Abstract
Objective
The present study aimed to examine the behavioral and dietary risk factors of recurrent urinary tract infection (RUTI) in postmenopausal patients in China.
Methods
We performed a population-based case–control study with 193 postmenopausal women with RUTI and 193 age-matched healthy female controls with no history of RUTI. The study was conducted between January 2016 and June 2018 in Changzhou, China. Data were collected using an interviewer-based questionnaire, including information on demographics, lifestyle behavior, and habitual diet. Conditional logistic regression analysis was conducted to examine the risk factors associated with RUTI.
Results
Wiping from back to front after toilet use, sedentary behavior >6 hours/day, delayed voiding, and chronic constipation were associated with an increased risk of RUTI. Drinking more than three cups of green tea per month showed an inverse association with RUTI. However, there was no evidence of dose dependency for overall consumption. Additionally, the three-cup association involved a small proportion of cases and may reflect statistical artifact.
Conclusions
Wiping from back to front after toilet use, sedentary behavior, delayed voiding, and chronic constipation are associated with an increased risk of RUTI in postmenopausal women.
Keywords: Postmenopause, recurrent urinary tract infection, risk factor, toileting habit, sedentary behavior, chronic constipation
Introduction
Urinary tract infection (UTI) is one of the most common bacterial infections that is globally encountered by women.1,2 Approximately 50% of women have reported at least one episode of UTI in their lifetime.3 A recent study indicated that, apart from lower respiratory tract infection, UTI accounts for 11.29% of infections, and is the second most frequent type of healthcare-associated infection in mainland China.4 Recurrent urinary tract infection (RUTI) is frequently defined as more than two episodes in the last 6 months or more than three episodes in the last 12 months.5 The prevalence of UTI increases with age, such that the prevalence rate increases to 8% to 10% in postmenopausal women, and of these, 5% of UTIs will recur within 1 year.6 Postmenopausal women are more likely to have anatomical and physiological risk factors, such as cystocele, incontinence, incomplete bladder emptying, and estrogen deficiency, predisposing them to RUTI, compared with premenopausal women.
Antibiotics can alleviate the symptoms of UTI, but cannot reduce the recurrence rate and improve the outcome. Moreover, antibiotic resistance has become an increasing concern, and it is attributed to the increased use of a post-coital or long-term, low-dose, daily antibiotic regimen administered as prophylaxis against RUTI.7 Recent studies have suggested that cranberry products, probiotics, the bacterial vaccine Uromune, D-mannose powder, and vitamin D have a role in preventing RUTI.8–13 However, the evidence for these non-antibiotic products are weak, and more studies on this issue are required.
RUTI leads to various negative effects on the quality of life and imposes a significant economic burden upon public healthcare.14–16 At present, China has a population of 1.34 billion, which is steadily increasing and rapidly aging at the same time.4 Therefore, RUTI is an important issue in the increasingly aging population in China. However, the risk factors associated with RUTI in postmenopausal women have not been widely described in China. Therefore, this study aimed to evaluate behavioral and dietary risk factors associated with RUTI in postmenopausal women, and to provide a basis of designing preventative strategies.
Material and methods
Study population
A total of 5867 postmenopausal women with suspected UTI who presented at the outpatient clinics of the Nephrology Department at Changzhou Affiliated Hospital of Nanjing University of Chinese Medicine between January 2016 and June 2018 were prospectively recruited. Individuals were included if they were women, menopausal (without menstruation for at least 12 months), and had a documented history of two or more culture-confirmed symptomatic episodes of uncomplicated UTI in the previous 6 months or three or more during the previous year. UTI was defined as major bacteriuria established by a urine culture ≥105 CFU/mL, and with the presence of at least two of the following clinical symptoms: frequency, urgency, and dysuria. Uncomplicated UTI was defined as normal anatomical function of the urinary system when UTI occurred, and there were no complications. Complicated UTI was defined as the presence of anatomical or functional abnormalities in the urinary system, such as urinary tract obstruction, an operation history of the urinary system, indwelling catheter, diabetes mellitus, and immune system diseases.
The exclusion criteria were patients aged >80 years, functional or anatomical abnormalities of the genitourinary tract, neurogenic bladder, interstitial cystitis, asymptomatic bacteriuria, an indwelling urinary catheter, hormone replacement therapy, diabetes mellitus, stroke, heart disease, respiratory disease, and tumors.
Controls were age-matched healthy postmenopausal women with no history of RUTI who attended the Hospital Healthy Examination Center at the same time interval as cases. Control subjects were subjected to urine culture and, if sterile, they were included in this study.
Urinalysis and clinical examination
Urinalysis and urine cultures were collected at enrollment in the study. All of the participants were routinely instructed on the proper collection technique for midstream clean-catch urine specimens, including cleansing of the external female genitalia two to three times with a prepackaged wipe and spreading the labia before voiding to reduce contamination of the specimen. Physical measurements, including body weight and height, were collected by standardized methods for anthropometrics. Body weight without heavy clothing to the nearest 0.05 kg was measured using a body fat and weight measurement device (GL-150P; G-Tech international Co. Ltd., Uijeongbu, Korea). Height was measured without shoes to the nearest 0.1 cm. Additionally, a thorough physical examination was performed by a clinician, and genitourinary ultrasonography was performed by an ultrasonologist using a color Doppler ultrasound diagnostic apparatus (Philips IU22; Philips, Amsterdam, Holland).
Questionnaire survey
Information on demographics, lifestyle behavior, and dietary behavior was collected face-to-face by trained interviewers using a self-made, structured questionnaire. Demographic data included age, body mass index (BMI), time since the last menstrual period, marital status, education levels, and number of deliveries. Information of lifestyle behavior during the previous year included smoking habits, sedentary behavior, wiping from front to back or back to front after toilet use, delayed voiding, and having a bath by standing or sitting. Bathing practice meant the women’s typical bathing behavior. Data on dietary behavior during the previous year were collected by using a validated food frequency questionnaire according to local dietary preference. The diet included fresh fruits, fresh vegetables, green tea, liquid, dairy products, soy bean products, spicy foods, and fatty foods.
Ethics statement
All participants fully understood the research contents and provided written informed consent for participation in the registry. This study was approved by the Ethics Committee of Changzhou Affiliated Hospital of Nanjing University of Chinese Medicine (Jiangsu, China).
Definitions
Smoking habit
Smoking habit was categorized into three groups of never, former (cessation of smoking, ≥6 months), and current (daily smoking, ≥6 months).
Delayed voiding and chronic constipation
Delayed voiding behavior was defined as behavior in which individuals inhibited the voiding urge and delayed voiding. Determination of delayed voiding behavior was achieved with the following question: “During the past year, have you delayed voiding when busy or in public?” Delayed voiding behavior was grouped into the three categories of never/rarely, sometimes, and always/often. Constipation was defined as infrequent defecation (less than three bowel movements per week), and/or the presence of frequent (more than twice a week) passage of hard stools and/or need to strain and/or sensation of incomplete evacuation. Participants were asked: “Have you had any of the following problems in the previous year?” Response options were listed as “never/rarely”, “sometimes”, or “frequent”.
Sedentary behavior
Self-reported sedentary behavior was measured using the following question: “During the past year, what was the average time per day you spent at the following types of activities: sitting, reclining, lying, watching TV, and spending time on the internet?” Sedentary behavior was classified into the following four groups: <2 hours/day, 2 to 4 hours/day, 4 to 6 hours/day, and >6 hours/day.
Dietary habits
The question “During the past year, did you eat fresh fruit?” pertaining to fruit intake included the following choices of answers: “never/rarely”, “1 to 2 times/week”, “3 to 4 times/week”, and “≥5 times/week”. The question pertaining to vegetable intake was “During the past year, did you eat fresh vegetables?” and the choice of answers included “<500 g/day” and “≥500 g/day”. Information on drinking green tea was obtained with the question “During the past year, did you consume green tea, and how often?” The choices of answers to this question were classified into the following five categories: never, 1 to 3 cups/month, 1 to 3 cups/week, 1 to 2 cups/day, and ≥3 cups/day. Liquid consumption was classified into the following four groups: ≤0.5 L/day, >0.5 L/day and ≤1.0 L/day, >1.0 L/day and ≤1.5 L/day, and >1.5 L/day. Liquid consumption included daily intake of green tea and other liquid consumption, such as water, coffee, and juice. Older people are accustomed to using “jin” (approximately 500 mL) as a common liquid measure of intake. We chose 0.5 L/day increments for liquids to make their choices more intuitive. The question pertaining to dairy products (e.g., milk, yogurt, and powdered milk) and soy bean products (e.g., soybean milk and tofu) was “During the past year, how often did you consume this food?” The participants were instructed to select from four consumption frequency categories (never/rarely, 1–2 times/week, 3–4 times/week, and ≥5 times/week). The question “How often did you eat this food?” was asked for eating spicy foods (e.g., hot peppers, dried chilies, pointed peppers, and chili sauce) and fatty foods (e.g., red meat, animal fats, and processed meat). The choices of answers included “never/rarely”, “1 to 2 times/month”, “3 to 4 times/month”, “2 to 3 times/week”, and “almost every day”.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Categorical variables are shown as number and percentage. Univariable conditional logistic regression models were initially used to estimate the associations of lifestyle behavior and dietary behavior on the risk of RUTI, with adjustment for BMI, time since the last menstrual period, marital status, education level, and number of deliveries. Associations were determined by calculating the odds ratios (ORs) and 95% confidence intervals (CIs). Variables with P ≤ 0.2 in this analysis were further subjected to multivariable conditional logistic regression models, and their associations with RUTI were estimated by additionally adjusting for smoking and liquid consumption. A two-sided P < 0.05 was considered significant.
Results
Characteristics
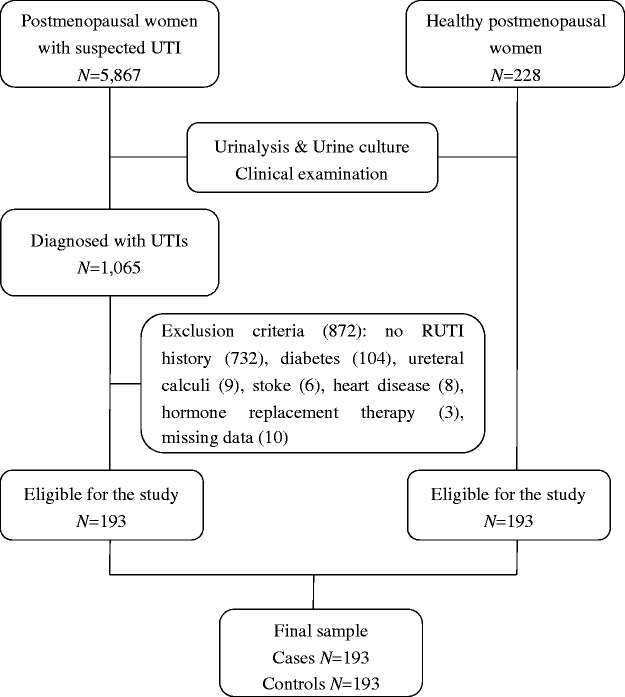
Of the 5867 female patients who were invited to participate in the study, 1065 were diagnosed as having UTIs. The remaining 4082 patients had negative urinalysis and urine culture results. They were diagnosed with lower urinary tract symptoms (e.g., overactive bladder, incomplete bladder emptying), neurogenic bladder, pelvic organ prolapse, and pelvic infection. Among the 1065 (18.15%) patients with UTI, 862 were not eligible for the study because they had no recurrent history of UTIs (n = 732), diabetes (n = 104), ureteral calculi (n = 9), stroke (n = 6), heart disease (n = 8), and hormone replacement therapy (n = 3). We also excluded 10 individuals because of missing data of height (n = 2), weight (n = 4), and the time since the last menstrual period (n = 4). Over the 30-month study period, 193 cases and 193 controls were included in the final analysis (Figure 1).
Figure 1.
Flowchart of the study population.
More than 60% of the participants were >60 years old and 75.39% did not have menstruation for >5 years. The mean age of cases was 61.61 ± 9.57 years and that in the controls was 62.93 ± 9.17 years, with no significant difference in age between cases and controls. Characteristics of the participants are shown in Table 1. There were no significant differences in age, marital status, time since the last menstrual period, education level, number of deliveries, and smoking habits between cases and controls. However, mean BMI was significantly higher in cases than in controls (26.64 ± 3.02 vs. 24.96 ± 3.26 kg/m2, P = 0.02) (Table 1).
Table 1.
Characteristics of cases and controls.
| Characteristics | Controls(n = 193) | Cases(n = 193) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age (years) | ||||
| 40–49 | 19 (9.84) | 12 (6.22) | Matched | |
| 50–59 | 57 (29.53) | 63 (32.64) | ||
| 60–69 | 68 (35.23) | 65 (33.68) | ||
| 70–80 | 49 (25.39) | 53 (27.46) | ||
| BMI | ||||
| <25 kg/m2 | 95 (49.22) | 68 (35.23) | Ref | 0.020 |
| ≥25 and <30 kg/m2 | 83 (43.01) | 104 (53.89) | 1.78 (1.15–2.74) | |
| ≥30 kg/m2 | 15 (7.77) | 21 (10.88) | 2.15 (1.00–4.65) | |
| Time since the last menstrual period (years) | ||||
| 1–5 | 52 (26.94) | 43 (22.28) | Ref | 0.475 |
| 6–10 | 44 (22.80) | 50 (25.91) | 1.44 (0.78–2.66) | |
| >10 | 97 (50.26) | 100 (51.81) | 1.32 (0.76–2.27) | |
| Marital status | ||||
| Single | 6 (3.11) | 5 (2.59) | Ref | 0.937 |
| Married | 146 (75.65) | 151 (78.24) | 1.24 (0.38–4.10) | |
| Divorced/separated | 25 (12.95) | 23 (11.92) | 1.09 (0.29–4.15) | |
| Widowed | 16 (8.29) | 14 (7.25) | 1.04 (0.27–3.93) | |
| Education level | ||||
| Illiteracy | 32 (16.58) | 40 (20.73) | Ref | 0.268 |
| Primary school | 89 (46.11) | 97 (50.26) | 0.86 (0.49–1.50) | |
| Middle school | 55 (28.50) | 37 (19.17) | 0.55 (0.29–1.03) | |
| High school | 13 (6.74) | 12 (6.22) | 0.72 (0.29–1.77) | |
| College and above | 4 (2.07) | 7 (3.63) | 1.35 (0.36–5.10) | |
| Number of deliveries | ||||
| 0–2 | 146 (75.65) | 138 (71.50) | Ref | 0.671 |
| 3–5 | 35 (18.13) | 41 (21.24) | 1.22 (0.75–1.98) | |
| ≥6 | 12 (6.22) | 14 (7.25) | 1.23 (0.56–2.69) |
Values are shown as n (%). OR, odds ratio; CI, confidence interval; BMI, body mass index; Ref, reference.
Clinical symptoms and distribution of organisms
In this study, the predominant clinical presentations in postmenopausal patients were frequency, urgency, painful voiding, and difficulty in emptying the bladder. Other generalized unspecific symptoms, such as lower abdominal pain, low back pain, and genital discomfort, were also observed. Among postmenopausal patients with a total of 193 pathogens, 162 Gram-negative bacteria, 27 Gram-positive bacteria, and four fungi were isolated. Among these women, the proportion of Escherichia coli was the largest (61.66%), followed by Klebsiella pneumoniae (15.03%) and Proteus mirabilis (7.25%) (Table 2).
Table 2.
Pathogens detected in patients with recurrent urinary tract infection.
| Microorganism | Number of patients (%) |
|---|---|
| Gram-negative | |
| Escherichia coli | 119 (61.66) |
| Klebsiella pneumoniae | 29 (15.03) |
| Proteus mirabilis | 14 (7.25) |
| Gram-positive | |
| Streptococcus agalactiae | 12 (6.22) |
| Enterococcus faecalis | 9 (4.66) |
| Staphylococcus epidermidis | 6 (3.11) |
| Fungus | |
| Candida albicans | 4 (2.07) |
Lifestyle and dietary behavior
There was a small number of current and former smokers (3.37% of the total women), with no significant difference between cases and controls (Table 3).
Table 3.
Comparison of lifestyle behavior and chronic constipation between cases and controls.
| Variables | Controls n (%) | Cases n (%) | OR (95% CI) | Adjusted ORa (95% CI) | P value |
|---|---|---|---|---|---|
| Smoking | |||||
| Never | 186 (96.37) | 187 (96.89) | Ref | Ref | 0.780 |
| Former | 5 (2.59) | 4 (2.07) | 0.80 (0.22–2.98) | 0.68 (0.16–2.84) | |
| Current | 2 (1.04) | 2 (1.04) | 1.00 (0.14–7.10) | 1.72 (0.18–16.06) | |
| Wiping after toilet use | |||||
| Front to back | 155 (80.31) | 109 (56.48) | Ref | Ref | <0.001 |
| Back to front | 38 (19.69) | 84 (43.52) | 3.71 (2.17–6.33) | 4.35 (2.38–7.94) | |
| Sedentary behavior | |||||
| <2 hours/day | 142 (73.58) | 112 (58.03) | Ref | Ref | <0.001 |
| 2–4 hours/day | 36 (18.65) | 37 (19.17) | 1.39 (0.80–2.41) | 1.20 (0.66–2.16) | |
| 4–6 hours/day | 9 (4.66) | 30 (15.54) | 4.02 (1.81–8.92) | 4.67 (2.01–10.87) | |
| >6 hours/day | 6 (3.11) | 14 (7.25) | 2.90 (1.07–7.87) | 2.90 (1.01–8.36) | |
| Delayed voiding | |||||
| Never/rarely | 158 (81.87) | 77 (39.90) | Ref | Ref | <0.001 |
| Sometimes | 25 (12.95) | 70 (36.27) | 5.91 (3.07–11.40) | 5.63 (2.83–11.20) | |
| Always/often | 10 (5.18) | 46 (23.83) | 7.18 (3.25–15.88) | 7.78 (3.34–18.12) | |
| Chronic constipation | |||||
| Never/rarely | 103 (53.37) | 33 (17.10) | Ref | Ref | <0.001 |
| Sometimes | 56 (29.02) | 82 (42.49) | 4.61 (2.55–8.33) | 4.47 (2.39–8.36) | |
| Frequent | 34 (17.62) | 78 (40.41) | 7.56 (3.91–14.63) | 8.98 (4.32–18.63) | |
| Having a bath | |||||
| By standing | 154 (79.79) | 161 (83.42) | Ref | Ref | |
| By sitting | 39 (20.21) | 32 (16.58) | 0.78 (0.46–1.32) | 0.86 (0.49–1.50) | 0.591 |
OR: adjusted for body mass index, time since the last menstrual period, marital status, education level, and number of deliveries. OR, odds ratio; CI, confidence interval; Ref, reference.
In univariable conditional logistic regression analysis, wiping from back to front after toilet use was a risk factor for RUTI (adjusted OR = 4.35, 95% CI = 2.38–7.94, P < 0.001). A significantly higher proportion of cases reported sedentary behavior ≥2 hours/day than did controls (41.97% vs. 26.42%, P < 0.001). A significantly higher rate of cases was more likely to delay voiding compared with controls (60.10% vs. 18.13%, P < 0.001). Chronic constipation was observed significantly more frequently in cases than in controls (82.90% vs. 46.63%, P < 0.001). The proportions of having a bath by standing or sitting were not statistically significant between cases and controls.
No significant differences in the consumption of fruits, vegetables, liquid, dairy products, soy bean products, spicy foods, and fatty foods were observed between cases and controls (Table 4). At baseline, 21.76% of controls reported consuming ≥1 cup/day of green tea and 88.08% of cases reported never/rarely consuming green tea. Because of the infrequency of green tea consumption of more than one to three cups per month in cases, we consolidated the three groups (1–3 cups/week, 1–2 cups/day, and ≥3 cups/day) of green tea drinking. Green tea drinking of >3 cups/month was observed more frequently in controls than in cases (29.53% vs 3.62%, P < 0.001).
Table 4.
Comparison of dietary behavior between cases and controls.
| Variables | Controls n (%) | Casesn (%) | OR (95% CI) | Adjusted ORa (95% CI) | P value |
|---|---|---|---|---|---|
| Fresh fruits | |||||
| Never/rarely | 35 (18.13) | 30 (15.54) | Ref | Ref | 0.535 |
| 1–2 times/week | 29 (15.03) | 25 (12.95) | 0.99 (0.48–2.06) | 0.74 (0.33–1.66) | |
| 3–4 times/week | 114 (59.07) | 118 (61.14) | 1.20 (0.69–2.07) | 1.09 (0.60–1.98) | |
| ≥5 times/week | 15 (7.77) | 20 (10.36) | 1.54 (0.68–3.46) | 1.48 (0.61–3.59) | |
| Fresh vegetables | |||||
| <500 g/day | 52 (26.94) | 60 (31.09) | Ref | Ref | 0.446 |
| ≥500 g/day | 141 (73.06) | 133 (68.91) | 0.80 (0.50–1.27) | 0.82 (0.50–1.36) | |
| Green tea drinking | |||||
| Never | 129 (66.84) | 170 (88.08) | Ref | Ref | <0.001 |
| 1–3 cups/month | 7 (3.63) | 16 (8.29) | 1.38 (0.53–3.62) | 1.18 (0.40–3.46) | |
| >3 cups/month | 57 (29.53) | 7 (3.62) | 0.06 (0.02–0.19) | 0.05 (0.01–0.16) | |
| Liquid consumption | |||||
| ≤0.5 L/day | 44 (22.80) | 47 (24.35) | Ref | Ref | 0.815 |
| >0.5 and ≤1.0 L/day | 51 (26.42) | 56 (29.02) | 1.00 (0.58–1.71) | 0.93 (0.52–1.64) | |
| >1.0 and ≤1.5 L/day | 72 (37.31) | 68 (35.23) | 0.88 (0.52–1.50) | 0.83 (0.46–1.49) | |
| >1.5 L/day | 26 (13.47) | 22 (11.40) | 0.79 (0.38–1.63) | 0.71 (0.33–1.53) | |
| Dairy products | |||||
| Never/rarely | 33 (17.10) | 29 (15.03) | Ref | Ref | 0.960 |
| 1–2 times/week | 37 (19.17) | 43 (22.28) | 1.35 (0.68–2.71) | 1.16 (0.55–2.42) | |
| 3–4 times/week | 66 (34.20) | 70 (36.27) | 1.21 (0.67–2.19) | 1.03 (0.54–1.95) | |
| ≥5 times/week | 57 (29.53) | 51 (26.42) | 1.03 (0.55–1.92) | 0.98 (0.50–1.90) | |
| Soy bean products | |||||
| Never/rarely | 23 (11.92) | 22 (11.40) | Ref | Ref | 0.954 |
| 1–2 times/week | 76 (39.38) | 79 (40.93) | 1.08 (0.54–2.17) | 1.01 (0.47–2.17) | |
| 3–4 times/week | 54 (27.98) | 55 (28.50) | 1.07 (0.51–2.24) | 0.98 (0.43–2.21) | |
| ≥5 times/week | 40 (20.73) | 37 (19.17) | 0.97 (0.44–2.13) | 0.86 (0.36–2.01) | |
| Spicy foods | |||||
| Never/rarely | 98 (50.78) | 101 (52.33) | Ref | Ref | 0.964 |
| 1–2 times/month | 26 (13.47) | 23 (11.92) | 0.86 (0.46–1.61) | 0.87 (0.45–1.72) | |
| 3–4 times/month | 37 (19.17) | 41 (21.24) | 1.06 (0.62–1.80) | 0.93 (0.52–1.67) | |
| 2–3 times/week | 21 (10.88) | 19 (9.84) | 0.87 (0.45–1.68) | 0.96 (0.46–2.03) | |
| Almost every day | 11 (5.70) | 9 (4.66) | 0.76 (0.29–2.00) | 0.68 (0.24–1.95) | |
| Fatty foods | |||||
| Never/rarely | 133 (68.91) | 126 (65.28) | Ref | Ref | 0.763 |
| 1–2 times/month | 14 (7.25) | 21 (10.88) | 1.50 (0.76–2.96) | 1.59 (0.75–3.35) | |
| 3–4 times/month | 20 (10.36) | 22 (11.40) | 1.11 (0.60–2.08) | 1.09 (0.55–2.13) | |
| 2–3 times/week | 19 (9.84) | 17 (8.81) | 0.95 (0.48–1.85) | 0.89 (0.43–1.83) | |
| Almost every day | 7 (3.63) | 7 (3.63) | 1.02 (0.35–2.96) | 0.80 (0.25–2.54) |
OR: adjusted for body mass index, time since the last menstrual period, marital status, education level, and number of deliveries. OR, odds ratio; CI, confidence interval; Ref, reference.
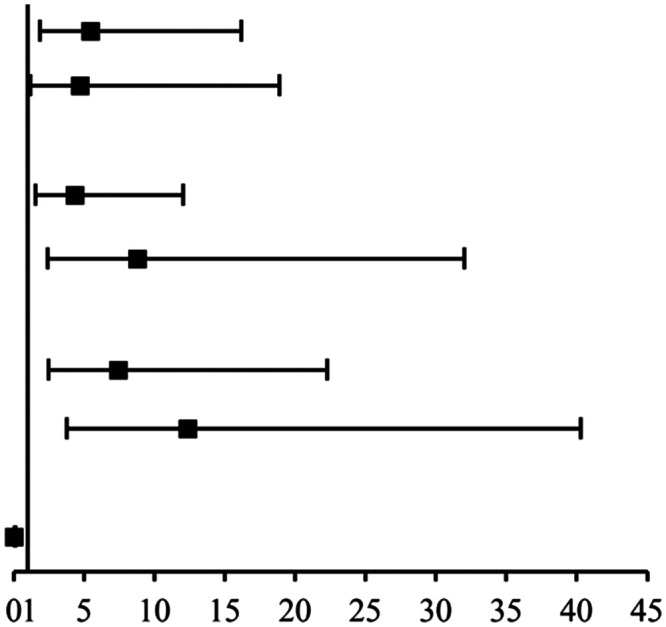
Multivariable conditional logistic regression analysis was adjusted for BMI, the time since the last menstrual period, marital status, education level, number of deliveries, smoking, and liquid consumption. The significant independent risk factors for RUTI were wiping from back to front after toilet use (P < 0.001), sedentary behavior >6 hours/day (P < 0.05), delayed voiding (P < 0.001), and chronic constipation (P < 0.001). In contrast, drinking >3 cups/month of green tea was associated with a decreased risk of RUTI (P < 0.001) (Table 5).
Table 5.
Attributable risk factors associated with recurrent urinary tract infection in multivariable analysis.
| Variables | OR (95% CI) | Adjusted ORa (95% CI) | P value | |
|---|---|---|---|---|
| Wiping from back to front | 3.86 (1.68–8.83) | 5.46 (1.84–16.20) |
|
<0.001 |
| Sedentary >6 hours/day | 4.59 (1.03–20.34) | 4.68 (1.16–18.89) | <0.05 | |
| Delayed voiding | ||||
| Sometimes | 4.85 (2.01–11.69) | 4.35 (1.57–12.04) | <0.001 | |
| Always/often | 7.22 (2.29–22.75) | 8.78 (2.41–32.00) | <0.001 | |
| Chronic constipation | ||||
| Sometimes | 4.91 (2.14–11.26) | 7.43 (2.48–22.26) | <0.001 | |
| Frequent | 6.88 (2.78–17.04) | 12.36 (3.80–40.26) | <0.001 | |
| Drinking green tea | ||||
| >3 cups/month | 0.06 (0.01–0.28) | 0.02 (0.01–0.15) | <0.001 |
OR: adjusted for body mass index, time since the last menstrual period, marital status, education level, number of deliveries, smoking, and liquid consumption. OR, odds ratio; CI, confidence interval. In the chart, symbols represent ORs and bars represent the 95% CIs.
Discussion
Postmenopausal women are biologically, physiologically, and behaviorally exposed to an increased risk of developing UTI. UTI is a common genitourinary tract complaint in postmenopausal women with a high rate of recurrence after menopause.17,18 The prevalence of RUTI significantly increases with age. UTI is often overdiagnosed in postmenopausal women, and a positive urine culture often leads to initiation of antibiotic treatment, regardless of the presence or absence of symptoms referable to infection.19 Asymptomatic bacteriuria (ASB) is common in postmenopausal women, without symptoms or signs of UTI. Treatment of asymptomatic bacteriuria is usually futile, and it increases the risk of subsequent symptomatic UTI episodes, and is thus not recommended for this patient group.20 The criteria used to diagnose symptomatic UTI or ASB are problematic. A quantitative count of ≥105 CFU/mL is appropriate for symptomatic UTI and ASB.19
In our study, the majority of patients had their RUTI caused by E. coli. E. coli is believed to cause 80% to 90% of all UTIs, and it ascends the urethra to the bladder causing cystitis, or even up the ureters into the kidneys, leading to pyelonephritis.21,22 Other pathogens that we identified were Klebsiella pneumoniae (15.03%), Proteus mirabilis (7.25%), Streptococcus agalactiae (6.22%), Enterococcus faecalis (4.66%), Staphylococcus epidermidis (3.11%), and Candida albicans (2.07%). These results are similar to those of other studies conducted in China.23 Treatment of E. coli with antibiotics is often ineffective because of acquisition of drug-resistant genes by the bacteria. Li et al.8 reported that more than 45% of E. coli isolated from UTIs were resistant to wide-spectrum antibiotics. Chen et al.24 demonstrated that E. coli strains harboring class 1 integron showed high resistance towards tobramycin.
The incidence of UTI might be higher in patients with diabetes because of multiple mechanisms, such as genetic susceptibility and a damaged immune response.25 In our study, patients with diabetes were excluded because their eating behavior and dietary patterns are influenced by this disease. These patients often consume a low-carbohydrate, low-fat diet for achieving tight control over blood glucose levels.
Estrogen deficiency in postmenopausal women has a strong association with RUTI.1,26 Lüthje et al.27 found that estrogen induced expression of antimicrobial peptides and promoted expression and redistribution of cell–cell contact-associated proteins. Postmenopausal women are more predisposed to RUTI because of their decreased production of antimicrobial peptides and diminished integrity of the urothelial lining. The available evidence suggests that estrogen is effective in reducing UTI episodes in some patients as a prophylactic therapy;27,28 however, this evidence is controversial.29 In contrast, supplementation with estrogen significantly increased the susceptibility of surgically menopausal mice to ascending UTI.30 In our study, the proportion of postmenopausal patients with RUTI who received estrogen therapy was limited. Generally, prescriptions for hormone replacement therapy for Chinese women are lagging behind those for Caucasian women in the postmenopausal period.
We observed that wiping from back to front after toilet use was associated with an increased risk of RUTI. Some studies have shown that wiping habits from back to front after a bowel movement or urination are associated with an increased risk of UTI.31,32 In women, the urethra is close to the genitalia and anus. The fecal microbiota is a primary source of E. coli causing UTIs via the fecal–perineal–urethral route. Whole-genome phylogenetic analysis of bacterial strains that were isolated from the same female vagina and bladder showed highly similar E. coli, suggesting an interlinked female genitourinary tract microbiota.33 Therefore, improving hygiene behavior after using the toilet is thought to reduce the risk of contaminating the urethra with E. coli.
In our study, the mean BMI of cases was significantly higher than that in controls. A significantly higher proportion of cases reported a sedentary behavior ≥2 hours/day than did controls. Sedentary behavior is associated with a greater increase in BMI in postmenopausal women.34 A recent cross-sectional study showed that obesity (BMI ≥30 kg/m2) was a risk factor for UTI, and the incidence of UTI was correlated with increasing degrees of BMI.35 In multivariable conditional regression analysis, sedentary behavior >6 hours/day was found to be associated with RUTI. We speculate that sedentary behavior might have a cause and effect relationship with elevated BMI and delayed voiding behavior.
We identified the toileting behavior of delayed voiding as a risk factor for RUTI, which is consistent with a previous study.36 Not emptying the bladder frequently enough can cause bacteria to accumulate inside the urinary tract. Furthermore, delayed voiding combined with the Valsalva maneuver can aggravate the bladder neck’s descent in women, leading to lower urinary tract symptoms.37 Emptying the bladder regularly helps flush away any bacteria.
In our study, the rate of chronic constipation in cases was significantly higher than that in controls. Retrospective and prospective studies in children and adults have suggested that constipation plays an etiological role in UTI.38,39 Compression of the bladder by the rectum or sigmoid colon loaded with feces may cause stimulation of stretch receptors. This results in uninhibited bladder contractions, which are common to vesico-ureteral reflux, RUTI, and enuresis.40 Clinicians should consider the use of stool softeners or a soluble fiber because of the high incidence of constipation and association with RUTI.
Interestingly, we found that drinking green tea was observed less frequently in cases than in controls. Green tea is derived from the leaves of Camellia sineneis, and has been consumed with a history of thousands of years in China. Green tea has a high quantity of tea polyphenols, and has an inhibitory effect on the growth of E. coli strains and a synergistic effect with antibiotics in in vitro experimental studies.41–44 We found that drinking >3 cups/month of green tea decreased the risk of RUTI. Notably, green tea was associated with a decreased risk of RUTI, but not fresh fruits or vegetables, which also contain plant polyphenols. An example of this situation is that strawberry and lemon extracts show an inhibition zone against bacteria causing UTI.45 Additionally, different green teas have varying polyphenol content and brewing conditions are known to affect phenolic content.46 These various factors of green tea need to be further studied.
The present study has several limitations. First, this was a single-center study and the sample size was small. Second, sexual intercourse was not queried. However, a previous study suggested that behavioral risk factors, such as sexual activity and contraceptive use, do not play a large role in RUTI after menopause as in premenopausal women.47 Third, internet use may not have been sedentary if people were accessing the internet on their mobile devices, which is frequently performed while standing or ambulatory. Fourth, with regard to the query of wiping practices after toilet use, only two modes of wiping were queried and the approach of dabbing was assumed to fall into the front to back wiping. Fifth, the food frequency questionnaire is not a broadly comprehensive reflection of diet because it does not include snack foods, carbohydrates, seafood, poultry, or alcohol. Additionally, although drinking more than three cups of green tea per month showed an inverse association with case status, there was no evidence of green tea dose-dependency for overall consumption. Furthermore, the three-cup association involved a small proportion of cases and may reflect statistical artifact. Sixth, many genetic associations and comorbid host factors that are thought to be risk factors for RUTI were not included in the survey. Liu et al.48 showed that the Ala19Val polymorphism of the SP-A1 gene and Lys223Gln polymorphism of the SP-A2 gene may be genetic factors that affect susceptibility of RUTI in Chinese women. The final limitation is recall bias in this case–control study, which is especially common in self-reporting and retrospective cohort studies.
Conclusion
Our study shows that postmenopausal women with chronic constipation and poor hygiene behavior or urination habits are at high risk of developing RUTI. Therefore, postmenopausal women with RUTI should undergo a comprehensive assessment to identify possible risk factors to reduce the incidence of RUTI. The inverse association of drinking more than three cups of green tea per month with RUTI could have been affected by the small proportion of cases.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Meifeng Zhu https://orcid.org/0000-0003-1760-054X
References
- 1.Aydin A, Ahmed K, Zaman I, et al. Recurrent urinary tract infections in women. Int Urogynecol J 2015; 26: 795–804. [DOI] [PubMed] [Google Scholar]
- 2.Waller TA, Pantin SAL, Yenior AL, et al. Urinary tract infection antibiotic resistance in the United States. Prim Care 2018; 45: 455–466. [DOI] [PubMed] [Google Scholar]
- 3.Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis 2000; 30: 152–156. [DOI] [PubMed] [Google Scholar]
- 4.Wang JC, Liu FF, Tartari E, et al. The prevalence of healthcare-associated infections in mainland China: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2018; 39: 701–709. [DOI] [PubMed] [Google Scholar]
- 5.Malik RD, Wu YF, Zimmern PE. Definition of recurrent urinary tract infections in women: which one to adopt? Female Pelvic Med Reconstr Surg 2018; 24: 424–429. [DOI] [PubMed] [Google Scholar]
- 6.Foxman B, Barlow R, D’Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 2000; 10: 509–515. [DOI] [PubMed] [Google Scholar]
- 7.Li XY, Chen YQ, Gao WG, et al. A 6-year study of complicated urinary tract infections in southern China: prevalence, antibiotic resistance, clinical and economic outcomes. Ther Clin Risk Manag 2017; 13: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luís Â, Domingues F, Pereira L. Can cranberries contribute to reduce the incidence of urinary tract infections? A systematic review with meta-analysis and trial sequential analysis of clinical trials. J Urol 2017; 198: 614–621. [DOI] [PubMed] [Google Scholar]
- 9.Nicolle LE. Cranberry for prevention of urinary tract infection? Time to move on. JAMA 2016; 316: 1873–1874. [DOI] [PubMed] [Google Scholar]
- 10.Ng QX, Peters C, Venkatanarayanan N, et al. Use of Lactobacillus spp. to prevent recurrent urinary tract infections in females. Med Hypotheses 2018; 114: 49–54. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Foley S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int 2018; 121: 289–292. [DOI] [PubMed] [Google Scholar]
- 12.Kranjčec B, Papeš D, Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: a randomized clinical trial. World J Urol 2014; 32: 79–84. [DOI] [PubMed] [Google Scholar]
- 13.Deng QF, Chu H, Wen Z, et al. Vitamin D and urinary tract infection: a systematic review and meta-analysis. Ann Clin Lab Sci 2019; 49: 134–142. [PubMed] [Google Scholar]
- 14.Flower A, Bishop FL, Lewith G. How women manage recurrent urinary tract infections: an analysis of postings on a popular web forum. BMC Fam Pract 2014; 15: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson I, Gustafson Y, Fagerström L, et al. Do urinary tract infections affect morale among very old women? Health Qual Life Outcomes 2010; 8: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagenlehner F, Wullt B, Ballarini S, et al. Social and economic burden of recurrent urinary tract infections and quality of life: a patient web-based study (GESPRIT). Expert Rev Pharmacoecon Outcomes Res 2018; 18: 107–117. [DOI] [PubMed] [Google Scholar]
- 17.Minardi D, d’Anzeo G, Cantoro D, et al. Urinary tract infections in women: etiology and treatment options. Int J Gen Med 2011; 4: 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman JA, Schreiber HL, Hooton TM, et al. From physiology to pharmacy: developments in the pathogenesis and treatment of recurrent urinary tract infections. Curr Urol Rep 2013; 14: 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicolle L. Symptomatic urinary tract infection or asymptomatic bacteriuria? Improving care for the elderly. Clin Microbiol Infect 2019; 25: 779–781. [DOI] [PubMed] [Google Scholar]
- 20.Storme O, Tirán Saucedo J, Garcia-Mora A, et al. Risk factors and predisposing conditions for urinary tract infection. Ther Adv Urol 2019; 11: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol 2010; 64: 203–221. [DOI] [PubMed] [Google Scholar]
- 22.Ejrnæs K, Stegger M, Reisner A, et al. Characteristics of Escherichia coli causing persistence or relapse of urinary tract infections: phylogenetic groups, virulence factors and biofilm formation. Virulence 2011; 2: 528–537. [DOI] [PubMed] [Google Scholar]
- 23.Qiao LD, Chen S, Yang Y, et al. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open 2013; 3: e004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen MX, Wu YQ, Yu SL, et al. Drug resistance and integron genes in Escherichia coli isolated from urinary tract infection. J Nanosci Nanotechnol 2019; 19: 5989–5993. [DOI] [PubMed] [Google Scholar]
- 25.Nitzan O, Elias M, Chazan B, et al. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes 2015; 8: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev 2008; 2: CD005131. [DOI] [PubMed] [Google Scholar]
- 27.Lüthje P, Brauner H, Ramos NL, et al. Estrogen supports urothelial defense mechanisms. Sci Transl Med 2013; 5: 190ra80. [DOI] [PubMed] [Google Scholar]
- 28.Schiavi MC, Pinto AD, Sciuga V, et al. Prevention of recurrent lower urinary tract infections in postmenopausal women with genitourinary syndrome: outcome after 6 months of treatment with ospemifene. Gynecol Endocrinol 2017; 34: 1–4. [DOI] [PubMed] [Google Scholar]
- 29.Dueñas-Garcia OF, Sullivan G, Hall CD, et al. Pharmacological agents to decrease new episodes of recurrent lower urinary tract infections in postmenopausal women. A systematic review. Female Pelvic Med Reconstr Surg 2016; 22: 63–69. [DOI] [PubMed] [Google Scholar]
- 30.Curran EM, Tassell AH, Judy BM, et al. Estrogen increases menopausal host susceptibility to experimental ascending urinary-tract infection. J Infect Dis 2007; 195: 680–683. [DOI] [PubMed] [Google Scholar]
- 31.Epp A, Larochelle A. Urogynaecology Committee; Family Physicians Advisory Committee. Recurrent urinary tract infection. J Obstet Gynaecol Can 2010; 32: 1082–1090. [DOI] [PubMed] [Google Scholar]
- 32.Persad S, Watermeyer S, Griffiths A, et al. Association between urinary tract infection and postmicturition wiping habit. Acta Obstet Gynecol Scand 2006; 85: 1395–1396. [DOI] [PubMed] [Google Scholar]
- 33.Thomas-White K, Forster SC, Kumar N, et al. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat Commun 2018; 9: 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hodson L, Harnden K, Banerjee R, et al. Lower resting and total energy expenditure in postmenopausal compared with premenopausal women matched for abdominal obesity. J Nutr Sci 2014; 3: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semins MJ, Shore AD, Makary MA, et al. The impact of obesity on urinary tract infection risk. Urology 2012; 79: 266–269. [DOI] [PubMed] [Google Scholar]
- 36.Foxman B, Frerichs RR. Epidemiology of urinary tract infection: II. Diet, clothing, and urination habits. Am J Public Health 1985; 75: 1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan XJ, Wu C, Xu DJ, et al. Toileting behaviours and lower urinary tract symptoms among female nurses: a cross-sectional questionnaire survey. Int J Nurs Stud 2017; 65: 1–7. [DOI] [PubMed] [Google Scholar]
- 38.Shaikh N, Hoberman A, Keren R, et al. Recurrent urinary tract infections in children with bladder and bowel dysfunction. Pediatrics 2016; 137: e20152982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter D, Beer-Gabel M. Lower urinary tract symptoms in chronically constipated women. Int Urogynecol J 2012; 23: 1785–1789. [DOI] [PubMed] [Google Scholar]
- 40.O'Regan S, Yazbeck S, Schick E. Constipation, bladder instability, urinary tract infection syndrome. Clin Nephrol 1985; 23: 152–154. [PubMed] [Google Scholar]
- 41.Reygaert W, Jusufi I. Green tea as an effective antimicrobial for urinary tract infections caused by Escherichia coli. Front Microbiol 2013; 4: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neyestani TR, Khalaji N, Gharavi A. Selective microbiologic effects of tea extract on certain antibiotics against Escherichia coli in vitro. J Altern Complement Med 2007; 13: 1119–1124. [DOI] [PubMed] [Google Scholar]
- 43.Noormandi A, Dabaghzadeh F. Effects of green tea on Escherichia coli as a uropathogen. J Tradit Complement Med 2015; 5: 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng QX. To investigate the antimicrobial effects of extracted polyphenols from green tea (Camellia sinensis) and banana (Musa sp.) leaves. PeerJ PrePrints 2015; 3: e1580v1. [Google Scholar]
- 45.Liya SJ, Siddique R. Determination of antimicrobial activity of some commercial fruit (apple, papaya, lemon and strawberry) against bacteria causing urinary tract infection. Eur J Microbiol Immunol 2018; 8: 95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liebert M, Licht U, Böhm V, et al. Antioxidant properties and total phenolics content of green and black tea under different brewing conditions. Z Lebensm Unters Forsch 1999; 208: 217–220. [Google Scholar]
- 47.Hannan TJ, Hooton TM, Hultgren SJ. Estrogen and recurrent UTI: what are the facts? Sci Transl Med 2013; 5: 190fs23. [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Hu F, Liang W, et al. Polymorphisms in the surfactant protein a gene are associated with the susceptibility to recurrent urinary tract infection in Chinese women. Tohoku J Exp Med 2010; 221: 35–42. [DOI] [PubMed] [Google Scholar]