Abstract
Objective
AT-rich interactive domain-containing protein 1A (ARID1A) is frequently mutated or deficient in various types of tumors. However, the role of ARID1A in bladder cancer remains unclear. We aimed to evaluate ARID1A expression and its biological role and correlation with prognosis in patients with urothelial bladder carcinoma (BUC).
Methods
ARID1A expression levels in BUC and normal tissues were assessed by immunohistochemistry and correlated with clinicopathological characteristics and patient outcomes. Downregulation of ARID1A was mimicked by transfection with small interfering RNA in T24 bladder cancer cells, and the effects on cell proliferation and migration were evaluated.
Results
ARID1A expression was significantly reduced in BUC tissues and was significantly associated with T stage and AJCC stage. Upregulation of ARID1A predicted a better prognosis in BUC patients. ARID1A expression and lymph node status were identified as independent prognostic factors for overall survival. Silencing of ARID1A promoted the proliferation of BUC cells.
Conclusions
ARID1A may represent a novel diagnostic and prognostic biomarker in patients with BUC.
Keywords: ARID1A, bladder cancer, tumor suppressor, proliferation, migration, prognosis
Introduction
Bladder cancer (BC) is the fourth most common malignant neoplasm worldwide, with a morbidity of 7% and mortality of 4%.1,2 Despite rapid advancements in the treatment of BC over recent years, the overall survival (OS) rate of patients remains unoptimistic.3 In the United States, BC poses a considerable burden on patients due to the long-term follow-up and monitoring required to detect progression and recurrence.4 The prognosis of patients with BC is mainly predicted by clinical and pathological stages, such as the American Joint Committee on Cancer (AJCC) and TMN staging. However, the main disadvantage of these systems is the lack of relevant tumor markers. In addition, the precision of forecasting by the current staging systems is low in the era of precision medical treatment.5 There is thus an urgent need for novel molecular biomarkers for therapeutic and prognostic prediction, to improve disease management and treatment efficacy.6
AT-rich interactive domain 1A (ARID1A) is tumor-suppressor that interacts with brahma-related gene 1 (BRG1, a component of the evolutionarily conserved SWI/SNF chromatin-remodeling complex) to form a switch/sucrose non-fermentable (SWI/SNF) chromatin remodeling protein complex. The ARID1A gene has been shown to be frequently mutated in ovarian clear cell carcinoma (57%),7 endometrioid carcinoma (23%–42%),8 liver cancer (10%–16.8%),9,10 and lung cancer (8%).11 These mutations, which are primarily frameshift or nonsense mutations resulting in mRNA decay, protein folding errors, or domain dysfunction, are the main cause of ARID1A deficiency.
Functional studies have revealed that ARID1A influences cancer cell proliferation and metastasis. Specifically, colony formation in soft agar was suppressed in a breast cancer cell line with re-expression of ARID1A.12 Silencing of ARID1A in gynecologic cancer cell lines enhanced cell proliferation and colony formation, while recovery of ARID1A expression exerted the opposite effect.13 Furthermore, ARID1A silencing increased the migration and invasion abilities of liver cancer cells.14 ARID1A regulated cell migration and invasion by mediating of E-cadherin signaling and epithelial-mesenchymal transition in gastric cancer.15 Together with p53, ARID1A regulated p21 and SMAD3 transcription and mediated tumor growth in gynecologic cancers.16 These results suggest that ARID1A suppresses cell proliferation by modulating the cell cycle.
Gui et al.17 discovered inactivation of ARID1A in 18.5% of patients with urothelial bladder carcinoma (BUC). Furthermore, a recent study7 established that low expression of ARID1A was associated with reduced recurrence in patients with non-muscular invasive BC treated by transurethral resection of the bladder. Low ARID1A expression tended to predict a better outcome. In addition, ARID1A expression gradually and significantly increased from normal through noninvasive urothelial carcinoma to invasive urothelial carcinoma.18 In a previous study, ARID1A inactivation was found to be a critical regulator in fibroblast growth factor receptor 3-wild-type bladder carcinoma tumors.19 However, the biological role of ARID1A in BC remains unclear.17,19 In this study, we assessed ARID1A expression in patients with BUC by immunohistochemistry (IHC). We also evaluated the associations between ARID1A expression and clinicopathological and prognostic features, and assessed the biological role of ARID1A in BC cells.
Materials and methods
Patient cohort
We collected surgical tissue specimens from patients undergoing radical cystectomy for BC at the Department of Urology, XinHua Hospital Affiliated with Shanghai Jiao Tong University School of Medicine between February 2012 and December 2018. The indications for radical cystectomy were based on European Association of Urology (EAU) guidelines. Follow-up information was collected by the authors from May 2012 to June 2019 at 3-month intervals after surgery. OS was calculated from the date of surgery to the date of death or final follow-up. Cancer-specific survival (CSS) was defined as the time from surgery to the date of death from BC. All pathological specimens were confirmed by a professional urological pathologist. All patients provided signed informed consent, and the study was approved by the ethics committee of XinHua Hospital Affiliated with Shanghai Jiao Tong University School of Medicine.
The inclusion criteria were: (1) age 18 years or above; (2) confirmed BUC; (3) no distant metastasis; (4) complete clinical characteristics and outcome data; (5) indicated for radical resection of BC according to EAU guidelines; and (6) underwent standardized radical surgery for BC. Exclusion criteria were incomplete clinical or follow-up data and non-urothelial carcinoma.
Most high-risk patients (pT3+ disease or with one positive lymph node) were treated with a standard postoperative cisplatin-based regimen (gemcitabine 1000 mg/m2 on days 1 and 8; cisplatin 70 mg/m2 on day 2; once every 3 weeks for 6 cycles) according to EAU recommendations. Some high-risk patients did not receive chemotherapy because of advanced age or poor general condition.
ARID1A IHC and scoring system
IHC was performed using a polyclonal rabbit anti-ARID1A antibody (HPA005456; Sigma-Aldrich, St Louis, MO, USA) at 1:6000 dilution.19 Antigen retrieval was carried out by submerging the tissue sections in hydrogen peroxide block for 10 to 15 minutes, followed by blocking with serum for 30 minutes and incubation with the rabbit antibody at a dilution of 1:1000 at 4°C overnight. Only nuclear staining was evaluated.
The staining intensities of the specimens were evaluated independently by two professional pathologists. A histochemistry score (H score) was applied to select the cutoff value for staining intensity. The H score is a method of scoring the results of immunohistochemical analyses. The number of positive cells and their staining intensity in each section were transformed into corresponding values to produce semi-quantitative staining scores as follows: percent stained area (0 = 0%; 1 = 1%–25%; 2 = 26%–50%; 3 = 51%–75%; 4 = 76%–100%) and staining intensity (0 = negative, 1 = weakly positive, 2 = moderately positive, and 3 = strongly positive).18
Proliferation assay
Cell proliferation was detected by Cell Counting Kit-8 (CCK8) assay. T24 BUC cells (Shanghai Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were transfected with small interfering RNA (siRNA) and a single cell suspension was prepared. Approximately 2000 cells were seeded in each well of a 96-well plate. Each experiment included at least three replicates. After 24 hours, CCK8 reagent (10 µL; GLPBIO, Montclair, CA, USA) was added to each plate and the absorbance of the dye at 450 nm was measured at different time points using an Epoch microplate spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
Cell migration assay
Cell migration was evaluated by Transwell assay. Briefly, T24 BUC cells were transfected with ARID1A siRNA (GCCCUAACAUGGCCAAUAUTT) and incubated in serum-free medium for 24 hours, and then used to prepare a cell suspension. The treated cells were seeded into the upper chamber of a Transwell plate (diameter 0.8 µm) and medium containing 10% fetal bovine serum as a chemotactic factor was then added to the lower chamber and cultured for 24 hours at 37°C. The chamber was fixed for 30 minutes and stained with 0.1% crystal violet for 20 minutes. Residual cells in the upper chamber were scraped off carefully with cotton swabs and residual dye was washed off using phosphate-buffered saline. The cells were then observed under a light microscope and cell numbers were calculated in three randomly selected fields.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA, USA). ARID1A expression levels in tumor and normal tissues were compared using unpaired Student’s t-test. Correlations between ARID1A expression and clinicopathological characteristics in patients with BC were evaluated by χ2 tests. Survival curves were calculated using the Kaplan–Meier algorithm and log-rank (Mantel–Cox) test. Independent prognostic variables were evaluated by univariate and Cox multivariate analyses. Hazard ratios (HRs) and 95% confidence intervals (CIs) of covariates were calculated. P < 0.05 was considered statistically significant.
Results
Patients
We collected surgical tissue specimens, pathological data, and clinical characteristics for 78 patients undergoing radical cystectomy for BC. Nine of these patients were excluded because of incomplete clinical or follow-up data, and three were excluded because of non-urothelial carcinoma. The remaining 66 patients included six cases of T1G3 non-muscle invasive BC, three bladder tumor in situ (Tis), and 57 cases of T2–T4 muscle invasive BC Table 1). The average follow-up time for the entire cohort was 51.0 months (range, 4–82 months).
Table 1.
Correlation between ARID1A expression and clinicopathological variables in patients with bladder urothelial carcinoma.
| Low ARID1A expression (n = 31) | High ARID1A expression (n = 35) | P-value | |
|---|---|---|---|
| Sex | |||
| Male | 23 | 31 | 0.131 |
| Female | 8 | 4 | |
| Age, years | 68.32 ± 10.989 | 69.86 ± 8.738 | 0.530 |
| Tumor grade | |||
| Low | 7 | 12 | 0.295 |
| High | 24 | 23 | |
| Tumor stage | |||
| T1–T2, Tis | 13 | 24 | 0.03 |
| T3–T4 | 18 | 11 | |
| Lymph node stage | |||
| N0 | 25 | 27 | 0.728 |
| N1 | 6 | 8 | |
| AJCC stage | |||
| 1–2 | 10 | 20 | 0.043 |
| 3–4 | 21 | 15 | |
| Tumor volume (cm3) | 12.43 ± 12.93 | 20.98 ± 21.72 | 0.086 |
Values given as number or mean ± standard deviation.
Thirty patients (18 T3–T4N0M0, 6 T3–4N1M0, and 6 T2N1M0) received adjuvant chemotherapy, with the dose calculated individually.
ARID1A was significantly downregulated in BC tissue
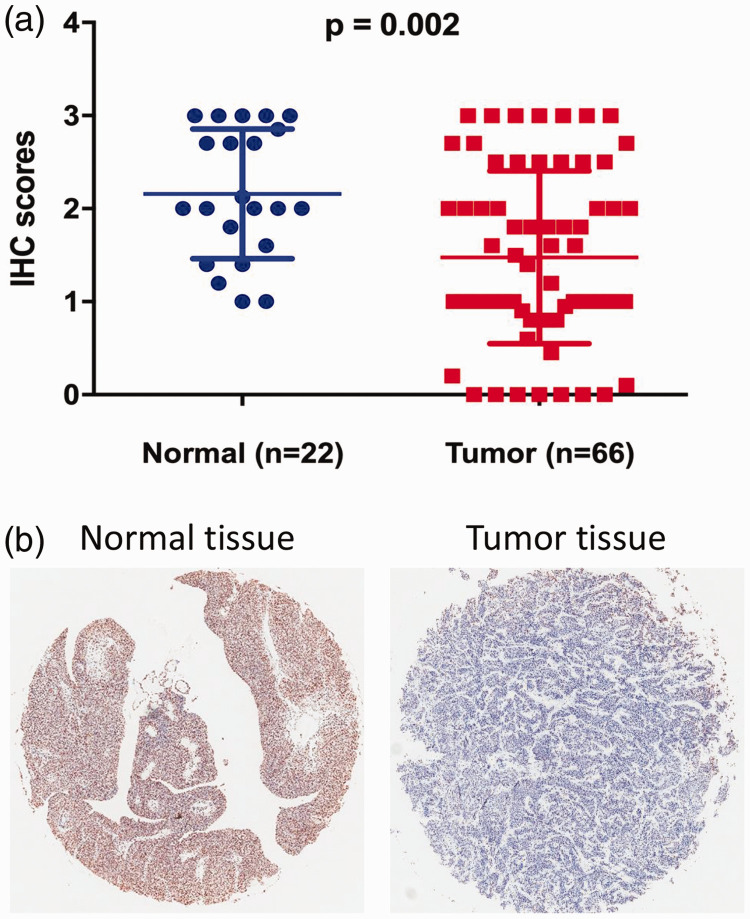
We investigated changes in ARID1A expression levels in 66 BUC and 22 normal urothelium samples from patients with BUC by IHC. ARID1A expression was significantly downregulated in BUC tissues (n = 66) compared with normal tissues (n = 22) (P = 0.002) (Figure 1a). ARID1A staining in BUC and normal tissues is shown in Figure 1b. This result suggested that ARID1A may act as a tumor suppressor in the development of BUC.
Figure 1.
ARID1A expression in bladder urothelial carcinoma (BUC) tissues and normal tissues. (a) ARID1A expression was decreased in 66 BUC tissue samples compared with 22 normal tissue samples. P-values were calculated by unpaired Student’s t-tests. (b) Representative immunohistochemical staining of ARID1A in tumor and normal tissues. IHC, immunohistochemistry.
Association of ARID1A expression with clinicopathological features in patients with BUC
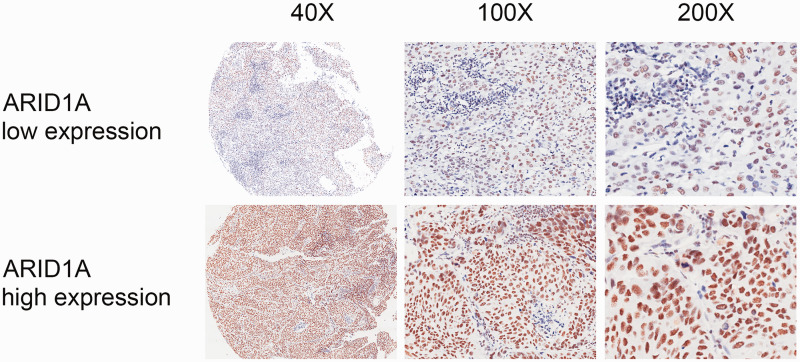
We analyzed the relationships between ARID1A expression levels and various clinicopathological characteristics in 66 patients undergoing radical cystectomy for BUC. Patients were divided into high-ARID1A expression (n = 35) and low-ARID1A expression groups (n = 31), according to the cutoff value for immunoreactivity score (H score). Representative high and low staining patterns of ARID1A in BUC tissues are presented in Figure 2. The coefficients of correlation between ARID1A expression and clinicopathological characteristics are listed in Table 1. ARID1A expression was significantly associated with T stage (P = 0.03) and AJCC stage (P = 0.043) in patients with BC (Table 1). These results suggested that ARID1A could serve as a histological screening and diagnostic biomarker.
Figure 2.
ARID1A expression in bladder urothelial carcinoma tissues. Representative immunohistochemistry staining of ARID1A in low-expression (upper panel) and high-expression groups (lower panel). ARID1A-positive staining was predominantly located in the nucleus.
High expression of ARID1A was associated with OS and CSS, and was an independent indicator of better OS in patients with BUC
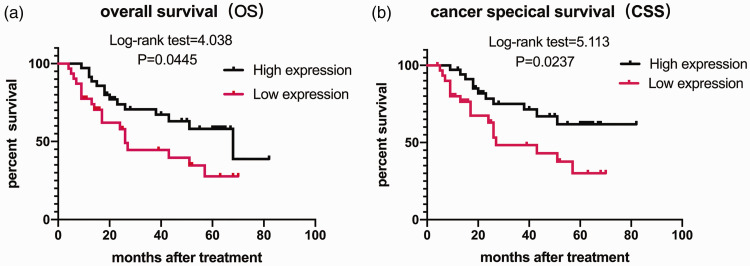
The relationships of ARID1A expression with OS and CSS in the 66 BUC patients were revealed by Kaplan–Meier analysis. Patients with high ARID1A expression had significantly better OS (log-rank test = 4.038, P = 0.0445) (Figure 3a) and CSS (log-rank test = 5.113, P = 0.0237) (Figure 3b) compared with patients with low ARID1A expression. Univariate analysis and multivariate Cox analysis of clinicopathological characteristics and ARID1A expression were used to identify independent prognostic factors for OS in BUC patients. The results of univariate analysis are shown in Table 2. A multivariate Cox proportional hazards model was subsequently performed involving all variables to identify independent prognostic factors. ARID1A expression (HR = 0.454, 95%CI: 0.224–0.920, P = 0.029) and lymph node status (HR = 2.996, 95% CI: 1.422-6.313, P = 0.004) were both identified as independent prognostic factors for OS (Table 2).
Figure 3.
Overall survival (OS) and cancer-specific survival (CSS) in patients with urothelial bladder carcinoma based on ARID1A expression. Kaplan–Meier analysis of OS (a) and CSS (b). P-values were calculated using the log-rank (Mantel–Cox) test.
Table 2.
Univariate and multivariate Cox regression analyses of overall survival.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| B | P-value | HR (95% CI) | B | P-value | HR (95% CI) | |
| ARID1A | −0.554 | 0.145 | 0.575 (0.273–1.210) | −0.789 | 0.029 | 0.454 (0.224–0.920) |
| Sex | −0.652 | 0.210 | 0.521 (0.188–1.443) | |||
| Age | 0.027 | 0.194 | 1.028 (0.986–1.071) | |||
| Tumor grade | −0.079 | 0.887 | 0.924 (0.341–2.508) | |||
| Tumor volume | −0.027 | 0.78 | 0.973 (0.944–1.003) | |||
| T stage | 1.198 | 0.123 | 3.313 (0.724–15.158) | |||
| Lymph node stage | 1.738 | 0.004 | 5.684 (1.753–18.429) | 1.097 | 0.004 | 2.996 (1.422–6.313) |
| AJCC stage | −0.483 | 0.589 | 0.617 (0.107–3.555) | |||
| Adjuvant chemotherapy | −1.195 | 0.067 | 0.303 (0.084–1.088) | |||
B, regression coefficient; HR, hazard ratio; CI, confidence interval.
Silencing of ARID1A enhanced the proliferation and migration of BC cells
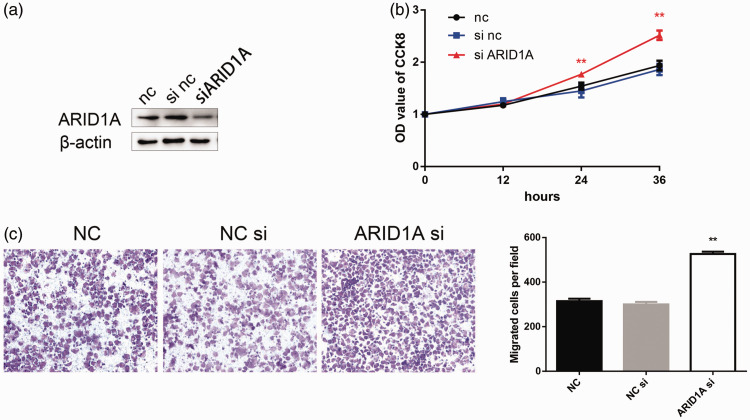
We analyzed the functional role of ARID1A by silencing its expression in T24 BC cells using siRNA. Western blot analysis confirmed that ARID1A was silenced (Figure 4a). CCK8 assay revealed that downregulation of ARID1A enhanced the proliferation of T24 cells compared with controls (Figure 4b). Loss of ARID1A also significantly promoted the migration of T24 cells, as determined by Transwell assay (Figure 4c).
Figure 4.
Silencing of ARID1A enhanced the proliferation and migration of bladder cancer (BC) cells. (a) ARID1A expression was reduced in cells transfected with ARID1A siRNA. (b) Silencing of ARID1A promoted the proliferation of BC T24 cells determined by CCK-8 assay (c) and promoted the migration of T24 BC cells determined by Transwell assay. **P<0.01. NC, negative control.
Discussion
Proteins involved in chromatin remodeling modifications have been indicated to be effective tumor suppressors inactivated in many cancers.7–11 A variety of human cancers contain ARID1A mutations.6,9,11 However, the biological functions and molecular mechanism of ARID1A in BC remain to be elucidated. The identification of cancer susceptibility associated with ARID1A deficiency provides multiple opportunities for cancer treatment, and a combination of different strategies might be an effective, if costly, clinical option.
p53 is involved in the regulation of a wide range of tumors. ARID1A mutations were related to a poor prognosis in TP53 wild-type tumors, suggesting that p53 and ARID1A functioned synergistically.20,21 Furthermore, ARID1A has been shown to regulate the expression of c-Myc by antagonizing Myc activity and promoting cell differentiation by binding to its target promoters.22 ARID1A mutations have been detected in numerous cancers, suggesting that these mutations could be exploited to identify a potential common vulnerability of human cancers. The lethality of ARID1A deficiency has been used in strategies targeting PI3K/Akt signaling.23–25 However, these strategies have only been evaluated in ovarian cancer cells, and there is thus an urgent need to identify or verify such strategies in different types of cancers, including BC.
We evaluated ARID1A expression in BUC tissue specimens and assessed its correlation with patients’ clinical characteristics and prognosis. ARID1A expression was significantly lower in BUC compared with normal tissues, and was significantly associated with both T and AJCC stages. These results suggest that ARID1A might play an important role during tumor suppression and tumor progression of BC. Furthermore, upregulation of ARID1A was associated with a better prognosis in patients with BC, and ARID1A expression level and lymph node stage were revealed as independent prognostic factors for OS in BUC patients.
ARID1A participates in cell-cycle arrest and interacts with p53,26 BRG1/BRM (the main catalytic subunits with ATPase activity in the BAF complex, which is in turn a component of the SWI/SNF chromatin-remodeling complex),16 and topoisomerase IIα27 to regulate tumor oncogenesis and development. Previous studies showed that ARID1A expression levels were significantly associated with prognosis in patients with tumors including lung,13 gastric,15,28 and breast cancer,29 with high ARID1A expression usually indicating a relatively better prognosis. This was confirmed by the current results for BC. Overall, these results suggest that ARID1A acts as a tumor suppressor with an important role in tumor prognosis, and may thus be a useful indicator of prognosis in patients with bladder tumors.
Silencing ARID1A enhanced the proliferation and migration of BC cells, indicating that ARID1A may be a potential molecular therapeutic target. ARID1A deficiency was previously shown to promote the proliferation, growth, and nutrient consumption of gastric cancer cells, accompanied by activation of PI3K/Akt signaling.15 ARID1A silencing also upregulated the phosphorylation of Akt and p70S6K in endometrial cancer and glioma.23,30 However, the direct targets of ARID1A in the PI3K/Akt pathway remain unknown, and global changes in phosphorylation of the PI3K/Akt signaling pathway in BUC cells remains to be elucidated. Further studies are also required to determine the mechanisms by which ARID1A mediates tumor progression, and how it modulates other tumor suppressors or cell factors, thus laying the foundations for effective BC therapies. Additional in vivo experiments are also needed to verify the biological function of ARID1A.
This study had some limitations, including its single-center, retrospective design and relatively small sample size. Further prospective cohort studies or clinical trials are therefore needed to confirm our results.
Conclusions
The current analysis suggests that ARID1A is involved in the tumorigenesis and malignancy of BC, and may thus serve as an independent diagnostic and/or prognostic biomarker in patients with BC.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Haibo Shen https://orcid.org/0000-0002-1183-6570
References
- 1.Oosterhuis JW, Schapers RF, Janssen-Heijnen MLet al. Histological grading of papillary urothelial carcinoma of the bladder: prognostic value of the 1998 WHO/ISUP classification system and comparison with conventional grading systems. J Clin Pathol 2002; 55: 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaux A, Karram S, Miller JSet al. High-grade papillary urothelial carcinoma of the urinary tract: a clinicopathologic analysis of a post-World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Hum Pathol 2012; 43: 115–120. DOI: 10.1016/j.humpath.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet 2009; 374: 239–249. DOI: 10.1016/s0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 4.Botteman MF, Pashos CL, Redaelli Aet al. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics 2003; 21: 1315–1330. DOI: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. DOI: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 6.Ayhan A, Mao TL, Seckin Tet al. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gynecol Cancer 2012; 22: 1310–1315. DOI: 10.1097/IGC.0b013e31826b5dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones S, Wang TL, Shih IeMet al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010; 330: 228–231. DOI: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang H, Cheung LW, Li Jet al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res 2012; 22: 2120–2129. DOI: 10.1101/gr.137596.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guichard C, Amaddeo G, Imbeaud Set al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet 2012; 44: 694–698. DOI: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto A, Totoki Y, Abe Tet al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet 2012; 44: 760–764. DOI: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 11.Imielinski M, Berger AH, Hammerman PSet al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012; 150: 1107–1120. DOI: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mamo A, Cavallone L, Tuzmen Set al. An integrated genomic approach identifies ARID1A as a candidate tumor-suppressor gene in breast cancer. Oncogene 2012; 31: 2090–2100. DOI: 10.1038/onc.2011.386. [DOI] [PubMed] [Google Scholar]
- 13.Wang DD, Chen YB, Pan Ket al. Decreased expression of the ARID1A gene is associated with poor prognosis in primary gastric cancer. PLoS One 2012; 7: e40364. DOI: 10.1371/journal.pone.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Deng Q, Wang Qet al. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet 2012; 44: 1117–1121. DOI: 10.1038/ng.2391. [DOI] [PubMed] [Google Scholar]
- 15.Yan HB, Wang XF, Zhang Qet al. Reduced expression of the chromatin remodeling gene ARID1A enhances gastric cancer cell migration and invasion via downregulation of E-cadherin transcription. Carcinogenesis 2014; 35: 867–876. DOI: 10.1093/carcin/bgt398. [DOI] [PubMed] [Google Scholar]
- 16.Guan B, Wang TL, Shih IeM. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res 2011; 71: 6718–6727. DOI: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gui Y, Guo G, Huang Yet al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet 2011; 43: 875–878. DOI: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraj SF, Chaux A, Gonzalez-Roibon Net al. ARID1A immunohistochemistry improves outcome prediction in invasive urothelial carcinoma of urinary bladder. Hum Pathol 2014; 45: 2233–2239. DOI: 10.1016/j.humpath.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Balbas-Martinez C, Rodriguez-Pinilla M, Casanova Aet al. ARID1A alterations are associated with FGFR3-wild type, poor-prognosis, urothelial bladder tumors. PLoS One 2013; 8: e62483. DOI: 10.1371/journal.pone.0062483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer G, Hanrahan AJ, Milowsky MIet al. Genome sequencing identifies a basis for everolimus sensitivity. Science 2012; 338: 221. DOI: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Kan J, Yuen STet al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet 2011; 43: 1219–1223. DOI: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 22.Romero OA, Setien F, John Set al. The tumour suppressor and chromatin-remodelling factor BRG1 antagonizes Myc activity and promotes cell differentiation in human cancer. EMBO Mol Med 2012; 4: 603–616. DOI: 10.1002/emmm.201200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samartzis EP, Gutsche K, Dedes KJet al. Loss of ARID1A expression sensitizes cancer cells to PI3K- and AKT-inhibition. Oncotarget 2014; 5: 5295–5303. DOI: 10.18632/oncotarget.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitler BG, Aird KM, Garipov Aet al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med 2015; 21: 231–238. DOI: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helming KC, Wang X, Wilson BGet al. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat Med 2014; 20: 251–254. DOI: 10.1038/nm.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagl NG, Jr., Patsialou A, Haines DSet al. The p270 (ARID1A/SMARCF1) subunit of mammalian SWI/SNF-related complexes is essential for normal cell cycle arrest. Cancer Res 2005; 65: 9236–9244. DOI: 10.1158/0008-5472.CAN-05-1225. [DOI] [PubMed] [Google Scholar]
- 27.Dykhuizen EC, Hargreaves DC, Miller ELet al. BAF complexes facilitate decatenation of DNA by topoisomerase IIalpha. Nature 2013; 497: 624–627. DOI: 10.1038/nature12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zang ZJ, Cutcutache I, Poon SLet al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet 2012; 44: 570–574. DOI: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Zhang Y, Yang Yet al. Frequent low expression of chromatin remodeling gene ARID1A in breast cancer and its clinical significance. Cancer Epidemiol 2012; 36: 288–293. DOI: 10.1016/j.canep.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Samartzis EP, Noske A, Dedes KJet al. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int J Mol Sci 2013; 14: 18824–18849. DOI: 10.3390/ijms140918824. [DOI] [PMC free article] [PubMed] [Google Scholar]