Abstract
The main objective of this research was to evaluate the impact of methanolic, ethanolic and aqueous extracts of Origanum majorana L., Origanum vulgare L., Teucrium chamaedrys L., Teucrium montanum L., Thymus serpyllum L. and Thymus vulgaris L. (Lamiaceae) on the effects of free radicals using different model systems. The extracts were characterized on the basis of the contents of total phenolics, phenolic acids, flavonoids and flavonols, and also using high-performance liquid chromatography with diode-array detection. Antioxidant activity in vitro was assessed using DPPH assay. The genoprotective properties were tested using plasmid relaxation assay on pUC19 E. coli XL1-Blue, while SOS/umuC assay on Salmonella typhimurium TA1535/pSK1002 and Comet assay on human lung fibroblasts were used to assess the antigenotoxicity of the extracts. Ethanolic extracts had the most phenolics (up to 236.20 mg GAE/g at 0.5 mg/mL), flavonoids (up to 42.47 mg QE/g at 0.5 mg/mL) and flavonols (up to 16.56 mg QE/g at 0.5 mg/mL), and they exhibited the highest DPPH activity (up to 92.16% at 0.25 mg/mL). Interestingly enough, aqueous extracts provided the best protection of plasmid DNA (the lowest IC50 value was 0.17 mg/mL). Methanolic extracts, on the other hand, most efficiently protected the prokaryotic DNA, while all the extracts had a significant impact against genomic damages inflicted on human fibroblasts. O. vulgare extracts are considered to be the most promising in preserving the overall DNA integrity against oxidative genomic damages. Moreover, HPLC-DAD analysis highlighted rosmarinic acid as the most abundant in the investigated samples (551.45 mg/mL in total in all the extracts), followed by luteolin-7-O-glucoside (150.19 mg/mL in total), while their presence correlates with most of the displayed activities. The novelty of this study is reflected in the application of a prokaryotic model for testing the antigenotoxic effects of Lamiaceae species, as no previous reports have yet been published on the genoprotective potential of these species.
Keywords: Lamiaceae, Phenolic components, Antioxidant activity, Cytotoxicity, Antigenotoxicity, Genoprotective effect
1. Introduction
In biological sense, oxidative stress is defined as a physiological imbalance between reactive oxygen species (ROS) and the ability of the body to discard free radicals (Gupta et al., 2014). Free radicals such as hydrogen peroxide can lead to oxidation of lipids and proteins, and even produce damages to the genetic material inside the cells. Nevertheless, this so-called genotoxic effect of free radicals might not influence only the DNA, but also the cellular components related to the function of chromosomes within the living cell (López-Romero et al., 2018). Hence, oxidative stress can be associated, besides ageing, with organ inflammatory diseases – cardiovascular and neurodegenerative diseases, chronic kidney and obstructive pulmonary diseases, as well as diabetes and cancer (Liguori et al., 2018, Kuciel-Lewandowska et al., 2020). In a healthy organism, the levels of free radicals are under firm control mechanisms, hence, oxidative stress can be annulled by the involvement of antioxidant response mechanisms normally occurring in the cells with the help of enzymatic and nonenzymatic endogenous antioxidants (Kuciel-Lewandowska et al., 2020). However, considering that people nowadays are exposed to excessive environmental pollution, chronic psychological stress and physical exertion, cigarette and alcohol usage, among others, this antioxidant protection might only alleviate the oxidative damage (Xu et al., 2017, Liguori et al., 2018). Nonetheless, additional protection factors, such as exogenous antioxidants, are required to alternate free radical reactions, cell signals transmission and also the activity of different proteins and genes involved in DNA repair mechanisms (Kuciel-Lewandowska et al., 2020). Exogenous antioxidants (externally supplied through foods or supplements) might be of artificial or natural origin (Gupta et al., 2014, Liguori et al., 2018). Since the basic knowledge of modern medicine and pharmacy relies on the insights of traditional medicine, it is not surprising that nearly a quarter of pharmaceuticals nowadays are developed using natural sources (Pandey and Rizvi, 2009, Nastić et al., 2018). Moreover, the previous fact justifies the ever-growing scientific interest in ethnobotanical studies.
Medicinal plants contain pharmacologically active secondary metabolites which exhibit beneficial health effects and are often used as therapeutic agents. Plant extracts containing metabolites such as polyphenols (phenolic acids, flavonoids, stilbenes and lignans) are proven as effective antioxidant agents. Since phenolic groups are able to accept an electron, relatively stable phenoxyl radicals can be formed, which consequently leads to the disruption of chain oxidation reactions in cellular components. Additionally, phenolic compounds may affect the activity of other endogenous antioxidants, or, on the other hand, absorb the pro-oxidative components of food (for example, iron). The consumption of plants rich in polyphenols may lead to the reduction of oxidative damage inflicted on DNA and also to protection of cell constituents against oxidative damage, which further reduces the risk of development of miscellaneous diseases related to oxidative stress (Pandey and Rizvi, 2009).
The assessment of the antioxidant capacity of natural products has been regarded as a basis for ranking the plants with antioxidant potential and recommending best antioxidant agents for both nutrition and treatment of various diseases (Carović-Stanko et al., 2016).
Serbia is distinguished by a remarkable diversity of vegetation and flora, being one of the 158 world centers of biodiversity. This biodiversity is also reflected in the number of medicinal species with about 700, of which 41 belong to the Lamiaceae family (Jarić et al., 2014). In the present study, extracts of six Lamiaceae species often used in traditional medicine of Serbia (Origanum majorana L. - Om, Origanum vulgare L. - Ov, Teucrium chamaedrys L. - Tc, Teucrium montanum L. - Tm, Thymus serpyllum L. - Ts and Thymus vulgaris L. - Tv) were extensively analyzed for their antioxidant activity with the emphasis on understanding their deleterious effects of oxidative stress in acellular, prokaryotic and eukaryotic models. These plants were selected for research due to their frequent use in Serbian traditional medicine as teas and tinctures and in that form they can often be spotted on the shelves in supermarkets and pharmacies. Om is previously reported by Serbian interviewees to be used fresh or as infusion against sore throat, pneumonia and as a sedative (Janaćković et al., 2019). Ov is usually used as tea in the treatment of minor digestive problems, insomnia, headaches, colds, bronchitis, as an antiseptic for the respiratory and urinary systems. Tc and Tm are reported to be used in Serbian folk medicine as a remedy for gastrointestinal ailments, improving digestion, as febrifuges and antipyretics, while Tm might also be used in ethnoveterinary medicine (Šavikin et al., 2013, Jarić et al., 2014). Several plants from the genus Thymus L. are among the most common cultivated medicinal and aromatic plants in Serbia, with Ts being one of the most used medicinal herbs in this country (Dajić Stevanović, 2011). It is often used in preparations of natural herbal remedies, such are infusions, decoctions, tea, tinctures, syrups and oils as disinfectant and sedative, to improve blood circulation and in treating illnesses and problems related to both respiratory and gastrointestinal systems, among others (Šavikin et al., 2013, Jarić et al., 2015). When used as an infusion, Tv might successfully treat digestive system disorders and bacterial infections (Janaćković et al., 2019).
In this research, the antigenotoxic effects of extracts of the examined Lamiaceae species are presented for the first time on a prokaryotic model. The knowledge gained on bacterial model systems can often be successfully applied in more complex higher organisms (Žegura et al., 2006, Rea et al., 2010), which is the reason of our assessing of the plant extracts’ potential on this model. The results are expected to contribute to further studies on the DNA protecting effects of plant extracts.
2. Materials and methods
2.1. Chemicals and reagents
All reagents and standards were of analytical, LC-S and HPLC grade. Hydrochloric acid, ethanol and methanol were purchased from Zorka Pharma, Šabac, Serbia. Acridine orange, aluminium chloride (AlCl3), aluminum nitrate nonahydrate (Al(NO3)3×9H2O), ascorbic acid, benzo[α]pyrene (BaP), bicarbonate buffer solution, caffeic acid, dimethyl sulfoxide (DMSO), EDTA, Folin–Ciocalteu reagent, gallic acid, low melting point agarose (LMP), normal melting point agarose (NMP), ortho-nitrophenyl-b-galactoside (ONPG), potassium acetate (CH3COOK), quercetin, sodium acetate (CH3COONa), sodium carbonate anhydrous (Na2CO3), TBE buffer, Tris, Triton X-100, trypsin, β-galactosidase, 2-tert-butyl-4-hydroxyanisole (BHA), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 3,5-di-tert-butyl-4-hydroxytoluene (BHT), 4-nitroquinoline N-oxide (4NQO) were obtained from Sigma-Aldrich, St. Louis, MO. Dulbecco’s Modified Eagle Medium (DMEM), foetal bovine serum, penicillin/streptomycin and 1 × phosphate buffered saline solution (PBS) were purchased from PAA Laboratories GmbH, Oberosterreich, Austria. Sodium molybdate and sodium nitrite were purchased from Dispo-chem, UK. Hydrogen peroxide (H2O2) was purchased from Farmanea Galenska Laboratorija, Belgrade, Serbia. Sodium hydroxide (NaOH) was purchased from Superlab, Belgrade, Serbia, sodium chloride (NaCl) was obtained from Carlo Erba Reagents, Milano, Italy, formic acid, acetonitrile, from Merck, Darmstadt, Germany and rosmarinic acid, caffeic acid, chlorogenic acid, quercetin, rutin, naringin, luteolin-7-O-glucoside from Extrasynthese, Genay, France.
2.2. Plant material and extracts preparation
The aerial plant parts were collected in the spring of 2018. Om and Tv have been cultivated on production fields of the Institute for Medicinal Plants Research “Dr Josif Pančić” in Pančevo, Serbia (N 44.872162, E 20.699931, 81 m a.s.l). The soil type was humogley and the plants were harvested in the full flowering stage in the third and fifth years of cultivation, for marjoram and thyme, respectively. Ov, Tc, Tm and Ts were collected from the Stara planina Mt., eastern Serbia (Ov – N 43.269969, E 22.766822, 1052 m a.s.l; Tc – N 43.158317, E 22.816939, 738 m a.s.l; Tm – N 43.307335, E 22.780980, 1392 m a.s.l and Ts – N 43.292139, E 22.767138, 1199 m a.s.l). The harvested plant material was subsequently dried in an industrial dryer at 50 °C. The specimens were deposited in the Herbarium of the Institute for Medicinal Plant Research “Dr Josif Pančić”, Belgrade (Om voucher specimen number – 302231, Ov – 302291, Tc – 302091, Tm – 302351, Ts – 302,321 and Tv – 302361).
Ten grams of ground plant material was subjected to the procedure of classical maceration (24 h, room temperature) using methanol and ethanol (70:30 alcohol:water), and hot distilled water as solvents (10% w/v). The prepared mixtures were exposed to ultrasound (30 °C, 2 h). The extracts were filtered twice through Whatman No. 1. The excess of solvent was evaporated under reduced pressure with a rotary evaporator (Buchi Rotavapor R-114). Until further analyses, the obtained extracts were stored at 4 °C, protected from light and moisture.
2.3. Determination of phytochemical contents
Experiments concerning the quantification of phytochemical contents of the extracts were finalized during the first month of extracts preparation in order to evade the possible degradation of their active substances. All measurements were done for extracts on three concentrations (0.1, 0.25 and 0.5 mg/mL).
The total phenolic (TPC) and total flavonoid contents (TFC) were measured using Perkin Elmer Lambda Bio UV/VIS spectrophotometer as described previously (Alimpić et al., 2015, Alimpić et al., 2017). TPC was calculated from the curve equation of gallic acid and expressed as gallic acid equivalents (mg GAE) per gram of dry extract. On the other hand, TFC was calculated from quercetin curve equation and expressed as quercetin equivalents (mg QE) per gram of dry extract.
Phenolic acids content (PAC) and flavonol content (FC) quantification was done as described earlier, with slight modifications (Mihailović et al., 2016), at 490 and 440 nm, respectively, using Perkin Elmer Lambda Bio UV/VIS spectrophotometer. PAC was expressed as mg of caffeic acid equivalents (CAE), while FC was expressed as mg of quercetin equivalents (QE) per gram of dry extract per gram of dry extract.
All the results were presented as mean ± standard error averaged from three measurements.
2.4. HPLC-DAD analysis
The phenolic compounds in the tested extracts were determined by comparing the retention times and absorption spectra (200–400 nm) of unknown peaks with the reference standards. HPLC-DAD analysis was performed on Agilent 1200 Series HPLC (Agilent Technologies, Palo Alto, CA, USA) equipped with Lichrospher® 100 RP 18e column (5 μm, 250 × 4 mm). The mobile phase A was formic acid in water (0.17%), while the mobile phase B was acetonitrile. The injection volume was 10 μL, and flow rate 0.8 mL/min with gradient program (0–53 min 0–100% B). Stopping time of the analysis was 55 min. Compounds were determined by comparing the retention times and absorption spectra (200–400 nm) of unknown peaks with the reference standards. The investigated samples were analyzed in triplicate.
2.5. Evaluation of DPPH scavenging activity
The antioxidant activity of extracts in vitro was evaluated using 2,2-diphenyl-1-picrylhydrazyl radical, a scavenging method that was suggested earlier, with minor modifications (Blois, 1958). BHA, BHT and ascorbic acid were used as positive controls, and the absorbance was measured at 517 nm using Perkin Elmer Lambda Bio UV/VIS spectrophotometer. The decrease in absorption of DPPH was calculated as follows:
| (1) |
where Ac represents the absorbance of the negative control and As is the absorbance of the test samples. The results are presented as averaged percentage of DPPH inhibition from three measurements ± standard error.
2.6. Genoprotective activity in a cell-free system
The effects of extracts on the protection of supercoiled DNA were studied against OH radical in a model system named plasmid relaxation assay (Russo et al., 2006). Briefly, plasmid pUC19 was isolated from E. coli XL1-blue (Birnboim, 1983) and was checked after 1 h electrophoresis on 1% agarose gel (0.5 × TBE, 80 V, 300 mA). About 80% of the isolated plasmid was present in the closed circular form, while about 20% was in the open circular (nicked) form.
All experiments were performed in a volume of 15 µL of 1 × PBS containing 800 ng of plasmid pUC19 and plant extracts in the final concentrations of 0.1, 0.5 and 1 mg/mL. Solvent controls were included in experiments where the extracts were substituted with ethanol and methanol (final concentration in the mixture was 7%). Immediately prior to the irradiation of the samples with UV light, hydrogen peroxide was added to a final concentration of 3 mM. The reaction volumes were held in a cover of 96 well plate, placed directly under the UV lamp and irradiated for 3 min (540 J/m2) at 254 nm. After irradiation, 5 µL of 6 × loading dye (30% (v/v) glycerol, 0.25% (w/v) bromophenol blue and 0.25% (w/v) xylene cyanol) was added to each reaction. The samples were analyzed by electrophoresis on a 1% agarose gel prepared in 0.5 × TBE buffer. The positions of open circular form and closed circular form of plasmid were compared to the ladder λ/PstI (0.5 µg/lane, Thermo Fisher Scientific, SAD). Electrophoresis was performed at a constant voltage of 80 V, 300 mA for about 1 h, whereas bromphenol blue did not exceed 75% of the gel. Gels were observed on a UV transilluminator under ultraviolet light at 312 nm and photographed with a digital camera. The images were analyzed using software ImageJ (National Institutes of Health, Bethesda, MD). Treatments were performed in three individual experiments.
2.7. Protective effect of the extracts against hydrogen peroxide-induced DNA damage in a prokaryotic model
The inhibition of DNA damage induced by hydrogen peroxide in co-treatment with selected extracts was assessed in SOS/umuC assay, following the protocol given earlier (Kolarević et al., 2019). Briefly, the treatment with hydrogen peroxide in the final concentration of 1.68 mM was performed for 2 h at 37 °C in incubation mixtures composed of 10 µL of plant extract (concentrations 0.125; 0.25 and 0.5 mg/mL) and 90 µL of bacterial culture of Salmonella typhimurium TA1535/pSK1002 in exponential phase. Sterile bidistilled water was used as a negative control, while methanol and ethanol (final concentration of 3.5%) were used as solvent controls. The determination of bacterial growth rate was done at 600 nm on a microtiter plate reader (Thermo Scientific, Waltham, MA, Multiscan FC). On the other hand, β-galactosidase activity (G) was determined using ONPG as a substrate (30 min at 25 °C). The absorption was measured at 405 nm using a reference solution without bacteria. All treatments were performed in triplicates in three individual experiments.
2.8. Protective effect of the extracts against hydrogen peroxide-induced DNA damage in eukaryotic model
2.8.1. Cell culture
MRC-5 cell line (normal human foetal lung fibroblast cell line; ECACC No. 84101801) was grown in cultivation medium (DMEM supplemented with foetal bovine serum in final concentration 10%, and 100 U/mL penicillin/streptomycin) at 37 °C in 5% CO2. The cells were used at passages between 21 and 26.
2.8.2. Screening of cytotoxicity of the extracts in MRC-5 cell line by MTT assay
MTT test was performed following the protocol previously described in details (Kolarević et al., 2019). The effect of the extracts (in three concentrations: 0.025, 0.05 and 0.1 mg/mL) on the viability of MRC-5 cells was measured during 1 h and 24 h. Ethanol and methanol in the final concentration of 0.35% were used as solvent controls. The medium was removed after the treatment, and the cell monolayer was washed with 1 × PBS. To each well was further added 200 µL of MTT solution (0.5 mg/mL). After the incubation of the plates (3 h at 37 °C, 5% CO2), the supernatant was extracted from the wells, and formazan crystals were dissolved by adding of 200 µL DMSO per well. The absorbance was measured at 570 nm.
2.8.3. Comet assay
The protective effect against hydrogen peroxide-induced DNA damage was assessed in co-treatment experiments based on the study of Yang et al. (1999). MRC-5 cells were seeded in 24 well plates (0.7 mL/well) in concentration 105 cells/mL and left for 24 h at 37 °C to attach. The cultivation medium was replaced with FBS-free DMEM containing 50 µM hydrogen peroxide and different concentrations of extracts. The concentrations of extracts were selected based on the results of the MTT assay (0.025; 0.05 and 0.1 mg/mL). To rule out the effect of solvents, methanol and ethanol were used in a final concentration of 0.35%. Treatments were performed, for at least 1 and no longer than 2 h. At the end of the incubation period, cells were trypsinized, centrifuged for 10 min at 1000 rpm and eluted in 1 × PBS.
Comet assay was performed in minigel format as described earlier (Azqueta et al., 2013), with some modifications. Aliquots of cell suspensions obtained after treatment (30 µL) were mixed with 70 µL of 1% low melting point agarose. For each sample, 15 µL of the prepared mix was placed on slides pre-coated with 1% normal melting point agarose. Each microscope slide contained duplicates of negative controls and three concentrations of extract positive controls. Comet assay conditions were the same as previously described in details (Kolarević et al., 2019). For visualization, the gels were stained with 20 µL of acridine orange (2 µg/mL) per slide. A total of 100 nucleoids per sample were scored by fluorescent microscope (Leica, DMLS, Austria, under magnification 400×, excitation filter 450–490 nm, barrier filter 510 nm) using Comet IV Computer Software (Perceptive Instruments, UK) software in order to obtain values for Tail Intensity parameter (TI%).
2.9. Statistical analysis
Statistical analyses were carried out using Statistica 10 Software. Kolmogorov-Smirnoff was used to determine if the data were normally distributed. As the data were in line for the usage of nonparametric tests, Kruskal–Wallis one-way ANOVA was applied followed by the Mann-Whitney U test for pairwise comparison of the treated groups with negative and solvent controls. The level of significance for all comparisons was set at p < 0.05. In order to evaluate the influence of extraction solvents on phytochemical contents and values obtained by DPPH assay, one-way ANOVA and Tukey’s post-hoc test were performed. Differences were considered as statistically significant if the p-value was<0.05. Pearson's correlation coefficients (r) were calculated among the results obtained from DPPH assay and phytochemical content, and presented according to Taylor (1990). For SOS/umuC assay the significance of DNA damage inhibition in co-treatments with extracts in comparison with hydrogen peroxide alone was assessed by t-test.
3. Results
In the present study, extracts of six Lamiaceae species traditionally used in Serbia were subjected to the in vitro examination of their potential to reduce the deleterious effects of free radicals in acellular, prokaryotic and eukaryotic model systems. Likewise, their total phenolic, phenolic acids, flavonoid and flavonol contents were quantified in order to link the exhibited biological activities with the respective groups of phytochemicals.
3.1. Phytochemical composition
The contents of total phenolics, phenolic acids, flavonoids and flavonols were calculated by applying appropriate calibration curves and the results are presented in Table 1. For each test, the results were screened on three concentrations (0.1, 0.25 and 0.5 mg/mL) in order to make the obtained results comparable with DPPH assay. Moreover, by using different concentrations, the methods underwent a verification, since a concentration gradient for the vast majority of samples was found. Furthermore, only the results obtained at the concentration of 0.25 mg/mL will be considered for further discussion, since a dose dependency was observed for the phytochemical contents, meaning that with the increase of extract concentration, there is a higher amount of polyphenolics found in them.
Table 1.
Phytochemical (total phenolic (TPC), phenolic acid (PAC), total flavonoid (TFC) and flavonol (FC)) content of the tested plant extracts presented at 0.1, 0.25 and 0.5 mg/mL, as means ± standard errors.
| Species | Extract | TPC (mg GAE/g) |
PAC (mg CAE/g) |
TFC (mg QE/g) |
FC (mg QE/g) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/mL) | 0.1 | 0.25 | 0.5 | 0.1 | 0.25 | 0.5 | 0.1 | 0.25 | 0.5 | 0.1 | 0.25 | 0.5 | |
| Om | Methanolic | 35.14 ± 1.02 | 82.60 ± 3.46 | 160.59 ± 0.61 | 2.70 ± 1.28ab | 20.85 ± 5.59ab | 81.59 ± 6.42ab | 4.54 ± 0.37 | 11.65 ± 0.78 | 25.13 ± 0.19 | 0.45 ± 0.09 | 1.96 ± 0.32 | 8.86 ± 0.46 |
| Ethanolic | 26.66 ± 1.25 | 64.01 ± 2.63 | 122.23 ± 4.11 | 25.67 ± 3.33a | 52.33 ± 4.01a | 117.15 ± 5.48a | 6.37 ± 0.79 | 17.83 ± 0.63 | 35.41 ± 0.75 | 2.02 ± 0.22 | 5.58 ± 0.19 | 9.88 ± 0.44 | |
| Aqueous | 23.59 ± 0.54 | 58.07 ± 1.81 | 105.60 ± 0.35 | nd | 9.37 ± 4.44b | 45.67 ± 4.44b | 6.03 ± 0.61 | 8.48 ± 0.28 | 21.03 ± 0.34 | 1.88 ± 0.29 | 3.09 ± 1.88 | 6.56 ± 0.53 | |
| Ov | Methanolic | 34.46 ± 1.09ab | 82.14 ± 2.66ab | 158.51 ± 1.82ab | 40.48 ± 4.63 | 96.04 ± 2.57 | 160.85 ± 6.12 | 8.36 ± 0.75 | 22.49 ± 0.55 | 47.03 ± 0.34 | 1.17 ± 0.06 | 4.73 ± 0.18 | 12.30 ± 0.47 |
| Ethanolic | 51.97 ± 2.79a | 129.25 ± 0.04a | 236.20 ± 0.34a | 27.89 ± 2.22 | 74.19 ± 2.80 | 137.89 ± 4.01 | 6.90 ± 0.39 | 19.36 ± 0.19 | 42.47 ± 0.89 | 3.36 ± 0.23 | 7.60 ± 0.24 | 16.56 ± 0.47 | |
| Aqueous | 25.09 ± 0.20b | 60.75 ± 1.13b | 106.05 ± 1.98b | 22.70 ± 1.71 | 36.04 ± 5.01 | 101.96 ± 5.01 | 4.82 ± 0.37 | 12.93 ± 0.80 | 32.49 ± 1.25 | 2.65 ± 0.15 | 5.43 ± 0.29 | 9.13 ± 0.61 | |
| Tc | Methanolic | 10.32 ± 0.49a | 25.84 ± 0.90a | 50.31 ± 1.67a | 5.30 ± 1.28 | 29.74 ± 2.80 | 77.89 ± 6.67 | 4.76 ± 0.11 | 15.57 ± 0.53 | 32.28 ± 1.07 | 3.23 ± 0.03 | 5.37 ± 0.23 | 8.34 ± 0.31 |
| Ethanolic | 23.17 ± 1.27ab | 51.25 ± 0.60ab | 99.04 ± 3.53ab | 3.81 ± 2.80 | 16.41 ± 3.39 | 72.33 ± 5.56 | 5.87 ± 0.87 | 14.82 ± 0.28 | 25.94 ± 0.79 | 3.36 ± 0.31 | 6.81 ± 0.12 | 11.76 ± 0.37 | |
| Aqueous | 25.06 ± 0.60b | 61.30 ± 1.69b | 110.23 ± 3.62b | nd | 11.22 ± 2.94 | 57.15 ± 7.23 | 6.00 ± 1.02 | 15.78 ± 0.42 | 30.51 ± 1.13 | 1.40 ± 0.59 | 3.98 ± 0.12 | 7.10 ± 0.81 | |
| Tm | Methanolic | 25.32 ± 3.04ab | 56.54 ± 4.68ab | 114.27 ± 1.28ab | 0.85 ± 0.64 | 18.63 ± 1.28 | 45.67 ± 4.01 | 4.69 ± 0.46 | 13.70 ± 0.23 | 29.36 ± 1.31 | 2.02 ± 0.12 | 3.94 ± 0.10 | 8.62 ± 0.49 |
| Ethanolic | 35.37 ± 1.72a | 79.21 ± 3.73a | 147.74 ± 3.68a | 10.11 ± 1.92 | 33.07 ± 6.70 | 43.81 ± 2.31 | 7.02 ± 1.40 | 17.37 ± 0.85 | 31.47 ± 1.46 | 2.32 ± 0.12 | 7.53 ± 0.17 | 12.81 ± 0.18 | |
| Aqueous | 10.19 ± 0.85b | 24.80 ± 0.37b | 26.95 ± 0.81b | 5.67 ± 1.11 | 31.96 ± 5.70 | 48.63 ± 6.79 | 2.39 ± 0.43 | 7.27 ± 0.05 | 15.29 ± 0.23 | 1.76 ± 0.20 | 3.88 ± 0.29 | 8.39 ± 0.06 | |
| Ts | Methanolic | 31.39 ± 1.91 | 77.15 ± 3.18 | 147.22 ± 1.20 | 20.85 ± 1.70 | 43.81 ± 5.59 | 56.78 ± 2.22 | 5.41 ± 0.19 | 15.29 ± 0.30 | 34.48 ± 0.79 | 3.52 ± 0.20 | 7.47 ± 0.55 | 16.29 ± 0.29 |
| Ethanolic | 26.92 ± 0.10 | 64.63 ± 1.26 | 121.87 ± 4.61 | nd | 21.96 ± 3.90 | 43.81 ± 2.80 | 5.72 ± 0.39 | 14.54 ± 0.75 | 32.65 ± 0.71 | 3.13 ± 0.17 | 5.91 ± 0.26 | 14.19 ± 0.27 | |
| Aqueous | 16.48 ± 0.06 | 43.98 ± 1.41 | 78.65 ± 2.48 | 5.67 ± 3.33 | 32.33 ± 5.56 | 34.19 ± 3.57 | 3.51 ± 0.09 | 7.68 ± 0.44 | 19.04 ± 0.43 | 2.36 ± 0.32 | 4.62 ± 0.37 | 9.84 ± 0.40 | |
| Tv | Methanolic | 23.59 ± 0.10 | 35.08 ± 1.18 | 68.38 ± 1.91 | 2.33 ± 1.11 | 21.96 ± 2.31 | 114.56 ± 2.22 | 5.59 ± 0.14 | 18.42 ± 0.61 | 36.87 ± 1.43 | 1.86 ± 0.21 | 6.83 ± 1.86 | 12.03 ± 0.49 |
| Ethanolic | 25.97 ± 2.02 | 59.80 ± 1.65 | 110.00 ± 5.42 | nd | 14.93 ± 2.31 | 68.63 ± 3.90 | 7.15 ± 0.16 | 19.57 ± 0.56 | 37.43 ± 1.29 | 3.88 ± 0.12 | 8.97 ± 0.06 | 14.33 ± 0.63 | |
| Aqueous | 16.25 ± 1.02 | 40.85 ± 0.59 | 75.42 ± 1.08 | nd | 6.78 ± 2.94 | 53.44 ± 8.01 | 2.71 ± 0.48 | 7.21 ± 0.28 | 16.03 ± 1.17 | 3.65 ± 0.15 | 5.39 ± 0.47 | 8.59 ± 0.38 | |
Values are presented as means ± standard errors (n = 3). For each assay and each tested species, mean values with different superscript letters within column (a–b) differ significantly (one-way ANOVA, Tukey's post hoc; p < 0.05); nd – not detected.
Generally, alcoholic extracts showed higher TPC, PAC, TFC and FC when compared with aqueous extracts (Table 1).
The most excessive amount of phenolics was found in ethanolic extract of Ov, followed by the methanolic extract of Om (129.25 and 82.60 mg GAE/g, respectively). The lowest TPC was observed in aqueous extract of Tm (24.80 mg/mL). Only in the cases of Ov, Tc and Tm ethanolic and methanolic extracts were significantly different from the aqueous ones (see Table 1).
On the other hand, when the PAC was considered, a different relationship between the extracts was observed i.e. PAC was higher in methanolic extracts compared to the ethanolic ones. Regardless, only in Om ethanolic and aqueous extracts PAC is shown to be significantly different. The highest PAC was observed in Ov methanolic extract, followed by Ov ethanolic extract (96.04 and 74.19 mg CAE/g, respectively). The lowest PAC was found in Tv aqueous extract (6.78 mg CAE/g), while it was not detected in several aqueous and ethanolic extracts at the concentration of 0.1 mg/mL.
Alcoholic extracts at 0.1 and 0.25 mg/mL showed the highest TFC, while aqueous ones had the lowest TPC overall, but with no observed significant differences. Specifically, methanolic and ethanolic extracts of Ov had the highest TFC (22.49 and 19.36 mg QE/g). Once again, the lowest amount of TFC was detected in aqueous extracts of Tm (7.27 mg QE/g).
Regarding FC, higher amount was found in ethanolic extracts compared to the methanolic and aqueous ones, however the observed differences are not considered as statistically significant. Specifically, the highest amounts of FC (7.60 and 7.53 mg QE/g) were detected in ethanolic extracts of Ov and Tm. On the other hand, the lowest amount of FC was detected in aqueous extracts of Om (3.09 mg QE/g).
According to Pearson's correlation analysis (Table 4), all of the tested contents were in strong correlation with each other (correlation coefficient, r, was found to be from 0.69 to 0.89).
Table 4.
Correlation coefficients between the assays used for analyzes of the phytochemical content (TPC, PAC, TFC and FC), RA-rosmarinic acid, CA-caffeic acid, CLA-chlorogenic acid, Q-quercetin, RUT-rutin, NAR-naringin, LG-luteolin-7-O-glucoside and antioxidant activity in vitro (DPPH assay).
| TPC | PAC | TFC | FC | RA | CA | CLA | Q | RUT | NAR | LG | DPPH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.75c | 0.85c | 0.81c | 0.47b | −0.34a | −0.06a | −0.08a | −0.26a | −0.26a | 0.60b | 0.79c |
| PAC | 1 | 0.85c | 0.69c | 0.58b | −0.34a | −0.36a | −0.15a | −0.07a | 0.05a | 0.69c | 0.75c | |
| TFC | 1 | 0.89c | 0.56b | −0.64a | −0.11a | 0.18a | 0.06a | −0.01a | 0.59b | 0.88c | ||
| FC | 1 | 0.59b | −0.57a | −0.09a | 0.34a | 0.06a | 0.17a | 0.55b | 0.84c | |||
| RA | 1 | −0.36a | −0.53a | 0.28a | −0.55a | 0.49b | 0.91c | 0.39b | ||||
| CA | 1 | −0.34a | −0.08a | 0.24a | 0.02a | −0.35a | −0.53a | |||||
| CLA | 1 | 0.20a | 0.01a | −0.40a | −0.36a | 0.04a | ||||||
| Q | 1 | −0.02a | 0.34a | 0.23a | 0.13a | |||||||
| RUT | 1 | −0.25a | −0.51a | 0.13a | ||||||||
| NAR | 1 | 0.39b | −0.26a | |||||||||
| LG | 1 | 0.46b | ||||||||||
| DPPH | 1 |
According to Taylor (1990): ar ≤ 0.35 weak correlation; b0.36 < r < 0.67 moderate correlation; c0.68 < r < 1 strong correlation.
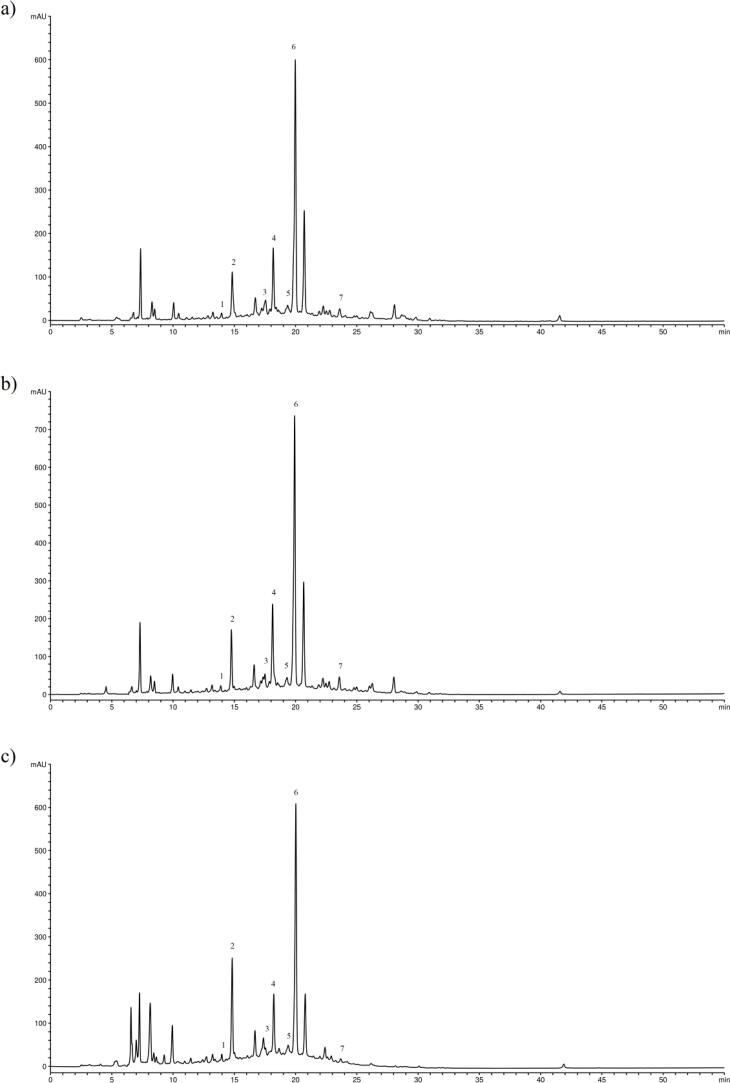
In this study, for each sample, seven chemical compounds were quantified using HPLC-DAD technology, and the results are presented in Table 2 and Supplementary Fig. 1. According to this analysis, the most abundant phenolic compound overall was rosmarinic acid, after which follows luteolin-7-O-glucoside and rutin, whereas the least abundant compound was chlorogenic acid. Among the phenolic acids, the highest amount of rosmarinic acid was found in Ov and Tv ethanolic extracts, 63.66 and 63.48 mg/mL, the most abundant quantity of caffeic acid was found in Tm ethanolic extract, 6.94 mg/mL, while Ts ethanolic extract had the highest amount of chlorogenic acid, 2.39 mg/mL. Furthermore, Tv ethanolic extract had the highest amount of quercetin, 6.95 mg/mL, and the most naringin was found in Tv methanolic extract, 8.84 mg/mL. The amount of rutin in the samples was below 10 mg/mL, however, interestingly, in Tc extracts was found up to 23.40 mg/mL (in methanolic extract). The highest value of luteolin-7-O-glucoside was 19.64 mg/mL and that was found in Ov ethanolic extract. The plant extracts with the most total phenolics, according to HPLC analysis, were Tv and Ov ethanolic extracts (100.21 and 92.05 mg/mL, respectively), while Tm aqueous extract had the least amount of the tested phenolics (11.42 mg/mL).
Table 2.
The results of the HPLC-DAD analysis presented as mg/g dry extract, as means ± standard deviation. RA – rosmarinic acid, CA – caffeic acid, CLA – chlorogenic acid, Q – quercetin, RUT – rutin, NAR – naringin, LG – luteolin-7-O-glucoside.
| HPLC-DAD analysis (mg/g dry extract) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Extract | RA | CA | CLA | Q | RUT | NAR | LG | Total |
| Om | Methanolic | 48.57 ± 2.47 | 5.94 ± 0.13 | tr | 5.18 ± 0.19 | 3.75 ± 0.18 | 4.15 ± 0.21 | 12.04 ± 0.52 | 79.63 ± 3.70 |
| Ethanolic | 37.71 ± 1.98 | 5.28 ± 0.11 | tr | 3.46 ± 0.13 | 3.94 ± 0.12 | 3.91 ± 0.13 | 12.76 ± 0.61 | 67.06 ± 3.08 | |
| Aqueous | 23.31 ± 1.02 | 4.95 ± 0.21 | 0.24 ± 0.01 | 3.17 ± 0.09 | 0.58 ± 0.21 | 2.68 ± 0.11 | 5.41 ± 0.31 | 40.34 ± 1.96 | |
| Ov | Methanolic | 58.68 ± 2.51 | 0.04 ± 0.00 | tr | 3.58 ± 0.10 | 2.69 ± 0.08 | 1.93 ± 0.03 | 15.91 ± 1.08 | 82.83 ± 3.80 |
| Ethanolic | 63.66 ± 3.18 | 0.70 ± 0.03 | 0.67 ± 0.02 | 2.99 ± 0.11 | 2.11 ± 0.09 | 2.28 ± 0.05 | 19.64 ± 1.69 | 92.05 ± 5.17 | |
| Aqueous | 35.61 ± 1.52 | 1.20 ± 0.04 | 1.36 ± 0.04 | 4.11 ± 0.15 | 2.07 ± 0.10 | 2.35 ± 0.11 | 6.99 ± 0.28 | 53.69 ± 2.24 | |
| Ts | Methanolic | 44.76 ± 2.32 | 1.62 ± 0.04 | 0.77 ± 0.30 | 5.62 ± 0.28 | 1.39 ± 0.06 | 4.95 ± 0.28 | 5.18 ± 0.21 | 64.29 ± 3.22 |
| Ethanolic | 50.94 ± 2.85 | 1.45 ± 0.05 | 1.23 ± 0.05 | tr | tr | 2.75 ± 0.08 | 6.97 ± 0.34 | 63.34 ± 3.37 | |
| Aqueous | 25.41 ± 1.53 | 5.07 ± 0.19 | 2.39 ± 0.11 | 6.59 ± 0.28 | 0.19 ± 0.01 | 2.49 ± 0.09 | 7.00 ± 0.31 | 49.14 ± 2.52 | |
| Tv | Methanolic | 60.12 ± 3.19 | 0.88 ± 0.04 | tr | 5.43 ± 0.31 | 4.91 ± 0.21 | 8.84 ± 0.41 | 14.19 ± 0.04 | 94.37 ± 4.20 |
| Ethanolic | 63.48 ± 3.22 | 2.36 ± 0.11 | 0.75 ± 0.03 | 6.95 ± 0.34 | 1.90 ± 0.09 | 7.88 ± 0.35 | 16.89 ± 0.09 | 100.21 ± 4.23 | |
| Aqueous | 30.03 ± 1.48 | 3.87 ± 0.17 | 0.90 ± 0.02 | 3.00 ± 0.14 | 2.29 ± 0.11 | 6.92 ± 0.33 | 7.98 ± 0.33 | 54.99 ± 2.58 | |
| Tc | Methanolic | 4.22 ± 0.68 | tr | 0.30 ± 0.02 | 6.07 ± 0.38 | 23.40 ± 0.48 | 2.90 ± 0.06 | 3.93 ± 0.15 | 40.85 ± 1.77 |
| Ethanolic | 2.71 ± 0.31 | 0.45 ± 0.09 | 1.39 ± 0.09 | 3.00 ± 0.17 | 20.04 ± 0.59 | 2.30 ± 0.05 | 5.40 ± 0.19 | 35.26 ± 1.49 | |
| Aqueous | 1.51 ± 0.45 | 0.34 ± 0.02 | 1.31 ± 0.22 | 2.18 ± 0.07 | 13.71 ± 0.64 | 2.84 ± 0.04 | 3.87 ± 0.11 | 25.76 ± 1.55 | |
| Tm | Methanolic | 0.51 ± 0.07 | 5.67 ± 0.23 | 1.41 ± 0.05 | 3.78 ± 0.38 | 7.01 ± 0.16 | 1.38 ± 0.08 | 1.53 ± 0.04 | 21.29 ± 1.01 |
| Ethanolic | 0.07 ± 0.00 | 6.94 ± 0.54 | tr | 5.71 ± 0.32 | 8.08 ± 0.25 | 1.95 ± 0.11 | 1.23 ± 0.03 | 23.98 ± 1.25 | |
| Aqueous | 0.15 ± 0.01 | 3.65 ± 0.08 | 1.18 ± 0.05 | 2.18 ± 0.12 | 0.99 ± 0.60 | tr | 3.27 ± 0.21 | 11.42 ± 0.53 | |
tr – traces.
3.2. DPPH scavenging activity
The in vitro antioxidant activity was studied for the plant extracts (concentrations of 0.1, 0.25 and 0.5 mg/mL) using DPPH assay. The results showed concentration dependency, however, it was noticed that at the concentration of 0.5 mg/mL the reaction mixture turned yellow showing strong reaction before the incubation time had expired, which was the case for almost all of the tested extracts (except for Tm aqueous extract). For this reason, the results obtained at this concentration will not be discussed hereinafter. The results of phytochemical contents of extracts and DPPH assay are presented at 0.25 mg/mL since at this concentration they are best distinguished among each other.
In general, ethanolic extracts were the most active ones at the concentrations of 0.1 and 0.25 mg/mL, while the aqueous ones were the least active against DPPH radicals. The extraction solvents did not influence significantly the DPPH activity of the tested plant species. This statement coincides with the results described regarding the presence of certain groups of phenolic components.
Altogether, the ethanolic extract of Ov exhibited the highest DPPH scavenging activity (92.16%), while the lowest activity against DPPH radicals showed the aqueous extract of Tm (22.55%). Three commercial antioxidants (BHA, BHT and ascorbic acid) at the same three concentrations were used in order to compare the results obtained when using plant extracts. Since ascorbic acid was proven to be extremely efficient against DPPH radical and it could not be measured, we only considered BHA and BHT for comparison with the extracts at the concentration of 0.25 mg/mL. Eleven extracts (alcoholic Om extracts, all three Ov extracts, ethanolic Tc extract, both alcoholic extracts of Tm and Ts and also Tv ethanolic extract) were more active than BHA and BHT, while the rest of them, mostly aqueous extracts, were less active than the applied standards (Table 3).
Table 3.
The activity of the selected plant extracts and positive controls against DPPH radical, presented as means of percentage of inhibition ± standard errors.
| DPPH (% of inhibition) | ||||
|---|---|---|---|---|
| Concentration (mg/mL) |
||||
| Species | Extract | 0.1 | 0.25 | 0.5 |
| Om | Methanolic | 32.66 ± 0.83 | 58.82 ± 0.71 | 93.55 ± 0.43 |
| Ethanolic | 32.29 ± 0.36 | 87.77 ± 1.42 | 95.02 ± 0.09 | |
| Aqueous | 25.46 ± 1.35 | 35.97 ± 1.56 | 92.58 ± 0.25 | |
| Ov | Methanolic | 40.16 ± 0.62 | 73.23 ± 2.15 | 94.48 ± 0.48 |
| Ethanolic | 51.84 ± 0.59 | 92.16 ± 0.17 | 95.37 ± 0.25 | |
| Aqueous | 42.38 ± 1.19 | 81.22 ± 2.34 | 93.47 ± 0.14 | |
| Tc | Methanolic | 33.74 ± 1.22 | 55.13 ± 2.53 | 95.25 ± 0.09 |
| Ethanolic | 35.09 ± 0.52 | 61.27 ± 0.66 | 95.25 ± 0.09 | |
| Aqueous | 26.38 ± 0.84 | 44.31 ± 1.54 | 93.86 ± 0.09 | |
| Tm | Methanolic | 30.29 ± 0.61 | 68.82 ± 0.85 | 95.29 ± 0.05 |
| Ethanolic | 27.66 ± 0.44 | 56.18 ± 0.35 | 94.17 ± 0.24 | |
| Aqueous | 21.07 ± 1.51 | 22.55 ± 1.24 | 89.73 ± 0.46 | |
| Ts | Methanolic | 31.54 ± 1.68 | 60.63 ± 0.79 | 94.63 ± 0.24 |
| Ethanolic | 34.95 ± 1.46 | 68.63 ± 0.58 | 94.75 ± 0.30 | |
| Aqueous | 27.39 ± 2.00 | 43.82 ± 0.88 | 92.55 ± 0.30 | |
| Tv | Methanolic | 32.56 ± 0.25 | 53.81 ± 0.68 | 94.40 ± 0.11 |
| Ethanolic | 33.33 ± 0.44 | 66.55 ± 0.86 | 94.59 ± 0.05 | |
| Aqueous | 28.03 ± 1.00 | 40.65 ± 0.82 | 92.08 ± 0.22 | |
| BHA | 36.20 ± 0.66 | 55.43 ± 1.38 | 75.43 ± 1.16 | |
| BHT | 32.73 ± 0.60 | 52.01 ± 0.53 | 71.90 ± 1.86 | |
| Ascorbic acid | 89.42 ± 0.17 | too high | too high | |
Values are presented as means ± standard errors (n = 3). For each tested species, mean values without superscript letters within column are not significantly different (one-way ANOVA, Tukey's post hoc; p < 0.05).
The results obtained in this assay correlated very well with the results of TPC, PAC, TFC and FC (Table 4). The correlation coefficient ranged from 0.75 to 0.88, which represents a strong correlation (Taylor, 1990). The presented results suggest that the phenolic compounds are responsible for the antioxidant activity of the investigated plants.
3.3. Genoprotective activity in a cell-free system
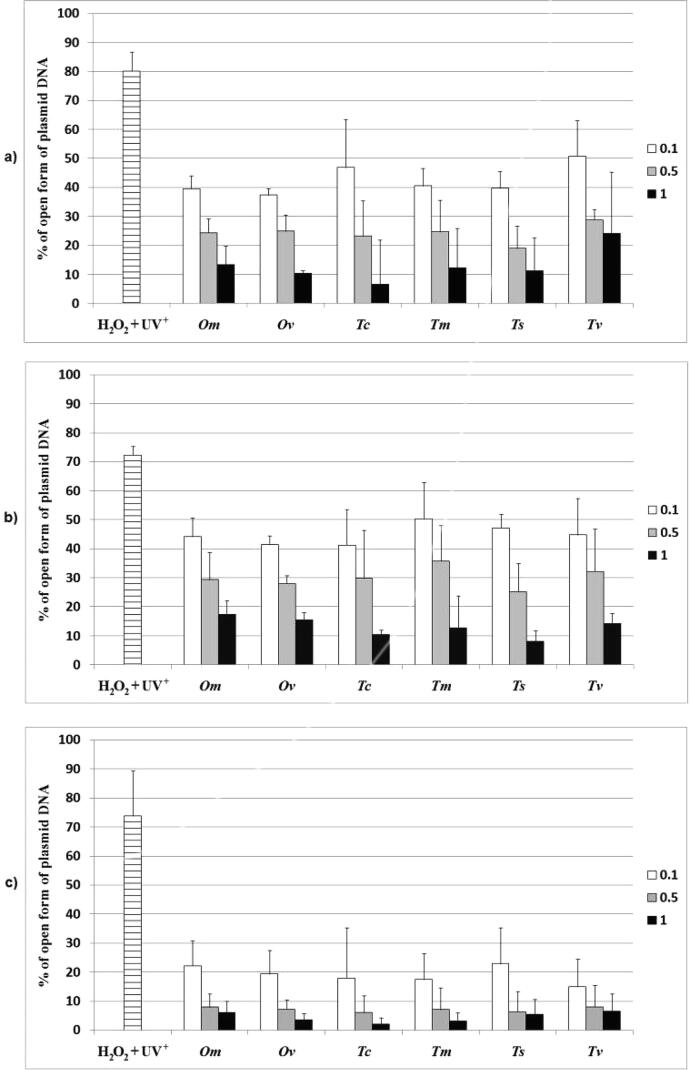
The genoprotective activity of extracts was tested on the plasmid DNA on three concentrations and in three individual experiments. Plasmid DNA was treated with hydrogen peroxide and UV light, which ultimately produced hydroxyl radicals. Positive control was not treated with a genoprotective agent; however, all the other probes were exposed to the effect of the plant extracts. Since the positive control was not treated with the extracts, it was expected to have the highest percentage of an open form of plasmid (the plasmid DNA was most damaged in this probe). Methanol and ethanol alone did not have an impact on DNA. All of the extracts, on the other hand, showed genoprotective activity against hydroxyl radicals, with aqueous extracts providing the best protection of DNA from free radicals (lower percentage of open plasmid DNA form on the chart) (Fig. 1). IC50 values of aqueous extracts varied from 0.17 (Tv) to 0.22 mg/mL (Om), of ethanolic extracts varied from 0.33 (Ts) to 0.40 mg/mL (Tv) and of methanolic extracts ranged from 0.36 (Tc) to 0.52 mg/mL (Tm), making Tv, Ts and Tc the most promising genoprotective agents in this assay (Table 5). All tested extracts showed statistically significant effects against plasmid DNA damage, nonetheless the best genoprotective potential of aqueous extracts, leaving methanolic and ethanolic ones less active, are not in accordance with the results obtained in DPPH test.
Fig. 1.
Percentage (%) of DNA damage (open form of plasmid DNA) in proportion to the concentrations of extracts (0.1, 0.5 and 1 mg/mL). (a) Methanolic extracts; (b) ethanolic extracts; c) aqueous extracts· H2O2 + UV+ – positive control (treated plasmid without the extracts).
Table 5.
IC50 values of the extracts at which they exhibited the highest genoprotective activity against plasmid DNA damage, expressed as mg/mL.
| Species | IC50 values for each extract |
||
|---|---|---|---|
| Methanolic | Ethanolic | Aqueous | |
| Om | 0.39 | 0.37 | 0.22 |
| Ov | 0.37 | 0.34 | 0.19 |
| Tc | 0.36 | 0.34 | 0.18 |
| Tm | 0.52 | 0.37 | 0.18 |
| Ts | 0.39 | 0.33 | 0.21 |
| Tv | 0.39 | 0.40 | 0.17 |
3.4. Antigenotoxic activity in a prokaryotic model
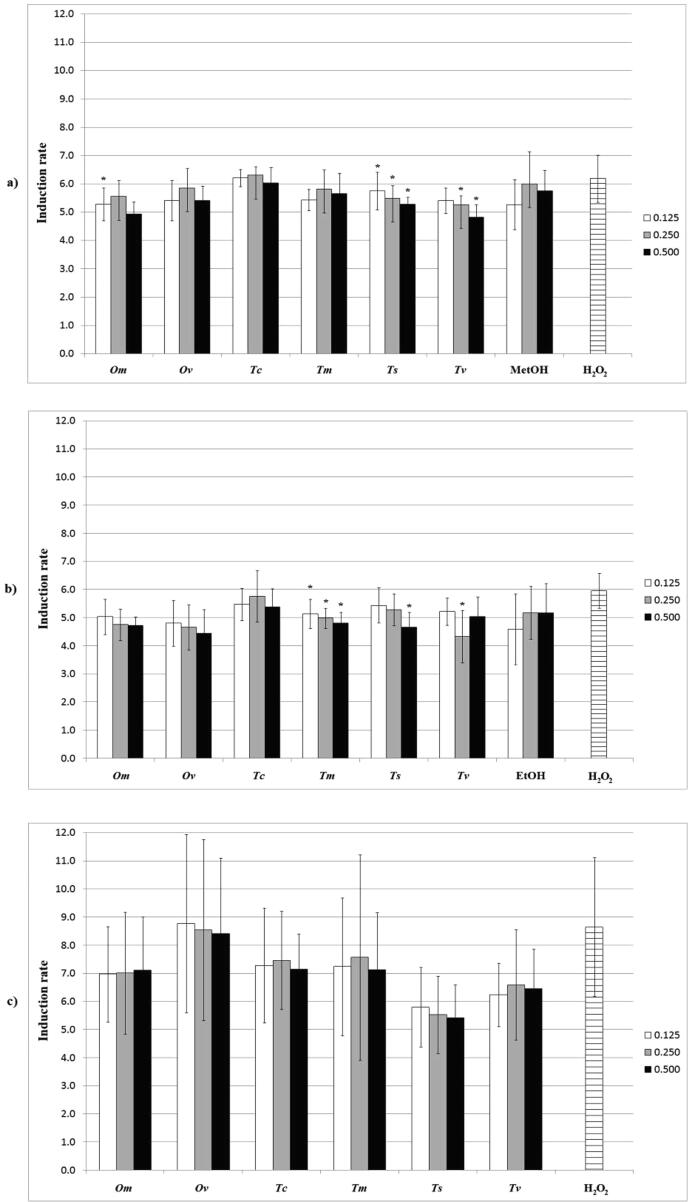
First of all, antigenotoxic effect of the extracts was tested on S. typhimurium TA1535/pSK1002. This bacterium is widely recognized as a sensitive system for the detection of genotoxic agents (Kolarević et al., 2019). The results of a co-treatment of extracts with hydrogen peroxide are shown in Fig. 2. The methanolic extracts of both Thymus species significantly reduced the DNA damage at all tested concentrations (the induction rate for Ts was 5.74, 5.49 and 5.28, while the induction rate for Tv was 5.40, 5.26 and 4.81, while the induction rate for the negative control was 6.18). Moreover, regarding ethanolic extracts, Tm significantly reduced the DNA damage at all three concentrations (the induction rate for Tm was 5.14, 4.98 and 4.81) while Ts and Tv reduced the damage only at the highest and intermediate applied concentrations, respectively (the induction rate for Ts was 4.65, while for Tv it was 4.33, while for the negative control it was 5.96). Aqueous extracts did not reduce significantly the hydrogen peroxide induced DNA damage (the induction rate ranged from 5.53 to 8.77, while for the negative control it was 8.65). Methanol and ethanol used as negative controls did not have an impact on DNA (the induction rate for the methanol control at the concentration of 0.125, 0.250 and 0.500 was 5.26, 6.00, 5.75, respectively, while for the negative control it was 6.18; whereas the induction rate for the ethanol control was 4.59, 5.17 and 5.16, and for the negative control it was 5.96). In addition, in the next step of analyzing the potential of the extracts to reduce DNA damage, they were tested with the comet assay using eukaryotic cells.
Fig. 2.
Impact on the induction ratio in SOS/umuC test of (a) methanolic extracts; (b) ethanolic extracts; (c) aqueous extracts, at the concentrations of 0.125, 0.25 and 0.5 mg/mL. MetOH and EtOH – controls for methanol and ethanol, respectively. * - statistically significant decrease of DNA damage compared with the negative control (H2O2).
3.5. Antigenotoxic activity in eukaryotic model
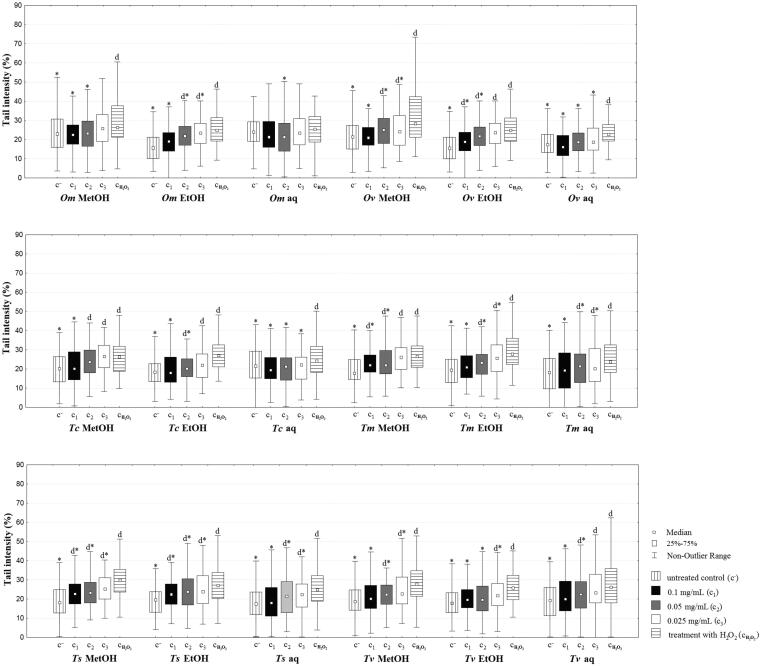
Before testing the antigenotoxic potential of extracts against MRC-5 cells, MTT assay was performed in order to determine the effect of the plant extracts on their viability. The extracts were tested on three concentrations, 0.025, 0.05 and 0.1 mg/mL. In most cases, the viability of the cells was found to be about 100%, hence, the extracts did not show an impact on the viability of the cells. Additionally, the same concentrations of the extracts were used for the assessment of the antigenotoxic effect against hydrogen peroxide-induced DNA damage in MRC-5 cells by comet assay (Fig. 3).
Fig 3.
Comet assay performed during a 24-h period, at the concentrations of 0.1, 0.05 and 0.025 mg/mL. MetOH, EtOH, aq – methanol, ethanol and aqueous extracts, respectively. * – statistically significant decrease of DNA damage compared with the treated control (cH2O2); d – statistically significant increase of DNA damage compared with the untreated control (c-).
A vast majority of the extracts showed concentration dependent results, where the highest administered concentration showed stronger activity in comparison with the other concentrations. All of the tested extracts exhibited significant antigenotoxic activity at the highest applied concentration, with the exception of Om aqueous extract, and also most of them had a significant antigenotoxic effect on 0.05 mg/mL. Additionally, Ov and Tc aqueous extracts showed statistically significant decrease of DNA damage in comparison with the treated control at all three concentrations (tail intensity values for Ov aqueous extract at the concentration of 0.1, 0.05 and 0.025 mg/mL were 16.96,19.09 and 20.37%, respectively, while for the untreated control it was 18.04% and for the control with hydrogen-peroxide it was 24.12%; whereas the tail intensity values for Tc aqueous extract at the concentration of 0.1, 0.05 and 0.025 mg/mL were 17.89, 21.21 and 22.11%, respectively, while for the untreated control it was 18.43% and for the control with hydrogen-peroxide it was 26.83%).
4. Discussion
Literature data imply that there is considerable interest in identifying natural antioxidants deriving from plants, with the ability to protect the human organism from free radical damage, as an alternative to synthetic drugs. Phenolic compounds are bioactive components which exhibit a wide range of biological effects, among others, they provide protection from the harmful impacts of free radicals (Spiridon et al., 2011).
The results of TPC presented in this paper showed that it was the highest in ethanolic samples, however, methanolic extracts of Tv and Om from Egypt are reported to have higher TPC in comparison with ethanolic ones (8.10 and 5.20 mg GAE/g for methanolic extracts of Tv and Om, respectively, and 7.30 and 4.65 mg GAE/g for their ethanolic extracts) (Roby et al., 2013). Recent findings indicate that methanolic extracts contain higher TPC in comparison to aqueous, which is in agreement with the results presented in this paper. Moreover, extracts of Ov from Romania and Greece had higher TPC than Tv/Thymus capitatus (about 68.00 mg GAE/g and 53.00 mg/g for the Romanian species, and 55.00 and 35.00 mg GAE/g for the Greek species) (Spiridon et al., 2011, Skendi et al., 2017) which is in accordance with the results presented in this paper. Proestos et al. (2005), on the other hand, found that Tv methanolic extract possesses a higher TPC than Om and Tc extracts (19.20, 16.90 and 9 mg GAE/g, respectively), while according to our results, Om contains the highest TPC, followed by Tv and Tc, with the lowest TPC. On the other hand, TPC calculated from the HPLC analysis of the Greek species showed that both methanolic and aqueous extracts of Ov have higher polyphenolic content compared to T. capitatus extracts, which is not in agreement with our results, probably due to the different number of compounds quantified in the analysis. The results of a study involving the TPC of methanolic and aqueous extracts of Tm from Serbia (Stankovic et al., 2011) are also in accordance with our results, methanolic extract showing a value of about 170.00 mg GAE/g, while the aqueous extract contained about 110.00 mg GAE/g phenolics. On the other hand, it was shown that the amount of TPC in methanolic and aqueous extracts of several Lamiaceae plants from Poland was as following: Tv > Om > Ov and Om > Tv > Ov, respectively (Ulewicz-Magulska and Wesolowski, 2019), which is not consistent with our results. This difference might be attributed to the climate conditions and geographical distance between the growth sites of the investigated plants.
There is not enough available literature data on spectrophotometrically determined PAC for the six Lamiaceae species that we have studied. However, the results of PAC analysis presented in this paper showed that these compounds have higher values in both methanolic and aqueous extracts of Ov in comparison with Tv methanolic and aqueous extracts, which is coherent with the results reported by Skendi et al., (2017) for Ov and T. capitatus extracts. Moreover, their HPLC analysis showed that the most abundant phenolic acid in these extracts was rosmarinic acid (12.36 and 3.26 mg/g for Ov and T. capitatus methanolic extracts, respectively, and 0.98 and 0.73 mg/g for Ov and T. capitatus aqueous extracts, respectively), however these results are not in accordance with our results, since we have obtained similar values of rosmarinic acid in these samples (about 60.00 mg/g for the methanolic extracts and about 30.00 mg/g for the aqueous extracts). Furthermore, Roby et al. (2013) reported a rather low amount of rosmarinic and caffeic acids in methanolic extracts of Om and especially in Tv from Egypt, which is different from the results reported in this study. This discordance might be present due to the different climate conditions and geographic locations where these plants were growing. In support of this it goes the fact that Vladimir-Knežević et al. (2014) after analyzing Ov, Tv, Tc and Tm, among other species from the Lamiaceae family from Croatia, reported similar value ratios for rosmarinic, caffeic and chlorogenic acids in their ethanolic extracts to the ones from the Table 2.
Methanolic extracts of Ov from Romania were reported to have higher TFC compared to Tv (43.6 mg rutin/g and around 39 mg rutin/g) (Spiridon et al., 2011), which is in agreement with our results. However, these results are not in accordance with the results obtained after summarizing the total flavonoids in HPLC analysis for methanolic and aqueous extracts of plants from Greece, where it was reported that methanolic Ov extract has 8.90 mg flavonoids/g and T. capitatus 9.08 mg/g, while aqueous Ov extract has 0.49 mg/g and T. capitatus extract has 0.66 mg/g (Skendi et al., 2017). Their HPLC analysis showed that both methanolic and aqueous Ov extracts have higher amount of rutin (0.50 and 0.04 mg/g) compared with the T. capitatus extracts (0.008 mg/g for methanolic extract, while in aqueous extract rutin was not found), which is not in accordance with our results regarding the methanolic extracts, however the results for aqueous extracts are in line. Methanolic extract of Tm from Serbia had higher TFC compared to the aqueous one (Stankovic et al., 2011), which is consistent with our results. On the other hand, regarding ethanolic extracts of Teucrium species from N. Macedonia, Tc had slightly higher TFC compared with Tm (0.18% and 0.15%, respectively) (Kadifkova Panovska et al., 2005), which is not coherent with the results presented in our research. They have also identified luteolin in Tc and Tm diethyl ether extracts while apigenin was identified only in T. polium extract. However, we have quantified similar flavonoids, luteolin-7-O-glucoside and quercetin in each of the Tc and Tm extract. Several factors are known to influence the content of phenolic compounds in plants such as climate, altitude, exposure and vegetation phase, to which the observed differences could be attributed.
Apart from a report that acetone extract of Om from Taiwan had higher FC than Ts (Lee et al., 2011), which is inconsistent with our results, extensive literature survey showed that there is no available literature data on FC assay for the six investigated Lamiaceae species. However, giving that quercetin is one of the most common flavonol in plants, there are studies related to its presence in the Lamiaceae species. Namely, Skendi et al. (2017) reported that neither Ov methanolic nor aqueous extracts contained quercetin, while T. capitatus aqueous extract had a higher amount of quercetin compared to its methanolic extract (0.022 and 0.012 mg/g). Our analysis shows that Tv methanolic extract has indeed more quercetin than Ov methanolic extract, however it also shows that Ov aqueous extract contains more quercetin than Ov extract. Moreover, regarding the plants from Egypt, Roby et al. (2013) reported 1.72% of quercetin in Tv and none in Om methanolic extract, which differs from our results only in the sense that Om methanolic extract had only a slightly lower quantity of quercetin than Tv extract. Proestos et al. (2005), on the other hand, did not find any trace of quercetin in their methanolic extracts of Om, Tv and Tc. Regarding the plants from Croatia, Kulišić et al. (2006), on the other hand, found that Ts aqueous extract had higher quercetin quantity than Tv (0.31 and 0.16 mg/g), while Ov aqueous extract had even higher amount (0.70 mg/g), which is partially in accordance with our results, giving that Ts was the most abundant in quercetin, followed by Ov and Tv aqueous extract. Once more, it might be hypothesized that different growing conditions (including insolation and other climate factors, as well as geographic region) play a major role in the chemical composition of these plants.
Considering that these compounds exhibit a wide range of biological activities, and that plants containing them are reported to be traditionally used in treating various pathological conditions in humans, our further research was aimed at the analysis of different aspects of their antioxidant activity. In order to analyze miscellaneous mechanisms of antioxidant action, it was necessary to take into account more than one aspect of the aforementioned activity, i.e. not only to investigate this activity using chemical assays, but also to test their potential using different model systems.
DPPH assay is a chemical test, which is based on the color change of the DPPH solution (from purple to yellow) as the DPPH radical receives an electron or hydrogen from the antioxidant agent. Therefore, DPPH assay evaluates the capacity of antioxidants to act as free radical scavengers or hydrogen donors. Briefly, ethanolic extracts showed the best activity in DPPH assay overall, highlighting the activity of Ov extract, whereas the aqueous ones had the least potential to inhibit the DPPH radicals, especially the aqueous extract of Tm. Methanolic extracts of Tv, Ov, Om from Poland, in their respected order (50.20, 46.60 and 41.6%), showed higher activity than the aqueous ones (27.20, 28.60 and 25.90%) (Ulewicz-Magulska and Wesolowski, 2019), which is consistent with the results of our research. Moreover, ethanolic extracts of several Lamiaceae species grown in Croatia were tested for their scavenging potential and the activity decreased in the following order: Ov > Tc > Tm > Tv (Vladimir-Knežević et l., 2014), which is partially in accordance with our results. Interestingly, it was previously reported that the aqueous extract of Tm originating from Serbia had a higher DPPH activity compared to its methanolic extract (IC50 for the aqueous extract was reported to be 0.029 mg/mL, while for the methanolic extract it was 0.045 mg/mL) (Stankovic et al., 2011), which is not consistent with our results.
Due to the obtained correlation, the results presented in Table 4 showed that the phenolic compounds contribute significantly to the exhibited antioxidant capacity of the studied species. What’s more, Ulewicz-Magulska and Wesolowski (2019) hypothesized that the displayed antioxidant activity might be due to the presence of rosmarinic acid, known for the ability to inhibit DPPH radical. Interestingly enough, according to our Pearson’s correlation analysis, DPPH assay shows a moderate correlation with rosmarinic acid (r = 0.39) and also luteolin-7-O-glucoside (r = 0.46). Each herb holds a different mixture of phenolic compounds with potential synergistic or antagonistic effects resulting in different antioxidant potential (Sonam and Guleria, 2017).
Hydroxyl radicals derived from hydrogen peroxide are known to induce oxidative damage in plasmid DNA (Salehi et al., 2019), resulting in strand breaks. However, this research proved the significance of in vitro genoprotective capacity of aqueous, ethanolic and methanolic extracts of the six Lamiaceae species traditionally used in Serbia. As far as the literature survey could confirm, no report was published on the DNA damage protection activity of the studied Lamiaceae species. Among the Lamiaceae species, a study on Teucrium polium showed that aqueous extracts at the concentration of even 40 mg/mL significantly protected the supercoiled DNA (Tepe et al., 2011). On the other hand, T. ramosissimum methanolic extract at the concentrations of 0.25 and 0.5 mg/mL showed a protective effect towards the DNA exposed to hydrogen peroxide and UV irradiation (Sghaier et al., 2016). Interestingly, aqueous extracts showed the highest genoprotective capacity in our study, in spite of being the least active in in vitro antioxidant assay. However, this is in accordance with the literature data, since in a study with methanolic and hexane extracts of two other Lamiaceae species, Marrubium parviflorum and Lamium amplexicaule from Turkey, hexane extracts showed weak activities in DPPH assay (0.74 and 1.59 mmol TE/g, respectively, for the extracts at the concentration of 2 mg/mL), whereas in the assay with the plasmid, it exhibited high activity at the concentration range of 0.02–0.04 mg/mL. This incidence was reported to also be found in other researches (Yumrutas and Saygideger, 2010). Yumrutas and Saygideger (2010) hypothesized that non-polar compounds in the hexane extracts might be responsible for the displayed activity in protecting the plasmid DNA, however it still remains unknown what might be the reason for the ability of aqueous extracts from our study to contribute to the protection of DNA, since the HPLC analysis was not able to clarify it.
SOS/umuC assay is based on the adjustment in the induction of SOS response in Salmonella typhimurium TA1535/pSK1002, as a consequence of DNA damage. Salmonella typhimurium TA1535/pSK1002 carries the plasmid pSK1002 with umuC operon fused with the lacZ gene responsible for ß-galactosidase activity, hence, by measuring the ß-galactosidase activity, umuC induction can be monitored (Žegura et al., 2006). The intensity of SOS response induction was quantified by the degree of color change in the system since it correlates with the amount of DNA damage in the cell caused by its exposure to hydrogen-peroxide. To the best of our knowledge, the results presented in this paper represent the first report on the antigenotoxic potential of Lamiaceae plant extracts tested in SOS/umuC assay, underlying the importance of these plants, especially Thymus methanolic and ethanolic extracts, as a source of antigenotoxic phytochemicals.
The examined extracts had no cytotoxic effect in MTT assay, which is in compliance with the literature data on healthy human cells (Fathy et al., 2016).
Moreover, comet assay, a widely accepted standard method for assessing DNA lesions in eukaryotic cells on one hand, and DNA repair activity on the other hand (Azqueta et al., 2014), was used to establish additional effects of the extracts of six Lamiaceae species in an eukaryotic model system. The results showed that since treatment with hydrogen-peroxide induced single-strand DNA breaks (SSB), MRC-5 cells, also treated with Lamiaceae extracts, activated SSB rejoining pathways concentration dependently. Since Ov and Tc extracts, especially aqueous ones, were the most successful in protecting the eukaryotic DNA from the hydrogen-peroxide inflicted damage, it might be hypothesized that chlorogenic acid might have had an influence in regard to this activity since in these extracts its quantity was rather high in comparison with the others. Moreover, flavonols like quercetin might have also influenced the displayed antigenotoxic activity, however the rather high amounts of rutin found in Tc extracts is not to be ignored since it has been previously shown that rutin prevents UV-induced changes in fibroblasts’ cell membrane function and it also has a cytoprotective effects on cells which are exposed to different physical factor (Gęgotek et al., 2020). Hence, this metabolite might have provided these extracts with the ability to prevent genotoxic damages of the DNA molecule. According to a review paper (Sabahi et al., 2018) and a comprehensive literature survey, only a handful of plants from the Lamiaceae family were studied for their antigenotoxic effect on normal cell lines. Previous research suggests that the antigenotoxic potential of these plants might be attributed to the presence of phenolic groups (Kapiszewska et al., 2005, Sghaier et al., 2016, Sabahi et al., 2018). Thymus piperella showed better antigenotoxic ability (around 40% of DNA was protected) in comparison to Origanum heracleoticum (around 5%) (Kapiszewska et al., 2005), while according to our results no significant difference between Thymus and Origanum plant extracts was observed.
The divergence between the results presented in this paper and the discussed literature data could be connected with the fact that the climate, geographical location of the plant origin, parts of plants used for the preparation of the extracts, as well as the extraction protocol could affect both phytochemical composition and the antioxidant activity of the plant extracts (Hamrouni-Sellami et al., 2013).
5. Conclusions
The data presented in this paper demonstrates that the ethanolic extracts of selected Origanum, Teucrium and Thymus species are the ones with the highest polyphenolic contents, with O. vulgare and T. vulgaris as the ones with the highest quantity of these compounds. Rosmarinic acid was found to be the most abundant in the investigated samples, followed by a flavone glucoside – luteolin-7-O-glucoside, both known to be excellent antioxidant agents, and their presence correlates with most of the displayed activities. Moreover, ethanolic extracts were the most active in DPPH assay, with special reference to O. vulgare extract. Aqueous extracts, particularly the ones of T. vulgaris and O. majorana, were shown to be the most potential genoprotective agents. Methanolic extracts of both Thymus species showed the most promising antigenotoxic effects in prokaryotic model. Furthermore, all of the examined extracts significantly reduced the DNA damage caused by hydrogen-peroxide activating SSB rejoining pathways, with the emphasis on O. vulgare and T. chamaedrys aqueous extracts, which exhibited antigenotoxic potential even at the lowest tested concentration. However, the chemical complexity of the extracts, their synergistic and antagonistic relations, as well as the dilution of the active compounds in the presence of the less active or rather inactive ones, make the conclusions about their activities more complicated. The obtained results on the antigenotoxic effects on Salmonella typhimurium TA1535/pSK1002 could be considered as the first report for the tested Lamiaceae species extracts. Finally, considering the growing demand for natural antioxidants on the global market nowadays, and also an academic interest in nutraceuticals with the potential to act against the deleterious effects of oxidative stress, the obtained results demonstrated promising properties of the investigated plants to improve human health. Consequently, this report provides experimental confirmation for the folk application of the tested plants, and also emphasizes the importance of preservation of traditional knowledge and biodiversity conservation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors are grateful to Luka Gačić and Lena Ninković (M.A. in English language, literature and culture), who provided improvements to our English.
Funding
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, No. 451-03-68/2020-14/200178 and 451-03-68/2020-14/200003.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsps.2020.10.006.
Contributor Information
Mariana M. Oalđe, Email: marianao@bio.bg.ac.rs.
Stoimir M. Kolarević, Email: stoimir.kolarevic@ibiss.bg.ac.rs.
Jelena C. Živković, Email: ksavikin@mocbilja.rs.
Branka S. Vuković-Gačić, Email: brankavg@bio.bg.ac.rs.
Jovana M. Jovanović Marić, Email: b3010_2016@stud.bio.bg.ac.rs.
Margareta J. Kračun Kolarević, Email: margareta.kracun@ibiss.bg.ac.rs.
Jelena Z. Đorđević, Email: b3013_2017@stud.bio.bg.ac.rs.
Ana Z. Alimpić Aradski, Email: alimpic.ana@bio.bg.ac.rs.
Petar D. Marin, Email: pdmarin@bio.bg.ac.rs.
Katarina P. Šavikin, Email: jzivkovic@mocbilja.rs.
Sonja N. Duletić-Laušević, Email: sduletic@bio.bg.ac.rs.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary Fig. 1.
References
- Alimpić A., Knežević A., Šavikin K., Ćurčić M., Veličković D., Stević T., Matevski V., Stajić M., Marković S., Marin P.D., Duletić-Laušević S. Composition and biological activities of different extracts of Salvia jurisicii-rare and endemic Macedonian species. Plant Biosyst. 2017;151:1002–1011. doi: 10.1080/11263504.2016.1219414. [DOI] [Google Scholar]
- Alimpić A., Pljevljakušić D., Šavikin K., Knežević A., Ćurčić M., Veličković D., Stević T., Petrović G., Matevski V., Vukojević J., Marković S., Marin P.D., Duletić-Laušević S. Composition and biological effects of Salvia ringens (Lamiaceae) essential oil and extracts. Ind. Crops Prod. 2015;76:702–709. doi: 10.1016/j.indcrop.2015.07.053. [DOI] [Google Scholar]
- Azqueta A., Gutzkow K.B., Priestley C.C., Meier S., Walker J.S., Brunborg G., Collins A.R. A comparative performance test of standard, medium- and high-throughput comet assays. Toxicol. In Vitro. 2013;27(2):768–773. doi: 10.1016/j.tiv.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Azqueta A., Slyskova J., Langie S.A., O'Neill Gaivão I., Collins A. Comet assay to measure DNA repair: approach and applications. Front Genet. 2014;5:1–8. doi: 10.3389/fgene.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H.C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Blois Marsden.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Carović-Stanko K., Petek M., Grdiša M., Pintar J., Bedeković D., Herak Ćustić M., Satovic Z. Medicinal plants of the family Lamiaceae as functional foods – a review Medicinal plants of the family Lamiaceae as functional foods – a review. Czech J. Food Sci. 2016;34(No. 5):377–390. doi: 10.17221/504/2015-CJFS. [DOI] [Google Scholar]
- Dajić Stevanović, Z., 2011. Herbal sector of Serbia: General Overview. [cited 29 April 2020]. Available from: http://www.amapseec.com/Herbal%20sector%20of%20Serbia%20overview.pdf.
- Fathy S.A., Emam M.A., Agwa S.A., Zahra F.A., Youssef F.S., Sami R.M. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: suppression of NF-kB. Cell Mol. Biol. 2016;62(10):80–84. doi: 10.14715/cmb/2016.62.10.13. [DOI] [PubMed] [Google Scholar]
- Gęgotek A., Jarocka-Karpowicz I., Skrzydlewska E. Cytoprotective effect of ascorbic acid and rutin against oxidative changes in the proteome of skin fibroblasts cultured in a three-dimensional system. Nutrients. 2020;12(4):1–15. doi: 10.3390/nu12041074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., Patel A.K., Shah N., Choudhary A.K., Jha U.K., Yadav U.C., Gupta P.K., Pakuwal U. Oxidative stress and antioxidants in disease and cancer: a review. Asian Pac. J. Cancer Prev. 2014;15(11):4405–4409. doi: 10.7314/APJCP.2014.15.11.4405. [DOI] [PubMed] [Google Scholar]
- Hamrouni-Sellami I., Rahali F.Z., Rebey I.B., Bourgou S., Limam F., Marzouk B. Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinalis L.) plants as affected by different drying methods. Food Bioprocess Technol. 2013;6(3):806–817. doi: 10.1007/s11947-012-0877-7. [DOI] [Google Scholar]
- Janaćković P., Gavrilović M., Savić J., Marin P.D., Dajić Stevanović Z. Traditional knowledge on plant use from Negotin Krajina (Eastern Serbia): an ethnobotanical study. Indian J. Tradit. Know. 2019;18(1):25–33. [Google Scholar]
- Jarić, S., Mitrović, M., Pavlović, P., 2014. An ethnobotanical and ethnomedicinal study on the use of wild medicinal plants in rural areas of Serbia. In: Pieroni, A., Quave, C.L. (Eds.), Ethnobotany and Biocultural Diversities in the Balkans: Perspectives on Sustainable Rural Development and Reconciliation. Springer, New York, pp. 87–111.
- Jarić S., Mitrović M., Pavlović P. Review of ethnobotanical, phytochemical, and pharmacological study of Thymus serpyllum L. Evidence-Based Complem. Alternative Med. 2015;2015:1–10. doi: 10.1155/2015/101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadifkova Panovska T., Kulevanova S., Stefova M. In vitro antioxidant activity of some Teucrium species (Lamiaceae) Acta Pharm. 2005;55:207–214. [PubMed] [Google Scholar]
- Kapiszewska M., Soltys E., Visioli F., Cierniak A., Zajac G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J. Physiol. Pharmacol. 2005;56(1):183–197. [PubMed] [Google Scholar]
- Kolarević S., Milovanović D., Kračun-Kolarević M., Kostić J., Sunjog K., Martinović R., Đorđević J., Novaković I., Sladić D., Vuković-Gačić B. Evaluation of genotoxic potential of avarol, avarone, and its methoxy and methylamino derivatives in prokaryotic and eukaryotic test models. Drug Chem. Toxicol. 2019;42(2):130–139. doi: 10.1080/01480545.2017.1413108. [DOI] [PubMed] [Google Scholar]
- Kuciel-Lewandowska J., Kasperczak M., Bogut B., Heider R., Laber W.T., Laber W., Paprocka-Borowicz M. The impact of health resort treatment on the nonenzymatic endogenous antioxidant system. Oxid. Med. Cell. Longevity. 2020;2020:1–9. doi: 10.1155/2020/8423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulišić T., Dragović-Uzelac V., Miloš M. Antioxidant activity of aqueous tea infusions prepared from oregano, thyme and wild thyme. Food Technol. Biotech. 2006;44(4):485–492. [Google Scholar]
- Lee C.-J., Chen L.-G., Chang T.-L., Ke W.-M., Lo Y.-F., Wang C.-C. The correlation between skin-care effects and phytochemical contents in Lamiaceae plants. Food Chem. 2011;124(3):833–841. doi: 10.1016/j.foodchem.2010.07.003. [DOI] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., Gargiulo G., Testa G., Cacciatore F., Bonaduce D., Abete P. Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757–772. doi: 10.2147/CIA.S158513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Romero D., Izquierdo-Vega J.A., Morales-González J.A., Madrigal-Bujaidar E., Chamorro-Cevallos G., Sánchez-Gutiérrez M., Betanzos-Cabrera G., Alvarez-Gonzalez I., Morales-González Á., Madrigal-Santillán E. Evidence of some natural products with antigenotoxic effects. Part 2: plants, vegetables, and natural resin. Nutrients. 2018;10(12):1–46. doi: 10.3390/nu10121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailović V., Kreft S., Benković E.T., Ivanović N., Stanković M.S. Chemical profile, antioxidant activity and stability in stimulated gastrointestinal tract model system of three Verbascum species. Ind. Crops Prod. 2016;89:141–151. doi: 10.1016/j.indcrop.2016.04.075. [DOI] [Google Scholar]
- Nastić N., Švarc-Gajić J., Delerue-Matos C., Barroso M.F., Soares C., Moreira M.M., Morais S., Mašković P., Gaurina Srček V., Slivac I., Radošević K., Radojković M. Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants. Ind. Crops Prod. 2018;111:579–589. [Google Scholar]
- Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longevity. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proestos C., Chorianopoulos N., Nychas G.-J.- E., Komaitis M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005;53(4):1190–1195. doi: 10.1021/jf040083t. [DOI] [PubMed] [Google Scholar]
- Rea S.L., Graham B.H., Nakamaru-Ogiso E., Kar A., Falk M.J. Bacteria, yeast, worms, and flies: exploiting simple model organisms to investigate human mitochondrial diseases. Dev. Disabil. Res. Rev. 2010;16(2):200–218. doi: 10.1002/ddrr.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby M.H.H., Sarhan M.A., Selim K.-H., Khalel K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013;43:827–831. doi: 10.1016/j.indcrop.2012.08.029. [DOI] [Google Scholar]
- Russo A., Cardile V., Lombardo L., Vanella L., Acquaviva R. Genistin inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells. J. Nutr. Biochem. 2006;17(2):103–108. doi: 10.1016/j.jnutbio.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Sabahi Z., Soltani F., Moein M. Insight into DNA protection ability of medicinal herbs and potential mechanisms in hydrogen peroxide damages model. Asian Pac. J. Tropic Biomed. 2018;8(2):120–129. doi: 10.4103/2221-1691.225616. [DOI] [Google Scholar]
- Salehi B., Armstrong L., Rescigno A., Yeskaliyeva B., Seitimova G., Beyatli A., Sharmeen J., Mahomoodally M.F., Sharopov F., Durazzo A., Lucarini M. Lamium plants – a comprehensive review on health benefits and biological activities. Molecules. 2019;24(10):1–23. doi: 10.3390/molecules24101913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šavikin K., Zdunić G., Menković N., Živković J., Ćujić N., Tereščenko M., Bigović D. Ethnobotanical study on traditional use of medicinal plants in South-Western Serbia, Zlatibor district. J. Ethnopharmacol. 2013;146(3):803–810. doi: 10.1016/j.jep.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Sghaier M.B., Ismail M.B., Bouhlel I., Ghedira K., Chekir-Ghedira L. Leaf extracts from Teucrium ramosissimum protect against DNA damage in human lymphoblast cell K562 and enhance antioxidant, antigenotoxic and antiproliferative activity. Environ. Toxicol. Pharmacol. 2016;44:44–52. doi: 10.1016/j.etap.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Skendi A., Irakli M., Chatzopoulou P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants. 2017;6:62–69. doi: 10.1016/j.jarmap.2017.02.001. [DOI] [Google Scholar]
- Sonam K.S., Guleria S. Synergistic antioxidant activity of natural products. Annal Pharmacol. Pharm. 2017;2:1–6. [Google Scholar]
- Spiridon I., Bodirlau R., Teaca C.A. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent. Eur. J. Biol. 2011;6(3):388–396. doi: 10.2478/s11535-011-0028-6. [DOI] [Google Scholar]
- Stankovic M.S., Niciforovic N., Topuzovic M., Solujic S. Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium montanum L. var. montanum, F. Supinum (L.) Reichenb. Biotechnol. Biotechnol. Equip. 2011;25(1):2222–2227. doi: 10.5504/BBEQ.2011.0020. [DOI] [Google Scholar]
- Taylor R. Interpretation of the correlation coefficient: a basic review. J. Diagnostic Med. Sonogr. 1990;6(1):35–39. doi: 10.1177/875647939000600106. [DOI] [Google Scholar]
- Tepe B., Degerli S., Arslan S., Malatyali E., Sarikurkcu C. Determination of chemical profile, antioxidant, DNA damage protection and antiamoebic activities of Teucrium polium and Stachys iberica. Fitoterapia. 2011;82(2):237–246. doi: 10.1016/j.fitote.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ulewicz-Magulska B., Wesolowski M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum. Nutr. 2019;74(1):61–67. doi: 10.1007/s11130-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimir-Knežević S., Blažeković B., Kindl M., Vladić J., Lower-Nedza A., Brantner A. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules. 2014;19(1):767–782. doi: 10.3390/molecules19010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D.P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., Zhang J.J., Li H.B. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 2017;18(1):1–32. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.-F., Shen H.-M., Ong C.-N. Protective effect of ebselen against hydrogen peroxide-induced cytotoxicity and DNA damage in HepG2 cells. Biochem. Pharmacol. 1999;57(3):273–279. doi: 10.1016/s0006-2952(98)00299-8. [DOI] [PubMed] [Google Scholar]
- Yumrutas O., Saygideger S.D. Determination of in vitro antioxidant activities of different extracts of Marrubium parviflorum Fish et Mey. and Lamium amplexicaule L. from South east of Turkey. J. Med. Plant Res. 2010;4(20):2164–2172. [Google Scholar]
- Žegura B., Heath E., Černoša A., Filipič M. Toxicity and genotoxicity studies of surface and waste water samples using a bacterial SOS/umu test and mammalian MTT and comet assay. Environ. Toxicol. 2006;1:159–168. doi: 10.2495/ETOX060161. [DOI] [Google Scholar]