Abstract
Millettia peguensis, popular for its ethnopharmacological uses, was employed to evaluate its different pharmacological properties in this study. The analgesic studies of the plant have been performed by acetic acid-induced writhing and formalin-induced licking tests respectively, whereas the antidiarrheal experiment was done by castor oil-induced diarrheal test. Besides, antioxidant, cytotoxic, antimicrobial, thrombolytic evaluations were performed by DPPH scavenging with phenol content determination, brine shrimp lethality, disc diffusion and clot lysis methods respectively. Moreover, in silico study of the phytoconstituents was carried out by molecular docking and ADME/T analysis.
The methanol extract of Millettia peguensis (MEMP) revealed significant biological activity in the analgesic and antidiarrheal test (p < 0.001) compared to the standards. Antioxidant assay displayed promising IC50 values (15.96 μg/mL) with the total phenol content (65.27 ± 1.24 mg GAE/g). In the cytotoxicity study, the LC50 value was found to be 1.094 μg/mL. Besides, MEMP was highly sensitive to the bacteria but less liable to clot lysis. Furthermore, phytoconstituents exposed potential binding affinity towards the selected receptors, whereas the ADME/T properties indicated the drug likeliness of the plant. The outcomes of these findings suggest the therapeutic potential of this plant against pain, diarrhea, inflammation, and tissue toxicity.
Keywords: Millettia peguensis, Analgesic, Antidiarrheal, Antioxidant, Antitumor
Abbreviations: MEMP, methanol extract of Millettia peguensis; ROS, reactive oxygen species; RONS, reactive oxygen and nitrogen species; TNF-alpha, tumour necrosis factor alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL-1, interleukin-1; COX 1, cyclooxygenase-1; COX 2, cyclooxygenase-2; 5-HT3, 5-hydroxytryptamine 3; GABA, gamma-Aminobutyric acid; BHT, butylated hydroxytoluene; IC50, half maximal inhibitory concentration; LC50, Lethal Concentration 50; ASA, acetyl salicylic acid; DPPH, 2,2-diphenyl-1-picryl-hydrazyl-hydrate; MMP 9, matrix metalloproteinase 9; CADD, computer-aided drug discovery
1. Introduction
Inflammation has been reported to happen via multiple mechanisms and by varying mediators resulting in a wide variety of deleterious effects including necrosis, degeneration and different types of exudation (Medzhitov, 2008). However, analgesic drugs are used to manage the pain, inflammation and categorized as opioids (morphine, fentanyl), NSAIDs and some newer approaches (gabapentin, carbamazepine, ketamine). Besides, glucocorticoids exert response by binding with receptors resulting in increased transcription of anti-inflammatory proteins (e.g., IL-1 antagonist) along with inhibition of activated transcription factors (e.g., NF-κB) (Barnes, 1998). NSAIDs also inhibit cyclooxygenase enzymes (COX-1 and COX-2) which are responsible for the biosynthesis of various inflammatory mediators. Though various NSAIDs are commercially available, they exhibit few major side effects including GI ulceration, liver toxicity, kidney disease etc. after prolonged uses (Sostres et al., 2010). Thus, new phytochemicals may be investigated to establish better alternatives (Liu, 2007).
Diarrhea is a familiar disease associated with an increased incidence of liquid defecation along with abdominal pain (Tadesse et al., 2014). It is the leading cause of malnutrition and mortality, particularly in poor countries (Zhao et al., 2018). Diarrhea is another disease leading to more than 5,000,000 child deaths per year (Agbor et al., 2014) although simple treatments like oral saline and antibiotics are available to manage diarrhea. Plant extracts have been reported to deliver antidiarrheal properties through stimulation of water reabsorption, decreasing electrolyte loss and reducing gastrointestinal peristalsis (Agbor et al., 2004, Shifah et al., 2020)
Antioxidants are molecules that can quench reactive oxygen species (ROS) (Hosaka et al., 2005). In addition, plant extracts also have been identified to display prominent antioxidant activities which are of special importance in inflammation, atherosclerosis, Alzheimer’s disease, cancer, parkinsonism, hypertension, psychological disordesr and diabetes mellitus (Digiesi et al., 2001, Mittler, 2002, Joseph et al., 2015). Infectious diseases are of another class of clinical conditions causing 25% of the hospital and 20% of the total deaths every year (Thabit et al., 2015). Bioactive phytoconstituents also have been found to have auspicious roles against these infections (Rios, 2005, Heinrich et al., 2004). Though a lot of antimicrobial agents are available commercially, increased resistance and associated mortality are forcing the researchers to discover new drugs continuously (Roberts et al., 2010). Besides, increased healthcare cost and mortality rate are forcing researchers to discover newer antimicrobials with fewer side effects to reduce the death rate (Roberts et al., 2009).
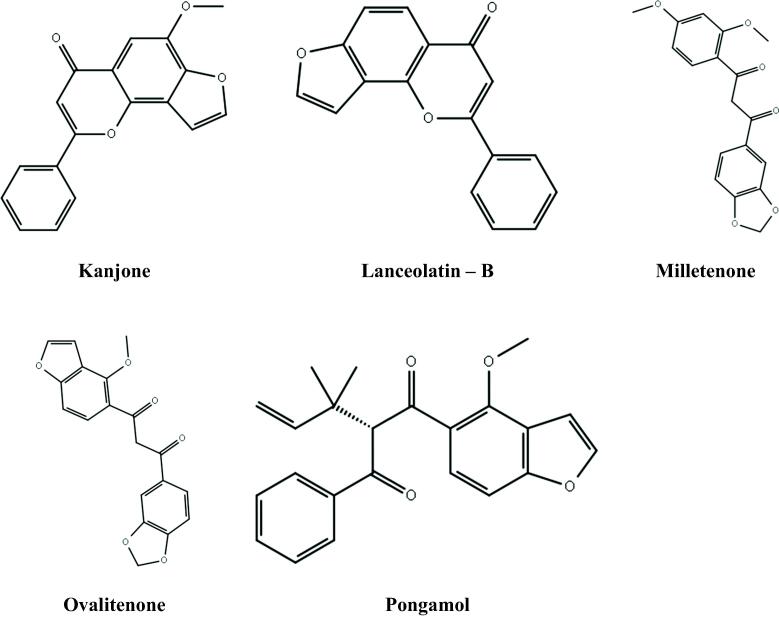
Due to the diverse pharmacological roles, medicinal plants have been appeared as potential sources of life-saving therapeutic agents worldwide. In line with this, Millettia peguensis has been taken for this study and evaluation was done for the analgesic, antidiarrheal, antioxidant, cytotoxic, thrombolytic and antimicrobial activity from the leaves. However, several phytoconstituents were isolated, most notably flavonoids including pongamol, lanceolatin-B, kanjone, milletenone, ovaliflavanone-A, ovalitenone, pongaglabol and other bioactive phytoconstituents (Ganapaty et al., 1998) and as the extended study an in silico study was done to get an overall idea of the binding interaction. The observations have been reported here.
Computational biology is reflecting a mammoth role as enormous data can be generated and validated by molecular and experimental biologists nowadays through this approach. To establish and investigate drug designing of a newer molecule, execution of computer-aided drug discovery (CADD) techniques and molecular docking has been proven as time-efficient as in silico process. An efficacious molecular docking should possess the ability to identify the native ligand pose with the binding site of the three-dimensional protein structure along with physicochemical interactions (Guedes et al., 2014).
Millettia is a genus of legume in the Fabaceae family. This genus has about 150 species, which are abundant in the tropical and subtropical regions of the world (Manikandan et al., 2017). Millettia peguensis is commonly called Moulmein Rosewood (Packiyalakshmi et al., 2017b) and known as Tuma found in Bangladesh which is native to lower Burma and Siam, but cultivated in Burma, India and Pakistan too. It is planted for ornamental purposes, and showed eye-catching beauty during full bloom. This blooms with racemes of mauve pea-like flowers along with pinnate leaves and oval-shaped leaflets. From Millettia peguensis several phytoconstituents were isolated, most notably flavonoids including pongamol, lanceolatin-B, kanjone, milletenone, ovaliflavanone-A, ovalitenone, pongaglabol and other bioactive phytoconstituents (Ganapaty et al., 1998). In addition, pongamol has been reported to have larvicidal activity against Aedesaegypti (Narkhede et al., 2016) and pongaglabol for antioxidant activity (Rao et al., 2020); lanceolatin - B has shown antibacterial and anti-inflammatory activity (Rao et al., 2020), whereas pongaglabol has shown antiulcer activity (Ahmad et al., 1999, Huo et al., 2015). Millettia peguensis is reported with other versatile phytochemicals too in a different report including 9-methylheptadecane, eicosane, heneicosane, dodecyl hexyl ester, 2- bromododecane and other phytoconstituents (Ganapaty et al., 1998, Packiyalakshmi et al., 2017). Moreover, the genus of Millettia plants has shown the potentiality to work as insecticidal and pesticide agents along with antimalarial and antioxidant candidates (Abiy et al., 2003). This study was conducted to evaluate the analgesic, antidiarrheal, antioxidant, cytotoxic, thrombolytic and antimicrobial activity of the leaves extract of M. peguensis by biological and computational approaches.
2. Materials and methods
2.1. Sample collection and preparation
The leaves of M. peguensis were gathered from Dhaka, Bangladesh and were ascertained by the experts of Bangladesh National Herbarium, Mirpur, Dhaka and a voucher specimen (DACB; Accession no: 62166) has been deposited for this collection. After cutting the leaves into pieces, they were dried in room temperature without the exposure of direct sunlight and then crushed with the help of a high-powered grinding machine. The final product sample was 0.7 kg M. peguensis coarse powder.
2.2. Drugs and chemicals
The Folin-Ciocalteu reagent was collected from Merck, St. Louis, MO, USA. Methanol and tween-80 were bought from Merck Darmstadt, Germany. Lyophilized Alteplase (Streptokinase) vial of 15,00,000 I.U. and vincristine sulfate were purchased from Beacon Pharmaceuticals, Dhaka, Bangladesh. Acetylsalicylic acid and loperamide had been procured from Sanofi Bangladesh Ltd. All chemicals were used as analytical grade.
2.3. Test microorganisms
For the antimicrobial assay, the gram-positive bacteria (Bacillus megaterium, Bacillus cereus, Candida albicans, Candida glabrata, Sarcina lutea, Bacillus subtillis and Staphylococcus aureus) and gram-negative bacteria (Pseudomonas aeruginosa, Escherichia coli, Salmonella typhi, Salmonella paratyphi, Shigella dysenteriae, Vibrio parahemolyticus, Vibrio mimicus, Klebsiella pneumonia, Shigella boydii) were utilized which were supplied from University of Dhaka, Bangladesh.
2.4. Experimental design
2.4.1. Extraction of plant material
The coarse powder of M. peguensis leaves was macerated in 1.5 L methanol in an amber glass container and kept in a dry and dark place and stirred occasionally. After two weeks, the mixture was filtered with cotton and Whatman filter paper #1, respectively. The filtrate was collected and evaporated to 30% to get the semi-solid mass for preparing the extract.
2.4.2. Test animal models
Swiss albino mice of 4–5 weeks old and either sex have been collected from International Center for Diarrheal Disease and Research, Bangladesh (ICDDR, B). The mice were kept under a controlled temperature of 24 ± 2 °C and relative humidity of 60–70% in a light-dark cycle of 12 h along with ad libitum water and ICDDR, B formulated rodent food. All experiments were conducted following guidelines for the care and use of laboratory animals which were approved by the institutional ethical committee (Zimmermann, 1983).
2.4.3. Acute toxicity test
Twenty five Swiss albino mice of 4–5 weeks old were given a single oral dose of either 500 mg/kg b.w., 1000 mg/kg b.w., 1500 mg/kg b.w., or 2000 mg/ kg b.w. of M. peguensis leave extract. The reported protocol was followed, Test No. 423 (OECD, 2001; Acute oral toxicity-acute toxic class method) (Walum, 1998) for this study. Feeding was ceased for 3–4 h. After dosing, all animals are kept under close observation for 30 min periodically for the first 24 h followed by 3 days to document any delayed toxicity including several changes in skin and fur, eyes and mucous membranes, respiratory and circulatory rate or autonomic and CNS function.
2.5. Analgesic assay
2.5.1. Acetic acid-induced writhing test
Acetic acid-induced writhing test was employed to investigate the analgesic activity of MEMP (Ahmad et al., 2010). Group I and II received tween-80 (10 mL/kg; b.w, p.o), and acetylsalicylic acid (10 mg/kg; b.w, i.p.) and were treated as negative and positive controls respectively, whereas group III and IV received MEMP 200 and 400 (mg/kg; b.w, p.o) respectively. After the administration of test samples, acetic acid solution (0.6% v/v) was injected intraperitoneally. The writhing was counted after 5 min of the injection of acetic-acid and counted over 25 min.
2.5.2. Formalin induced paw licking test
This study was performed by subcutaneous injection of 20 µL of formalin solution (2.5% v/v) into the right hind paws of all mice (Ahmad et al., 2010). During this test, the mice were divided into 4 groups consisting 6 mice in each group. Group I and II received tween-80 (10 mL/kg; b.w, p.o), and acetylsalicylic acid (10 mg/kg; b.w, i.p.) and were treated as negative and positive controls respectively, whereas the group III and IV received MEMP 200 and 400 (mg/kg; b.w, p.o) respectively. Licking was observed during initial 5 min and subsequent 15–30 min.
2.6. Antidiarrheal assay
2.6.1. Castor oil-induced diarrhea test
The antidiarrheal effect of MEMP was performed by castor oil-induced method (Shoba & Thomas, 2001) with minor modification. In this study, mice were randomly allocated to four groups comprising of six mice in each group. The test animals fastened overnight with free access to water. 0.5 mL castor oil was administered to the mice and then only those indicating diarrhea were chosen for the experiment. Besides, the animals of group I (control) received vehicles (distilled water containing 1% Tween-80); Group-II (positive control/standard drug) received a standard anti-motility drug named loperamide (3 mg/kg body weight). Group III, group IV, (test groups) were treated with a suspension of MEMP at the oral dose of 200 and 400 (mg/kg body weight) respectively. One hour after administering test samples, all mice received 0.5 mL of castor oil, and afterward, they were independently placed in the enclosure’s floor of which was fixed with transparent paper. During the observational period, the onset of diarrhea, number and weight of wet stools, the total number and the total weight of feces yields were recorded. The count continued until the four hours before the administration of castor oil, and the average values were considered final results.
2.7. Antioxidant assay
2.7.1. DPPH scavenging test
According to the reported method (Islam et al., 2020), to the test sample, containing 2 mL of solution with serially diluted different concentrations (500 μg/mL to 0.977 μg/mL), 3 mL of a DPPH methanol solution (20 μg/mL) was mixed. The test tubes were kept in a dark place for 30 min at 25 °C and then the absorbance values were measured by a UV spectrophotometer at 517 nm. BHT (butylated hydroxytoluene) was used as positive control.
2.7.2. Total phenol content (TPC) analysis
The total phenolic content of M. peguensis leaves was estimated by following the previously described technique of Skerget et al (Škerget et al., 2005). The Folin-Ciocalteu reagent was employed as an oxidizing agent, whereas gallic acid was considered as a reference. In addition, 2 mg M. peguensis leaves extract was used to prepare a sample solution, vehicle (2 mg/mL), MEMP (0.5 mL) and FCR (2.5 mL) and sodium bicarbonate (2 mL) solution were mixed and incubated for 20 min at room temperature. Then the absorbance values were measured by a UV spectrophotometer at 760 nm and from this data the total phenol content values were measured. The standard curve was also prepared from gallic acid solution using different concentrations. The unit of phenol content is expressed as mg of GAE (gallic acid equivalent)/gm of the extract.
2.8. Anti-tumor assay
2.8.1. Brine shrimp lethality bioassay
The brine shrimp lethality bioassay was performed to evaluate the possible cytotoxicity using related techniques (Meyer et al., 1982). In this study, 38 g NaCl salt was dissolved in 1000 mL of distilled water to make simulated seawater and pH (8.0) was maintained by adding NaOH. The plant extract was dissolved in DMSO (50 μL in 5 mL solution) to prepare the test sample with simulated seawater followed by the preparation of serially diluted concentrations of 50, 100, 200, 400, 600 and 800 μg/mL. Vincristine sulfate was used as a positive control in a serial concentration dilution of 0.125, 0.25, 0.5, 1, 5 and 10 μ /mL as the preceding form. Then ten matured live shrimp were put in all the test tubes at ambient temperature (25 ± 1 °C) and each test tube was measured after 24 h. The number of death nauplii was counted as well as recorded.
where, N0 = Number of nauplii taken; Nl = Number of nauplii death
2.9. Thrombolytic assay
2.9.1. Clot lysis test
The clot lysis bioassay was conducted following previously used techniques (Prasad et al., 2006). In this study, 100 mg plant extract was used to prepare 10 mL solution with distilled water and the solution was kept at room temperature overnight. 5 mL venous blood was drawn from each of the six healthy volunteers who were medication-free for seven days. For each sample, 0.5 mL blood was taken in a pre-weighted micro-centrifuge tube and then it was incubated at 37 °C for 45 min to form a clot. Serum was utterly removed from that and 100 µL of the crude extract was added. 100 µL distilled water and 100 µL streptokinase (30 000 I.U.) were used as negative and positive controls respectively. After that, each micro-centrifuge tube was incubated for 90 min at 37 °C and then % clot lysis was measured by the following formula:
2.10. Antimicrobial assay
2.10.1. Disc diffusion test
To evaluate the antimicrobial property of methanol fractions of M. peguensis, the disc diffusion method (Huys et al., 2002) was employed. To conduct the susceptibility assay, two antibiotic candidates available in the market were considered as standard drugs: streptomycin (for gram-negative) and amoxicillin (for gram-positive). The zones of the nutrient agar medium plates were pre-inoculated with test bacteria and fungi, whereas sample discs, standard antibiotic discs and the control discs were placed gently and the plates were then incubated at 37 °C for 24 h. The diameters of the clear zones were measured carefully.
2.11. In silico study
2.11.1. Molecular docking: Ligand preparation
The structure of five previously isolated compounds from M. peguensis leaves namely kanjone (PubChem CID: 12305449), lanceolatin – B (PubChem CID: 689051), milletenone (PubChem CID: 42607652), ovalitenone (PubChem CID: 627910), pongamol (PubChem CID: 101936575) have been retrieved from the PubChem database exposed in Fig. 1 (Ganapaty et al., 1998). The ligands were downloaded in the 2DSDF format and minimized and converted into pdbqt format throughout PyRx tools to quest of best optimal hit against these mentioned targets. PyRx (Herowati & Widodo, 2014) from MGL Tools (https://ccsb.scripps.edu/mgltools/) was used for virtual screening using default settings.
Fig. 1.
Structure of selected phytoconstituents.
2.11.2. Molecular docking: Protein preparation
Three dimensional crystal structure includes prostaglandin – 1 (PDB ID: 2OYE) (Harman et al., 2007), prostaglandin – 2 (PDB ID: 6COX) (Kurumbail et al., 1996), 5-HT3 receptor (PDB ID: 5AIN) (Price et al., 2015), urate oxidase (PDB ID: 1R4U) (Retailleau et al., 2004), protein tyrosine kinase (PDB: 1XKK) (Wood et al., 2004), E.coli exonuclease I (PDB ID: 1FXX) (Breyer & Matthews, 2000) and human tissue-type plasminogen activator (PDB ID: 1A5H) (Renatus et al., 1997) have been culled from RCBS Protein Data Bank (https://www.rcsb.org/structure) in PDB format. All the water and the heteroatom were removed from the proteins throughout using Discovery studio 2020. Proteins were arranged by combining non-polar hydrogens and assigning the Gasteiger charge. Besides, all the proteins were brought down to the least energy state with keeping standard residues in AMBER ff14sB and other residues in Gasteiger mode in UCSF Chimera and processed for further analyses (Shapovalov and Dunbrack Jr., 2011).
2.11.3. Molecular docking analysis
PyRx Autodock Vina has been used for the protein-ligand linking process of the chosen protein-ligand complexes (Herowati & Widodo, 2014). The docking study was employed with a semi-flexible docking system. Using PyRx AutoDock software, PDB files of phytochemicals and proteins have been transformed into PDBQT format. This study has maintained the rigidity of proteins and the flexibility of ligands. Ligand molecules had 10 degrees of liberty. AutoDock defines the steps to transform molecules into pdbqt format, sort of box, grid box creation, etc. The grid box was generated in the center of the box with an active site. Finally, BIOVIA Discovery Studio Visualizer 2020 (Biovia, 2017) has been accelerated to assess docking positions for the best linking strategies.
2.11.4. Ligand based pharmacokinetics and toxicity measurement
Here for determining the pharmacokinetic properties (ADME) of three major compounds, the online tool SwissADME (http://www.swissadme.ch/) was used. Lipinski’s rule of five (M.W not more than 500; H-bond donors ≤ 5; H-bond acceptors ≤ 10; Lipophilicity < 5 and molar refractivity ranging from 40 to 130) were considered to evaluate favorable drug-like properties of all compounds (Lipinski et al., 1997). Moreover, the toxicological properties of all the compounds were determined by the web tool admetSAR (http://lmmd.ecust.edu.cn/admetsar2).
2.12. Statistical analysis
Statistical analysis was interpreted as mean ± SEM. The values obtained were compared with the control group and considered statistically significant (***p < 0.001, **p < 0.01 and *p < 0.05) followed by One-way analysis of variance (ANOVA) with Dunnett's test. All statistical analyses were performed using GraphPad Prism Version 5.2 (San Diego, CA).
3. Results
3.1. Effect of MEMP on acetic acid-induced writhing in mice model
In this study, both doses of MEMP reported significant activity with the standard. The MEMP (200 mg/kg and 400 mg/kg) exhibited the abdominal writhing of 52.33% and 67.44% respectively and standard (ASA) appeared 80.23% of inhibition, whereas control did not report any inhibitory response in Table 1.
Table 1.
Analgesic effect of methanol extract of M. peguensis leaves (MEMP) in acetic acid induced writhing test in mice.
| Treatments | Number of abdominal writhings | Inhibition (%) |
|---|---|---|
| TWN – 80 (10 mL/kg) | 17.20 ± 1.30 | – |
| ASA (10 mg/kg) | 3.40 ± 0.24*** | 80.23 |
| MEMP (200 mg/kg) | 8.20 ± 0.37*** | 52.33 |
| MEMP (400 mg/kg) | 5.60 ± 0.24*** | 67.44 |
Values were presented as Mean ± SEM (n = 6); one-way analysis of variance (ANOVA) was followed by Dunnett’s test. *p < 0.05, **p < 0.01 and ***p < 0.001 was considered as significant, compared with standard. TWN = 1% Tween 80, and ASA = Acetylsalicylic acid, SEM = Standard error mean.
3.2. Effect of MEMP on formalin-induced paw licking study in mice model
In the formalin-induced test, the MEMP of both doses produced a moderate anti-inflammatory response. The percent of inhibition of paw licking in the early phase showed 37.39%, 63.13% and in late phase 35.32%, 56.78% at both dose (200, 400) mg/kg compared to standard (71.33%, 70.91%) respectively, whereas control did not report any significant inhibition. Besides, the dose-dependent relationship between inhibition of paw-licking of standard and MEMP was comprehensible and expressed in Table 2.
Table 2.
Analgesic effect of methanol extract of M. peguensis leaves (MEMP) formalin-induced paw licking test in mice.
| Treatment | Early phase (0–5 min) | Inhibition (%) | Late phase (15–30 min) | Inhibition (%) |
|---|---|---|---|---|
| TWN-80 (10 mL/kg) | 61.23 ± 1.34 | – | 47.11 ± 3.13 | – |
| ASA (10 mg/kg) | 17.55 ± 2.23*** | 71.33 | 13.70 ± 2.45*** | 70.91 |
| MEMP (200 mg/kg) | 38.33 ± 1.37*** | 37.39 | 30.47 ± 2.67** | 35.32 |
| MEMP (400 mg/kg) | 22.57 ± 3.21*** | 63.13 | 20.36 ± 1.97*** | 56.78 |
Values were presented as Mean ± SEM (n = 6); one-way analysis of variance (ANOVA) was followed by Dunnett’s test. *p < 0.05, **p < 0.01 and ***p < 0.001 was considered as significant, compared with standard. TWN = 1% Tween 80, and ASA = Acetylsalicylic acid, SEM = Standard error mean.
3.3. Effect of MEMP on castor oil-induced diarrhea
In the castor oil-induced diarrheal method, the methanol extract of M. peguensis leaves prolonged the onset of diarrhea and reduced the number and weight of diarrhoeal feces in a dose-dependent manner. The antidiarrhoeal effect of loperamide and MEMP has been determined by comparing it with the effect of a negative control (tween – 80) presented in Table 3. However, the data revealed that, MEMP 200 and 400 (mg/kg; b.w, p.o) delayed the diarrhoeal time till 139.33 min (p < 0.001) and 175.67 min (p < 0.001) respectively. MEMP 400 (mg/kg) restrained the average number of wet feces (3.67; p < 0.001), the average number of total feces (5.53; p < 0.001), the average weight of wet feces (0.16; p < 0.001), and the average weight of total feces (0.23; p < 0.001) which is comparatively close to the standard drug loperamide.Table 4.
Table 3.
Antidiarrheal effect of methanol extract of M. peguensis leaves on castor oil induced test in mice.
| Treatment | Dose, route (p.o) | Onset of diarrhea (min) | Average number of wet feces | Total number of feces | Average weight of wet feces (g) | Average weight of total feces (g) |
|---|---|---|---|---|---|---|
| Group-Ⅰ | 1% tween 80–10 mL/kg | 77.16 ± 1.83 | 8.67 ± 2.19 | 11.17 ± 3.79 | 0.47 ± 0.09 | 0.57 ± 0.03 |
| Group-Ⅱ | Loperamide-3 mg/kg | 189.16 ± 3.91*** | 2.33 ± 2.16*** | 4.16 ± 2.30*** | 0.09 ± 0.01*** | 0.13 ± 0.12*** |
| Group-III | MEMP-200 mg/kg | 139.33 ± 2.93*** | 4.67 ± 3.30** | 6.67 ± 2.13*** | 0.24 ± 0.03*** | 0.32 ± 0.05*** |
| Group-Ⅳ | MEMP-400 mg/kg | 175.67 ± 2.46*** | 3.67 ± 1.21*** | 5.53 ± 3.15*** | 0.16 ± 0.07*** | 0.23 ± 0.07*** |
Values were presented as Mean ± SEM (n = 6); one-way analysis of variance (ANOVA) was followed by Dunnett’s test. *p < 0.05, **p < 0.01 and ***p < 0.001 was considered as significant, compared with control. TWN = 1% Tween 80, and ASA = Acetylsalicylic acid, SEM = Standard error mean.
Table 4.
Quantitative analysis of total phenol content of methanol extract of M. peguensis leaves.
| Plant extract | Total phenol content (mg GAE/g) | Regression Line |
|---|---|---|
| MEMP | 65.27 ± 1.24 | y = 0.0383x + 0.0892, R2 = 0.9446 |
3.4. Effect of MEMP on DPPH free radical scavenging activity
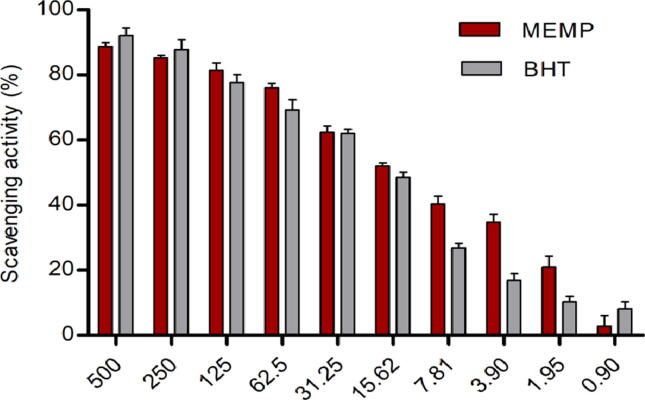
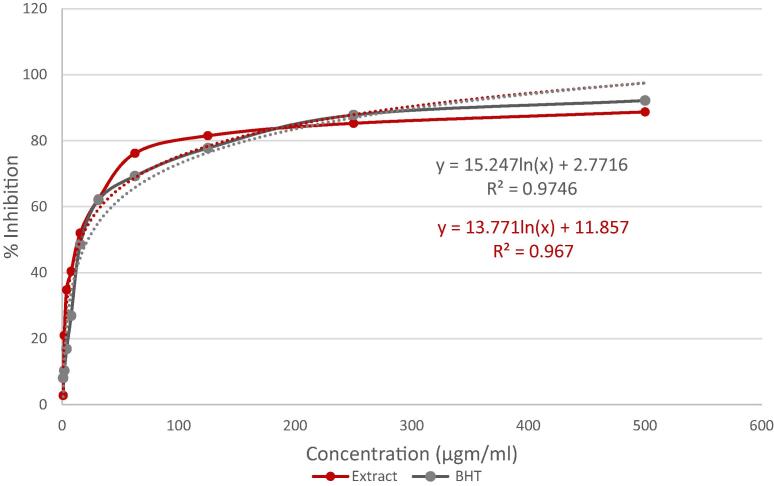
In the antioxidant study by DPPH scavenging method, M. peguensis crude extract displayed a dose-dependent radical scavenging activity in compared to standard. MEMP exhibited potent scavenging activity (88.71%) while BHT value was (92.15%) at 500 μg/mL. Besides, the IC50 values of BHT and MEMP were 20.07, 15.96 μg/mL respectively and calculated by the linear regression equation, summarized in Fig. 2 and Fig. 3.
Fig. 2.
Percentage of radical scavenging activities of methanol extract of M. peguensis leaves and standard drug.
Fig. 3.
IC50 value of butylated hydroxytoluene (BHT) and methanol extract of M. peguensis leaves.
3.5. Effect of MEMP on brine shrimp lethality bioassay
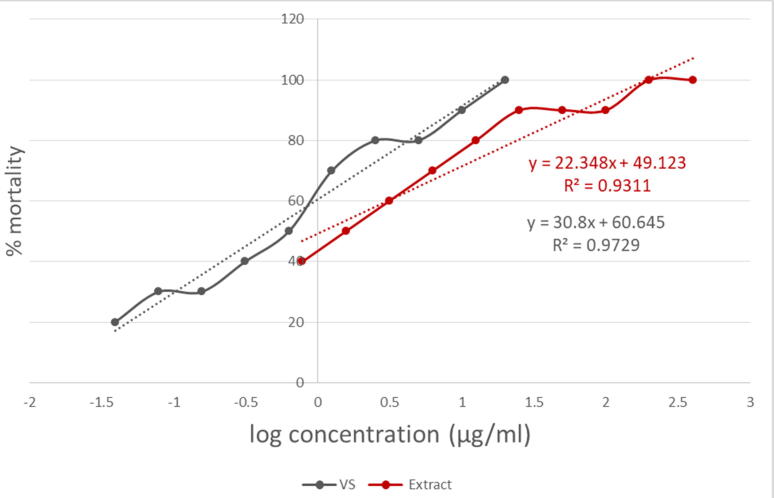
In this test, vincristine sulfate and DMSO (dimethyl sulfoxide) were used as the positive control and the negative control respectively to measure the cytotoxicity. The LC50 values for the positive control and the methanol extract of M. peguensis leaves were found to be 0.451 μg/mL and 1.094 μg/mL respectively and were represented in Fig. 4.
Fig. 4.
% Mortality and predicted regression line of vincristine sulphate and methanol extract of M. peguensis leaves.
3.6. Effect of MEMP on thrombolytic study
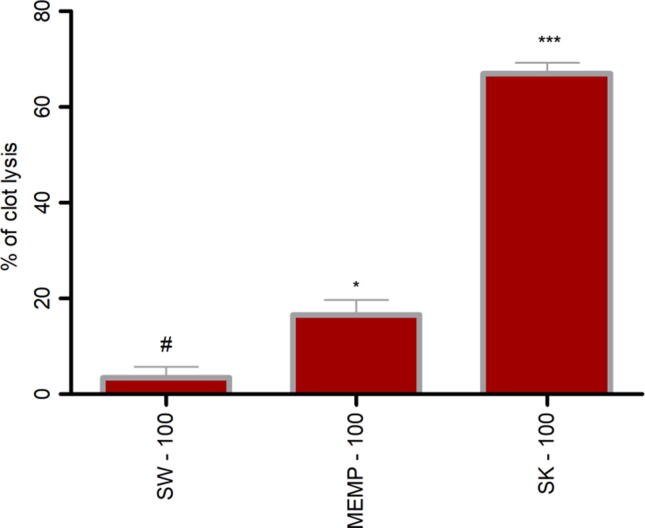
The MEMP showed low thrombolytic activity by clot lysis method. Methanol extract of M. peguensis leaves (MEMP) caused lysis of clot by 16.59%, whereas standard displayed 66.98% which were documented in Fig. 5.
Fig. 5.
Clot lysis effects of methanol extract of M. peguensis leaves. Clot lysis values are presented as mean ± SEM (n = 5); One-way analysis of variance (ANOVA) was followed by Dunnett’s test. *p < 0.05, ** p < 0.01, ***p < 0.001 were considered as significant compared with the control, where # is designated as control. SW = Saline water, SK = streptokinase and MEMP = methanol extract of M. peguensis leaves.
3.7. Effect of MEMP on disc diffusion assay
Antimicrobial activity of MEMP was tested by disc diffusion assay procedure against seven gram-positive and nine gram-negative bacteria where amoxicillin and streptomycin (30 µg/disc) were used as the standard along with 100 µg/disc of MEMP as test sample. The result is displayed in Table 5. From the result, it is vibrant that MEMP was notably effective against all the bacterial strains.
Table 5.
Antimicrobial activity of M. peguensis leaves and standard against gram-positive and gram-negative bacterial strains.
| Diameter of Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|
| Test Microorganisms |
MEMP (100 µg/disc) |
Amoxicillin (30 µg/disc) |
Test Microorganisms |
MEMP (100 µg/disc) |
Streptomycin (30 µg/disc) |
| Gram positive bacteria | Gram negative bacteria | ||||
| Bacillus cereus | 23 | 38 | Escherichia coli | 30 | 37 |
| Bacillus megaterium | 28 | 34 | Pseudomonas aeruginosa | 32 | 39 |
| Bacillus subtilis | 32 | 40 | Salmonella paratyphi | 29 | 38 |
| Staphylococcus aureus | 29 | 39 | Salmonella typhi | 31 | 40 |
| Sarcina lutea | 28 | 41 | Shigella dysenteriae | 25 | 41 |
| Candida albicans | 27 | 36 | Vibrio mimicus | 26 | 35 |
| Candida glabrata | 30 | 37 | Vibrio parahemolyticus | 29 | 37 |
| Klebsiella pneumoniae | 28 | 36 | |||
| Shigella boydii | 27 | 36 | |||
3.8. In silico molecular docking analysis
3.8.1. Molecular docking study for analgesic and anti-inflammatory activity
The five selected phytoconstituents of M. peguensis have been docked against the prostaglandin – 1 (PDB ID: 2OYE) and prostaglandin – 2 (PDB ID: 6COX) receptors. The docking score and glide energy has been determined and presented in Table 6. Besides, the best docking figures are presented in Fig. 6, Fig. 7, Fig. 8. From the table, it can be distinguished that, pongamol uncovered the highest (−10.42 kcal/mol) docking score through the binding of residues (ser353, ile523, gly526, tyr385, trp347 and ala527). Besides, milletenone showed the lowest (−7.43 kcal/mol) docking score with the interaction of 2OYE receptor. The ranking of the docking is as follows: pongamol > Kanjone > Lanceolatin–B > Ovalitenone > milletenone. Similarly, the interaction of the selected compounds and the 6COX enzymes yields the docking score ranging from −6.27 kcal/mol to −8.33 kcal/mol. The docking scores are ranked as follows: Lanceolatin– B > Kanjone > Ovalitenone > Milletenone > Pongamol. Lanceolatin – B binds with the receptor through the glu43, asp40, arg35, tyr113, leu89, asp83 and arg87 residues.
Table 6.
Docking score and glide energy of kanjone, lanceolatin – B, milletenone, ovalitenone, pongamol with prostaglandin – 1 (PDB ID: 2OYE), prostaglandin – 2 (PDB ID: 6COX), 5-HT3 receptor (PDB ID: 5AIN), urate oxidase (PDB ID: 1R4U), protein tyrosine kinase (PDB: 1XKK), E.coli exonuclease I (PDB ID: 1FXX) and human tissue-type plasminogen activator (PDB ID: 1A5H) in kcal/mol.
| Compounds | Analgesic and Anti-inflammatory |
Antidiarrheal |
Antioxidant |
Cytotoxic |
Antibacterial |
Thrombolytic |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2OYE |
6COX |
5AIN |
1R4U |
1XKK |
1FXX |
1A5H |
||||||||
| Docking score | Glide energy | Docking score | Glide energy | Docking score | Glide energy | Docking score | Glide energy | Docking score | Glide energy | Docking score | Glide energy | Docking score | Glide energy | |
| Aspirin | −6.69 | −27.55 | −5.91 | −27.00 | – | – | – | – | – | – | – | – | – | – |
| Loperamide | – | – | – | – | −6.15 | −45.41 | – | – | – | – | – | – | – | – |
| Ascorbic acid | – | – | – | – | – | – | −4.32 | −27.36 | – | – | – | – | – | – |
| Doxorubicin | – | – | – | – | – | – | – | – | −7.47 | −63.00 | ||||
| Doxycycline | – | – | – | – | – | – | – | – | – | – | −6.35 | −41.23 | – | – |
| Streptokinase | – | – | – | – | – | – | – | – | – | – | – | – | −6.00 | −26.24 |
| Kanjone | −9.38 | −37.96 | −7.70 | −32.28 | −6.01 | −36.57 | – | – | −5.28 | −38.33 | −5.80 | −28.41 | −6.62 | −37.29 |
| Lanceolatin - B | −8.83 | −35.71 | −8.33 | −33.24 | −6.26 | −38.24 | −4.03 | −22.80 | −6.32 | −35.33 | −4.66 | −28.60 | −6.85 | −38.38 |
| Milletenone | −7.43 | −45.15 | −6.41 | −36.58 | −6.94 | −52.45 | −3.92 | −29.64 | −5.34 | −43.25 | −4.11 | −31.33 | −5.81 | −46.49 |
| Ovalitenone | −8.76 | −50.15 | −6.96 | −39.84 | −6.10 | −50.86 | −3.77 | −28.21 | −5.24 | −43.08 | −6.54 | −46.13 | −6.41 | −49.04 |
| Pongamol | −10.42 | −54.91 | −6.27 | −9.64 | −4.68 | −38.49 | −2.96 | −29.87 | −4.83 | −43.73 | −3.31 | −31.08 | −6.07 | −44.02 |
Fig. 6.
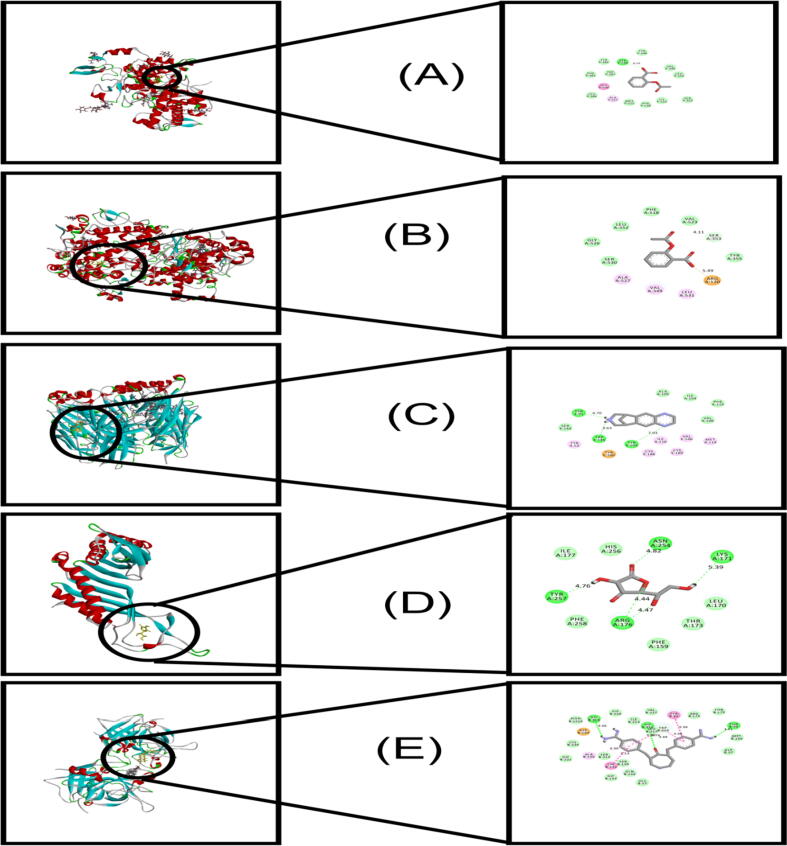
3D and 2D representation of docking interaction with standard drugs.A. Aspirin interaction with 2OYE; B. Aspirin interaction with 6COX; C. Loperamide interaction with 5AIN; D. Doxorubicin interaction with 1XKK; E. Streptokinase interaction with 1A5H.
Fig. 7.
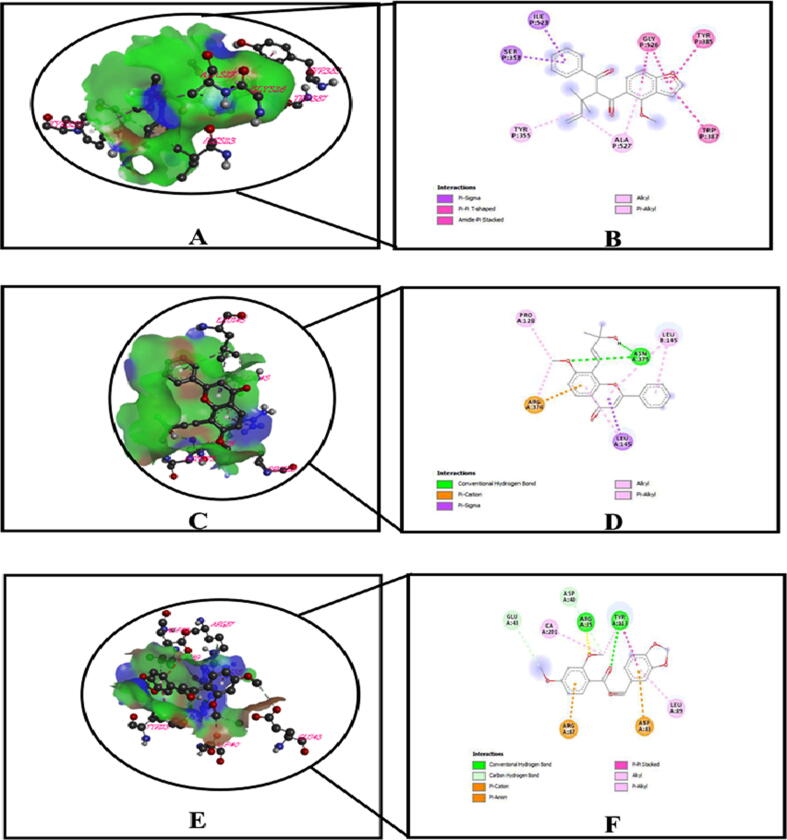
3D and 2D representation of the best key interactions in the binding pocket for selected ligands and receptors whether, (A and B) represents prostaglandin – 1 (PDB ID: 2OYE), pongamol; (C and D) represents prostaglandin – 2 (PDB ID: 6COX), lanceolatin – B; (E and F) represents 5-HT3 receptor (PDB ID: 5AIN) and milletenone; respectively.
Fig. 8.
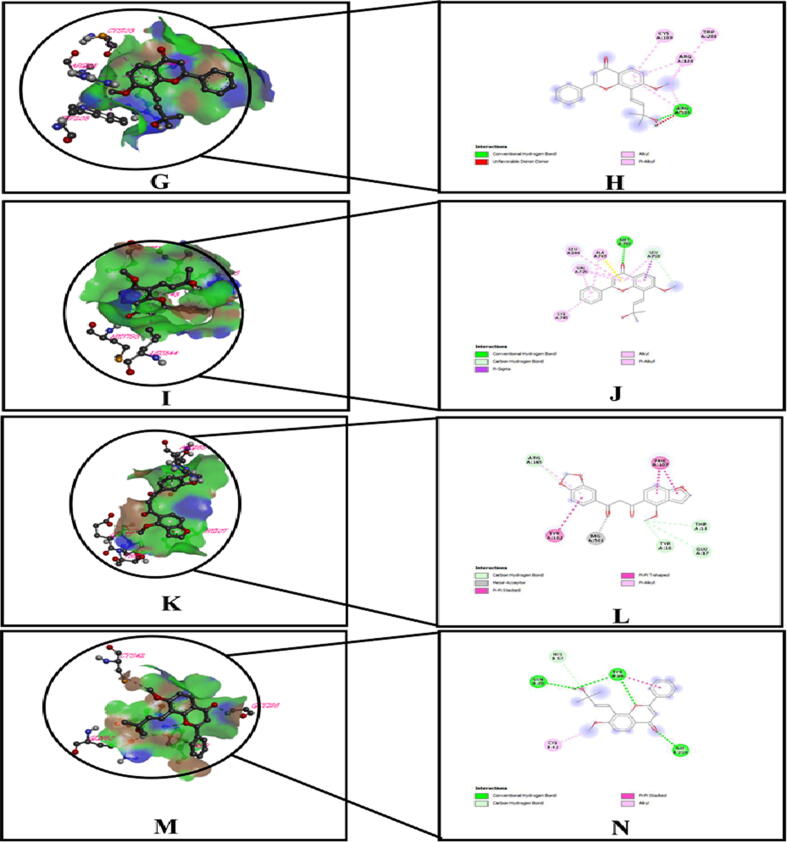
3D and 2D representation of the best key interactions in the binding pocket for selected ligands and receptors whether, (G and H) represents urate oxidase (PDB ID: 1R4U), lanceolatin – B; (I and J) represents protein tyrosine kinase (PDB: 1XKK), lanceolatin – B; (K and L) represents E. coli exonuclease I (PDB ID: 1FXX), ovalitenone; (M and N) represents human tissue-type plasminogen activator (PDB ID: 1A5H) and lanceolatin –B, respectively.
3.8.2. Molecular docking study for antidiarrheal activity
In this study, the selected phytoconstituents have been docked against 5-HT3 receptor (PDB ID: 5AIN). The ranking of the molecular docking is: Milletenone > Lanceolatin–B > Ovalitenone > Kanjone > Pongamol. The docking score range from −4.68 kcal/mol to −6.94 kcal/mol. Milletenone showed highest docking score through the binding of hydrogen bond by the series of residues namely arg87, glu43, asp40, arg35, tyr113, leu83, asp83 and arg87 which are represented in Table 6.
3.8.3. Molecular docking study for antioxidant activity
Antioxidant docking study was implemented by the docking of selected constituents with urate oxidase (PDB ID: 1R4U). The results of the docking score have been presented in Table 6 and docking interaction has been showing in Fig. 6, Fig. 7, Fig. 8. From the observation, the present study enumerated that the lanceolatin – B and pongamol have the highest and lowest binding affinity to the 1R4U receptor and achieved docking score ranging from −2.96 kcal/mol to −4.03 kcal/mol by binding with the series of hydrogen and hydrophobic bond namely: cys103, trp208, arg128 and arg105. Besides, kanjone showed no binding affinity to the 1R4U enzyme.
3.8.4. Molecular docking study for cytotoxic activity
The result of the cytotoxic docking study of five phytoconstituents of M. peguensis has been presented in Table 6. The lanceolatin – B showed the upmost docking score (−6.32 kcal/mol) and the lowest score (−4.83 kcal/mol) was observed for pongamol. Lanceolatin – B binds with the protein tyrosine kinase (PDB: 1XKK) through a series of a bond via the residue named lys745, val726, leu844, ala743, met793, and leu718 in Fig. 6, Fig. 7, Fig. 8.
3.8.5. Molecular docking study for antibacterial activity
The selected compounds were docked with the E. coli exonuclease I (PDB ID: 1FXX). From the result, it was observed that ovalitenone and pongamol showed the uppermost and lowermost binding affinity to the 1FXX receptor. The ranking of the docking score is: Ovalitenone > Kanjone > Lanceolatin–B > Milletenone > Pongamol. Ovalitenone showed the binding affinity to the 1FXX receptor by binding with the residues (mg501, tyr102, arg165, phe107, thr18, glu17 and tyr16) of hydrogen and hydrophobic bond. The summary of antibacterial docking study has been enumerated in Table 6 and the best docking interaction by ovalitenone and 1FXX has been shown in Fig. 6, Fig. 7, Fig. 8.
3.8.6. Molecular docking study for thrombolytic activity
In this experiment, lanceolatin – B revealed upmost (−6.85 kcal/mol) and milletenone demonstrated lowermost (−5.81 kcal/mol) binding affinity to the human tissue-type plasminogen activator (PDB ID: 1A5H). Lanceolatin – B binds with the 1A5H receptor through thecys42, gln60, his57, tyr99 and gly216 residues of hydrogen and hydrophobic bond. The best binding affinity with the 1A5H receptor has been shown in the Fig. 6, Fig. 7, Fig. 8 and the summary of the docking score has been given in Table 6.
3.9. Ligand based pharmacokinetics and toxicity property analysis
The Lipinski’s rules as well as the level of human intestinal absorption, AMES carcinoma, acute oral toxicity in human has been found within the limit according to this rule. Hereafter, kanjone; lanceolatin – B; milletenone; ovalitenone; and pongamol meet the requirement of Lipinski’s five rules which might be measured as drug-likeliness and are represented in Table 7.
Table 7.
Absorption, distribution, metabolism, excretion, and toxicological (ADME/T) properties of the compounds for good oral bioavailability.
| Molecules | PID | MW (g/mol) | HBD | HBA | LogP (o/w) | HIA | AM | CAR (binary) | PPB (100%) | AOT (kg/moL) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kanjone | 12,305,449 | 292.30 | 0 | 4 | 4.21 | 0.9928 | 0.6700 | 0.5400 | 0.893 | 2.129 |
| Lanceolatin - B | 10,978,265 | 262.26 | 0 | 3 | 4.21 | 0.9906 | 0.6100 | 0.8888 | 1.021 | 2.313 |
| Milletenone | 42,607,652 | 328.32 | 1 | 6 | 3.21 | 0.9881 | 0.6500 | 0.8672 | 1.028 | 2.007 |
| Ovalitenone | 627,910 | 338.32 | 0 | 6 | 3.63 | 0.9765 | 0.6500 | 0.9745 | 0.847 | 1.703 |
| Pongamol | 101,936,575 | 362.43 | 0 | 4 | 5.34 | 0.9904 | 0.6300 | 0.9143 | 0.927 | 3.148 |
PID = PubChem ID, MW = Molecular Weight (acceptance range: <500), HBD = Hydrogen Bond Donor (acceptance range: ≤ 5), HBA = Hydrogen Bond Acceptor: (acceptance range: ≤ 10), LogP = High Lipophilicity (acceptance range: < 5), HIA = Human Intestinal Absorption, AM = AMES Mutagenesis, CAR = Carcinogens, PPB = Plasma Protein Binding, AOT = Acute Oral Toxicity.
4. Discussion
Medicinal plants have the greatest source to discover new bioactive molecules with novel therapies (Abe & Ohtani, 2013). Thus, plant based natural therapies are very common in developing countries and get notable priorities due to their protective properties and numerous benevolent properties in human health. Even in developing countries, about 80% of people use conventional medicines (Kim, 2005). As therapeutic agents with distinct biological activities, many plant-derived chemicals serve potential responses such as thrombolytic, antioxidant, cytotoxicity, anxiolytics, antidepressants, neuroprotective and hepatoprotective agents (Okwu & Uchenna, 2009).
The human body produces thousands of chemical reactions through various catabolism and anabolism processes. These different reactions that encourage pain, inflammation, oxidative stress are triggered by inflammatory mediators, reactive oxygen and nitrogen species (RONS) (Wilhelm et al., 2016). Sources of inflammation like microbial attack or tissue injury cause the rush of defense cells like leukocytes to the site of injury which is set off by receptors like TLRs (toll-like receptors) and NLRs (NOD-like receptors) (Abdel Motaal & Abdel Maguid, 2005). Various inflammatory mediators including eicosanoids, cytokines, chemokines and vasoactive amines are also produced which can result in inflammation (Sun et al, 2014). Flavonoids have exhibited the ability to reduce the number of inflammatory cells along with the synthesis of MMP-9 (matrix metalloproteinase) and other mediators of inflammation (Li et al., 2012). Besides, various flavonoids had been reported possessing anti-inflammatory characteristics by inhibiting COX-2 expression gene (Chen et al., 2000). In this current study, MEMP showed significant (p < 0.001) inhibition of writhing and licking compared to standard. The result exhibited that increasing the dose of MEMP can promote better inhibition of writhing as well as paw licking in mice model which indicated a potential dose-dependent relationship. Thus, it can be assumed that the secondary metabolites specially flavonoids, present in MEMP may suppress the analgesia by reducing inflammatory response.
Diarrhea is an alteration of intestinal motility and accumulation of fluids along with heightened peristalsis by interfering with the electrolyte permeability of the intestinal membrane (Bristy et al., 2020). Infectious diarrhea is caused by the colonization and spreading of infectious agents, mostly Salmonella and Shigella (Panda et al., 2012), and traditional approaches have been found effective against these pathogens (Kone et al., 2004). Phytochemicals extracted from various plants have been reported to function as antidiarrheal agents by increasing the absorption of liquids from the intestine and decreasing gastric motility (Agbor et al., 2004). The MEMP study reported promising antidiarrheal activity due to the presence of several phytoconstituents, namely flavonoids which can display antidiarrheal potentiality (Otshudi et al., 2000). Flavonoids may also express this activity through the reduction of small and large intestinal motility (Capasso et al., 1991, Meli et al., 1990) and bowel contraction. Methanol extract of M. peguensis exhibited prominent antidiarrheal property at higher doses (p < 0.001) and revealed a dose-dependent pattern. The leaves displayed notable antidiarrheal efficacy by alleviating the average weight of both total and wet faeces along with a total number of faeces at 400 mg/kg dose which is close to loperamide introduced as a standard drug to treat diarrheal state in this study.
Antioxidants function via counteracting reactive oxygen species (ROS), including superoxide radicals, hydroxyl radicals and peroxy radicals (Wong et al., 2006). ROS is independent and contains one or more unpaired electrons which can be the result of various exogenous and endogenous effects such as radiation and microbial contamination (Krishnaiah et al., 2007). These ROS oxidants can cause lipid peroxidation, DNA oxidation leading to mutation (Nakabeppu et al., 2006) and protein oxidation causing degradation, denaturation and loss of function of cell (Davies, 2001). Ascorbic acid, a common antioxidant can scavenge ROS and chelating metals, whereas vitamin E can function by delaying oxidation of poly-unsaturated fatty acids (PUFA) (Krishnaiah et al., 2007). In addition, various medicinal plants containing antioxidants including flavonoids, phenolic acids, tannins and other phytochemicals have been studied (Cai et al., 2004) and found to be promising against aging, coronary heart disease and cancer (La Vecchia et al., 2001, Stephens et al., 1996). Besides, flavonoids have the ability to donate hydrogen atoms or electrons to demonstrate strong antioxidant activity (Agati et al., 2012). Flavonoids can also play a pivotal role as antitumorigenic agents in different stages of cancer development and progression of cancer following different mechanisms including induction of apoptosis in tumor cells (Han et al., 2007, Babu et al., 2013). In this quantitative test of MEMP, the total phenol content was found 65.27 mg GAE/g. Besides, from the DPPH scavenging test, MEMP revealed remarkable antioxidant activity with IC50 value of 15.96 μg/mL while standard BHT exhibited IC50 value of 20.07 μg/mL. Furthermore, the brine shrimp bioassay displayed moderate toxicity (LC50 = 1.094 μg/mL) in comparison to vincristine sulfate (LC50 = 0.451 μg/mL). Thus, research concern is increased to evaluate phytochemicals to establish new natural cytotoxic and antioxidant molecules.
The necessity to explore medicinal plants emerges to discover new potential therapeutic leads against bacterial resistance and reemerging contagious diseases (Nostro et al., 2000). Bacteria have a genetic ability to show drugs resistance (Nascimento et al., 2000) and spread this ability from progeny to progeny (Ertürk et al., 2006). In spite of marketizing a variety of antibiotics in the last few decades by pharmaceutical industries, newer molecules are still required to combat microbes of the various spectrum (Vital & Rivera, 2009). Antibiotics can exhibit their actions in different mechanism including targeting bacterial cell wall by beta-lactam antibiotics and inhibiting protein synthesis (DNA and RNA formation) (Ulanowska et al., 2006). Moreover, phytochemicals like flavonoids are also notable for showing bactericidal properties (Cushnie et al., 2003). In addition, M. peguensis had been reported to contain several phytochemicals along with flavonoids and bioactive phytochemicals (Ganapaty et al., 1998) and can be assumed that antimicrobial properties of MEMP against all selected bacterial strains (gram-positive and gram-negative) compared to standard (amoxicillin and streptomycin) can be attributed by the presence of these flavonoids (Oladeji et al., 2020).
Among the docking study of the 5 selected compounds i.e. flavonoids suggest that kanjone, lanceolatin – B, milletenone, ovalitenone and pongamol may be the responsible bioactive phytochemicals for potential analgesic and anti-inflammatory activities. kanjone, lanceolatin – B, milletenone, ovalitenone are highly accountable for the antidiarrheal activity. Lanceolatin - B is modestly responsible for the antioxidant activity. kanjone, lanceolatin – B, milletenone, and ovalitenone are significantly responsible for the cytotoxic activity. kanjone and ovalitenone are responsible for the antibacterial potentiality. Furthermore kanjone, lanceolatin – B, milletenone, ovalitenone and pongamol and highly liable for the thrombolytic potentiality of the plant. According to the Glide Docking score and Glide energy we can see that among these five compounds lanceolatin – B, ovalitenone, kanjone, and pongamol are the potent compounds, although further biological studies are needed to explore their in-depth mechanism of action. All the compounds obsessed a higher fascination towards the ligand binding domain of prostaglandin – 1, prostaglandin – 2, 5-HT3 receptor, urate oxidase, crystal structure of GABA (B), E.coli exonuclease I and human tissue-type plasminogen activator enzyme and strong ligand-receptor complex creation may be responsible for the antagonism of these receptors. For naturally occurring product, virtual testing is important to search for active principles with attractive ADME/T profiles that have formerly been separated but not analyzed for activity against specific drug targets (Liu et al., 2015). The ADME and toxicity profiles of the compounds kanjone, lanceolatin – B, milletenone, ovalitenone and pongamol are found good with very mild toxicity which is essential to be a possible drug.
5. Conclusion
In vivo and in vitro studies of MEMP showed drug like activity which may be due to their phytochemicals i.e. flavonoids and in silico phytoconstituent analysis of Millettia peguensis validated the pharmacological insights of the extract through computer aided approaches. Hence, Millettia peguensis leaves extract may provide a new dimension in the treatment of pain, diarrhea, inflammation, oxidative stress and further study is required to identify these mechanisms.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
All biological activity screenings were conducted in accordance with the ethical standards laid down in the Declaration of Helsinki 2013. Animal models were handled and treated according to the principles of the Swiss Academy of Medical Sciences and Swiss Academy of Sciences and were euthanized following the Guidelines for the Euthanasia of Animals: 2013 edition.
Author contribution
SA and AG conceptualized and designed the study protocol; SA and FTR prepared the plant extract, designed protocols, collected and calculated the data. NUE and SAS designed, conducted the computational analysis; NUE, SA, SS, MNI and SAS - wrote the manuscript. AG and MRH supervised, monitored the research. SA, AG, MRH, MNI, SAS and NUE along with writing, reviewed and edited manuscript. All authors read and approved the final manuscript for publication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Safaet Alam is really grateful to his beloved mother Shahanaj Parven for her continuous inspiration towards the successful completion of the research work.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammad Rashedul Haque, Email: haquemr@du.ac.bd.
Amlan Ganguly, Email: gangulyamlan@du.ac.bd.
References
- Abdel Motaal N.A., Abdel Maguid A. Effect Of Fractionated And Single Doses Gamma Irradiation On Certain Mammalian Organs. Egypt. J. Hospital Med. 2005;19(1):111–122. [Google Scholar]
- Abe R., Ohtani K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J. Ethnopharmacol. 2013;145(2):554–565. doi: 10.1016/j.jep.2012.11.029. [DOI] [PubMed] [Google Scholar]
- Abiy Y., Hellen A.O.R., Solomon J. Anti-plasmodial activities and X-ray crystal structures of rotenoids from Millettia usaramensis. Phytochemistry. 2003;64(3):773–779. doi: 10.1016/s0031-9422(03)00373-x. [DOI] [PubMed] [Google Scholar]
- Agati G., Azzarello E., Pollastri S., Tattini M. Flavonoids as antioxidants in plants: location and functional significance. Plant Sci. 2012;196:67–76. doi: 10.1016/j.plantsci.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Agbor G.A., Léopold T., Jeanne N.Y. The antidiarrhoeal activity of Alchornea cordifolia leaf extract. Phytotherapy Res.: Int. J. Devoted Pharmacol. Toxicol. Eval. Natural Product Derivatives. 2004;18(11):873–876. doi: 10.1002/ptr.1446. [DOI] [PubMed] [Google Scholar]
- Agbor G.A., Longo F., Makong E.A., Tarkang P.A. Evaluation of the antidiarrheal and antioxidant properties of Justicia hypocrateriformis. Pharm. Biol. 2014:1–6. doi: 10.3109/13880209.2013.879189. [DOI] [PubMed] [Google Scholar]
- Ahmad N.S., Waheed A., Farman M., Qayyum A. Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J. Ethnopharmacol. 2010;129(2):250–253. doi: 10.1016/j.jep.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Ahmad V.U., Ali Z., Hussaini S.R., Iqbal F., Zahid M., Abbas M., Saba N. Flavonoids of Tephrosia purpurea. Fitoterapia. 1999;70:443–445. [Google Scholar]
- Barnes P.J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. 1998;94(6):557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Biovia D.S. San Diego; CA, USA: 2017. Discovery studio visualizer; p. 936. [Google Scholar]
- Breyer W.A., Matthews B.W. Structure of Escherichia coli exonuclease I suggests how processivity is achieved. Nat. Struct. Biol. 2000;7(12):1125–1128. doi: 10.1038/81978. [DOI] [PubMed] [Google Scholar]
- Bristy T.A., Barua N., Montakim Tareq A., Sakib S.A., Etu S.T., Chowdhury K.H. Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, Vitro and In Silico Approaches. Pharmaceuticals. 2020;13(8):183. doi: 10.3390/ph13080183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74(17):2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso A., Pinto A., Sorrentino R., Capasso F. Inhibitory effects of quercetin and other flavonoids on electrically-induced contractions of guinea pig isolated ileum. J. Ethnopharmacol. 1991;34(2–3):279–281. doi: 10.1016/0378-8741(91)90048-i. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Yang L.-L., Lee T.J. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochem. Pharmacol. 2000;59(11):1445–1457. doi: 10.1016/s0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- Cushnie T.T., Hamilton V.E., Lamb A.J. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiol. Res. 2003;158(4):281–289. doi: 10.1078/0944-5013-00206. [DOI] [PubMed] [Google Scholar]
- Davies K.J. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83(3–4):301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- Digiesi V., Lenuzza M., Digiesi G. Prospects for the use of antioxidant therapy in hypertension. Annali italiani di medicina interna: organo ufficiale della Società italiana di medicina interna. 2001;16(2):93–100. [PubMed] [Google Scholar]
- Ertürk Ö., Kati H., Yayli N., Demirbağ Z. Antimicrobial properties of Silene multifida (Adams) Rohrb. plant extracts. Turkish J. Biol. 2006;30(1):17–21. [Google Scholar]
- Ganapaty S., Pushpalata V., Babu G.J., Naidu K.C., Waterman P.G. Flavonoids from Millettia peguensis Ali (Fabaceae) Biochem. Syst. Ecol. 1998:125–126. [Google Scholar]
- Guedes I.A., de Magalhães C.S., Dardenne L.E. Receptor–ligand molecular docking. Biophys. Rev. 2014;6(1):75–87. doi: 10.1007/s12551-013-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Sun M., Cui Y., Wang T., Zhang W., Guo M. Kushen flavonoids induce apoptosis in tumor cells by inhibition of NF-κB activation and multiple receptor tyrosine kinase activities. Phytother. Res. 2007;21(3):262–268. doi: 10.1002/ptr.2065. [DOI] [PubMed] [Google Scholar]
- Harman C.A., Turman M.V., Kozak K.R., Marnett L.J., Smith W.L., Garavito R.M. Structural basis of enantioselective inhibition of cyclooxygenase-1 by S-α-substituted indomethacin ethanolamides. J. Biol. Chem. 2007;282(38):28096–28105. doi: 10.1074/jbc.M701335200. [DOI] [PubMed] [Google Scholar]
- Heinrich M., Barnes J., Gibbons S., Williamson E. Churchill Livingstone; Edinbrugh: 2004. Fundamentals of Pharmacognosy and Phytotherapy. [Google Scholar]
- Herowati R., Widodo G.P. Molecular Docking studies of chemical constituents of Tinospora cordifolia on glycogen phosphorylase. Procedia Chem. 2014;13:63–68. [Google Scholar]
- Hosaka S., Obuki M., Nakajima J., Suzuki M. Comparative study of antioxidants as quenchers or scavengers of reactive oxygen species based on quenching of MCLA-dependent chemiluminescence. Luminescence: J. Biol. Chem. Luminescence. 2005;20(6):419–427. doi: 10.1002/bio.867. [DOI] [PubMed] [Google Scholar]
- Huo X., Zhang L., Gao L., Guo Y., Zhang L., Li L., Si J., Cao L. Antiinflammatory and Analgesic Activities of Ethanol Extract and Isolated Compounds from Millettia pulchra. Biol. Pharm. Bull. 2015;XXXVII I(9):1328–1336. doi: 10.1248/bpb.b15-00187. [DOI] [PubMed] [Google Scholar]
- Huys, G., D'haene, K., & Swings, J. (2002). Influence of the culture medium on antibiotic susceptibility testing of food‐associated lactic acid bacteria with the agar overlay disc diffusion method. Lett. Appl. Microbiol., 34(6), 402-406. [DOI] [PubMed]
- Islam M.N., Tasnim H., Arshad L., Haque M.A., Tareq S.M., Kamal A.T.M.M. Stem extract of Albizia richardiana exhibits potent antioxidant, cytotoxic, antimicrobial, anti-inflammatory and thrombolytic effects through in vitro approach. Clin. Phytosci. 2020;6(1):60. doi: 10.1186/s40816-020-00212-w. [DOI] [Google Scholar]
- Joseph N., Zhang-James Y., Perl A., Faraone S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Attent. Disorders. 2015;19(11):915–924. doi: 10.1177/1087054713510354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-S. Do not put too much value on conventional medicines. J. Ethnopharmacol. 2005;100(1–2):37–39. doi: 10.1016/j.jep.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Kone W., Atindehou K.K., Terreaux C., Hostettmann K., Traore D., Dosso M. Traditional medicine in North Côte-d’Ivoire: screening of 50 medicinal plants for antibacterial activity. J. Ethnopharmacol. 2004;93(1):43–49. doi: 10.1016/j.jep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Krishnaiah D., Sarbatly R., Bono A. Phytochemical antioxidants for health and medicine–A move towards nature. Biotechnol Mol. Biol. Rev. 2007;1(4):97–104. [Google Scholar]
- Kurumbail R.G., Stevens A.M., Gierse J.K., McDonald J.J., Stegeman R.A., Pak J.Y. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- La Vecchia C., Altieri A., Tavani A. Vegetables, fruit, antioxidants and cancer: a review of Italian studies. Eur. J. Nutr. 2001;40(6):261–267. doi: 10.1007/s394-001-8354-9. [DOI] [PubMed] [Google Scholar]
- Li L., Bao H., Wu J., Duan X., Liu B., Sun J. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int. Immunopharmacol. 2012;13(1):15–22. doi: 10.1016/j.intimp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23(1–3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Liu R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007;46(3):207–219. [Google Scholar]
- Liu Z.H., Yan H., Liu H.Y. Chemical constituents and their bioactivities of plants of Taccaceae. Chem. Biodivers. 2015;12(2):221. doi: 10.1002/cbdv.201300353. [DOI] [PubMed] [Google Scholar]
- Manikandan G., Vimana R.A., Divya C., Ramasubbu R. GC-MS Analysis Of Phytochemical Constituents In The Petroleum Ether Leaf Extracts Of Millettia peguensis. Int. Res. J. Pharm. 2017;8(9):144–150. [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Meli R., Autore G., Di Carlo G., Capasso F. Inhibitory action of quercetin on intestinal transit in mice. Phytother. Res. 1990;4(5):201–202. [Google Scholar]
- Meyer, B., Ferrigni, N., Putnam, J., Jacobsen, L., Nichols, D. j., & McLaughlin, J. L. (1982). Brine shrimp: a convenient general bioassay for active plant constituents. Planta medica, 45(05), 31-34. [PubMed]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7(9):405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Sakumi K., Sakamoto K., Tsuchimoto D., Tsuzuki T., Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol. Chem. 2006;387(4):373–379. doi: 10.1515/BC.2006.050. [DOI] [PubMed] [Google Scholar]
- Narkhede, C. P., Koli, S. H., Suryawanshi, R. K., Patil, C. D., Borase, H.P., Patil, S. V. (2016). Potentiation of Bacillus thuringiensis by using some natural products: Novel preparations against dengue vector Aedis aegypti larvae. Indian J. Nat. Prod. Resources, VII(3), 229-233.
- Nascimento G.G., Locatelli J., Freitas P.C., Silva G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000;31(4):247–256. [Google Scholar]
- Nostro, A., Germano, M., D’angelo, V., Marino, A., & Cannatelli, M. (2000). Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol., 30(5), 379-384. [DOI] [PubMed]
- Okwu D.E., Uchenna N.F. Exotic multifaceted medicinal plants of drugs and pharmaceutical industries. Afr. J. Biotechnol. 2009;8(25) [Google Scholar]
- Oladeji O.S., Odelade K.A., Oloke J.K. Phytochemical screening and antimicrobial investigation of Moringa oleifera leaf extracts. African J. Sci., Technol., Innovat. Dev. 2020;12(1):79–84. [Google Scholar]
- Otshudi A.L., Vercruysse A., Foriers A. Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhoea in Lomela area, Democratic Republic of Congo (DRC) J. Ethnopharmacol. 2000;71(3):411–423. doi: 10.1016/s0378-8741(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Packiyalakshmi, D., Athilakshmi, P., Gayathri, S., Karthiga, P., & Thiri, R. (2017). Antimicrobial potential of different solvents leaf extract of Millettia peguensis against selected pathogens.
- Packiyalakshmi, D. A., P., Gayathri, S., Karthiga, P., Thiri Bhuvaneswari, R., Manikandan, G. (2017). Antimicrobial potential of different solvents leaf extract of Millettia peguensis against selected pathogens. Pharma Innovation J., VIII(1), 109-124.
- Panda S.K., Patra N., Sahoo G., Bastia A.K., Dutta S.K. Anti-diarrheal activities of medicinal plants of Similipal Biosphere Reserve, Odisha, India. Int. J. Med. Aromatic Plants. 2012;2(1):123–134. [Google Scholar]
- Prasad S., Kashyap R.S., Deopujari J.Y., Purohit H.J., Taori G.M., Daginawala H.F. Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thrombosis J. 2006;4 doi: 10.1186/1477-9560-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K.L., Lillestol R.K., Ulens C., Lummis S.C. Varenicline interactions at the 5-HT3 receptor ligand binding site are revealed by 5-HTBP. ACS Chem. Neurosci. 2015;6(7):1151–1157. doi: 10.1021/cn500369h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A.S., Yadav S.S., Singh P., Nandal A., Singh N., Ganaie S.A., Yadav N., Kumar R., Bhandoria M.S., Bansal P. A comprehensive review on ethnomedicine, phytochemistry, pharmacology, and toxicity of Tephrosia purpurea (L.) Pers. Phytother. Res. 2020:1–24. doi: 10.1002/ptr.6657. [DOI] [PubMed] [Google Scholar]
- Renatus M., Bode W., Huber R., Stürzebecher J., Prasa D., Fischer S. Structural Mapping of the Active Site Specificity Determinants of Human Tissue-type Plasminogen Activator IMPLICATIONS FOR THE DESIGN OF LOW MOLECULAR WEIGHT SUBSTRATES AND INHIBITORS. J. Biol. Chem. 1997;272(35):21713–21719. doi: 10.1074/jbc.272.35.21713. [DOI] [PubMed] [Google Scholar]
- Retailleau P., Colloc'h N., Vivarès D., Bonnete F., Castro B., El Hajji M. Complexed and ligand-free high-resolution structures of urate oxidase (Uox) from Aspergillus flavus: a reassignment of the active-site binding mode. Acta Crystallogr. D Biol. Crystallogr. 2004;60(3):453–462. doi: 10.1107/S0907444903029718. [DOI] [PubMed] [Google Scholar]
- Rios, J. L. a. R., M.C. (2005). Medicinal plants and antimicrobial activity. J. Ethnopharmacol., 80-84. [DOI] [PubMed]
- Roberts R.R., Hota B., Ahmad I., Scott R.D., Foster S.D., Abbasi F. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin. Infect. Dis. 2009;49(8):1175–1184. doi: 10.1086/605630. [DOI] [PubMed] [Google Scholar]
- Roberts R.R., Scott R.D., Hota B., Kampe L.M., Abbasi F., Schabowski S. Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med. Care. 2010:1026–1035. doi: 10.1097/MLR.0b013e3181ef60a2. [DOI] [PubMed] [Google Scholar]
- Shapovalov M.V., Dunbrack R.L., Jr A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure. 2011;19(6):844–858. doi: 10.1016/j.str.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifah, F., Tareq, A. M., Sayeed, M. A., Islam, M. N., Emran, T. B., Ullah, M. A., et al. (2020). Antidiarrheal, cytotoxic and thrombolytic activities of methanolic extract of Hedychium coccineum leaves. Measurement, 11, 12.
- Shoba F.G., Thomas M. Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhoea. J. Ethnopharmacol. 2001;76(1):73–76. doi: 10.1016/s0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- Škerget M., Kotnik P., Hadolin M., Hraš A.R., Simonič M., Knez Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89(2):191–198. [Google Scholar]
- Sostres C., Gargallo C.J., Arroyo M.T., Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010;24(2):121–132. doi: 10.1016/j.bpg.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Stephens N.G., Parsons A., Brown M., Schofield P., Kelly F., Cheeseman K., Mitchinson M. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) The Lancet. 1996;347(9004):781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- Sun X., Sit A., Feinberg M.W. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc. Med. 2014;24(3):105–112. doi: 10.1016/j.tcm.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse W.T., Hailu A.E., Gurmu A.E., Mechesso A.F. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Complement. Alternative Med. 2014;14(1):460. doi: 10.1186/1472-6882-14-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabit A.K., Crandon J.L., Nicolau D.P. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opinion Pharmacotheapy. 2015;16(2):159–177. doi: 10.1517/14656566.2015.993381. [DOI] [PubMed] [Google Scholar]
- Ulanowska K., Tkaczyk A., Konopa G., Węgrzyn G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2006;184(5):271–278. doi: 10.1007/s00203-005-0063-7. [DOI] [PubMed] [Google Scholar]
- V Babu, B., K Konduru, N., Nakanishi, W., Hayashi, S., Ahmed, N., & M Mitrasinovic, P. (2013). Experimental and theoretical advances in functional understanding of flavonoids as anti-tumor agents. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 13(2), 307-332. [DOI] [PubMed]
- Vital P.G., Rivera W.L. Antimicrobial activity and cytotoxicity of Chromolaena odorata (L. f.) King and Robinson and Uncaria perrottetii (A. Rich) Merr. Extracts. J. Med. Plants Res. 2009;3(7):511–518. [Google Scholar]
- Walum E. Acute oral toxicity. Environ. Health Perspect. 1998;106(suppl 2):497–503. doi: 10.1289/ehp.98106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm, J., Vytášek, R., Uhlík, J., & Vajner, L. (2016). Oxidative stress in the developing rat brain due to production of reactive oxygen and nitrogen species. Oxidative Medicine and Cellular Longevity, 2016. [DOI] [PMC free article] [PubMed]
- Wong C.-C., Li H.-B., Cheng K.-W., Chen F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006;97(4):705–711. [Google Scholar]
- Wood E.R., Truesdale A.T., McDonald O.B., Yuan D., Hassell A., Dickerson S.H. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64(18):6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- Zhao S.S., Ma D.X., Zhu Y., Zhao J.H., Zhang Y., Chen J.Q., Shenig Z.L. Antidiarrheal effect of bioactivity-guided fractions and bioactive components of pomegranate (Punica granatum L.) peels. Neurogastroenterol Motil. 2018 doi: 10.1111/nmo.13364. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]