Abstract
Cytoplasmic incompatibility is a selfish reproductive manipulation induced by the endosymbiont Wolbachia in arthropods. In males Wolbachia modifies sperm, leading to embryonic mortality in crosses with Wolbachia-free females. In females, Wolbachia rescues the cross and allows development to proceed normally. This provides a reproductive advantage to infected females, allowing the maternally transmitted symbiont to spread rapidly through host populations. We identified homologs of the genes underlying this phenotype, cifA and cifB, in 52 of 71 new and published Wolbachia genome sequences. They are strongly associated with cytoplasmic incompatibility. There are up to seven copies of the genes in each genome, and phylogenetic analysis shows that Wolbachia frequently acquires new copies due to pervasive horizontal transfer between strains. In many cases, the genes have subsequently acquired loss-of-function mutations to become pseudogenes. As predicted by theory, this tends to occur first in cifB, whose sole function is to modify sperm, and then in cifA, which is required to rescue the cross in females. Although cif genes recombine, recombination is largely restricted to closely related homologs. This is predicted under a model of coevolution between sperm modification and embryonic rescue, where recombination between distantly related pairs of genes would create a self-incompatible strain. Together, these patterns of gene gain, loss, and recombination support evolutionary models of cytoplasmic incompatibility.
Keywords: Wolbachia, cytoplasmic incompatibility, cif genes evolution
Introduction
Maternally transmitted bacteria in the genus Wolbachia commonly manipulate the reproduction of their arthropod hosts by inducing cytoplasmic incompatibility (CI). In the simplest case, CI causes embryonic mortality in crosses between symbiont-infected males and uninfected females (unidirectional CI). Because Wolbachia-infected females can still reproduce successfully with infected males, this provides them with a fitness advantage. Above a certain threshold in Wolbachia frequency, CI allows the infection to rapidly spread in the host population, even if it induces moderate fitness costs (Turelli et al. 1992). This is thought to have contributed to the remarkable evolutionary success of Wolbachia, which is estimated to infect around half of terrestrial arthropod species (Weinert et al. 2015).
Cytological studies have revealed that CI results from cytogenetic defects affecting the paternal chromosomes in early embryogenesis (Callaini et al. 1997; Tram and Sullivan 2002). As Wolbachia is not present in mature sperm, this suggests that Wolbachia modifies the sperm of infected males during spermatogenesis (the modification function). This leads to development failing unless the zygote inherits a Wolbachia from the mother that is able to rescue embryonic development (the rescue function) (Callaini et al. 1997; Tram and Sullivan 2002). When the male and female parents are infected with different Wolbachia strains, it is also common to find that the cross is incompatible (bidirectional CI) (O’Neill and Karr 1990; Bordenstein et al. 2001; Atyame et al. 2014). This suggests the modification and rescue factors must match each other for development to proceed normally (Poinsot et al. 2003).
Recent work on the Wolbachia strains wMel and wPip from Drosophila melanogaster and Culex pipiens has found that the bacterial genes cifA and cifB are sufficient to induce CI (Beckmann et al. 2017; Lepage et al. 2017; Chen et al. 2019; Shropshire and Bordenstein 2019). In both strains cifA is located directly upstream of cifB, and it is thought that cifA and cifB are transcribed as a single operon (Beckmann et al. 2017) (although this has been questioned by Shropshire and Bordenstein [2019]). In both Wolbachia genomes the genes are found within a prophage called WO, and the proteins encoded by the two genes bind each other (Beckmann et al. 2017; Chen et al. 2019).
In Drosophila, expressing cifAwMel in the female germline rescues CI in crosses with wMel-infected males (Shropshire et al. 2018; Shropshire and Bordenstein 2019). Unexpectedly, in transgenic flies the modification of sperm requires both cifAwMel and cifBwMel to be expressed in the male germline (Lepage et al. 2017; Shropshire and Bordenstein 2019; Shropshire et al. 2020). This suggests that both genes are required for the modification function but only one gene for rescue and has been referred to as the “two-by-one” model of CI by some authors (Shropshire and Bordenstein, 2019). One hypothesis to explain this is that cifA and cifB together modify sperm in a way that is lethal unless reversed by cifA in the early embryo. Other authors have proposed that cifB may be the only toxin in sperm and that cifA could act as an antitoxin both in embryos and in sperm (Beckmann, Bonneau, et al. 2019). In this model, the requirement of cifA in sperm would be due to the fact that it protects maturing sperm cells from the toxic effect of cifB (Beckmann, Bonneau, et al. 2019). This model requires further validation as experiments have so far failed to detect cifB being transferred to females on mating (Beckmann and Fallon 2013).
There appear to be at least two distinct molecular mechanisms by which these genes cause CI, which are illustrated by two paralogous pairs of cifA–cifB genes in wPip. In one case cifB has two PD-(D/E)XK domains, which encode DNase activity and are required for the modification of sperm (Chen et al. 2019). The other cifB paralog in wPip has two PD-(D/E)XK domains that lack the residues required for nuclease activity (Chen et al. 2019). Instead, a deubiquitylating domain, which cleaves ubiquitin from proteins, is required for the sperm modification function (Beckmann et al. 2017; Beckmann, Sharma, et al. 2019). Based on these distinct molecular functions, cif genes with DNase activity are also known as cinA and cinB, and cif genes with deubiquitinase activity are known as cidA and cidB (Beckmann, Bonneau, et al. 2019). Following Beckmann, Bonneau, et al. (2019), we use the cif terminology to refer to CI factors regardless of their mode of action.
Homologs of cifA and cifB have been discovered in other Wolbachia genomes, allowing us to address questions around their evolution (Lepage et al. 2017; Lindsey et al. 2018). The cifA and cifB phylogenies are strongly congruent, which is compatible with a model where modification and rescue factors must be matched (Lindsey et al. 2018). In contrast, the cif gene and Wolbachia phylogenies are incongruent indicating that these genes are often transferred between Wolbachia genomes (Lindsey et al. 2018). CI genes are also commonly associated with prophage sequences on the Wolbachia chromosome, suggesting that phage-mediated transfer may underlie their mobility (Lepage et al. 2017; Lindsey et al. 2018). Additionally, Wolbachia genomes often contain multiple pairs of the cifA and cifB genes, which may explain complex patterns of bidirectional incompatibility (Bonneau et al. 2018). Finally, homologs of cifA and cifB frequently display signs of pseudogenization, carrying mutations that disrupt their open reading frame (ORF) or introduce premature stop codons (Asselin et al. 2018; Turelli et al. 2018; Meany et al. 2019). Therefore, the evolution of CI genes appears to be highly dynamic, being punctuated with acquisition events through horizontal transfer and losses by pseudogenization. However, the rate at which these events occur and to what extent they are governed by neutral processes or selection acting on the CI phenotype remains to be explored.
The identification of the cifA and cifB genes allows us to revisit theoretical predictions about the evolution of CI. A curious feature of CI is that it involves sperm being modified in males, and yet Wolbachia is not transmitted from males to future generations. This leads to the prediction that in randomly mating populations the ability to modify sperm is selectively neutral, so the genes involved can be lost by mutation and genetic drift (Turelli 1994; Hurst and McVean 1996). Once sperm modification has been lost, the rescue function will be free to suffer a similar fate (Turelli 1994; Hurst and McVean 1996). It has been suggested that the long-term maintenance of CI can be explained by kin selection in structured populations (Wolbachia in females benefits from related males inducing CI [Frank 1997]), but theoretical analyses suggest that this may be a weak force that acts only under specific circumstances (Haygood and Turelli 2009). This has led to the suggestion that CI may be maintained by a process of clade selection where CI is necessary for the horizontal transfer of Wolbachia to new species (Hurst and McVean 1996). There is an analogy between these models, as here Wolbachia populations are structured depending on which species they infect (clade selection model) as opposed to structure in space (kin selection model). Theory also predicts that multiple Wolbachia strains with different rescue and modification factors can invade populations (Frank 1998; Vautrin et al. 2007). A similar logic predicts that Wolbachia variants that acquire novel CI crossing types will invade infected populations provided they retain compatibility with the resident strain (Charlat et al. 2001). At the molecular level, this could be achieved by Wolbachia genomes accumulating cifA–cifB paralogs.
Here, we explore the evolution of cifA and cifB using publicly available and newly sequenced Wolbachia genomes. We found the genes in most Wolbachia genomes, often in multiple copies. Although the protein domains required for CI are widely conserved, the domain structure of divergent homologs found in some Wolbachia strains and related bacterial genera suggests that they may play a diverse range of molecular functions. The cif genes are lost on relatively short time scales, with the modification function usually being lost before the rescue function. This is compensated by pervasive horizontal transfer of functional cif genes between symbiont genomes, in many instances across long phylogenetic distances. Finally, recombination between cif genes is largely restricted to closely related homologs, supporting the hypothesis that genetic divergence leads to the diversification of CI compatibility types.
Results
New Genome Sequences of Wolbachia from Drosophila
We sequenced 11 Wolbachia genomes from 11 different Drosophila hosts, with mean sequencing depths ranging from 27× to 40× (table 1). Assembly sizes were within the range of typical Wolbachia genomes, with the genomes of the strains wStv and wNik forming a single scaffold. The number of near-universal, single-copy genes from the BUSCO proteobacteria database in the assemblies was similar to published reference genomes of Wolbachia, indicating that genomes are near complete (table 1; repeating the analysis on published genomes of wMel, wAu, wHa, wNo, and wRi yielded 181, 184, 183, 182, and 182 complete BUSCO genes, respectively). One of these strains, wTri, has been previously sequenced by Turelli et al. (2018). Our sequence differed by 114 single nucleotide polymorphisms was more intact and contained an additional pair of cif genes. We named our strain wTri-2. The newly sequenced strains all cluster within Wolbachia supergroup A, like most Wolbachia isolated from Drosophila hosts in our data set (fig. 1).
Table 1.
Summary Statistics of Newly Sequenced Wolbachia Genomes.
| Host | Strain | Mean Sequencing Depth | Genome Size (bp)a | GC Content (%) | N Scaffolds | N Contig | Scaffold N50 | Contig N50 | BUSCO Complete | BUSCO Fragment | BUSCO Missing |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Drosophila arawakana | wAra | 30× | 1,290,535 | 35.3 | 10 | 17 | 185,314 | 171,449 | 179 | 2 | 39 |
| Drosophila baimaii | wBai | 40× | 1,119,646 | 35.3 | 26 | 123 | 76,530 | 13,921 | 180 | 2 | 38 |
| Drosophila bicornuta | wBic | 30× | 1,177,727 | 35.1 | 24 | 34 | 71,402 | 49,062 | 182 | 1 | 37 |
| Drosophila bifasciata | wBif | 33× | 1,187,580 | 35.1 | 17 | 26 | 122,861 | 113,299 | 183 | 3 | 34 |
| Drosophila borealis | wBor | 36× | 1,210,092 | 35.3 | 16 | 39 | 146,013 | 52,110 | 183 | 2 | 35 |
| Drosophila neotestacea | wNeo | 40× | 1,353,942 | 35.2 | 19 | 26 | 124,304 | 93,317 | 182 | 3 | 35 |
| Drosophila nikananu | wNik | 27× | 1,137,710 | 35.3 | 1 | 7 | — | 583,015 | 183 | 2 | 35 |
| Drosophila orientacea | wOrie | 27× | 1,359,726 | 35.2 | 19 | 30 | 103,124 | 73,532 | 181 | 4 | 34 |
| Drosophila sturtevanti | wStv | 30× | 1,183,448 | 35.3 | 1 | 3 | — | 207,642 | 185 | 2 | 33 |
| Drosophila triauraria b | wTri-2 | 33× | 1,284,908 | 35.2 | 9 | 19 | 265,635 | 124,454 | 183 | 1 | 36 |
| Drosophila tropicalis | wTro | 30× | 1,214,296 | 35.2 | 13 | 17 | 106,850 | 67,971 | 184 | 2 | 34 |
Ungapped genome size.
This stock was identified in Mateos et al. (2006) and Miyake and Watada (2007) as Drosophila quadraria but Watada et al. (2011) later concluded that quadraria is a junior synonym for triauraria.
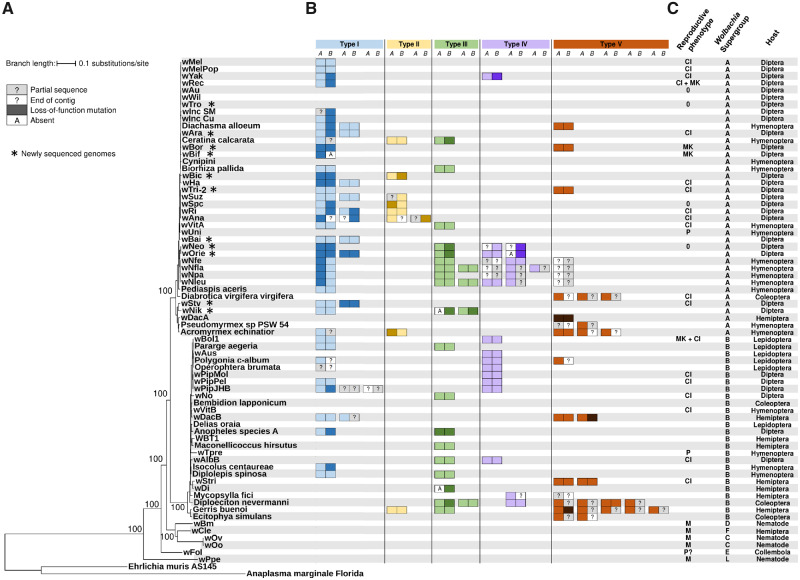
Fig. 1.
Distribution of cifA and cifB homologs across Wolbachia strains. (A) Maximum likelihood phylogeny of Wolbachia reconstructed from the concatenated sequences of 28 genes. Bootstrap values were estimated from 1,000 replicates. (B) cifA and cifB homologs. Each box represents a single gene and contiguous boxes indicate adjacent genes in the genome. Genes are organized according to Type, as defined from their genealogy (fig. 2 and supplementary fig. S1, Supplementary Material online). (C) Reproductive phenotypes (CI, cytoplasmic incompatibility; MK, male-killing; P, parthenogenesis; M, mutualism; 0, no reproductive phenotype), Wolbachia supergroups, and hosts in which the Wolbachia was found. The reproductive phenotype is not shown for strains for which conflicting phenotypic data exist (wYak and wSuz).
The Cif Genes Are Widespread in the Genomes of Wolbachia and Related Rickettsiales
To examine the evolution of cif genes, we combined our newly sequenced genomes with published sequences (supplementary table S1, Supplementary Material online). This gave 71 Wolbachia genome sequences, in which we identified 129 and 128 homologs of cifA and cifB, respectively (fig. 1; supplementary table S2, Supplementary Material online). Synteny was highly conserved—in 115 cases (∼89%) cifA was located immediately upstream of cifB (fig. 1). A single cifA homolog and three cifB homologs broke this pattern and were present without their partner. Interestingly, all four of these genes carry mutations that disrupt their ORF suggesting that they are pseudogenes. The remaining genes not found in pairs were located at the end of contigs and/or were partially sequenced, preventing us from drawing conclusions about synteny. As the operon status of these genes is disputed (Beckmann, Bonneau, et al. 2019; Shropshire et al. 2019), we will refer to the 115 syntenic pairs of genes as cifA–cifB genes.
Seventy-three percent (52/71) of Wolbachia strains carry at least one homolog of cifA or cifB (fig. 1), and almost half the Wolbachia genomes carry multiple cifA–cifB homologs (35/71). The largest number was in the strains infecting Diploeciton nevermanni and Gerris buenoi, both of which had seven syntenic cifA–cifB genes (fig. 1). The counts of gene pairs across strains did not differ from a Poisson distribution (Cameron–Trivedi test for Poisson equidispersion on per strain counts of intact syntenic cifA–cifB genes in supergroups A and B: z = 1.38, P = 0.16 [Cameron and Trivedi 1990]).
To examine the distribution of cifA–cifB genes across bacterial strains, we reconstructed the Wolbachia phylogeny using a set of 28 single-copy genes that were present in over 95% of the genomes. The cifA and cifB genes are widespread across the Wolbachia supergroups A and B, which contain most of the genomes analyzed (fig. 1). Six symbiont strains belong to other Wolbachia supergroups, and none of their genomes contained cifA or cifB. However, syntenic cifA–cifB genes were identified in the genomes of Rickettsia gravesii, R. amblyommatis, and Occidentia massiliensis which infect ticks, and in a plasmid found in R. felis strain LSU-Lb, which infects the booklouse Liposcelis bostrychophila (fig. 2 and supplementary fig. S1 and table S2, Supplementary Material online).
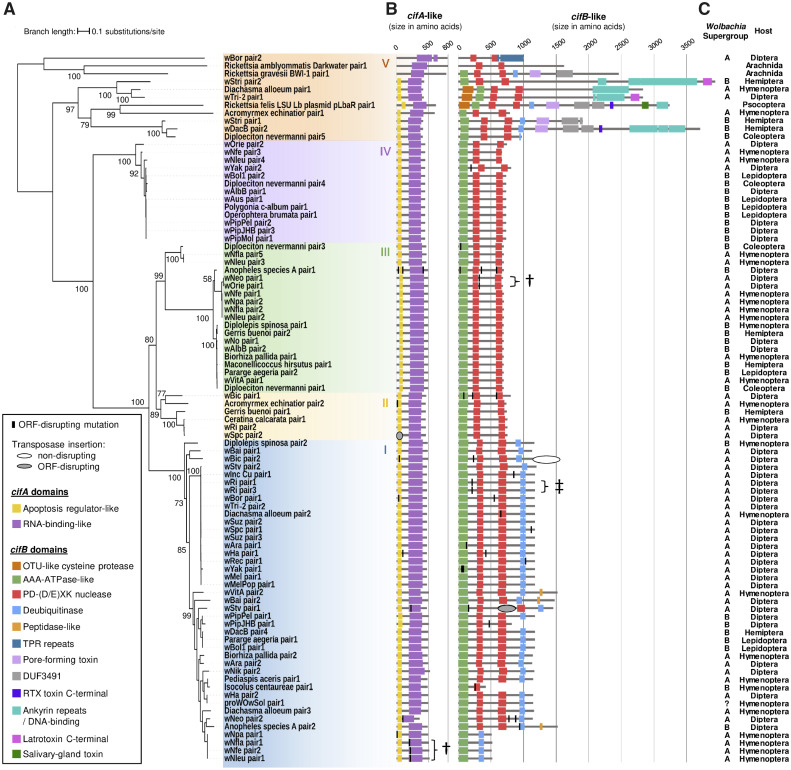
Fig. 2.
Phylogeny and protein domain architecture of cifA and cifB. (A) Maximum likelihood tree of concatenated cifA and cifB nucleotide sequences. Partially sequenced cif homologs were excluded. The tree is midpoint rooted. Bootstrap values were estimated from 1,000 replicates. (B) Protein domains. Mutations that disrupt the ORF are indicated by a vertical bar. Symbols indicate where the same ORF-disrupting mutation is found in two homologs due to speciation (†) or duplication (‡) events. All domains had a HHpred probability of being true positives >75% in at least one sequence. The suffix “-like” at the end of the domain name indicates that there were no sequences where the probability of the domain being true was >95%. Details of the domains are in supplementary table S5, Supplementary Material online. (C) Wolbachia supergroups and arthropod hosts from which the cif sequences were isolated.
The Cif Genes Are Associated with CI
To examine the association between cif genes and phenotype, we compiled a list of the effects that each Wolbachia strain has on host reproduction (fig. 1C, supplementary table S1, Supplementary Material online). We found that there was a significant association between the presence of cifA–cifB genes (without ORF-disrupting mutations) and published reports of a strain inducing CI (table 2; Fisher’s exact test: P < 0.0001). The only case of CI-inducing strain without these genes was wVitB; however, this may be an omission from the genome sequence. Indeed, we found what appeared to be fragments of cifA and cifB on very small contigs in the wVitB genome assembly. Another CI-inducing strain, wRec, carries a syntenic cifA–cifB pair where cifB has a frameshift mutation toward its 3′-end, downstream of predicted protein domains known to have a role in sperm modification (fig. 2, supplementary table S2, Supplementary Material online). Although we counted this as a pseudogene, it is possible that it encodes a functional protein. Finally, wYak induces weak CI (Cooper et al. 2017) and both copies of cifB in the genome contain stop codons (Cooper et al. 2019; fig. 2).
Table 2.
Association between CI and the Presence of cifA–cifB Genes in Wolbachia Genomes.
Wolbachia strains for which there is no phenotypic information available or there are contradictory reports in the literature (wSuz) were discarded.
“Present” stands for cif genes without loss-of-function mutations; partially sequenced cifA–cifB pairs were discarded. wAna and the strain found in Diabrotica virgifera virgifera induce CI but are excluded from the table as they only have partially sequenced cif genes, preventing us from inferring their pseudogenization status.
There were 14 strains that are reported not to induce CI (table 2). Among them, the strain wBor was the only one to carry a pair of cifA–cifB genes without ORF-disrupting mutations, although this strain is only known to induce male-killing. As described below, both of these genes lack domains that are conserved across all CI-inducing strains. Among the other non-CI strains, the genes were absent from two Wolbachia strains that induce parthenogenesis (wTpre and wUni), one strain suspected to induce parthenogenesis (wFol), two strains that do not induce CI or other clear phenotypic effects (wAu and wTro), and five strains thought to be mutualists (wCle in bed bugs and the four strains from nematodes) (fig. 1). Finally, the male-killing strain wBif and two strains with no clear reproductive phenotype (wSpc and wNeo) were only found to carry cif gene pairs with ORF-disrupting mutations. Overall, the strong association between syntenic cif genes and the CI phenotype suggests a single evolutionary origin of this reproductive manipulation in Wolbachia (table 2).
A New and Diverse Group of Cif Genes is Found in Wolbachia and Other Rickettsiales
Reconstruction of the cifA–cifB gene tree revealed new diversity among these genes. Although most sequences fell into four clades that have been named Types I–IV, the genes from two Rickettsia species, a Rickettsia plasmid, O. massiliensis and several Wolbachia strains were basal to these four types on the midpoint rooted tree (fig. 2; supplementary fig. S1, Supplementary Material online). The divergence among these sequences is frequently greater than between Types I–IV, and we are unable to root the phylogeny with high confidence without a reliable outgroup. However, midpoint rooting suggests that this may be a paraphyletic group with the other cif genes nested within it. We propose to call this diverse assemblage of cifA–cifB homologs “Type V.”
We found multiple protein domains, including the cifB nuclease and deubiquitinase domains known to be involved in sperm modification (see below for a detailed description of protein domain conservation). Type V cifB genes tend to be longer and possess a diverse array of domains, suggesting that they may perform a variety of molecular functions (fig. 2; supplementary fig. S1B, Supplementary Material online). Among these genes we identified toxin domains (Latrotoxin, RTX, pore-forming, and salivary-gland toxins), a protease domain (OTU-like cysteine protease), and domains involved in protein–protein interactions (tetratricopeptide and ankyrin repeats). The ankyrin repeat domain also shows strong similarities with DNA-binding proteins (probabilities ∼100% in the HHpred search).
All five cifA–cifB types are associated with CI. Type I genes from wMel and wPip, and Type IV genes from wPip have been experimentally linked to CI (Beckmann et al. 2017; Lepage et al. 2017; Chen et al. 2019). Additionally, we found CI-inducing strains such as wNo and wStri that carry only Type III or Type V genes. Finally, in the CI-inducing strain wRi the only cifA–cifB genes without signs of pseudogenization belong to Type II.
The cifA and cifB Genes Codiverge with Recombination Restricted to Closely Related Genes
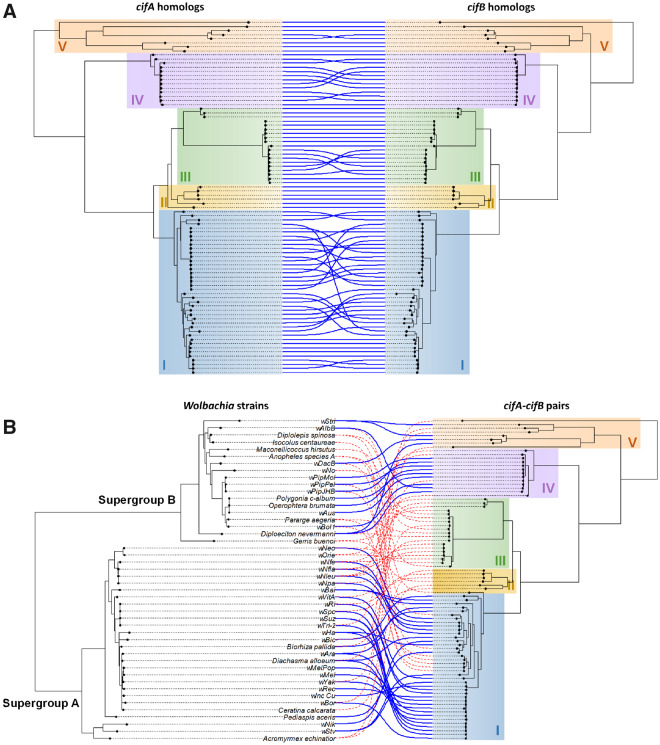
The cifA and cifB proteins bind each other, and in a comparison of two Wolbachia strains the proteins encoded by syntenic pairs of genes bound more strongly than heterologous proteins (Beckmann et al. 2017; Chen et al. 2019). This led to the suggestion that coevolution of binding affinities between the proteins could underlie the divergence of CI crossing types (Beckmann, Bonneau, et al. 2019). Consistent with this and in agreement with earlier studies (Lepage et al. 2017; Lindsey et al. 2018), syntenic cifA and cifB genes show strong phylogenetic congruence (Mantel test P-value < 0.0001; supplementary fig. S2A and B, Supplementary Material online; fig. 3A). Strikingly, there is no case where recombination has brought together cifA and cifB genes from different Types (fig. 3A). Nonetheless, the two trees are not identical. Using multiple approaches to recombination detection on the concatenated alignment of cifA and cifB, we identified 83 well-supported recombination events (supplementary table S4, Supplementary Material online; note that some events may have been counted multiple times). Manual inspection of the sequences frequently revealed clear recombination breakpoints (supplementary fig. S3A–C, Supplementary Material online). However, all but one of these events involved sequences of the same Type (82/83 events; supplementary table S4, Supplementary Material online). This pattern of recombination tending to occur between closely related sequences was strongly supported, as the mean genetic distance between inferred parental sequences was significantly lower than expected by chance (supplementary fig. S4, Supplementary Material online). Manual inspection of the only recombination event involving parental sequences belonging to different types revealed no clear breakpoint in the recombinant sequence, suggesting that this event could be a false-positive created from low-quality alignment between highly divergent homologs (supplementary fig. S3D, Supplementary Material online). Together, these results indicate that cifA and cifB recombination is largely restricted to closely related sequences, perhaps because their binding affinities have coevolved.
Fig. 3.
Phylogenetic congruence between cif genes and Wolbachia strains. (A) Cophylogeny of syntenic cifA and cifB genes. (B) Cophylogeny of Wolbachia genomes and the cifA–cifB genes. Links between phylogenies indicate (A) syntenic genes and (B) Wolbachia strain–cif gene associations, respectively. Blue links have a significant contribution to the global cophylogenetic signal in the Parafit test and red links do not.
A Conserved Protein Domain Architecture Is Associated with CI
To gain insights into the molecular basis of CI and its evolution, we used a comparative approach to identify the protein domains associated with the trait. A complication is that closely related domains may be annotated in some sequences but not others depending on whether they meet an arbitrary significance threshold. To avoid this, we first searched for domains using conventional tools, and then used these sequences to create a Wolbachia-specific Hidden Markov Model (HMM) profile for each domain that was used to repeat the search.
The induction of CI by the Type IV cifB paralog in wPip (cinB) requires DNAse activity due to its PD-(D/E)XK nuclease domains (Chen et al. 2019). However, the Type I cifB paralog in wPip (cidB) has lost the catalytic residues associated with DNase activity and instead CI requires a deubiquitinase domain that functions to deconjugate ubiquitin from proteins (Beckmann et al. 2017; Beckmann, Sharma, et al. 2019). We found that the two PD-(D/E)XK nuclease domains were highly conserved across the cifB tree (fig. 2; supplementary fig. S1B and table S5, Supplementary Material online). As is normally the case, these domains were associated with a 5′ AAA-ATPase domain (Knizewski et al. 2007). The catalytic D–E–K residues were conserved throughout cifB evolution until they were lost in the common ancestor of the Type I genes (supplementary fig. S6, Supplementary Material online, Lindsey et al. 2018; Chen et al. 2019). This coincided with the Type I genes acquiring the deubiquitinase domain, and this domain is conserved across Type I. This suggests that the molecular basis of CI has been conserved across the Type II, III, and IV genes, and then changed (Chen et al. 2019) in the ancestor of the Type I sequences. Interestingly, the deubiquitinase domain is also present in some but not all Type V cifB homologs, suggesting that these might induce CI through a mechanism similar to that of the Type I genes (fig. 2; supplementary fig. S1B and table S5, Supplementary Material online). The sparse distribution of the deubiquitinase domain raises questions about its origin. A phylogenetic reconstruction of the deubiquitinase domain’s amino acid sequence grouped Type I and V deubiquitinase domains as two monophyletic clades (supplementary fig. S7, Supplementary Material online), so the domain does not appear to have been exchanged by recombination. Instead, the deubiquitinase domain must either have been acquired independently by the Type I and V genes or have been present in the common ancestor of the cif genes and subsequently lost multiple times.
The cifA gene has a more conserved domain structure than cifB (fig. 2; supplementary fig. S1A and table S5, Supplementary Material online). The two domains that we identified were annotated as functioning in apoptosis regulation and RNA-binding, but we caution that the support for these annotations being true positives was <85%. There are six cifA genes that have lost the apoptosis regulator-like domain (including two putative pseudogenes), and none of these is known to induce CI (supplementary fig. S1A, Supplementary Material online). If some of these strains were found to induce CI, it would be of interest to test whether they can also rescue this effect, to assess whether this domain is required for the rescue activity of cifA proteins.
The Cif Genes Frequently Transfer between Distantly Related Wolbachia Genomes and Insect Hosts
The cif genes are often found in the vicinity of prophage genes, a cluster of genes known to undergo intense genomic rearrangements and horizontal transfer between Wolbachia genomes (Bordenstein and Wernegreen 2004). The link between cif genes and mobile genetic elements is reinforced by the presence of the genes on a plasmid found in Rickettsia. There are numerous cases of the genes being exchanged between distantly related bacterial hosts, including multiple instances of transfer between the Wolbachia supergroups A and B (fig. 3B). This suggests that horizontal transfer of cif genes between distantly related symbionts is common, sometimes even crossing the bacterial genus boundaries as exemplified by the presence of cifA–cifB homologs in Rickettsia genomes and plasmids (fig. 2; supplementary fig. S1, Supplementary Material online). Nonetheless, there is partial congruence between the phylogenies of Wolbachia and their cif genes (Mantel test P-value < 0.0001; fig. 3B;supplementary fig. S2C and D, Supplementary Material online). This could result from cif genes being maintained for long enough to be coinherited with the Wolbachia genome during speciation events or the genes frequently transferring between closely related bacterial strains.
As the cifA and cifB proteins interact with targets in the arthropod host, their distribution may be constrained by the arthropod phylogeny rather than the Wolbachia phylogeny. Despite the cifA and cifB genes frequently transferring between distantly related arthropods, there is a highly significant tendency for closely related cif genes to be found in the same host order (Mantel test comparing cifA–cifB pairwise distances and insect orders: P-value < 0.0001; supplementary fig. S2E and F, Supplementary Material online). However, we would caution that this association could be the result of the Wolbachia phylogeny being correlated with the arthropod phylogeny (Mantel test comparing Wolbachia pairwise distances and insect orders: P-value < 0.0001; supplementary fig. S2G and H, Supplementary Material online) and these effects are difficult to disentangle.
Pseudogenes Are Common and the Loss of Sperm Modification Usually Predates the Loss of the Rescue
In randomly mating populations there is no selection for CI-inducing Wolbachia to modify sperm (Turelli 1994), so a gene whose only function is sperm modification (cifB) is predicted to eventually lose its function. Once this occurs there is no selection to maintain the rescue function, so cifA can also be lost. Excluding partially sequenced genes, putative loss-of-function mutations were found in 12.1% of cifA sequences (15/124) and 27.8% of cifB sequences (27/97), suggesting that the modification of sperm function is lost more frequently than the rescue function (supplementary fig. S1A and B, Supplementary Material online; Fisher’s exact test: P = 0.02). The fixation rate of loss-of-function mutations per site was not significantly different between the two genes (cifA: 2.4 × 10−4 mutations/amino acid; cifB: 3 × 10−4 mutations/amino acid; Fisher’s exact test: P = 0.64).
To examine the order in which cifA and cifB become pseudogenes, we visually inspected the 87 fully sequenced cifA–cifB pairs. We identified 38 independent mutational events that cause the ORF to be disrupted (i.e., not double-counting mutations inherited through speciation or duplication events; fig. 2). These putative loss-of-function mutations include single-base pair substitutions introducing premature stop codons, indels producing frameshifts, insertion of transposable elements (wStv pair 1), and short inversions (wYak pair 1) (supplementary table S2, Supplementary Material online). The majority of cifA–cifB gene pairs shows no signs of pseudogenization (63/87), whereas six carry ORF-disrupting mutations in both genes. In cases where just one gene carried a mutation, it was more often cifB than cifA (14 vs. 4 instances; fig. 2; binomial test: P = 0.03). This suggests that sperm modification function is usually being lost before the rescue function. Interestingly, in two cases where only cifA appears to be pseudogenized (Wolbachia in Nomada bees), the cifB genes are ∼50% shorter than their close relatives and lack the nuclease domains conserved in all other cifB genes (fig. 2). Therefore, these cifB genes may be nonfunctional despite the absence of ORF-disrupting mutations. In a third case, cifA is pseudogenized in wSpc, a strain that does not modify sperm in its host D. subpulchrella despite carrying an intact cifB gene, meaning that there should be no selection acting to maintain the rescue function in this strain (fig. 1).
Once the CI genes lose their function, it has been predicted that the Wolbachia infection will also be lost from the population. This should happen unless other phenotypes such as the provision of fitness benefits to the host are maintaining the symbiont, or the Wolbachia genomes harbor additional cif genes that are still functional. If the infection is maintained, nonfunctional CI genes may accumulate further loss-of-function mutations or be eliminated from the Wolbachia genomes, for instance, by excision of the prophage harboring the CI genes. By looking at the phylogenetic distribution of putative loss-of-function mutations, it is possible to infer whether nonfunctional cif genes slowly degenerate or are quickly eliminated. The majority of loss-of function mutations is located on the terminal branches of the CI gene phylogenies (fig. 2; supplementary fig. S1A and B, Supplementary Material online). The only exceptions are a few mutations coinherited between closely related Wolbachia strains (wNeo/wOrie; Wolbachia from Nomada bees) or through a putative duplication in the wRi genome (pairs 1 and 3 are identical). This suggests that pseudogenized cif genes are rarely horizontally transmitted between Wolbachia genomes or maintained for long enough to be inherited through speciation events.
Closely Related Cif Genes Commonly Coexist in the Same Genome
CI favors female hosts that have the greatest reproductive compatibility with males in the population. Therefore, Wolbachia strains that induce a new CI crossing type can invade Wolbachia-infected populations provided they are compatible with the resident strain (Charlat et al. 2001). Such a mutant could arise if Wolbachia acquires an additional pair of cif genes, and this may explain why Wolbachia genomes commonly harbor multiple cif paralogs. However, additional cif genes should only spread if they induce a different crossing type from the genes already present in the genome. If closely related genes confer the same crossing type, then this would mean that closely related cif homologs are unlikely to be found within the same genome. However, we found no support for this as in our data set—putatively functional cif homologs in the same genome have very similar levels of divergence to cif homologs found in different genomes (supplementary fig. S5, Supplementary Material online). A similar argument applies to the loss of cif genes—if a genome contains paralogs that induce the same crossing type then one of the paralogs could be lost by mutation. However, again there is no evidence for this process. Looking within genomes, the genetic distance between intact cif genes and putative pseudogenes was similar to the genetic distance between pairs of functional genes (genetic distance calculated from fig. 2: 1.15 and 1.28, respectively).
Discussion
CI is the most commonly observed reproductive manipulation induced by Wolbachia, and its evolution has been investigated for over 60 years through phenotypic experiments (Laven 1957; Sinkins et al. 1995; Charlat et al. 2003; Zabalou et al. 2008) and evolutionary models (Caspari and Watson 1959; Turelli 1994; Hurst and McVean 1996; Vautrin et al. 2007). The discovery of the genes underlying CI now makes it possible to reconstruct the trait’s evolution at the molecular level and infer the selection pressures acting on CI using the tools of molecular evolution. Here we analyzed 71 Wolbachia genome sequences to investigate the importance of recombination, pseudogenization, and horizontal gene transfer in the evolution of CI.
The cif genes are widespread across the Rickettsiales. Our large data set supported the observation of Lindsey et al. (2018) that cif genes are common in Wolbachia supergroups A and B. These supergroups contain most of the Wolbachia strains that have been described, and here the genes are tightly linked to the CI phenotype. The genes were absent from a small sample of Wolbachia strains from other supergroups. This may explain why, to our knowledge, there are no reports of strains from these supergroups inducing CI. However, divergent Type V cif genes are found in other Rickettsiales, and here their phenotypic effects are uncertain (Gillespie et al. 2018). Gillespie et al. (2018) proposed that cif homologs found in Rickettsia may induce other reproductive phenotypes. For instance, R. felis strain LSU-Lb, which carries Type V cif genes on its plasmid, infects a parthenogenetic insect. Accurate rooting of the cif gene tree together with functional characterization of these genes will be needed to confirm whether the ancestral function of these genes was to induce CI. However, the presence of a CI-inducing Wolbachia strain that has only Type V genes suggests that CI evolved very early in the evolution of cif genes.
It has long been observed that crosses between insects carrying different Wolbachia strains are frequently incompatible, suggesting that modification and rescue factors must be matched to produce viable offspring. A possible molecular cause of this phenomenon comes from the observation that cifA and cifB bind each other (Beckmann et al. 2017; Chen et al. 2019), and so binding affinities may be greatest between coevolved genes. If this is the case, then there would be strong selection against recombination between the genes as this could generate symbionts that are unable to rescue crosses with infected females (Charlat et al. 2001). Furthermore, even if the symbiont retained the ability to rescue the cross, for example, if genes are swapped among paralogous pairs within the genome, the pair of genes might be eliminated in the long term if it generated self-incompatibility when transferred to a new genome. Despite this, clear evidence of recombination has been observed among cif genes from wPip, which infects C. pipiens mosquitoes (Bonneau et al. 2018). wPip strains can carry multiple copies of Type I cif genes, and recombination between closely related paralogs correlates with different CI crossing types. Whether recombination itself created these incompatibilities or whether crossing types arose due to sequence divergence following recombination is unknown. We found that recombination is frequent across the cif gene phylogeny, but it almost exclusively occurs between related syntenic cifA–cifB gene pairs within the same Type. As Wolbachia genomes often carry divergent cif homologs, the absence of recombination between these genes is compatible with recombination being constrained by selection. This can be explained by the coevolution of binding affinities between the proteins encoded by cifA and their cognate cifB gene.
The evolution of sperm modification by Wolbachia poses an evolutionary puzzle—the trait is only expressed in males and yet symbionts in males are not passed on to the next generation. In randomly mating populations, it will be at best selectively neutral (Prout 1994; Turelli 1994). Although kin selection can act to maintain CI in structured populations (Frank 1997), this is a weak force that operates only under specific circumstances (Haygood and Turelli 2009). Therefore, theory predicts that a gene involved exclusively in sperm modification, such as cifB, will accumulate loss-of-function mutations. Once these have been fixed within a population, the gene required for the rescue function (cifA) will then degenerate by mutation. It has already been reported that cif genes often carry putative loss-of-function mutations (Asselin et al. 2018; Lindsey et al. 2018; Cooper et al. 2019; Meany et al. 2019). We found cifB carries loss-of-function mutations more often than cifA, and that cifA generally acquires such mutations after cifB. This confirms a key prediction of theory and supports the hypothesis that lack of selection to maintain sperm modification may lead to the loss of CI in some populations (Turelli 1994; Hurst and McVean 1996). Although evolutionary predictions were based on a mechanistic model that assumes sperm modification and rescue were encoded by different genes, the same pattern of gene loss would be expected if both cifA and cifB are required to modify sperm (Shropshire and Bordenstein 2019). This is because a mutant that lost cifA, and therefore the ability to both induce and rescue CI, would initially be rare in a population of CI-inducing symbionts and should be counterselected. By contrast, mutating cifB will only cause the loss of sperm modification and will therefore not be exposed to selection. Therefore, even in the two-by-one mechanistic model, the spread of a symbiont carrying a pseudogenized cifA and a functional cifB is unlikely. Nonetheless, how the two-by-one model affects details of the dynamics, such as the effects of population structure, remains to be explored theoretically.
The degeneration of cif genes could also occur if the CI phenotype is lost first, for instance, due to the host evolving to suppress the trait (Koehncke et al. 2009), leaving the genes free to degenerate by mutation. This is plausible as artificial transfers of Wolbachia between species have shown that host genetic background can affect the expression of CI (Poinsot et al. 1998; Jaenike 2007; Zabalou et al. 2008). As this model makes no predictions about the order of gene loss, it may explain why we found at least one Wolbachia strain that does not induce any reproductive incompatibility and whose genome contains a cifA pseudogene alongside an intact cifB. As cifB is typically longer than cifA, even under this model of evolution cifB may tend to acquire loss-of-function mutations first. Indeed, the number of loss-of-function mutations per amino acid is similar between cifA and cifB, which is compatible with the two genes evolving under similar selective pressures. However, this is a rather weak test as after a loss-of-function mutation in cifB, under any evolutionary model both genes are free to degenerate at the same rate. Therefore, to separate these models it is necessary to know whether strains carrying cifB pseudogenes alongside functional cifA genes are found in hosts that can express CI. An example is wRi. This strain induces CI, and its genome contains an intact pair of cif genes alongside two distantly related pairs of genes where cifB is a pseudogene. Another apparent example of this is wYak, which has two copies of cifB in its genome, both of which contain internal stop codons (fig. 1; Cooper et al. 2019). When its natural host D. yakuba was experimentally infected with a different Wolbachia strain, it induced strong CI (Zabalou et al. 2004). However, the recent discovery that wYak itself induces weak CI means that it is unclear whether the mutations in the two cifB genes reduced the ability of wYak to modify sperm (Cooper et al. 2017). Together these examples provide tentative support for the model that sperm modification may degenerate by mutation, even in hosts expressing CI.
In the absence of other phenotypic effects on the host, theory predicts that the loss-of-sperm modification will lead to the loss of Wolbachia from the host population (Turelli 1994; Hurst and McVean 1996). We found that the majority of loss-of-function mutations appears to have occurred recently. Firstly, these mutations tend to be on terminal branches of the gene tree. Secondly, pseudogenes rarely accumulate many loss-of-function mutations. Although this suggests that cifA and cifB pseudogenes rarely codiverge with the Wolbachia genome for long periods, it is unclear whether this results from the loss of the Wolbachia infection or through the genes being deleted from the genome, for instance, by phage excision. Since the publication of theoretical studies on CI evolution (Turelli 1994; Hurst and McVean 1996), it has become increasingly clear that many Wolbachia strains can persist in host populations without inducing any reproductive manipulation by providing fitness benefits (Dedeine et al. 2001; Taylor et al. 2005; Hosokawa et al. 2010; Kriesner et al. 2013; Martinez et al. 2014; Kriesner and Hoffmann 2018). Therefore, it remains to be demonstrated whether the degeneration of cif genes causes the loss of Wolbachia from populations.
The frequent loss of cif genes raises a paradox when trying to explain the high prevalence of CI across Wolbachia strains. Hurst and McVean (1996) argued that CI-inducing Wolbachia infections were more likely to transfer horizontally between host species, so symbiont strains that induce CI are more likely to persist over an evolutionary timescale. Consistent with this process of clade selection, Wolbachia frequently jumps between host species (Zhou et al. 1998; Vavre et al. 1999; Baldo et al. 2008). Our observations suggest that clade selection may also be acting at the level of the cif genes as Wolbachia genomes appear to be frequently recolonized by cif genes from other symbionts. The pervasive horizontal transfer of cif genes may allow them to evade inevitable extinction within symbiont lineages by escaping into a new symbiont population. This process is analogous to the evolution of transposable elements, which frequently go extinct within host species, but persist long term by jumping into new species (Schaack et al. 2010).
Horizontal transfer of cif genes occurs frequently and sometimes over large phylogenetic distances, sometimes even crossing the bacterial genus boundaries. High rates of horizontal transfer likely result from the cif genes being associated with mobile genetic elements, such as WO prophage sequences, transposons, and plasmids (Gillespie et al. 2018; Lindsey et al. 2018; Cooper et al. 2019). Interestingly, Lepage et al. (2017) found no significant association between the phylogenies of cif genes and genes found in the structural module of phages. Prophage regions often rearrange (Bordenstein and Wernegreen 2004) which likely explains this pattern. However, this does not mean that phages are not a major route of horizontal transmission of cif genes, and the association between cif genes and mobile genetic elements supports the idea that the ability to move horizontally between genomes may be an important adaptation of these elements. Indeed, cif genes that lose their association with mobile elements may ultimately go extinct as they would be less likely to undergo horizontal transfer. As argued by Beckmann, Bonneau, et al. (2019), in some cases CI may be best viewed as an adaptation of mobile genetic elements to spread within Wolbachia populations.
Wolbachia genomes often carry multiple cifA–cifB gene pairs. This is analogous to the frequent occurrence of multiple Wolbachia strains within the same host individuals. Both experimental and theoretical studies have demonstrated that such multiple infections can invade host populations provided the strains carry different modification and rescue factors (Sinkins et al. 1995; Rousset et al. 1999). This is because females harboring additional Wolbachia strains will be compatible with all males in the population. An equivalent process will promote the invasion and maintenance of cif paralogs within the same genome, provided that they encode bidirectionally incompatible modification and rescue factors. If bidirectionally incompatibility evolves gradually, this hypothesis predicts that paralogous cif genes within a genome might be distantly related. However, we found no evidence for this, and frequently paralogs within the same genome are closely related. We would argue that this does not mean that we should discount the hypothesis that cif paralogs accumulate within the same genome because they encode bidirectionally incompatible CI factors. In particular, our analysis assumes that genetic divergence can be used as a proxy for the divergence of crossing types and this may not be the case. For example, the evolution of new crossing types could occur following cif gene duplication events (Beckmann, Bonneau, et al. 2019), meaning that a single genome could harbor closely related paralogs that encode incompatible crossing types, as observed in the wPip-Culex system described above (Bonneau et al. 2018). Although sequence divergence following duplication is thought to have led to new compatibility types (Beckmann, Bonneau, et al. 2019), an open question is whether carrying multiple identical copies of a cif gene pair might be sufficient to produce new crossing types, perhaps by “dose effects.”
There is both direct and indirect evidence that homologs from all the main cif gene types can induce CI, and analysis of protein domains suggests that the molecular basis of CI is conserved. The cifA domain structure varies little across the gene family. All cifB genes associated with CI had one of the domains that has been experimentally linked to CI—a functional PD-(D/E)XK nuclease domain or a deubiquitinase domain (Beckmann et al. 2017; Chen et al. 2019). As previously reported, Type I cifB genes lack the catalytic residues in their PD-(D/E)XK nuclease domains, and instead have the deubiquitinase domain (Beckmann et al. 2017). Unexpectedly, this domain is also present in some divergent Type V sequences, making its evolutionary origins unclear. Alongside these conserved core domains, we found a diverse range of other cifB domains, notably in the long Type V genes. It is of interest whether these additional cifB domains are associated with CI. Given the relative homogeneity of cifA domains and the similarities of these additional cifB domains with known toxins and eukaryotic-like ankyrin proteins, it is tempting to hypothesize that they are linked to the modification function by interacting with the host rather than the binding to cifA. It would be interesting to test whether these domains allow manipulation of reproduction across a broader host range. Interestingly, one of these domains, the ovarian tumor domain (OTU), is also found in a toxin involved in male-killing induced by the symbiont Spiroplasma poulsonii, raising the possibility that some domains could be involved in multiple forms of reproductive manipulations.
In conclusion, our study illustrates the dynamic evolution of CI genes and highlights the high rates of gene loss and horizontal gene transfer. Further functional analysis will open new avenues of research and allow us to reconstruct the full evolutionary history of CI. In particular, the identification of the insect factors targeted by the sperm modification may soon allow us to study the coevolution of cif genes with the insect reproductive system (Beckmann, Sharma, et al. 2019). Finally, a deeper analysis of divergent cif homologs found outside Wolbachia should allow us to address questions around the deep evolutionary origin of CI and may reveal novel functions of these genes.
Materials and Methods
Sequencing of New Wolbachia Genomes
New Wolbachia genomes were obtained from 11 Drosophila species (listed in table 1) using the protocol described in Ellegaard et al. (2013). Briefly, Wolbachia cells were purified from 20 to 30 fly embryos that were dechorionated in bleach and homogenized in phosphate-buffered saline. The homogenate was then centrifuged, passed through 5- and 2.7-µm pore size filters and multiple-displacement amplification was performed directly on the bacterial pellet using the Repli-g midi kit (Qiagen) as in Ellegaard et al. (2013). The amplified DNA was finally cleaned using the QIAamp DNA mini kit prior to sequencing. From each DNA sample, 3-kb paired-end and 50-bp paired-end DNA libraries were prepared. These were multiplexed and sequenced on one plate of 454 Roche FLX (University of Cambridge, Department of Biochemistry, United Kingdom) and one lane of Illumina HiSeq2000 instruments (The Genome Analysis Centre, Norwich, United Kingdom), respectively.
454 and Illumina reads were used to perform hybrid de novo assemblies in Newbler v2.6 (454 Life Sciences Corp., Roche, Branford, CT). Non-Wolbachia contigs were then removed from each assembly by aligning the contigs to the wMel reference genome (GenBank accession number: NC_002978.6) using Mauve v2.3.1 (Darling et al. 2004) and visual comparisons in the Artemis Comparison Tool (Carver et al. 2005). A BlastN analysis of the discarded contigs revealed positive matches with Drosophila mitochondrial and nuclear genomes, as well as with Saccharomyces cerevisiae (yeast used to collect the fly embryos), suggesting low levels of contamination during the DNA extraction process. Scaffolding was refined using SSPACE v2 (Boetzer et al. 2011) and gaps were filled with Gapfiller v1.11 (Boetzer and Pirovano 2012). Additionally, for strains wStv, wAra, and wBor, Illumina reads were assembled separately using Abyss v1.3.5 (Simpson et al. 2009) and the contigs generated as well as the Illumina reads were mapped onto the hybrid assemblies using Consed (Gordon et al. 1998) in order to manually edit the scaffolds. Final assemblies were annotated as in Ellegaard et al. (2013) using a custom pipeline. In brief, gene and pseudogene predictions were performed using Prodigal (Hyatt et al. 2010) and GenePrimp (Pati et al. 2010), respectively. Domain prediction was done using hmmsearch implemented in pfam_scan.pl with the PFAM database (Bateman et al. 2002). Finally, annotations were manually edited through visual inspection in Artemis (Carver et al. 2012). The completeness of the assemblies was assessed using BUSCO v3 by searching the genomes against the near-universal, single-copy genes of the proteobacteria database (Simão et al. 2015).
BLAST Search of CI Genes and Annotation
Previously identified cif gene homologs have been categorized into four phylogenetic clusters denominated as Type I–IV, as well as an uncharacterized type of more divergent homologs found in the Wolbachia strain wStri (Lindsey et al. 2018). The presence of cif gene homologs in publicly available and newly sequenced Wolbachia genomes (supplementary table S1, Supplementary Material online) was searched with TBlastN (https://blast.ncbi.nlm.nih.gov/Blast.cgi; last accessed August 20, 2020) using cifA and cifB amino acid sequences representative of each CI type as queries (supplementary table S2, Supplementary Material online). Some of the genomes assembled in Pascar and Chandler (2018) were excluded from our analysis as they showed signs of multiple infections based on the assembly size, the sequencing coverage, and/or the presence of duplicated BUSCO reference genes. Additionally, when more than one genome were sequenced from the same host species and we found no evidence in the literature that they correspond to different Wolbachia strains, only one of them was included in the analysis. Default parameters and an e-value threshold of 0.05 were used. TBlastN hits across the Wolbachia genomes were then visually inspected in Artemis. Hits that were at least 40% of the length of the smallest query sequence and/or hits displaying the typical cifA–cifB synteny were considered as positive matches. Where sequences did not span the entirety of an ORF, they were manually extended to include the closest start and stop codons. The presence of stop codons and frameshifts within the reannotated sequences were interpreted as indicative of a putative pseudogenization event. DNA sequences were aligned with Clustal Omega using default parameters (https://www.ebi.ac.uk/Tools/msa/clustalo/; last accessed August 20, 2020), and putative “loss-of-function” mutations were examined by comparing closely related sequences and were defined as unique mutational events or, when found in more than one homolog, as coinherited.
Using BlastP online tool (https://blast.ncbi.nlm.nih.gov/Blast.cgi; last accessed August 20, 2020) with cifAwMel and cifBwMel amino acid sequences and the more divergent sequences found in wStri as queries, we also found additional homologs in prophage WOSol (AGK87106 and AGK87078) and in non-Wolbachia taxa (Rickettsial plasmid genes pLbAR_36/38: WP_039595309.1/WP_081996388.1; R. gravesii: NZ_AWXL00000000.1; R. amblyommatis: GCA_000964995.1; O. massiliensis: CANJ01000001). These cif homologs were searched again and manually annotated as above from the original nucleotide sequences present in GenBank (https://www.ncbi.nlm.nih.gov/genbank/; last accessed August 20, 2020).
CI Genes Phylogeny and Recombination
Following the manual reannotation, cifA and cifB DNA sequences were aligned separately based on their amino acid translations using TranslatorX (Abascal et al. 2010). The alignment program MAFFT implemented in the TranslatorX pipeline was used along with GBlocks in order to filter out weakly conserved regions from the alignment. The same was done for concatenated cifA and cifB sequences. PhyML v3.0 (Guindon et al. 2010) was used with the GTR GAMMA substitution model of evolution and 1,000 bootstrap replicates. All phylogenies were first reconstructed with all sequences, including partial ones (located at the end of genome contigs or showing bases called as Ns within their ORF) to classify the genes into phylogenetic types. Phylogenies were then rerun without partial sequences for the remaining analyses.
Recombination was analyzed using the recombination detection program RDP4 (Martin et al. 2015). The alignment of the concatenated cifA and cifB linear sequences was used to detect putative recombination events with the default parameters of the six methods implemented in RDP4 (RDP, GENECONV, Bootscan, MaxChi, Chimaera, and SiScan). Recombination events with a phylogenetic support and a Bonferroni-corrected P-value <0.05 with at least four of the detection methods were interpreted as reliable evidence of recombination. Out of 83 significant events, 22 were randomly selected and visually inspected to ensure that they were genuine. This was done by realigning the amino acid sequences of the putative recombinant and the two inferred parents.
Prediction of Functional Domains
Protein domains were predicted for fully sequenced cifA and cifB homologs using the HHpred webserver (https://toolkit.tuebingen.mpg.de/#/tools/hhpred/ (last accessed August 20, 2020); Söding et al. 2005) with defaults parameters as in Lindsey et al. (2018). Premature stop codons were first removed manually from putative pseudogenes and DNA sequences translated into amino acid sequences. Amino acid sequences were then queried individually against the following databases: SCOPe70 (v.2.07), Pfam (v.32.0), SMART (v6.0), and COG/KOG (v1.0). Only hits with probabilities >75% in at least one cif homolog were considered as putative functional domains. In many cases, predicted domains on one sequence were not detected by HHpred on closely related homologs, although their presence could be suspected by visual inspection of the sequence alignments. In order to refine our domain search, we used representative amino acid sequences of each domain to build HMM profiles using hmmbuild implemented in HHMMER v3.2.1 (Eddy 2011). Representative domain selection was conducted by choosing domains distributed across the CI gene phylogenies, encompassing the maximum genetic divergence between reference homologs. Basically, one domain copy per phylogenetic type as well as all copies from non-Wolbachia taxa were chosen as references where available. When domains were missing from some types, a maximum of five domain copies were selected across the different types or all copies if there were fewer than five copies in total.
All CI homologs were then scanned using hmmscan and the HMM profile created from each functional domain with an e-value inclusion threshold of 0.0001. The amino acid sequences of hmmscan hits were extracted and reused to build new HMM profiles that were used to scan the CI genes again. Three search iterations were performed in this way which allowed us to retrieve many domains that HHpred failed to detect. Finally, domain coordinates were extracted and, in the case of putative pseudogenes, they were manually edited to take into account the presence of loss-of-function mutations in the original DNA sequences.
Wolbachia Strain Phylogeny
Twenty-eight single copy genes that were present in >95% of the bacterial strains were identified using Phylosift v1.0.1 (Darling et al. 2014) (supplementary table S3, Supplementary Material online). The genomes of Anaplasma marginale (NC_012026.1) and Ehrlichia muris (NC_023063.1) were included as outgroups. For each genome, DNA sequences were concatenated and aligned with MAFFT v7. A bacterial phylogeny was then built with PhyML v3.0 using the GTR GAMMA substitution model with 1,000 bootstrap replicates.
Data Visualization and Statistical Analysis
The visualization of phylogenies and their related information (host taxonomy, bacterial strain, Wolbachia supergroup, protein domains) was done using the online tool iTOL v4.3.3 (Letunic and Bork 2007, https://itol.embl.de/; last accessed August 20, 2020). All statistical analyses were performed in the R software (R Core Team 2013). The congruence between the phylogenies of cifA and cifB homologs as well as their concatenation, and their respective Wolbachia strains were tested using Mantel tests on the pairwise patristic distances between sequences (1,000 permutations). Additionally, ParaFit with 9,999 permutations implemented in CopyCat v2.0 (Meier-Kolthoff et al. 2007) was used to examine cophylogenetic signals between trees and visualize the contribution of individual links between them. Finally, we compared the observed mean phylogenetic distances between cif gene pairs occurring within the same genome to a random distribution of mean distances generated by randomly permuting cif genes between genomes (1,000 permutations).
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Supplementary Material
Acknowledgments
We thank Professor John Jaenike, Professor Greg Hurst, and Dr Bryant McAllister for providing some of the Drosophila lines used for sequencing. This work was supported by the Wellcome Trust (Grant Nos. WT094664MA and WT202888/Z/16/Z) and the European Research Council (Grant No. 281668, DrosophilaInfection). Raw sequencing reads are available at the Sequence Read Archive (Bioproject PRJNA603900), and the genome assemblies at GenBank (CP050530, CP050531, JAATKZ000000000, JAATLA000000000, JAATLB000000000, JAATLC000000000, JAATLD000000000, JAATLE000000000, JAATLF000000000, JAATLG000000000, JAATLH000000000).
References
- Abascal F, Zardoya R, Telford MJ.. 2010. TranslatorX: multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 38(Suppl 2):W7–W13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin AK, Villegas-Ospina S, Hoffmann AA, Brownlie JC, Johnson KN.. 2018. Contrasting patterns of virus protection and functional incompatibility genes in two conspecific Wolbachia strains from Drosophila pandora. Appl Environ Microbiol. 85:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atyame CM, Labbé P, Dumas E, Milesi P, Charlat S, Fort P, Weill M.. 2014. Wolbachia divergence and the evolution of cytoplasmic incompatibility in Culex pipiens. PLoS One 9(1):e87336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo L, Ayoub NA, Hayashi CY, Russell JA, Stahlhut JK, Werren JH.. 2008. Insight into the routes of Wolbachia invasion: high levels of horizontal transfer in the spider genus Agelenopsis revealed by Wolbachia strain and mitochondrial DNA diversity. Mol Ecol. 17(2):557–569. [DOI] [PubMed] [Google Scholar]
- Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer ELL.. 2002. The Pfam protein families database. Nucleic Acids Res. 30(1):276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann J, Ronau J, Hochstrasser M.. 2017. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat Microbiol. 2:17007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JF, Bonneau M, Chen H, Hochstrasser M, Poinsot D, Merçot H, Weill M, Sicard M, Charlat S.. 2019. The toxin–antidote model of cytoplasmic incompatibility: genetics and evolutionary implications. Trends Genet. 35(3):175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JF, Fallon AM.. 2013. Detection of the Wolbachia protein WPIP0282 in mosquito spermathecae: implications for cytoplasmic incompatibility. Insect Biochem Mol Biol. 43(9):867–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JF, Sharma GD, Mendez L, Chen H, Hochstrasser M.. 2019. Wolbachia cytoplasmic incompatibility enzyme CidB targets nuclear import and protamine-histone exchange factors. Elife 8:e50026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W.. 2011. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics 27(4):578–579. [DOI] [PubMed] [Google Scholar]
- Boetzer M, Pirovano W.. 2012. Toward almost closed genomes with GapFiller. Genome Biol. 13(6):R56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau M, Atyame C, Beji M, Justy F, Cohen-gonsaud M, Sicard M, Weill M.. 2018. Culex pipiens crossing type diversity is governed by an amplified and polymorphic operon in Wolbachia genome. Nat Commun. 9(1):319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein SR, O’Hara FP, Werren JH.. 2001. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 409(6821):707–710. [DOI] [PubMed] [Google Scholar]
- Bordenstein SR, Wernegreen JJ.. 2004. Bacteriophage flux in endosymbionts (Wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol. 21(10):1981–1991. [DOI] [PubMed] [Google Scholar]
- Callaini G, Dallai R, Riparbelli MG.. 1997. Wolbachia-induced delay of paternal chromatin condensation does not prevent maternal chromosomes from entering anaphase in incompatible crosses of Drosophila simulans. J Cell Sci. 110:271–280. [DOI] [PubMed] [Google Scholar]
- Cameron AC, Trivedi PK.. 1990. Regression-based tests for overdispersion in the Poisson model. J Econom. 46(3):347–364. [Google Scholar]
- Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA.. 2012. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28(4):464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver TJ, Rutherford KM, Berriman M, Rajandream M-A, Barrell BG, Parkhill J.. 2005. ACT: the Artemis comparison tool. Bioinformatics 21(16):3422–3423. [DOI] [PubMed] [Google Scholar]
- Caspari E, Watson GS.. 1959. On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution 13(4):568–570. [Google Scholar]
- Charlat S, Calmet C, Merçot H.. 2001. On the mod resc model and the evolution of Wolbachia compatibility types. Genetics 159(4):1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlat S, Le Chat L, Merçot H.. 2003. Characterization of non-cytoplasmic incompatibility inducing Wolbachia in two continental African populations of Drosophila simulans. Heredity 90(1):49–55. [DOI] [PubMed] [Google Scholar]
- Chen H, Ronau JA, Beckmann JF, Hochstrasser M.. 2019. A Wolbachia nuclease and its binding partner comprise a novel mechanism for induction of cytoplasmic incompatibility. Proc Natl Acad Sci U S A. 116(44):22314–22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BS, Ginsberg PS, Turelli M, Matute DR.. 2017. Wolbachia in the Drosophila yakuba complex: pervasive frequency variation and weak cytoplasmic incompatibility, but no apparent effect on reproductive isolation. Genetics 205(1):333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper BS, Vanderpool D, Conner WR, Matute DR, Turelli M.. 2019. Wolbachia acquisition by Drosophila yakuba-clade hosts and transfer of incompatibility loci between distantly related Wolbachia. Genetics 212(4):1399–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A, Mau B, Blattner F, Perna N.. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14(7):1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AE, Jospin G, Lowe E, Matsen IF, Bik HM, Eisen JA.. 2014. PhyloSift: phylogenetic analysis of genomes and metagenomes. PeerJ 2:e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeine F, , Vavre F, , Fleury F, , Loppin B, , Hochberg ME, , Bouletreau M. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci. 98(11):6247–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol. 7(10):e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegaard KM, Klasson L, Näslund K, Bourtzis K, Andersson SGE.. 2013. Comparative genomics of Wolbachia and the bacterial species concept. PLoS Genet. 9(4):e1003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA. 1997. Cytoplasmic incompatibility and population structure. J Theor Biol. 184(3):327–330. [DOI] [PubMed] [Google Scholar]
- Frank SA. 1998. Dynamics of cytoplasmic incompatibility with multiple Wolbachia infections. J Theor Biol. 192(2):213–218. [DOI] [PubMed] [Google Scholar]
- Gillespie JJ, Driscoll TP, Verhoeve VI, Rahman MS, Kevin R, Azad AF.. 2018. A tangled web: origins of reproductive parasitism. Genome Biol Evol. 10(9):2292–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Abajian C, Green P.. 1998. Consed: a graphical tool for sequence finishing. Genome Res. 8(3):195–202. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O.. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 59(3):307–321. [DOI] [PubMed] [Google Scholar]
- Haygood R, Turelli M.. 2009. Evolution of incompatibility-inducing microbes in subdivided host populations. Evolution 63(2):432–447. [DOI] [PubMed] [Google Scholar]
- Hosokawa T, , Koga R, , Kikuchi Y, , Meng X-Y, , Fukatsu T. 2010. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 107(2):769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst L, McVean GT.. 1996. Clade selection, reversible evolution and the persistence of selfish elements: the evolutionary dynamics of cytoplasmic incompatibility. Proc R Soc Lond B Biol Sci. 263:97–104. [Google Scholar]
- Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, Hauser LJ.. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J. 2007. Spontaneous emergence of a new Wolbachia phenotype. Evolution 61(9):2244–2252. [DOI] [PubMed] [Google Scholar]
- Knizewski L, Kinch LN, Grishin NV, Rychlewski L, Ginalski K.. 2007. Realm of PD-(D/E)XK nuclease superfamily revisited: detection of novel families with modified transitive meta profile searches. BMC Struct Biol. 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehncke A, Telschow A, Werren JH, Hammerstein P.. 2009. Life and death of an influential passenger: Wolbachia and the evolution of CI-modifiers by their hosts. PLoS One 4(2):e4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesner P, Hoffmann AA.. 2018. Rapid spread of a Wolbachia infection that does not affect host reproduction in Drosophila simulans cage populations. Evolution 72(7):1475–1487. [DOI] [PubMed] [Google Scholar]
- Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR.. 2013. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog. 9(9):e1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven H. 1957. Vererbung durch Kerngene und das Problem der ausserkaryotischen Vererbung bei Culex pipiens. Z Vererbungsl. 88(4):443–477. [Google Scholar]
- Lepage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, Shropshire JD, Layton EM, Funkhouser-Jones LJ, Beckmann JF, Bordenstein Seth R.. 2017. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543(7644):243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Bork P.. 2007. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23(1):127–128. [DOI] [PubMed] [Google Scholar]
- Lindsey ARI, Rice D, Bordenstein S, Brooks A, Bordenstein S, Newton ILG.. 2018. Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB in prophage WO of Wolbachia. Genome Biol Evol. 10(2):434–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DP, Murrell B, Golden M, Khoosal A, Muhire B.. 2015. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 1:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, , Longdon B, , Bauer S, , Chan Y-S, , Miller WJ, , Bourtzis K, , Teixeira L, , Jiggins FM. 2014. symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of wolbachia strains. PLoS Pathog. 10(9):e1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos M, Castrezana SJ, Nankivell BJ, Estes AM, Markow TA, Moran NA.. 2006. Heritable endosymbionts of Drosophila. Genetics 174(1):363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meany MK, Conner WR, Richter SV, Bailey JA, Turelli M, Cooper BS.. 2019. Loss of cytoplasmic incompatibility and minimal fecundity effects explain relatively low Wolbachia frequencies in Drosophila mauritiana. Evolution 73(6):1278–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Kolthoff JP, Auch AF, Huson DH, Göker M.. 2007. CopyCat: cophylogenetic analysis tool. Bioinformatics 23(7):898–900. [DOI] [PubMed] [Google Scholar]
- Miyake H, Watada M.. 2007. Molecular phylogeny of the Drosophila auraria species complex and allied species of Japan based on nuclear and mitochondrial DNA sequences. Genes Genet Syst. 82(1):77–88. [DOI] [PubMed] [Google Scholar]
- O’Neill SL, Karr TL.. 1990. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 348(6297):178–180. [DOI] [PubMed] [Google Scholar]
- Pascar J, Chandler CH.. 2018. A bioinformatics approach to identifying Wolbachia infections in arthropods. PeerJ 6:e5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati A, Ivanova NN, Mikhailova N, Ovchinnikova G, Hooper SD, Lykidis A, Kyrpides NC.. 2010. GenePRIMP: a gene prediction improvement pipeline for prokaryotic genomes. Nat Methods. 7(6):455–457. [DOI] [PubMed] [Google Scholar]
- Poinsot D, Bourtzis K, Markakis G, Savakis C, Merçot H.. 1998. Wolbachia transfer from Drosophila melanogaster into D. simulans: host effect and cytoplasmic incompatibility relationships. Genetics 150(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinsot D, Charlat S, Merçot H.. 2003. On the mechanism of Wolbachia- induced cytoplasmic incompatibility: confronting the models with the facts. BioEssays 25(3):259–265. [DOI] [PubMed] [Google Scholar]
- Prout T. 1994. Some evolutionary possibilities for a microbe that causes incompatibility in its host. Evolution 48(3):909–911. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/. Accessed August 20, 2020. [Google Scholar]
- Rousset F, Braig HR, O’Neill SL.. 1999. A stable triple Wolbachia infection in Drosophila with nearly additive incompatibility effects. Heredity 82(6):620–627. [DOI] [PubMed] [Google Scholar]
- Schaack S, Gilbert C, Feschotte C.. 2010. Promiscuous DNA: horizontal transfer of transposable elements and why it matters for eukaryotic evolution. Trends Ecol Evol. 25(9):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire JD, Bordenstein SR.. 2019. Two-By-One model of cytoplasmic incompatibility: synthetic recapitulation by transgenic expression of cifA and cifB in Drosophila. PLOS Genet. 15(6):e1008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire JD, Kalra M, Bordenstein SR.. 2020. Evolution-guided mutagenesis of the cytoplasmic incompatibility proteins: Identifying CifA’s complex functional repertoire and new essential regions in CifB. bioRxiv: 2020. DOI: 10.1101/2020.05.13.093815 [DOI] [PMC free article] [PubMed]
- Shropshire JD, Leigh B, Bordenstein SR, Duplouy A, Riegler M, Brownlie JC, Bordenstein SR.. 2019. Models and nomenclature for cytoplasmic incompatibility: caution over premature conclusions. Trends Genet. 35(6):397–399. [DOI] [PubMed] [Google Scholar]
- Shropshire JD, On J, Layton EM, Zhou H, Bordenstein SR.. 2018. One prophage WO gene rescues cytoplasmic incompatibility in Drosophila melanogaster. Proc Natl Acad Sci U S A. 115(19):4987–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM.. 2015. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31(19):3210–3212. [DOI] [PubMed] [Google Scholar]
- Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I.. 2009. ABySS: a parallel assembler for short read sequence data. Genome Res. 19(6):1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkins SP, Braig HR, O’Neill SL.. 1995. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc Biol Sci. 261(1362):325–330. [DOI] [PubMed] [Google Scholar]
- Söding J, Biegert A, Lupas AN.. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33(Web Server):W244–W248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MJ, , Bandi C, , Hoerauf A. 2005. Wolbachia bacterial endosymbionts of filarial nematodes. Adv Parasitol. 60:245–284. [DOI] [PubMed] [Google Scholar]
- Tram U, Sullivan W.. 2002. Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296(5570):1124–1126. [DOI] [PubMed] [Google Scholar]
- Turelli M. 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48(5):1500–1513. [DOI] [PubMed] [Google Scholar]
- Turelli M, Cooper BS, Richardson KM, Ginsberg PS, Peckenpaugh B, Antelope CX, Kim KJ, May MR, Abrieux A, Wilson DA, et al. 2018. Rapid global spread of wRi-like Wolbachia across multiple Drosophila. Curr Biol. 28(6):963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Hoffmann AA, McKechnie SW.. 1992. Dynamics of cytoplasmic incompatibility and mtDNA variation in natural Drosophila simulans populations. Genetics 132(3):713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautrin E, Charles S, Genieys S, Vavre F.. 2007. Evolution and invasion dynamics of multiple infections with Wolbachia investigated using matrix based models. J Theor Biol. 245(2):197–209. [DOI] [PubMed] [Google Scholar]
- Vavre F, Fleury F, Lepetit D, Fouillet P, Boulétreau M.. 1999. Phylogenetic evidence for horizontal transmission of Wolbachia in host-parasitoid associations. Mol Biol Evol. 16(12):1711–1723. [DOI] [PubMed] [Google Scholar]
- Watada M, Matsumoto M, Kondo M, Kimura MT.. 2011. Taxonomic study of the Drosophila auraria species complex (Diptera: Drosophilidae) with description of a new species. Entomol Sci. 14(4):392–398. [Google Scholar]
- Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ.. 2015. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc R Soc B. 282(1807):20150249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalou S, Apostolaki A, Pattas S, Veneti Z, Paraskevopoulos C, Livadaras I, Markakis G, Brissac T, Merçot H, Bourtzis K.. 2008. Multiple rescue factors within a Wolbachia strain. Genetics 178(4):2145–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalou S, Charlat S, Nirgianaki A, Lachaise D, Merçot H, Bourtzis K.. 2004. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics 167(2):827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O’Neill S.. 1998. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci. 265(1395):509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.