Abstract
Natural bioflavonoids are an essential component of dietary supplements possessing antimicrobial properties. Many of the bioflavonoids have resulted in positive antitumor, anticancer, antibacterial, antifungal, anti-inflammatory properties, but the efficacy remains low due to toxicity at the molecular level whereas antiviral property limits to negative. The synergistic link between nanoscience and flavonoid chemistry enhances the epidemiological properties of flavonoid and also diminish the antimicrobial resistivity (AMR) by forming their hybrid nanocomposites. Nanochemistry uses various nanocomposite and nanomaterials for biosensing the flavonoids and their delivery as a drug. The quercetin flavonoid and its derivatives such as rutin, and myricetin are used for sensing and drug delivery. Quercetin with 15Carbon-5Hydroxyl chemical scaffold has been explored for a few decades for the development of hybrid nanocomposite and nanomaterial with metallic as well as organic nano co-composites. This quercetin flavonoid based hybrid nanocomposites seemed to show a significant effect on In vitro and some animal model processes along with attenuating lipid peroxidation, platelet aggregation, and capillary permeability actions. This review mainly focused on the hybrid nanoscience of quercetin bioflavonoid and its application in numerous biological, material fields with a future perspective.
Keywords: Quercetin, Flavonoid, Nanocomposites, Biomedical applications, Antimicrobial resistivity
1. Introduction
Flavonoids are an important class of bioactive compounds with effective antimicrobial activities (Berkessel, n.d.). These flavonoids are commonly known as bioflavonoids because these are extracted from natural dietary sources like onions, grapes, orange, celery (Panche et al., 2016, Boots et al., 2008). The first bioflavonoid is isolated and identified by Szent-Gyorgyi (de la Rosa et al., 2018). Out of more than 6000 flavonoids, experimental science has been explored for few for the determination of epidemiological properties (Docheva et al., 2012).
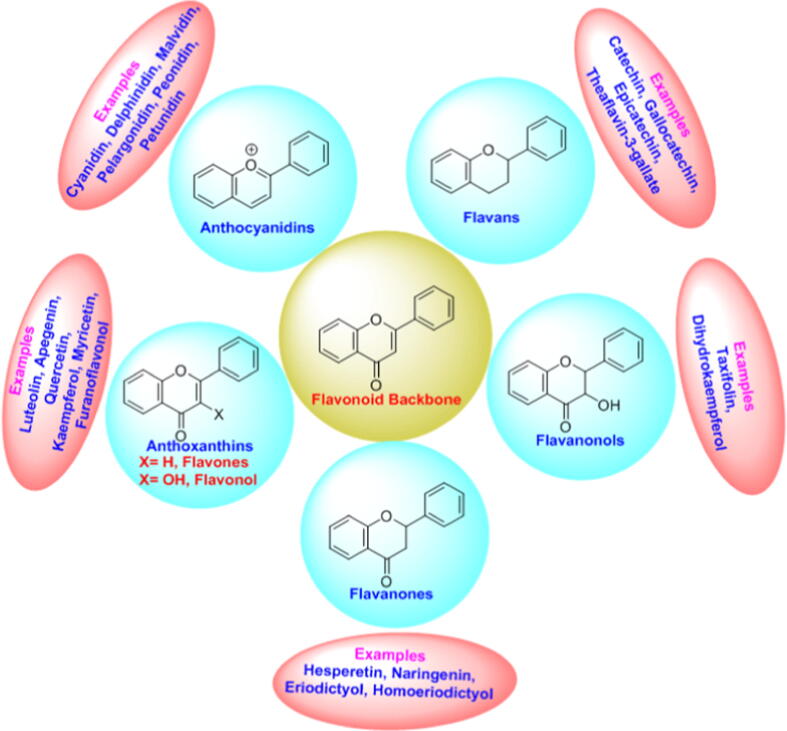
Bioflavonoids are mainly classified into five subgroups namely anthocyanidins, flavans, flavonols, anthoxanthins, and, flavanones. Fig. 1 represents the classification of bioflavonoids. The major flavonols found from numerous natural sources are quercetin (QCN), myricetin, morin, kaempferol, galangin. Chemically flavonols are polyphenolic compounds with two phenolic rings attached to a pyranone ring with a total of five hydroxyl groups along with fifteen carbon atoms.
Fig. 1.
Classification of bioflavonoid backbones.
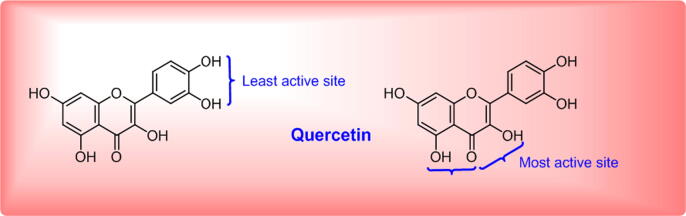
Initially, nanotechnology has been used for sensing flavonols and their derivatives like quercetin, myricetin, rutin, morin due to their high antioxidant, anti-inflammatory (Gomes et al., 2008), and, antiproliferative properties (Bello et al., 2018, Saha et al., 2016, Lozano et al., 2019, Zhang et al., 2019, Rosas et al., 2019). The name of the polyphenolic compound quercetin is derived from quercetum which means the “oak forest”. The natural flavonol quercetin is found in many leafy vegetables, fruits, vegetables, herbs. The examples are radish leaves, fennel leaves, pear, apple, cranberry, grapes, onions, tomato, kale, capers, and red wine. Among all, capers contain a high amount of quercetin up to 234 mg per 100 gm (Bhagwat et al., 2011). Out of all available natural sources, the culinary vegetable onion with quercetin 30 mg/100 g is self-sufficient to fulfill the average daily consumption limit of quercetin i.e. 25–50 mg. Even in the crude form along with other components, onion has shown a high range of medicinal value. Research has proven that the extract of onion peel (OPE) is a great source of antioxidants. It is tested for the storage of animal meat with the inhibition of microorganism growth in the medium (Ifesan, 2017). In 2016, Kim et al. have reported OPE, which is a rich source of quercetin significantly decrease obesity in women (Kim and Yim, 2016). Since ancient, it has been used in Chinese medicine (Li et al., 2019). The influential properties of such flavonoids based composites on hormone levels, resistivity against bacterial and cancer cell lines are the key steps to overcome the challenges of the nano biotechnogy research field. Such technology has proven its essentiality for developed medical care at the nanoscale level. Literature also gave an idea of such bio-based nanomaterials in the medical areas via many processes like stents, drugs, tablets and accessories. The chemical structure of quercetin is represented in Scheme 1. In 2006, Leopoldini et al. have demonstrated the active binding site of quercetin flavonol. The two binding sites 3-hydroxyl-Carbonyl and 5-hydroxyl-Carbonyl are more active than the catechol part. Mira et al. reported the antioxidant properties of flavonoids like quercetin through the reducing activity towards copper and iron ions (Mira et al., 2002, Leopoldini et al., 2006).
Scheme 1.
Active sites of bioflavonol quercetin.
In this review, we focused on the applications of quercetin based nanocomposite through different techniques along with brief characterization and uses of quercetin in different perspectives including sensing, drug delivery through a different medium (oral, skin-based), encapsulation, anti-bacterial, anti-cancer, antioxidant, and anti-inflammatory properties. We will be discussing the quercetin base NPs, nanocomposites for such applicative purposes in detail in Section 3. In 2014, Nikita et al. have extracted and isolated the quercetin particles by reverse-phase high performance liquid chromatographic (RP-HPLC) method from Tridaxprocumbens L (Tridax daisy, a plant family) simply and cost-effectively. The reverse-phase HPLC technique is found to be more precise for the determination of quercetin (Sanghavi et al., 2014). We will be discussing the sensitive and selective determination of quercetin based materials via different techniques in a detailed way in the later section. The understanding of the presence of quercetin in nanomaterials is very important due to the sensitive application processes. In 2015, Yun et al. have demonstrated the alginate-based quercetin NPs for heavy metal Pb(II) ionic adsorption in the aqueous solution. These particles have shown a better recyclability property with a maximum adsorption capacity of 140.37 mg L−1 with 0.2 g adsorbents in 1000 mg L−1of Pb(II) at pH 7 due to a partial inducement by the presence of few metal ions like Mn(II), Co(II), and Cd(II) (Qi et al., 2015). Lauren et al. have developed a newly designed nano dispersed quercetin NPs by different nanoformulations. The study had revealed the potential bioavailability of quercetin NPs due to the external parameters of surfactant type, surfactant amount, and stirring speed (Lefevre et al., 2016).
In 2018, Sunoqrot et al. have performed a unique approach for the development of surface-modified quercetin NPs for drug delivery approach. In that study, they revealed the synthesis of polyphenol quercetin NPs functionalized with ligands and drugs. Spherically modified NPs sized 30–40 nm has shown a drug loading of 35.6 ± 4.9% w/w with a loading efficiency of 88.9 ± 12.4% which is remarkably good for cell susceptibility tests. Hence, the modified NPs seem to be nontoxic in nature (Sunoqrot et al., 2018). Several reports about quercetin have been established with a new pathway to inhibit the microbial, cancer growth in the animal models. Quercetin and its composites have many potential applications in the treatment of a variety of diseases. The simple, eco-friendly, green synthetic methods for the development of quercetin based composites with less time consuming and cost-effective ways those possess higher therapeutic efficacy in terms of antioxidant (Mittal et al., 2014), antimicrobial and anticancer activities (Milanezi et al., 2019) has attracted many scientists to work on such biologically active materials (Kumari et al., 2012).
Recent years witnessed the use of quercetin bioflavonoids to develop hybrid nanocomposite (HNC) for biomedical applications. The major challenge to design HNC using quercetin with other nanomaterials is the toxicity of quercetin at the molecular level. Synergistic effect between nanochemistry and flavonol chemistry triggers the rapid growth in this particular area. Irrespective of rapid globalization with modern medical facilities, the present mortality rate per annum caused due to microorganisms like bacteria, viruses, fungi, the amoeba is too high. The major reason for this high death is the antimicrobial resistivity (AMR). 21st-century world mainly suffers from antibiotic resistivity (ABR) which is more synonymously used for antibacterial resistivity. The age for this period may be regarded as “the age of superbugs”. Lack of awareness for low use of antibiotics in animal husbandry, plant growth, and misuse of antibiotics in humans without a proper diagnosis is the main cause for the growth of antibacterial resistivity. The world health organization (WHO) reports suggesting that in 2050, the death due to AMR will be 10 million each year. The main concern for science and technology is to inhibit the AMR before it rules nature by endangering other lives. In this regard, nanotechnology researchers have been working with their complete effort for contributing the best. Few reports have been explored in this field in a particular direction to deal with anti-bacterial resistivity (ABR). Quercetin flavonol based hybrid nanocomposite has been the most focused case due to the broad range of antimicrobial properties (Abdellah et al., 2018, Sun et al., 2016). Several useful applications of quercetin based hybrid nanocomposite and their initiative journey towards suppressing antimicrobial resistivity have been well discussed in this review (Lee and Park, 2019, Sathishkumar et al., 2021, Din et al., 2017, Verma et al., 2013).
2. Characterizations
2.1. Physical, chemical, and biological experiments
Earlier research on quercetin has developed the knowledge of such scaffold for the betterment of human purpose. But the significance of such flavonol is proved by several characterization techniques. Such techniques are involved in FTIR, XRD, TEM, SEM, TGA, Antioxidant behaviour, Antibacterial susceptibility, anticancer property, and many more which we will be exploring in the later section of the discussion.
In general, dynamic scattering light measurement is performed to observe the particle size in a solution of the suspended matrix at different solvent. Rahmanand et al. have developed the quercetin nanomaterial in ethanol–water mixture by using a very simple nanoprecipitation technique. The observed particle size analysis for quercetin is revealed with an average hydrodynamic diameter of 16–17 nm (Abd and El-Rahmanand Suhailah, 2014, Fang et al., 2011). Previous research suggested the hydrodynamic radius and ζ potential of flavonoid-based Ag-Se NPs in aqueous solution are observed to be 40 nm and −19 mV, respectively. This report has mentioned the uniform dispersion of capped QCN phytochemicals by negatively charged metallic group nanocomposites (Mittal et al., 2014). The capping mechanism of QCN phytochemical is the prime reason for the hindrance towards the formation of aggregation. Saha et al. performed the ζ potential and DLS measurements for quercetin loaded PLGA NPs. The ζ potential and the hydrodynamic radius are observed of −10.0 mv and 180 nm respectively for such NPs which proved to be very much potential for the biomedical purpose (Saha et al., 2016).
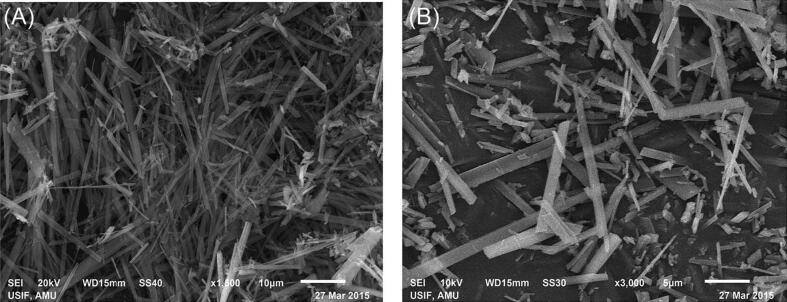
The scanning electron microscopic analysis is an ideal technique for the quantitative morphological study of the macro and nanometric dimensional surface structure of the desired synthesized particles. SEM micrograph of original QCN particles showed the lack of uniformity in size than newly developed quercetin with less crystallinity and agglomeration of bigger particles (see Fig. 2). The particle diameter of the QCN NPs is significantly smaller and more uniform than that of the virgin quercetin. The controlled environmental parameters like stirring rate, time, and the concentration of the quercetin led to a beautiful morphology which is described by Rahmanand et al. (Abd and El-Rahmanand Suhailah, 2014, Fang et al., 2011).
Fig. 2.
illustrates the SEM image for quercetin NPs at two different magnification range [31]. [Reproduced with the permission from Elsevier].
The transmission electron microscopic is the best technique to analyze fringes, the d-spacing pattern on the surface of the synthesized nanoparticles at the minimal range. Qi et al. have discussed the surface morphology of quercetin loaded alginate (Q-AN) NPs. In this work, Q-AN NPs showed a spherical diametre of only 1–2 nm with the hydroxyl group interaction and intermolecular bonding between quercetin and alginate NPs (Qi et al. 2015). The TEM images (Alam et al.) revealed the successful synthesis of QCN NPs at the nano range. The particles seemed to be rod-like structure with the diameter ranges from 50 to 500 nm and length to be in some micron size. The similar morphological structures of QCN NPs hence described the uniform behaviour of such particles at different dimensions. The arranged and uniform structure of QCN based biomaterial could be used as a potential candidate for drug delivery and other biological therapy in the future study. Sunoqrot et al. have reported the particle size of pQCT@PEG and pQCT@FITC-PEG based nanocomposites with an average diameter of 30 and 40 nm with the spherical shape. Both the nanocomposites have seemed to possess the unchanged and uniform morphology. Mittal et al. also have suggested about the capping morphology formation by quercetin on the surface of bimetallic (Ag-Se) nanoparticles make the nanomaterial a potential candidate for antitumor and antimicrobial application (Mittal et al., 2014). Depending on the morphological behaviour of such composites seems to act as a smart candidate for numerous application in biotechnology.
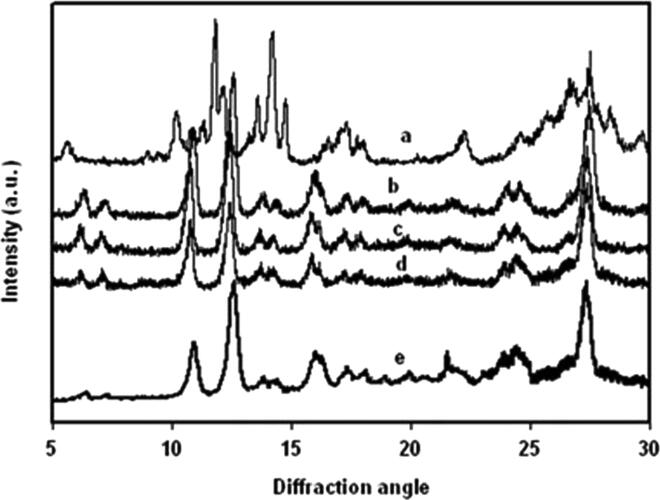
XRD is a technique to describe the crystallinity, size of the formed crystallites along with the d-spacing patterns of the samples in a precise manner. Performing physical characterizations like XRD and TG-DTA, scientists have preferred to study the crystallinity and thermal behaviour of quercetin. XRD is performed at 2θ between 10 and 80°. X-ray diffraction analysis for quercetin showed peaks at 2θ of 12.6803°, 14.6068°, 16.0944°, 16.6714°, 18.6049°, 20.1795°, 20.9740°, 23.2009°, 25.5010°, 27.9646°, and 28.9808°. The quercetin also showed peaks at 5.2°, 10.06°, 11.76°, 13.52°, 26.38°, and 27.38° which are very similar to virgin quercetin material (Abd and El-Rahmanand Suhailah, 2014, Moreira da Costa et al., 2002, Sahoo et al., 2011). Fig. 3 represents the XRD of original quercetin (a) and quercetin nanocrystals at different concentrations (b–e).
Fig. 3.
Illustrates the XRD of original quercetin (a), and quercetin nanocrystals at different concentration of 2 wt% (b), 5 wt% (c), 10 wt% (d), and 2 wt% after 90 days (e) respectively [34]. [Reproduced with permission from Elsevier].
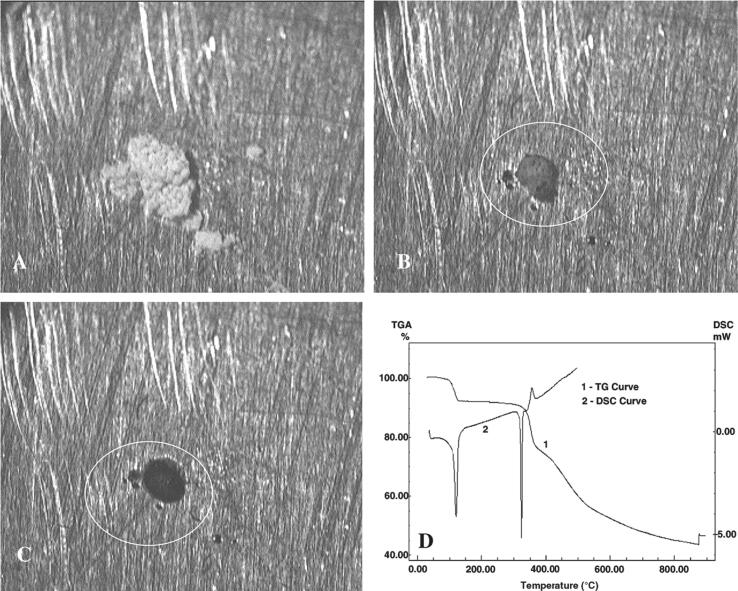
The thermogravimetric analysis is an ideal technique for studying the thermal stability, heat flow, and the structural deformation of the all-dimensional synthesized particles in an inert environment. TGA study of quercetin at 116 °C, has shown the decomposition of water molecules from the system. Picture A, B and C have shown the quercetin thermal visual study at room temperature, 125 °C and 314 °C respectively. The DSC curve for quercetin presented three-phase transition peaks. The third peak at 344 °C, an exothermal peak, corresponds to the initial decomposition process for the quercetin (Fig. 4) (Moreira da Costa et al., 2002, Sahoo et al., 2011, Rodríguez-Félix et al., 2019, Bruno et al., 2010). Qi et al. have discussed the thermal stability of quercetin based alginate NPs to be stable up 540.9 °C range (Qi et al. 2015).
Fig. 4.
represents the thermal state of QCN at (A) room temperature (RT), (B) 125 °C, (C) 314 °C, and (D) TG/DSC curve of the quercetin under nitrogen atmosphere [33].[Reproduced with the permission from Elsevier].
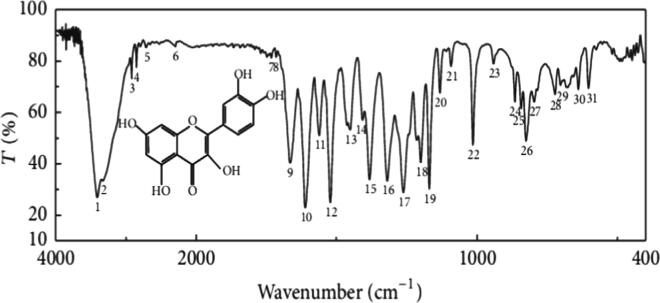
FTIR measurement is used to identify the chemical structure and the functional group of the prepared materials from 400 cm−1 to 4000 cm−1. Bruno et al. have performed the FTIR experiment to know the functionality and chemical groups present in the quercetin. They have mentioned about the two significant peaks associated with quercetin. The FTIR spectra suggested a significant OH stretch band of at 3400 cm−1 due to the presence of H-bonded OH groups. The large presence of OH groups accounts for the solubility of the polymer in the ethanol/water solvent system. Another prominent peak is observed in the 1550–1650 cm−1 regions due to the quinone molecule stretching spectra (see Fig. 5) (Bruno et al., 2010, Catauro et al., 2015). Milanezi et al. in his report suggested the presence of significant functional groups like at 3248 cm−1 (O—H stretching), 1670 cm−1 (C O stretching), and 1500 cm−1 (C—C stretching) which belong to capped QCN based Au NPs with the most important absorption bands in between lower region of 650 and 1000 cm−1 (aromatic compounds) (Milanezi et al., 2019).
Fig. 5.
illustrates the FTIR spectra of the monomer (quercetin) and the polyuercetin [37]. [Reproduced with the permission from Elsevier].
The position of carbon and hydrogen environment can be easily observed by NMR analysis of any organic-based material in the required solvents. Slimestal et al. have described a detailed NMR study of bioflavonoids including quercetin (Slimestad et al., 2007). The predicted NMR report of is suggested by Fossen et al. in 1998. They have reported the 1H NMR and 13C NMR of quercetin isolated from red onion. The 1H chemical shifts of quercetin taken in deuterated methanol (CD3OD) are 6.27, 6.47, 7.82, 6.97, 7.72 ppm for 6, 8, 2′, 5′, 6′ position respectively. The 13C NMR chemical shifts of quercetin in deuterated methanol are 148.00, 137.21, 177.33, 162.50, 99.25, 165.34, 94.40, 158.22, 104.52, 124.15, 115.99, 148.75, 146.21, 116.22, 121.67 ppm for 2, 3, 4, 5, 6, 7, 8, 9, 10, 1′, 2′, 3′, 4′, 5′, 6′ position respectively.
The percentage of viable cells is determined by MTT assay and it helps to find the cytotoxicity of the material against cancerous and noncarcinoma cells in a particular environment. The Hela cells are cultured for 24 h in DMEM medium. In vitro cytotoxicity assay is performed for quercetin. Saraswat et al. have reported the incorporation of quercetin in stealth liposomal formulations for the treatment of cervical cancer. It has been found that liposomal formulations enhanced the solubility of quercetin NPs than the virgin quercetin NPs by 44-fold and increased the liposomal encapsulation. Hence, it is concluded that quercetin NPs are great biocompatible agents for cytotoxicity and acted as a promising drug carrier (Slimestad et al., 2007, Michalski et al., 2020, Saraswat and Maher, 2020). Milanezi et al. in his work have mentioned about the excellent anticancer, antioxidant and antimicrobial activity possessed by Au based quercetin NPs. The cytotoxicity experiment is performed against L929 fibroblasts cells at a concentration of 7 × 105 cells/. The physiochemical and biological application of quercetin based nanocomposites are very much useful for biomedical applications.
2.2. Sensitive and selective determination of quercetin based materials via different techniques
To check the superfluity of these components for the benefit of mankind, various techniques have been used for sensing purposes. These components can be broadly classified into two sections namely inorganic, and organic components for sensing. Another component section which rarely considers for sensing purposes can be categorized as complex of these two. The minerals like the ions of sodium, magnesium, iron, potassium, fluoride, mercury, arsenic have a tremendous impact on human health (Alva-Ensastegui et al., 2018). The lack and over-accumulation of these minerals cannot be ignored due to its involvement in physiological processes. Both can be fatal if the concentration does not fall under the normal range. Each country has its normal range for these minerals which can be found from different sources. WHO also recommends the normal range for such minerals especially the minerals from a water source as water is the most abundant requirement for humans. For sensing these minerals many reports have been produced in the past by using different techniques (Hsu and Lin, 2016). The last decade witnessed the rapid need for sensing heavy minerals like arsenic, mercury. In this regard, many publications came, out of which the sensing using fluorescence technique remains a preferred choice due to its cost-effective broad range sensing. Recently, our group also report the sensing of mercury using silver-based nanocomposite via fluorescence technique (Sahu et al., 2019a, Sahu et al., 2019b).
Similarly, the bioactive molecules and drugs have their normal beneficial range for human wellbeing, and over range can lead to various health problems. Out of all the flavonoids molecules, the penta hydroxy polyphenol quercetin which plentily available from dietary source possesses a wide range of medicinal properties as it scavenges the radical species. But with high concentration when the dose is high with quercetin drug, it shows nephrotoxicity leading to kidney cancer (Liu et al., 2017a). To know the concentration of such flavonoid based QCN especially the threshold concentration which is beneficial for medical use, various techniques have been used for the detection of this miraculous phytochemical. These techniques involve spectrophotometry, capillary electrophoresis, high-performance liquid chromatography (HPLC), pulse, and wave voltammetry.
2.2.1. Derivative spectrophotometric method
The derivative spectra of UV spectrometric analysis mainly follow two measurements “graphical” and “zero crossing”. It possesses several gains including precise determination of maximum λ, quantitative separation of merged absorption peaks, turbidity determination, and minor spectral increment observed on a broad background. Graphical measurements include ‘peak to peak’ and ‘peak to baseline’ measurement. As far as the literature concern, the first report for sensing the quercetin is published by Nikolovska et al. in 1996 using first and second derivative spectrophotometric methods for chrysin and quercetin (Nikolovska-Čoleska et al., 1996). The advantage of this method is that without the interference of primary purification theory, the mixture can be used for the analysis. The derivative spectrophotometric technique has been used for the dose analysis and determination of pharmaceutical drugs like quercetin due to its simple, sensitive, and rapid technology.
2.2.2. Capillary electrophoresis (CE)
The capillary electrophoresis method is useful for the separation of samples which are electroactive. The detector used in this case uses UV or UV–Vis range of absorbance for the spectral outcomes. Quercetin being one of the electroactive flavonoids at moderate oxidation potential has been used for sensing purposes using the CE method. In 2000, Jiannong Ye group have reported the capillary electrophoresis with electrochemical detection of quercetin and rutin in plants (Chen et al., 2000). The quantitative detection of quercetin is reported by Sun et al. in 2003 using the electrophoresis method (Sun et al., 2003).
2.2.3. High performance liquid chromatography
High performance liquid chromatographic technique is the most sophisticated technology which provides high-quality analysis with absolute precision. The analytes are determined by observing the retention time with the amplitude. This method separates samples as well as identifies them quantitatively using a solid stationary phase column with the mobile liquid phase. Generally, the detectors used in this technique are IR and UV. The first publication for the determination of quercetin like flavonoids using HPLC is reported by Ward et al. (Ward, 1974). But the fractionation and preliminary purification of analyte make this analysis technique less appreciable. The first report of the detection of quercetin in human plasma and urine using the HPLC technique is reported by Ishii et al. (Ishii et al., 2003). Berrueta team has published online characterization of QCN using HPLC coupled UV and mass spectrometry (Alonso-Salces et al., 2004). The reversed-phase HPLC method with diode array detection is introduced for the detection quercetin by Zu et al. (Zu et al., 2006). Simultaneous detection of quercetin along with rutin is reported by Lin et al. by reversing differential pulse voltammetry (Lin et al., 2006). Another report came by Wu et al. for the detection of quercetin using capillary electrophoresis (Wu et al., 2012). A tandem technique using ionic liquid-based pressurized liquid extraction-liquid chromatography-chemiluminescence is reported by Zhu group for detection of trace amount of quercetin, rutin in Chinese herbal medicine (Xu et al., 2007). Solak and Shen teams have reported the detection of quercetin using the square wave voltammetry method [(Yola et al., 2013). Solak et al. used a novel voltammetric sensor based on p-Aminophenol functionalized graphene oxide/gold nanoparticles for the detection of quercetin whereas Shen group used a novel graphene oxide-ionic liquid modified electrode for such purpose (Guo et al., 2013, Lawrence et al., 1984).
3. Applications of quercetin and quercetin based nanomaterials
The abundant dietary flavonol quercetin possesses many pharmacological properties like anti-oxidant, anti-inflammatory, anti-cancer, anti-proliferative, anti-bacterial, and anti-allergic. It has been used for the treatment of cardio-protective diseases, osteoporosis, and obesity. Due to the above mentioned bioactive properties, quercetin has been widely used in the field of science and technology for a range of applications. The prominent applications in which quercetin has been used for sensing, drug delivery purpose, as an enhancer. Other than these applications, the recent area of interest is to design quercetin based hybrid nanocomposite for biomedical applications.
3.1. As a sensing parameter
The need for the concept of sensing arises to counter the consequences of the quote “Excess of everything is bad”. The necessity of minerals, vitamins, proteins as well as bioactive molecules to living healthy is highly commendable. But the excess of these components can change the physiological health condition drastically which can lead to various fatal diseases. He et al. have studied the in situ sensing behaviour of Al3+ residues in food by natural quercetin-based AIEgen composite film with significant antibacterial and antioxidant properties (He et al., 2018). Being an aggregation-induced emission (AIE) active organic compound, quercetin has also indicated the food quality and enhanced the life span of the storage capacity of food material by novel sensing parameter. Yang et al. in his report mentioned quercetin as a fluorescent sensor to detect the presence of excess Cu2+ ions in aqueous media. In this report, the binding stoichiometry of the quercetin based Cu(II) the complex is found to be a very stable value of 3.56 × 107 at pH 7.4 (Yang et al., 2015). Literature is also proven the quercetin based materials as very good fluorescence promoters to study the spectrofluorometric quantification in the water medium using several surfactants and similar flavonoids (Alva-Ensastegui et al., 2018). Yao et al. have discussed the quercetin sensor-based RGO/Ag nanocomposite designed electrochemically. The quercetin-based modified electrode has shown its sensor efficiency by measuring the drug and food residue in medicinal tablets practically (Yao et al., 2018). This sensor application seemed to be quite useful for the biomedical industry. In a recent study, it is found that the quercetin-based bismuth nanocomposite has been effectively used as a sensor for the detection of dihydroxybenzene isomers in the sea and tap water (Patel et al., 2020, Tohidinia et al., 2018). Quercetin coated Fe3O4 nanomaterials also acted as a sensor detector to remove the excess of Pb2+ and Cu2+ ions in a low-filed NMR phase by coordination reaction mechanism (Jiang et al., 2018a). Table 1 represents a list of useful work based on quercetin materials and its numerous sensing applications.
Table 1.
List of reports associated with the sensing applications of quercetins based materials.
| Material type | Loaded with composition | Sensing Application | Reference |
|---|---|---|---|
| Nanoparticles | CNT/PS@quercetin | Flos Sophorae Immaturus. | Wen et al. |
| Polymer | PMMA@quercetin | Drug delivery | Lee et al. |
| Nanocomposite | QACF based AIEgen quercetin | Al3+ along with antioxidant property | He et al. |
| Natural | querecetin | Cu+2 and fluorescent sensor | Yang et al. |
| Nanocomposite | RGO-Ag@quercetin | Electrochemical | Yao et al. |
| Nanocomposite | Bismuth@quercetin | dihydroxybenzene isomers and nitrite | Tohidina et al. |
| NPs | Fe3O4@quercetin | Cu+2 and Pb+2 in biological samples | Jiang et al. |
3.2. Drug delivery
Drugs are bioactive compounds used for healing diseases in animals and humans. Synthetic as well as naturally isolated drug possesses numerous pharmacological properties to deal with various medical conditions. Naturally, isolated drugs have proven to be safer than synthetic drugs due to a lack of hazardous synthetic site (Sarkar et al., 2018). But the complication remains with most of the synthetic and naturally isolated drugs because of the bioavailability at the molecular level. Bioavailability data relay on different parameters like solubility, pH, and stability along with other physiological conditions. For resolving the complication of bioavailability, drug modifications, prodrug enhancers, and suitable drug carriers especially nanocarriers for delivery have been implemented for different classes of drugs. Last decades witness, the rapid growth of drug delivery in nanoscience. Drugs like doxorubicin, ofloxacin, taxol, iloprost, dopamine, chlorpromazine being selected mostly for drug delivery. Nanocarriers such as hybrid nanocomposite hydrogel (Jain and Mehata, 2017) and hybrid nanocomposites (Prusty and Swain, 2018, Prusty and Swain, 2019, Prusty et al., 2019) are proven to be most suitable for such drugs.
Quercetin with numerous wondering pharmacological properties has moderate bioavailability due to its poor water solubility (Gugler et al., 1975). To solve the issue of solubility, quercetin has first interacted with DMSO for the improvement of solubility but the process remains unsuccessful due to the toxicity of DMSO (Gugler et al., 1975). Previous research suggested the excellent solubility of quercetin in ethanol than other organic polar as well as non-polar solvents (Sunoqrot et al. 2018); (Mulholland et al., 2001) have also designed a water-soluble prodrug of quercetin by a derivatizing three –OH group of quercetin with a (N-Carboxymethyl) carbamoyl group to increase the solubility (Muther and Bennett, 1980). But the bioavailability also remains low 20–25% after hydrolysis. Kale et al. tried the cyclodextrin-quercetin complex system to increase bioavailability for in-vivo anticancer activity (Kale et al., 2006). Danihelova et al. have reported various derivatives of quercetin for the enhancement of bioavailability of quercetin to check the antioxidant activity and cytotoxicity on HeLa and NIH 3T3 cells (Danihelová et al., 2013). To interpret the drug delivery method and biological applications in a wide range, various methods with numerous drugs have been used. The techniques include skin-based drug delivery, oral drug delivery, antioxidant study, antitumor and microbial performance, and drug encapsulation theory.
3.2.1. Drug delivery application of quercetin based nanomaterials through the skin
Focusing on the drug delivery behaviour of quercetin, a lot of research has been performed for the betterment of humans. Lee et al. have discussed the quercetin loaded polymethyl methacrylate (PMMA) microcapsules (Lee et al., 2007). They even mentioned the smooth morphology of such spherical microcapsules with a diameter of 1.03 ± 0.12 mm to 2.39 ± 0.42 mm, and the encapsulation efficiency from 12.7% to 26.9%. With the attachment of PMMA, free uercetin has developed controlled diffusion behaviour towards the in-vitro study. There are also reports available with quercetin loaded material shown promising bacterial degradation in the large intestine of the rat by improving the biocompatibility (Matsukawa et al., 2009) and antioxidant property by microemulsion method for skin (Kitagawa et al., 2009, Sangai and Verma, 2011). Kitagawa et al. have discussed the enhanced intradermal delivery by microemulsion of quercetin/polyoxyethylene sorbitan monooleate /isopropyl myristate via micropig skin. The breaking of emulsified vesicles while approaching the surface of the skin is the prime reason for the stimulation of quercetin from the emulsion into the surface of the stratum corneum (Kitagawa et al., 2009). Abhijit et al. have mentioned in his work about the therapeutic efficacy of quercetin by the interaction of novel phospholipid based cationic nanocarriers (LeciPlex) (Date et al., 2011) quercetin-based LeciPlex nanocarriers have shown a prominent role by improving oral delivery of hydrophobic drugs including those with poor solubility in oils and solid lipids.
3.2.2. Antioxidant and antibacterial study of quercetin based nanomaterial in drug delivery
Being a phenolic aromatic compound, quercetin possesses natural antioxidant properties due to which the use of quercetin based materials has been extensively reported previously. The quercetin loaded chitosan NPs have shown a promising drug delivery and antioxidant pathway. Zhang et al. have evaluated the in vitro experiment by two different methods of free radical scavenging activity test and reducing power test to show the inclusion of quercetin in chitosan NPs and the usefulness of bioavailability (Zhang et al., 2008). Barbosa et al. have mentioned the strong antioxidant property and controlled release study of quercetin-loaded fucoidan@chitosan NPs under a simulated gastrointestinal environment, which has prevented the quercetin degradation by increasing the oral drug (Barbosa et al., 2019). There are also many reports associated with quercetin related to a different type of drug delivery which includes the encapsulation of quercetin based silicon quantum dots for monitored delivery (Wang et al., 2013b), protective effects of quercetin on rats due to potent antioxidant properties (Aktoz et al., 2010), PLGA based co-encapsulated/tamoxifen@quercetin (QT) polymeric nanocomposite (Jain et al., 2013) encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems impact on quercetin bioaccessibility (Pool et al., 2013). Jain et al. have reported PLGA based co-encapsulated/tamoxifen@quercetin polymeric nanocomposite and its encapsulation formation which enhanced the drug delivery efficacy in the medium with the particle diameter of 185 nm and entrapment efficiency of drugs 67%. The encapsulation theory of drug-loaded quercetin nanomaterials improved the solubility of quercetin particles and maximized the stability with significant biocompatible properties. The study confirmed the smart behaviour of quercetin based nanomaterials found to be more remarkable along with polymeric nanomaterials compared to metal oxide composites. The encapsulation mechanism has signified numerous paths for drug delivery of quercetin based materials. Sun et al. in his work have discussed the significant antibacterial activity along with the insertion of PLGA/quercetin (PQTs) composites with several incubation periods. He gave a solid foundation that novel PQTs biomaterials are very much suitable for disinfection in vivo (Sun et al. 2016).
3.2.3. Oral drug delivery by quercetin based materials
Oral drug delivery has remained a suitable and easy technique in the practice of drug delivery of numerous nanomaterials. Tran et al. have mentioned about the flavonoid quercetin with its useful chemoprotective effect and low bioavailability because of low solubility in aqueous solution (Tran et al., 2014). They have demonstrated that Q-SNEDDS formulation increased the transport of quercetin across the Caco-2 cell monolayer and enhanced the surface absorption and oral bioavailability of quercetin in rats. Piyasi et al. have discussed the encapsulation efficiency of quercetin loaded NSC/alginate microparticles (MPs) which is found to be 94% (Mukhopadhyay et al., 2016). The loaded MPs have shown a significant hypoglycemic response following oral delivery in diabetic rats. Wang et al. have discussed the oral drug delivery of quercetin particles in very detail. They developed a non-aqueous self-double-emulsifying drug delivery system (SDEDDS) for the enhancement of oral bioavailability of low water-soluble quercetin and formulated for both in vitro and in vivo. The pharmacokinetic studies have mentioned an increased relative bioavailability about 5 times of QT-SDEDDS compared to quercetin suspension in rats after oral administration of 26.67% (Wang et al., 2016). The significant oral drug delivery system remained a great scope for quercetin in future use.
3.2.4. Antitumour and microbial performance of materials composed of quercetin in drug delivery
Though quercetin has many prominent biological applications, the study of different NPs, metal oxides, and polymer embedded quercetin materials have shown a better scope in the practical applications. Here the discussion will be based on the drug delivery application of such materials embedded with quercetin. Polyethylene Glycol has been used for numerous applications and very good for the bowel movement. Wang et al. in his work, suggested about the PEG 2000-DPSE-coated quercetin NPs and its glioma C6 cells tumor cell killing efficiency. His team also mentioned the dose-dependent cytotoxicity to C6 glioma cells and enhanced ROS (Reactive Oxygen Species) accumulation with an increase in cytochrome c and caspase-3 protein level proving enhanced anticancer effect on C6 glioma cells (Wang et al., 2013a). Another report has also mentioned about the liposomal formulation acted as a suitable carrier for co-delivery of chemotherapeutic drugs to overcome multidrug-resistant effect (MDR) of PEG-based quercetin and suitable to use for in vitro and in vivo applications (Zhang et al., 2016, El-Gogary et al., 2014). Zhang et al. in his report have mentioned the worldwide problem of multidrug resistance (MDR) property against cancer therapy. The development of Biotin based PEG@quercetin loaded polymeric composites easily addressed these issues with the highest cytotoxicity against MCF-7/adr cell lines and this is due to the liposomal formation of a polymer with the natural quercetin. Sunoqrot et al. have followed a greener synthetic route for the plant polyphenol quercetin loading with a hydrophobic anticancer drug, curcumin, and functionalized with PEG for drug delivery application against a cancer cell line with higher antiproliferative properties (Sunoqrot et al., 2019). It is reported that many factors are depending on which the designed materials showed its efficacy against bacterial, viral, and cancer cell lines. The factors are including of the morphology, nature of the synthesized material, solution behaviour, and solubility. In this discussion, we have observed that the quercetin based nanomaterials are seemed to be well soluble in aqueous medium with a spherical morphology in most of the cases.
But here we will discuss different forms of quercetin based nanomaterials and its response towards the anticancer activity. Hydrogels (Quagliariello et al., 2017), lipid NPs (Liu et al., 2015), biopolymer (Zhang et al., 2018), Germanium NPs (Guo et al., 2014), hyaluronic acid (Barbarisi et al., 2018), CuS@MOF (Jiang et al., 2018b), Ag NPs (Sun et al., 2017), Au NPs (Yilmaz et al., 2019) and many more polymeric gels(Yu et al., 2020, Xu et al., 2018) loaded quercetin nanomaterials are responsible for drug delivery therapy for anti-ovarian cancer, articulate cartilage repair, antibacterial molecular mechanism. Liu et al. have reported that the nanoemulsion of quercetin/Laden lipid particles protects eye cells from oxidative damage by encapsulation theory. Possessing a higher cytotoxicity value also, this material helps the eye to suffer from cancer attacks (Liu et al. 2015). Zhang et al. also have reported about a pH-sensitive and biocompatible quercetin/GO/ poly-ether amine /hyaluronic acid nanocarrier to inhibit the growth of cancer cell line from an infected tumor (Zhang et al. 2018). Multifunctional nanoplatform of quercetin/CuS@ZIF/ folic acid-bovine serum albumin has actively targeted both in vivo and in vitro cancer therapy by near-infrared (NIR) fluorescent imaging. Following the literature study, it is clear that quercetin based nanomaterials are found to be the most efficient flavonoids for the treatment of various cancers with virtually no toxic side effects.
3.2.5. Drug encapsulation and anti-inflammatory study of quercetin based nanomaterials
The encapsulation study of quercetin in surfactant polymeric micelles in aqueous media (Fraile et al., 2014) has improved the solubility via non-covalent interaction in the organic network (Vyas et al., 2016, Park et al., 2017). The schematic diagram of the encapsulation process has been attached in Fig. 6. Pedro et al. have demonstrated the pH-responsive amphiphilic chitosan loaded NPs to encapsulate quercetin (QCN) for sustained drug release in cancer therapy. The spherical sized micelles ranged from 140 to 300 nm have inhibited MCF-7 cell growth with a good hemocompatibility resulted as a very good carrier for hydrophobic drugs in cancer therapy (Pedro et al., 2017), Huang et al. (Huang et al., 2017) and Wen et al. (Wen et al., 2018b) in their work also stated the encapsulation of flavonoids in liposomes and antiproliferative effects on cancer cells. Pedro et al. also reported that the quercetin based NPs can encapsulate up to 78% of the drug in it for biological purposes.
Fig. 6.
represents a schematic for a general representation of the encapsulation process.
There are many reports associated with quercetin based encapsulation drug delivery in in-vitro digestion model (Masrul et al., 2018, Salehi et al., 2020, Ni et al., 2017, Marcolin et al., 2012) against few cancer cells including HeLa cell lines (Ni et al., 2017) and its encapsulation efficacy for anti-inflammatory and antioxidant behaviour (Guazelli et al., 2013, Rodriguez et al., 2019, Wen et al., 2018a). Table 2 represents a detailed list of significant work based on quercetin based materials and its wide drug delivery application
Table 2.
List of detailed reports of quercetins and its composites with several drug delivery and other biomedical applications.
| Material type | Loaded with composition | Application | Reference |
|---|---|---|---|
| Nanoparticle | PTX@Chitosan | Pulmonary drug delivery | Liu et al. |
| Nanocomposite | quercetin @Cyclodextrin | Cytotoxicity against human erythroleukaemia and cervix cancer cells | Kale et al. |
| Derivative | quercetin | Antioxidant action and cytotoxicity on HeLa and NIH-3T3 | Danihelova et al. |
| Emulsion | quercetin -pMMA | Model Falvonid Drug | Lee et al. |
| Natural | quercetin aglycon | Bacreial degradation in large intenstine and loss of glucoside in rats | Mastukawa et al. |
| Synthetic | LeciPlex@QCN | Improve therapeutic efficacy on oral administatrion | Date et al. |
| Nanoparticle | Chitosan- quercetin | Antioxidant | Zhang et al. |
| Polymer | PEG-quercetin | Oral drug delivery | Tran et al. |
| Polymeric nanocapsules | PEG@quercetin | Folate expressing anticancer drugs | Riham et al. |
| Polymer | PEG-quercetin | In vivo cancer activity | Sunoqrot et al. |
| Nanoparticle | Ag@ quercetin | Antibacterial | Sun et al. |
| pH responsive | Chitosan@quercetin | Drug delivery | Pedro et al. |
| Liposomes | quercetin | Encapsulation of Drugs | Huang et al. |
3.2.6. Quercetin and its nanomaterials as an enhancer
Kim et al. have reported about the novel quercetin Flavonoids and its effect on bone metabolism. Previous literature involved the controversial statement toward the effect of these flavonoids on osteoblastogenesis (Kim et al., 2006). The differentiation and proliferation of quercetin on human adipose tissue-derived stromal cells (hADSC) are discussed and observed to increase osteogenic differentiation in a dose-dependent manner. To satisfy the enhancing and stimulating property of quercetins, his team also focused on a few other flavonoids like chrysin, kaempferol, etc. and studied the increased osteogenic differentiation of hADSC. But surprisingly, the stimulatory effect of quercetin is higher than the above-mentioned flavonoids. Even it is also confirmed that the quercetin helped to inhibit the proliferation of hADSC, but is not that much reproducible for the survival of cells. The pre-treatment of quercetin, in hADSC enhanced in vitro osteogenic differentiation, and the transplantation of such cells also helped in bone regeneration in a skull defect model of nude mice efficiently.
Following his work, many works have been established but among them, Zhou et al. reported the overall effect of quercetin on proliferation and osteogenic differentiation of mouse adipose stem cells (mASCs) in vitro system (Zhou and Lin, 2014). A similar result is also obtained by this team with the less active proliferation study and enhanced osteogenesis processes by up-regulating gene expression. Anti-inflammatory and anti-cancer properties of quercetin are performed on mice model by Chen and his team in 2017 by using A549 cells. These findings demonstrated that a diet containing 0.1% or 1% quercetin enhances the antitumor effect of TSA and prevents TSA-induced muscle isting (Chan et al., 2018). There are limitless research is going on the natural vitamins, flavonoids, steroids carbohydrates, etc. for their naturally inbuilt antimicrobial and anticancer properties (Zhou et al., 2017). Table 3 represented a detailed list of outstanding work based on quercetin and its enhanced property application.
Table 3.
List of detailed reports of quercetins and its nanomaterials role in enhancing properties.
| Material type | Loaded with composition | Application | Reference |
|---|---|---|---|
| Liposome | quercetin | 44 fold cytotoxicity against Cervical cancer | Saraswat et al. |
| Microemulsion | QCN | Enhanced Skin delivery | Kitagawa et al. |
| Nanoparticle | Chitosan-quercetin | Amplified oxidant property | Zhang et al. |
| Nanoparticle | Au@ quercetin | Enhanced Tumor targeting tendency | Yilmaz et al. |
| Derivative | Pluronic@ quercetin | Enhanced Delivery in Poloxamers by Antisolvent Process | Fraile et al. |
| Free quercetin | Quercetin | Inhibit Proliferation and acts as an enhancer | Kim et al. |
| Free quercetin | quercetin with protein | Anti tumor and anticancer (enhancer) | Chen et al. |
3.3. New aspects of quercetin in nanoscience: quercetin based hybrid composite and its applications
There are few reports available particularly with the quercetin based hybrid nanocomposites concerning the application towards sensing, drug delivery, etc. In this report, we have mentioned a detailed list of nanocomposites associated with quercetin particles and showed the applicative proforma for a better understanding of future research. The current time is dependent on composite materials due to the stability, durability, strengthening behaviour, low cost with multipurpose use. Although, quercetin is a naturally occurring material it has shown its great effect on the non-biological application due to the stabilizing property (Arrigo et al., 2015). Arrigo et al. have proved that quercetin is an excellent anti-oxidant (Arrigo et al., 2016) for polyethylene with a very stable thermo-oxidation behaviour with a good radical scavenging activity (Lu et al., 2018). Fabricated Au NPs@quercetin is synthesized by green and environmentally safe reducing and capping agents by Osonga et al. (Osonga et al., 2019). The team has performed and calculated a very good catalytic activity for quercetin-loaded triangular AuNPs of kinetic rate constants (k) of 3.44 × 10−2 s−1 whereas, spherical NPs possessed of k, 1.11 × 10−2 s−1 respectively. Silica has been acted as a very smart material. Silica@quercetin NPs also resembled their smartness in dental transplants (Jadeja and Devkar, 2013), drug loading, and anticancer property (Liu et al., 2017b). Quercetin nanocomposites also act as a very novel anticancer therapeutic which improved anticancer efficiency and reduced toxicity in the last decade (Cirillo et al., 2013). Orazio et al. have mentioned that CNTs@PMMAlaoded quercetin NPs acted as a very good candidate for CP synergistic treatment against neuroblastoma (Vittorio et al., 2014) In the last decade, many works have been performed for quercetin loaded composites and its usefulness against chronic diseases (Alban et al., 2020, Chu et al., 2018, Rajkumar et al., 2019, Sanchez-Rexach et al., 2019, Ma et al., 2018). Table 4 represented a detailed list of good work based on quercetin-based composite and its numerous applications. In a very recent study, Satishkumar group has developed ZnO-quercetin nanocomposite by fabricated technology. They have mentioned about the successful loading of quercetin into ZnO NPs with 210 μg mg−1. They also observed an excellent anticancer activity against MCF-7 cancer cells with the protection of ZnO NPs cytotoxicity effect after uercetin attachment. Hence the literature suggests that quercetin based nanocomposite has been proving itself as an essential medium between materials science and biotechnology (Sathishkumar et al. 2021).
Table 4.
List of detailed reports of quercetins based composites and other applications.
| Material type | Loaded with composition | Application | Reference |
|---|---|---|---|
| Microparticles | Chitosan/alginate @ quercetin | Oral delivery to diabetic animals | Mukhopadhyay et al. |
| Nanocomposites | DOX/QUE BPL @quercetin | Multi drug resistant breast cancer | Zhang et al. |
| Polymer composite | PEG- quercetin @Curcumin | Invivo fate | Sunoqrot et al. |
| Composite | CNT@ quercetin | Antioxidant | Arrigo et al. |
| Composite | Au@ quercetin NPs | Catalytic behaviour | Osongaet al. |
| Composite | MMA-CNT- quercetin | Anticancer against neuroblastoma | Orazioet al. |
| Composite | GO-PEG- quercetin | Diabetic Wound Healing | Chu et al. |
| Composite | PCL@ quercetin | biomimetic bacterial S-layer coatings | Eva et al. |
| Composite | quercetin @zein@chitosan NPs | Antioxidant | Zuan et al. |
4. Significance of future work
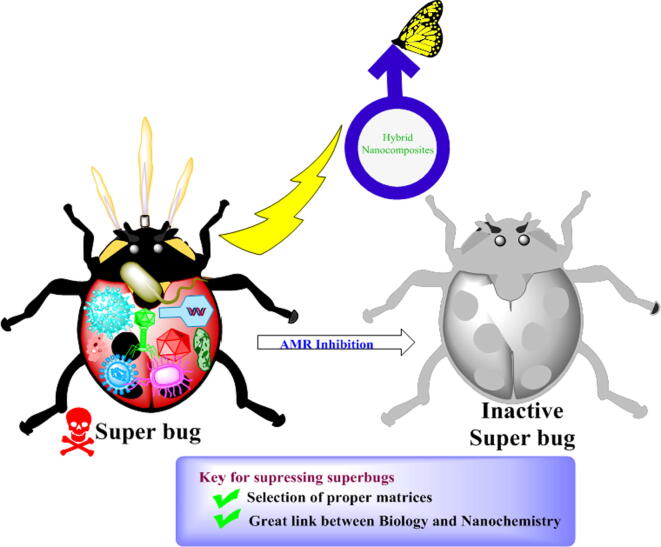
After all the heat and trial methods, it is thought to design a biocompatible carrier that can deliver the precious drug. Many reports have been successfully established for such but the efficacy remains less prominent which is troubleshot with the introduction of hybrid nanocomposites. Few reports have been explored with a moderate selection of matrices which could be used for the betterment of human health but the betterment can be questionable with the adventurous growth of AMR. To overcome this issue, the selection of matrices for the design of new hybrid nanocomposites through the biological study will be the key. Understanding the biology along with nanochemistry can resolve this issue by inhibiting the AMR. We are strongly working in this area for resolving the issue of AMR growth. Fig. 7 represents the potential application of quercetin based materials.
Fig. 7.
represents the schematic view for the use of quercetin hybrid nanocomposites to inhibit AMR.
5. Conclusions
In conclusion to this review, quercetin the natural bioflavonoid with multi-pharmacological properties has been widely used in drug delivery applications. As the concentration of quercetin based nanomaterials has a greater role in the physiological impact of life, sensing for quercetin also been explored with a similar frequency. Nanoscience which is the most dynamic field of research uses quercetin in different matrices for the development of composites with bio-importance. These composites have been used for various biomedical and sensing applications. Towards the end of this review, recent works including nanoscience of quercetin have been discussed along with the most vital issue for human health. The issue of antimicrobial resistance more often known for antibacterial resistance (ABR) will be the leading killing devil shortly as mentioned by WHO. This issue can be resolved with continuous research in science especially in the field of nanoscience. In this regard, we are working regularly for designing composites with different matrices of quercetin for the inhibition of AMR which could be used to service mankind. Presently, few composites have been under examination for Antibacterial study along with AMR study and other interesting compositions are with the characterization units.
Acknowledgments
Authors acknowledge the research support grants awarded by UGC India to DSK Kothari Post-Doctoral fellowship to Dr.Biswajit Parhi. Dr. BP and Dr. DB acknowledge Veer Surendra Sai University of Technology, Burla, India for the support in research facilities.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd Soheir N., El-Rahmanand Suhailah S.A.-J. Quercetin nanoparticles: preparation and characterization. Indian J. Drugs. 2014;2:96–103. [Google Scholar]
- Abdellah A.M., Sliem M.A., Bakr M., Amin R.M. Green synthesis and biological activity of silver-curcumin nanoconjugates. Future Med. Chem. 2018;10:2577–2588. doi: 10.4155/fmc-2018-0152. [DOI] [PubMed] [Google Scholar]
- Aktoz T., Kanter M., Aktas C. Protective effects of Quercetin on testicular torsion/detorsion-induced ischaemia-reperfusion injury in rats. Andrologia. 2010;42:376–383. doi: 10.1111/j.1439-0272.2010.01044.x. [DOI] [PubMed] [Google Scholar]
- Alban L., Monteiro W.F., Diz F.M., Miranda G.M., Scheid C.M., Zotti E.R., Morrone F.B., Ligabue R. New Quercetin-coated titanate nanotubes and their radiosensitization effect on human bladder cancer. Mater. Sci. Eng. C. 2020;110 doi: 10.1016/j.msec.2020.110662. [DOI] [PubMed] [Google Scholar]
- Alonso-Salces R.M., Ndjoko K., Queiroz E.F., Ioset J.R., Hostettmann K., Berrueta L.A., Gallo B., Vicente F. On-line characterisation of apple polyphenols by liquid chromatography coupled with mass spectracometry and ultraviolet absorbance detection. J. Chromatogr. A. 2004;1046:89–100. doi: 10.1016/j.chroma.2004.06.077. [DOI] [PubMed] [Google Scholar]
- Alva-Ensastegui J.C., Palomar-Pardavé M., Romero-Romo M., Ramírez-Silva M.T. Quercetin spectrofluorometric quantification in aqueous media using different surfactants as fluorescence promoters. RSC Adv. 2018;8:10980–10986. doi: 10.1039/c8ra01213j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo R., Dintcheva N.T., Guenzi M., Gambarotti C., Filippone G., Coiai S., Carroccio S. Thermo-oxidative resistant nanocomposites containing novel hybrid-nanoparticles based on natural polyphenol and carbon nanotubes. Polym. Degrad. Stab. 2015;115:129–137. doi: 10.1016/j.polymdegradstab.2015.02.014. [DOI] [Google Scholar]
- Arrigo R., Dintcheva N.T., Guenzi M., Gambarotti C. Nano-hybrids based on Quercetin and carbon nanotubes with excellent anti-oxidant activity. Mater. Lett. 2016;180:7–10. doi: 10.1016/j.matlet.2016.05.096. [DOI] [Google Scholar]
- Barbarisi M., Iaffaioli R.V., Armenia E., Schiavo L., De Sena G., Tafuto S., Barbarisi A., Quagliariello V. Novel nanohydrogel of hyaluronic acid loaded with Quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018;233:6550–6564. doi: 10.1002/jcp.26238. [DOI] [PubMed] [Google Scholar]
- Barbosa A.I., Costa Lima S.A., Reis S. Application of pH-responsive fucoidan/chitosan nanoparticles to improve oral Quercetin delivery. Molecules. 2019;24 doi: 10.3390/molecules24020346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello O.A., Ayanda O.I., Aworunse O.S., Olukanmi B.I. Phytotherapeutics of polyphenolic-loaded drug delivery systems: a review. Pharmacogn. Rev. 2018;1:8–15. doi: 10.4103/phrev.phrev. [DOI] [Google Scholar]
- Berkessel, H.G., n.d. Asymmetric Organocatalysis: From Biomimetic Concepts to Applications in Asymmetric Synthesis. Textbook. 10.1155/2013/162750. [DOI]
- Bhagwat S., Haytowitz D.B., Holden J.M. USDA database for the flavonoid content of selected foods release 3 prepared by USDA database for the flavonoid content of selected foods release 3 prepared by. U.S. Dep. Argic. 2011:1–156. [Google Scholar]
- Boots A.W., Haenen G.R.M.M., Bast A. Health effects of Quercetin: from antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Bruno F.F., Trotta A., Fossey S., Nagarajan S., Nagarajan R., Samuelson L.A., Kumar J. Enzymatic synthesis and characterization of polyQuercetin. J. Macromol. Sci. Part A Pure Appl. Chem. 2010;47:1191–1196. doi: 10.1080/10601325.2010.518839. [DOI] [Google Scholar]
- Catauro M., Papale F., Bollino F., Piccolella S., Marciano S., Nocera P., Pacifico S. Silica/Quercetin sol-gel hybrids as antioxidant dental implant materials. Sci. Technol. Adv. Mater. 2015;16 doi: 10.1088/1468-6996/16/3/035001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.T., Chuang C.H., Lin Y.C., Liao J.W., Lii C.K., Yeh S.L. Quercetin enhances the antitumor effect of trichostatin A and suppresses muscle isting in tumor-bearing mice. Food Funct. 2018;9:871–879. doi: 10.1039/c7fo01444a. [DOI] [PubMed] [Google Scholar]
- Chen G., Zhang H., Ye J. Determination of rutin and Quercetin in plants by capillary electrophoresis with electrochemical detection. Anal. Chim. Acta. 2000;423:69–76. doi: 10.1016/S0003-2670(00)01099-0. [DOI] [Google Scholar]
- Jing Chu, Panpan Shi, Wenxia Yan, Jinping Fu, Zhi Yang, Chengmin He, X.D., nd H.L., 2018. PEGylated graphene oxide-mediated Quercetin modified collagen hybrid scaffold for enhancement of MSCs differentiation potential and diabetic wound healing. Nanoscale 6, 1–12. 10.1039/x0xx00000x. [DOI] [PubMed]
- Cirillo G., Vittorio O., Hampel S., Iemma F., Parchi P., Cecchini M., Puoci F., Picci N. Quercetin nanocomposite as novel anticancer therapeutic: improved efficiency and reduced toxicity. Eur. J. Pharm. Sci. 2013;49:359–365. doi: 10.1016/j.ejps.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Danihelová M., Veverka M., Šturdík E., Jantová S. Antioxidant action and cytotoxicity on HeLa and NIH-3T3 cells of new Quercetin derivatives. Interdiscip. Toxicol. 2013;6:209–216. doi: 10.2478/intox-2013-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date A.A., Nagarsenker M.S., Patere S., Dhawan V., Gude R.P., Hassan P.A., Aswal V., Steiniger F., Thamm J., Fahr A. Lecithin-based novel cationic nanocarriers (Leciplex) II: improving therapeutic efficacy of Quercetin on oral administration. Mol. Pharm. 2011;8:716–726. doi: 10.1021/mp100305h. [DOI] [PubMed] [Google Scholar]
- de la Rosa J.D.P., Ruiz-Palomino P., Arriola-Guevara E., García-Fajardo J., Sandoval G., Guatemala-Morales G.M. A green process for the extraction and purification of hesperidin from mexican lime peel (Citrus aurantifolia Swingle) that is extendible to the citrus genus. Processes. 2018;6:3–13. doi: 10.3390/pr6120266. [DOI] [Google Scholar]
- Din M.I., Arshad F., Hussain Z., Mukhtar M. Green adeptness in the synthesis and stabilization of copper nanoparticles: catalytic, antibacterial, cytotoxicity, and antioxidant activities. Nanoscale Res. Lett. 2017;12 doi: 10.1186/s11671-017-2399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docheva M., Dagnon S., St. Statkova D.D. Isolation of bioflavonoids from tobacco. Trakia J. Sci. 2012;10:79–83. [Google Scholar]
- El-Gogary R.I., Rubio N., Wang J.T.W., Al-Jamal W.T., Bourgognon M., Kafa H., Naeem M., Klippstein R., Abbate V., Leroux F., Bals S., Van Tendeloo G., Kamel A.O., Awad G.A.S., Mortada N.D., Al-Jamal K.T. Polyethylene glycol conjugated polymeric nanocapsules for targeted delivery of Quercetin to folate-expressing cancer cells in vitro and in vivo. ACS Nano. 2014;8:1384–1401. doi: 10.1021/nn405155b. [DOI] [PubMed] [Google Scholar]
- Fang R., Jing H., Chai Z., Zhao G., Stoll S., Ren F., Liu F., Leng X. Design and characterization of protein-Quercetin bioactive nanoparticles. J. Nanobiotechnol. 2011;9:1–14. doi: 10.1186/1477-3155-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraile M., Buratto R., Gómez B., Martín Á., Cocero M.J. Enhanced delivery of Quercetin by encapsulation in poloxamers by supercritical antisolvent process. Ind. Eng. Chem. Res. 2014;53:4318–4327. doi: 10.1021/ie5001136. [DOI] [Google Scholar]
- Gomes A., Fernandes E., Lima J., Mira L., Corvo M. Molecular mechanisms of anti-inflammatory activity mediated by flavonoids. Curr. Med. Chem. 2008;15:1586–1605. doi: 10.2174/092986708784911579. [DOI] [PubMed] [Google Scholar]
- Guazelli C.F.S., Fattori V., Colombo B.B., Georgetti S.R., Vicentini F.T.M.C., Casagrande R., Baracat M.M., Verri W.A. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J. Nat. Prod. 2013;76:200–208. doi: 10.1021/np300670w. [DOI] [PubMed] [Google Scholar]
- Gugler R., Leschik M., Dengler H.J. Disposition of Quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975;9:229–234. doi: 10.1007/BF00614022. [DOI] [PubMed] [Google Scholar]
- Guo J., Kong W., Wang L., Ren H., Sun X., Ye B., Shen Q. Simultaneous determination of rutin and Quercetin at a graphite oxide and ionic liquid modified electrode by square wave voltammetry. Sens. Lett. 2013;11:603–606. doi: 10.1166/sl.2013.2823. [DOI] [Google Scholar]
- Guo Y.J., Yang F., Zhang L., Pi J., Cai J.Y., Yang P.H. Facile synthesis of multifunctional germanium nanoparticles as a carrier of Quercetin to achieve enhanced biological activity. Chem. - An Asian J. 2014;9:2272–2280. doi: 10.1002/asia.201402227. [DOI] [PubMed] [Google Scholar]
- He T., Wang H., Chen Z., Liu S., Li J., Li S. Natural Quercetin AIEgen composite film with antibacterial and antioxidant properties for in situ sensing of Al3+ residues in food, detecting food spoilage, and extending food storage times. ACS Appl. Bio Mater. 2018;1:636–642. doi: 10.1021/acsabm.8b00128. [DOI] [PubMed] [Google Scholar]
- Hsu N.Y., Lin Y.W. Microwave-assisted synthesis of bovine serum albumin-gold nanoclusters and their fluorescence-quenched sensing of Hg2+ ions. New J. Chem. 2016;40:1155–1161. doi: 10.1039/c5nj02263k. [DOI] [Google Scholar]
- Huang M., Su E., Zheng F., Tan C. Encapsulation of flavonoids in liposomal delivery systems: the case of Quercetin, kaempferol and luteolin. Food Funct. 2017;8:3198–3208. doi: 10.1039/c7fo00508c. [DOI] [PubMed] [Google Scholar]
- Ifesan B.O.T. Chemical composition of onion peel (allium cepa) and its ability to serve as a preservative in cooked beef. Int. J. Sci. Res. Methodol. 2017;7:25–34. [Google Scholar]
- Ishii K., Furuta T., Kasuya Y. High-performance liquid chromatographic determination of Quercetin in human plasma and urine utilizing solid-phase extraction and ultraviolet detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003;794:49–56. doi: 10.1016/S1570-0232(03)00398-2. [DOI] [PubMed] [Google Scholar]
- Jadeja, R.N., Devkar, R. V., 2013. Polyphenols and Flavonoids in Controlling Non-Alcoholic Steatohepatitis, Polyphenols in Human Health and Disease. Elsevier Inc. 10.1016/B978-0-12-398456-2.00047-5. [DOI]
- Jain S., Mehata M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-15724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A.K., Thanki K., Jain S. Co-encapsulation of tamoxifen and Quercetin in polymeric nanoparticles: Implications on oral bioavailability, antitumor efficacy, and drug-induced toxicity. Mol. Pharm. 2013;10:3459–3474. doi: 10.1021/mp400311j. [DOI] [PubMed] [Google Scholar]
- Jiang Weina, Yang S., Sun X., Lu W., Jiang D., Xu L., Xu H., Gao B., Ma M., Cao F. Quercetin-coated Fe3O4 nanoparticle sensors based on low-field NMR for determination and removal of Pb2+ and Cu2+ in biological samples. Anal. Methods. 2018;10:2494–2502. doi: 10.1039/c8ay00598b. [DOI] [Google Scholar]
- Jiang Wei, Zhang H., Wu J., Zhai G., Li Z., Luan Y., Garg S. CuS@MOF-Based Well-Designed Quercetin Delivery System for Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces. 2018;10:34513–34523. doi: 10.1021/acsami.8b13487. [DOI] [PubMed] [Google Scholar]
- Kale R., Saraf M., Juvekar A., Tayade P. Decreased B16F10 melanoma growth and impaired tumour vascularization in BDF1 mice with Quercetin-cyclodextrin binary system. J. Pharm. Pharmacol. 2006;58:1351–1358. doi: 10.1211/jpp.58.10.0008. [DOI] [PubMed] [Google Scholar]
- Kim Y.J., Bae Y.C., Suh K.T., Jung J.S. Quercetin, a flavonoid, inhibits proliferation and increases osteogenic differentiation in human adipose stromal cells. Biochem. Pharmacol. 2006;72:1268–1278. doi: 10.1016/j.bcp.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Kim K.-A., Yim J.-E. The effect of onion peel extract on inflammatory mediators in Korean overweight and obese women. Clin. Nutr. Res. 2016;5:261. doi: 10.7762/cnr.2016.5.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S., Tanaka Y., Tanaka M., Endo K., Yoshii A. Enhanced skin delivery of Quercetin by microemulsion. J. Pharm. Pharmacol. 2009;61:855–860. doi: 10.1211/jpp/61.07.0003. [DOI] [PubMed] [Google Scholar]
- Kumari A., Kumar V., Yadav S.K. Plant extract synthesized PLA nanoparticles for controlled and sustained release of Quercetin: a green approach. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence Marshall, P.E. camp and S.B., 1984. Dimethyl Sulfoxide for the treatment of intracranial hypertension: A preliminary Trail. [DOI] [PubMed]
- Lee Y.J., Park Y. Green synthetic nanoarchitectonics of gold and silver nanoparticles prepared using quercetin and their cytotoxicity and catalytic applications. J. Nanosci. Nanotechnol. 2019;20:2781–2790. doi: 10.1166/jnn.2020.17453. [DOI] [PubMed] [Google Scholar]
- Lee D.-H., Sim G.-S., Kim J.-H., Lee G.-S., Pyo H.-B., Lee B.-C. Preparation and characterization of Quercetin-loaded polymethyl methacrylate microcapsules using a polyol-in-oil-in-polyol emulsion solvent evaporation method. J. Pharm. Pharmacol. 2007;59:1611–1620. doi: 10.1211/jpp.59.12.0002. [DOI] [PubMed] [Google Scholar]
- Lefevre, L., M. Ferreira, A., C.E. Vilhena, J., C. Florentino, A., A.S. Cruz, R., Bereau, D., Robinson, J.-C., R. R. Amado, J., C.T. Carvalho, J., P. Fernandes, C., 2016. Development of Quercetin Based Nanodispersions. Curr. Top. Med. Chem. 16, 2051–2056. 10.2174/1568026616666160215161333. [DOI] [PubMed]
- Leopoldini M., Russo N., Chiodo S., Toscano M. Iron chelation by the powerful antioxidant flavonoid Quercetin. J. Agric. Food Chem. 2006;54:6343–6351. doi: 10.1021/jf060986h. [DOI] [PubMed] [Google Scholar]
- Li H., Tan L., Zhang J.W., Chen H., Liang B., Qiu T., Li Q.S., Cai M., Zhang Q.H. Quercetin is the active component of yang-yin-qing-fei-tang to induce apoptosis in non-small cell lung cancer. Am. J. Chin. Med. 2019;47:879–893. doi: 10.1142/S0192415X19500460. [DOI] [PubMed] [Google Scholar]
- Lin X.Q., He J.B., Zha Z.G. Simultaneous determination of Quercetin and rutin at a multi-wall carbon-nanotube paste electrodes by reversing differential pulse voltammetry. Sensors Actuators, B Chem. 2006;119:608–614. doi: 10.1016/j.snb.2006.01.016. [DOI] [Google Scholar]
- Liu Z., Balasubramanian V., Bhat C., Vahermo M., Mäkilä E., Kemell M., Fontana F., Janoniene A., Petrikaite V., Salonen J., Yli-Kauhaluoma J., Hirvonen J., Zhang H., Santos H.A. Quercetin-based modified porous silicon nanoparticles for enhanced inhibition of doxorubicin-resistant cancer cells. Adv. Healthc. Mater. 2017;6:1–11. doi: 10.1002/adhm.201601009. [DOI] [PubMed] [Google Scholar]
- Liu K., Chen W., Yang T., Wen B., Ding D., Keidar M., Tang J., Zhang W. Paclitaxel and Quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. Int. J. Nanomed. 2017;12:8239–8255. doi: 10.2147/IJN.S147028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.H., Huang Y.C., Jhang J.W., Liu Y.H., Wu W.C. Quercetin delivery to porcine cornea and sclera by solid lipid nanoparticles and nanoemulsion. RSC Adv. 2015;5:100923–100933. doi: 10.1039/c5ra17423f. [DOI] [Google Scholar]
- Lozano O., Lázaro-Alfaro A., Silva-Platas C., Oropeza-Almazán Y., Torres-Quintanilla A., Bernal-Ramírez J., Alves-Figueiredo H., García-Rivas G. Nanoencapsulated Quercetin improves cardioprotection during hypoxia-reoxygenation injury through preservation of mitochondrial function. Oxid. Med. Cell. Longev. 2019;2019:1–14. doi: 10.1155/2019/7683051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N., Sui Y., Zeng L., Tian R., Peng Y.Y. Generation of a diligand complex of bovine serum albumin with quercetin and carbon nanotubes for the protection of bioactive quercetin and reduction of cytotoxicity. J. Agric. Food Chem. 2018;66:8355–8362. doi: 10.1021/acs.jafc.8b02327. [DOI] [PubMed] [Google Scholar]
- Ma J.J., Yu Y.G., Yin S.W., Tang C.H., Yang X.Q. Cellular uptake and intracellular antioxidant activity of zein/chitosan nanoparticles incorporated with quercetin. J. Agric. Food Chem. 2018;66:12783–12793. doi: 10.1021/acs.jafc.8b04571. [DOI] [PubMed] [Google Scholar]
- Marcolin E., San-Miguel B., Vallejo D., Tieppo J., Marroni N., González-Gallego J., Tuñón M.J. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis1–3. J. Nutr. 2012;142:1821–1828. doi: 10.3945/jn.112.165274. [DOI] [PubMed] [Google Scholar]
- Masrul M.Z., Suprayogi T., Diantoro M., Fuad A., Latifah E., Hidayat A. The effect of light irradiation on performance of photo-supercapacitor of FTO/TiO2-ZnO-β Carotene-Quercetin/Carbon/Al/PVDF-BaTiO3/Al. IOP Conf. Ser. Mater. Sci. Eng. 2018;515 doi: 10.1088/1757-899X/515/1/012077. [DOI] [Google Scholar]
- Matsukawa N., Matsumoto M., Shinoki A., Hagio M., Inoue R., Hara H. Nondigestible saccharides suppress the bacterial degradation of Quercetin aglycone in the large intestine and enhance the bioavailability of Quercetin glucoside in rats. J. Agric. Food Chem. 2009;57:9462–9468. doi: 10.1021/jf9024079. [DOI] [PubMed] [Google Scholar]
- Michalski J., Deinzer A., Stich L., Zinser E., Steinkasserer A., Knippertz I. Immunobiology Quercetin induces an immunoregulatory phenotype in maturing human dendritic cells. Immunobiology. 2020;151929 doi: 10.1016/j.imbio.2020.151929. [DOI] [PubMed] [Google Scholar]
- Milanezi F.G., Meireles L.M., de Christo Scherer M.M., de Oliveira J.P., da Silva A.R., de Araujo M.L., Endringer D.C., Fronza M., Guimarães M.C.C., Scherer R. Antioxidant, antimicrobial and cytotoxic activities of gold nanoparticles capped with Quercetin. Saudi Pharm. J. 2019;27:968–974. doi: 10.1016/j.jsps.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira L., Fernandez M.T., Santos M., Rocha R., Florêncio M.H., Jennings K.R. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic. Res. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- Mittal A.K., Kumar S., Banerjee U.C. Quercetin and gallic acid mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014;431:194–199. doi: 10.1016/j.jcis.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Moreira da Costa E., Filho José Maria Barbosa, Ticiano Gomes R.O.M., do Nascimento Thermal characterization of the Quercetin and rutin flavonoid. Thermochim. Acta. 2002;392–393:79–84. [Google Scholar]
- Mukhopadhyay P., Maity S., Chakraborty S., Rudra R., Ghodadara H., Solanki M., Chakraborti A.S., Prajapati A.K., Kundu P.P. Oral delivery of Quercetin to diabetic animals using novel pH responsive carboxypropionylated chitosan/alginate microparticles. RSC Adv. 2016;6:73210–73221. doi: 10.1039/c6ra12491g. [DOI] [Google Scholar]
- Mulholland P.J., Ferry D.R., Anderson D., Hussain S.A., Young A.M., Cook J.E., Hodgkin E., Seymour L.W., Kerr D.J. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of Quercetin. Ann. Oncol. 2001;12:245–248. doi: 10.1023/A:1008372017097. [DOI] [PubMed] [Google Scholar]
- Muther R.S., Bennett W.M. Effects of dimethyl sulfoxide on renal function in man. JAMA J. Am. Med. Assoc. 1980;244:2081–2083. doi: 10.1001/jama.1980.03310180047034. [DOI] [PubMed] [Google Scholar]
- Ni S., Hu C., Sun R., Zhao G., Xia Q. Nanoemulsions-based delivery systems for encapsulation of quercetin: preparation, characterization, and cytotoxicity studies. J. Food Process Eng. 2017;40:1–13. doi: 10.1111/jfpe.12374. [DOI] [Google Scholar]
- Nikolovska-Čoleska Ž., Klisarova L., Šuturkova L., Dorevski K. First and second derivative spectrophotometric determination of flavonoids chrysin and Quercetin. Anal. Lett. 1996;29:97–115. doi: 10.1080/00032719608000395. [DOI] [Google Scholar]
- Osonga F.J., Kariuki V.M., Wambua V.M., Kalra S., Nweke B., Miller R.M., Çeşme M., Sadik O.A. Photochemical synthesis and catalytic applications of gold nanoplates fabricated using quercetin diphosphate macromolecules. ACS Omega. 2019;4:6511–6520. doi: 10.1021/acsomega.8b02389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:1–15. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.H., Choi J.M., Cho E., Jeong D., Shinde V.V., Kim H., Choi Y., Jung S. Enhancement of solubility and bioavailability of quercetin by inclusion complexation with the cavity of mono-6-deoxy-6-aminoethylamino-β-cyclodextrin. Bull. Korean Chem. Soc. 2017;38:880–889. doi: 10.1002/bkcs.11192. [DOI] [Google Scholar]
- Patel B.R., Noroozifar M., Kerman K. Review—nanocomposite-based sensors for voltammetric detection of hazardous phenolic pollutants in water. J. Electrochem. Soc. 2020;167 doi: 10.1149/1945-7111/ab71fa. [DOI] [Google Scholar]
- Pedro R.de O., Pereira S., Goycoolea F.M., Schmitt C.C., Neumann M.G. Self-aggregated nanoparticles of N-dodecyl, N′-glycidyl(chitosan) as pH-responsive drug delivery systems for Quercetin. J. Appl. Polym. Sci. 2017;135 doi: 10.1002/app.45678. 45678-1–12. [DOI] [Google Scholar]
- Pool H., Mendoza S., Xiao H., McClements D.J. Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: impact of physical form on Quercetin bioaccessibility. Food Funct. 2013;4:162–174. doi: 10.1039/c2fo30042g. [DOI] [PubMed] [Google Scholar]
- Prusty K., Swain S.K. Nano silver decorated polyacrylamide/dextran nanohydrogels hybrid composites for drug delivery applications. Mater. Sci. Eng. C. 2018;85:130–141. doi: 10.1016/j.msec.2017.11.028. [DOI] [PubMed] [Google Scholar]
- Prusty K., Swain S.K. Release of ciprofloxacin drugs by nano gold embedded cellulose grafted polyacrylamide hybrid nanocomposite hydrogels. Int. J. Biol. Macromol. 2019;126:765–775. doi: 10.1016/j.ijbiomac.2018.12.258. [DOI] [PubMed] [Google Scholar]
- Prusty K., Biswal A., Bhusan S., Swain S.K. Synthesis of soy protein / polyacrylamide nanocomposite hydrogels for delivery of ciprofloxacin drug. Mater. Chem. Phys. 2019 doi: 10.1016/j.matchemphys.2019.05.038. [DOI] [Google Scholar]
- Qi Y., Jiang M., Cui Y.L., Zhao L., Zhou X. Synthesis of quercetin loaded nanoparticles based on alginate for Pb(II) adsorption in aqueous solution. Nanoscale Res. Lett. 2015;10:1–10. doi: 10.1186/s11671-015-1117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliariello V., Iaffaioli R.V., Armenia E., Clemente O., Barbarisi M., Nasti G., Berretta M., Ottaiano A., Barbarisi A. Hyaluronic acid nanohydrogel loaded with quercetin alone or in combination to a macrolide derivative of rapamycin RAD001 (Everolimus) as a new treatment for hormone-responsive human breast cancer. J. Cell. Physiol. 2017;232:2063–2074. doi: 10.1002/jcp.25587. [DOI] [PubMed] [Google Scholar]
- Rajkumar C., Nehru R., Chen S.M., Kim H., Arumugam S., Sankar R. Electrosynthesis of carbon aerogel-modified AuNPs@Quercetin: via an environmentally benign method for hydrazine (HZ) and hydroxylamine (HA) detection. New J. Chem. 2019;44:586–595. doi: 10.1039/c9nj05360c. [DOI] [Google Scholar]
- Rodriguez E.B., Almeda R.A., Vidallon M.L.P., Reyes C.T. Enhanced bioactivity and efficient delivery of Quercetin through nanoliposomal encapsulation using rice bran phospholipids. J. Sci. Food Agric. 2019;99:1980–1989. doi: 10.1002/jsfa.9396. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Félix F., Del-Toro-Sánchez C.L., Javier Cinco-Moroyoqui F., Juárez J., Ruiz-Cruz S., López-Ahumada G.A., Carvajal-Millan E., Castro-Enríquez D.D., Barreras-Urbina C.G., Tapia-Hernández J.A. Preparation and characterization of quercetin-loaded zein nanoparticles by electrospraying and study of in vitro bioavailability. J. Food Sci. 2019;84:2883–2897. doi: 10.1111/1750-3841.14803. [DOI] [PubMed] [Google Scholar]
- Rosas E.C., Correa L.B., das Graças Henriques M. Antiinflammatory properties of schinus terebinthifolius and its use in arthritic conditions. Bioact. Food as Diet. Interv. Arthritis Relat. Inflamm. Dis. 2019;489–505 doi: 10.1016/b978-0-12-813820-5.00028-3. [DOI] [Google Scholar]
- Saha C., Kaushik A., Das A., Pal S., Majumder D. Anthracycline drugs on modified surface of Quercetin-loaded polymer nanoparticles: a dual drug delivery model for cancer treatment. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0155710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo, N.G., Kakran, M., Shaal, L.A., Li, L., M¨uller, R.H., Pal, M.L.P.T., 2011. Preparation and Characterization of Quercetin Nanocrystals. J. Pharm. Sci. 100, 2379–2390. 10.1002/jps. [DOI] [PubMed]
- Sahu D., Sahoo G., Mohapatra P., Swain S.K. Dual activities of nano silver embedded reduced graphene oxide using clove leaf extracts: Hg 2+ sensing and catalytic degradation. ChemistrySelect. 2019;4:2593–2602. doi: 10.1002/slct.201803725. [DOI] [Google Scholar]
- Sahu D., Sarkar N., Mohapatra P., Swain S.K. Nano gold hybrid polyvinyl alcohol films for sensing of Cu2+ ions. ChemistrySelect. 2019;4:9784–9793. doi: 10.1002/slct.201902167. [DOI] [Google Scholar]
- Salehi B., Machin L., Monzote L., Sharifi-Rad J., Ezzat S.M., Salem M.A., Merghany R.M., El Mahdy N.M., Klllç C.S., Sytar O., Sharifi-Rad M., Sharopov F., Martins N., Martorell M., Cho W.C. Therapeutic potential of quercetin: new insights and perspectives for human health. ACS Omega. 2020;5:11849–11872. doi: 10.1021/acsomega.0c01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Rexach E., Iturri J., Fernandez J., Meaurio E., Toca-Herrera J.L., Sarasua J.R. Novel biodegradable and non-fouling systems for controlled-release based on poly(∊-caprolactone)/Quercetin blends and biomimetic bacterial S-layer coatings. RSC Adv. 2019;9:24154–24163. doi: 10.1039/c9ra04398e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangai N.P., Verma R.J. Quercetin alleviates bisphenol A-induced changes in nucleic acid and protein contents in mice. Acta Pol. Pharm. - Drug Res. 2011;68:867–873. [PubMed] [Google Scholar]
- Sanghavi N., Bhosale S.D., Malode Y. RP-HPLC method development and validation of Quercetin isolated from the plant Tridax procumbens L. J. Sci. Innov. Res. 2014;3:594–597. [Google Scholar]
- Saraswat A.L., Maher T.J. Development and optimization of stealth liposomal system for enhanced in vitro cytotoxic effect of Quercetin. J. Drug Deliv. Sci. Technol. 2020;55 doi: 10.1016/j.jddst.2019.101477. [DOI] [Google Scholar]
- Sarkar N., Sahoo G., Das R., Swain S.K. Three-dimensional rice straw-structured magnetic nanoclay-decorated tripolymeric nanohydrogels as superadsorbent of dye pollutants. ACS Appl. Nano Mater. 2018;1:1188–1203. doi: 10.1021/acsanm.7b00358. [DOI] [Google Scholar]
- Sathishkumar P., Li Z., Govindan R., Jayakumar R., Wang C., Long Gu F. Zinc oxide-Quercetin nanocomposite as a smart nano-drug delivery system: Molecular-level interaction studies. Appl. Surf. Sci. 2021;536 doi: 10.1016/j.apsusc.2020.147741. [DOI] [Google Scholar]
- Slimestad R., Fossen T., Vågen I.M. Onions: a source of unique dietary flavonoids. J. Agric. Food Chem. 2007;55:10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- Sun Y., Guo T., Sui Y., Li F. Quantitative determination of rutin, Quercetin, and adenosine in Flos Carthami by capillary electrophoresis. J. Sep. Sci. 2003;26:1203–1206. doi: 10.1002/jssc.200301437. [DOI] [Google Scholar]
- Sun D., Li N., Zhang W., Yang E., Mou Z., Zhao Z., Liu H., Wang W. Quercetin-loaded PLGA nanoparticles: a highly effective antibacterial agent in vitro and anti-infection application in vivo. J. Nanoparticle Res. 2016;18:1–21. doi: 10.1007/s11051-015-3310-0. [DOI] [Google Scholar]
- Sun D., Zhang W., Mou Z., Chen Y., Guo F., Yang E., Wang W. Transcriptome analysis reveals silver nanoparticle-decorated quercetin antibacterial molecular mechanism. ACS Appl. Mater. Interfaces. 2017;9:10047–10060. doi: 10.1021/acsami.7b02380. [DOI] [PubMed] [Google Scholar]
- Sunoqrot S., Al-Shalabi E., Messersmith P.B. Facile synthesis and surface modification of bioinspired nanoparticles from Quercetin for drug delivery. Biomater. Sci. 2018;6:2656–2666. doi: 10.1039/c8bm00587g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunoqrot S., Al-Debsi T., Al-Shalabi E., Hasan Ibrahim L., Faruqu F.N., Walters A., Palgrave R., Al-Jamal K.T. Bioinspired polymerization of quercetin to produce a curcumin-loaded nanomedicine with potent cytotoxicity and cancer-targeting potential in vivo. ACS Biomater. Sci. Eng. 2019;5:6036–6045. doi: 10.1021/acsbiomaterials.9b01240. [DOI] [PubMed] [Google Scholar]
- Tohidinia M., Farsadrooh M., Bahmanzadeh S., Sabbaghi N., Noroozifar M. Poly(Quercetin)-bismuth nanowires as a new modifier for simultaneous voltammetric determination of dihydroxybenzene isomers and nitrite. RSC Adv. 2018;8:1237–1245. doi: 10.1039/c7ra11132k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.H., Guo Y., Song D., Bruno R.S., Lu X. Quercetin-containing self-nanoemulsifying drug delivery system for improving oral bioavailability. J. Pharm. Sci. 2014;103:840–852. doi: 10.1002/jps.23858. [DOI] [PubMed] [Google Scholar]
- Verma N.K., Crosbie-Staunton K., Satti A., Gallagher S., Ryan K.B., Doody T., McAtamney C., MacLoughlin R., Galvin P., Burke C.S., Volkov Y., Gun’ko Y.K. Magnetic core-shell nanoparticles for drug delivery by nebulization. J. Nanobiotechnology. 2013;11:1–12. doi: 10.1186/1477-3155-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittorio O., Brandl M., Cirillo G., Spizzirri U.G., Picci N., Kavallaris M., Iemma F., Hampel S. Novel functional cisplatin carrier based on carbon nanotubes-Quercetin nanohybrid induces synergistic anticancer activity against neuroblastoma in vitro. RSC Adv. 2014;4:31378–31384. doi: 10.1039/c4ra03331k. [DOI] [Google Scholar]
- Vyas V.S., Vishwakarma M., Moudrakovski I., Haase F., Savasci G., Ochsenfeld C., Spatz J.P., Lotsch B.V. Exploiting noncovalent interactions in an imine-based covalent organic framework for quercetin delivery. Adv. Mater. 2016;28:8749–8754. doi: 10.1002/adma.201603006. [DOI] [PubMed] [Google Scholar]
- Wang Q., Bao Y., Ahire J., Chao Y. Co-encapsulation of biodegradable nanoparticles with silicon quantum dots and quercetin for monitored delivery. Adv. Healthc. Mater. 2013;2:459–466. doi: 10.1002/adhm.201200178. [DOI] [PubMed] [Google Scholar]
- Wang Qiang, Caibiao Hu, Qian Airui, Liu Tian, Zhang Hong, Zhang Yali, Xia Qiang. Enhanced oral bioavailability of Quercetin by a new non-aqueous self-double-emulsifying drug delivery system. Eur. J. Lipid Sci. Technol. 2016 [Google Scholar]
- Wang G., Wang J., Luo J., Wang L., Chen X., Zhang L., Jiang S. PEG2000-DPSE-coated Quercetin nanoparticles remarkably enhanced anticancer effects through induced programed cell death on C6 glioma cells. J. Biomed. Mater. Res. - Part A. 2013;101:3076–3085. doi: 10.1002/jbm.a.34607. [DOI] [PubMed] [Google Scholar]
- Ward, R.S., A.P., 1974. High-performance liquid chromatography of 34 selected flavonoids. J. Chromatogr. Sci 571, 613–616. 10.1007/BF02976930. [DOI]
- Wen P., Hu T.G., Li L., Zong M.H., Wu H. A colon-specific delivery system for Quercetin with enhanced cancer prevention based on co-axial electrospinning. Food Funct. 2018;9:5999–6009. doi: 10.1039/c8fo01216d. [DOI] [PubMed] [Google Scholar]
- Wen P., Zong M.H., Hu T.G., Li L., Wu H. Preparation and characterization of electrospun colon-specific delivery system for quercetin and its antiproliferative effect on cancer cells. J. Agric. Food Chem. 2018;66:11550–11559. doi: 10.1021/acs.jafc.8b02614. [DOI] [PubMed] [Google Scholar]
- Wu H., Chen M., Fan Y., Elsebaei F., Zhu Y. Determination of rutin and Quercetin in Chinese herbal medicine by ionic liquid-based pressurized liquid extraction-liquid chromatography- chemiluminescence detection. Talanta. 2012;88:222–229. doi: 10.1016/j.talanta.2011.10.036. [DOI] [PubMed] [Google Scholar]
- Xu G., Li B., Wang T., Wan J., Zhang Y., Huang J., Shen Y. Enhancing the anti-ovarian cancer activity of Quercetin using a self-assembling micelle and thermosensitive hydrogel drug delivery system. RSC Adv. 2018;8:21229–21242. doi: 10.1039/c8ra03274b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhang H., Chen G. Carbon nanotube/polystyrene composite electrode for microchip electrophoretic determination of rutin and Quercetin in Flos Sophorae Immaturus. Talanta. 2007;73:932–937. doi: 10.1016/j.talanta.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Yang S., Yin B., Xu L., Gao B., Sun H., Du L., Tang Y., Jiang W., Cao F. A natural Quercetin-based fluorescent sensor for highly sensitive and selective detection of copper ions. Anal. Methods. 2015;7:4546–4551. doi: 10.1039/c5ay00375j. [DOI] [Google Scholar]
- Yao Z., Yang X., Liu X., Yang Y., Hu Y., Zhao Z. Electrochemical Quercetin sensor based on a nanocomposite consisting of magnetized reduced graphene oxide, silver nanoparticles and a molecularly imprinted polymer on a screen-printed electrode. Microchim. Acta. 2018;185:1–9. doi: 10.1007/s00604-017-2613-5. [DOI] [PubMed] [Google Scholar]
- Yilmaz M., Karanastasis A.A., Chatziathanasiadou M.V., Oguz M., Kougioumtzi A., Clemente N., Kellici T.F., Zafeiropoulos N.E., Avgeropoulos A., Mavromoustakos T., Dianzani U., Karakurt S., Tzakos A.G. Inclusion of quercetin in gold nanoparticles decorated with supramolecular hosts amplifies its tumor targeting properties. ACS Appl. Bio Mater. 2019;2:2715–2725. doi: 10.1021/acsabm.8b00748. [DOI] [PubMed] [Google Scholar]
- Yola M.L., Atar N., Üstündaǧ Z., Solak A.O. A novel voltammetric sensor based on p-aminothiophenol functionalized graphene oxide/gold nanoparticles for determining Quercetin in the presence of ascorbic acid. J. Electroanal. Chem. 2013;698:9–16. doi: 10.1016/j.jelechem.2013.03.016. [DOI] [Google Scholar]
- Yu W., Zhu Y., Li H., He Y. Injectable quercetin-loaded hydrogel with cartilage-protection and immunomodulatory properties for articular cartilage repair. ACS Appl. Bio Mater. 2020 doi: 10.1021/acsabm.9b00673. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Huang X., Pu Y., Yi Y., Zhang T., Wang B. pH-sensitive and biocompatible Quercetin-loaded GO–PEA–HA carrier improved antitumour efficiency and specificity. Artif. Cells, Nanomedicine Biotechnol. 2018;46:S28–S37. doi: 10.1080/21691401.2018.1489261. [DOI] [PubMed] [Google Scholar]
- Zhang J., Luo Y., Zhao Xiufeng, Li X., Li K., Chen D., Qiao M., Hu H., Zhao Xiuli. Co-delivery of doxorubicin and the traditional Chinese medicine Quercetin using biotin-PEG2000-DSPE modified liposomes for the treatment of multidrug resistant breast cancer. RSC Adv. 2016;6:113173–113184. doi: 10.1039/c6ra24173e. [DOI] [Google Scholar]
- Zhang J., Shen L., Li X., Song W., Liu Y., Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13:12511–12524. doi: 10.1021/acsnano.9b02875. [DOI] [PubMed] [Google Scholar]
- Zhang Yuying, Yang Yan, Tang Kai, Xing Hu G.Z. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci. 2008;107:891–897. doi: 10.1002/app. [DOI] [Google Scholar]
- Zhou C., Lin Y. Osteogenic differentiation of adipose-derived stem cells promoted by Quercetin. Cell Prolif. 2014;47:124–132. doi: 10.1111/cpr.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wu Y., Ma W., Jiang X., Takemra A., Uemura M., Xia L., Lin K., Xu Y. The effect of Quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J. Mater. Chem. B. 2017;5:612–625. doi: 10.1039/c6tb02312f. [DOI] [PubMed] [Google Scholar]
- Zu Y., Li C., Fu Y., Zhao C. Simultaneous determination of catechin, rutin, Quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. J. Pharm. Biomed. Anal. 2006;41:714–719. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]