
Of the 8 patients with abnormal thyroid hormone levels, serum TSH levels were modestly increased (by 5–9 μU/mL) in 3 (14%) patients, FT4 levels were increased in 5 (23%) patients, and FT3 levels were decreased in 6 (27%) patients; hence, the FT4/FT3 ratio was high in all 8 patients. A positive, but not significant, correlation was observed between the decrease rate of selenium level and FT4/FT3 ratio (r = 0.42, p = 0.050; Fig. 1). No significant correlation was seen between the decrease rate of selenium levels and the TSH, FT4, or FT3 levels; however, 2 patients (Cases 21 and 22) revealed no symptoms of selenium deficiency despite having undetectable selenium levels, suggesting that the condition could present with varied symptoms. Regarding the association between thyroid function and the other symptoms of selenium deficiency, three patients with abnormal thyroid hormone levels (Cases 1, 4, and 6) presented three other symptoms of selenium deficiency, such as whitened nail and macrocythemia (Table 1A); however, no significant correlation was observed between the other selenium deficiency symptoms and the presence of abnormal thyroid hormone levels.
Fig. 1.
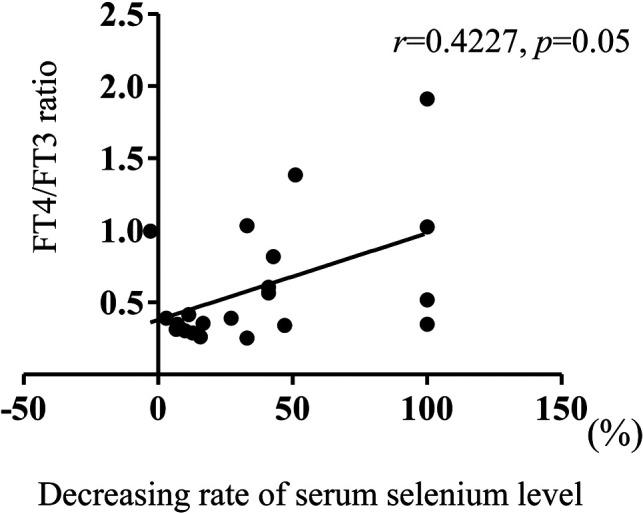
Relationship between decreased selenium concentration and free T4 (FT4)/ free T3 (FT3) ratio in patients with selenium deficiency. The relationship between the severity of selenium deficiency and the FT4/FT3 ratio was analyzed. The severity of selenium deficiency was assessed by comparing the decrease rate with the lower limits of the standard serum selenium levels for each age group. The decrease rate was calculated as follows: (1 − serum selenium level/lower limit of the standard serum selenium level for that age group) × 100 (%). Spearman correlation coefficient was used to evaluate the relationship, and p < 0.05 was defined as statistically significant.
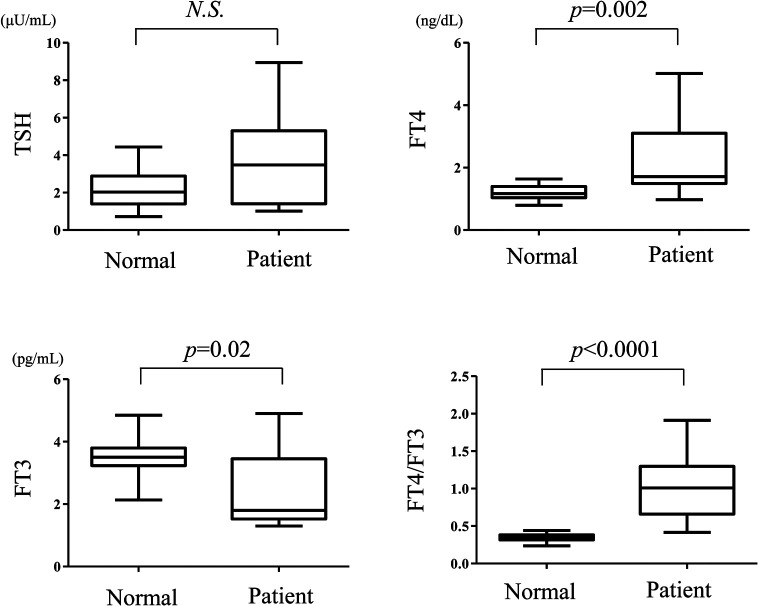
The comparison between thyroid hormone levels in the selenium deficiency group and those of the control group revealed that the FT3 levels were significantly lower and FT4/FT3 ratio was significantly higher (FT3 and FT4/FT3 ratio; p = 0.005 and 0.04, respectively); however, no significant differences were observed in the other parameters (TSH and FT4; p = 0.43 and 0.47, respectively). Moreover, comparison of the TSH, FT4, FT3 levels and the FT4/FT3 ratio between the abnormal thyroid group and the control group is illustrated in Fig. 2. The FT4 levels and FT4/FT3 ratio were significantly higher (p = 0.002 and p < 0.0001, respectively), whereas the FT3 levels were significantly lower (p = 0.02), and no significant differences were found between the TSH levels in the two groups (p = 0.15).
Fig. 2.
Comparison of thyroid hormone levels between the abnormal thyroid group in selenium-deficient patients and normal controls. Serum thyroid stimulating hormone (TSH), free T4 (FT4), and free T3 (FT3) levels as well as FT4/FT3 ratio of the abnormal thyroid group in patients with selenium deficiency were compared with those of the control group. The Mann-Whitney U-test was used for between-group comparisons. The median values are depicted within the boxes. The boxes end at the 25th and 75th percentiles, and the whiskers extend to the farthest points. Statistically significant differences were defined as p < 0.05. N.S., not significant.
Changes in thyroid hormone levels after selenium supplementation
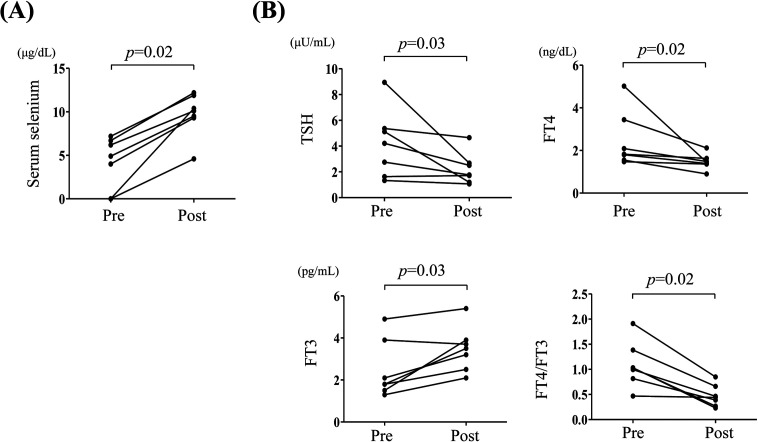
Changes in thyroid hormone levels after selenium supplementation in seven patients in the abnormal thyroid group were analyzed (selenium supplementation was not provided to one of the eight patients due to death, which was not associated with selenium deficiency or thyroid dysfunction). The route of selenium supplementation was: i) intravenous infusion for Cases 1 and 2, ii) enteral nutrient was changed to that containing selenium (ENEVO®) for Cases 3 and 6, and iii) addition of nutritional supplements containing trace elements (V-ACCEL®, NUTRI Co., Ltd., Mie, Japan, Multi Mineral®, DHC Corporation, Tokyo, Japan) for Cases 4, 5, and 7. Serum selenium levels were clearly elevated in 6 of the 7 patients who received selenium supplementation (Fig. 3A). In Case 4, selenium supplementation was first attempted using a supplement containing trace elements (Tezon®, Terumo Corporation, Tokyo, Japan), but selenium levels remained undetectable despite there being no problems with digestion and/or absorption; however, the serum levels of other trace elements present in Tezon® were increased. Selenium levels slightly increased due to the change to V-ACCEL®, but it was not sufficient. After selenium supplementation, thyroid function enhanced along with elevation of serum selenium levels in all patients (Fig. 3B). Selenium supplementation significantly reduced the TSH, FT4 levels, and FT4/FT3 ratio, and increased FT3 levels (p = 0.03, 0.02, 0.02, and 0.03, respectively).
Fig. 3.
Changes in serum selenium and thyroid hormone levels following selenium supplementation in abnormal thyroid group of selenium-deficient patients. (A): Selenium levels were compared before and after selenium supplementation. (B): Serum thyroid stimulating hormone (TSH), free T4 (FT4), and free T3 (FT3) levels, and FT4/FT3 ratio were compared before and after selenium supplementation. The Wilcoxon rank-sum test was used to compare the changes. Statistically significant differences are defined as p < 0.05. Pre, Presupplementation; Post, Postsupplementation.
Discussion
On average, Japanese people consume ~100 μg/d of selenium through their diet (11); thus, their chances of selenium deficiency are scarce (13); however, the risk of selenium deficiency is high in patients who have difficulty with oral feeding and are dependent on enteral feeding via a feeding tube, particularly those who are administered selenium-free enteral nutrients such as ELENTAL®/ELENTAL-P® (EA Pharma Co., Ltd., Tokyo, Japan) or enteral nutrients containing low selenium content such as Twinline® (1.2 μg/100 mL; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan) or RACOL®(2.5 μg/100 mL; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). This deficiency may cause remarkable issues for patients receiving parenteral nutrition because the trace element preparations used for parenteral nutrition lack selenium. Due to the availability of selenium-sufficient enteral nutrient ENEVO® (8 μg/100 mL), the number of patients receiving selenium-sufficient nutrition has been increasing. Nevertheless, this deficiency may occur in tube-fed patients who cannot tolerate ENEVO® due to milk allergy, digestive diseases (inflammatory bowel disease, short bowel syndrome, etc.), old age (14), and hemodialysis patients (15). Patients with selenocysteine insertion sequence-binding protein 2 abnormalities (16) are also at risk.
Selenium-deficient patients present with various clinical symptoms, which include serious myocardial disorders such as cardiomyopathy and ischemic heart disease. These conditions are rare and potentially fatal; thus, selenium deficiency has to be diagnosed and treated early. In Japan, the deaths of some patients with selenium deficiency have been attributed to heart failure (11). Franczek-Jucha et al. (17) reported that patients complicated with myocardial infarction and heart failure presented remarkably lower levels of serum selenium, FT3, and FT3/FT4 ratio than those in the control group. Moreover, the study suggested a weak correlation between serum selenium and FT4 as well as FT3 levels. We found that not only low FT3, defined as diagnostic criteria, but high FT4 and high FT4/FT3 ratio could be useful as markers for selenium deficiency; however, no patients had myocardial disorders. Importantly, selenium deficiency can be diagnosed by discovering the abnormal thyroid hormone status prior to the onset of heart disease; this early detection and management could positively influence the prognosis of patients with selenium deficiency.
Selenium deficiency is treated via selenium supplementation through intravenous injection, enteral nutrients, and supplements such as V-ACCEL® (50 μg/1 sac) and Tezon® (16 μg/100 mL). In general, the recommended rate of supplementation for selenium deficiency is 2−5 μg/kg/d (11). Selenium concentration increases relatively quickly after supplementation, but it is often found that blood concentration does not rise despite patients having a normal digestive absorption. In the present study, one patient did not present increased serum concentration despite receiving supplementation with Tezon®. Various approaches can be used for supplementation, that is, supplements, nutrients, and foods; however, the efficiency of absorption may differ for each. It is necessary, therefore, to monitor the blood concentration of selenium to select the appropriate route of supplementation. More recently, selenium intravenous formulations, sodium selenite (ASELEND®, 100 μg/2 mL; Fujimoto Pharmaceutical Corporation, Osaka, Japan) and ENORAS® (8.96 μg/100 mL; Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan), have also become available for enteral nutrition. Presently, various methods are used to effectively treat selenium deficiency, if it is detected early.
The outcomes related to changes in thyroid function after selenium supplementation remain controversial according to certain reports (10). Kawai et al. (18) supplemented selenium in 11 severely deficient patients (aged 1–24 yr) with concentration of < 2 μg/dL, resulting in a decrease in FT4 levels. Barcza Stockler-Pinto et al. (19) supplemented selenium in 40 hemodialysis patients (aged ≥ 18 yr, concentration 1.8 ± 1.2 μg/dL), resulting in a decrease in their FT4/FT3 ratios and an increase in their FT4 levels. Calomme et al. (20) supplemented selenium in 10 patients with phenylketonuria (aged 6–18 yr, 2.1 ± 1.0 μg/dL), resulting in a decrease in their T4 and T3 levels. Some studies reported that T3 and TSH levels remained unchanged; however, T4 was significantly reduced due to supplementation in selenium-deficient patients (21, 22). Winther et al. (23) also reported that the addition of selenium to healthy subjects decreased the TSH and FT4 levels, but the FT3 levels and the FT3/FT4 ratio remained unchanged.
In contrast, some studies reported that thyroid function was not influenced by selenium supplementation. Omrani et al. (24) reported that thyroid function in 32 selenium-deficient patients with hemodialysis was not improved by selenium supplementation, although the selenium concentration in the blood increased. Thomson et al. (25) demonstrated that selenium supplementation in selenium-deficient patients did not significantly affect the T4 levels and T4/T3 ratios. Rayman et al. (26) also reported that 501 healthy volunteers (mean selenium concentration 9.13 μg/dL) who received supplements did not experience changes in any of the parameters of thyroid function. Collectively, the influence of selenium supplementation on thyroid function remains controversial. In the present study, FT3 was significantly lower and FT4/FT3 ratio was significantly higher in selenium-deficiency group than that in the control group, and moreover, FT4 in the subgroup of patients with selenium deficiency accompanied by thyroid dysfunction was higher than that in the control group. This result suggests that some cases with severe selenium deficiency might remarkably affect the conversion of T4 to T3 and result in higher FT4 levels. Drutel et al. (10) also reported that severe deficiency might affect thyroid synthesis (T3 synthesis) and that a small amount of supplementation was sufficient for DIO expression, which is in accordance with our study.
In the present study, the increase in TSH was mild (5–9 µU/mL) despite low FT3 levels. T4 is synthesized in the thyroid, whereas bulk daily T3 production occurs in various extrathyroidal tissues via 5’ deiodination catalyzed by DIO1 and DIO2 (9). In contrast, certain studies have reported that the selenium concentration and DIO activity in selenium-deficient patients differ across various tissues (9, 27). Selenium is retained in the brain, pituitary gland, and thyroid even when selenium intake is insufficient, whereas selenium content in plasma, liver, and skeletal muscle rapidly decreases, that is, in case of selenium deficiency in blood, the selenium concentration and DIO activity is maintained in the cerebrum, pituitary gland, and thyroid gland. Therefore, since the conversion from T4 to T3 in the pituitary gland was maintained, a negative feedback effect was diminished, presumably resulting in the mild increase of TSH despite the low FT3 level. Thus, individual differences may occur between selenium intake/serum concentration and the appearance of selenium deficiency-associated symptoms, including abnormal thyroid hormone status. Differences in the absorption efficiency of selenium and the degree of DIO activity in each organ may also be influenced.
The limitations of this study are as follows: (i) the small number of cases, (ii) standard values of thyroid function differed between the two participating facilities, (iii) patients with epilepsy were included in both groups, and it could be denied that antiepileptic drugs might have affected thyroid function, and (iv) cases without intake of iodine and selenium may affect thyroid function. To solve the problem of standard values, age and facility were matched between both groups, as described in the Methods. As selenium was supplemented and iodine was not, and no switch in antiepileptic drugs was observed, changes in the thyroid function due to increased selenium concentrations alone could be examined. As only few studies have assessed the effect of selenium supplementation on thyroid function in Japanese population, the present study could prove valuable. Increasing the number of cases and adding iodine assessments could provide a more detailed and accurate assessment of the selenium effects on thyroid function.
Conclusion
Some cases of selenium deficiency could exhibit abnormal thyroid hormone levels, which present with the characteristics of high FT4 and high FT4/FT3 ratio as well as low FT3 (which is already a diagnostic criterion). Moreover, we need to consider selenium deficiency when encountering unique thyroid hormone status such as those in clinical practice.
Conflict of interests
The authors declare no conflicts of interest.
References
- 1.Weeks BS, Hanna MS, Cooperstein D. Dietary selenium and selenoprotein function. Med Sci Monit 2012;18: RA127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayman MP. Selenium and human health. Lancet 2012;379: 1256–68. [DOI] [PubMed] [Google Scholar]
- 3.Demirdas S, van Spronsen FJ, Hollak CEM, van der Lee JH, Bisschop PH, Vaz FM, et al. Micronutrients, Essential Fatty Acids and Bone Health in Phenylketonuria. Ann Nutr Metab 2017;70: 111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etani Y, Nishimoto Y, Kawamoto K, Yamada H, Shouji Y, Kawahara H, et al. Selenium deficiency in children and adolescents nourished by parenteral nutrition and/or selenium-deficient enteral formula. J Trace Elem Med Biol 2014;28: 409–13. [DOI] [PubMed] [Google Scholar]
- 5.Vinton NE, Dahlstrom KA, Strobel CT, Ament ME. Macrocytosis and pseudoalbinism: manifestations of selenium deficiency. J Pediatr 1987;111: 711–7. [DOI] [PubMed] [Google Scholar]
- 6.Shenkin A. Selenium in intravenous nutrition. Gastroenterology 2009;137(Suppl): S61–9. [DOI] [PubMed] [Google Scholar]
- 7.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, et al. Characterization of mammalian selenoproteomes. Science 2003;300: 1439–43. [DOI] [PubMed] [Google Scholar]
- 8.Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol 2005;184: 455–65. [DOI] [PubMed] [Google Scholar]
- 9.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 2002;23: 38–89. [DOI] [PubMed] [Google Scholar]
- 10.Drutel A, Archambeaud F, Caron P. Selenium and the thyroid gland: more good news for clinicians. Clin Endocrinol (Oxf) 2013;78: 155–64. [DOI] [PubMed] [Google Scholar]
- 11.Kodama H, Asagiri K, Ida S, Etani Y, Koyama H, Soh H, et al. Diagnosis and Treatment of Selenium Deficiency. J Jpn Soc Clin Nutr 2015;3: 182–217(In Japanese). [Google Scholar]
- 12.Iwaku K, Noh JY, Minagawa A, Kosuga Y, Suzuki M, Sekiya K, et al. Determination of pediatric reference levels of FT3, FT4 and TSH measured with ECLusys kits. Endocr J 2013;60: 799–804. [DOI] [PubMed] [Google Scholar]
- 13.Overview of Dietary Reference Intakes for Japanese (2015) [published 2014]. Available from https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf.
- 14.Kondo M, Aiba N, Higashi E, Oka J, Kajimoto M. Changes due to ageing in the concentrations of trace elements in the whole blood that assist with the process of antioxidation. Biomed Res Trace Elements 2004;15: 342–4. [Google Scholar]
- 15.Suzuki T, Kato K, Hatafuku F, Konda R, Fujioka T, Itai K, et al. Low serum concentration of selenium in hemodialysis patients. J Jpn Soc Dial Ther 2004;37: 1487–92(In Japanese). [Google Scholar]
- 16.Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, et al. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 2005;37: 1247–52. [DOI] [PubMed] [Google Scholar]
- 17.Frączek-Jucha M, Kabat M, Szlósarczyk B, Czubek U, Nessler J, Gackowski A. Selenium deficiency and the dynamics of changes of thyroid profile in patients with acute myocardial infarction and chronic heart failure. Kardiol Pol 2019;77: 674–82. [DOI] [PubMed] [Google Scholar]
- 18.Kawai M, Shoji Y, Onuma S, Etani Y, Ida S. Thyroid hormone status in patients with severe selenium deficiency. Clin Pediatr Endocrinol 2018;27: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcza Stockler-Pinto M, Carrero JJ, De Carvalho Cardoso Weide L, Franciscato Cozzolino SM, Mafra D. Effect of selenium supplementation via Brazil nut (Bertholletia excelsa,HBK) on thyroid hormones levels in hemodialysis patients: a pilot study. Nutr Hosp 2015;32: 1808–12. [DOI] [PubMed] [Google Scholar]
- 20.Calomme M, Vanderpas J, François B, Van Caillie-Bertrand M, Vanovervelt N, Van Hoorebeke C, et al. Effects of selenium supplementation on thyroid hormone metabolism in phenylketonuria subjects on a phenylalanine restricted diet. Biol Trace Elem Res 1995;47: 349–53. [DOI] [PubMed] [Google Scholar]
- 21.Olivieri O, Girelli D, Azzini M, Stanzial AM, Russo C, Ferroni M, et al. Low selenium status in the elderly influences thyroid hormones. Clin Sci (Lond) 1995;89: 637–42. [DOI] [PubMed] [Google Scholar]
- 22.Contempré B, Duale NL, Dumont JE, Ngo B, Diplock AT, Vanderpas J. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin Endocrinol (Oxf) 1992;36: 579–83. [DOI] [PubMed] [Google Scholar]
- 23.Winther KH, Bonnema SJ, Cold F, Debrabant B, Nybo M, Cold S, et al. Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. Eur J Endocrinol 2015;172: 657–67. [DOI] [PubMed] [Google Scholar]
- 24.Omrani HR, Rahimi M, Nikseresht K. The effect of selenium supplementation on acute phase reactants and thyroid function tests in hemodialysis patients. Nephrourol Mon 2015;7: e24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomson CD, McLachlan SK, Grant AM, Paterson E, Lillico AJ. The effect of selenium on thyroid status in a population with marginal selenium and iodine status. Br J Nutr 2005;94: 962–8. [DOI] [PubMed] [Google Scholar]
- 26.Rayman MP, Thompson AJ, Bekaert B, Catterick J, Galassini R, Hall E, et al. Randomized controlled trial of the effect of selenium supplementation on thyroid function in the elderly in the United Kingdom. Am J Clin Nutr 2008;87: 370–8. [DOI] [PubMed] [Google Scholar]
- 27.Bates JM, Spate VL, Morris JS, St Germain DL, Galton VA. Effects of selenium deficiency on tissue selenium content, deiodinase activity, and thyroid hormone economy in the rat during development. Endocrinology 2000;141: 2490–500. [DOI] [PubMed] [Google Scholar]