Abstract
Objective
This study aimed to investigate the effects of fructo-oligosaccharides (FOS) on serum lipid levels and to determine the mechanisms underlying these effects and the potential role of inflammation.
Methods
Male C57BL/6 mice received a normal diet, a high-fat/high-sugar (HFS) diet, or an HFS diet supplemented with 10% FOS for 10 weeks. In vivo intestinal and serum short-chain fatty acid (SCFA) levels were measured by gas chromatography. In vivo serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and malonaldehyde (MDA) were also measured. Lipid accumulation was visualized. Reactive oxygen species (ROS) generation was evaluated and apoptosis was quantified.
Results
FOS reversed in vivo HFS-induced lipid accumulation in the liver. An HFS diet increased ALT, AST, TC, TG, and LDL serum levels, decreased HDL serum levels, and increased IL-6, TNF-α, 8-OHdG, and MDA levels. These changes were reduced by FOS. FOS also increased intestinal and serum levels of short chain fatty acids (SCFAs). In vitro, SCFAs ameliorated palmitic acid-induced ROS production and apoptosis of HepG2 cells.
Conclusion
FOS supplementation lowers serum lipid levels and ameliorates HFS-induced inflammation by upregulating SCFAs.
Keywords: Fructo-oligosaccharide, short-chain fatty acids, lipid, inflammation, apoptosis, reactive oxygen species
Introduction
Over the past two decades, food and lifestyle choices have dramatically changed. Because of the rising incidence of metabolic disorders, including obesity, diabetes, hypertension, and dyslipidemia, studies have aimed to identify new sources of dietary fiber and have attracted increasing attention.1,2 Epidemiological and clinical studies have shown that dietary fiber significantly affects the intestinal microbiota and intestinal inflammatory diseases.3,4 In addition to promoting a more diverse/balanced intestinal microbiota, dietary fiber is effective for preventing and treating obesity, diabetes, and cardiovascular diseases.4–6 In recent years, the idea of the gut as an endocrine organ has been increasingly accepted. Alterations in the microflora can affect production of useful gut hormones, such as glucagon-like peptide-1, which affects insulin sensitivity and systemic inflammation.7 Variations in intestinal microbiota in conjunction with specific nutrition can increase intestinal permeability, which promotes insulin resistance by sustaining a low-grade inflammatory state.8
Fructo-oligosaccharides (FOS) are plant-derived polysaccharides comprising fructose monomers connected via β (2-1) glycosidic bonds linked to a terminal glucose residue.9 FOS, which are naturally present in various fruits and vegetables (including chicory), can be produced from beet sugar and are frequently used to replace sugars in production of low-sugar foods to lower the postprandial glycemic response.9 Dietary fibers, including FOS, are resistant to degradation in the upper gut, but fermented in the colon by resident microflora.10 During fermentation, FOS are metabolized to short-chain fatty acids (SCFAs) and selectively stimulate the growth of Bifidobacteria.11–13 Inflammation plays a crucial role in insulin resistance, oxidative stress, and other diseases. Inflammation is not only the primary factor in occurrence and development of non-alcoholic fatty liver disease (NAFLD), but also affects progression and treatment of NAFLD. SCFAs inhibit liver inflammation, and many agents can be used to treat high-fat-induced liver diseases through the anti-inflammatory effect of SCFAs.14,15
We hypothesized that FOS affect serum lipid levels and the inflammatory response simultaneously owing to the close relationship between inflammation and lipid metabolism.16 Therefore, we evaluated the effects of FOS on serum lipid levels and the mechanisms underlying these effects to determine whether FOS ameliorate the negative effect of a high-fat/high-sugar (HFS) diet.
Materials and methods
Animals and dietary treatments
This study was approved by the ethical review board for laboratory animal use at The Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University. Sixty 8-week old male C57BL/6 mice were purchased from Yangzhou University and acclimated for 1 week before starting the experiments. The room temperature was 18 to 22°C and humidity was 60%. The mice were randomly divided into three groups of 20 mice in each group and they received a normal diet (control group), an HFS diet (HFS group, 30% of energy derived from fat and 50% from sugar; purchased from Model Animal Research Centre of Nanjing University), or an HFS diet supplemented with 10% FOS (HFS + FOS group; FOS were purchased from Shanghai General Pharmaceutical Co., Ltd., Shanghai, China). Mice were provided ad libitum access to food and water and kept on a 12:12-hour light:dark cycle in wire-bottomed cages to reduce coprophagic activity with groups of 3 to 4 mice/cage. After 10 weeks of continuous feeding, mice were anesthetized with 1% pentobarbital sodium (40 mg/kg) by intraperitoneal injection. Blood samples (0.5–1.0 mL) were collected from the orbital sinuses of the mice after removing the eyeball and blood samples were preserved at −80°C. Livers and epididymal white adipose tissues were obtained from the mice and fixed in 10% buffered formalin. All mice were then euthanized by an overdose of pentobarbital sodium.
Gut SCFA analysis
Colon contents (1 mL) were added to a microfuge tube containing 20 µL of 50% H2SO4. The sample was incubated at 18 to 22°C and centrifuged for 3 minutes at 1500 ×g. A volume of 600 µL of supernatant was then transferred to a new microfuge tube, 120 µL of 25% metaphosphoric acid was added, and the sample was allowed to stand for 30 minutes at 37°C. The samples were centrifuged again and the supernatant was transferred to a gas chromatograph vial. The concentrations of individual SCFAs were measured using a PerkinElmer Autosystem gas chromatography device (Waltham, MA, USA) by using 4% Carbowax 20 M on 80/120 mesh Carbopack B-DA, with a 1.8 × 2-mm column. Nitrogen was used as a carrier gas, the injector temperature was 170°C, and the detector temperature was 175°C. The column temperature was maintained at 115°C for 45 minutes. Nitrogen flow was maintained at 25 mL/minute.
Assessment of serum parameters
Blood samples were centrifuged (10,000 ×g at 4°C) for 10 minutes and serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and malonaldehyde (MDA) were determined by enzyme-linked immunosorbent assay (ELISA) (Abcam, Cambridge, UK). SCFA serum levels were measured by gas chromatography.
Histopathological analysis
To evaluate the histopathological changes in lipid accumulation caused by FOS, liver and white adipose tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Standard 3-µm sections were cut and stained with hematoxylin and eosin (H&E). Random fields in the stained sections were observed with a microscope (Olympus, Tokyo, Japan). Images of the stained sections in each group were examined with a medical image analysis system (Medical 5.0 Digital Medical Image Analysis System, Canon, Tokyo, Japan).
Cell culture and treatment
Human hepatoblastoma HepG2 cells were purchased from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). HepG2 cells were plated in tissue culture dishes that were coated with type 1 collagen (at a density of 200,000 cells/cm2) in hepatocyte basal growth medium (Lonza, Basel, Switzerland) supplemented with growth factors and 2% fetal bovine serum. The plates were incubated at 37°C in a tissue culture incubator (5% CO2) for 4 to 5 hours. The medium was changed to hepatocyte maintenance media during the experiment. HepG2 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B. For the experiments, HepG2 cells were first incubated with 0.5 mM palmitic acid (PA) complexed with bovine serum albumin for 24 hours to induce fat accumulation.17 The cells were then incubated with or without 0.5 mM acetate, which is an SCFA, for 24 hours.
Cellular reactive oxygen species production
Reactive oxygen species (ROS) generation in HepG2 cells was evaluated by the dichlorodihydrofluorescein diacetate (DCFDA) method. Briefly, the cells were incubated with 10 µM DCFDA for 30 minutes. After removing excess DCFDA, the cells were washed and then exposed to 100 ng/mL lipopolysaccharide for 4 hours at 37°C. The cells were collected and lysed in phosphate-buffered saline (PBS) containing 0.5% Triton X-100. Fluorescence of the samples was monitored at an excitation wavelength of 485 nm and an emission wavelength of 538 nm.18
Quantification of apoptosis by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
DNA fragmentation of apoptotic cells was detected by the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay (Roche Applied Science, Basel, Switzerland). For the TUNEL assay, acetate treated and untreated cells were fixed with 4% paraformaldehyde in PBS for 20 minutes at room temperature. Cells were then washed with PBS for 30 minutes and incubated with permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate) for 2 minutes on ice. The cells were washed twice and incubated in a humidified atmosphere with TUNEL reaction mixture for 60 minutes at 37°C in the dark. A volume of 50 µL peroxidase was then added and incubated for 20 to 30 minutes at 37°C. After washing three times with PBS, 75 µL diaminobenzidine was added and incubated for 10 minutes. After washing with PBS, the cells were stained with hematoxylin for 30 seconds, followed by washing. Finally, the cells were analyzed by optical microscopy.
Statistical analysis
IBM SPSS 22.0 (IBM Co., Armonk, NY, USA) was used for statistical analyses and all figures were created in GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Data are expressed as mean ±standard deviation. Differences between two groups were analyzed by one-way analysis of variance followed by Tukey’s test. P values < 0.05 were considered statistically significant.
Results
FOS reverse the effect of HFS on fat deposition and liver function
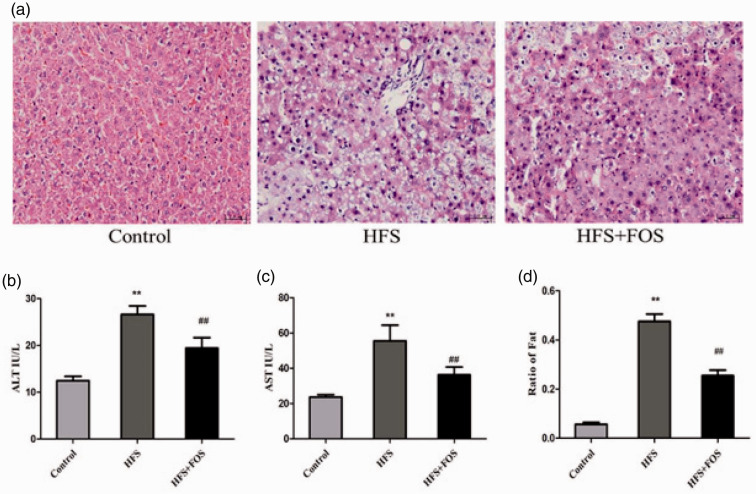
FOS ameliorated HFS diet-induced histological and functional changes in the mouse liver (Figure 1). Liver sections from the HFS group showed tissue inflammation compared with the control group. This inflammation was much less in the HFS+FOS group compared with the HFS group. Mice in the HFS group showed a large amount of lipid deposition and microvesicular steatosis in the liver, with small lipid droplets in the cells. However, FOS supplementation significantly reduced hepatic steatosis (P < 0.01 vs. the HFS group), which suggested a lipid-lowering effect (Figure 1a and 1d). Mice in the HFS group showed significantly higher ALT and AST levels than those in the control group (both P < 0.01), and FOS reversed this change (P < 0.01 vs. the HFS group). Serum ALT and AST levels were significantly lower in the control group compared with the other two groups (both P < 0.01 Figure 1b–c).
Figure 1.
FOS supplementation ameliorates HFS diet-induced histological and functional changes in the mouse liver. (a) H&E-stained liver tissue at 40× magnification. (b) Serum ALT levels (IU/L). (c) Serum AST levels (IU/L). (d) Ratio of fat in the liver. Data (b and c) are presented as mean ± standard deviation (n = 20 in each group). **P < 0.01 vs. the control group; ##P < 0.01 vs. the HFS group. FOS, fructo-oligosaccharides; H&E, hematoxylin and eosin; ALT, alanine transaminase; AST, aspartate aminotransferase; HFS, high-fat/high-sugar.
Effect of FOS on serum lipid levels
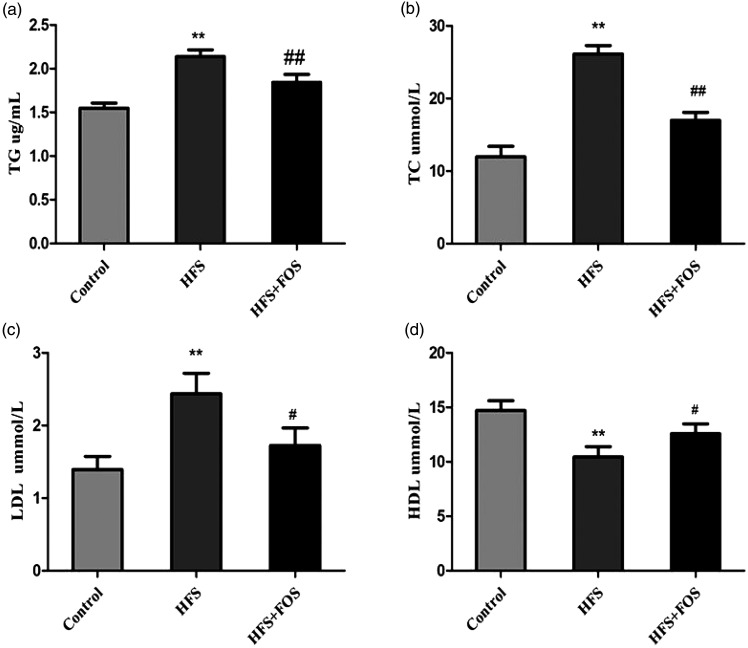
The ELISA assay showed large differences in serum lipids levels among the three groups of mice. Mice in the HFS group showed significantly higher TC, TG, and LDL levels, and lower HDL levels compared with the control group (all P < 0.01). However, dietary FOS reversed these changes. Serum TC, TG, and LDL levels were significantly lower and HDL levels were higher in the FOS group compared with the HFS group (all P < 0.01, Figure 2a–d).
Figure 2.
FOS supplementation improves serum lipid levels in HFS-fed mice. (a) TG levels (mmol/L); (b) TC levels (mmol/L); (c) LDL levels (mmol/L); and (d) HDL levels (mmol/L). Data are presented as mean ± standard deviation (n = 20 in each group). **P < 0.01 vs. the control group; #P < 0.05, ##P < 0.01 vs. the HFS group. FOS, fructo-oligosaccharides; TG, triglycerides; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HFS, high-fat/high-sugar.
Effect of FOS on inflammation and oxidative stress
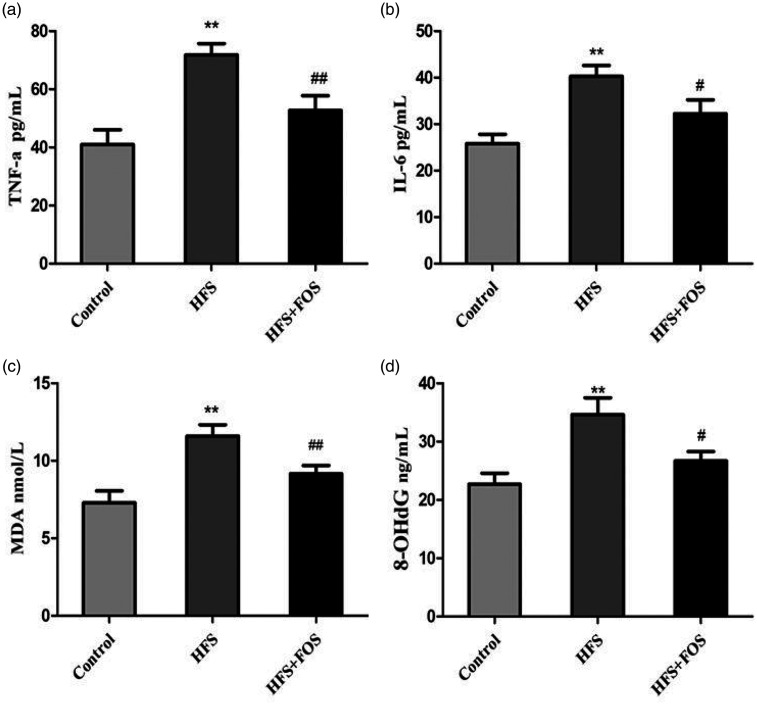
The ELISA assay also showed that serum IL-6, TNF-α, 8-OHdG, and MDA levels in mice in the HFS group were significantly higher than those in the control group (all P < 0.01). FOS significantly reduced these high levels of inflammatory cytokines and oxidative stress (all P < 0.01 vs. the HFS group, Figure 3a–d).
Figure 3.
FOS supplementation reduces serum levels of inflammatory cytokines and oxidative stress in HFS-fed mice. (a) TNF-α levels (ng/mL); (b) IL-6 levels (ng/mL); (c) MDA levels (mmol/L); and (d) 8-OHdG levels (ng/mL). Data are presented as mean ± standard deviation (n = 20 in each group). **P < 0.01 vs. the control group; #P < 0.05, ##P < 0.01 vs. the HFS group. FOS, fructo-oligosaccharides; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; MDA, malonaldehyde; HFS, high-fat/high-sugar.
Effect of FOS on gut and serum SCFA levels
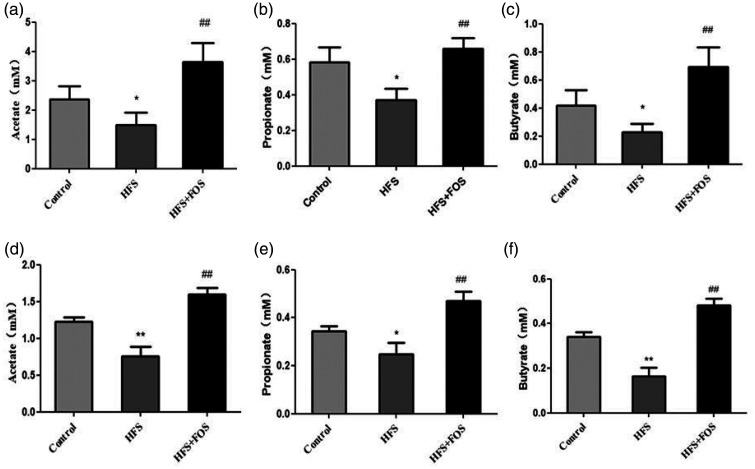
Mice in the HFS group had significantly lower intestinal (Figure 4a–c) and serum levels (Figure 4d–f) of acetate, propionate, and butyrate than did those in the control group (all P < 0.05). Mice in the HFS+FOS group had significantly higher intestinal and serum levels of acetate, propionate, and butyrate than did those in the HFS group (all P < 0.01).
Figure 4.
FOS supplementation increases intestinal and serum short-chain fatty acid (SCFA) levels in HFS-fed mice. (a) Intestinal acetate levels (mM); (b) intestinal propionate levels (mM); (c) intestinal butyrate levels (mM); (d) serum acetate levels (mM); (e) serum propionate levels (mM); and (f) serum butyrate levels (mM). Data are presented as mean ± standard deviation (n = 20 in each group). *P < 0.05, **P < 0.01 vs. the control group; ##P < 0.01 vs. the HFS group. FOS, fructo-oligosaccharides; HFS, high-fat/high-sugar.
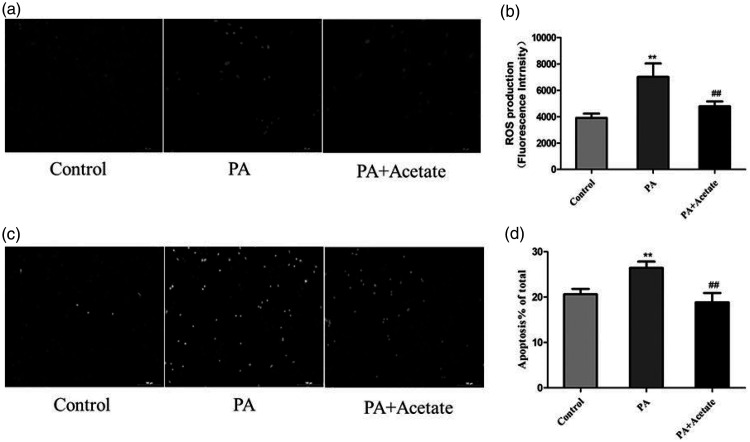
SCFAs inhibit ROS production and apoptosis in HepG2 cells
Stimulation of HepG2 cells with PA led to significantly higher ROS production compared with controls (P < 0.01), while 0.5 mM acetate (SCFA) significantly reduced the PA-induced increase in ROS levels in HepG2 cells (P < 0.01, Figure 5a–b). The number of TUNEL-positive apoptotic cells in the PA-treated group was significantly higher than that in the control group (P < 0.01). After treatment with acetate, the number of TUNEL-positive cells was significantly lower in PA-treated cells (P < 0.01, Figure 5c–d).
Figure 5.
SCFAs (acetate, 0.5 mM) inhibit palmitic acid (PA)-induced ROS production and apoptosis in HepG2 cells. (a) Representative fluorescence images of ROS. (b) Cellular ROS levels. (c) Representative images of apoptosis. (d) The rate of apoptosis. Data (b and d) are presented as mean ± standard deviation (n = 20). **P < 0.01 vs. the control group; ##P < 0.01 vs. the PA group. SCFAs, short chain fatty acids; PA, palmitic acid; ROS, reactive oxygen species.
Discussion
A significant effect of gut microbiota on the body’s weight and insulin resistance was recently shown in patients with type 2 diabetes.19 Supplementation with probiotics or prebiotics modulates the immune response and oxidative stress in patients with diabetes or mice with irritable bowel syndrome.5,20 In this study, we examined the effect of FOS supplementation on serum lipid levels and inflammation by HFS feeding in mice and found that SCFAs may have an important role in this process. This is the first study to demonstrate that the FOS diet can not only increase intestinal SCFAs, but also inhibit inflammation and reduce oxidative stress, eventually protecting the liver from apoptosis or lipid deposition and reducing systemic lipid levels.
The critical role of gastrointestinal microbiota in human health, including metabolic syndrome and inflammatory bowel disease, has attracted increasing attention.19 There is growing interest in using dietary approaches to modulate the composition and metabolic function of gut microbial communities, which eventually affect gastrointestinal health and disease prevention or treatment.19 Consumption of dietary fiber and prebiotics is the most commonly used strategy for modulating gut microbiota.19 The most complex carbohydrates and plant polysaccharides that are consumed in the diet are metabolized by microbes, which generate SCFAs, particularly acetate, propionate, and butyrate, and gases (H2 and CO2). SCFAs have been reported to greatly affect gastrointestinal epithelial integrity, glucose homeostasis, lipid metabolism, appetite regulation, and immune function.21 Because of differences in fiber and prebiotic consumption, individual responses to dietary intervention vary and are related to a combination of host genetics,22 adequate dosages of the dietary polysaccharide of interest,23 and the unique microbiota composition of the individual.24 In the current study, the HFS diet decreased intestinal concentrations of SCFAs, including acetate, propionate, and butyrate. However, mice supplemented with the FOS diet showed greater cecal concentrations of acetate, propionate, and butyrate compared with mice fed regular chow or the HFS diet. This finding indicated that FOS maintained homeostasis of intestinal SCFAs during exposure to HFS.
The anti-obesity effect of probiotic or prebiotic administration on metabolic syndrome has been evaluated in clinical studies and obese rats or diabetic mice.25,26 Decreased body weight gain, adipocyte size, adiposity, and insulin resistance were observed after prebiotic supplementation in several obese animal models.27,28 In a recent report, high-fat diet-induced accumulation of large adipocytes promoted peroxisome proliferator-activated receptor gamma-activated differentiation factors, which resulted in elevated G-protein-coupled receptor 43 expression in subcutaneous adipose tissue.29 However, prebiotic supplementation paradoxically counteracted G-protein-coupled receptor 43 overexpression in male C57BL/6J mice that were fed a high-fat diet.27 The role of prebiotic consumption on improving lipid metabolism and preventing cardiovascular disease has become a research hotspot.28 Synbiotic foods containing prebiotics can reduce serum TG and LDL levels and increase HDL levels.28 Our study showed that FOS reversed the high serum levels of TC, TG, and LDL and low HDL levels in HFS-fed mice. Additionally, serum ALT and AST levels in the HFS group were significantly higher than those in the control group and the FOS diet partially reversed this change. Histological changes in the liver showed that FOS inhibited fat deposition. The results of our study regarding the effect of FOS diet on liver function and lipid metabolism are consistent with those of previous research showing a close relationship between diets containing probiotics or prebiotics and development of NAFLD.30,31
We also evaluated the effect of FOS on inflammation in mice fed an HFS diet. We found that the FOS diet reduced high levels of inflammation and oxidative stress in mice fed the HFS diet. An HFS diet elevated levels of IL-6, TNF-α, 8-OHdG, and MDA, which were significantly reduced by FOS supplementation. A previous study showed that a prebiotic blend composed of FOS, galactooligosaccharide, inulin, and anthocyanins significantly decreased pro-inflammatory cytokines levels in infected Caco-2 cells and mice with irritable bowel syndrome. Loss of body weight, reduced expression of the tight junction protein occludin, and changes in the microbiota composition induced by infections are significantly improved by prebiotic blend intervention. These changes are associated with the peroxisome proliferator-activated receptor pathway.20
A previous study showed a close relationship between FOS and intestinal SCFA production in a stress-induced irritable bowel syndrome mouse model.20 In this previous study, FOS administration in mice subjected to water avoidance stress led to higher intestinal production of individual (acetic, propionic, and butyric acids), as well as total, SCFAs. To further examine whether FOS affected serum lipid and inflammation via SCFAs, we evaluated ROS production and apoptosis in HepG2 cells. In vitro experiments showed that SCFAs significantly inhibited ROS production and apoptosis of HepG2 cells. This finding suggested that FOS lowered serum lipid levels and suppressed apoptosis of liver cells by inhibiting oxidative stress and inflammation.
In summary, FOS supplementation protects against inflammation and decreases serum lipid levels in mice fed an HFS diet by decreasing ROS production and liver cell apoptosis. Therapeutic FOS supplementation may inhibit inflammation and lower serum lipid levels by regulating SCFAs.
Acknowledgement
We would like to thank Editage for providing English language editorial assistance.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by the Wuxi Municipal Science and Education Strengthening Health Engineering Medical Key Discipline Construction Program (ZDXK003) and Young Talent Project (QNRC039).
ORCID iDs
Renqiang Yu https://orcid.org/0000-0001-6529-0158
Qin Zhou https://orcid.org/0000-0002-0126-8741
Yingzi Mei https://orcid.org/0000-0002-8593-4114
References
- 1.Amani H, Habibey R, Shokri Fet al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep 2019; 9: 6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amani H, Mostafavi E, Alebouyeh MRet al. Would colloidal gold nanocarriers present an effective diagnosis or treatment for ischemic stroke? Int J Nanomedicine 2019; 14: 8013–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seksik P, Sokol H, Lepage Pet al. Review article: the role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment Pharmacol Ther 2010; 24: 11–18. [DOI] [PubMed] [Google Scholar]
- 4.Tucker LA, Thomas KS. Increasing total fiber intake reduces risk of weight and fat gains in women. J Nutr 2009; 139: 576–581. [DOI] [PubMed] [Google Scholar]
- 5.Consortium IA. Dietary fibre and incidence of type 2 diabetes in eight European countries: the EPIC-InterAct study and a meta-analysis of prospective studies. Diabetologia 2015; 58: 1394–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarpace PJ, Matheny M, Tümer Net al. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia 2005; 48: 1075–1083. [DOI] [PubMed] [Google Scholar]
- 7.Shane-Mcwhorter L. Dietary supplements and probiotics for diabetes. Am J Nurs 2012; 112: 47–53. [DOI] [PubMed] [Google Scholar]
- 8.Bekkering P, Jafri I, van Overveld FJet al. The intricate association between gut microbiota and development of type 1, type 2 and type 3 diabetes. Expert Rev Clin Immunol 2013; 9: 1031–1041. [DOI] [PubMed] [Google Scholar]
- 9.Stone-Dorshow T, Levitt MD. Gaseous response to ingestion of a poorly absorbed fructo-oligosaccharide sweetener. Am J Clin Nutr 1987; 46: 61–65. [DOI] [PubMed] [Google Scholar]
- 10.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001; 81: 1031–1064. [DOI] [PubMed] [Google Scholar]
- 11.Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr 2009; 63: 1277–1289. [DOI] [PubMed] [Google Scholar]
- 12.Gibson GR, Probert HM, Loo JVet al. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 2004; 17: 259–275. [DOI] [PubMed] [Google Scholar]
- 13.Roberfroid MB. Fructo-oligosaccharide malabsorption: benefit for gastrointestinal functions. Curr Opin Gastroenterol 2000; 16: 173–177. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Zhang K, Yang H. Pectin alleviates high fat (lard) diet-induced nonalcoholic fatty liver disease in mice: possible role of short-chain fatty acids and gut microbiota regulated by pectin. J Agric Food Chem 2018; 66: 8015–8025. [DOI] [PubMed] [Google Scholar]
- 15.Sawin EA, De Wolfe TJ, Aktas Bet al. Glycomacropeptide is a prebiotic that reduces Desulfovibrio bacteria, increases cecal short-chain fatty acids, and is anti-inflammatory in mice. Am J Physiol Gastrointest Liver Physiol 2015; 309: G590–G601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol 2006; 290: G852–G858. [DOI] [PubMed] [Google Scholar]
- 17.Joshi‐Barve S, Barve SS, Amancherla Ket al. Palmitic acid induces production of proinflammatory cytokine interleukin‐8 from hepatocytes. Hepatology 2007; 46: 823–830. [DOI] [PubMed] [Google Scholar]
- 18.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med 2003; 35: 9–16. [DOI] [PubMed] [Google Scholar]
- 19.Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017; 8: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Ren Y, Lu Jet al. A novel prebiotic blend product prevents irritable bowel syndrome in mice by improving gut microbiota and modulating immune response. Nutrients 2017; 9: E1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh A, De Vadder F, Kovatcheva-Datchary Pet al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016; 165: 1332–1345. [DOI] [PubMed] [Google Scholar]
- 22.Goodrich JK, Waters JL, Poole ACet al. Human genetics shape the gut microbiome. Cell 2014; 159: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis LM, Martínez I, Walter Jet al. Barcoded pyrosequencing reveals that consumption of galactooligosaccharides results in a highly specific bifidogenic response in humans. PLoS One 2011; 6: e25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker AW, Ince J, Duncan SHet al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 2011; 5: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cano PG, Santacruz A, Trejo FMet al. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high‐fat diet‐fed mice. Obesity (Silver Spring) 2013; 21: 2310–2321. [DOI] [PubMed] [Google Scholar]
- 26.Kadooka Y, Sato M, Imaizumi Ket al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 2010; 64: 636–643. [DOI] [PubMed] [Google Scholar]
- 27.Dewulf EM, Cani PD, Neyrinck AMet al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem 2011; 22: 712–722. [DOI] [PubMed] [Google Scholar]
- 28.Neyrinck AM, Possemiers S, Druart Cet al. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 2011; 6: e20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delzenne NM, Neyrinck AM, Cani PD. Gut microbiota and metabolic disorders: how prebiotic can work? Br J Nutr 2013; 109: S81–S85. [DOI] [PubMed] [Google Scholar]
- 30.Ganji SH, Kashyap ML, Kamanna VS. Niacin inhibits fat accumulation, oxidative stress, and inflammatory cytokine IL-8 in cultured hepatocytes: impact on non-alcoholic fatty liver disease. Metabolism 2015; 64: 982–990. [DOI] [PubMed] [Google Scholar]
- 31.Sharifov OF, Nayyar G, Ternovoy VVet al. Cationic peptide mR18L with lipid lowering properties inhibits LPS-induced systemic and liver inflammation in rats. Biochem Biophys Res Commun 2013; 436: 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]