Abstract
The genus Millettia belongs to Fabaceae includes 200 species which are distributed in tropical and subtropical regions of the world. Plants belong to this genus are used as folkloric medicine, for the treatment of different ailments like in wound healing, boil, sores, skin diseases, snake bite, muscle aches, pains, rheumatic arthritis, and gynaecological diseases. The aim of the review is to provide updated, comprehensive and categorized information on the aspects of ethnobotanical, phytochemical, pharmacological uses and toxicity of genus Millettia in order to identify their therapeutic potential and generate space for future research opportunities. The present study comprises of isolated flavonoids, phenolic compounds, phytosterols, saponins, alkaloids, polysaccharides, terpenoids and resins and pharmacological activities of various Millettia species. The relevant data were searched by using the keyword “Millettia” in different scientific databases like, “Google Scholar”; “NISCAIR repository”; “Pub Med”; “Science Direct”; “Scopus” and the taxonomy is validated by “The Plant List”. This review discusses the existing information of the traditional evaluation as well as phytochemical and pharmacological evaluation of the extract and active constituents of the genus “Millettia”. This review confirms that several Millettia species have emerged as a high-quality medicine in a traditional system for arthritis, wound healing, inflammation, skin diseases. Numerous conventional uses of Millettia species have been validated by modern pharmacology research. Intensive investigations of the genus Millettia relating to phytochemistry and pharmacology, especially their mechanism of action, safety, and efficacy could be the future research interests by the researcher in the area of phytomedicine.
Abbreviations: EtOAc, ethyl acetate; MeOH, CH3OH, methanol; n-BuoH, n-butanol; CH2Cl2, dichloromethane; MDR, multidrug resistance; CNS, central nervous system; MIC, minimum inhibitory concentration; DPPH, 2,2-diphenyl-picyrlhydrazyl; NO, nitric oxide; TNF-α, tumour necrosis factor; IL-6, interleukin; iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; LPS, lipopolysaccharide; HepG2, hepatocellular carcinoma; MCF7, breast cancer cell line; HCT116, colon cancer; KG-1, acute myelogenous leukemia cell line; Raji, lymphoma cell line; MTT assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; ORAC assay, oxygen radical absorption capacity; TLR4, toll-like receptor4; SRA, scavenger receptor type A and GR, glucagon receptor; COX, cycloxygenase
Keywords: Millettia, Fabaceae, Ethnomedicine, Biological activities, Chemical constituents
1. Introduction
The genus Millettia belongs to family Fabaceae consists of more than 200 species plants which are grown in tropical and subtropical regions of the world (Chen et al., 2018). The family Fabaceae is positioned as the major group of angiosperms and the genus was earlier known as Pongamia, which is a large distributed genus of flowering plants. The plant list includes 363 scientific plant names of species rank, for the genus Millettia, out of which 202 are acknowledged species (www.theplantlist.org).
The traditional use of Millettia species includes antibacterial, anti-tumour, insecticidal, pesticidal, piscicidal (Sritularak and Likhitwitayawuid, 2006), antispasmodial, chemopreventive (Marco et al., 2017) joint pain, rheumatoid arthritis, amenorrhea, tuberculosis, etc. Most of them are used in the production of biodiesel (Madhu et al., 2016).
A number of distinctive studies have been performed by validating the usefulness in conventional medicine during the past few years by putting efforts to characterize both the chemical and pharmacological properties of Millettia species. A variety of phytochemicals have been identified from different species of Millettia such as flavonoids, phenolic compounds, phytosterols, saponins, alkaloids, polysaccharides, terpenoids and resins, etc as its secondary metabolites (Manikandan et al., 2017).
Some of the well-known species of the genus Millettia are Millettia extensa (Benth.) Baker, Millettia pinnata (L.) Panigrahi, Millettia ovalifolia Kurz, Millettia auriculata Brandis, Millettia speciosa Champ., Millettia laurentii De Wild. as per report collected from electronic search (using Pub med, Science Direct, Google Scholar, Scopus and NISCAIR repository) by using the keyword Millettia. A complete library search was done for the available data in different published articles and also in locally surveyed folkloric claims.
This review targets a comprehensive and significant assessment based on the existing access data in the area of the ethno-medicinal uses, phytochemistry, biological activities and toxicological research of different Millettia species in order to analyze their therapeutic potential and help the researchers in marking out of the strength and opportunities present in the plant species.
2. Distribution and botanical description
The genus Millettia belongs to the family Fabaceae (Leguminosae) which is comprised of more than 200 species (Chen et al., 2018). These include mostly trees, shrubs and woody climbers having cultured distribution. All the species are distributed broadly all over the tropical parts of the globe i.e. continents like Asia, Africa, and Australia are presented in Table 1 (Dat et al., 2019). African Millettia contributes about 24% and is found in East Africa and more than 25 species of Millettia available in Tanzania and also available in Republic of Congo, Cameroon, and Pacific Islands. From the literature, it is found that near about 30 different species of Millettia are found in a different region of India. Most of the species are found in eastern India, Western Ghats, and Himalayan foot hills. In tropical South-East Asia, this genus is distributed from Bhutan, China, India, Pakistan, Nepal, Myanmar, Bangladesh, Thailand, and Taiwan to Malaysia.
Table 1.
Traditional uses of plant parts belonging to Millettia species.
| Plant Species | Plant parts | Traditional uses and method of preparation/administration | Geographical distribution | References |
|---|---|---|---|---|
| M. extensa (Benth.) Baker | Stem, root, leaves, bark | Infected wound, tonic, skin infection, cough, boils, sores The juice of bark is applied over the wounded area. |
India | (Sharma et al., 2014) |
| M. ovalifolia Kurz | Bark | Hypotension, malaria | Pakistan | (Rahman et al., 2015) |
| M. conraui Harms | Stem, bark | Insecticidal, molluscicidal, pesticidal | France, Cameroon, Nigeria | (Fuendjiep et al., 1998) |
| M. pachycarpa Benth. | Seed | Pain, bruises, skin disorders, anthelmintic. The seeds are useful for the treatment of worm infection. | South-East Asia, China, India, Bhutan, Nepal, Myanmar, Bangladesh, Thailand, Taiwan | (Tu et al., 2019) |
| M. auriculata Brandis | Leaves, stem | Toothache, insecticide, vermifuge, fishing poison The stem and leaves are boiled with water and used in bath for its tonic property. |
India | (Das and Ganapaty, 2014) |
| M. dielsiana Harms ex Diels | Leaves, stem | Muscle ache, pain, rheumatoid arthritis | China, Vietnam, Laos | (Dat et al., 2019) |
| M. dura Dunn | Stem bark, seed pod, root | Insecticidal, pesticidal, larvicidal, diarrhoea, hernia, wound healing, menstrual irregularities | Kenya | (Marco et al., 2017) |
| M. pachyloba Drake | Vine stem, leaves | Pain, relieving stasis, promoting blood circulation, irregular menstruation | China | (Yan et al., 2019) |
| M. pervilleana | Root bark | Cancer | Italy | (Palazzino et al., 2003) |
| M. laurentii De Wild. | Stem bark | Commercial timber | France, Africa, Republic of Congo, Cameroon | (Kamnaing et al., 1994) |
| M. pinnata (L.) Panigrahi | Seed, flower | Diarrhoea, ulcer, diabetes The flower is used for the treatment of piles as well as bleeding disorders. Fruits are meant for the treatment of abdominal ulcer and tumour. Leaf juice is meant for the treatment of cough, colds leprosy, diarrhoea and whole leaves are used as digestive and also for inflammation and wounds. Stem extract is used as a sedative in CNS. The root juice is mixed with coconut milk and lime water for the treatment of gonorrhoea. |
Australia, Pacific islands. In Tropical Asia it extends from India, Japan, and Thailand to Malaysia including Himalayan foothills. | (Bora et al., 2014) |
| M. speciosa Champ. | Root | Joint pain, rheumatoid arthritis, amenorrhea, hepatitis, tuberculosis, chronic bronchitis | China | (Fu et al., 2016) |
| M. brandisianai Kurz | Root | Haematonic, inflammation, ulcer | Thailand | (Pailee et al., 2019) |
| M. dorwardii Collett & Hemsl. | Vine stem | Cancer, inflammation | China | (Chen et al., 2018) |
| M. duchesnei De Wild. | Twig | Fish poison, insecticide | Cameroon | (Ngandeu et al., 2008) |
| M. griffithii Dunn | Stem | Inflammation, joint pain, skin disease | China | (Tang et al., 2016) |
| M. nitita Var. hirsutissima | Vine stem | Promotes blood circulation, relieving stasis | China | (Zhang et al., 2009) |
| M. pulchra Kurz | Whole plant | Memory improvement, anti-ageing | China | (Lin et al., 2014) |
| M. barteri (Benth.) Dunn | Stem, bark root, twig | Vermifuge, purgative, feverish aches, cough, dysmenorrheal, cardiac pain As a rectal formulation, the macerated twigs are used to treat the purgative disorder. The stem bark (decoction) extract is taken for feverish aches, cough, and dysmenorrhea. The decoction of bark root helps to lower the cardiac pains. |
Cameroon | (Havyarimana et al., 2012) |
| M. versicolor Baker | Stem bark, leaves | Relieve pain, parasitosis, cough, female sterility, headache, rheumatism In Congo, the aqueous decoction of leaves and stem bark is taken to treat intestinal parasitoses, feverish aches, kidney pains, cough, and female sterility. For the treatment of the syphilitic wounds, the infusion of stem bark is used on the bath. The trunk barks having potent anthelmintic property. |
Congo | (Ongoka et al., 2008) |
The leaves are opposite and imparipennates, stipellae. The inflorescence is paniculate with different colour flowers; the calyx is campanulate, adherent stamen, vexillary filament, ovate and dorsifixed anther, pubescent and sessile ovary. The seeds are kidney-shaped having funicle. The pod is flat, coriaceous and woody (Banzouzi et al., 2008).
3. Traditional uses and ethnopharmacology
A few species of Millettia has evidence of values as a drug in the indigenous system of medicine for a number of ailments. In different countries of Asia, Africa and Australia such as India, Pakistan, Burma, China, Thailand, France, Kenya, etc in which Millettia species are also grown naturally and used as ethnomedicine by local people (Dat et al., 2019). These species have been accessed for the treatment of various ailments. A number of studies have reported their use as traditional medicine by local people and have been widely used to treat the infected wounds, skin disorders, cough, rheumatoid pain, ulcer, menstrual disorder, inflammation, bronchititis, toothache, muscle ache, tuberculosis, hepatitis and bruises, etc presented in Table 1. The roots of M. auriculata Brandis, M. racemosa (Roxb.) Benth., M. pachycarpa Benth. and M. piscidia (Roxb.) Wight were reported to have insect repellent, larvicidal activity, antitumor, anti-inflammatory, antiviral, and antibacterial and pest destroying activity. These are also used as a fish poison and applied to sores of cattle to kill vermin. A different community of Cameroon has used this Millettia species for the treatment of intestinal parasites in case of children as a potent inhibitor (Rahman et al., 2015). In India, the Gujjar tribe follows the use of M. extensa (Benth.) Baker barks paste for the treatment of wound (Sharma et al., 2013). The Bhilla tribe in Maharashtra, India also used its bark juice for the wound by applying it once a day for a time period of 4–5 days (Kamble et al., 2010). The tribal community of Odisha (Kalahandi District) used the root paste of M. extensa (Benth.) Baker with liquor for the treatment of piles. They used this herbal formulation by applying it over the piles for 5 min in a time period of 2 days and also the root paste is used for the treatment of patient bitten by a mad dog (Panda and Padhy, 2008). It is also used for the treatment of cough and skin infection (Padhi et al., 2017). The Tai Yai community in Northern Thailand used the leaves and stem of M. auriculata Brandis by boiling it with water and used in the bath due to its tonic property (Khuankaew et al., 2014). M. pachycarpa Benth. seeds are promptly used for the treatment of worm infection, pain and bruises (Tu et al., 2019). As a rectal formulation the macerated twigs of M. barteri (Benth.) Dunn is used to treating the purgative disorder. The stem bark (decoction) extract is taken for feverish aches, cough, and dysmenorrhea. Also, the decoction of bark root is taken to lower the cardiac pains (Havyarimana et al., 2012). In Congo, the aqueous decoction of M. versicolor Baker leaves and stem bark is taken to treat intestinal parasitoses, feverish aches, kidney pains, cough, and female sterility. For the treatment of the syphilitic wounds, the infusion of stem bark is used on the bath. The trunk barks having potent anthelmintic property (Ongoka et al., 2008). M. pinnata (L.) Panigrahi flower is used for the treatment of piles as well as bleeding disorders. Its fruits are meant for the treatment of abdominal ulcer and tumor. The leaf juice is meant for the treatment of cough, colds, leprosy, diarrhoea. The whole leaves are used as digestive, inflammation and also for wounds. Stem extract is used as sedative for CNS. The root juice is mixed with coconut milk and lime water for the treatment of gonorrhea (Pulipati et al., 2018). However, to the best of our knowledge, there are a number of traditional uses of the genus Millettia are available in the literature as folkloric claims and these findings raised the possibility of researchers to undertake the exploration as well as scientific assessment of the activities.
4. Phytochemical constituents
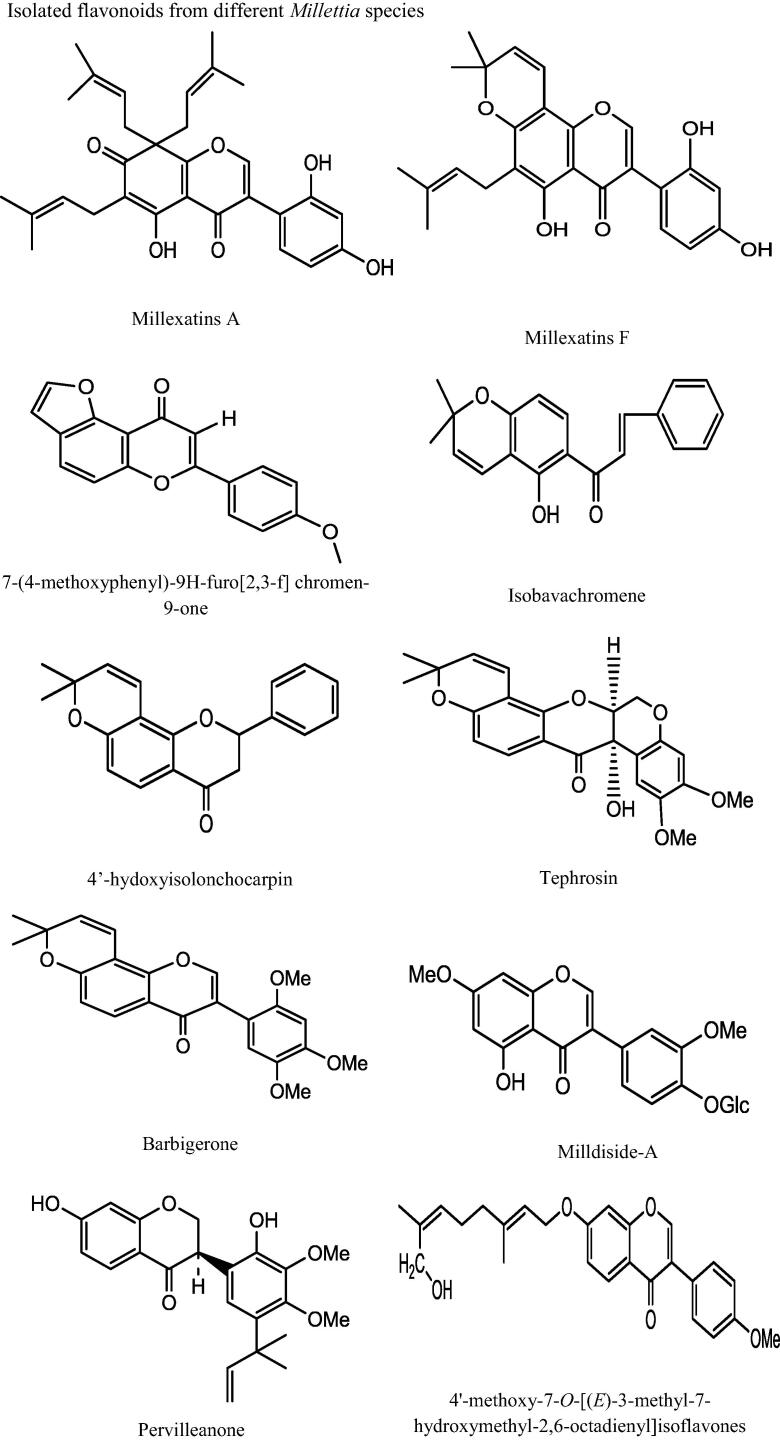
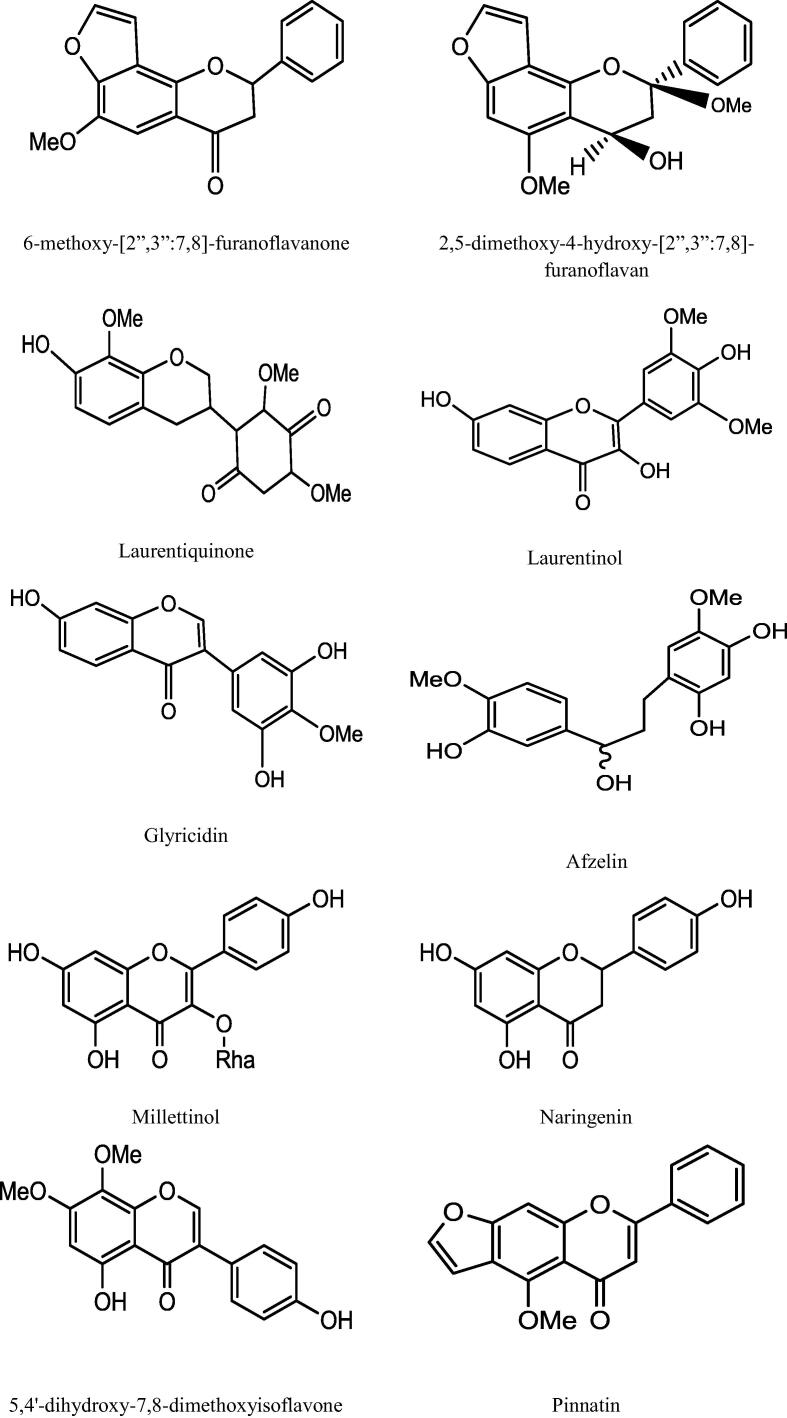
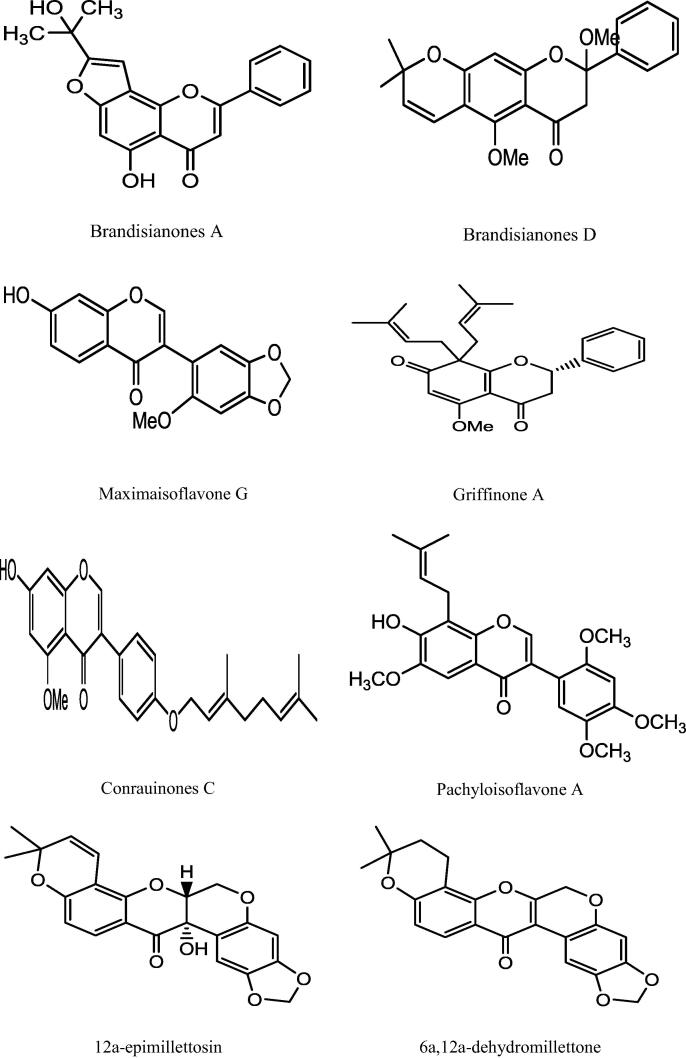
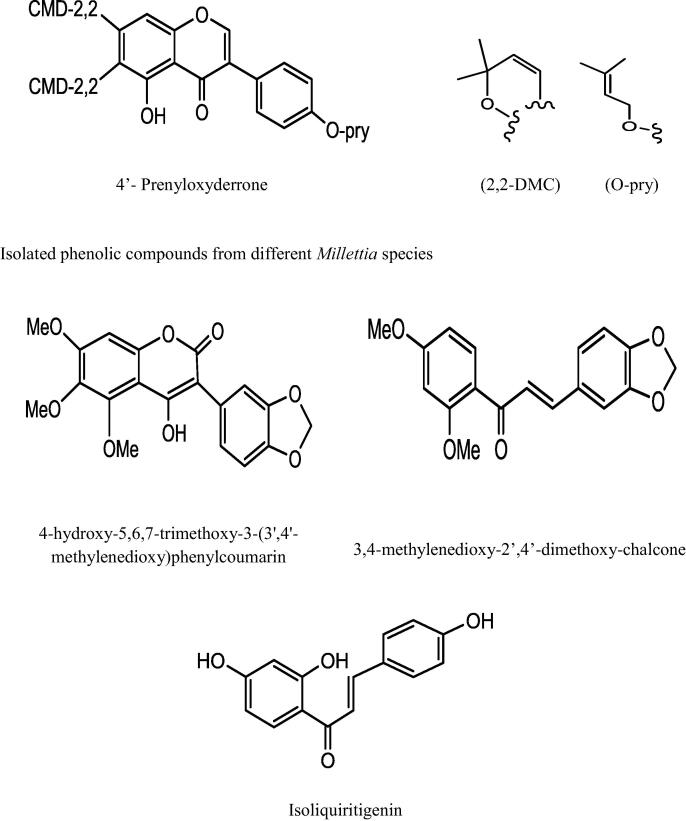
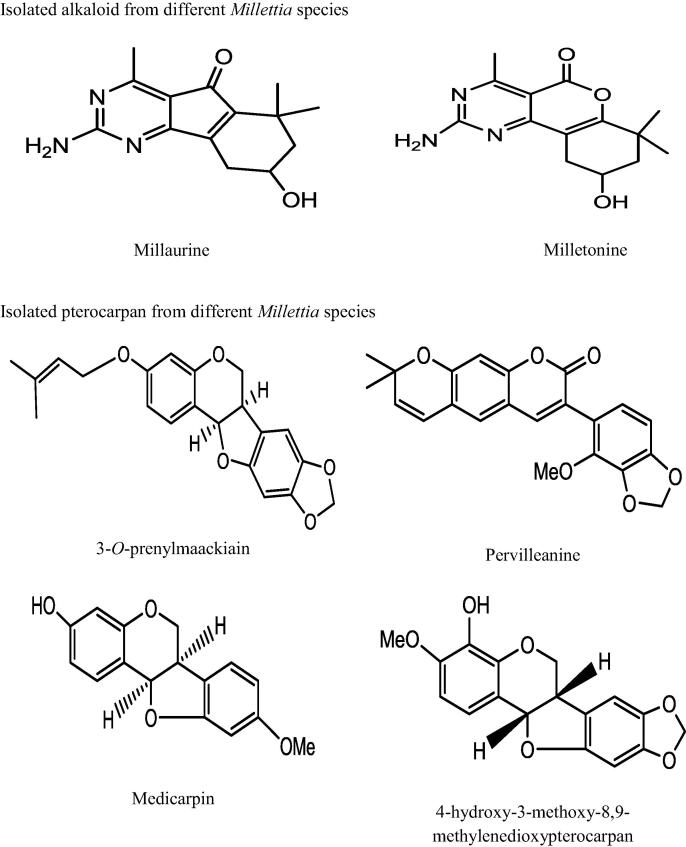
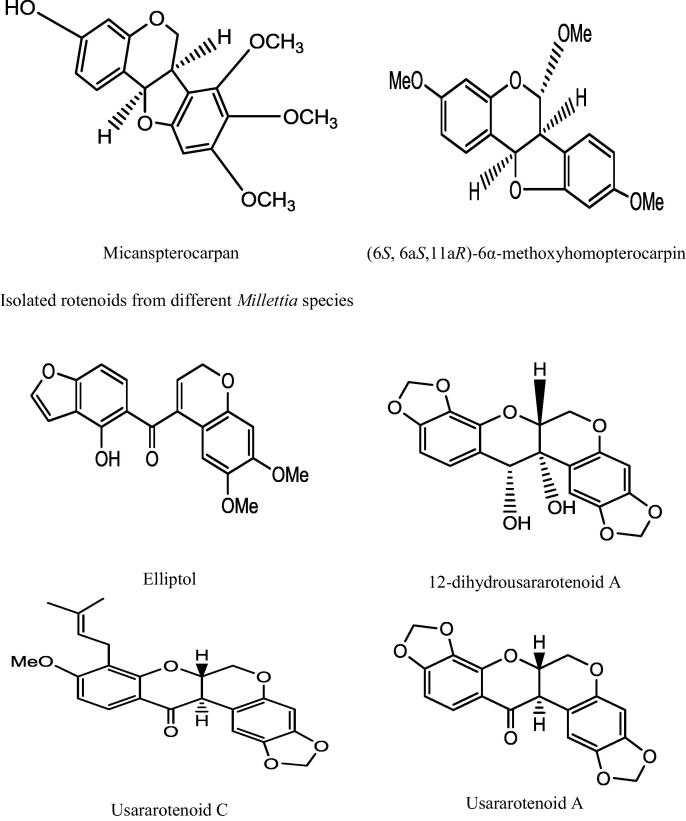
This genus “Millettia” is well recognized for its medicinal priority due to presence of a number of secondary metabolites. An extensive and depth investigations of different Millettia species have derived isolation and characterization of various secondary metabolites belongs to alkaloids, triterpenoids, coumarins, flavonoids, isoflavonoids, phenols, and phytosterols (Havyarimana et al., 2012) presented in Table 2 and the important chemical structure are described (Fig. 1).
Table 2.
Isolated phytoconstituents of evaluated extracts from Millettia species.
| Plant Species | Phytoconstituents | Chemical class | Extracts | References |
|---|---|---|---|---|
| M. extensa (Benth.) Baker | Millexatins A, Millexatins B, Millexatins C, Millexatins D, Millexatins E, Millexatins F, Auriculatin, Scandenone, Elongatin, Auriculasin, Isoauriculasin, isoauriculatin, 3′-methylorobol, 7,4′-di-O-prenylgenistein, 2′-deoxyisoauriculatin, 2′-O-methylisoauriculatin, 4′-methylalpinumisofiauone, 5-O-methyl-4′-O-(3-methyl-2-utenyl)alpinumisoflavone, 5-hydroxy-7-methoxy-4′-O-(3-methylbut-2-enyl) isoflavones | Isoflavones | Acetone | (Padhi et al., 2017, Raksat et al., 2018) |
| (−)-maackiain | Pterocarpan | |||
| (−)-sumatrol (−)-6a,12a-dehydrotoxicarol |
Rotenoids | |||
| M. ovalifolia Kurz | 7-(4 methoxyphenyl)-9H-furo[2,3-f]chromen-9-one, | Flavonoids | Chloroform fraction of methanol extract | (Rahman et al., 2015) |
| Pervilleanone (Potent hypotensiveagent) | Prenylated isoflavanone | |||
| M. pachycarpa Benth. | 4-methoxylonchocarpin, dorspoinsettifolin, isobavachromene, deguelin, tephrosin, barbigerone, 4′,5′-dimethoxy-6,6-dimethylpyrano isoflavones, 4′-hydroxyisolonchocarpin, 12a-hydroxyrotennone | Flavonoids | EtOAc extract | (Tu et al., 2019, Su et al., 2012) |
| 3-hydroxy4-methoxylonchocarpin | Prenylated chalcone | Petroleum ether fraction of ethanol extract | ||
| 4-methoxylonchocarpin Isobarachromene Dorspoinsettifolin |
Flavonoids | Petroleum ether fraction of ethanol extract | ||
| M. dielsiana Harms ex Diels | Mildiside-A | Isoflavonoe glycoside | Chloroform and EtOAc fraction of ethanol extract | (Dat et al., 2019) |
| Formononetin, ononin, isoliquiritigenin, liquiritigenin, naringenin, gallocatechin, catechin, (3S)-vestitol, tupichinol C, (+)-epicatechin, (−)-epicatechin, protocatechuic acid, trans-ferulic acid, trans-3-O-p-hydroxycinnamoyl ursolic acid | Phenolic derivatives | |||
| M. dura Dunn | 3-O-prenylmaackiain | Pterocarpan | Chloroform: methanol(1:1) | (Marco et al., 2017) |
| Calopogonium isoflavone B, Maximaisoflavone B, durmillone, isoerythrin A-4′-(3-methylbut-2-enyl) ether, 7-hydroxy-8,3′,4′-trimethoxyisoflavone, 7,2′-dimethoxy-4′,5′-methylenedioxyisoflavone | Isoflavones | |||
| Butein | Chalcone | |||
| M. pervilleana | 3-phenylcoumarin or pervilleanine | Prenylated isoflavanone | Chloroform | (Palazzino et al., 2003) |
| Pervilline, Pervillinine | Pterocarpans | |||
| 3α-hydroxyrotenone | Rotenone | |||
| Pervilleanone, 3′-O-demethylpervilleanone | Isoflavanones | |||
| M. griffoniana Baill. | 4′-methoxy-7-O-[(E)-3-methyl-7-hydroxymethyl-2,6-octadienyl]isoflavone, 3′,4′-dihydroxy-7-O-[(E)-3,7-dimethyl-2,6-octadienyl]isoflavone, | O-geranylated isoflavones | n-hexane, chloroform | (Yankep et al., 1998) |
| 4-hydroxy-5,6,7-trimethoxy-3-(3′,4′-methylenedioxy)phenylcoumarin | 3-phenylcoumarin | |||
| M. erythrocalyx Gagnep. | 6-methoxy-[2″,3″:7,8]-furanoflavanone, 2,5-dimethoxy-4-hydroxy-[2″,3″:7,8]-furanoflavan | Flavonoid | n-hexane, chloroform, methanol | (Sritularak and Likhitwitayawuid, 2006) |
| 3,4-methylenedioxy-2′,4′-dimethoxychalcone | Chalcone | |||
| M. laurentii De Wild. | 3′, 6 '-diketo-7-hydroxy-8,2′,4′-trimethoxyisoflavan or laurentiquinone | Isoflavan-quinone | n-hexane, chloroform, EtOAc, methanol | (Kamnaing et al., 1994, Kamnaing et al., 1999) |
| 3,7,4 '-trihydroxy-3′,5′-dimethoxyflavone or laurentinol | Flavonol | |||
| Calycosin, Glyricidin | Isoflavones | |||
| M. oblate ssp. teitensis | 4- prenyloxyderrone, durmilone, 8-O-methylretusin, Maximaisoflavone B, Maximaisoflavone H, Maximaisoflavone J | Isoflavone | CH2Cl2:MeOH (1:1) | (Derese et al., 2014) |
| Tephrosin | Rotenoid | |||
| Lupeol | Triterpene | |||
| M. leucantha Kurz | 1-(3-hydroxy-4-methoxyphenyl)-3-(2,4-dihydroxy-5-methoxyphenyl)propan-1-ol (millettinol) physcion, (R)-(-)-mellein, isoliquiritigenin | Phenolic compound | n-BuOH, EtOAc fraction of ethanol extract | (Rayanil et al., 2011) |
| 4-hydroxy-3-methoxy-8,9-methylenedioxypterocarpan 3-hydroxy-9-methoxypterocarpan or medicarpin |
Pterocarpan | |||
| 5,4′-dihydroxy-7,8-dimethoxyisoflavone | Isoflavone | |||
| M. pinnata (L.) Panigrahi | Resin, flavonoids, terpenoids, phenols, saponins, alkaloids, alkyd resin | (Bora et al., 2014) | ||
| Palmitic acid, stearic acid, oleic acid, linoleic acid, lignoceric acid, arachidic acid and behenic acid | Fatty acid | Oil extract | ||
| M. speciosa Champ. | Naringenin, liquiritigenin, garbanzol | Flavonoid | n-BuOH, EtOAc fraction of ethanol extract | (Fu et al., 2016) |
| Calycosin, 2′-hydrroxybiochanin 7-hydroxy-6,4′-dimethoxyisoflavone, 2′5′7-trihydroxy-4′-methoxyisoflavone, 6-methoxycalopogonium isoflavones A |
Isoflavones | |||
| Secoisolariciresinol, polystachyol | Lignan | |||
| 4,4′-dihydroxy-2′-methoxychalcone, 2,4′-dihydroxy-4-methoxychalcone | Chalcones | |||
| Rhododendrol | Phenolic compound | |||
| Polysaccharide(MSCP2) | Aqueous | (Huang et al., 2020) | ||
| M. brandisiana Kurz | Brandisianones A, Brandisianones B, Brandisianones C, Brandisianones D, Brandisianones E | Flavonoids | Dichloromethane | (Pailee et al., 2019) |
| M. conraui Harms | Conrauinones C, Conrauinones D, 7- hydroxyl-6-methoxy-3′,4′-methylenedioxy isoflavone | O-geranylated isoflavones | Benzene | (Fuendjiep et al., 1998) |
| M. duchesnei De Wild. | Elliptol, 12-deoxo-12-α-methoxyelliptone, 6-methoxy-6a,12a-dehydrodeguelin, 6a,12a-dehydrodeguelin, 6-hydroxy-6a,12a-dehydrodeguelin, 6-oxo-6a,12a-dehydrodeguelin, elliptone, 12a-hydroxyelliptone, eriodictyol | Prenylated rotenoids | CH2Cl2:MeOH (1:1) | (Ngandeu et al., 2008) |
| M. micans Taub. | Micanspterocarpan | Pterocarpan | CH2Cl2:MeOH (1:1) | (Marco et al., 2017) |
| M. griffithii Dunn | Griffinones A, Griffinones B, Griffinones C, Griffinones D, Griffinones E | Flavonoids | n-hexane, EtOAc | (Tang et al., 2016) |
| Griffiliganan A | Biphenylneolignan | |||
| M. manni Baker | 5-hydroxy-4,7′-dimethoxyisoflavone | Isoflavones | Methanol | (Kamto et al., 2012) |
| Furanonaphthoquinone | Naphthalene | |||
| M. pachyloba Drake | Pachyloisoflavone A, Pachyloisoflavone B | Prenylated isoflavones | Ethanol | (Na et al., 2017) |
| Pachylobin A | Pterocarpan | |||
| 6-methoxycalogonium isoflavones A, durallone, genistein, millesianin C, millesianin D, afromosin, hernancorizin, ichthynone, 5-hydroxy-2′,4′,5′,7-tetramethoxyflavone | Flavonoids | |||
| M. pulchra Kurz | (2S)-5,7,4′-trihydroxy-8,3′,5′-triprenylflavanone, (2R,3R)-7,4′dihydroxy-8,3′,5′-triprenyldihydroflavanol, 5,7,2′,4′-tetrahydroxy-6,3′-diprenylisoflavone, 5,7,4′-trihydroxy-2′-methoxy-6,3′-diprenylisoflavone. | Isoflavones | Chloroform, methanol | (Baruah et al., 1984) |
| (6S, 6aS, 11aR)-6a-methoxypterorpin, (6S, 6aS,l1aR)-6α-methoxyhomopterocarpin | Pterocarpan | |||
| M. usaramensis Taub. | (6aR,12aS)-2,3-methylenedioxy-9-methoxy-8-(3,3-dimethylallyl)-12a-hydroxyrotenoid (usararotenoid C) | Rotenoids | Dichloromethane | (Yenesew et al., 2003) |
| 12a-epimillettosin, 6a,12a-dehydromillettone, barbigerone, 4′-O-geranylisoliquiritigenin | Flavonoids | |||
| M. barteri (Benth.) Dunn | Millaurine, milletonine | Guanidine alkaloids | Methanol | (Havyarimana et al., 2012) |
| Afzelin | Flavonoid | |||
| β-sitosterol, β-sitosterol glucoside, a mixture of stigmasterol and β-sitosterol, palmitates | Sterols | |||
| M. peguensis Ali | Pentadecane, Tetradecane, Octadecane, Undecane, 9-methylheptadecane, Heptadecane, 2,6,10,15-tetramethyl- 2-Bromo dodecane | Alkane hydrocarbon | Petroleum ether | (Manikandan et al., 2017) |
| Eicosane, Heneicosane | Acyclic alkane |
Fig. 1.
Chemical structure of isolated compounds from various Millettia species.
These flavonoids and isoflavonoids mainly play the important mechanism as wound healing, insecticidal, piscicidal and molluscicidal activity. From M. ovaifolia Kurz., flavonoids chalcones were isolated showing antimalarial activity and are also used as hypotensive agent (Rahman et al., 2015). It is reported that the activities of murine retroviral reverse transcriptase and human DNA polymerases are inhibited by M. pachycarpa Benth. (Ye et al., 2008).
Phytoconstituents like rotenone and 3α-hydroxyrotenone identified from M. pervilleana showed of TPA-induced ornithine decarboxylase inhibition at the level of its m RNA expression and act as a potent cancer chemo-preventive agents (Manikandan et al., 2017). Anticancer agents like prenylated isoflavanone, pervilleanone were screened from M. pervilleana. 4- prenyloxyderrone, isoflavones, chalcone, pterocarpan were isolated from M. dura Dunn and another compound i.e. 6α, 12α-didehydro-6-oxodeguelin was also screened having an insecticidal activity which inhibits phytotoxins of ornithine decarboxylase (Marco et al., 2017). Isoflavonoids, maximaisoflavone, griffonianone were screened from M. griffoniana Baill. showing significant cytotoxicity whereas, another compound i.e. griffonianone C showed promising estrogenic activity (Yankep et al., 1998). An anti-tumour agent, known as millepurone isolated from M. atropurpurea (Wall.) Benth. and osajin from M. auriculata Brandis showed antioxidant activity (Manikandan et al., 2017). M. duchusnei De Wild. contains rotenones, which is a potent insecticide (Ngandeu et al., 2008).
An extensive and thorough review of the various available species of Millettia genus have emerged in the isolation and identification of numerous isolates belongs to flavonoids, alkaloids, pterocarpan, phenols, rotenoids and steroids. Approximately 148 phytoconstituents have been isolated from 24 Millettia species of which 73 are flavonoids, 29 are phenolics, and 18 are rotenoids thus, making flavonoids and phenolic compounds i.e. the chief phytochemical class of Millettia genus.
5. Pharmacological activities
The genus Millettia is the target of different pharmacological investigations, which is evaluated for their various ethno-medicinal uses. From the literature, it was found that Millettia genus exhibit an extensive variety of biological activities, such as antibacterial, antiviral, anthelmintic, anti-inflammatory, antioxidant, antiplasmodial, anti-allergy, anti-tumour, cognitive activity and cytotoxicity. The active phytoconstituents have been isolated from the solvents like ethanol, butanol, n-hexane, chloroform, acetone, ethyl acetate, dichloromethane, etc. followed by characterization. A summary of the curative activity assessments performed on this Millettia has been represented in Table 3. These findings endorse the traditional uses of plants with respect to the pharmacological activity.
Table 3.
Reported pharmacological activities of plant extracts and compounds of Millettia species.
| Plant species | Plant parts | Activity studies | In- vivo/in- vitro | Tested extracts/active constituent | Observed effect | Mechanism | References |
|---|---|---|---|---|---|---|---|
| M. barteri (Benth.) Dunn | Stem bark | Antimicrobial | In-vitro | Hexane, EtOAc | Both extracts showed a MIC value of 64 to 512 µg/mL. | Not studied | (Havyarimana et al., 2012) |
| M. extensa (Benth.) Baker | Leaves | Antimicrobial | In-vitro | Acetone, ethanol | Both extracts showed significant MIC value i.e. 140 µg/mL and 210 µg/mL against S. aureus. | Not studied | (Padhi et al., 2017) |
| M. pinnata (L.) Panigrahi | Seed, bark, leaves | Antimicrobial | In-vitro | EtOAc, petroleum ether, green synthesized silver nanoparticles |
EtOAc, petroleum ether extract showed maximum zone of inhibition against B. subtilis. A maximum growth inhibition observed i.e the inhibition zone is more than 10 mm (10 mL/disc) particularly against E. coli (20.25 ± 0.91 mm), P. aeruginosa (17.13 ± 0.80 mm), S. aureus (15.09 ± 0.17 mm), K. pneumonia (14.81 ± 0.34 mm) |
Flavones, flavans, chalcone, triterpenes and aromatic carboxylic acids are responsible for the antimicrobial property. | (Pulipati et al., 2018) (Rajakumar et al., 2017) |
| M. pinnata (L.) Panigrahi | Leaves | Antioxidant | In-vivo | Ethanol | Upon the oral administration of the extract (300 mg/kg body mass), there was a significant increase in the reduced glutathione, glutathione peroxidase, catalase, superoxide dismutase level and decrease in the level of conjugative dines, hydrooxyperoxide, thiobarbituric acid reactive substances in albino rats by using ammonium chloride-induced model. | Free radical scavenging activity | (Pulipati et al., 2018) |
| M. barteri (Benth.) Dunn | Stem bark | Antioxidant | In-vitro | Hexane, EtOAc, millaurine | Both extracts showed IC50 62.74 and 77.23 µg/mL in DPPH assay. | Free radical scavenging activity | (Havyarimana et al., 2012) |
| M. usaramensis Taub. | Stem bark | Antiplasmodial | In-vitro | Rotenoid, flavonoids | Dichloromethane against the chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of P. falciparum showed potent activity with an IC50 value of 21.1 and 28 μg/mL each. | Not studied | (Yenesew et al., 2003) |
| M. oblate ssp. teitensis | Stem bark | Antiplasmodial | In-vitro | CH2Cl2/CH3OH (1:1) | The extract showed significant activity against chloroquine-resistant Indochina 1 (W2) and chloroquine-sensitive Sierra Leone 1 (D6) strains of P. falciparum with an IC50 value of 10.0 ± 2.3 and 12.0 ± 1.2 μM each. | Presence of isoflavones is responsible | (Derese et al., 2014) |
| M. dura Dunn | Root bark | Antiplasmodial | In-vitro | CH2Cl2/CH3OH (1:1) | The minimal activity was found for compounds viz. calopogonium isoflavone B, maximaisoflavone B and 7, 2′-dimethoxy-4′, 5′-methylenedioxyisoflavone against the chloroquine-sensitive 3D7 and the chloroquine-resistant Dd2 P. falciparum strain where the activity found in between 70 and 90% inhibition at 40 μM. | Isoflavones like Calopogonium isoflavone B, Maximaisoflavone B are responsible for this activity | (Marco et al., 2017) |
| M. Speciosa Champ. | Root | Immunomodulatory | In-vitro | Polysaccharide fraction (MSCP2) | MSCP2 was confirmed to have significant immunomodulatory activity by improving the pinocytic capacity and increasing the levels of NO, TNF-α, and IL-6. | The molecular mechanism of MSCP2 is executed by macrophage activation through TLR4, SRA and GR mediated signalling pathways. | (Huang et al., 2020) |
| M. pulchra Kurz | Whole plant | Cognitive | In-vivo | Polysaccharide | The animal behavioural study showed that polysaccharide significantly reversed D-galactose induced learning and memory impairments with distinctively decreasing of the content and deposition of β-amyloid peptide increase the level of acetylcholine but decreased the cholinesterase activity. | The mechanisms of this action are the reduction of oxidative stress as well as the suppression of inflammatory responses | (Zhang et al., 2009) |
| M. pinnata (L.) Panigrahi | Flower | Anti-cholinesterase | In-vitro | Green synthesized nanoparticles (AgNPs) | The IC50 value of AgNPs was 24.03 ± 1.01 mg/mL for acetylcholinesterase and 171.69 ± 0.98 mg/mL for butyrylcholinesterase which is comparatively higher than the activity of flower extract. | Not studied | (Rajakumar et al., 2017) |
| M. pachycarpa Benth. | Seed | Anti-cholinesterase | In-vitro | n- BuOH, EtOAc | The isolates were evaluated by Ellman’s methods where they result moderate to weak acetylcholinesterase activity with an IC50 value ranging from 17.14 to 131.17 μM. | Flavonoids exhibited Anti-cholinesterase activity. | (Y. Tu et al., 2019) |
| M. auriculata Brandis | Stem, leaves | Anthelmintic | In-vitro | Chloroform | Extract possessed a significant anthelmintic activity (in-vitro) using Pheretima posthuma at a dose of 10 mg/mL against the standard drug albendazole, where the leaves extract was found to be more potent as compared with stem and standard. | Phenolic compounds and flavonoids may have a direct effect on the pre-parasitic stages which hampers the viability of parasite. | (Das and Ganapaty, 2014) |
| M. extensa (Benth.) Baker | Leaves | Anthelmintic | In-vitro | Acetone, ethanol, aqueous | After treatment of 1 μL of extract, the % of inhibition on C. elegans was observed where the aqueous extract was found to be more potent (% of inhibition is 88.0 ± 3.0) | Due to the presence of flavonoids as its major phytoconstituents. | (Padhi et al., 2017) |
| M. pinnata (L.) Panigrahi | Seed, root | Anti-ulcer | In-vivo | Methanol | The extract showed optimal effective dose at a dose of 25 mg/kg in pyloric ligation, aspirin and duodenal induced ulcer model. | Not studied | (Pulipati et al., 2018) |
| M. griffithii Dunn | Whole plant | Anti inflammatory | In-vitro | EtOAc, n-hexane, griffinone B | Isolates were evaluated on LPS-induced NO production in RAW 264.7 cell resulting significant activity and 3 isolates showed more than 50% inhibition having IC50 value 20.4, 2.1 and 35.7 μM. griffinone B exhibits the best activity. | The possible mechanism is the suppression of the expression of iNOS protein and inhibition of NO production. | (Tang et al., 2016) |
| M. dielsiana Harms ex Diels | Stem | Anti-inflammatory | In-vitro | Ethanol | Isolates were evaluated for nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated murine RAW264.7 macrophage cells where (3S)-vestitol showed the highest inhibitory effect (IC50 value 16.0 ± 1.5 μM). | The mechanism is based on a decrease in NO production and TNF-α secretion. | (Dat et al., 2019) |
| M. pinnata (L.) Panigrahi | Leaves | Anti-inflammatory | In-vivo | 70% ethanol | An oral dose of 100, 300 and 1000 mg/kg did not show any gastric lesion and ulcerogenic activity in the both acute and chronic model which implied a significant anti-inflammatory activity. | COX-2 inhibition resulting decrease in PGE-2 synthesis | (Pulipati et al., 2018) |
| Pongamia piñata (synonym of M. pinnata (L.) Panigrahi) | Leaves | Antidiabetic | In-vivo | Petroleum ether, chloroform, ethanol, aqueous | An oral dose of 500 mg/kg of extracts was evaluated for in alloxan-induced diabetic rat model against standard drug Glibenclamide. It was observed that the aqueous and ethanol extract showed less potential but significant than the standard Glibenclamide and at the same time both of the extracts decrease the blood glucose and increase the body weight. | The possible mechanism may be for the increase insulin secretion which improves the rate of glucose utilization. Different bioactive constituents like flavonoids, phenolics, steroids also play an important role in antidiabetic study | (Sikarwar and Patil, 2010). |
| M. leucantha Kurz | Wood | Cytotoxicity, anti-cancer | In-vitro | Ethanol | Extracts were evaluated against NCI-H187, BCA-1and KB tumour cell lines resulting physcion and millettinol having potent activity against the NCI-H187 cell lines (IC50 value 4.30 μg/mL) and BCA-1 cell lines (IC50 value 3.44 μg/mL) respectively. | Not studied | (Rayanil et al., 2011) |
| M. speciosa Champ. | Root | Cytotoxicity | In-vitro | 70% ethanol, petroleum ether | The extract showed moderate activity against MCF-7, HCT-116, A549, HepG-2 cell lines. | Not studied | (Chen et al., 2015) |
| M. pinnata (L.) Panigrahi | Flower | Cytotoxicity, anti-cancer | In-vitro | Green synthesized nanoparticles | Several concentrations i.e. 11.11, 33.33, 100, 300 μg/mL of extract were evaluated using brine shrimp lethality assay resulting lowered LD50 value of 36.41 μg/mL. | This lowered LD50 value suggests the cytotoxicity activity green silver nanoparticles synthesized. This enhanced activity justifies the presence of toxic molecules which is a probable mechanism for anticancer activity. | (Rajakumar et al., 2017) |
| M. dorwardi Collett & Hemsl. | Vine stem | Cytotoxicity | In-vitro | EtOAc, n-BuOH, millepurpan, Medicarpin | Millepurpan showed moderate activity against four cancer cell lines, HepG2, HCT116, Raji and KG-1 cell lines (IC50 values 52.03, 68.89, 40.17 and 61.22 μM respectively). Medicarpin exhibited the best cytotoxic activity as compared to other compounds having IC50 value 38.07, 46.85, 36.13 and 30.11 μM, respectively. | Not studied | (Chen et al., 2018) |
| M. brandisiana Kurz | Root | Cytotoxicity, antioxidant, anti-cancer, aromatase inhibition activity, | In-vitro | Dichloromethane | MTT assay of the extract showed significant cytotoxicity against HepG2, A549, HuCCA-1, HeLa cell lines. The flavonoid isolates also showed good aromatase inhibition activity. | Bioactive flavonoids like lanceolatin B, isoloncho may be responsible to execute cytotoxicity as well as anti-cancer activity. | (Pailee et al., 2019) |
| M. pachyloba Drake | Stem | Cytotoxicity | In-vitro | Ethanol, durmillone | Preliminary screening study resulted in 10 compounds having cytotoxicity against HeLa and MCF-7 cells and further evaluation against HeLa, HepG2, MCF-7, Hct116, MDA-MB-231 and HUVEC (normal cell line) showed specific cytotoxicity having an IC50 range from 5 to 40 μM among of them durmillone showed potent activity by inducing apoptosis. | The isolates may induce apoptosis and autophagy in a concentration-dependent manner. | (Yan et al., 2019) |
5.1. Antimicrobial activity
The antimicrobial activity of various extracts of Millettia species viz. M. extensa (Benth.) Baker, M. pachycarpa Benth., M. pinnata (L.) Panigrahi, M. speciosa Champ. have been reported against gram-positive, gram-negative and fungal strains. Both agar well diffusion and disk diffusion in vitro methods were adopted for the evaluation of the antimicrobial property.
The antimicrobial activity of hexane and EtOAc extract of the stem bark of M. barteri (Benth.) Dunn was evaluated by determining the minimum inhibitory concentration against bacterial and fungal strains where, the reported MIC value is 64–512 µg/mL (Havyarimana et al., 2012). The isolates millaurine and milletonine were tested for the first time for its antimicrobial activity. Both of the extracts showed antimicrobial where, EtOAc extract showed a significant result.
The antimicrobial activities of different extracts of leaves of M. extensa (Benth.) Baker were evaluated against various multidrug-resistant (MDR) bacterial strains i.e. Staphylococcus aureus, Escherichia coli, Candida albicans using the agar cup plate method and MIC. Both aqueous and methanol extracts were evaluated and the methanol extract was shown to have the zone of inhibition ≥12 mm whereas, the MIC (minimum inhibitory concentration) is ≤1 mg/mL against both gram-positive and gram-negative organisms (Padhi et al., 2017). The root and leaf extract (methanol) of M. extensa (Benth.) Baker are having antifungal activity against Mycobacterium phlei (Gautam et al., 2007).
The ethyl acetate and petroleum extract of M. pinnata (L.) Panigrahi seed, bark and leaves showed good antimicrobial activity against different strains in agar well diffusion method. Extracts showed maximum zone of inhibition against Bacillus subtilis. The presence of triterpenes, chalcones, flavones and carboxylic acid may be responsible for the antimicrobial activity. The oil extracted from its seed showed significant antifungal activity against Aspergillus niger. Ethanol extract of stem of M. pinnata (L.) Panigrahi also showed significant antimicrobial activity against selected gram-positive and gram-negative organism by agar diffusion method at a concentration ranging from 250 to 1000 μg (Pulipati et al., 2018).
The leaf extract of M. pinnata (L.) Panigrahi and green synthesized silver nanoparticles possessed potent antibacterial activity in disc diffusion method against different microbial strains where, it showed maximum inhibition against the E. coli (Rajakumar et al., 2017). This inhibitory activity may be due to the presence of silver cations which is responsible for the structural changes of the membrane of microbes and alteration of membrane permeability leading to cell death.
The probable mechanism behind the antimicrobial activity is somehow linked with the presence of prenylated isoflavones, chalcones, and pterocarpans. The prenyl groups help in the attachment to the outer membrane of the cell of the bacteria resulting in membrane apoptosis.
5.2. Antioxidant activity
The antioxidant activity of ethanol extract of leaves of M. pinnata (L.) Panigrahi may be due to the presence of polyphenols and flavonoid contents which was performed upon the oral administration of the extract (300 mg/kg body mass) in the albino rat. There was a significant increase in the reduced glutathione, glutathione peroxidase, catalase, superoxide dismutase levels whereas, a decrease in the levels of conjugative dines, hydrooxyperoxide, thiobarbituric acid reactive substances were found. This results showed a protective role of the extract against lipid peroxidation which draws a probable mechanism for antioxidant activity (Pulipati et al., 2018).
The evaluation of the antioxidant activity of hexane and EtOAc extract of the stem bark of M. barteri (Benth.) Dunn was done by using the DPPH assay technique and revealed that millaurine showed maximum activity in comparison with other compounds (Havyarimana et al., 2012).
Ethyl acetate and ethanol extracts/fractions contain most of the free radical scavenger and in other hands the phenolic contents directly related to the antioxidant effect, which may be a possible mechanism for this activity.
5.3. Antiplasmodial activity
It was investigated on the antiplasmodial activity of isolated rotenoid and flavonoids which was extracted from the stem bark of M. usaramensis Taub. using dichloromethane. This study was carried out against the chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of P. falciparum with an IC50 value of 21.1 and 28 μg/mL each (Yenesew et al., 2003).
The CH2Cl2/MeOH (1:1) extract of the stem bark of M. oblate ssp. teitensis was evaluated, which confirmed about the in vitro antiplasmodial activity using non-radioactive assay against the chloroquine-sensitive Sierra Leone 1 (D6) and chloroquine-resistant Indochina 1 (W2) strains of P. falciparum with an IC50 value of 12.0 ± 1.2 and 10.0 ± 2.3 μM each (Derese et al., 2014).
The antiplasmodial activity of CH2Cl2/CH3OH (1:1) extract of the root bark of M. dura Dunn was investigated and the minimal activity was found for compounds viz. calopogonium isoflavone B, maximaisoflavone B and 7, 2′-dimethoxy-4′, 5′-methylenedioxyisoflavone against the chloroquine-sensitive 3D7 and the chloroquine-resistant Dd2 P. falciparum strain where the activity found in between 70 and 90% inhibition at 40 μM (Marco et al., 2017).
5.4. Immunomodulatory activity
A novel polysaccharide fraction (MSCP2) was extracted and isolated from the roots of M. Speciosa Champ. MSCP2 (molecular weight = 2.85 × 104 Da) was purified from the aqueous root extract and confirmed to have significant immunomodulatory activity by improving the pinocytic capacity and increasing the levels of NO, TNF-α, and IL-6. TLR4, SRA and GR were found to be the major PRRs for MSCP2 to trigger signalling transcription and macrophage activation. These results indicated that MSCP2 might be a potent agent in medical science and food industries (Huang et al., 2020).
5.5. Cognitive activity
A polysaccharide isolated and purified by DEAE-cellulose and Sephadex G-75 chromatography from M. pulchra Kurz was investigated for its cognitive activity. The polysaccharide was evaluated in experimental animals (Kunming mice) by performing the behavioural study (Lin et al., 2014). From this study, it was found that polysaccharide significantly reversed D-galactose induced learning and memory impairments by reducing the oxidative stress along with suppression of inflammatory response. It was also found that polysaccharide distinctly lowers the content and deposition of β-amyloid peptide increase the level of acetylcholine but decreased the cholinesterase activity. These outcomes suggested that polysaccharide may be the active constituent to exert a protective effect on cognitive impairment in mice (Zhang et al., 2018).
5.6. Anti-cholinesterase activity
It was reported that a stronger inhibitory activity of both acetylcholinesterase and butyrylcholinesterase found for the green synthesized silver nanoparticles (AgNPs) as compared with flower extract of M. pinnata (L.) Panigrahi (Rajakumar et al., 2017).
Studies were carried out to observe the anti-cholinesterase activity of the ethanol n- BuOH extracts of seed of M. pachycarpa Benth. which showed potent activity where n- BuOH extract also showed significant anti-cholinesterase activity. The bio-assay guided isolates of EtOAc extract was evaluated by Ellman’s methods and also showed better anticholinesterase activity which showed moderate to weak acetylcholinesterase activity with an IC50 value ranging from 17.14 to 131.17 μM, which is the future prospect to analyze as a definite agent for Alzheimer’s therapy (Tu et al., 2019).
5.7. Anthelmintic activity
Assessment of the anthelmintic activity of chloroform extract of leaves and stems of M. auriculata Brandis was carried out using Pheretima posthuma at a dose of 10 mg/mL against the standard drug Albendazole, where, the leaves extracts were found to be more potent as compared with stem extract and standard. Here Albendazole plays an inhibitory mechanism on the helminthic β-tubulin polymerization by interfering with microtubule-dependent functions such as uptake of glucose and depletion of glycogen (Das and Ganapaty, 2014). The probable mechanism behind the anthelmintic activity maybe due to the availability of the rich source like flavonoids, triterpenes, and phenolic contents, which is responsible for the death of parasite by damaging the glycoprotein.
It was observed that different extracts (ethanol, acetone and aqueous) of M. extensa (Benth.) Baker leaves (1 μL) were having anthelmintic property against the activity of C. elegans where the aqueous extract showed significant result i.e. 80.0 ± 3.0 in terms of % of inhibition of relative movement (Padhi et al., 2017).
5.8. Anti-ulcer activity
The in-vivo assay was performed to investigate the methanol extract of seeds of M. pinnata (L.) Panigrahi and observed anti-ulcerogenic activity at a dose of 25 mg/kg in pyloric ligation and aspirin-induced ulcer model (Pulipati et al., 2018). Generally, secondary metabolites like terpenoids, quinines, flavonoids, etc. exhibits some kind of cytoprotective as well as anti-secretory property. Flavonoids play a vital role in anti-ulcer activity by amplifying the mucosal defence system and also improve the capillary resistance by raising the gastric defensive factors (Mohod and Bodhankar, 2013).
5.9. Anti-inflammatory activity
Studies were carried out to investigate the in vitro anti-inflammatory activity of the isolated compounds of the n-hexane and EtOAc extract of M. griffithii Dunn on LPS-induced NO production in RAW 264.7 cell, where, griffinone B showed the best activity. Further, it was selected for the evaluation of iNOS and COX-2 expression with 1400 W and revealed the suppression of iNOS protein and mRNA as the positive control (Tang et al., 2016).
The anti-inflammatory activity of ethanol extracts of stems of M. dielsiana Harms ex Diels was evaluated. The nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated murine RAW264.7 macrophage cells showed the highest inhibitory effect (IC50 value 16.0 ± 1.5 μM). Isoliquiritigenin and tupichinol C showed the modest inhibitory effects on NO production having IC50 values 31.2 ± 2.5 and 38.4 ± 1.9 μM respectively (Dat et al., 2019).
It was also evaluated that M. pinnata (L.) Panigrahi leaves extract (70% ethanol) having potent anti-inflammatory activity in three different models (acute, sub-acute and chronic) in both Wister rat and Swiss mice. There were no signs of gastric lesion as well as ulcerogenic activity in both acute and chronic models which draw a possible mechanism for inflammatory activity (Pulipati et al., 2018).
The direct mechanism involved in anti-inflammatory activity is the inhibition of pro inflammatory mediators like keratinocytes, leukocytes endothelial cells and also prostaglandin release and synthesis by flavonoids (inhibit COX-1 and COX-2) (Schäfer et al., 2006), alkaloids (inhibit the metabolic pathway of arachidonic acid) (Ayal et al., 2019) and biphenylneolignans.
5.10. Antidiabetic activity
Different solvent extracts of leaves of Pongamia piñata (synonym of M. pinnata (L.) Panigrahi) was evaluated for antidiabetic activity in alloxan-induced diabetic rats against standard drug Glibenclamide. It was observed that the aqueous and ethanol extract showed less potential but significant than the standard Glibenclamide at an oral dose of 500 mg/kg. Both of the extracts also decrease blood glucose and increase body weight. So the possible mechanism may be for the increase insulin secretion which improves the rate of glucose utilization. Different bioactive constituents like flavonoids, phenolics and steroids also play an important role in the antidiabetic study (Sikarwar and Patil, 2010).
5.11. Cytotoxicity
The cytotoxicity and anti-cancer activity of the isolates of the wood of M. leucantha Kurz was evaluated against NCI-H187, BCA-1and KB tumour cell lines where, physcion showed potent anti-cancer activity against the NCI-H187 cell lines (IC50 value 4.30 μg/mL) and millettinol also showed significant activity against BCA-1 cell lines (IC50 value 3.44 μg/mL) (Rayanil et al., 2011).
Studies were carried out to evaluate the cytotoxicity of two new petroleum ether soluble isolates from the ethanol (70%) extract of the root of M. speciosa Champ. by using four different human cancer cell lines viz. MCF-7, HCT-116, A549, HepG-2 and showed moderate activity (Chen et al., 2015).
In another research, the cytotoxicity activity of green silver nanoparticles synthesized product of M. pinnata (L.) Panigrahi flower extract was evaluated of particular concentration using brine shrimp lethality assay where the LD50 value was 36.41 μg/mL. This revealed the presence of toxic constituents in green silver nanoparticles synthesized product which links a mechanism towards an anticancer activity as the nanoparticles are a good substitute for a cancer drug. Anticancer activity is due to the presence of flavonoid which is chemopreventive in nature and plays an important role in the cell signalling (i.e. cell proliferation and angiogenesis phase). It is the fact that phytoconstituents from flavonoid group may show potent anticancer activity (Rajakumar et al., 2017). Pongamia piñata (synonym of M. pinnata (L.) Panigrahi) was reported earlier for its sub-acute toxicity of its isolated compound “Pongamol” using long Evan’s rats at a dose of 300 μg/kg/day for a period of 14 days. Different parameters like biochemical, haematological, body weight and histopathology were evaluated in both the control and experimental group. It was found that Pongamol is safe for clinical trial as it showed no significant changes in any parameters (Baki et al., 2007). Again, the acute oral toxicity study of this same plant was also evaluated by using female Wistar rat. A dose of 2000 mg/kg/day of crude extract were administrated to the animals (100–120 gm, 4–8 week old) for 14 days. As a result, there were no toxicity signs and death found in any groups (Aneela et al., 2011). Later, another isolated compound known as “Karanjin” was also isolated from the methanol extract of the same plant and evaluated for its toxicity. Male Wistar rats (180–200 gm) were administered at a dose of 20 mg/kg/day of Karanjin for a time period of 14 days. The result showed no major changes in the biochemical profile indicating no lethal effect (Vismaya et al., 2011). Different solvent extracts like ethanol, aqueous, chloroform, and petroleum ether of Pongamia piñata (synonym of M. pinnata (L.) Panigrahi) was evaluated for acute oral toxicity for a dose range of 50–5000 mg/kg/day for a period of 14 days. It was observed that there is no significant change in the toxicity parameters of all the extracts (Sikarwar and Patil, 2010).
The seeds, root, and bark extract of M. ferruginea darasana were evaluated for in-vitro toxicity against Amblyomma variegatum Fabricius larvae (both male and female) at a different concentration ranging from 20 to 100% in Petri plates. Based on the mortality rate, it was observed that no toxic constituents were present in leaves extract where, the highest toxicity is observed in seed followed by root extract (Choudhury et al., 2015).
It was reported that different compounds were identified from EtOAc and n-BuOH extracts of vine stems of M. dorwardi Collett & Hemsl. and evaluated for the cytotoxicity effect against five cancer cell lines viz. HepG2, HCT116, MCF7, Raji and KG-1. These compounds have no evident activity but one of the compound known as millepurpan showed modest growth inhibitor activity against Raji and KG-1 cell line. From the advance study, it was revealed that millepurpan could induce G1 arrest and apoptosis in KG-1 cells (Chen et al., 2018).
The dichloromethane root extracts of M. brandisiana Kurz was reported for having significant cytotoxicity activity against mammalian cancer cell lines such as HepG2, A549, HuCCA-1, HeLa using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT assay). This study revealed the reports about the efficacy of isolated flavonoids having good aromatase inhibition activity and antioxidant activity (ORAC assay) as well. These results draw a probable mechanism that the bioactive flavonoids could be of importance for advance studies as prospective cytotoxicity and cancer chemopreventive agents (Pailee et al., 2019).
Studies were conducted to investigate the cytotoxicity activity of different fractionated isolates of ethanol extracts of stems of M. pachyloba Drake on HeLa and MCF-7cell lines by MTT assay. Total of 10 compounds showed potent cytotoxicity activity for which these were again subjected, to study the activity against different cancer cell lines viz. HeLa, HepG2, MCF-7, Hct116, and MDA-MB-231 and one normal cell line HUVEC. This revealed that these compounds have specific cytotoxicity potential in a cancer cell line whereas, there are no such results obtained in case of the normal cell line. Again, the autophagic effect was investigated by using GFP-LC3-HeLa cells and confirmed about the compound known as durmillone, which significantly induces autophagy. From the PI/Annexin V double staining assay, it was confirmed that durmillone, also induces apoptosis in HeLa and MCF-7 cells. These findings suggest the compound, as a potent anti-cancer agent demonstrated by highest cytotoxic activity, through the combined action of apoptosis and autophagy (Yan et al., 2019). Significant cytotoxicity is observed due to the presence of bioactive rotenoids.
6. Conclusions
Millettia species have been used as a conventional medicine across the globe as it a rich source of isoflavonoids, rotenoids, sterols, phenolic compounds, coumarin, terpenoids, resin, saponins, etc (Derese et al., 2014). The present review reports traditional uses, phytochemical constituents and pharmacological activities based on ethnopharmacological claims of genus “Millettia”. Extensive literature survey revealed that most of the species are used traditionally in different African and Asian countries including India, Pakistan, China, France, Burma, Malaysia, Thailand, Kenya, etc where, only a few species were scientifically evaluated for their phytochemical constituents that could mediate particular pharmacological activities. M. pinnata (L.) Panigrahi has been the most investigated species and it plays an important role in biodiesel production (Ruhul et al., 2017, Madhu et al., 2016). The major traditional use of Millettia species as reviewed involved in the treatment of joint pain, rheumatoid arthritis, hepatitis amenorrhea, tuberculosis, chronic bronchitis, the fish poison, insecticide, skin disease, vermifuge which have been validated scientifically. It is observed that most of the pharmacological activity studies were limited to both in-vitro and in-vivo screening where, the mechanisms of action, bioavailability and pharmacokinetics are not explored clearly. Furthermore, a number of studies were done for the bioassay-guided extraction and isolation of phytochemical constituents. Further research should target on the exploration and validation of traditional claims of other species by focusing the bioassay-guided drug discovery along with the formulation and mode of administration of drugs which we found lacking in most of the reviewed literature. An additional extensive use that requires to be evaluated is in the menstrual irregularities. Even though these species have ethno claims to be used for the female disease but it has not been evaluated significantly. So, further well designed and more clinical in-depth studies are required by focusing on the mechanism-based in vitro and in vivo studies for understanding the underlying mechanisms linked to ethnopharmacological uses.
7. Future perspective
The feature of this review based on traditional uses, phytochemicals and significant pharmacological activities of different Millettia species. Therefore, it would be essential to carry out a comprehensive investigation to identify their individual phytochemicals and possible mechanism of pharmacological activity for the development of new formulations in drug discovery.
Millettia species imply dynamic medicinal as well as pharmacological activity. So researchers should focus on the exploration of these species having potent activity against various ailments to figure out the probable mechanism in the molecular ground. However, the traditional uses are validated by performing different animal studies and the clinical trial is required to measure the safety and efficacy in the human being which is a part of the drug development process.
8. Author’s contributions
Rasmita Jena: Collection and compilation of data, writing of the manuscript. Diptirani Rath: Review and editing of the manuscript. Sudhanshu Sekhar Rout: Correction and analysis of chemical compounds. Durga Madhab Kar: Designing, supervising and editing of the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are thankful to the Siksha ‘O’ Anusandhan Deemed to be University for providing continuous support and encouragement in the promotion of scientific works.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aneela S., De S., Kanthal L., Choudhury N.S.K., Lohi B., Sagar K.V. Acute oral toxicity studies of Pongamia pinnata and Annona squamosa on albino wister rats. Int. J. Res. Pharm. Chem. 2011;1:820–824. [Google Scholar]
- Ayal G., Belay A., Kahaliw W. Evaluation of wound healing and anti-inflammatory activity of the leaves of Calpurnia aurea (Ait.) Benth (fabaceae) in mice. Wound Med. 2019;25(1):100151. doi: 10.1016/j.wndm.2019.100151. [DOI] [Google Scholar]
- Baki, Md. Abdullahil, Khan, Alam, Al-Bari, M. Abdul Alim, Mosaddik, Ashik, Sadik, G., Mondal, K.A.M.S.H., 2007. Sub-acute toxicological studies of pongamol isolated from Pongamia pinnata. Res. J. Med. Med. Sci. 2, 53–57.
- Banzouzi J.T., Prost A., Rajemiarimiraho M., Ongoka P. Traditional uses of the African Millettia species. Int. J. Bot. 2008;4:406–420. [Google Scholar]
- Baruah Putul, Barua Nabin C., Sharma Ram P., Baruah Jogendra N., Kulanthaivel Palaniappan, Herz Werner. Flavonoids from Millettia pulchra. Phytochemistry. 1984;23(2):443–447. [Google Scholar]
- Bora Montu Moni, Deka Riblu, Ahmed Nuruddin, Kakati Dilip Kumar. Karanja (Millettia pinnata (L.) Panigrahi) seed oil as a renewable raw material for the synthesis of alkyd resin. Ind. Crops Prod. 2014;61:106–114. [Google Scholar]
- Chen De-Li, Liu Yang-Yang, Ma Guo-Xu, Zhu Nai-Liang, Wu Hai-Feng, Wang De-Li, Xu Xu-Dong. Two new rotenoids from the roots of Millettia speciosa. Phytochem. Lett. 2015;12:196–199. [Google Scholar]
- Chen Kai, Tang Huan, Zheng Li, Wang Lun, Xue Linlin, Peng Ai-hua, Tang Ming-hai, Long Chaofeng, Chen Xiaoxin, Ye Hao-yu, Chen Li-juan. Identification of compounds with cytotoxic activity from Millettia dorwardi Coll. Et. Hemsl. Phytochem. Lett. 2018;25:60–64. [Google Scholar]
- Choudhury Manas Kumar, Yoseph Shiferaw, Hussen Ahmed. Toxicity of Millettia ferruginea darasana (family : Fabaceae) against the larvae and adult ticks of Amblyomma variegatum Fabricius a three-host tick in cattle. J. Parasite Dis. 2015;39:298–302. doi: 10.1007/s12639-013-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sanjoy, Ganapaty Seru. In vitro anthelmintic activity of Millettia auriculata leaves and stems. Asian Pacific Journal of Tropical Disease. 2014;4:S870–S873. [Google Scholar]
- Dat Le Duc, Tu Nguyen Thi Minh, Duc Ngo Viet, Luyen Bui Thi Thuy, Huyen Chu Thi Thanh, Jang Hyun Jae, Thu Dang Thi, Huong Tran Thu, Tram Le Huyen, Thong Nguyen Van, Hung Nguyen Duc, Kim Young Ho, Thao Nguyen Phuong. Anti-inflammatory secondary metabolites from the stems of Millettia dielsiana Harms ex Diels. Carbohydr. Res. 2019;484:107778. doi: 10.1016/j.carres.2019.107778. [DOI] [PubMed] [Google Scholar]
- Derese Solomon, Barasa Leonard, Akala Hoseah M., Yusuf Amir O., Kamau Edwin, Heydenreich Matthias, Yenesew Abiy. 4′-Prenyloxyderrone from the stem bark of Millettia oblata ssp. teitensis and the antiplasmodial activities of isoflavones from some Millettia species. Phytochem. Lett. 2014;8:69–72. [Google Scholar]
- Fu Man-qin, Xiao Geng-sheng, Xu Yu-juan, Wu Ji-jun, Chen Yu-long, Qiu Samuel-X. Chemical Constituents from Roots of Millettia speciosa. Chinese Herbal Med. 2016;8(4):385–389. [Google Scholar]
- Fuendjiep, Victorine, Augustin, E., Nkengfack, Z.T.F., 1998. Conrauinones C and D, Two isoflavones from stem bark of Millettia conraui. Phytochem 47, 113–115.
- Gautam Raju, Saklani Arvind, Jachak Sanjay M. Indian medicinal plants as a source of antimycobacterial agents. J. Ethnopharmacol. 2007;110(2):200–234. doi: 10.1016/j.jep.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Havyarimana Léopold, Ndendoung Simplice Tatsimo, de Dieu Tamokou Jean, de Théodore Atchadé Alex, Tanyi Joseph Mbafor. Chemical constituents of Millettia barteri and their antimicrobial and antioxidant activities. Pharm. Biol. 2012;50(2):141–146. doi: 10.3109/13880209.2011.579618. [DOI] [PubMed] [Google Scholar]
- Huang Zhi, Zeng Ying-Jie, Chen Xi, Luo Si-Yuan, Pu Lei, Li Fang-Zhou, Zong Min-Hua, Lou Wen-Yong. A novel polysaccharide from the roots of Millettia Speciosa Champ: preparation, structural characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2020;145:547–557. doi: 10.1016/j.ijbiomac.2019.12.166. [DOI] [PubMed] [Google Scholar]
- Kamble S.Y., Patil S.R., Sawant P.S., Sawant S., Pawar S.G., Singh E.A. Studies on plants used in traditional medicine by Bhilla tribe of Maharashtra. Indian J. Tradit. Knowl. 2010;9:591–598. [Google Scholar]
- Kamnaing P., Fanso Free S.N.Y., Nkengfack A.E., Folefoc G., Tanee Fomum Z. An isoflavan-quinone and a flavonol from Millettia laurentii. Phytochemistry. 1999;51:829–832. doi: 10.1016/S0031-9422(99)00043-6. [DOI] [Google Scholar]
- Kamnaing Pierre, Yurika Fanso Free S.N., Tanee Fomum Z., Martin Marie-Thérèse, bodo Bernard. Millettonine, a guanidine alkaloid from Millettia laurentii. Phytochemistry. 1994;36(6):1561–1562. [Google Scholar]
- Kamto Eutrophe Le Doux, Atchadé Alex de Théodore, Marston Andrew, Pegnyemb Dieudonné Emmanuel, van der Westhuizen Jan H. Chemical constituents from bark of Millettia mannii Baker (Papilionoideae−Leguminosae) Biochem. Syst. Ecol. 2012;45:98–101. [Google Scholar]
- Khuankaew Sunee, Srithi Kamonnate, Tiansawat Pimonrat, Jampeetong Arunothai, Angkhana Inta P.W. Ethnobotancal study of medicinal plants used by Tai Yai in Northern Thailand. J. Ethnopharmacol. 2014;151:829–838. doi: 10.1016/j.jep.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Lin Xing, Huang Zhongshi, Chen Xiaoyu, Rong Yanping, Zhang Shijun, Jiao Yang, Huang Quanfang, Huang Renbin. Protective effect of Millettia pulchra polysaccharide on cognitive impairment induced by d-galactose in mice. Carbohydr. Polym. 2014;101:533–543. doi: 10.1016/j.carbpol.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Madhu Devarapaga, Chavan Supriya B., Singh Veena, Singh Bhaskar, Sharma Yogesh C. An economically viable synthesis of biodiesel from a crude Millettia pinnata oil of Jharkhand, India as feedstock and crab shell derived catalyst. Bioresour. Technol. 2016;214:210–217. doi: 10.1016/j.biortech.2016.04.055. [DOI] [PubMed] [Google Scholar]
- Manikandan G., Rani A.V., Divya C., Ramasubbu R. Gc-Ms analysis of phytochemical constituents in the petroleum ether leaf extracts of Millettia Peguensis. Int. Res. J. Pharm. 2017;8:144–150. doi: 10.7897/2230-8407.089170. [DOI] [Google Scholar]
- Marco Makungu, Deyou Tsegaye, Gruhonjic Amra, Holleran John, Duffy Sandra, Heydenreich Matthias, Firtzpatrick Paul A., Landberg Göran, Koch Andreas, Derese Solomon, Pelletier Jerry, Avery Vicky M., Erdélyi Máté, Yenesew Abiy. Pterocarpans and isoflavones from the root bark of Millettia micans and of Millettia dura. Phytochem. Lett. 2017;21:216–220. [Google Scholar]
- Mohod Smeeta M., Bodhankar Subhash L. Antiulcer activity of aqueous extract of leaves of Madhuca indica J. F. Gmel against naproxen induced gastric mucosal injury in rats. J. Acute Dis. 2013;2(2):127–133. [Google Scholar]
- Na Zhi, Fan Qing-Fei, Song Qi-Shi, Hu Hua-Bin. Three new flavonoids from Millettia pachyloba. Phytochem. Lett. 2017;19:215–219. [Google Scholar]
- Ngandeu François, Bezabih Merhatibeb, Ngamga Dieudonne, Tchinda Alembert T., Ngadjui Bonaventure T., Abegaz Berhanu M., Dufat Hanh, Tillequin François. Rotenoid derivatives and other constituents of the twigs of Millettia duchesnei. Phytochemistry. 2008;69(1):258–263. doi: 10.1016/j.phytochem.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Ongoka P.R., Banzouzi J.T., Poupat C., Ekouya A., Ouamba J.M., Moudachirou M. Steroids isolated from Millettia versicolor Baker (Fabaceae) African J. Biotechnol. 2008;7:1727–1730. doi: 10.5897/AJB08.194. [DOI] [Google Scholar]
- Padhi L., Panda S.K., Leyssen P., Neyts J., Luyten W., Liu M. Antimicrobial, anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the similipal biosphere reserve, Odisha, India. Front. Pharmacol. 2017;8:1–15. doi: 10.3389/fphar.2017.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailee Phanruethai, Mahidol Chulabhorn, Ruchirawat Somsak, Prachyawarakorn Vilailak. Diverse flavonoids from the roots of Millettia brandisiana. Phytochemistry. 2019;162:157–164. doi: 10.1016/j.phytochem.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Palazzino Giovanna, Rasoanaivo Philippe, Federici Elena, Nicoletti Marcello, Galeffi Corrado. Prenylated isoflavonoids from Millettia pervilleana. Phytochemistry. 2003;63(4):471–474. doi: 10.1016/s0031-9422(02)00489-2. [DOI] [PubMed] [Google Scholar]
- Panda T., Padhy R. Ethnomedicinal plants used by tribes of Kalahandi district, Orissa. Indian J. Tradit. Knowl. 2008;07:242–249. [Google Scholar]
- Pulipati S., Navyasri Harshini N.Y., Vyshnavi J., Sowjanya Pulipati C., Srinivasa Babu P., Neelima Lakshmi D., Navyasri N., Harshini Y., Prasanth M. A phyto pharmacological review on a versatile medicinal plant: Pongamia pinnata (L.) pierre. 459. J. Pharmacogn. Phytochem. 2018;7:459–463. [Google Scholar]
- Rahman Taj Ur, Arfan Mohammad, Mahmood Tariq, Liaqat Wajiha, Gilani Mazhar Amjad, Uddin Ghias, Ludwig Ralf, Zaman Khair, Choudhary M. Iqbal, Khattak Khanzadi Fatima, Ayub Khurshid. Isolation, spectroscopic and density functional theory studies of 7-(4-methoxyphenyl)-9H-furo[2,3-f]chromen-9-one: A new flavonoid from the bark of Millettia ovalifolia. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;146:24–32. doi: 10.1016/j.saa.2015.03.061. [DOI] [PubMed] [Google Scholar]
- Rajakumar Govindasamy, Gomathi Thandapani, Thiruvengadam Muthu, Devi Rajeswari V., Kalpana V.N., Chung Ill-Min. Evaluation of anti-cholinesterase, antibacterial and cytotoxic activities of green synthesized silver nanoparticles using from Millettia pinnata flower extract. Microb. Pathog. 2017;103:123–128. doi: 10.1016/j.micpath.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Raksat Achara, Maneerat Wisanu, Andersen Raymond J., Pyne Stephen G., Laphookhieo Surat. Antibacterial prenylated isoflavonoids from the stems of Millettia extensa. J. Nat. Prod. 2018;81(8):1835–1840. doi: 10.1021/acs.jnatprod.8b00321. [DOI] [PubMed] [Google Scholar]
- Rayanil Kanok-on, Bunchornmaspan Pastraporn, Tuntiwachwuttikul Pittaya. A new phenolic compound with anticancer activity from the wood of Millettia leucantha. Arch. Pharm. Res. 2011;34(6):881–886. doi: 10.1007/s12272-011-0603-4. [DOI] [PubMed] [Google Scholar]
- Ruhul A.M., Kalam M.A., Masjuki H.H., Shahir S.A., Alabdulkarem Abdullah, Teoh Y.H., How H.G., Reham S.S. Evaluating combustion, performance and emission characteristics of Millettia pinnata and Croton megalocarpus biodiesel blends in a diesel engine. Energy. 2017;141:2362–2376. [Google Scholar]
- Schäfer A., Chovanová Z., Muchová J., Sumegová K., Liptáková A., Ďuračková Z., Högger P. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol) Biomed. Pharmacother. 2006;60:5–9. doi: 10.1016/j.biopha.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Sharma Jyotsana, Gairola Sumeet, Sharma Yash Pal, Gaur R.D. Ethnomedicinal plants used to treat skin diseases by Tharu community of district Udham Singh Nagar, Uttarakhand, India. J. Ethnopharmacol. 2014;158:140–206. doi: 10.1016/j.jep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Sharma J., Gaur R.D., Gairola S., Painuli R.M., Siddiqi T.O. Traditional herbal medicines used for the treatment of skin disorders by the Gujjar tribe of Sub-Himalayan tract, Uttarakhand. Indian J. Tradit. Knowl. 2013;12:736–746. [Google Scholar]
- Sikarwar Mukesh S., Patil M.B. Antidiabetic activity of Pongamia pinnata leaf extracts in alloxan-induced diabetic rats. Int. J. Ayurveda Res. 2010;1(4):199. doi: 10.4103/0974-7788.76780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritularak, B., Likhitwitayawuid, K., 2006. Flavonoids from the pods of Millettia erythrocalyx. Phytochemistry 67, 812–817. https://doi.org/10.1016/j.phytochem.2006.01.013. [DOI] [PubMed]
- Su, Xue-Hui, Li, Cong-Ying, Zhong, Yu-Jiao, Yuan, Zhi-Peng, Li, Yan-Fang, Liang, B., 2012. A new prenylated chalcone from the seeds of Millettia Pachycarpa. Chin. J. Nat. Med. 10, 222–225.
- Tang Huan, Pei He-Ying, Wang Tai-Jin, Chen Kai, Bo Wu., Yang Qiu-Nan, Zhang Qiqng, Yang Jian-Hong, Wang Xiao-Yan, Tang Ming-Hai, Peng Ai-Hua, Hao-Yu Ye L.-J. Flavonois and biphenylneolignans with anti-inflammatory activity from the stems of Millettia griffithii. Bioorga. 2016;26:4417–4422. doi: 10.1016/j.bmcl.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Tu, Yanbei, Wu, Chuanhi, Kang, Yunyao, Li, Qin, Zhu, Chao, Y.L., 2019. Bioactivity-guided identification of flavonoids with cholinesterase and beta-amyloid peptide aggregation inhibitory effects from the seeds of Millettia Pachycarpa. Bioorg. Med. Chem. Lett. 29, 1194–1198. [DOI] [PubMed]
- Vismaya, Belagihally Srikanta M. Sindhu, Rajashekhar B., Jayaram Vinay M., Dharmesh Shylaja C., Thirumakudalu Sindhu Kanya, 2011. Gastroprotective properties of Karanjin from Karanja (Pongamia pinnata) Seeds; Role as Antioxidant and H +, K + -ATPase Inhibitor. Evidence-based Complement. Altern. Med. 2011, 1–10. https://doi.org/10.1093/ecam/neq027. [DOI] [PMC free article] [PubMed]
- Yan Wei, Yang Jianhong, Tang Huan, Xue Linlin, Chen Kai, Wang Lun, Zhao Min, Tang Minghai, Peng Aihua, Long Chaofeng, Chen Xiaoxin, Ye Haoyu, Chen Lijuan. Flavonoids from the stems of Millettia pachyloba Drake mediate cytotoxic activity through apoptosis and autophagy in cancer cells. J. Adv. Res. 2019;20:117–127. doi: 10.1016/j.jare.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankep Emanuel, Fomum Zacharias T., Bisrat Daniel, Dagne Ermis, Veronika Hellwig W.S. O-geranylated isoflavones and a 3-phenylcoumarin from Millettia Griffoniana. Phytochemistry. 1998;49:2521–2523. [Google Scholar]
- Ye Haoyu, Chen Lijuan, Li Yanfang, Peng Aihua, Fu Afu, Song Hang, Tang Minghai, Luo Houding, Luo Youfu, Xu Yongbin, Shi Jianyou, Wei Yuquan. Preparative isolation and purification of three rotenoids and one isoflavone from the seeds of Millettia pachycarpa Benth by high-speed counter-current chromatography. J. Chromatogr. A. 2008;1178(1-2):101–107. doi: 10.1016/j.chroma.2007.11.060. [DOI] [PubMed] [Google Scholar]
- Yenesew Abiy, Derese Solomon, Midiwo Jacob O, Oketch-Rabah Hellen A, Lisgarten John, Palmer Rex, Heydenreich Matthias, Peter Martin G, Akala Hosea, Wangui Julia, Liyala Pamela, Waters Norman C. Anti-plasmodial activities and X-ray crystal structures of rotenoids from Millettia usaramensis subspecies usaramensis. Phytochemistry. 2003;64(3):773–779. doi: 10.1016/s0031-9422(03)00373-x. [DOI] [PubMed] [Google Scholar]
- Zhang Hongfang, Chen Junjie, Cen Ying. Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. Int. J. Biol. Macromol. 2018;112:862–867. doi: 10.1016/j.ijbiomac.2018.01.214. [DOI] [PubMed] [Google Scholar]
- Zhang Shuyu, Cheng Jun, Chen Wenjing, Ling Xiaomei, Zhao Yuying, Feng Jie, Xiang Cheng, Liang Hong. Interactions between thrombin and natural products of Millettia nitita var. hirsutissima using capillary zone electrophoresis. J. Chromatogr. B. 2009;877(32):4107–4114. doi: 10.1016/j.jchromb.2009.10.033. [DOI] [PubMed] [Google Scholar]