Abstract
The main aim of the topically applied drugs is to provide local drug contact to the skin and minimize general absorption of drugs. Ocimum basilicum (OB) is popular for folk medicines, having official acceptance in many countries. The aim of this study was to formulate and evaluate the efficacy of topical application of OB-based emulgel on wound healing in animal model. The prepared formulations (OB emulgel) were assessed for FTIR analysis, stability studies, physical appearance, rheological behavior, spreadability, patch/sensitivity test and in vitro drug release. The in vivo wound healing effect was evaluated and compared with commercially available Silver Sulfadiazine cream Quench® in wound-induced rabbits by macroscopic and histopathological evidence. The OB extract/drug was compatible with the selected polymer and other excipients and indicated the suitability of the polymers/excipients for preparation of topical emulgel. The formulated OB emulgel exhibited good physical properties. The release profile of emulgel was satisfactory and released 81.71 ± 1.7% of the drug in 250 min. In vivo wound healing studies showed that OB emulgel exhibited the highest percent wound contraction similar to the commercial product (p > 0.05). This activity was statistically significant (p < 0.05) in comparison to control. Histopathological assessment showed marked improvement in the skin histological architecture after 16 days of OB emulgel treatment. In conclusion, the data demonstrated here signify the prospective of 5% OB emulgel as an innovative therapeutic approach in wound healing.
Keywords: Burn wound, Emulgel, Ocimum basilicum, Histopathology
1. Introduction
Wound may be defined as breaking or loss of anatomic and cellular or functional connection of living tissue (Kased et al., 2017). It is an unavoidable part of the human life which is caused by chemical, physical, thermal, immunological or microbial damages to the tissue (Mehmet et al., 2020). Wound healing is natural process, obtained through four highly programmed stages: hemostasis, inflammation, proliferation and remodeling. For a wound to be healed effectively, all of these stages must occur in the appropriate sequence and time frame (Okur et al., 2018, Ayla et al., 2019). Healing involves platelets aggregation, blood clotting, formation of fibrin, a response of inflammation to injury, alteration in the ground substances, angiogenesis and re-epithelialization (Kokane et al., 2009). The healing process of wound gets worse and time for healing increases in burn injuries due to the obstruction of vascular bed. Due to this obstruction, which hold back the transportation of systemically administered antibiotics; the topical delivery becomes an important component of the burn therapy. Overall the goal of burn therapies is to promote the healing process by preventing the infection effectively (Kumar and Gosh, 2017).
Gellified emulsions (either of oil-in-water or water in-oil type) is called emulgel and formed by the addition of a gelling agent (Alexander et al., 2011). In emulsion the entrapped drugs slowly release from internal phase through external phase and slowly get absorbed through the skin. Thus, emulsion itself is controlled release systems. The same case is for the gel that forms cross linked network in which small particle of drugs are captured. Its release occurs in a controlled manner (Jain et al., 2011). Since, emulgel possesses the property of equally emulsions and gel; it acts as dual control release system (Elbayoumi and Torchilin, 2008). Gel is incorporated into emulsion to improve its penetration ability and stability. Further, gel for external use has many good characteristics such as being thixotropic, easily removable, spread easily, greaseless, soothing, and compatible with numerous excipients, non-staining and miscible or solubilized in water (Sarisozen et al., 2012). Emulgels have good stability than other available topical preparation. For example creams have the problem of phase inversion and breaking; hygroscopic nature shown by powders and rancidity is the problem in ointments (Singh et al., 2014).
Ethno-pharmacological use of crude drugs provided major evidence in drug discovery. Data proposed that>50% of medicines used during last 30 years were of natural origin (Aitazaz et al., 2020).
OB is popular both in Unani and Ayurvedic medicine system. In Pakistan, India, Bangladesh and several other Asian countries OB grows naturally. Keeping in view the tremendous pharmacological activities and available literature, OB may be utilized to relieve the symptoms of a variety of diseases as evident from pre-clinical data (Somashekar et al., 2008). Thus it appears that different mechanisms like free radical scavenging, metal chelation as well as immune modulation may act at different levels individually or in combination to bring about the wound healing effects of this medicinal plant.
2. Materials and methods
2.1. Plant materials
Fresh leaves of Ocimum basilicum were collected from different location of Dera Ismail Khan, Pakistan and were authenticated by Professor Mushtaq, Department of Botany, QAU Islamabad. A voucher specimen (No. pp1281) has been deposited. The leaves were shade dried for five days and the dried leaves were then grinded in a grinder and stored in an air resistant container.
2.2. Extraction procedure
Grinded OB leaves (850 g) powder were macerated in 6 L of 70% methanol. The mixture was stirred at an interval of 6–8 hrs for 24 hrs. The mixture was then allowed to keep for 6 days at room temperature. After that, the mixture was coarsely filtered using a muslin cloth followed by a filter paper. The solvent was evaporated in rotary evaporator at 45 °C. The extract was then stored in air-tight bottle. The yield was about 17–19% (Sekar et al., 2009, Amzad et al., 2010).
2.3. Formulation of emulgel
Formulations with different quantity of ingredient were made as shown in Table 1. The gel portion of the emulgel was made by dissolving carbopol-934 in cold water with constant stirring at a moderate speed until uniform mixture was made. The pH was then adjusted to 6–6.5 using triethanolamine (TEA). Tween 80 was dissolved in distilled water to prepare the aqueous phase of the emulsion while for the preparation of the oil phase of the emulsion; span 80 was dissolved in liquid paraffin. To preserve the emulsion, methyl parabene was dissolved in propylene glycol and the extract was dissolved in ethanol then both solutions were mixed with the aqueous phase. Both the aqueous and the oil phase were heated in a water bath at 70 °C separately. Then the oil phase was added drop wise to the aqueous phase with continuous stirring using homogenizer (WiseStir® HS-120A, Daihan Scientific, Korea) at speed of 3000 rpm for 10 min then cold to room temperature. At the end the gel and emulsion portions were mixed in 1:1 ratio with moderately stirring to prepare emulgel (Jain et al., 2011).
Table 1.
Composition of various emulgel formulations.
| Ingredient (%w/w) | FB1 | FB2 | FB3 | FB4 | FB5 | FB6 | FB 7 | FB8 |
|---|---|---|---|---|---|---|---|---|
| OB extract | – | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Carbopol 934 | 0.25 | 0.50 | 0.50 | 0.50 | 0.25 | 0.75 | 0.75 | 0.75 |
| Liquid paraffin | 3.75 | 2.50 | 3.75 | 2.50 | 3.75 | 3.75 | 3.75 | 2.50 |
| Tween 80 | 0.30 | 0.50 | 0.30 | 0.50 | 0.30 | 0.50 | 0.50 | 0.30 |
| Span 80 | 0.45 | 0.75 | 0.45 | 0.75 | 0.75 | 0.45 | 0.75 | 0.45 |
| Propylene glycol | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 |
| Methyl parabene | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Distilled water QS | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Triethanolamine few drops | pH = Adjusted to 6–6.5 | |||||||
*FB1 = Formulation 1.
2.4. Characterization of emulgel
2.4.1. Lambda max (λmax) determination by UV/Visible spectrophotometer
The lambda max of the plant extract was determined according to the previous studies reported using UV/Visible spectrophotometer (CECIL, CE 2021 Germany) (Bueno et al., 2012, Joseph et al., 2018). For this purpose 20 mg of OB extract was taken and dissolved in 20 ml of methanol (Stock solution). Then one ml of the stock solution prepared was taken and dissolved in volumetric flask. The volume was made up to the mark with methanol. Similarly the stock solution was serially diluted up to three concentrations. Aliquots of various solutions prepared were taken and scanned in UV range from 190 to 400 nm for the determination of wavelength of maximum absorbance for major constituent of OB extract (Linalol). The methanol was used as blank.
2.4.2. Fourier transform infrared spectrophotometer analysis (FTIR)
FTIR analysis was performed according to previous study reported by Burki et al to check the compatibility of drug/extract of Osimum basilicum with that of other excipients in the formulation (Burki et al., 2020). First of all, infrared spectral analysis of the extract of osimum basilicum was carried out using Fourier Transform Infrared Spectrophotometer (Tencor Series 7. Shimadzu, Germany). Then spectral analysis of the formulation was performed. The spectra were recorded in the region of 4000 cm−1-400 cm−1.
2.4.3. Stability study
Initially eight different formulations (FB1-FB8) were prepared and placed in an incubator at 25 °C for three days. Among eight formulations, the formulation FB8 was comparatively stable. FB8 was further selected for stability study. Formulation FB8 was divided into four samples and kept in four different incubators at 8, 25, 40 and 40 °C + 75% RH (relative humidity) respectively. These were observed organolaptically with respect of color, homogeneity, phase separation and liquefaction for period of one month at different time interval. The samples were also analyzed for pH (Jain et al., 2011).
2.4.4. Spreadability study
Spreadability was determined by apparatus suggested by Mutimer et al. It consists of a block of wood at one end of which, a pulley was connected. On the basis of ‘drag’ and ‘sleep’ method, spreadability was determined. A ground glass slide was fixed on this block. Test emulgel (2 g) was placed on this slide. The emulgel was then sandwiched between these slides and another glass slide having the same dimension of fixed ground side and provided with hook. Weight (40 g) was then placed on the top of this slide. The time required (in seconds) by the top slide to cover a distance of 6 cm was noted. Then spreadability was calculated using the following formula.
| (1) |
where S = spreadability, M = Weight tied to upper slide, L = Length of glass slides T = Time taken to separate the slides completely from each other.
2.4.5. Viscosity/Rheology
Initially viscosities of freshly prepared eight formulations (FB1-FB8) were determined using brook field viscometer with spindle no 04. The spindle was lowered perpendicular into the center of emulgel formulation placed in a beaker taking care that spindle did not touch the bottom of the beaker and rotated at the speed of 2.5 rpm for 5 min. The viscosity reading was noted (Basha et al., 2011). The stable formulation FB8 was then divided into four samples (i.e. FB8A, FB8B, FB8C and FB8D). The four samples were further subjected to viscosity study and they were checked from time to time for one month time period.
2.4.6. Patch Test/Sensitivity test
Human volunteers (n = 3) were selected for patch test. Formulation (1 g) was applied on the forearm of the volunteers in the form of bandage disc and then covered with surgical dressing. After 24 h, the patches were removed and the areas were washed with saline fluids. The volunteers were asked for any irritation and the areas of application were observed for the presence/absence of edema and erythema (redness of skin) (Rasul and Akhtar, 2011).
2.4.7. In vitro drug release analysis
Franz diffusion cell was used for in vitro drug release studies using a dialysis membrane as described in literature (Aitazaz et al., 2020). Donor compartment was filled with emulgels whereas recipient compartment contained phosphate buffer of pH 6.0. Magnetic bar was put in recipient chamber and rotated at 75 rpm. Temperature of system was kept at 37 °C by circulating hot water in the outer jacket. Samples were withdrawn at predetermined time intervals. First sample was taken at time 0 and then after 50 min, 100 min, 150 min, 200 min and 250 min. Each time the sample removed from the recipient compartment was filled with fresh buffer. After this the absorbance was determined using UV visible spectrophotometer at 320 nm. Drug released data of 250 min analysis were fitted to Korsmeyer-Peppas kinetic model which illustrates the mechanism by which the drug was released from the emulgel, The equation for this model is given below,
| (2) |
Where
Mt/M∞ is the fractional release of drug in time t
K is a constant characteristics of the drug/polymer system
n is an exponent that indicates the mechanism of release.
2.5. In vivo evaluation (animal study)
2.5.1. Ethical standards
This study was approved by the ethical review board of Gomal University, Dera Ismail Khan Pakistan under reference no (ERB/GUDIK/09/2019. All the protocols were followed in accordance to Helsinki declaration (For human study and NIH guidelines for lab animals).
2.5.2. Animals
New Zealand adult white rabbits having 1.5 Kg average weight were used for the study. All the rabbits were separately caged and subjected to standard food and environmental conditions. Rabbits were divided into three groups each containing 5 rabbits (n = 5) (Dua et al., 2016).
Group 1 Served as test group and was treated with emulgel containing 5% OB extract.
Group 2 Served as standard group and received cream containing silver sulfadiazine 1% cream (SSD) USP cream (Quench®)
Group 3 was a control group (base treated) group.
2.5.3. Wound’s infliction
The animal study was carried out as per the guidelines of National institute of health (NIH). The dorsal hairs of the rabbits were shaved and the skin was sterilized with 70% ethanol. Burn wound was inflicted on overnight starved animals under ketamine (35 mg/Kg i.m) anesthesia. A 1 × 1 cm plastic cylinder was placed on the shaven back of the animal. Melted wax at 80 °C was poured into the cylinder and the wax was allowed to solidify. After eight minutes, when the wax was completely solidified, the plastic cylinder containing wax adhering to the skin was gently removed. After 24 h the wound was inflicted (Dua et al., 2016).
2.5.4. Evaluation of wound healing
Emulgel formulation and the standard silver sulfadiazine 1% cream, USP (500 mg each) were topically applied on the wound areas of rabbits twice a day from day 1 to 16. Wound healing was assessed by measuring the percent wound contraction from day 1 to day 16. The contraction of the wound was calculated in percentage of original wound size of for each group of animal (Dua et al., 2016).
| (3) |
2.5.5. Histopathology of wounds
Histopathology of wound tissues was carried out according to the studies reported by Ashraf et al., 2018 with some modifications. After the process of euthanasia the wounds tissues from the rabbits were removed for 16 days (Day 4, 8, 12, 16). 10% solution of formaldehyde was made and the wounds tissues were fixed in the solution. Then using ethanol the specimens were dehydrated. After this procedure the samples/specimens collected were embedded in paraffin wax. With the help of microtome the specimens were cut into sections of 4 µm. Using Hematoxylin-Eosin, the sections of specimens were stained and then examined under microscope and photographs were recorded (Ashraf et al., 2018).
2.6. Statistical analysis
The results were analyzed using one way analysis of variance (ANOVA) with post hoc Scheffe's test to check out statistical significance. P-values 5% (p < 0.05) were considered statistically significant.
3. Results and discussion
3.1. Characterization of emulgel
3.1.1. FTIR analysis
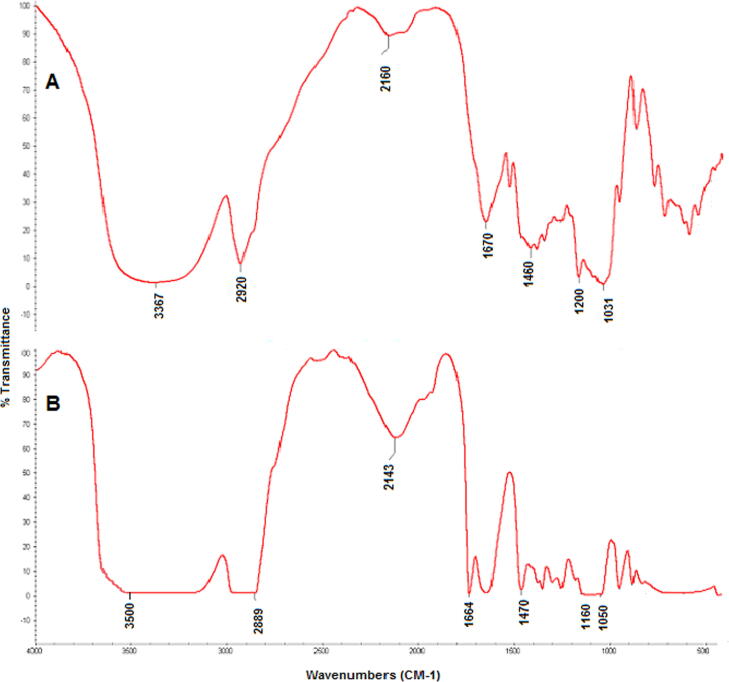
FTIR spectral analysis gives an idea about compatibility of the drugs/extract and polymers (excipients) used in the formulations of pharmaceutical dosage forms (Burki et al., 2020). The FTIR spectra of Ocimum basilicum (OB) extract and extract loaded formulation are presented in Fig. 1. The FTIR spectrum of OB extract (Fig. 1 A) showed characteristic peaks at 3367 CM-1 which is O-H carboxylic acid stretching, 2920 CM-1 which is CH2 a methylene stretching. The spectrum of extract showed peak at 1670.37 CM-1 which represents C = O carbonyl stretching. Similarly the peaks 1460.11 CM-1, 1200.40 CM-1 and 1030 CM-1 are due to C= (aromatic rings), CH3 Methyle bond and C-oH stretching respectively. In Fig. 1b, the FTIR spectrum of the OB extract and other excipients (Polymers) is presented. The characteristic peak at 3500 CM-1 indicates the presence of O-H stretching (Carboxylic acid). The 2889 CM-2 peak shows the CH2 a methylene stretching. The peaks 1664 CM-1 and 1470 CM-1 represents the presence of C=O (carbonyl stretch) and C=C (Aromatic ring). Similarly the peaks 1160 CM-1, and 1050 CM-1 are due to CH3, C-OH and phenolic stretching in the extract loaded formulation. There was no significant difference between the characteristics peaks of pure extract when compared with the peaks of extract loaded formulation. All the peaks were found intact indicating the compatibility of extract with other excipients used in the formulation of emulgel. Characteristic bands and peaks of the extract were identified in the spectra of prepared emulgel, so this study revealed that the drug/extract is compatible with the selected polymer and other excipients.
Fig. 1.
a) FTIR spectrum of crude extract of OB b) FTIR Spectrum of emulgel formulation.
3.1.2. Stability study
All four samples of the stable formulation FB8 (i.e. FB8A, FB8B, FB8C and FB8D) were kept at different storage conditions for one month and were examined physically from time to time for color, phase separation, homogeneity, consistency and liquefaction. Initially all formulations were yellowish green in color, viscous in consistency, smooth elegance and phase separation was not seen. pH of freshly prepared formulations was 6.1 which is an accepted value for skin which ranges from 5 to 6 (Helal et al., 2012). All the samples were found stable in respect to the mention parameters. The sample kept at 40 °C + 75% RH showed a slight phase separation and its color changed to slightly blackish. This change in color may be due to separation of oily phase promoted at higher temperatures (Khan et al., 2016). The four samples were also analyzed for pH at zero time, after 12, 24 h, one week, two week, three week and four week intervals. All the pH values were subjected to student t test. No significant (p > 0.05) change in pH was noted. The average pH of all the four samples of the formulation were in the normal range of 5–6 which is the accepted range to avoid the skin irritation risk upon skin use (Helal et al., 2012). A slight decrease in the pH was observed with the passage of time till one month, but this variation was with in the normal skin pH range. According to (Khan et al), the decrease in pH with the passage of time may be due to diffusion of water (pH 5 to 7) from internal phase to external phase or due to the production of highly acidic by-products from any of the oil ingredients (Khan et al., 2016).
The average pH change has been shown in Table 2.
Table 2.
Average pH values of the four samples placed at different temperature.
| Formulation codes | Average pH (Mean + SD |
|---|---|
| FB8A | 6.1 ± 0.57 |
| FB8B | 5.39 ± 0.88 |
| FB8C | 5.9 ± 0.57 |
| FB8D | 5.6 ± 0.48 |
3.1.3. Spreadability study
Spreadability indicates that the emulgel is easily spreadable and comes out of containers by small amount of shear (Helal et al., 2012). Average spreadability values of different formulations have been given in Table 3. Larger the value of spreadability coefficient better is its spreadability on the skin. Spreadability values of all sampled formulations were in the following order FB8C > FB8A > FB8B > FB8D. There was no significant (p > 0.05) change in spreadability values among all the formulations. FB8C showed highest average spreadability value that was 34 and the second highest value showed by FB8A that was 31.33. The high value of spreadability is due to low level of gelling agent (Joshi et al). Low level of liquid paraffin may also be responsible for high spreadability value (Jain et al., 2010).
Table 3.
Average spreadability values (mean + SD).
| Formulation codes | Average spreadability values (mean + SD) |
|---|---|
| FB8A | 31.33 ± 2.62 |
| FB8B | 29.33 ± 0.94 |
| FB8C | 34 ± 0.81 |
| FB8D | 26.33 ± 1.24 |
3.1.4. Viscosity and rheological study
Viscosity is an important parameter to be evaluated because consistency of dosage form and drug content release mainly depend upon viscosity (Ghada et al., 2014). Viscosities of all formulations were performed by using brook field viscometer. Values have been given in Table 4. The most viscous formulation was FB8 (12500 cp). This is due to high level of gelling agent, low level of emulsifying agent and low level of liquid paraffin (Jain et al., 2010). Among the four samples of formulation FB8 that were kept at different temperatures for one month period, the three formulations FB8B (kept at 25 °C) FB8C (kept at 40 °C), FB8D (kept at 40 °C + 74% RH) showed no significant (p > 0.05) change in viscosities with respect to each other, but change in viscosities of formulation FB8A with respect to FB8c and FB 8D was significant (p < 0.05). Decrease in viscosities was higher at 40 °C and 40 °C + 75% RH. Such decrease in viscosities is always associated with increase in temperature as mentioned by Li et al. According to his study, decline in viscosities was provoked by increase in temperature (from 25 °C to 32 °C). It means that when the emulgel was applied at skin surface (application site temperature, 32 °C), fluidity and spreadability would increase comparing with the condition of 25 °C. Such property of viscosity is important to insure if the formulation is acceptable to patients (Li et al., 2011). Two reasons are mentioned here for inverse relation of viscosities with temperature, first reason is that water molecules defuse from the dispersed aqueous phase to the continuous aqueous phase, the second reason is that the bursting of multiple globules due to osmotic pressure (Khan et al., 2016).
Table 4.
Viscosity of four samples of most stable formulation during storage for 30 days and their SD.
| Time |
Viscosities in centipoises |
|||
|---|---|---|---|---|
| FB8A | FB8B | FB8C | FB8D | |
| 0 h | 12,500 | 12,500 | 12,500 | 12,500 |
| 12 h | 12490 ± 21.5 | 12400 ± 16.10 | 11390 ± 17.3 | 11990 ± 16.1 |
| 24 h | 12480 ± 21.2 | 12000 ± 16.20 | 11370 ± 17.25 | 11300 ± 16.5 |
| 1 week | 12400 ± 21.32 | 11488 ± 16.25 | 10100 ± 17.10 | 10000 ± 16.3 |
| 2 week | 12380 ± 21.15 | 11400 ± 16.05 | 9900 ± 17.20 | 9890 ± 16.10 |
| 3 week | 12380 ± 21.10 | 11300 ± 16.07 | 9890 ± 17.32 | 9860 ± 16.40 |
| 4 week | 12350 ± 16.30 | 11300 ± 17.01 | 9850 ± 17.45 | 9803 ± 16.25 |
| Average | 7480 ± 16.33 | 12480 ± 21.60 | 5867 ± 17.02 | 5020 ± 16.32 |
FB8A = formulation kept at 8 °C, FB8B = Formulation kept at 25 °C, FB8C = Formulation kept at 40 °C, FB8D = Formulation kept at 40 °C + 75% RH
3.1.5. Patch test
For confirming the safety of topical preparations, the important point is that they must not cause any contact dermatitis when applied to the skin. For this purpose patch test was conducted (Khan et al., 2016). The skin areas of application were observed for the presence of edema and erythema. No erythema and edema on applied areas were observed. The results have been presented in Table 5.
Table 5.
Observation of erythema and edema.
| No of volunteers |
Erythema |
Edema |
||
|---|---|---|---|---|
| 0 hr | 24 hrs | 0 hrs | 24 hrs | |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 |
n = 3
3.1.6. Drug release analysis
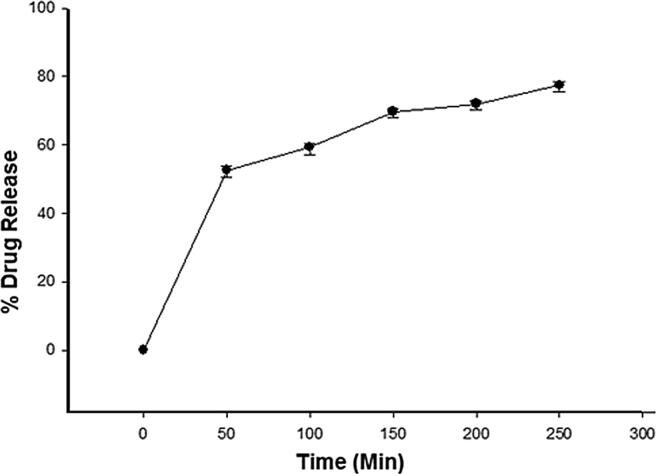
Various mathematical models were considered to correlate drug release profile with time intervals. The findings in the Fig. 2 showed that the optimized formulation FB8 released drug constantly over the period of time (250 min) and better release rates were observed. The drug released from prepared emulgel loaded with crude extract was observed for 0 time, 50 min, 100 min, 150 min, 200 min and 250 min and the percent drug release was recorded as 0, 50%, 57.3%, 65.7%, 73.2% and 81.71% respectively. The release of drug from pharmaceutical dosage form is important for therapeutic efficacy and it depends mainly upon the viscosity (Khan et al., 2020, Ghada and Yassin, 2014)). For this purpose the release of drug from the prepared emulgel was determined. As the formulation FB8 (Optimized formulation) was the most viscous formulation with higher concentration of Carbopol-934. These results are in agreement to the study conducted by the Yen et al (Yen et al., 2015). They proposed that drug release is inversely proportion to the percentage of Carbopol amount. In their study drug release was reduced significantly with increasing the Carbopol percentage to 1.00% w/w, 2.00% w/w, and 5.00% w/w. This reduction in drug release can be correlated with the higher complexity of gel network, produced by the higher polymer (Carbopol) content which caused longer diffusion pathway of drug to be permeated through the membrane (Yen et al., 2015).
Fig. 2.
% Drug release from the OB emulgel at different time intervals.
3.1.7. Drug release kinetics
The data achieved from in vitro drug release study was fitted into Power law Kinetic model. The r2 (co-efficient of determination) and “n” (drug release exponent) values obtained for the formulation are given in Table 6. Power law kinetic model (Korsmeyer-Pappas Kinetic Model) was applied to drug release data from formulation and the respective r2 value was 0.9371 that indicated linearity while the respective “n” value was 0.8266. As value of “n” was higher than 0.5, this showed the release of drug happened by anomalous non fickian diffusion mechanism.
Table 6.
Results of power law.
| Formulation Code |
Power law |
|
|---|---|---|
| r2 | N | |
| FB8 | 0.9372 | 0.8267 |
3.2. In vivo evaluation of wound area
Skin is the largest organ in the body that has key role as a primary protection against infection by acting as a physical barrier (Okur et al., 2018). When it damaged, body lose fluid, heat, and nutrient, and pathogens are capable to inter the body and induce infection. A wound is characterized by the breakage of the anatomic and cellular continuity of the skin tissue and can be caused by microbial, thermal, immunological, chemical or physical tissue trauma (Okur et al., 2018, Ayla et al., 2019). Wound healing is fundamental, dynamic, coordinated, interdependent, and overlapping cellular and immunologic response of tissue injury. These overlapping steps involve inflammation, cell migration and proliferation, angiogenesis, neovascularization, extracellular matrix production, and remodeling (Okur et al., 2018, Mehmet et al., 2020).
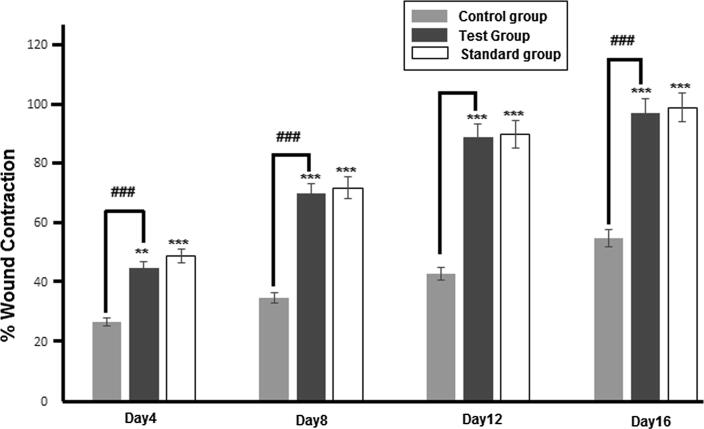
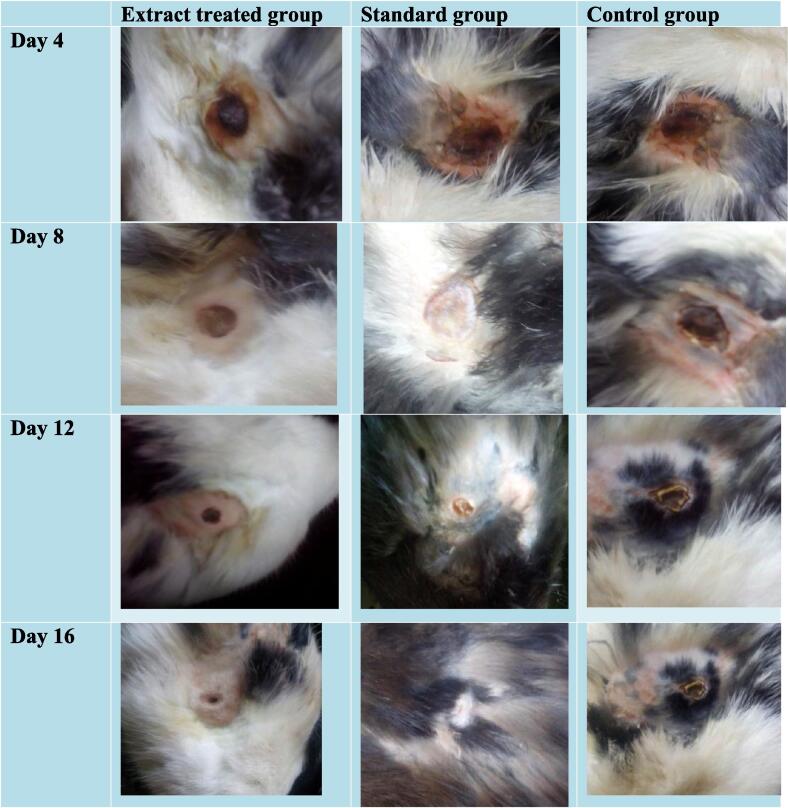
The wound healing activity OB extract was evaluated in rabbits from day 1 to 16. The percent wound contraction of the three groups has been shown in Fig. 3. The photographs of partial thickness burn wound (2nd degree) in rabbits have been taken at the distance of 10 cm from wound site using digital camera (Sony. 7.2 megapixel, Japan) and illustrated in Fig. 4.
Fig. 3.
% Wound contraction of Control, Test and Standard groups at Day4, Day8, Day12 and Day 16. The data is presented as the mean ± SD and analyzed using ANOVA. (###) indicates comparison of test group with control while **p < 0.01 and ***p < 0.001 refers to statistical significance of both groups from the control.
Fig. 4.
Photographs of wounds in extract treated group, standard group and control group from day 4 to 16 (Digital camera used (Sony, 7.2 megapixel), Japan.
All results were subjected to one way ANOVA with 0.05 as level of significance. The percent wound contractions measured on 4th, 8th, 12th and 16th day in extract treated group were 43.5 ± 5.84%, 75 ± 0%, 89 ± 1.2% and 98.78 ± 0.16% respectively. In base treated control group, values were 27.5 ± 6.6%, 51 ± 0%, 75 ± 1.85% and 89 ± 1.2% respectively. Results of test and standard treated animal models shows significant (p < 0.05; ANOVA) increase in wound contraction rate. The percent wound contraction rate in standard treated group was greater throughout the treatment period that was 51 ± 0%, 69.75 ± 4.29%, 91 ± 0%, 99 ± 0.24%, but on the day 8, the rate in the extract treated group was higher than standard treated group that was 69.75% ± 4.29%. But this increase was insignificant (p > 0.05; ANOVA). Over all, wound contraction rate between extract treated and standard treated group was insignificant (p > 0.05; ANOVA). The high rate of wound healing in test animal model may be due three factors, (a) due to presence of various phytochemicals; having the potential of wound healing and also possess anti-inflammatory action (Daniel et al., 2011, Bairy and Rao, 2001); (b) due to enhanced epithelialization (Priya et al., 2002); (c) due to enhanced angiogenesis, as the alcoholic extract of O. basilicum promote angiogenesis (Fatemeh et al., 2016). All these statement cited from literature and the results of this study show that crude extract of Ocimum basilicum has wound healing potential, but further research is needed to explore it standard topical preparation for wound healing purposes.
3.2.1. Histopathological evaluation
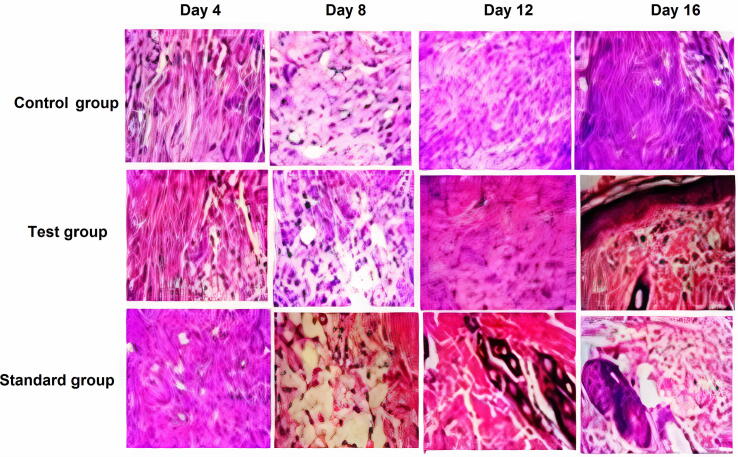
Wound healing is a complex and continuous process that can be classified into three overlapping stages: inflammation, proliferation, and tissue regeneration (Mehmet et al., 2020). Extensive tissue damaged during burns impairs angiogenesis, collagen re-organization, granulation tissue formation and induces free radical-mediated damage which results in delayed tissue repair (Heidari et al., 2018).
The tensile strenghth of the wound tissue is increased by the newly synthesized collagen deposition at the wound site. The tissue and cellular level interpretitions of the healing process of wounds are provided by the histopathological analysis (Ashraf et al., 2018). The hemotoxylin/Eosin stained wounds sections are presented in Fig. 5. It can be observed that on the day 8 of post wound infliction, gradual granulation tissue formation and inflammatory rersponse have been exibited by the inflicted burn wounds. Similarly granulation tissue formation with onset of collagen remodelling was observed on day 12 and day 16. The hispathology indicates that on day 16 of post wound infliction in rabbits, the fibroblasts were higher in the extract treated group (OB loaded emulgel) as compared to the control group which was treated with blank emulgel without extract. In both standard treated group (Quench®, Silver sulphadiazine) and OB extract loaded emulgel, there was boosted reepithelization and significant proliferation of fibroblasts and marked collegen deposition as compared to the control group.
Fig. 5.
Histopathological views of burn wounds sections stained with H&E, collected on days 4, 8, 12 and 16 in control, test and standard groups. Control group is treated with blank emulgel, test group is treated with OB loaded emulgel and standard group is treated with marketed product (Quench®; Silver sulfadiazine). Original magnification 400× (the scale bar is 100 μm).
4. Conclusion
The findings of this study reveal that OB emulgel contains a variety of phytochemicals such as tannins, sesquiterpenes that are responsible for its in vivo activities including wound healing. It can also be concluded that this study presents a better strategy for the formulation of emulgel with significant wound healing potential. Further studies are required to determine formulation of emulgel containing isolated constituents that are responsible for wound healing activity and to make standardized the extract, using standard and sophisticated phytochemical analysis techniques that would enable us to reach a conclusive stage.
5. Consent for publication
Not applicable
6. Availability of data and materials
It can be obtained from the corresponding author on demand.
Funding
There was no financial support for this research study.
8. Author’s contributions
BAK designed and supervised the study plan. SU performed all the experiment. MKK helped in formulation development, in vivo studies and writing of the manuscript. SMA edited the manuscript and figures. VAB performed histopathological analysis. All authors have read and approved the manuscript.
Declaration of Competing Interest
All the authors declare that they have no competing interest
Acknowledgement
The authors are thankful to Ferozsons lab, Nowshehra, Pakistan for providing us some of the active ingredients and excipients. We are also thankful to Dr. Nauman Rahim Khan (Lecturer at Faculty of Pharmacy, Gomal University, D. I. Khan, Pakistan) for helping us in FTIR analysis.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amzad H.M., Kabir S.M., Salehuddin S.M. Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimum basilicum occurring in Bangladesh. Pharma Biol. 2010;48:504–511. doi: 10.3109/13880200903190977. [DOI] [PubMed] [Google Scholar]
- Aitazaz A., Ghulam A.M., Humaira N., Masood U.R. Formulation, characterization and wound-healing potential of emulgel and in-situ gel containing root extract of Saussurea lappa Clarke (Asteraceae) Trop. Pharm. J. Res. 2020;19:1–9. [Google Scholar]
- Alexander A., Ajazuddin A., Tripathi D.K., Verma S.T., Maurya J., Patel S. Mechanism responsible for mucoadhesion of mucoadhesive drug delivery system: a review. Int. J. Appl. Biol. Pharm. Technol. 2011;2:234–246. [Google Scholar]
- Ashraf U.K., Muhammad Z.U., Ruqqaya A. Antinociceptive properties of 25-methoxy hispidol A, a triterpinoid isolated from Poncirus trifoliata (Rutaceae) through inhibition of NF-κB signalling in mice. Phytother Res. 2018;33:327–341. doi: 10.1002/ptr.6223. [DOI] [PubMed] [Google Scholar]
- Ayla S., Okur M.E., Günal M.Y., Özdemir E.M. Wound healing effects of methanol extract of Laurocerasus officinalis roem. Biotech. Histochem. 2019;94:180–188. doi: 10.1080/10520295.2018.1539242. [DOI] [PubMed] [Google Scholar]
- Bairy K.L., Rao C.M. Wound healing profile of Ginko biloba. J. Nat. Remed. 2001;1:25–27. [Google Scholar]
- Basha B.N., Prakasam K., Goli D. Formulation and evaluation of Gel containing Fluconazole-Antifungal Agent. Int. J. Drug. Dev. Res. 2011;3:119–127. [Google Scholar]
- Bueno F.G., Machareth A.D., Panizzon G.P., Lopes G.C. Development of a UV/Vis spectrophotometric method for analysis of total polyphenols from Caesalpinia peltophoroides Benth. Química Nova. 2012;35:822–826. doi: 10.1590/S0100-40422012000400031. [DOI] [Google Scholar]
- Burki I.K., Khan M.K., Khan B.A., Uzair B., Braga V.A., Jamil Q.A. Formulation development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS PharmSciTech. 2020;21:211. doi: 10.1208/s12249-020-01760-7. [DOI] [PubMed] [Google Scholar]
- Daniel V.N., Daniang I.E., Nimyel N.D. Phytochemical analysis and mineral elements composition of Ocimum basilicum Obtained in JOS metropolis, plateau state, Nigeria. Int. J. Eng. Tech. 2011;11:135–137. [Google Scholar]
- Dua K., Malipeddi V.R., Madan J., Gupta G. Norfloxacin and metronidazole topical formulations for effective treatment of bacterial infections and burn wounds. Interven. Med. App. Sci. 2016;8:68–76. doi: 10.1556/1646.8.2016.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbayoumi T.A., Torchilin V.P. Liposomes for targeted delivery of anti-thrombotic-drugs. Expert Opin. Drug Deliv. 2008;5:1185–1198. doi: 10.1517/17425240802497457. [DOI] [PubMed] [Google Scholar]
- Fatemeh N., Maryam T., Khadijeh S. The effects of alcoholic leaf extract Ocimum basilicum on angiogenesis in chick chorioallantoic membrane. Arak Med. University J. 2016;7:91–98. [Google Scholar]
- Ghada, E., Yassin., 2014. Formulation and evaluation of optimized clotrimazole emulgel formulations. Brit. J. Pharma Res. 4, 1014-1030.
- Heidari M., Bahramsoltani R., Abdolghaffari A.H., Rahimi R., Esfandyari M., Baeeri M., Hassanzadeh G., Abdollahi M., Farzaei M.H. Efficacy of topical application of standardized extract of Tragopogon graminifolius in the healing process of experimental burn wounds. J. Tradit. Complement. Med. 2018;9(1):54–59. doi: 10.1016/j.jtcme.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helal D.A., Abdel-rehman A.D., Abdel-halim S.A., Nabarawi M.A. Formulation and evaluation of fluconazole topical gel. Int. J. Pharm. Pharm. Sci. 2012;4:176–183. [Google Scholar]
- Jain A., Deveda P., Chauhan J. Development of antifungal emulsion based gel for topical fungal infection. Int. J. Pharm. Res. Dev. 2011;2:18–25. [Google Scholar]
- Jain A., Gautam S.P., Gupta Y., Khambete H., Jain S. Development and characterization of ketoconazole emulgel for topical drug delivery. Der. Pharm. Sinica. 2010;1:221–231. [Google Scholar]
- Joseph, A., Cedric, K., Amengor, Emmanuel, O., et al., 2018. Development and validation of uv-visible spectrophotometric method for the determination of 5-hydroxymethyl furfural content in canned malt drinks and fruit juices in Ghana. J. Food. Quality. 2019, 8 pages.
- Kased R.F., Amer R.I., Attia D., Elmazar M.M. Honey-based hydrogel. In Vitro and comparative In vivo evaluation for burn wound healing. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-08771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H., Akhtar N., Ali A., Khan H.M. Physical and chemical stability analysis of cosmetic multiple emulsions loaded with ascorbyl palmitate and sodium ascorbyl phosphate salts. Acta Polo. Pharma. Drug Res. 2016;5:1339–1349. [PubMed] [Google Scholar]
- Khan B.A., Shahid U., Muhammad K.K. Fabrication, physical characterizations and in vitro, in vivo evaluation of ginger extract-loaded gelatin/poly(vinyl alcohol) hydrogel films against burn wound healing in animal model. AAPS PharmScitech. 2020 doi: 10.1208/s12249-020-01866-y. [DOI] [PubMed] [Google Scholar]
- Kokane D.D., More R.Y., Kale M.B., Nehete M.N., Mehendale P.C., Gadgoli C.H. Evaluation of wound healing activity of root of Mimosa pudica. J. Ethno. Pharmacol. 2009;124:311–315. doi: 10.1016/j.jep.2009.04.038. [DOI] [PubMed] [Google Scholar]
- Kumar M.P., Gosh A. Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Euro. J. Pharm. Sci. 2017;96:243–254. doi: 10.1016/j.ejps.2016.09.038. [DOI] [PubMed] [Google Scholar]
- Li C., Liu C., Liu J., Fang L. Correlation between rheological properties, in vitro release, and percutaneous permeation of tetrahydropalmatine. Pharm. Sci. Tech. 2011;12(3):1002–1009. doi: 10.1208/s12249-011-9664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmet E.O., Şule A., Vildan Y., Neşe B.A., Ayşegül Y. Evaluation of burn wound healing activity of novel fusidic acid loaded microemulsion based gel in male Wistar albino rats. Saudi. Pharm. J. 2020;28:338–348. doi: 10.1016/j.jsps.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya K.S., Gnanamani N.A., Radhakrishnan B.M. Healing potential of Datura albaon burn wounds in albino rats. J. Ethnopharma. 2002;83:193–199. doi: 10.1016/s0378-8741(02)00195-2. [DOI] [PubMed] [Google Scholar]
- Rasul A., Akhtar N. Formulation and in vivo evaluation for anti-aging effects of an emulsion containing basil extract using non- invasive biophysical techniques. DARU. 2011;19:344–350. [PMC free article] [PubMed] [Google Scholar]
- Sarisozen C., Vural I., Levchenko T., Hincal A.A., Torchilin V.P. PEG-PE-based micelles co-loaded with paclitaxel and cyclosporine A or loaded with paclitaxel and targeted by anticancer antibody overcome drug resistance in cancer cells. Drug Deliv. 2012;19:169–176. doi: 10.3109/10717544.2012.674163. [DOI] [PubMed] [Google Scholar]
- Sekar K., Thangaraj S., Babu S., Harisaranraj R., Suresh K. Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum basilicum. J. Phytol. 2009;1:408–413. [Google Scholar]
- Singh R.P., Parpani S., Narke R., Chavan R. Emulgel: A recent approach for topical drug delivery system. A. J. Pharm. Res. 2014;2:112–123. [Google Scholar]
- Somashekar S., Saraswati U., Laxminarayan U. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of ocimum sanctum linn in rats. Evid. Based. Complement. Alternat. Med. 2008;5:95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okur M.E., Ayla Ş., Çiçek Polat D., Günal M.Y., Yoltaş A., Biçeroğlu Ö. Novel insight into wound healing properties of methanol extract of Capparis ovata Desf. var. palaestina Zohary fruits. J. Pharm. Pharmacol. 2018;70:1401–1413. doi: 10.1111/jphp.12977. [DOI] [PubMed] [Google Scholar]
- Yen, W.F., Mahiran, B., Mansor, A., Maznah, I., 2015. Formulation and Evaluation of Galantamine Gel as Drug Reservoir in Transdermal Patch Delivery System. Sci. World. J. 2015, 7 pages. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
It can be obtained from the corresponding author on demand.