Abstract
Objective
To compare the fracture risk in postmenopausal Asian women with or without type 2 diabetes mellitus (T2DM).
Methods
The study cohort comprised data from consecutive postmenopausal women with T2DM that were retrieved from a prospectively maintained institutional database from 2001 to 2009. Postmenopausal women without DM from the Medical Examination Center from 2001 to 2009 formed the control cohort. The primary endpoint was the World Health Organization Fracture Risk Algorithm (FRAX, revised 2013) score. The secondary endpoint was bone mineral density (BMD).
Results
There were 1014 individuals included for the assessment (T2DM, n=500 and non-DM, n=514). Based on the FRAX model, the risk of major osteoporotic fractures and hip fractures over the next 10 years was higher in the T2DM group compared with the non-DM group. Compared with the T2DM group, the non-DM group had a lower BMD. After adjusting for age, gender, history of alcohol consumption, smoking status, body mass index, and low-density lipoprotein, the differences were statistically significant.
Conclusions
Compared with postmenopausal women without DM, postmenopausal women with T2DM had a significantly higher fracture risk calculated using the FRAX model. Early intervention for postmenopausal women with T2DM may be necessary, although T2DM is associated with a high BMD.
Keywords: Diabetes mellitus, fracture risk assessment tool, bone mineral density, postmenopausal, osteoporotic fracture, FRAX, osteoporosis, hip fracture
Introduction
Type 2 diabetes mellitus (T2DM) tends to be associated with higher bone mineral density (BMD) and, illogically, with an increase in bone fragility.1–3 It is increasingly recognized that postmenopausal women with T2DM have a high fracture risk that is associated with osteoporosis1,4 and that previously established methods for predicting fracture events can be very variable in patients with T2DM because a higher BMD is associated with increased fracture events in these patients.5 Conceptually, osteoporosis is defined as a systemic skeletal disease that is characterized by bone mass impairment and micro-architectural deterioration of bone tissue with a consequent decrease in bone strength and susceptibility to fracture.1,6,7 With an aging population, the incidence of osteoporotic fractures in postmenopausal women is higher than that in the non-DM population, which is the main cause of long-term severe pain and/or dysfunction and it seriously affects patients’ quality of life.8 Therefore, preventive identification and prompt intervention for the risk of osteoporotic fracture in these patients are needed.
Although BMD measured by dual-energy X-ray absorptiometry (DXA) is the foremost determinant of bone strength and fracture risk, BMD values in many patients with fragility fractures tend to be in the osteopenic or even normal range.1,9,10 Multiple studies have also shown that BMD in T2DM patients is higher than that in non-DM individuals of the same age and gender, which does not seem to explain the increased risk of fracture.1,4,11 BMD can only reflect 70% of the bone strength and it ignores the risk factors for osteoporosis, such as gender, age, previous fracture history, and smoking history, although studies have consistently demonstrated BMD to be associated with fracture risk.1,8 Additionally, previous studies have shown that some patients did not meet the diagnostic criteria of osteoporosis but still had osteoporotic fractures.12,13 Consequently, some factors other than BMD (age, bone mineralization, bone microdamage, bone turnover, and fracture history) that are captured by the Fracture Risk Algorithm (FRAX) contribute to the overall assessment of fracture risk.6,10 However, the FRAX model estimating the 10-year probability of hip and major osteoporotic fracture, which is based on the individual risk factor profile, fails to capture all skeletal determinants of bone strength that tend to be independent of BMD.6,11
A consideration of the manner whereby FRAX-generated probabilities should warrant intervention, both for an intervention threshold and for an assessment threshold, is necessary for the application of the FRAX model in clinical decision making.6,13 To overcome specific limitations of FRAX, relatively straightforward arithmetic procedures have been applied to conventional FRAX estimates of fracture probabilities to adjust the probability assessment (e.g. information on trabecular bone score [TBS], hip structural analysis [HSA], concurrent data on lumbar spine BMD, and high moderate and low exposure to glucocorticoids).2 Currently, no guidelines for the prediction or assessment of fracture probabilities in postmenopausal women with T2DM have been reported. In our study, the FRAX model (revised 2013) was applied to compare the risk of fractures between T2DM and non-DM postmenopausal women over the next 10 years to verify the effectiveness of the FRAX model in estimating the 10-year probability of hip and major osteoporotic fractures in postmenopausal women with T2DM. To the best of our knowledge, previous studies do not appear to be available on fracture prediction in postmenopausal women with T2DM using BMD, although preliminary data have demonstrated an incremental improvement in fracture prediction when BMD is used in combination with other risk factors.6 Additionally, there is no FRAX model that is currently available in a Chinese clinical setting, mainly because of significant differences in fracture rates that are described among the different studies.1,7,11
Methods
Study population
This study was reviewed and approved by the review board at Pu’ai Hospital, Tongji Medical College, Huazhong University of Science and Technology. Consent to participate was not applicable because this was a retrospective study, and an exemption was obtained from the Investigational Ethical Review Board. All clinical investigations were conducted in accordance with the Declaration of Helsinki. Individual-level data regarding the general condition of postmenopausal women with T2DM from February 1, 2001 to February 28, 2009 were retrieved from a prospectively maintained institutional database with adjudicated fracture outcomes, and these patients formed the study cohort (the T2DM group). The data with adjudicated fracture outcomes regarding postmenopausal women without DM who underwent a physical examination at the Medical Examination Center, Pu’ai Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China) from February 1, 2001 to February 28, 2009 formed the control cohort (the non-DM group). Women had a postmenopausal status based on the Straw classification.14 The main inclusion criteria were as follows: women ≥55 years old; women with a clinically confirmed diagnosis of T2DM or women without DM; and women with T2DM who were treated with antihyperglycemic medications (e.g., metformin, glimepiride, pioglitazone, acarbose, and/or insulin). The main exclusion criteria were as follows: premature menopause (<45 years old); poor medical data with regard to the FRAX risk factors; repeat admissions for the same fracture; a history of tumor; women with pituitary, thyroid, parathyroid, adrenal, or gonadal diseases; serious infections; organ failure; severe circulatory or metabolic diseases; long-term use of calcium, vitamin D, or other drugs that affect bone metabolism or that are associated with increased fracture risk; a history of drug or alcohol abuse; a New York Heart Association classification of 3;15 or vascular cognitive impairment (VCI), as reported previously.16 The primary endpoint was FRAX fracture risk. The secondary endpoint was BMD.
Outcomes and assessments
According to the 2012 American Diabetes Association (ADA) definition,17 the diagnostic criteria for DM and impaired glucose regulation (IGR) include the following: patients with typical hyperglycemia or hyperglycemic crisis, with random blood glucose ≥200 mg/dL (11.1 mmol/L); women with fasting blood glucose (FPG) ≥126 mg/dL (7.0 mmol/L); oral glucose tolerance test (OGTT) 2-h results of blood glucose ≥200 mg/dL (11.1 mmol/L); or hemoglobin A1c (HbA1c) ≥6.5%. If there was no definite hyperglycemia, the test was repeated to verify the results. DXA examination was performed using a Discovery type A dual-energy X-ray bone density analyzer (CV <1%) (Hologic, Bedford, MA, USA). The DXA measurements included the lumbar spine (L1–L4) and bilateral proximal femur (total hip, femoral neck).
Osteoporosis was diagnosed based on the WHO criteria for osteoporosis diagnosis (1994 version), as follows:18,19 T value ≤−2.5 for osteoporosis; −2.5 < T value < 1 for bone mass reduction; T value ≥−1 for normal bone mass (of which the Z-value evaluation was applied to postmenopausal women, and the diagnostic standard was the same as the T value). Major osteoporotic fractures were defined as clinical spine, hip, forearm, and humeral fractures.11 Fractures were calculated based on radiograph confirmation. Glucose and lipid metabolism indicators were collected, including FPG, total cholesterol (TC), triacylglycerol (TG), HDL cholesterol (HDL-c), and LDL cholesterol (LDL-c).
The collection of fracture risk factors involved in the FRAX model was based on the previous description.9,11 The WHO 10-year absolute risk of hip and osteoporotic fracture (FRAX scores) was calculated based on the Chinese FRAX model6 (a computer-based algorithm), which is available online at the Web site http://www.shef.ac.uk/FRAX. A uniform survey form was used to inquire about and document the fracture risk factors that are involved in the FRAX model, including age, BMD T score, height, weight, BMI, previous history of brittle fracture, parental history of fracture, oral history of adrenal corticosteroids, current smoking status, drinking more than three alcoholic beverages per day, a history of rheumatoid arthritis, and diseases closely related to osteoporosis (including type 1 DM, adult osteogenesis immaturity, long-term treatment-naive hyperthyroidism, hypogonadism or premature menopause, chronic malnutrition or malabsorption, and chronic liver disease).10 A sensitivity analysis was performed to exclude patients, and the results were consistent with previous reports.9,20
Statistical Analysis
Variables were compared using the chi-squared test for categorical variables and the Mann–Whitney U test for continuous variables. In the univariate and multivariate analyses, hazard ratios (HRs) and appropriate 95% confidence intervals (CIs) were calculated using a logistic regression model and a Cox proportional hazard model, respectively. Interactions of the T score with age in patients with T2DM were assessed. All statistical tests were two-sided, and the significance level was set at 0.05. Ten-year cumulative risks were estimated using the Cox proportional hazards regression model baseline survival function, which was evaluated at 10 years, raised to the power of the relative hazard. Patient-related data extracted from a prospectively maintained database were initially stored in Excel software and subsequently analyzed using SPSS software, v 24.0 (IBM Corp., Armonk, NY, USA).
Results
Comparison of baseline data
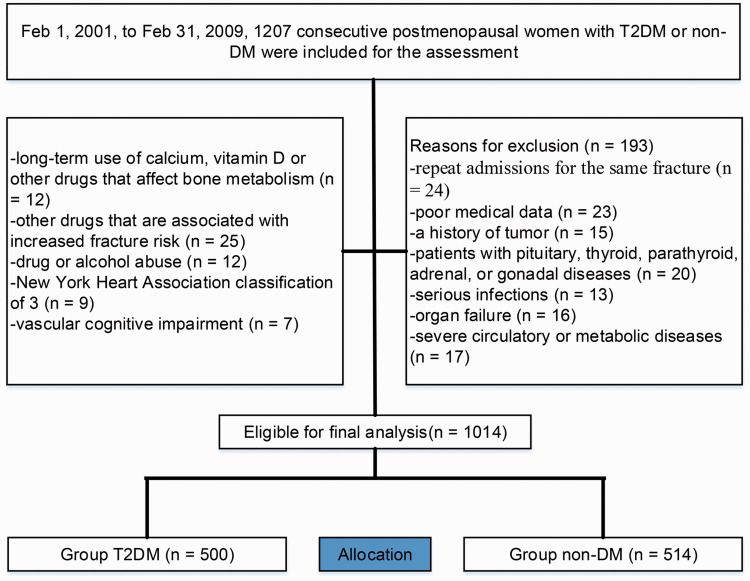
Overall, 1207 individuals were enrolled in the study, 193 of whom were considered to be ineligible based on the exclusion criteria, and 1014 patients (T2DM, n=500, mean age, 68.7 years [standard deviation (SD) 13.5] and non-DM, n=514, 68.5 years [SD 12.7]) were eligible to be included in the study (Figure 1), suggesting that there was no bias in data availability between the groups. At the time of our analyses, patient demographics and other characteristics of the individuals with available data, which were used for validation, are summarized in Table 1. There were statistically significant differences in FPGHbA1c, TC, HDL-c, and LDL-c between the groups. Considering that bone metabolism is greatly influenced by age, patients were divided into four groups based on age for comparative analysis. In this study, the non-DM group was collected from a physical examination cohort, and there were no individuals over 90 years old, so this group was combined with patients aged 80 to 89 years for analysis. The complete distribution of FRAX clinical risk factors is showed in Table 2.
Figure 1.
Flow diagram demonstrating the methods for identifying studies to compare the fracture risk in postmenopausal Asian women with type 2 diabetes mellitus (T2DM) or without diabetes mellitus (DM).
Table 1.
Demographic and baseline characteristics between groups.
| Variable | T2DM (n=500) | Non-DM (n=514) | p-value |
|---|---|---|---|
| Age (years) | 68.7±13.5 | 68.5±12.7 | 0.214 |
| Menopause age (years) | 50.2±2.2 | 50.6±2.7 | 0.107 |
| Age structure, n (%) (years) | 0.095 | ||
| 55–59 | 152 (30.4) | 143 (27.8) | |
| 60–69 | 197 (39.4) | 189 (36.8) | |
| 70–79 | 102 (20.4) | 117 (22.8) | |
| 80–89 | 49 (9.8) | 65 (12.6) | |
| Height (cm) | 162.1±7.3 | 162.3±6.8 | 0.335 |
| Weight (kg) | 67.4±9.3 | 67.6±8.8 | 0.126 |
| BMI (kg/m2) | 23.5±2.4 | 23.3±2.8 | 0.251 |
| FPG (mmol/L) | 8.6±2.1 | 4.5±1.1 | 0.001* |
| HbA1c, % (mean, range) | 7.7 (6.1–9.4) | 4.7 (4.3–5.8) | 0.031* |
| Blood lipid | |||
| TC | 4.3±0.7 | 4.8±1.4 | 0.013* |
| TG | 1.8±1.3 | 1.8±0.8 | 0.274 |
| HDL-c (mmol/L) | 1.2±1.1 | 1.4±0.7 | 0.038* |
| LDL-c (mmol/L) | 2.7±0.5 | 2.4±0.4 | 0.003* |
| Time from diagnosis of T2DM (months), n (%) | 0.054 | ||
| <6 | 135 (27.0) | 126 (24.5) | |
| 6–12 | 215 (43.0) | 200 (38.9) | |
| >12 | 150 (30.0) | 188 (36.6) | |
| Smoking status | 0.149 | ||
| Never a smoker | 387 (77.4) | 380 (73.9) | |
| Former smokers | 78 (15.6) | 82 (16.0) | |
| Current smokers | 35 (7.0) | 52 (10.1) | |
| Alcohol consumption, n (%) | 0.994 | ||
| Never | 15 (3.0) | 17 (3.3) | |
| Former | 344 (68.8) | 351 (68.3) | |
| Current (3 ≥units/day) | 141 (28.2) | 146 (28.4) | |
| Parental fractured hip, n (%) | 10 (2.0) | 10 (1.9) | 0.950 |
*Statistically significant values.
T2DM, type 2 diabetes mellitus; non-DM, non-diabetes mellitus; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; TC, total cholesterol; TG, Total triglyceride; HDL-c, high density lipoprotein cholesterol; LDL-c, low density lipoprotein cholesterol.
Table 2.
The complete distribution of FRAX clinical risk factors.
|
T2DM (n=500) |
Non-DM (n=514) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 50–59 (n = 152) | 60–69 (n =197) | 70–79 (n = 102) | 80–89 (n = 49) | 50–59 (n = 143) | 60–69 (n = 189) | 70–79 (n = 117) | 80–89 (n = 65) | |
| BMD (%) | 45.0 | 34.0 | 25.0 | 23.0 | 36.0 | 44.0 | 14.0 | 16.0 |
| BMI (%) | 10.0 | 8.0 | 13.0 | 21.0 | 11.0 | 2.0 | 6.0 | 22.0 |
| Previous fracture (%) | 61.0 | 47.0 | 51.0 | 45.0 | 53.0 | 33.0 | 43.0 | 54.0 |
| Parental hip fracture (%) | 18.0 | 6.0 | 5.0 | 14.0 | 16.0 | 3.0 | 3.0 | 3.0 |
| Corticotherapy (%) | 6.4 | 15.0 | 16.0 | 7.0 | 5.0 | 7.0 | 16.0 | 11.0 |
| RA (%) | 0.0 | 2.0 | 5.0 | 0.0 | 0.0 | 4.0 | 4.0 | 0.0 |
| Alcohol (%) | 47.0 | 36.0 | 11.0 | 6.0 | 35.0 | 332.0 | 15.0 | 2.0 |
| Tobacco(%) | 46.0 | 45.0 | 21.0 | 6.0 | 43.0 | 41.0 | 33.0 | 7.0 |
T2DM, type 2 diabetes mellitus; non-DM, non-diabetes mellitus; BMD, bone mineral density; BMI, body mass index; RA, rheumatoid arthritis.
Comparison of fracture risk
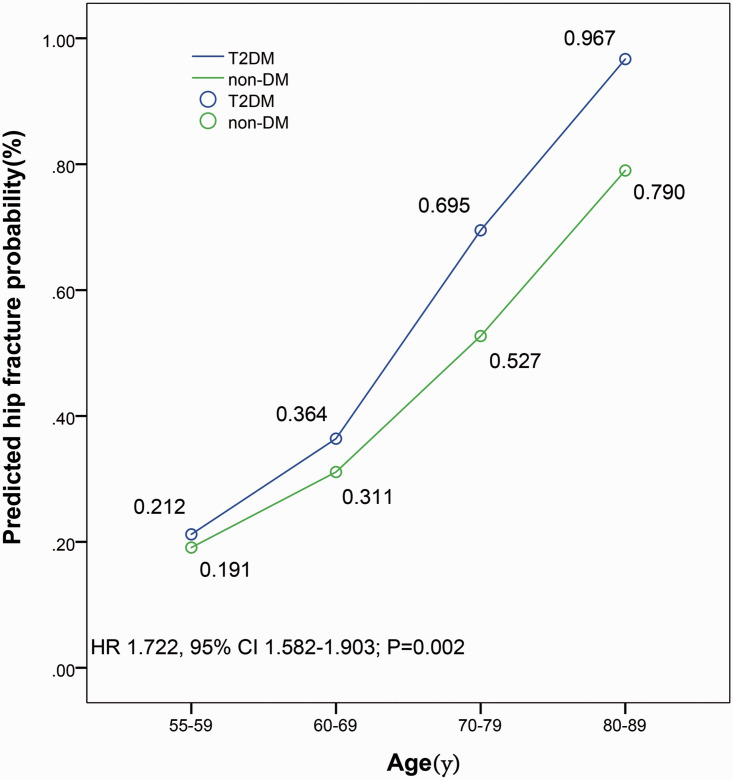
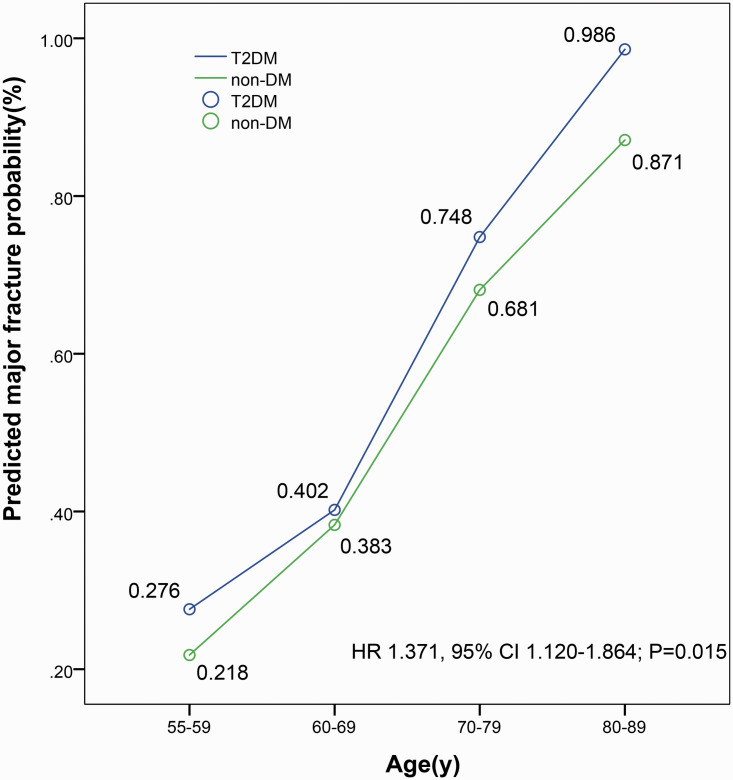
Among 500 postmenopausal women with T2DM, 65 had a hip fracture and 163 had an osteoporotic fracture during a mean (SD) follow-up of 14.2 (6.8) years. Among 514 postmenopausal women without DM, 31 had a hip fracture, and 72 had an osteoporotic fracture during a mean (SD) follow-up of 14.4 (7.8) years. The risk of hip fractures and major osteoporotic fractures over the next 10 years was assessed based on the FRAX model. The risk of hip fracture was higher in the T2DM group compared with the non-DM group (HR 1.722, 95% CI 1.582–1.903; P=0.002) (Figure 2). The risk for major osteoporotic fractures was higher in the T2DM group compared with the non-DM group (HR 1.371, 95% CI 1.120–1.864; P=0.015) (Figure 3). In different age groups, patients with T2DM, based on the FRAX model, had a higher risk of hip fractures and major osteoporotic fractures in the next 10 years compared with patients in the non-DM group. For these types of fractures, the risk of fracture increases with age. Additionally, this trend was significant for the risk of major osteoporotic fractures, especially in the group aged 60 years or older.
Figure 2.
Comparison of the FRAX fracture risk in predicted hip fracture probability between groups. Ten-year probability of a predicted hip fracture in Asian postmenopausal women with type 2 diabetes mellitus (T2DM). The risk of hip fracture is higher in the T2DM group compared with the non-DM group (HR 1.722, 95% CI 1.582–1.903; P=0.002). FRAX, Fracture Risk Assessment Tool; T2DM, type 2 diabetes mellitus; non-DM, non-diabetes mellitus; HR, Hazard ratio; CI, Confidence interval.
Figure 3.
Comparison of the FRAX fracture risk in predicted major fracture probability between groups. Ten-year probability of a predicted major fracture in Asian postmenopausal women with type 2 diabetes mellitus (T2DM). The risk of hip fracture is higher in the T2DM group than in the non-DM group (HR 1.371, 95% CI 1.120–1.864; P=0.015). FRAX, Fracture Risk Assessment Tool; T2DM, type 2 diabetes mellitus; non-DM, non-diabetes mellitus; HR, Hazard ratio; CI, Confidence interval.
Comparison of BMD
The differences in BMD corresponded to an HR of 1.463 (95% CI 1.103–1.917; P=0.024) for the femoral neck, 1.572 (95% CI 1.201–2.136; P=0.033) for the total hip, and 1.303 (95% CI 1.124–1.567; P=0.016) for lumbar vertebrae 1 to 4 (Table 3). BMD in the T2DM group was higher than that in the non-DM group, especially in individuals aged 80 to 89 years. There were significantly more individuals with bone mass reduction in the T2DM group compared with the non-TD group (154 [95% CI, 101.3–182.7] vs. 89 [95% CI, 73.5–122.4]; HR 1.573, 95% CI 1.313–1.839; P=0.021). The risk of bone mass reduction in patients with T2DM was further analyzed using the logistic regression model (Table 4), which showed that the risk of bone mass reduction in the T2DM group was similar to that in the non-DM group (OR = 0.748, P = 0.237). After adjusting for age, the results were OR = 0.655 and P = 0.004; after further adjustment for alcohol consumption, smoking status, and BMI, the results were OR = 0.811 and P = 0.002. After adjusting for LDL-c, the results were OR = 0.872 and P = 0.003. The differences were statistically significant for each adjustment.
Table 3.
Comparison of BMD between groups.
| BMD (g/cm2) | T2DM (n=500) | non-DM (n=514) | p-value |
|---|---|---|---|
| Femoral neck | |||
| 55–59 | 0.71 ± 0.11 | 0.70 ± 0.16 | 0.052 |
| 60–69 | 0.69 ± 0.08 | 0.64 ± 0.13 | 0.026* |
| 70–79 | 0.65 ± 0.08 | 0.60 ± 0.12 | 0.033* |
| 80–89 | 0.61 ± 0.09 | 0.53 ± 0.14 | 0.011* |
| 55–89 | 0.67 ± 0.12 | 0.62 ± 0.17 | 0.024* |
| Total hip | |||
| 55–59 | 0.98 ± 0.15 | 0.94 ± 0.13 | 0.023* |
| 60–69 | 0.95 ± 0.14 | 0.93 ± 0.11 | 0.041* |
| 70–79 | 0.90 ± 0.12 | 0.86 ± 0.10 | 0.025* |
| 80–89 | 0.82 ± 0.13 | 0.77 ± 0.08 | 0.014* |
| 55–89 | 0.92 ± 0.16 | 0.89 ± 0.14 | 0.033* |
| Lumbar 1–4 | |||
| 55–59 | 1.03 ± 0.09 | 1.01 ± 0.12 | 0.043* |
| 60–69 | 0.97 ± 0.08 | 0.92 ± 0.13 | 0.021* |
| 70–79 | 0.90 ± 0.11 | 0.84 ± 0.15 | 0.013* |
| 80–89 | 0.82 ± 0.03 | 0.77 ± 0.05 | 0.017* |
| 55–89 | 0.95 ± 0.15 | 0.90 ± 0.18 | 0.016* |
*Statistically significant values.
T2DM, type 2 diabetes mellitus; non-DM, non-diabetes mellitus; BMD, bone mineral density.
Table 4.
The risk of bone mass reduction for T2DM patients.
| Factors | OR (95%CI) | P-value |
|---|---|---|
| Crude | 0.748 (0.693–1.216) | 0.237 |
| Model 1a | 0.655 (0.531–0.857) | 0.004* |
| Model 2b | 0.811 (0.714–0.922) | 0.002* |
| Model 3c | 0.872 (0.785–0.916) | 0.003* |
*Statistically significant values. T2DM, type 2 diabetes mellitus
aAdjusted for age
bFurther adjustment for drinking, smoking status and body mass index (BMI)
cFurther adjustment for low-density lipoprotein cholesterol (LDL-c).
Discussion
Our study demonstrates that compared with postmenopausal women without DM, postmenopausal women with T2DM had significantly higher fracture risk, as calculated using the FRAX model. The applicability of the FRAX model in the clinical setting tends to be positive, which is consistent with previous studies involving postmenopausal women with T2DM.21–24 Although the fracture probability can be underestimated in the FRAX model, particularly for hip fractures because treatment effects were not included in the model, it is commonly accepted as a way of characterizing discriminatory models and as a good predictive tool in postmenopausal women with T2DM. This may be related to the contribution of treatment-induced changes in BMD.1,20 Additionally, although it may be influenced by the distribution of risk factors in dissimilar populations, the fracture risk can change frequently based on the timeframe or it can remain consistent across studies.9,10 A lack of appreciation of these facts can result in a faulty comparison among predictive tools across study populations.6
Our findings are consistent with those of previous studies.1,23–25 Whereas previous studies did not focus on the differences in the study population with T2DM, such as estrogen interference, our study provides an independent analysis of a postmenopausal cohort. With the increasing prevalence of T2DM and osteoporosis, the relationship between T2DM and bone metabolism has increasingly become the focus of research.2,4 Metabolic disorders induced by T2DM tend to affect bone metabolism in different ways.4,26 However, there is no consensus about the changes in BMD in patients with T2DM.27 An association between BMD and the fracture risk in T2DM failed to be detected.1,27 Additionally, the relationship between BMD and fracture risk in T2DM was not completely consistent because of the study design, measurement method, case selection, diabetes complications, and other problems.28,29 Currently, several studies have shown that T2DM patients with poor blood glucose control have higher BMD compared with T2DM patients with good blood glucose control.28,30–32 Potential explanations for the worse-than-expected BMD performance could be the choice of the study population or that understanding of how estrogen affects BMD should not be ignored. Regardless of the patient’s BMI, the power to draw dependable conclusions may be reduced because several studies have shown a positive correlation between BMI and BMD.28,31,32 Although the effects of estrogen on BMD are not yet acknowledged,33,34 a significant effect of estrogen on BMD was of interest in the early stages of T2DM and it showed little effect thereafter.34 An interaction in the early stages of T2DM appeared to be associated with superior BMD benefit in patients, in whom insulin resistance and hyperinsulinemia may be common.29,35 The mechanisms underlying the relationship between LDL and bone loss have not been fully elucidated.36 The LDL receptor-related protein 5 (LRP5) is considered to be a candidate susceptibility gene for osteoporosis that regulates BMD and/or fracture risk in the general population.36,37 LRP5 loss-of-function mutations, which were associated with impaired insulin sensitivity and dysregulated lipid metabolism, have been shown to lead to extreme osteoporosis.36
Frequent debate occurs about the group of postmenopausal women who tend to have an increased risk of fracture, especially hip fracture. BMD was consistently an independent contributor to the assessment of fracture risk.1,36,37 Previous studies have shown that although BMD increases in patients with T2DM, the probability of osteoporotic fractures increases.1,38 Additionally, osteoporotic fractures tend to occur in some T2DM patients without reaching the diagnostic criteria of osteoporosis, indicating that BMD does not accurately reflect the risk of fractures in these cases.4,39 Considering the high disability and mortality rates of osteoporosis, the FRAX model was recommended by the WHO in 2008.11 Currently, no guidelines have been proposed for the prediction or assessment of fracture risk in postmenopausal women with T2DM,6,38 and the application of the FRAX model in the assessment of fracture risk in the cohort has been reported less frequently.11 Schwartz et al.1 found that in patients with T2DM, both femoral neck T-value and FRAX score are associated with the risk of hip and non-hip fractures, but these patients actually have a higher risk of fracture for the same femoral neck T-value and FRAX score. Although the ability of the FRAX score to predict fracture is undeniable, the FRAX score underestimates the fracture risk in T2DM patients compared with the actual risk of major osteoporotic fractures and hip fractures that are observed.1,40 Bridges et al.40 reported 770 women with T2DM who had brittle fractures and found that 39.1% (770/1969) of participants were determined to have a low risk of fracture based on the FRAX model (HRs for a one-unit increase in FRAX score, 1.05; 95% CI, 1.03–1.07), suggesting that the FRAX score may underestimate the fracture risk of participants with T2DM. A multicentric cross-sectional study by Carnevale et al.22 showed that DM had a significantly lower FRAX-estimated probability of both major osteoporotic fracture and hip fracture compared with control subjects (6.35 ± 5.07% vs. 7.75 ± 6.93%, p<0.001, and 2.17 ± 3.07% vs. 2.91 ± 4.56%, p=0.023, respectively).
As predicted, according to the FRAX model, the risk of major osteoporotic fractures and hip fractures in the T2DM cohort in the next 10 years was higher compared with the non-DM cohort. Additionally, in previous cohort studies, fracture risk was higher in patients with T2DM compared with non-DM individuals.1,7 Although the FRAX model can estimate the risk of major osteoporotic fractures and hip fractures in the next 10 years in patients with T2DM, there is a concern that the model for predicting fracture risk might be performed inadequately in patients with T2DM. This may be related to some of the risk factors in the FRAX model (e.g., smoking, which is less common in patients with T2DM) and to other common risk factors in the cohort (e.g., falls), which are not included in the FRAX model. It is also suggested that the FRAX model should be further improved to evaluate the risk of major osteoporotic fractures and hip fractures in the next 10 years.
Some limitations should be acknowledged in the present study. First, the retrospective nature of this study has inherent limitations and potential confounding variables (i.e., underlying diseases) that were not addressed. Additionally, because this is a descriptive study with a lack of follow-up data, it is impossible to analyze the time variable and the power tends may be underestimated. Second, generalizability is lacking because our study population included only postmenopausal women. Third, our data were gathered from several institutions, where differences in the number of diagnostic procedures could introduce statistical errors. However, these data were merged based on standardized methods, which, to some extent, provides reliability.
In conclusion, the results reported here provide additional evidence that postmenopausal women with T2DM had a significantly higher fracture risk compared with postmenopausal women without DM, as calculated using the FRAX model, and that application of this model in these patients requires further improvement. Some variables, such as HbA1c, the number of hypoglycemic seizures and diabetes complications, should be considered in future studies.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 814721255).
References
- 1.Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 Diabetes. JAMA 2011; 305: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanis JA, Harvey NC, Cooper C, et al. A systematic review of intervention thresholds based on FRAX A report prepared for the National Osteoporosis guideline group and the International Osteoporosis Foundation. Arch Osteoporos 2016; 11(1): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shan PF, Wu XP, Zhang H, et al. Age-related bone mineral density, osteoporosis rate and risk of vertebral fracture in mainland Chinese women with type 2 diabetes mellitus. J Endocrinol Invest 2011; 34: 190–196. [DOI] [PubMed] [Google Scholar]
- 4.Miyake H, Kanazawa I, Sugimoto T, et al. Association of bone mineral density, bone turnover markers, and vertebral fractures with all-cause mortality in type 2 Diabetes Mellitus. Calcif Tissue Int 2018; 102: 1–13. [DOI] [PubMed] [Google Scholar]
- 5.Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 Diabetes Mellitus. J Clin Endocrinol Metab 2016; 101: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCloskey EV, Oden A, Harvey NC, et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 2016; 31: 940–948. [DOI] [PubMed] [Google Scholar]
- 7.Middleton RG, Shabani F, Uzoigwe CE, et al. FRAX and the assessment of the risk of developing a fragility fracture. J Bone Joint Surg Br 2012; 94B: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 8.Viegas M, Costa C, Lopes A, et al. Prevalence of osteoporosis and vertebral fractures in postmenopausal women with type 2 diabetes mellitus and their relationship with duration of the disease and chronic complications. J Diabetes Complications 2011; 25: 216–221. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA, Oden A, Johansson H, et al. FRAX (R) and its applications to clinical practice. Bone 2009; 44: 734–743. [DOI] [PubMed] [Google Scholar]
- 10.Kanis JA, Johnell O, Oden A, et al. FRAX (TM) and the assessment of fracture probability in men and women from the UK. Osteoporos Int 2008; 19: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int 2011; 22: 2395–2411. [DOI] [PubMed] [Google Scholar]
- 12.Giangregorio LM, Leslie WD, Lix LM, et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res 2012; 27: 301–308. [DOI] [PubMed] [Google Scholar]
- 13.Johansson H, Azizieh F, al Ali N, et al. FRAX-vs. T-score-based intervention thresholds for osteoporosis. Osteoporos Int 2017; 28: 3099–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop+10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012; 97: 1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raphael C, Briscoe C, Davies J, et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart 2007; 93: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopchak O. Efficacy of citicoline in the treatment of patients with vascular cognitive impairment. J Neurol 2010; 257: S128–S. [Google Scholar]
- 17.Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016; 39: 2065–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014; 25: 2359–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black DM, Rosen CJ. Postmenopausal Osteoporosis. N Engl J Med 2016; 374: 254–262. [DOI] [PubMed] [Google Scholar]
- 20.Kanis JA, McCloskey EV, Johansson H, et al. Case finding for the management of osteoporosis with FRAX (R) - assessment and intervention thresholds for the UK. Osteoporos Int 2008; 19: 1395–1408. [DOI] [PubMed] [Google Scholar]
- 21.de Liefde II, van der Klift M, de Laet C, et al. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int 2005; 16: 1713–1720. [DOI] [PubMed] [Google Scholar]
- 22.Carnevale V, Morano S, Fontana A, et al. Assessment of fracture risk by the FRAX algorithm in men and women with and without type 2 diabetes mellitus: a cross- sectional study. Diabetes Metab Res Rev 2014; 30: 313–322. [DOI] [PubMed] [Google Scholar]
- 23.Bhattoa HP, Onyeka U, Kalina E, et al. Bone metabolism and the 10-year probability of hip fracture and a major osteoporotic fracture using the country-specific FRAX algorithm in men over 50 years of age with type 2 diabetes mellitus: a case-control study. Clin Rheumatol 2013; 32: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 24.Bonaccorsi G, Fila E, Messina C, et al. Comparison of trabecular bone score and hip structural analysis with FRAX (R) in postmenopausal women with type 2 diabetes mellitus. Aging Clin Exp Res 2017; 29: 951–957. [DOI] [PubMed] [Google Scholar]
- 25.Poiana C, Capatina C. Fracture risk assessment in patients with diabetes mellitus. J Clin Densitom 2017; 20: 432–443. [DOI] [PubMed] [Google Scholar]
- 26.Leslie WD, Johansson H, McCloskey EV, et al. Comparison of methods for improving fracture risk assessment in diabetes: the Manitoba BMD registry. J Bone Miner Res 2018; 33: 1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutrim D, Pereira FA, de Paula FJA, et al. Lack of relationship between glycemic control and bone mineral density in type 2 diabetes mellitus. Braz J Med Biol Res 2007; 40: 221–227. [DOI] [PubMed] [Google Scholar]
- 28.Shan PF, Wu XP, Zhang H, et al. Bone mineral density and its relationship with body mass index in postmenopausal women with type 2 diabetes mellitus in mainland China. J Bone Miner Metab 2009; 27: 190–197. [DOI] [PubMed] [Google Scholar]
- 29.Arikan S, Tuzcu A, Bahceci M, et al. Insulin resistance in type 2 Diabetes Mellitus may be related to bone mineral density. J Clin Densitom 2012; 15: 186–190. [DOI] [PubMed] [Google Scholar]
- 30.Cakmak HA, Cakmak BD, Yumru AE, et al. The relationships between blood pressure, blood glucose, and bone mineral density in postmenopausal Turkish women. Ther Clin Risk Manag 2015; 11: 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui RT, Zhou I, Li ZH, et al. Assessment risk of osteoporosis in Chinese people: relationship among body mass index, serum lipid profiles, blood glucose, and bone mineral density. Clin Interv Aging 2016; 11: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang YP, Gong Y, Zeng QY, et al. A long-term, observational cohort study on the safety of low-dose glucocorticoids in ankylosing spondylitis: adverse events and effects on bone mineral density, blood lipid and glucose levels and body mass index. BMJ Open 2015; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density - The Women’s Health Initiative randomized trial. JAMA 2003; 290: 1729–1738. [DOI] [PubMed] [Google Scholar]
- 34.Villareal DT, Binder EF, Williams DB, et al. Bone mineral density response to estrogen replacement in frail elderly women - A randomized controlled trial. JAMA 2001; 286: 815–820. [DOI] [PubMed] [Google Scholar]
- 35.Watson SL, Weeks BK, Weis LJ, et al. High-intensity resistance and impact training improves bone mineral density and physical function in postmenopausal women with osteopenia and osteoporosis: The LIFTMOR randomized controlled trial. J Bone Miner Res 2018; 33: 211–220. [DOI] [PubMed] [Google Scholar]
- 36.Balemans W, Van Hul W. Minireview: The genetics of low-density lipoprotein receptor-related protein 5 in bone: A story of extremes. Endocrinology 2007; 148: 2622–2629. [DOI] [PubMed] [Google Scholar]
- 37.Bansal S, Pecina JL, Merry SP, et al. US preventative services task force FRAX threshold has a low sensitivity to detect osteoporosis in women ages 50-64 years. Osteoporos Int 2015; 26: 1429–1433. [DOI] [PubMed] [Google Scholar]
- 38.Compston J, Bowring C, Cooper A, et al. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 2013; 75: 392–396. [DOI] [PubMed] [Google Scholar]
- 39.Clark P, Denova-Gutierrez E, Zerbini C, et al. FRAX-based intervention and assessment thresholds in seven Latin American countries. Osteoporos Int 2018; 29: 707–715. [DOI] [PubMed] [Google Scholar]
- 40.Bridges MJ, Ruddick SA. Do FRAX/NOGG guidelines predict fractures in post - menopausal women with Type 2 diabetes? Diabet Med 2012; 29: 555–556. [DOI] [PubMed] [Google Scholar]