Abstract
Objective
We aimed to develop a simple and user-friendly scoring system to predict all-cause hospital-acquired infections (HAIs) after acute ischemic stroke (AIS) in the Chinese population.
Methods
AIS patients from a retrospective cohort study at our center were included from January 2016 to December 2018. HAIs were diagnosed based on the current criteria from Ministry of Health of the People’s Republic of China. Stepwise logistic regression models were performed to screen independent predictors of HAI after AIS. A scoring system was developed by including each of the above significant predictors.
Results
Among 1211 patients, 76 patients (6.28%) developed HAI. Age, baseline National Institute of Health stroke scale (NIHSS) score, and dysphagia were independent predictors of HAI. For the AND score, A refers to age, N refers to NIHSS, and D refers to dysphagia. The AND score showed a high area under the receiver operating characteristics (AUROC) curve (0.679), which comprised age (65–74 years was 4 points, 75–84 years was 6 points, ≥85 years was 8 points), NIHSS score ≥10 (5 points), and dysphagia (6 points).
Conclusions
We developed a simple scoring system to predict all-cause infections after AIS patients without a ventilator in the Chinese population.
Keywords: Acute ischemic stroke, hospital-acquired infection, prediction, AND score, NIHSS score, dysphagia
Introduction
A hospital-acquired infection (HAI) in the American Healthcare Safety Network is defined as a “localized or systemic condition that is preventable and results from an adverse reaction to the presence of an infectious agent with no evidence that the infection was present or incubating at the time of admission to the acute care setting”.1 HAI directly affects the course and prognosis of acute ischemic stroke (AIS) patients,2 extends the length of hospitalization, and increases the financial burden on the patients.3 It is necessary to identify high-risk patients so that we can take prevention measures and effective management.
Although several prediction models for hospital-acquired pneumonia (after ischemic stroke) have been generated, most of the scales require many variables and have limited clinical application.4–11 It is also necessary to try to prevent all types of infections rather than only preventing pneumonia. In contrast to studies aimed at predicting pneumonia, only two studies aimed at predicting all types of infections: one study enrolling 568 patients focused on ischemic stroke in New Orleans but the exclusion criteria were not very strict,7 and the other study focused on ischemic stroke and intracerebral hemorrhage in The Netherlands.11 Additionally, except for one study10 that excluded ventilated patients but restricted their analysis to only pneumonia after AIS, most studies were performed regardless of ventilator-associated infections, which have a different mechanism compared with other infections. Additionally, the infection rate and features of infection vary greatly from place to place, and there are no proper and practical all-cause infection prediction models for the Chinese population. Our study aimed to develop a simple risk-prediction model that focused on all-cause infections and that was based on baseline characteristics in AIS patients without a ventilator in the Chinese population.
Methods
Study population
Patients in this study were from our stroke registry database, which was a retrospective single-center hospital-based cohort study of consecutive patients who had transient ischemic attack (TIA) or AIS and who were admitted to the Department of Neurology at the First Affiliated Hospital in Sun Yat-Sen University from January 2016 to December 2018. The following routine clinical baseline factors were extracted from the patients’ medical records: age; sex; National Institute of Health stroke scale (NIHSS) score on admission; smoking; baseline comorbidities including hypertension, diabetes mellitus, hyperlipemia, deep vein thrombosis (DVT), myocardial infarction (MI), valvular heart disease (VHD), congestive heart failure (CHF; reported or documented past history and/or receiving relevant prescribed medication on admission), atrial fibrillation (AF; reported or documented past history and/or documentation of standard electrocardiographic findings on admission), previous stroke or TIA (reported or documented past history preceding the index admission stroke), and Trial of Org 10172 in Acute Stroke Treatment (TOAST) subtype,12 which included large-artery atherosclerosis (LAA), cardio-embolism (CE), small-artery occlusion (SAO), stroke of other determined etiology (SOE), and stroke of undetermined etiology (SUE).
Dysphagia, which refers to an abnormal water swallowing test, was recorded. A trained doctor performed the water swallowing test on admission by asking the patient to swallow 50 mL of water in 5 mL aliquots, and a meta-analysis showed that single sips offer good specificity.13 Dysphagia was verified if the patient developed choking, coughing, or an alteration in voice quality, at which point the test was discontinued and the amount that the patient swallowed was noted. Patients were considered to swallow normally if 50 mL was ingested.14
Infections following AIS were diagnosed by experienced doctors via clinical, laboratory, pathogen, and imaging modalities using current criteria of Ministry of Health of the People’s Republic of China in 2001.15 Infection sites were categorized as follows: respiratory system, cardiovascular system, blood system, abdomen and digestive system, central nervous system, urinary system, surgical site, skin and soft tissue, bone and joint, genital tract, oral cavity, and other sites.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients with AIS; age ≥18 years; time between stroke onset and arriving at the hospital was ≤7 days; and all patients were diagnosed by head CT or head MRI, based on the diagnostic criteria from the guidelines for healthcare professionals from the American Heart Association/American Stroke Association.16
The exclusion criteria were as follows: Patients were excluded if they were ventilated during their hospitalization; had an in-hospital stroke; had an infection that was present at admission; had malignant tumors; had incomplete medical data; or admissions were <48 hours in duration.
Ethics approval
This study was conducted in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the Declaration of Helsinki. The Institution of Committee for Ethics (ICE) for Clinical Research and Animal Trials of the First Affiliated Hospital of Sun Yat-sen University approved the study and informed consent was waived.
Statistical analysis
Descriptive statistics were calculated, which included the mean (standard deviation; SD) or median (interquartile range, IQR) for continuous variables, and the frequencies and percentages for categorical variables. Multivariable logistic regression was performed to screen independent predictors of HAI. Univariate logistic regression analysis was performed to determine whether the variables that were collected on admission were significant. Variables at a significance level of 0.1 in the univariate analysis were included in the multivariable logistic regression model by performing a backward elimination procedure. Thus, significant factors were obtained based on the beta estimates and a score was assigned for each risk factor based on the Framingham Study.17 Age was a continuous variable and should be changed to a categorizable variable when the score is assigned. In our study, age was divided into four stages, including <65 years, 65 to 74 years, 75 to 84 years, and ≥85 years. The following three risk categories for HAI were determined by the scores: low, medium, and high risk. The resulting scoring system was then validated by assessing model discrimination and calibration.18 Discrimination was assessed by calculating the area under receiver operating characteristic (ROC) curve. Calibration was assessed by performing the Hosmer–Lemeshow goodness-of-fit test and was graphically depicted in the plot of observed versus predicted HAI risk based on ten deciles of predicted risk. All hypotheses were two-tailed, and a P value of ≤0.05 was considered to be significant. We performed data analyses using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA), the statistical package R 3.50 (www.r-project.org), and MedCalc software (MedCalc Software Company, Oostende, Belgium).
Results
Baseline characteristics
There were 1344 patients enrolled into the stroke registry system, and among them, 59 were diagnosed as having a TIA. Based on the inclusion and exclusion criteria, 1211 patients were finally included in this study, and 66.14% of the patients were male. Additionally, 76 patients had infections during admission (35 patients were diagnosed with lower respiratory tract infections, 26 patients with upper respiratory tract infections, 15 patients with urinary tract infections, and five with other types of infections; overall, five patients had two kinds of infections). The incidence of HAI was 6.28% (76/1211). Baseline characteristics of the patients in the study are shown in Table 1.
Table 1.
Baseline characteristics of patients in the study.
| Clinical characteristics | Total |
Group |
P | |
|---|---|---|---|---|
| HAI group(n = 76) | Non-HAI group(n = 1135) | |||
| Age, mean (SD), years | 63.46 (13.74) | 68.87 (11.19) | 63.10 (13.82) | <0.001 |
| Age group, no. (%), years | 0.02 | |||
| <65 | 611 (50.45) | 585 (51.54) | 26 (34.21) | |
| 65–74 | 318 (26.26) | 295 (25.99) | 23 (30.26) | |
| 75–84 | 226 (18.66) | 206 (18.15) | 20 (26.32) | |
| ≥85 | 56 (4.62) | 49 (4.32) | 7 (9.21) | |
| Sex, no. (%) | 0.85 | |||
| Male | 801 (66.14) | 51 (67.11) | 750 (66.08) | |
| Female | 410 (33.86) | 25 (32.89) | 51 (67.11) | |
| TOAST subtype | 0.01 | |||
| LAA, no. (%) | 621 (51.36) | 42 (55.26) | 579 (51.1) | |
| CE, no. (%) | 116 (9.59) | 15 (19.74) | 101 (8.91) | |
| SAO, no. (%) | 332 (27.46) | 13 (17.11) | 319 (28.16) | |
| SOE, no. (%) | 56 (4.63) | 2 (2.63) | 54 (4.77) | |
| SUE, no. (%) | 84 (6.95) | 4 (5.26) | 80 (7.06) | |
| Medical history (state = “yes”), no. (%) | ||||
| Hypertension | 899 (79.49) | 57 (80.28) | 842 (79.43) | 0.86 |
| Hyperlipidemia | 313 (29.39) | 14 (21.88) | 299 (29.87) | <0.001 |
| Diabetes mellitus | 446 (41.49) | 32 (50.00) | 414 (40.95) | 0.35 |
| Atrial fibrillation | 125 (12.03) | 18 (28.13) | 107 (10.97) | <0.001 |
| Myocardial infarction | 135 (12.92) | 13 (20.31) | 122 (12.44) | 0.07 |
| Congestive heart failure | 35 (3.39) | 10 (16.13) | 25 (2.58) | <0.001 |
| Valvular heart disease | 67 (6.44) | 7 (10.94) | 60 (6.15) | 0.13 |
| Admission NIHSS score, median (IQR) | 4 (2–8) | 5 (3–13) | 4 (2–8) | <0.001 |
| Dysphagia (state = “yes”), no. (%) | 209 (18.05) | 28 (38.89) | 181 (16.67) | <0.001 |
| Smoking (state = “yes”), no. (%) | 469 (39.71) | 34 (48.57) | 435 (39.15) | 0.12 |
| Length of stay, mean (SD), days | 11.93 (6.58) | 19.85 (14.2267) | 11.36 (5.20) | <0.0001 |
| Hospitalization expenses, median (IQR), CNY | 12500 (17254–24121) | 31163 (20203–48964) | 16858 (12247–22807) | <0.0001 |
HAI, hospital acquired infection; TOAST, Trial of Org 10172 in Acute Stroke Treatment; LAA, large-artery atherosclerosis; CE, cardio-embolism; SAO, small-artery occlusion; SOE, stroke of other determined etiology; SUE, stroke of undetermined etiology; IQR, interquartile range; SD, standard deviation; CNY, Chinese Yuan.
Univariate and multivariate logistic analysis
Age, subtype of CE or SAO, baseline NIHSS, dysphagia, DVT, and heart disease (AF, CHF, MI and VHD) were associated with HAI in univariate analysis, but diabetes, hypertension, hyperlipemia, smoking, and sex were not associated with HAI. Age, dysphagia, and the baseline NIHSS score remained associated with HAI in a multivariate logistic regression model. An age of ≥65 years on admission was a significant predictor of HAI (RR = 1.03, 95% confidence interval [CI] 1.01–1.05, P < 0.01). AIS patients with baseline NIHSS score of ≥10 points had a significantly higher risk of HAIs compared with their counterparts with baseline NIHSS score of <10 points (RR = 1.90, 95%CI 1.04–3.48, P = 0.04). Additionally, patients with dysphagia had a significantly higher risk of HAIs compared with patients without dysphagia (RR = 2.17, 95%CI 1.20–3.94, P = 0.01). The results of logistic regression analyses are summarized in Table 2.
Table 2.
Predictors of hospital-acquired infection after stroke: univariate and multivariate logistic analysis.
| Variables | unadjusted RR (95%CI) | unadjusted P | adjusted RR (95%CI) | adjusted P |
|---|---|---|---|---|
| Age | 1.03 (1.02–1.05) | <0.01 | 1.03 (1.01–1.05) | <0.01 |
| Sex (male vs. female) | 0.96 (0.58–1.57) | 0.85 | ||
| Smoking (yes vs. no) | 1.40 (0.85–2.31) | 0.19 | ||
| CE (yes vs. no) | 2.52 (1.38–4.59) | <0.01 | – | 0.72 |
| SAO (yes vs. no) | 0.53 (0.29–0.97) | 0.04 | – | 0.11 |
| Hypertension (yes vs. no) | 1.05 (0.58–1.93) | 0.86 | ||
| Hyperlipidemia (yes vs. no) | 0.78 (0.44–1.40) | 0.41 | ||
| Diabetes mellitus (yes vs. no) | 1.41 (0.86–2.31) | 0.17 | ||
| Heart disease (yes vs. no) | 2.50 (1.50–4.16) | <0.01 | – | 0.15 |
| Admission NIHSS score (<10 vs. ≥10) | 2.62 (1.59–4.31) | <0.01 | 1.9 (1.04–3.48) | 0.04 |
| Dysphagia (yes vs. no) | 3.18 (1.93–5.25) | <0.001 | 2.17 (1.2–3.94) | 0.01 |
CE, cardio-embolism; CI, confidence interval; SAO, small-artery occlusion; NIHSS, National Institute of Health stroke scale.
AND score
The final prediction score for HAIs is shown in Table 3. Ranging from 0 to 19, the AND score (A refers to age, N refers to NIHSS, and D refers to dysphagia) consists of age (65–74 years conferred 4 points, 75–84 years conferred 6 points, ≥85 years conferred 8 points), NIHSS score ≥10 (5 points), and dysphagia (6 points). The three-level classification system for HAI after AIS was as follows: low (0–5 points), medium (6–10 points), and high risk (>10 points) (Table 4). AND score on admission can determine the HAI incidence; for example, a patient with a score of 1 has a 3.5% chance, while a patient with a score of 19 has a 31.2% chance, of having a HAI (Table 5).
Table 3.
Basic prognostic score (Ordinal scale 0–19 points).
| Predictors | Categories | Assigned Points |
|---|---|---|
| Age | ||
| <65 | 0 | |
| 65–74 | 4 | |
| 75–84 | 6 | |
| ≥85 | 8 | |
| NIHSS on admission | ||
| <10 | 0 | |
| ≥10 | 5 | |
| Dysphagia | ||
| 0 | 0 | |
| 1 | 6 |
NIHSS, National Institute of Health stroke scale.
Table 4.
Total scores and associated incidence of hospital-acquired infection.
| Total point | Risk category | Incidence of HAI no. (%) |
|---|---|---|
| 0–5 | Low | 24 (3.33) |
| 6–10 | Moderate | 23 (8.13) |
| 11–19 | High | 26 (14.77) |
Table 5.
Total scores and estimated risk of hospital-acquired infection.
| Point total | Estimated risk |
|---|---|
| 0 | 0.00 |
| 1 | 0.03 |
| 2 | 0.04 |
| 3 | 0.05 |
| 4 | 0.05 |
| 5 | 0.06 |
| 6 | 0.07 |
| 7 | 0.08 |
| 8 | 0.09 |
| 9 | 0.10 |
| 10 | 0.11 |
| 11 | 0.13 |
| 12 | 0.14 |
| 13 | 0.16 |
| 14 | 0.18 |
| 15 | 0.20 |
| 16 | 0.23 |
| 17 | 0.25 |
| 18 | 0.28 |
| 19 | 0.31 |
Validation of AND score in discrimination and calibration
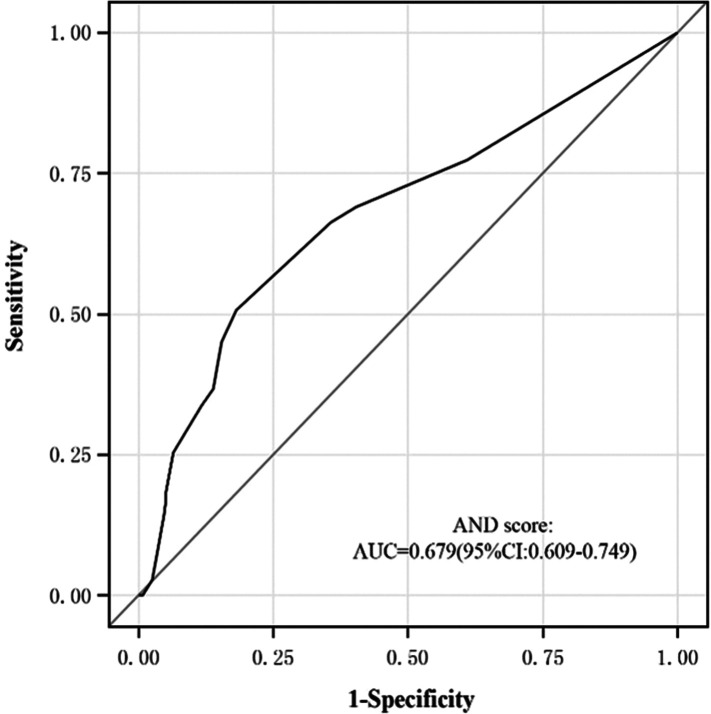
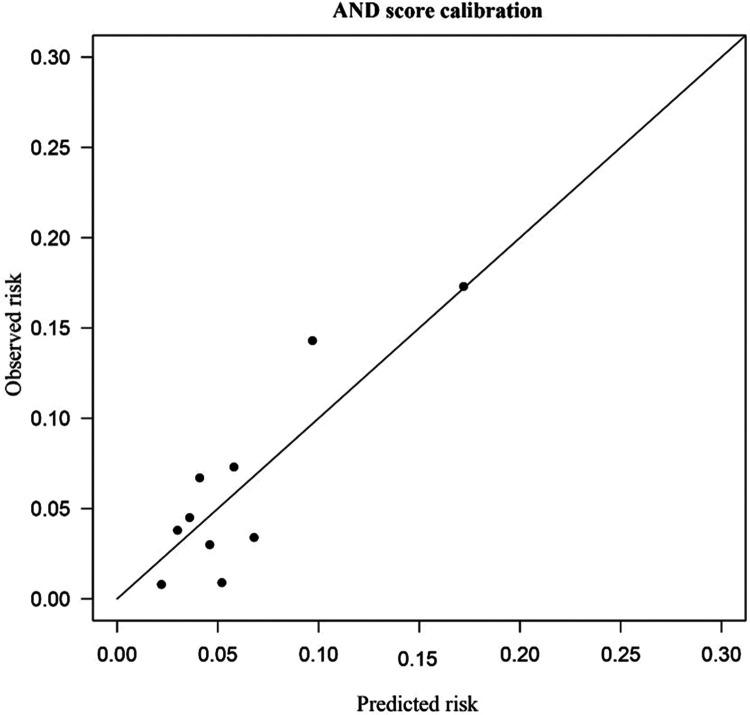
The results are shown in Figure 1a and 1b. Our scoring system achieved a high area under the ROC curve (AUROC) of 0.679 (95%CI 0.609–0.749) for predicting HAI. The calibration test (Hosmer–Lemeshow test) of the AND score showed a good fit. The predicted and observed risk were in close agreement.
Figure 1a.
ROC of AND score. ROC, receiver operating characteristics.
Figure 1b.
AND score calibration plots.
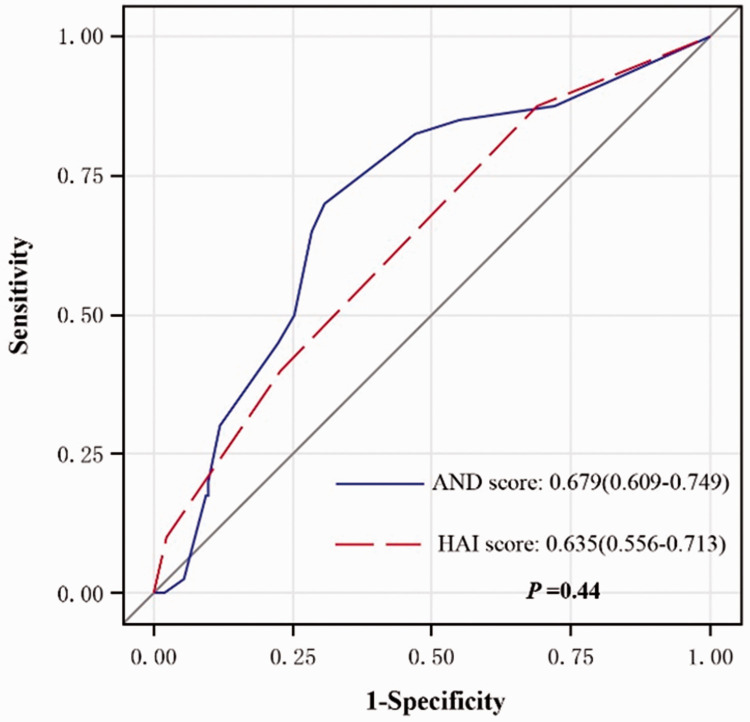
We used the MedCalc software to compare the AND model with the HAI score, which is a previously published model; the ROC was 0.679 for the AND score in our study compared with 0.635 for the HAI score in the 2015 study7. Our prediction model had better performance with a Net Reclassification Index (NRI) of 0.254 (95%CI 0.101–0.407, Z = 3.067, P = 0.001) (See Appendix).
Discussion
In this current cohort study, a simple risk score for HAI in AIS patients without a ventilator using clinical and demographic variables was developed. In our stroke registry, infection of the respiratory system (80.26%) was the most frequent infection and the most common cause was dysphagia. Our results showed the significance of advanced age, admission NIHSS score, and dysphagia in HAI after AIS, which was similar to the meta-analysis of predictors of post-stroke fever and infections in 2018.19 The AND score was then introduced.
Most of the previous predictive scores for pneumonia risk after AIS have shown good calibration and discrimination, but they are less applicable in clinical practice because of their complexity. The A2DS2 score4 from the Berlin Stroke Register cohort in 2012 included age, history of AF, dysphagia, sex, and NIHSS score on admission. The PANTHERIS score in 2013 included age, Glasgow Coma Score (GCS), early blood pressure increase, and early leukocytosis.20 AISAPS in 20145 from the China National Stroke Registry, is a 35-point score that includes eight variables (age, OCSP subtype, dysphagia, admission GCS score, admission NIHSS score, admission glucose, pre-stroke dependence, and medical history). The ISAN score8 was developed in 2015 in European populations, and it included pre-stroke independence, sex, age, and admission NIHSS. In 2018, the PASS score for both ischemic stroke and hemorrhagic stroke included age, male sex, diabetes, medical history of COPD, stroke severity, dysphagia, bladder catheter, and intracerebral hemorrhage.11 The prediction model for all-cause infections after AIS is very rare. Only one study in 20157 had three variables with a seven-point scoring system to predict all-cause infections after AIS, but the exclusion criteria were not very strict.
Because of a different pathogenesis in ventilator-associated infections, one feature of the AND score is that we initially used only nonventilated ischemic stroke patients, while the ACDD4 score includes both ischemic and hemorrhagic stroke patients.10
Infection rate varies greatly depending on the region. The infection rate was low in our study (6.28%), but the reported incidence of infection rate ranges from 5% to 65%.21 One reason for the low rate in our study is that the rate of NIHSS greater than 16 points was 8.17% in our study, but it was 12.4% in the A2DS2 study.4 Additionally, age less than 70 years was 64.5%, but it was 34.1% in the ISAN study.8 We also had strict exclusion criteria. Patients on a ventilator were excluded. We also excluded patients with malignant tumors who have a high risk of infection, but the PASS study did not exclude these patients.11 Additionally, our prediction model has better performance.
Compared with the existing models, the AND score included multiple HAI subtypes, and needed only three variables without lab values. It was supported by robust statistical methods, and both discrimination and calibration were evaluated. The AND score seems to be a practical and promising tool for predicting HAI after AIS.
Our study has some limitations. It was a single-center and retrospective study. Some items with several missing values, such as nursing status or nutritional status, were not included in our study because they may affect the results. We will collect more data and conduct a prospective study in the future. Another important limitation is that we did not use an independent cohort to perform external validation. Despite its limitations, our study is desirable. Patients who are at ten-fold higher odds of developing a HAI after an ischemic stroke could be identified using the AND score, which is helpful for prevention and treatment.
In conclusion, we have developed a simple score to predict all-cause infections after AIS in patients without a ventilator in the Chinese population. This score can assist clinicians in predicting infections and informing effective management.
Appendix
Comparison of ROC between the AND score and the HAI score. ROC, receiver operating characteristics; HAI, hospital acquired infection
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from the National Natural Science Foundation of China (81671132); National Natural Science Foundation of China for Young Scientists Fund (81701142, 81601004); Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Major Neurological Diseases (2017B030314103); The Southern China International Cooperation Base for Early Intervention and Functional Rehabilitation of Neurological Diseases (2015B050501003); Guangdong Provincial Engineering Center for Major Neurological Disease Treatment; and Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease.
ORCID iD
Wenli Sheng https://orcid.org/0000-0001-6867-371X
References
- 1.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309–332. [DOI] [PubMed] [Google Scholar]
- 2.Willeke FW, Jan-Dirk V, Elles Z, et al. The Preventive Antibiotics in Stroke Study (PASS): A pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015; 385: 1519–1526. [DOI] [PubMed] [Google Scholar]
- 3.Smith CJ, Heal C, Vail A, et al. Antibiotic class and outcome in post-stroke infections: An individual participant data pooled analysis of VISTA-acute. Front Neurol 2019; 10: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann S, Malzahn U, Harms H, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke 2012; 43: 2617–2623. [DOI] [PubMed] [Google Scholar]
- 5.Ji R, Shen H, Pan Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke 2013; 44: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 6.Ji R, Shen H, Pan Y, et al. Risk score to predict hospital-acquired pneumonia after spontaneous intracerebral hemorrhage. Stroke 2014; 45: 2620–2628. [DOI] [PubMed] [Google Scholar]
- 7.Friedant AJ, Gouse BM, Boehme AK, et al. A simple prediction score for developing a hospital-acquired infection after acute ischemic stroke. J Stroke Cerebrovasc Dis 2015; 24: 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CJ, Bray BD, Hoffman A, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc 2015; 4: e001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmy TA, Abd-Elhady MA, Abdou M. Prediction of ischemic stroke-associated pneumonia: A comparison between 3 scores. J Stroke Cerebrovasc Dis 2016; 25: 2756–2761. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Marchina S, Massaro J, et al. ACDD 4 score: a simple tool for assessing risk of pneumonia after stroke. J Neurol Sci 2017; 372: 399–402. [DOI] [PubMed] [Google Scholar]
- 11.Westendorp WF, Vermeij J, Hilkens NA, et al. Development and internal validation of a prediction rule for post-stroke infection and post-stroke pneumonia in acute stroke patients. Eur Stroke J 2018; 3: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams HJ, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 13.Brodsky MB, Suiter DM, Gonzalez-Fernandez M, et al. Screening accuracy for aspiration using bedside water swallow tests: A systematic review and meta-analysis. Chest 2016; 150: 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidd D, Lawson J, Nesbitt R, et al. Aspiration in acute stroke: A clinical study with videofluoroscopy. QJM 1993; 86: 825–829. [PubMed] [Google Scholar]
- 15.Ministry of Health of the People’s Republic of China. Diagnostic criteria for hospital-acquired infection. Chin Med J 2001; 81: 314–320. [Google Scholar]
- 16.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan LM, Massaro JM, D’Agostino RS. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 2004; 23: 1631–1660. [DOI] [PubMed] [Google Scholar]
- 18.Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA 2017; 318: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 19.Wästfelt M, Cao Y, Ström JO. Predictors of post-stroke fever and infections: A systematic review and meta-analysis. BMC Neurol 2018; 18: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harms H, Grittner U, Droge H, et al. Predicting post-stroke pneumonia: The PANTHERIS score. Acta Neurol Scand 2013; 128: 178–184. [DOI] [PubMed] [Google Scholar]
- 21.Amelia KB, Erin RK, Michelle C, et al. Infections increase the risk of 30-day readmissions among stroke survivors. Stroke 2018; 49: 2999–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]