Abstract
Background
Immune checkpoint inhibition (ICI) represents a novel treatment modality for refractory cancers, and improving prediction of potential responders is critical.
Method
We hypothesized that ICI is a systemic-effecting mechanism. The objective response rate (ORR) for anti-PD-1, anti-PD-L1, anti-CTLA-4, or combination therapy was plotted against the corresponding all-grade and grade 3–4 (G3/4) treatment-related adverse events (TRAEs) across several cancer types using an extensive literature search (MEDLINE and Google Scholar; December 1, 2012–December 30, 2017).
Results
Sixty-six eligible studies comprised 76 cohorts and 25 cancer types. A significant correlation was present between all-grade or G3/4 TRAEs and the ORR. The correlation coefficient was 0.5 for all-grade and 0.4 for G3/4 TRAEs, suggesting that >50% of the differences in the ORR across cancer types may be reflected by TRAEs and 40% of ORR differences may be predicted by G3/4 TRAEs. Hodgkin’s lymphoma and Merkel cell carcinoma showed a better response, while adrenocortical cancer, breast cancer, and uveal melanoma showed a worse response, compared with that predicted by TRAE.
Conclusion
There is a strong relationship between TRAEs and ICI activity across multiple cancers. The toxicity profile compared with the ORR to ICIs should be investigated in phase I trials.
Keywords: Cancer immunity, immune checkpoint inhibition, treatment-related adverse events, objective response rate, refractory cancer, toxicity profile
Introduction
Immune checkpoint inhibitors (ICIs) that target programmed cell death 1 (PD-1), its ligand (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) have demonstrated remarkable clinical benefit in various cancers.1 These three molecules represent the most critical and druggable targets for the inhibitory immune response and tumor cells hijack these molecules to exert immune exclusion.2 One of the greatest dilemmas ICIs face clinically is the bipolar phenomenon showing satisfactory and durable effect in responders, but the overall objective response rate (ORR) remains less than 50% across all applicable cancers.3 Given its high cost and potential adverse effects, identifying potential responders is of great interest. However, reliable predictors have yet to be validated.4
Recent studies suggest that immune-related adverse events (IrAEs) can predict the response to PD-1/PD-L1 inhibition in some cancers.5–7 IrAEs are generated by increased activity of the immune system in response to ICIs, and they can occur in multiple systems including, most commonly, the gastrointestinal tract, endocrine glands, skin, and liver, and less often in the central nervous system and cardiovascular, pulmonary, musculoskeletal, and hematologic systems.8 Although the precise mechanism of IrAEs remains unclear, the response represents a global alteration in immune status in the body by enhancing immune reactions in both tumor microenvironment and normal tissue.9
However, the definition of IrAE varies based on the different criteria and agents that are used. Some IrAEs can be misclassified as treatment-related adverse events (TRAEs). Reports of TRAEs, however, are relatively uniform across studies. Thus, we speculate that TRAEs may be a surrogate marker for the response to ICIs. In the current study, we aimed to globally examine the correlation between the prevalence of TRAEs, in particular high-grade TRAEs and the ORR of ICI across various types of cancer.
Materials and methods
We conducted an extensive literature search via MEDLINE and Google Scholar for published trials (December 1, 2012 to December 30, 2017) that modified the established criteria.3 Studies with anti-PD-1, anti-PD-L1, or anti-CTLA-4 monotherapy or a combination of any two of these therapies that enrolled at least ten patients who had no PD-L1 tumor expression were included in our analysis. We aimed to identify clinical studies that reported an objective response data for PD1, PD-L1, or anti-CTLA-4 inhibitor monotherapy or a combination of these inhibitors in major solid tumor types or subtypes for which TRAEs, grade 3–4 (G3/4) TRAEs, and ORR were reported. Because IrAEs are encompassed by TRAEs, studies that only reported IrAEs were also allowed. Databases including MEDLINE and Google Scholar, as well as abstracts presented at the American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the American Association for Cancer Research (AACR) were also used to identify clinical data for anti-PD1 or anti-PD-L1 therapy in each of these cancer types or subtypes. Abstracts that were later published as articles were merged. We searched for clinical trials using the following specific search terms: nivolumab, BMS-936558, pembrolizumab, MK-3475, atezolizumab, MPDL3280A, durvalumab, MEDI4736, avelumab, MSB0010718C, BMS-936559, cemiplimab, and REGN2810. Generally, only the largest published study for each anti-PD1 therapy was included in the final assessment of the pooled ORR for each cancer type or subtype. In tumor types for which phase 3 studies of any anti-PD1 therapy had been conducted, we also excluded other studies enrolling fewer than 40 patients or dose-finding studies with other anti-PD1 agents. Data on the response to treatment and TRAEs were pooled from each included study. We plotted the ORR for anti-PD-1, anti-PD-L1, anti-CTLA-4, or combination therapy against the corresponding all-grade and G3/4 TRAEs across a variety of cancer types. The correlations between ORR and all-grade and G3/4 TRAEs were analyzed using the Pearson’s correlation test and Spearman’s correlation test, respectively. Correlation across all cancer types was fit with the linear regression model.
Results
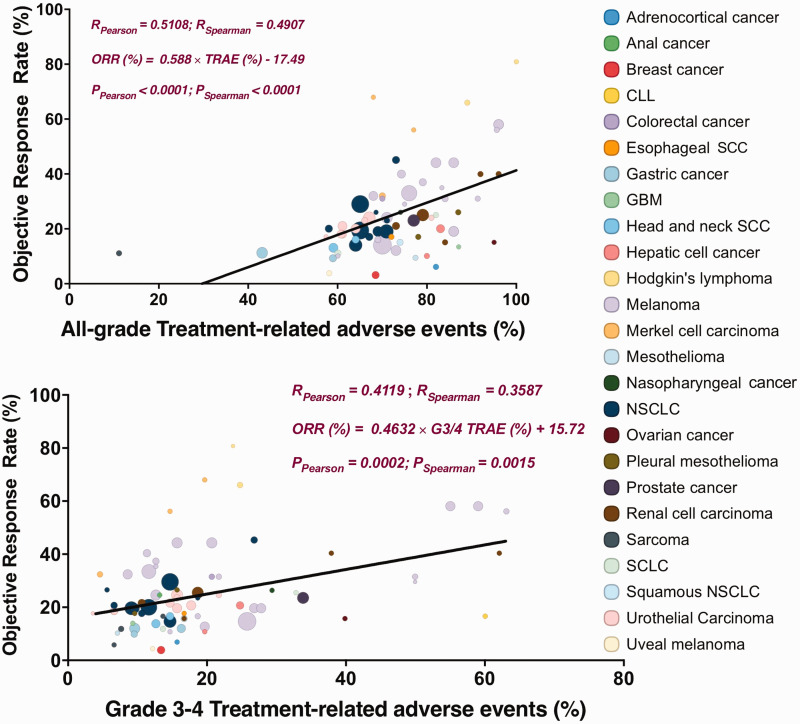
We identified 66 eligible studies, among which 76 cohorts encompassed 25 types of cancers including non-small cell lung cancer (NSCLC), renal cell carcinoma, melanoma, urothelial carcinoma, squamous NSCLC, Hodgkin’s lymphoma, head and neck squamous cell carcinoma (SCC), Merkel cell carcinoma, urothelial carcinoma, esophageal squamous-cell carcinoma, anal cancer, nasopharyngeal carcinoma, colorectal cancer, chronic lymphocytic leukemia, Hodgkin’s lymphoma, prostate cancer, adrenocortical carcinoma, breast cancer, gastric cancer, glioblastoma multiforme (GMB), hepatic cell cancer, pleural mesothelioma, mesothelioma, ovarian cancer, sarcoma, and uveal melanoma. We first identified seven types of cancer that were involved in more than three trials (Table 1). TRAEs were significantly correlated with the response in melanoma and urothelial carcinoma, while G3/4 TRAEs were significantly correlated with the response in melanoma, Merkel cell carcinoma, and urothelial carcinoma (Table 1). G3/4 TRAEs showed an almost linear match with the response in Merkel cell carcinoma. When all cancer types were included, we observed a significant correlation between ORR and all-grade TRAEs or G3/4 TRAEs (Figure 1).
Table 1.
Cancer types that were investigated in three or more trials were analyzed individually to determine the correlation between the ORR and all-grade and grade 3/4 TRAEs.
|
ORR |
||
|---|---|---|
| TRAE | G3/4TRAE | |
| Gastric Cancer | ||
| p value | – | 0.658 |
| r | – | 0.511 |
| Melanoma | ||
| p value | 0.0003 | 0.023 |
| r | 0.73 | 0.506 |
| Merkel Cell Carcinoma | ||
| p value | 0.9853 | <0.0001 |
| r | −0.023 | 1 |
| NSCLC | ||
| p value | 0.4097 | 0.055 |
| r | 0.277 | 0.593 |
| Renal Cell Carcinoma | ||
| p value | 0.1202 | 0.06 |
| r | 0.779 | 0.862 |
| Sarcoma | ||
| p value | – | 0.282 |
| r | – | 0.903 |
| Urothelial Carcinoma | ||
| p value | 0.0334 | 0.008 |
| r | 0.704 | 0.812 |
TRAEs, treatment-related adverse events; ORR, objective response rate; SCC, squamous cell carcinoma; NSCLC, non-small-cell lung carcinoma.
Figure 1.
Treatment-related adverse events (TRAEs) for all grades and grade 3–4 AEs among patients who received inhibitors of programmed death 1 (PD-1) protein or its ligand (PD-L1) or CTLA-4, as described in published studies where data on the objective response rate are available. Equations were determined using linear regression. R represents the correlation coefficients using Pearson’s and Spearman’s test, respectively. Circle size represents the number of patients in each cohort. CLL, chronic lymphocytic leukemia; SCC, squamous cell carcinoma; NSCLC, non-small-cell lung carcinoma; GBM, glioblastoma multiforme.
The correlation coefficient was 0.5 for all-grade and 0.4 for G3/4 TRAEs in both tests, suggesting that over half of the differences in the ORR across cancer types may be reflected by TRAEs and 40% of ORR differences may be predicted by G3/4 TRAEs. Generally, Hodgkin’s lymphoma and Merkel cell carcinoma had a better response compared with the prediction based on TRAEs, while adrenocortical cancer, breast cancer, and uveal melanoma had a worse response compared with the prediction based on TRAEs.
Discussion
In the current study, we showed that, from a global perspective, there was a correlation between ICI response and TRAEs. Whether TRAEs or IrAEs can predict drug response is an important clinical question because it pertains to decision-making in patients who are undergoing ICI therapy and who have AEs that suggest the response before a treatment response is noted.
The precise mechanism of ICI-induced AEs remains unclear and current understanding includes increasing T-cell activity against antigens that are present in tumors and healthy tissue, increasing levels of preexisting autoantibodies,10 increasing level of inflammatory cytokines,11 and enhanced complement-mediated inflammation resulting from direct binding of an antibody against CTLA-4, which is expressed on normal tissue.12 These AEs usually start within the first few weeks to months after treatment but they can occur anytime, even after treatment discontinuation. IrAEs are often managed with glucocorticoids, and if there is no response, other immunosuppressive agents are used. Such use does not appear to affect the ICI effect on tumor control while it mitigates AEs.13 It remains controversial whether IrAEs are related to the drug effect because there are many antigen specificities, and nonspecific activation of the immune system is not required to obtain the benefit from ICIs. This was also consistent with a study of ipilimumab showing that treatment outcomes were similar in patients with or without immune-related adverse events.14 However, CTLA-4 blockade entails a more global immune modulation than PD-1/PD-L1 blockade. To date, the only IrAE that is universally accepted as indicating an ICI response is the presence of vitiligo in melanoma patients.15 This is also consistent with our findings that both all-grade and G3/4 TRAEs correspond well with the response in melanoma. Consistent with our findings, a recent study supports the notion that IrAEs predict the response to monotherapy using PD-1 or PD-L1 blockade.16 AEs that indicate the response can be prevented by steroids, and they may still predict the future response in melanoma.17 However, the response to ICI monotherapy in most cancers is relatively low, and combinations of ICI and cancer-intrinsic genetic targeted therapy are now being used frequently in a variety of solid tumors.18 Because targeted therapies are associated with AEs by a different mechanism, they may overlap in organs or tissues, our the AEs may not fit well with the combination treatment.
In our study, the lower-than-anticipated ORR for adrenocortical cancer (ACC) suggests that a high prevalence of hormone excess may impair the response rate to ICI.19 This was intriguing because cortisol excess accounts for over 50% of the cases and very few tumor infiltrating lymphocytes (TIL) were present in ACC. Thus, for steroid management of IrAEs, we suggest that initial and delayed steroid use may impact differently on the ICI response. Additionally, while all studies for melanoma fall near the regression curve, uveal melanoma has a poor response to ICI, which was lower than anticipated based on the AEs. Such findings support a difference between uveal cancer and cutaneous melanoma, and a caveat for further testing of ICIs in these patients is proposed because of the high rate of AEs.20 There is agreement with the predictive effect between all-grade and G3/4 TREAs in most cancer types. The consistency of the response to ICI combination treatments (e.g. nivolumab/ipilimumab) with TRAEs in renal cell carcinoma and melanoma reflect the mechanistic similarity and functional supplementation of both agents.
Although linear correlation formulas were generated for all-grade and G3/4 TRAEs (Figure 1), this implication requires further insight. One suggestion is to predict ORR to ICIs using the toxicity profile that was reported in phase I trials. However, AEs develop over time and may present even after the response. The accessible data from the pooled studies did not allow analysis of the timeline for AE development. Whether early emergence of certain TRAEs is related to response warrants further investigation. Additionally, correlation between G3/4 TRAEs and the response suggests that prolonged treatment in patients who already have substantial AEs may be required, which may generate questions about the trade-off between the would-be benefit under prolonged AE tolerance and the timely discontinuation of treatment for safety reasons in cancer patients.
Conclusion
In the current study, we investigated relationship between TRAEs and the response to ICI treatment. Our findings highlight the strong relationship between the TRAEs and ICI activity across multiple cancers. One pragmatic use is to predict the ORR to ICIs using the toxicity profile that was reported in phase I trials.
List of abbreviations
TRAE, treatment-related adverse event; ICI, immune checkpoint inhibitor; G3/4, grade 3 to 4; ORR, object response rate; CTLA-4, cytotoxic T-lymphocyte antigen 4; IrAE, immune-related adverse effects; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1.
Supplemental Material
Supplemental material, IMR886454 Supplemental Material for Treatment-related adverse events and response rate to immune checkpoint inhibition by Yanmin Li, Zhengping Wang, Ting Guo, Shenghua Liu and Chenchen Feng in Journal of International Medical Research
Acknowledgement
This study was sponsored in parted by National Natural Science Foundation of China (No. 81874123).
Authors’ contributions
YL and TG wrote the manuscript, YL and SL performed the statistical analysis, and CF supervised all protocols.
Availability of data and material
The data and materials are not available to be deposited.
Consent for publication
All authors consent for the publication.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
This study did not require ethics approval or consent to participate.
Funding
This study was supported in part by the National Natural Science Foundation of China (Grant No. 81874123).
ORCID iD
Chenchen Feng https://orcid.org/0000-0002-1854-356X
References
- 1.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways. Am J Clin Oncol 2016; 39: 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Front Oncol 2018; 8: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377: 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins AM, Rowland A, Kichenadasse G, et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer 2017; 117: 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schadendorf D, Wolchok JD, Hodi FS, et al. Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: A pooled analysis of randomized phase II and III trials. J Clin Oncol 2017; 35: 3807–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018; 4: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: Characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016; 22: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Postow MA, Longo DL, Sidlow R, et al. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018; 378: 158–168. [DOI] [PubMed] [Google Scholar]
- 9.Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 2016; 375: 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small cell lung cancer. Ann Oncol 2017; 28: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbour SN, Maynard CL, Zindl CL, et al. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 2015; 112: 7061–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwama S, De Remigis A, Callahan MK, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014; 6: 230ra45. [DOI] [PubMed] [Google Scholar]
- 13.Weber JS, Yang JC, Atkins MB, et al. Toxicities of immunotherapy for the practitioner. J Clin Oncol 2015; 33: 2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering cancer center. J Clin Oncol 2015; 33: 3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 2017; 123: 2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogado J, Sánchez-Torres JM, Romero-Laorden N, et al. Immune-related adverse events predict the therapeutic efficacy of anti–PD-1 antibodies in cancer patients. Eur J Cancer 2019; 109: 21–27. [DOI] [PubMed] [Google Scholar]
- 17.Glutsch V, Grän F, Weber J, et al. Response to combined ipilimumab and nivolumab after development of a nephrotic syndrome related to PD-1 monotherapy. J Immunother Cancer 2019; 7: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choueiri TK, Larkin J, Oya M, et al. Preliminary results for avelumab plus axitinib as first-line therapy in patients with advanced clear-cell renal-cell carcinoma (JAVELIN Renal 100): an open-label, dose-finding and dose-expansion, phase 1b trial. Lancet Oncol 2018; 19: 451–460. [DOI] [PubMed] [Google Scholar]
- 19.Zheng S, Cherniack AD, Dewal N, et al. Comprehensive pan-genomic characterization of adrenocortical carcinoma. Cancer Cell 2016; 29: 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson AG, Shih J, Yau C, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 2018; 33: 151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, IMR886454 Supplemental Material for Treatment-related adverse events and response rate to immune checkpoint inhibition by Yanmin Li, Zhengping Wang, Ting Guo, Shenghua Liu and Chenchen Feng in Journal of International Medical Research
Data Availability Statement
The data and materials are not available to be deposited.