Abstract
Objective
A systematic review and meta-analysis was carried out to evaluate the efficacy and safety of Botulinum Toxin Type A in painful knee osteoarthritis.
Methods
The EMBASE and MEDLINE databases were searched to identify randomized controlled trials (RCTs) of Botulinum Toxin Type A in the treatment of painful knee osteoarthritis. The references of included literature were also searched.
Results
Five articles involving 5 RCTs including 314 patients were included in this analysis. There was a significant difference between Botulinum Toxin Type A and placebo in the visual analog scale (VAS) pain scale and Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC) questionnaire score in both the short-term (≤4 weeks) and long-term (≥8 weeks) treatment period. There were no serious adverse events in the Botulinum Toxin Type A groups.
Conclusions
This meta-analysis suggests that Botulinum Toxin Type A is effective and safe in the painful knee OA treatment. However, high-quality randomized controlled studies are still needed to further confirm our findings.
Keywords: Botulinum Toxin Type A, knee osteoarthritis, knee pain, randomized controlled trial, systematic review, meta-analysis
Introduction
Osteoarthritis (OA) is one of the most common forms of arthritis in patients, and is a common type of joint disease in the elderly. The knee is a common cause of chronic pain.1 Knee OA is a chronic and progressive disease that is one of the most common joint disorders around the world.2 It is characterized by articular cartilage degeneration, bony changes, and osteophyte formation. Knee OA often leads to swelling and joint pain and dysfunction, which may affect patients’ quality of life and contribute to depression.3
The primary aims in the treatment of knee OA are relieving pain, reducing the inflammatory response, restoring function, and slowing the progression of the disease. The American College of Rheumatology has recommended an initial noninvasive and nonoperative treatment plan for knee OA that includes rest, weight loss, physical modalities, bracing and therapeutic exercises, assistive devices, and pharmacological interventions.4 The most common pharmacological interventions include oral and topical analgesics, cyclooxygenase-2 inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), and opioids.5 Besides, if orally administered drugs cannot control symptoms, intraarticular (IA) injections can be a final nonoperative option. However, the therapeutic effect of IA injections is uncertain.6 Limited evidence has suggested that corticosteroids in knee OA therapy may be effective, especially in controlling pain, but only in the short term.7
Botulinum toxin type A (BoNT-A) has been used clinically for its paralytic effects, while increasing evidence suggests that it may have a role in pain modulation. Two previous meta-analyses published in 2017 and 2018 indicated that compared with placebo, BoNT-A IA injections have beneficial effects, with improved pain and WOMAC score in adult patients with refractory joint pain.8,9 However, thus far no meta-analysis or systematic review has been performed that has focused on the efficacy and safety of BoNT-A in painful knee OA.
The purpose of our study was to perform a systematic review and meta-analysis to evaluate the efficacy and safety of BoNT-A in treating painful knee OA.
Materials and methods
We searched Medline, Cochrane Controlled Trials Register databases, and Embase for randomized controlled trials (RCTs) published before Jul 1, 2019 using the following search criteria: Botulinum Toxin Type A, knee osteoarthritis, and RCTs. We limited our search to published studies in English only and obtained certain essential information directly from the authors for some studies. Besides, we screened the relevant references of included studies to identify other possible studies for inclusion.
Inclusion criteria
In our search, accepted studies were to include the following characteristics: (1) BoNT-A therapy and placebo therapy analyzed for patients with knee osteoarthritis; (2) full text available; and (3) visual analog scale (VAS) pain scale and Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC) questionnaire score.
Quality assessment
We used the Jadad scale to assess the quality of individual studies.10 We evaluated the quality of the studies based on allocation sequence generation, blinding method, and concealment of the allocation process. We divided the quality of each study into three levels: quality degree “A” if the study satisfied all quality criteria; quality degree “B” if the study had one or more ambiguous quality criteria; and quality degree “C” if the study had a high risk of bias and met few of the quality criteria. All of the authors assessed the quality of the RCTs and agreed with the final results.
Data extraction
We recorded the following information from the studies: (1) the authors’ first names and year of publication; (2) intervention method; (3) sample size; (4) inclusion criteria; (5) follow-up time, and (6) changes in the VAS pain scale and WOMAC questionnaire score.
Statistical analysis and meta-analysis
We used the RevMan (Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) to analyze differences between the variables.11 Using the included studies' data, we summarized changes in VAS pain scale and WOMAC questionnaire score. We used the mean difference (MD) to evaluate the continuous data, and we used the odds ratio (OR) with 95% confidence intervals (CI) to evaluate dichotomous data. A fixed-effects model was considered suitable for studies with p > 0.05, which was recognized as homogeneous. Inconsistent results were analyzed using the I2 statistic, which represents the proportion of heterogeneity among the studies. We used a random-effects model for studies with a p < 0.05 and where I2 > 50%. We considered p < 0.05 to indicate statistical significance.12
Results
Characteristics of individual studies
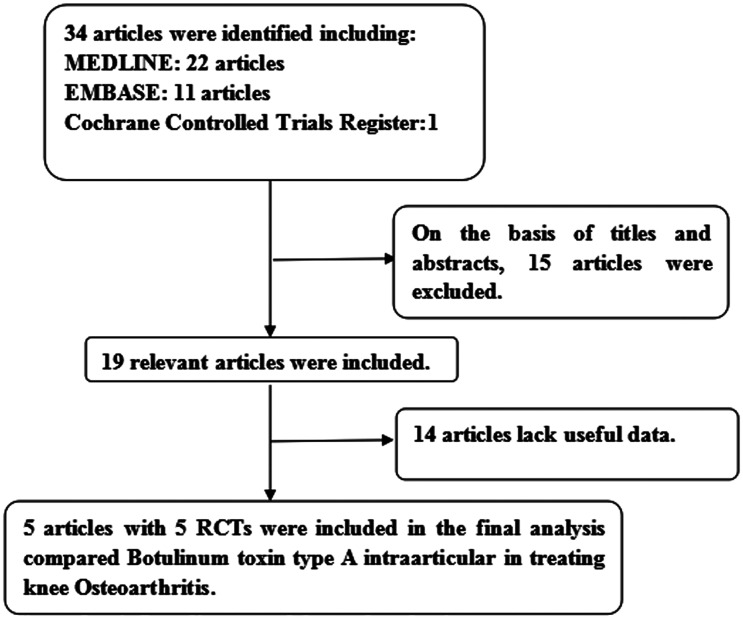
Our search identified 34 studies, and after reviewing their abstracts we excluded 15 studies. Among the remaining 19 studies, 14 studies were excluded for lack of useful data. Finally, 5 RCTs13–17 were used to evaluate the efficacy and safety of BoNT-A in painful knee OA (Figure 1 describes the search process in detail). Table 1 shows the baseline characteristics of these 5 studies.
Figure 1.
A flow diagram of the study selection process. RCT, randomized controlled trial.
Table 1.
Study and patient characteristics
| Study | Therapy in experimental group | Therapy in control group | Country |
Sample size |
Administration method | Duration of treatment | Dosage | Inclusion population | |
|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | ||||||||
| Mahowald ML 2009 | BoNT-A | Placebo | USA | 21 | 21 | Intraarticular injection | 4/12 w | 100 U | Arthritis knee pain ≥4.5 NRS; had not responded adequately to oral analgesic medications or intra-articular injections of corticosteroids and/or viscosupplements. |
| Arenelt-Nielsen L 2016 | BoNT-A | Placebo | Denmark | 61 | 60 | Intraarticular injection | 4/8 w | 200 U | Idiopathic knee OA who have had stable knee pain for ≥ 6 months before visit 1, with an ADWP score of 4.0–9.0. |
| Hsieh LF 2016 | BoNT-A | Placebo | Taiwan | 21 | 20 | Intraarticular injection | 1/18 w | 100 U | Knee OA disease duration ≥ 3 months with a radiographic OA severity grade between 2 and 3, VAS score ≥ 4, and failure of previous conservative treatments. |
| Bao X 2018 | BoNT-A | Placebo | China | 20 | 20 | Intraarticular injection | 4/8 w | 100 U | Radiographic OA severity grade ≥2 and pain VAS score ≥6; failure of physical therapy and/or medical treatment in the last 3 months; involvement of unilateral knee joint through clinical check and bilateral X-ray of the knees. |
| Mendes JG 2019 | BoNT-A | Placebo | Brazil | 35 | 35 | Intraarticular injection | 4/8 w | 100 U | Mild to moderate OA according to the Kellgren-Lawrence classification (grades II or III), knee pain for more than six months, knee pain at rest between 3 and 8 cm. |
BoNT-A: Botulinum toxin type A, OA: Osteoarthritis, ADWP: average daily worst pain, VAS: visual analog scale, w: week
Quality of the individual studies
All five of the included studies were RCTs. Each of the included studies had a scientific calculation of sample size. The intention-to-treat analysis was shown in one study.17 All of the included studies were of high quality, with a Jadad scores rating A (Table 2). The plot was highly symmetrical and no evidence of bias was found (Figure 2).
Table 2.
Quality assessment of individual studies
| Study | Allocation sequence generation | Allocation concealment | Blinding | Loss to follow-up | Calculation of sample size | Statistical analysis | Level of quality |
|---|---|---|---|---|---|---|---|
| Mahowald ML 2009 | A | A | A | 0 | YES | Not mentioned | B |
| Arenelt-Nielsen L 2016 | A | A | A | 1 | YES | Analysis of variance | A |
| Hsieh LF 2016 | A | A | A | 0 | YES | Mann-Whitney U test | A |
| Bao X 2018 | A | A | A | 0 | YES | Analysis of variance | A |
| Mendes JG 2019 | A | A | A | 6 | YES | Analysis of variance | A |
A – all quality criteria met (adequate): low risk of bias.
B – one or more of the quality criteria only partly met (unclear): moderate risk of bias.
C – one or more criteria not met (inadequate or not used): high risk of bias.
Figure 2.
Funnel plot of the studies represented in the meta-analysis.
MD: mean difference, SE: standard error.
Efficacy
We studied the changes in the measurement parameters in both the short-term (≤4 weeks) and long-term (≥8 weeks) treatment period to determine the efficacy of BoNT-A in painful knee OA.
VAS pain scale
Measurements at short-term (≤4 weeks)
Five studies involving 314 knee OA patients (158 in the BoNT-A therapy group and 156 in the placebo group) contained meaningful data on VAS pain scale. A random-effects model was used to evaluate changes between the two groups, which showed a MD of −1.21 (95% CI: −1.88 to −0.55, P=0.0004). Patients who received BoNT-A IA injection therapy had obvious improvement in the short-term VAS pain scale (Figure 3a).
Figure 3.
Forest plots showing changes in (a) VAS pain scale short-term (≤4 weeks) and (b) VAS pain scale long-term (≥8 weeks) in the treatment studies.
BoNT-A: Botulinum toxin type A, SD: standard deviation, IV: inverse variance, CI: confidence interval VAS: visual analog scale.
Measurements at long-term (≥8 weeks)
Four studies enrolling 272 knee OA patients (137 in the BoNT-A IA injection therapy group and 135 in the placebo group) were included, and a random-effects model revealed a significant decrease in the long-term VAS pain scale (MD: −1.40, 95% CI: −2.21 to −0.60, P = 0.0006). (Figure 3b).
WOMAC questionnaire score
Measurements at short-term (≤4 weeks)
Four studies including four comparisons were used to analyze results for WOMAC questionnaire score at the short-term. A fixed-effects model including 193 knee OA patients (97 in the BoNT-A therapy group and 96 in the placebo group) revealed an MD of −5.37 (95% CI: −7.18 to −3.57 (P<0.00001) and indicated that BoNT-A IA injection therapy resulted in lower WOMAC questionnaire scores (Figure 4a).
Figure 4.
Forest plots showing changes in (a) WOMAC questionnaire score short-term (≤4 weeks) and (b) WOMAC questionnaire score long-term (≥8 weeks) in the treatment studies.
BoNT-A: Botulinum toxin type A, SD: standard deviation, IV: inverse variance, CI: confidence interval, WOMAC: Western Ontario & McMaster Universities Osteoarthritis Index.
Measurements at long-term (≥8 weeks)
Five studies including five comparisons were used to analyze results for WOMAC questionnaire score at long-term. A random-effects model including 314 knee OA patients (158 in the BoNT-A therapy group and 156 in the placebo group) revealed an MD of −7.10 (95% CI: −10.89 to −3.31 (P = 0.0002) and indicated that BoNT-A IA injection therapy revealed a significant decrease in the long-term WOMAC questionnaire scores (Figure 4b).
Adverse events
No serious adverse events, such as death, sensory dysfunction or new lower limb motor dysfunction, anaphylactic reaction to the injection, or inflammation at the injection site occurred during these studies. No transient muscle weakness was found in any of the groups.
Discussion
Knee OA is a common joint disease that affects 250 million patients around the world.18 However, the health organizations have not approved any single therapeutic method as the standard treatment method for knee OA. Joint replacement surgery is an option for patients with advanced stages of the disease.19 Recently, there is increased interest in the new medical applications of BoNT-A. Although the clinical application of BoNT-A seems promising, there is still insufficient evidence on its therapeutic effects.
Our meta-analysis included five studies including 314 patients comparing the efficacy and safety of BoNT-A IA injection (100U/200U) to a placebo in treating men with knee OA in both the short-term (≤4 weeks) and long-term (≥8 weeks) periods. The analysis found that BoNT-A IA injection had a greater improvement than placebo in terms of the VAS pain scale and WOMAC questionnaire score in both the short-term and long-term treatment periods. Additionally, in the studies of Bao et al.16 and Mendes et al.,17 BoNT-A IA injection demonstrated an acceptable safety profile, with improvements in the Physical Component Summary-36, Mental Component Summary-36, and ultrasound measurement of synovial hypertrophy.
The mechanism of action of BoNT-A is to inhibit the release of acetylcholine from the exocytosis of motor nerve endings.20 This makes it useful for the treatment of several pathological conditions involving excessive muscle contraction, such as paralysis, painful dyskinesia, and other pain conditions.21 However, increasing evidence has indicated that BoNT-A can relieve pain by inhibiting the release of selective neuropeptide transmitters, thus directly reducing peripheral sensitization and indirectly reducing central sensitization.22
For safety, the study showed that no serious adverse events occurred, such as death, sensory dysfunction or new lower limb motor dysfunction, anaphylactic reaction to the injection, or inflammation of the injection site. No transient muscle weakness was found in any of the groups. Other adverse events were well tolerated and the relevant data were lacking from the included studies. However, Dutra23 found that BoNT-A injection in the masseter of mice can significantly damage the mandibular condylar cartilage and subchondral bone and the damage is not transient. Therefore, whether long-term use of BoNT-A will lead to histological changes still requires additional high-quality RCTs to prove. As for the injection route, all of the included RCTs injected BoNT-A into the articular cavity, and there have been no studies focusing on the difference between intra-articular injection and intra-muscular injection.
This meta-analysis includes findings only from RCTs. From a scientific point of view, the results of this analysis are very important. However, the number of included studies is small. Selection bias, subjective factors, and publication bias may also affect the final results of our study. These factors may lead to bias. More high-quality trials with larger sample sizes are needed to verify the efficacy and safety of BoNT-A IA injection therapy for painful knee OA.
Conclusion
This meta-analysis suggests that Botulinum Toxin Type A is an effective and safe approach for the treatment of painful knee OA. However, large-scale multicenter RCTs are still needed to further confirm our findings.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med 2010; 26: 355–369. DOI: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolf AD, Akesson K. Understanding the burden of musculoskeletal conditions. The burden is huge and not reflected in national health priorities. BMJ 2001; 322: 1079–1080. DOI: 10.1136/bmj.322.7294.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikeuchi M, Izumi M, Aso Ket al. Clinical characteristics of pain originating from intra-articular structures of the knee joint in patients with medial knee osteoarthritis. Springerplus 2013; 2: 628. DOI: 10.1186/2193-1801-2-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birmingham TB, Kramer JF, Kirkley Aet al. Knee bracing for medial compartment osteoarthritis: effects on proprioception and postural control. Rheumatology (Oxford) 2001; 40: 285–289. DOI: 10.1093/rheumatology/40.3.285. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg MC, Altman RD, April KTet al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012; 64: 465–474. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Lawrence RC, Dieppe PAet al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000; 133: 635–646. DOI: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 7.Juni P, Hari R, Rutjes AWet al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015; 10: Cd005328. DOI: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courseau M, Salle PV, Ranoux Det al. Efficacy of intra-articular botulinum toxin in osteoarticular joint pain: a meta-analysis of randomized controlled trials. Clin J Pain 2018; 34: 383–389. DOI: 10.1097/ajp.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 9.Wu T, Song HX, Dong Yet al. Intra-articular injections of botulinum toxin a for refractory joint pain: a systematic review and meta-analysis. Clin Rehabil 2017; 31: 435–443. DOI: 10.1177/0269215516644951. [DOI] [PubMed] [Google Scholar]
- 10.Jadad A. Randomised controlled trials. London: BMJ Publishing Group, 1998. [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1998; 3: 177–188. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJet al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. DOI: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahowald ML, Krug HE, Singh JAet al. Intra-articular Botulinum Toxin Type A: a new approach to treat arthritis joint pain. Toxicon 2009; 54: 658–667. DOI: 10.1016/j.toxicon.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh LF, Wu CW, Chou CCet al. Effects of botulinum toxin landmark-guided intra-articular injection in subjects with knee osteoarthritis. PM R 2016; 8: 1127–1135. DOI: 10.1016/j.pmrj.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Arendt-Nielsen L, Jiang GL, DeGryse Ret al. Intra-articular onabotulinumtoxinA in osteoarthritis knee pain: effect on human mechanistic pain biomarkers and clinical pain. Scand J Rheumatol 2017; 46: 303–316. DOI: 10.1080/03009742.2016.1203988. [DOI] [PubMed] [Google Scholar]
- 16.Bao X, Tan JW, Flyzik Met al. Effect of therapeutic exercise on knee osteoarthritis after intra-articular injection of botulinum toxin type A, hyaluronate or saline: a randomized controlled trial. J Rehabil Med 2018; 50: 534–541. DOI: 10.2340/16501977-2340. [DOI] [PubMed] [Google Scholar]
- 17.Mendes JG, Natour J, Nunes-Tamashiro JCet al. Comparison between intra-articular Botulinum toxin type A, corticosteroid, and saline in knee osteoarthritis: a randomized controlled trial. Clin Rehabil 2019; 33: 1015–1026. DOI: 10.1177/0269215519827996. [DOI] [PubMed] [Google Scholar]
- 18.Mills K, Hubscher M, O'Leary Het al. Current concepts in joint pain in knee osteoarthritis. Schmerz 2019; 33: 22–29. DOI: 10.1007/s00482-018-0275-9. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen FK, Egund N, Jorgensen Aet al. Risk factors for joint replacement in knee osteoarthritis; a 15-year follow-up study. BMC Musculoskelet Disord 2017; 18: 510. DOI: 10.1186/s12891-017-1871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev 1981; 33: 155–188. [PubMed] [Google Scholar]
- 21.Lew HL, Lee EH, Castaneda Aet al. Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: a pilot study. Arch Phys Med Rehabil 2008; 89: 75–80. DOI: 10.1016/j.apmr.2007.08.133. [DOI] [PubMed] [Google Scholar]
- 22.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 2005; 26: 785–793. DOI: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Dutra EH, Yadav S. The effects on the mandibular condyle of Botox injection into the masseter are not transient. Am J Orthod Dentofacial Orthop 2019; 156: 193–202. DOI: 10.1016/j.ajodo.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]