White-footed mice are thought to be the most important reservoir host for the deer tick-transmitted pathogens that cause Lyme disease and human babesiosis because they are the primary host for immature ticks. Transmission would be reduced, however, if ticks feed on deer, which are not capable of infecting ticks with either pathogen. By directly measuring whether ticks had fed on either mice or deer using a new quantitative PCR (qPCR) assay to detect remnants of host DNA leftover from the larval blood meal, we demonstrate that host utilization by ticks varies significantly over time and space and that mice often feed fewer ticks than expected. This finding has implications for our understanding of the ecology of these diseases and for the efficacy of control measures.
KEYWORDS: blood meal analysis, retrotransposons, Lyme disease, deer ticks, Peromyscus leucopus, Odocoileus virginianus
ABSTRACT
Deer tick-transmitted Borrelia burgdorferi sensu stricto (Lyme disease) and Babesia microti (babesiosis) increasingly burden public health across eastern North America. The white-footed mouse is considered the primary host for subadult deer ticks and the most important reservoir host for these and other disease agents. Local transmission is thought to be modulated by less reservoir-competent hosts, such as deer, diverting ticks from feeding on mice. We measured the proportion of mouse-fed or deer-fed host-seeking nymphs from 4 sites during 2 transmission seasons by blood meal remnant analysis using a new retrotransposon-based quantitative PCR (qPCR) assay. We then determined the host that was associated with the infection status of the tick. During the first year, the proportion of mouse-fed ticks ranged from 17% on mainland sites to 100% on an island, while deer-fed ticks ranged from 4% to 24%. The proportion of ticks feeding on mice and deer was greater from island sites than mainland sites (on average, 92% versus 43%). Mouse-fed ticks decreased significantly during year 2 in 3 of 4 sites (most were <20%), while deer-fed ticks increased for all sites (75% at one site). Overall, ticks were more likely to be infected when they had fed on mice (odds ratio [OR] of 2.4 and 1.6 for Borrelia and Babesia, respectively) and were less likely to be infected if they had fed on deer (OR, 0.8 and 0.4). We conclude that host utilization by deer ticks is characterized by significant spatiotemporal diversity, which may confound efficacy tests of interventions targeting reservoir hosts.
IMPORTANCE White-footed mice are thought to be the most important reservoir host for the deer tick-transmitted pathogens that cause Lyme disease and human babesiosis because they are the primary host for immature ticks. Transmission would be reduced, however, if ticks feed on deer, which are not capable of infecting ticks with either pathogen. By directly measuring whether ticks had fed on either mice or deer using a new quantitative PCR (qPCR) assay to detect remnants of host DNA leftover from the larval blood meal, we demonstrate that host utilization by ticks varies significantly over time and space and that mice often feed fewer ticks than expected. This finding has implications for our understanding of the ecology of these diseases and for the efficacy of control measures.
INTRODUCTION
In the United States, deer ticks, Ixodes dammini (“northern” clade of Ixodes scapularis), transmit more than 5 different agents that cause disease in humans, including Borrelia burgdorferi sensu stricto, the agent of Lyme disease, and Babesia microti, the agent of human babesiosis. Lyme disease and human babesiosis were first described in the 1970s, and cases were restricted to areas of coastal New England (1, 2). Since then, the incidence of disease has steadily increased, and both have expanded their geographic range (3), with Lyme disease cases now being described in Canada as well as in the prairie states of the Midwest. An estimated 40,000 cases of tick-borne disease were reported in 2018 to the CDC (4), but there may be >300,000 cases each year (5). Tick-borne disease can be a significant public health burden in parts of the United States, and this burden continues to increase as the vectors increase their range and density within diverse landscapes.
Interventions to reduce the public health burden of tick-borne infection require an understanding of the local enzootic cycle of the agents and what influences the bridge from the enzootic to the zoonotic condition, as well as the mode of human exposure. Early ecological studies, conducted soon after the discovery of these infections, identified the white-footed mouse (Peromyscus leucopus) as the main reservoir of both B. microti and B. burgdorferi. The evidence, however, was largely circumstantial. Peromyscus was the most common small mammal in zoonotic sites and was infested with more ticks than other small mammals, leading to the conclusion that this mouse was the main host for subadult deer ticks (6–11). These mice also were universally infected with both agents and appeared to remain infected for long durations (11, 12). Laboratory studies demonstrated that white-footed mice were easily infected by tick bite and efficiently transmitted both infections to ticks (13–15). Much of the seminal field work, however, was conducted on islands (such as Nantucket Island) that have a less diverse mammalian fauna, as these were among the sites where zoonotic transmission was intense (6, 8, 11). More than 160 species of vertebrates are infested by deer ticks, however, including rabbits, squirrels, chipmunks, voles, birds, shrews, deer, and many medium-sized mammals (16); some of these animals may also infect ticks with either agent. Proportionally, mice appeared to have the greatest reservoir capacity; xenodiagnosis measured mouse infectivity, and they were more abundant in study sites than other hosts (10, 17). Greater host diversity could divert ticks from reservoir-competent mice to less-effective reservoir hosts (zooprophylaxis [10, 18, 19], also known as dilution effect [20]), providing a rationale for why insular sites tended to have intense zoonotic transmission. The paradigm of mouse reservoir capacity modified by the availability of alternative hosts, however, rests on descriptive evidence from snapshots of a dynamic natural process. Identification of the host from which a host-seeking tick acquired its infection, by host blood meal analysis, would provide direct evidence of that host’s contribution and whether that contribution varied over space and time.
Host blood meal analysis is a key tool for analyzing the maintenance of mosquito-borne infections (21). Even simple methods, such as immunoprecipitation, can be used because mosquitoes are relatively short-lived and a partially digested blood meal may be present in their guts (22). In contrast, ixodid ticks require one blood meal during each developmental stage, and several months may elapse before they molt and seek hosts in the next instar. Blood meal analysis for ticks, then, attempts to detect whatever may remain after the tick has molted, which can be as much as a year after the blood meal was taken. In addition, digestion in ticks occurs by hemolysis within the gut lumen and intracellularly within gut epithelial endosomes during the blood meal and continues through the molt, with most of the components assimilated or excreted before the next host-seeking event (23). Only small remnants of the blood meal remain. Diverse reports have described blood meal identification from host-seeking ticks (24–33), primarily utilizing a PCR assay targeting a conserved mammalian mitochondrial gene paired with either blotting using specific probes or high-resolution melting temperature analysis to assign identity to amplicons. With field-collected ticks, most studies reported a capacity to identify blood meal hosts for only 50% to 60% of the samples. The most sensitive assay published to date (reported at 88%) utilized mass spectroscopy of the proteins in the tick, using a database made from known hosts as a comparison (34, 35). Unfortunately, this method has not yet been shown to be effective in field-collected ticks and would not likely be routine or cost-effective. Interestingly, host blood meal analysis has not become a routine method in tick ecology despite the many published reports, leading us to suspect that issues of cost, ease, sensitivity, or reproducibility have hindered their adoption by researchers. Our understanding of locally adapted enzootic cycles of the deer tick-transmitted agents would be enhanced by the routine use of a tick host blood meal remnant assay that is more sensitive than previous assays, as well as simple to use, easily reproducible, and cost-effective. In addition, such an assay must leave enough nucleic acid template to allow determination of tick infection status.
Retrotransposons, or “jumping genes,” are genetic elements with the ability to integrate into the genome and replicate themselves via an RNA intermediate. These elements are ancient in origin and have been associated with their host genomes for millions of years, evolving family-specific lineages. Because they leave behind a copy after every replication, hundreds of thousands of copies have accumulated in their host genomes; for example, retrotransposons apparently make up about 30% of the human genome (36). Retrotransposons have 2 major forms, namely, long interspersed repetitive elements (LINEs), which are up to 6 kb in length and have the ability to replicate themselves, and short interspersed repetitive elements (SINEs), which are shorter sequences, usually 80 to 400 bp, and rely on the presence of LINEs for their replication mechanisms. We sought to leverage the extreme number of copies of retrotransposons within mammalian genomes to maximize the probability that a few copies of the target gene will remain undigested and available for amplification by PCR. Accordingly, we developed a real-time PCR assay which targets retrotransposons of the white-tailed deer (Odocoileus virginianus) and of white-footed mice, the two hosts that are thought to disproportionately contribute to the development of intense zoonotic transmission of the deer tick-transmitted agents in the northeastern United States (19, 37). Although many more species may serve as hosts for deer ticks, we focused on these two important ones as a proof of concept; deer are the reproductive host for the deer tick, the sine qua non of dense tick infestations (38), and mice have been implicated as the main reservoir for infection (8, 39). Deer are considered incompetent reservoirs for the two most common deer tick-transmitted agents of infection, namely, B. burgdorferi and B. microti (40), and are thought to be zooprophylactic. We measured the proportions of host-seeking nymphal deer ticks that fed on mice and on deer within 4 different field sites in southern New England (2 on islands and 2 on the mainland). In addition, we analyzed the relationship of these hosts to the infection status of the ticks.
RESULTS
Sensitivity and specificity.
Using real-time PCR of specific retrotransposon targets, we were able to detect mouse or deer DNA down to the lowest dilution tested, 10−8 ng/μl. None of the other species tested positive in duplicate samples at the highest concentration tested, 10−4 ng/μl. There were random samples that did give a spurious signal in individual samples, but they were not repeatable; the threshold cycle (CT) (i.e., the cycle number at which the samples crossed the baseline) was greater than our cutoff (>45), and the amplification peaks were not exponential. Therefore, it was easy to distinguish a real positive from nonspecific amplification. DNA from unfed larval ticks derived from hunter-killed deer did not yield any amplification. All of the Peromyscus-fed lab-reared ticks that were promptly processed tested positive with our assay (100% of 16). Although of limited use because of the small size of the amplicons, sequence data from 5 field-collected nymphs from each assay were obtained and demonstrate that both assays yielded the expected results (see supplemental data).
The age of the ticks affected the sensitivity of the assay (Table 1). Using lab-reared nymphs that had fed on Peromyscus as larvae, there was no significant difference in the number of ticks that tested positive for mouse (16 out of 16 promptly processed ticks versus 9 out of 10 aged ticks, P = 1.0). However, holding the field-collected ticks 6 months in the lab before processing caused the number of ticks testing positive for mouse to drop by half compared with those from the same collection that had been promptly extracted (100% versus 51%, P = 0.000) (Table 1). Furthermore, the CT was significantly greater in the aged ticks than in the promptly processed ticks for both the lab-reared and the field-collected ticks (Fig. 1). Because of this possibility of degradation of the blood meal signal, in the second year, we froze the ticks promptly after collection. The mean CT of ticks testing positive for either mouse or deer, however, was not significantly different between the promptly processed ticks from year one and the frozen ticks from the second year (see Table S1 in the supplemental material).
TABLE 1.
Comparison of the number of deer ticks testing positive for mouse from promptly processed field collected ticks versus those that were allowed to age in the lab for 6 months
| Sample processing group | No. of ticks tested | No. of ticks positive for mouse | Percenta |
|---|---|---|---|
| Prompt | 49 | 49 | 100 |
| Delayed | 35 | 18 | 51 |
P = 0.00.
FIG 1.
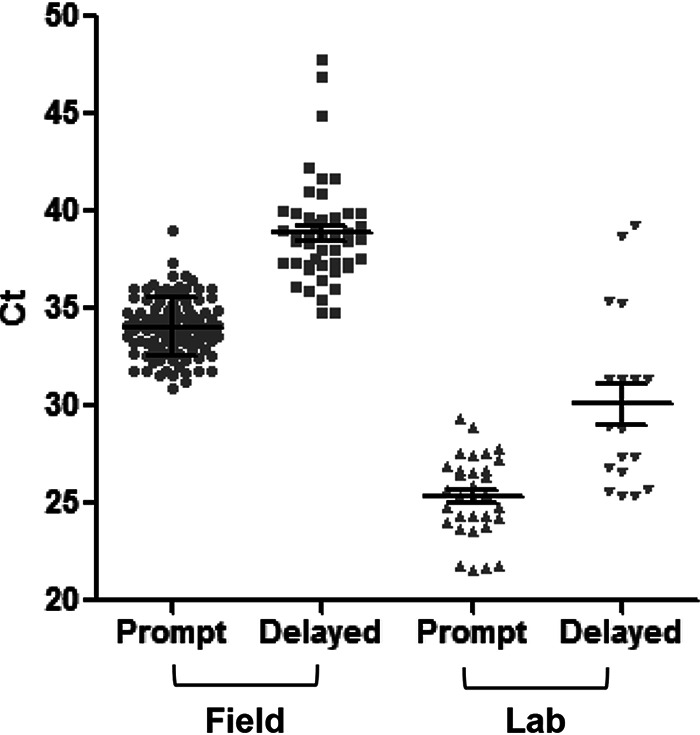
The mean PCR amplification threshold cycle (CT) for laboratory-derived or field-collected deer ticks that were promptly processed compared with those allowed to age for 6 months in the laboratory (delayed).
Blood meal identification.
The contributions of mice and deer as hosts for ticks varied by year as well as by site. Island sites with limited fauna demonstrated a high success rate of blood meal remnant identification using our two primer sets. In 2018, we were able to identify the blood meal source from all the ticks from Nantucket and 95% from Robins Island (Table 2; Fig. 2). We were less successful in identifying blood meals from ticks of mainland sites, with only 19% of blood meals identified from RI Crew and 64% from RI Trust. Overall, the success rate for blood meal identification for each site remained the same in the second year, with the exception of Nantucket, where it dropped to 81%.
TABLE 2.
The proportion of deer ticks that fed on mouse or deer and the prevalence of B. burgdorferi and B. microti in nymphal ticks at 4 sites in northeastern United States
| Site | Yr | Total no. of ticks tested | Blood meal PCR result (n [%]) |
Pathogen PCR result |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mouse | Deer | Mixed | Undetermined |
B. burgdorferi |
B. microti |
|||||
| n | % (95% CI) | n | % (95% CI) | |||||||
| NFSa | 2018 | 65 | 65 (100) | 6 (9) | 6 (9) | 0 | 14 | 22 (12, 33) | 10 | 15 (8, 26) |
| 2019 | 88 | 16 (18) | 65 (74) | 10 (11) | 17 (19) | 20 | 23 (14, 33) | 15 | 17 (10, 27) | |
| P valueb | 0.00 | 0.00 | ||||||||
| RI Crew | 2018 | 63 | 11 (17) | 2 (4) | 1 (2) | 51 (81) | 19 | 30 (19, 43) | 14 | 22 (13, 34) |
| 2019 | 86 | 16 (18) | 15 (17) | 3 (3) | 58 (67) | 19 | 22 (14, 32) | 11 | 13 (7, 22) | |
| P valueb | 0.01 | |||||||||
| RI Trust | 2018 | 64 | 33 (52) | 9 (14) | 1 (2) | 23 (36) | 19 | 30 (19, 42) | 19 | 30 (19, 42) |
| 2019 | 90 | 15 (17) | 35 (39) | 0 | 40 (44) | 17 | 19 (11, 29) | 14 | 16 (9, 25) | |
| P valueb | 0.00 | 0.001 | 0.05 | |||||||
| Robins Island | 2018 | 55 | 50 (91) | 13 (24) | 11 (20) | 3 (5) | 36 | 65 (51, 78) | 8 | 15 (7, 27) |
| 2019 | 77 | 41 (53) | 58 (75) | 28 (36) | 6 (8) | 38 | 50 (38, 62) | 4 | 5 (1, 13) | |
| P valueb | 0.00 | 0.000 | ||||||||
NFS, Nantucket Field Station.
The P values are included only for statistically significant differences between years.
FIG 2.

Blood meal identification from questing nymphal deer ticks collected in 2018 and 2019 from the 4 field sites. The bars are divided into the percentage of ticks testing positive for mouse (black), deer (gray), mixed blood meal (black and gray checked), and to be determined (TBD; white).
On both island sites, mice were the main blood meal source for the majority of ticks in 2018, namely, 100% from Nantucket and 91% from Robins Island. In contrast, on the mainland, mice apparently fed only 17% of ticks from RI Crew and 52% from RI Trust (Table 2; Fig. 2). Deer were less likely to have served as the blood meal source that year, although a number of ticks tested positive for both animals, suggesting that ticks took partial blood meals. On Nantucket, 9% of the ticks tested positive for both species. On Robins Island, 4% of the ticks tested positive for deer only, while 20% were positive for both. Once again, this was less likely to occur on the mainland sites, where 2% of ticks fed on deer alone from RI Crew and 2% fed on both; at the RI Trust site, 12% fed on deer alone and only 2% fed on both animals. The CTs from ticks with mixed blood meals did not reliably indicate which species had provided the majority of the blood meal (Table S1).
In 2019, the mouse contribution to feeding ticks dropped dramatically for 3 of the 4 sites and that of deer increased. On Nantucket, the proportion of ticks fed on mice diminished from 100% in 2018 to 18% in 2019 (P = 0.00) and the proportion from deer increased from 9% to 74% (P = 0.00). On Robins Island, a 38% drop was detected in mouse-fed ticks (P = 0.00), with a corresponding 51% increase in those from deer (P = 0.00). A similar trend was detected at the RI Trust site, where mouse-fed ticks decreased by 35% (P = 0.00) and those from deer increased by 25% (P = 0.001). The RI Crew site had the least amount of year-to-year variation; the proportions of ticks feeding on mice were not significantly different, but there was an increase in the contribution of deer from 4% to 17% (P = 0.01), and the overall success rate of blood meal identification remained low at that site (67% did not amplify with either mouse or deer assays in 2019). We conclude that the contributions of deer and mice to feeding larval deer ticks vary greatly between sites and by year even within some sites. At the RI Crew site, neither mice nor deer appeared to feed the majority of larval deer ticks.
The prevalence of infection detected in ticks did not appear to be driven by whether they had fed primarily on mice or deer and did not significantly differ during our study for 3 of the 4 field sites, namely, RI Crew, Nantucket, and Robins Island, despite the dramatic change from mostly mouse-fed ticks in 2018 to deer-fed ticks in 2019 (Table 3 and 4; Fig. 3 and 4). The proportion of ticks fed on mice for the RI Crew site remained unchanged during our study. RI Trust was the only site with a change in the prevalence of infection in ticks. Although the prevalence of B. burgdorferi fell from 30% in 2018 to 19% in 2019, this decrease was not statistically significant (P = 0.1) (Table 3; Fig. 3). However, the change in the prevalence of B. microti in ticks was significant (30% to 16%, P = 0.05) (Table 4; Fig. 4). This site experienced the same change in tick-feeding profile from mice to deer as Nantucket and Robins Island.
TABLE 3.
Blood meal identification from B. burgdorferi-infected deer ticks
| Site | Yr | Total no. of ticks tested | No. (%) of ticks testing positive for: |
|||
|---|---|---|---|---|---|---|
| Mouse | Deer | Mixeda | Undeterminedb | |||
| NFS | 2018 | 14 | 14 (100) | 0 | 0 | 0 |
| 2019 | 20 | 3 (15) | 16 (80) | 3 (15) | 4 (20) | |
| RI Crew | 2018 | 19 | 8 (37) | 1 (5) | 1 (5) | 11 (58) |
| 2019 | 19 | 4 (21) | 2 (11) | 0 | 13 (68) | |
| RI Trust | 2018 | 19 | 15 (79) | 0 | 0 | 4 (21) |
| 2019 | 17 | 6 (35) | 1 (6) | 0 | 10 (51) | |
| Robins Island | 2018 | 36 | 33 (92) | 5 (14) | 5 (14) | 3 (8) |
| 2019 | 38 | 21 (56) | 31 (82) | 17 (45) | 3 (7) | |
Positive for both mouse and deer.
“Undetermined” refers to ticks that did not test positive with either assay.
TABLE 4.
Blood meal identification from B. microti-infected deer ticks
| Site | Yr | Total no. of ticks tested | No. (%) of ticks testing positive for: |
|||
|---|---|---|---|---|---|---|
| Mouse | Deer | Mixeda | Undeterminedb | |||
| NFS | 2018 | 10 | 10 (100) | 0 | 0 | 0 |
| 2019 | 15 | 3 (20) | 12 (80) | 2 (13) | 2 (13) | |
| RI Crew | 2018 | 14 | 4 (28) | 1 (7) | 1 (7) | 10 (71) |
| 2019 | 11 | 0 | 1 (9) | 0 | 10 (91) | |
| RI Trust | 2018 | 19 | 15 (79) | 0 | 0 | 4 (21) |
| 2019 | 14 | 5 (36) | 0 | 0 | 9 (64) | |
| Robins Island | 2018 | 8 | 8 (100) | 0 | 0 | 0 |
| 2019 | 4 | 4 (100) | 4 (100) | 4 (100) | 0 | |
Positive for both mouse and deer.
“Undetermined” refers to are ticks that did not test positive with either assay.
FIG 3.

Blood meal identification from questing nymphal deer ticks infected with Borrelia burgdorferi, collected in 2018 and 2019 from 4 field sites. The bars are divided into the percentage of ticks testing positive for Peromyscus (black), white-tailed deer (gray), mixed blood meal (black and gray checked), and to be determined (TBD; white).
FIG 4.

Blood meal identification from questing nymphal deer ticks infected with Babesia microti, collected in 2018 and 2019 from 4 field sites. The bars are divided into the percentage of ticks testing positive for mouse (black), deer (gray), mixed blood meal (black and gray checked), and to be determined (TBD; white).
Overall, ticks that had fed on mice were more likely to become infected than if they had fed on a deer or some unknown animal (odds ratio [OR] of 2.4 and P = 0.000 for B. burgdorferi; OR of 1.6 and P = 0.04 for B. microti) (Fig. 5). Feeding on deer was negatively associated with both infections, although this effect was more pronounced for B. microti than for B. burgdorferi (OR of 0.8 and 0.4 and P values of 0.3 and 0.000 for B. burgdorferi and B. microti, respectively). Ticks that had fed on undetermined animals were unlikely to be infected with B. burgdorferi (OR, 0.6; P = 0.01); however, the undetermined ticks were positively associated with infection with B. microti, although the association was not statistically significant (OR, 1.2; P = 0.5).
FIG 5.

The likelihood that a deer tick infected with either Borrelia burgdorferi or Babesia microti had fed on a mouse, deer, or another host yet to be determined (TBD). Odds ratios are plotted with 95% confidence intervals around them. Data from all sites are combined.
DISCUSSION
Deer ticks can feed on many kinds of animals, but mice and deer have thus far been thought to be those contributing most to the enzootic cycle and to zoonotic overflow. We sought to determine the extent to which white-footed mice or deer serve as hosts for larval deer ticks by directly detecting residual host DNA in host-seeking nymphs. We designed an assay that is a simple, specific, and sensitive real-time PCR that can be done in any laboratory that has a real-time PCR machine. Our assay was able to consistently amplify either mouse or deer DNA at a concentration of 10−8 ng/μl, the lowest concentration that we tested. Our primers/probe did not cross-react with any of the animals on our panel of positive controls, which included many rodent species, such as vole, rat, and house mouse, as well as ruminants like sheep and cow. However, at this point, we have not determined the actual sensitivity of the assay in ticks because our sensitivity calculations were done with serially diluted DNA that remains in relatively large pieces. This does not simulate the highly digested fragments of DNA that are inside the gut cells of a molted tick.
We were able to identify mouse from all of the promptly processed lab-reared nymphs, as well as identify a high proportion of blood meals from ticks from our island sites, giving us confidence that our assay has sufficient sensitivity to give reliable results. We did find that assay sensitivity declined when ticks were allowed to age in the lab. Aged ticks produced significantly higher CT values for amplification and, consequently, a higher rate of failure in field-collected ticks than in those that were promptly processed. This finding is consistent with the paradigm that components of the blood meal remain in gut cells after the molt in some ixodid ticks, such as I. ricinus, and may serve as a continuing source of energy (23). Tick age, therefore, may confound our results; for samples testing negative, we cannot know for certain if this is because the ticks were old or because they simply had not fed on either of the two species for which we assayed. At this point, there are no practical assays for tick age, and we cannot directly test this possibility. We note, however, that we were able to successfully identify the blood meal source for almost all the ticks from both island sites, where there are limited numbers of species available to serve as hosts. This result leads us to believe that the high rate of unidentified ticks from the mainland sites is largely due to the limited species for which we tested and not due to either a lack of sensitivity of the assay or the presence of old ticks. We are currently working to validate new primer sets that identify other likely hosts for subadult deer ticks.
The white-footed mouse has long been considered the main reservoir for the deer tick microbial guild (8, 17, 41–43). Other animals (certain birds, raccoons, shrews, chipmunks, and squirrels) are known to have various degrees of reservoir capacity for the agent of Lyme disease (44–46), but mice are generally considered the main source of infected ticks during years when this mouse is at typical densities (17). Its incrimination as the main reservoir rests mainly on descriptive and inferential analyses. The general argument is that white-footed mice (i) are usually the most common small mammal in zoonotic sites, (ii) are densely infested by subadult deer ticks, and (iii) have great reservoir competence (8, 47). Statements such as “transmission of the Lyme disease spirochete appears to depend on the specific association between white footed mice and immature I. dammini” (48) continue to be common in the literature. Consistent with such a hypothesis, mouse-targeted interventions, such as Damminix tick tubes or doxycycline baits, greatly reduced the prevalence of infected nymphal ticks (49, 50). However, an equal number of other types of field experiments targeting mice (51–55) failed to substantively reduce the force of transmission. The mixed results obtained with mouse-targeted interventions have led to thoughtful questioning of the mouse dogma using modeling (17) or associations with B. burgdorferi genotypes (56) to demonstrate the likelihood of “rescue effects” (57) by hosts, such as short-tailed shrews, which might replace mice as the main B. burgdorferi reservoir when mice are scarce.
Our results help to clarify the role of mice as hosts for subadult deer ticks and as reservoirs. In 2017 (note that the larvae fed in 2017 but were sampled as nymphs in 2018), mice served as hosts for almost all the larval ticks from our island sites. Although the island site mammalian fauna are not as diverse as the mainland mammalian fauna, possible alternative hosts on which larvae could feed are abundant, including voles, rabbits, shrews, birds, rats, and, of course, deer (41). Mice were also identified as the host for the majority of the ticks sampled at one of the mainland sites, RI Trust. Over all our sampled sites and the 2 years of sampling, infected ticks were 2.4 times more likely to have derived from mice than from deer or unidentified hosts (Fig. 5). Although we might have expected a greater association of mice with infection, we do note that not all ticks feeding on infected mice become infected; also, if larvae fed on recruits not yet exposed to an infected nymph, the resulting nymphs would not be infected either.
Deer, on the other hand, were responsible for feeding only a minor portion of ticks during 2017. In the second year of our study (2019; ticks fed during 2018), the proportion of mouse-fed ticks dropped dramatically for 3 of our sites and that from deer greatly increased. Although we handled the ticks differently in the second year of the study (in year 1, we held the ticks at 9°C), we believe that this change should have increased the success of our assay, not decreased it. The most parsimonious hypothesis is that mice were scarce during 2018, perhaps due to a wide-spread population crash. We did not estimate the density of either mice or deer during this study, and hence, this hypothesis remains untested. Although 20% more deer were taken during the 2018 hunting season than in 2017 (https://www.mass.gov/service-details/deer-harvest-data; Nantucket is Zone 14), the harvest was influenced by the Nantucket Board of Health’s initiative to reduce the deer herd that year. We cannot exclude the possibility that there were more deer within our Nantucket sampling sites during 2018. We suggest that in an insular site such as Nantucket with typical densities of mice and deer, mice are preferred hosts, but when mice are scarce, deer may compensate for their absence.
Although deer are the reproductive host for deer ticks (58), their contribution to feeding subadult ticks is less well studied. Telford et al. (40) collected an average of 342 larvae (median, 126) from 19 deer, with a range of 18 to 2,008. Of the 6,500 larvae that were collected, only 185 were engorged enough to molt to nymphs. Whether all ticks would have fed to repletion is not known, but we do note that sufficient numbers must do so because we detected good proportions of host-seeking nymphs that contained only evidence of remnants of deer blood. Surprisingly, we detected ticks that tested positive for both mouse and deer, suggesting that partial blood meals occur more commonly than previously appreciated. Indeed, about 20% of identified blood meals from host-seeking I. ricinus were from mixed hosts (24). Larvae may be groomed off (or detach from dead hosts) and then attach to another host to complete feeding. As many as a third of deer tick larvae were groomed off experimentally infested mice (59), although such ticks are only rarely partially fed (60). Grooming and tick feeding success are poorly studied for most deer tick hosts, and the factors that influence these processes may be complex (61); for example, infestation by other ectoparasites may increase tick grooming by deer (62).
We considered the possibility that due to the great sensitivity of PCR, our detection of mixed feeding reflected contamination of the forceps used to manipulate ticks. Our initial validation of the assay included laboratory-reared nymphs that were derived from larvae from engorged females that fed on deer; we found no evidence that deer blood meal remnants persisted into the nymphal stage (data not shown). We excluded that possibility for laboratory forceps because they are not used to manipulate deer-derived ticks. However, forceps used in field sites might have been exposed. We tested 4 different pairs of forceps, all held within the same console of the field vehicle used for both Nantucket and Robins Island; one was known to have been used to remove ticks from hunter-killed deer 3 months previously. All lab-reared nymphs manipulated with that known contaminated pair of forceps, but only 1 of 15 other nymphs handled by the other 3 pairs (5 ticks per pair), were considered to have deer DNA (data not shown). Although possible, we reject the hypothesis that our field forceps contaminated our samples because (i) Nantucket and Robins Island sites were sampled within days of each other using the same sets of forceps and yet had differing proportions of mixed blood meals; (ii) the greater proportion of deer blood meals was observed for 2019, during which we made sure to dedicate forceps for collecting ticks intended for blood meal analysis; (iii) the Rhode Island collections were made independently by the Rhode Island laboratory; and (iv) a pair of forceps from the Tufts field vehicle would have been exposed to 7 months of sunlight after being used for removing ticks from hunter-killed deer in November and then used for handling host-seeking nymphs the following June. Accordingly, we consider our results to be evidence of mixed blood meals, indicating that some larvae may attach to a host, be groomed off or otherwise detach, and then attach to another host to complete feeding.
Another surprising finding was the presence of B. burgdorferi infection in ticks that contained evidence of remnants of deer blood, because deer are considered incompetent reservoirs (40, 63–65). Host complement is a main determinant of host associations for B. burgdorferi genospecies (66, 67), with some spirochetes being resistant and others susceptible to a given source of complement. Deer serum lyses diverse B. burgdorferi genospecies (66, 68). The paradigm of “species-specific, complement-associated host selectivity” (69) seems to be well supported from laboratory and field evidence for deer reservoir incompetence. However, in vitro studies of complement sensitivity are influenced by technical parameters, such as serum collection and storage (70), and additional analyses of the susceptibility of B. burgdorferi to deer serum may be desirable. Then, too, there may be factor H binding protein variants of B. burgdorferi lineages that may be selected to allow for host complement evasion. It may also be that the deer-fed ticks acquired their Borrelia infection through cofeeding with infected nymphs, similar to the maintenance of B. burgdorferi by sheep, which do not support systemic infection (71); the sera of these hosts also lyse B. burgdorferi sensu stricto (67). Deer are also said to be incompetent reservoirs for B. microti (72), and overall, deer-fed ticks were unlikely to be infected with B. microti (Fig. 5). Despite this, we identified a number of B. microti-infected ticks from Nantucket that contained evidence of having fed on a deer. Cofeeding is very unlikely to be an explanation for these babesial infections unless sporozoites liberated within tick saliva are totipotent and can become gametes. Although it is possible that we were unable to detect mouse DNA from a larva that had fed only briefly, the most likely explanation is that B. microti-infected ticks that appear to have fed solely on deer derive from a partial blood meal and that the source of the other blood meal was not a mouse but a shrew or any of a number of small mammals that are known to be infected by B. microti. Our ongoing research validating host blood meal remnant detection from hosts other than deer or mice will soon allow us to clarify these paradoxical results.
The lower proportion of mouse-fed ticks found in most of our 2019 collections remains to be explained, although the most likely cause is low mouse density in our sites. However, the RI Crew site was characterized by <20% of the host-seeking nymphs having fed as larvae on mice for both years, yet the prevalence of infection remained consistent with that of the other RI sites and Nantucket for either year. Indeed, even on Nantucket, the prevalence of infection remained stable between the 2 years despite fewer nymphs having fed on mice as larvae in the second year. We note, however, that our sample sizes for host-seeking nymphs provide a wide confidence interval (CI) for infection prevalence, and we recognize that it is possible that the effects of host association might be detected with a greater sample size. Nonetheless, we expected to have easily found unambiguous evidence for the role of mice, given the extensive literature that supports the widely accepted dogma that “mice are the main reservoir.” We can conclude only that where and when mice are not available to serve as hosts for larval deer ticks, other hosts can effectively maintain B. burgdorferi and B. microti. Additional sampling from diverse geographic areas and over multiple years will be needed to better define the spatiotemporal variability for mouse-driven enzootic cycles, but it is clear that mice are not required for the perpetuation of B. burgdorferi and B. microti. This conclusion is not new; early studies of B. burgdorferi ecology demonstrated that woodrats, chipmunks, and Norway rats maintain the enzootic cycle (46, 73, 74) in the United States. As we validate and use host blood meal remnant assays for additional species, we expect to find great diversity for the maintenance of B. burgdorferi, with locally adapted enzootic cycles driven by temporal variations in host abundance, thereby explaining the strong ecological success of this bacterial agent.
The spatiotemporal differences in the contribution of mice to the production of infected deer tick nymphs help to explain inconsistencies in the literature describing the outcomes of mouse-targeted interventions. Our findings do not broadly negate such an approach but suggest that interventions need to be informed by site-specific knowledge of the enzootic cycle; the failure of a short-term mouse-targeted approach, such as tick tubes (see, e.g., reference 75), may be due to heterogeneity in mouse contributions, perhaps even at the level of individual yards. Failure to observe a reduction in entomological risk indices (prevalence of infection in host-seeking ticks multiplied by a measure of tick density) may not reflect the failure of the method but rather just the failure to detect evidence of the prevailing infecting host in a specific site at a specific time. Then, too, conflation of enzootic (maintaining the basic reproduction number of B. burgdorferi at >1 over long durations) and zoonotic (human risk) conditions tends to obscure the critical focus for intervention, namely, to reduce overflow, or basic reproductive number (BRN) greatly exceeding 1 over multiple transmission seasons. Low-level enzootic transmission may not translate to measurable zoonotic risk, but in most sites with human cases, enzootic transmission is at a force that greatly exceeds what is needed to perpetuate B. burgdorferi. The relevant question for a site then becomes one of “to what extent can we reduce zoonotic transmission if mouse reservoir capacity is diminished?,” realizing that driving BRN to <1 is unlikely. Our assay for host blood meal remnant analysis may become integral for evaluating the efficacy of host-targeted interventions.
MATERIALS AND METHODS
Tick collection.
Host-seeking nymphal deer ticks were collected in June of 2018 and 2019 by dragging vegetation in 4 different field sites. Two of the sites were on islands, Nantucket, MA, and Robins Island, near the town of New Suffolk in Suffolk County, NY. The 300-ha Nantucket Field Station site has been previously described, with dense brush (bayberry, highbush blueberry, and poison ivy) and mown trails; sampling was undertaken mainly at the edge of trails (11). Robins Island is a privately owned, 1-square-mile island in Peconic Bay, Long Island, NY. The unpopulated island is a wildlife reserve and contains mature stands of coastal hardwood (oak-hickory) with greenbrier and low shrub understory interspersed with mown fields. More than 50 deer are present. White-footed mice are common on both islands but neither has medium-sized mammals, such as skunk, raccoon, opossum, or fox; both have gray squirrels and cottontail rabbits. Chipmunks are not present on Nantucket. Two sites were on the mainland, namely, “Crew” and “Trust” in Washington County, Rhode Island. Both are mixed hardwood forests with abundant wildlife typical of such mainland landscapes in the northeastern United States; the former has a fern and greenbrier understory, whereas the latter has greenbrier and a mix of low shrubs. All sampling was undertaken in habitat where deer ticks are dense and did not solely comprise collections from “hot spots,” but rather are the result of hours of dragging within >5 ha or 1,000-m transects. During the first year of sampling, ticks were held at 9°C until processing. The second year, ticks were frozen immediately after they arrived in the laboratory.
Screening ticks for infection.
Tick DNA was extracted using a modified HOTSHOT protocol. Briefly, ticks were homogenized individually in 25 μl phosphate-buffered saline (PBS). A total of 25 μl 2× concentrated NaOH-EDTA lysis solution was added, ticks were boiled for 30 minutes and cooled on ice, and 50 μl Tris neutralizing solution was added. Ticks were screened individually for evidence of infection with B. burgdorferi and B. microti using a multiplex real-time PCR assay with primers and probes as described elsewhere (76). Samples were amplified in 15-μl reaction mixtures containing 1.5 μl of tick DNA using Multiplex Powermix (Bio-Rad Laboratories, Hercules, CA). Primer and probe concentrations were the same as those used in the retrotransposon blood meal assay (see below).
Retrotransposon blood meal assay.
The consensus sequence for rodent B1 SINE was downloaded from SINEBase (77). GenBank was searched for matching sequences using a BLAST search specifically targeting sequences from Peromyscus, Microtus, Mus, and Rattus, which are all possible small rodent hosts in our study sites. Approximately 50 sequences were downloaded from each search and were aligned using Geneious (Biomatters Ltd., Auckland, New Zealand). Primer pairs and probes targeting Peromyscus (hereafter referred to as mouse or white-footed mouse) were designed using Geneious to areas that appeared to be unique (Table 5). They were then tested against a panel of positive-control DNA at a concentration of 10−4 ng/μl and redesigned as needed (see Table 6). DNA from unfed larval deer ticks was used to test for cross-reactions with tick DNA. Sensitivity of the assay was estimated by serial dilution of control DNA from 10−4 ng/μl to 10−8 ng/μl. Finally, the primers and probe were tested against lab-reared nymphs that had fed on Peromyscus as larvae.
TABLE 5.
Panel of positive-control DNA used to test for cross-reactions
| Host | Species | Sample name | Source/site | Yr |
|---|---|---|---|---|
| Dog | Canis familiaris | Dog | Tufts Hospital, Grafton, MA | 2009 |
| Raccoon | Procyon lotor | Rac0503-5 | Martha’s Vineyard, MA | 2003 |
| Meadow vole | Microtus pennsylvanicus | MpX001 | Martha’s Vineyard, MA | 2003 |
| Bank vole | Myodes gapperi | Cg05 | Otis, MA | 2005 |
| Rabbit | Sylvilagus floridanus | Rabb821 | Martha’s Vineyard, MA | 2002 |
| White-footed mouse | Peromyscus leucopus | Pl909 | Martha’s Vineyard, MA | 2009 |
| Deer mouse | Peromyscus maniculatus | PmLF3 | Quabbin, MA | 2014 |
| House mouse | Mus musculus | Mus | Cuttyhunk, MA | 2018 |
| Deer | Odocoileus virginianus | Deer1 | Hunter killed, MA | 2015 |
| Rat | Rattus norvegicus | Rat | Vermont | 2007 |
| Cat | Felis catus | Cat | Tufts Hospital, Grafton, MA | 2010 |
| Muskrat | Ondatra zibethicus | MuskVT | Vermont | 2007 |
| Jumping mouse | Zapus hudsonius | ZhX001 | Nantucket, MA | 2014 |
| Chipmunk | Tamias striatus | Ts01 | Otis, MA | 2015 |
| Opossum | Didelphis virginiana | PossVT | Vermont | 2007 |
| Shrew | Blarina brevicauda | BbX001 | Nantucket, MA | 2016 |
| Squirrel | Sciurus carolinensis | MV690 | Martha’s Vineyard, MA | 2004 |
| Skunk | Mephitis mephitis | Sk0503-4 | Martha’s Vineyard, MA | 2004 |
| Mallard | Anas platyrhynchos | Duck | MassWildlife | 2010 |
| Sparrow | Passer domesticus | Sparrow | MassWildlife | 2010 |
| Turkey | Meleagris gallopavo | Turkey | Meat counter, grocery store | 2015 |
| Chicken | Gallus gallus | Chicken | Meat counter, grocery store | 2015 |
| Mole | Parascalops breweri | Mole | Shrewsbury, MA | 2019 |
| Sheep | Ovis aries | Sheep | Meat counter, grocery store | 2018 |
| Cow | Bos taurus | Beef | Meat counter, grocery store | 2018 |
| Pig | Sus scrofa | Pork | Meat counter, grocery store | 2018 |
| Fox | Vulpes vulpes | Fox | Shrewsbury, MA | 2017 |
| Groundhog | Marmota monax | Groundhog | Shrewsbury, MA | 2017 |
TABLE 6.
Primers and probe used in the studya
| Target | Primer/probe | Sequence | Concn (μM) | Primer/probe Tm (°C) | Amplicon Tm (°C) |
|---|---|---|---|---|---|
| Peromyscus | PlSINE-41F | GATCTCTGTGAGTTCGAGG | 0.2 | 60.2 | NAb |
| PlSINE-114R | GTTTCTCTGTGTAGCTTTGC | 0.5 | 60.7 | ||
| PlSINE-66P | FAM-TGGGCTACCAAGTGAGCTCCAGG-ZEN-IowaBlackFQc | 0.33 | 70.4 | ||
| Odocoileus | WTDrep10-167F | GATCTGTTTCACCCTAGATAAT | 0.2 | 59.1 | 71.5–72.5 |
| WTDrep10-220R | ATGTTTCAAGAGAACAGCATC | 0.2 | 60.6 |
Tm, melting temperature.
NA, not applicable.
FAM, 6-carboxyfluorescein.
A portion of the white-tailed deer (hereafter referred to as deer) genome sequence was downloaded from GenBank (accession no. NC_015247.1) and was searched for repeated units of >1,000 bp using Geneious. The entire genome was then screened using BLAST to determine whether the resulting repeats were rare or common. Primers were designed against the repeat units that appeared to have many copies throughout the genome, although exact copy number was not determined. Candidate primer sets were then tested for specificity against other ungulates (cow, sheep, and pig; meat samples from the local supermarket; see Table 5) as well as humans and sensitivity using diluted positive-control DNA and then redesigned as needed. The inclusion of a probe in the assay appeared to decrease the sensitivity, so it was not used in the final assay. Specificity is measured by determining the melting temperature of the amplicon. The final primer set was tested against positive and negative controls to ensure there was no cross-reaction with tick DNA from unfed larval ticks or other animals known to be present in our study sites (Table 5).
Samples were amplified in 15-μl reaction mixtures using ssofast probe master mix (Bio-Rad Laboratories, Hercules, CA) for the mouse assay and the ssofast Evagreen master mix (Bio-Rad Laboratories) for the deer assay in a Light Cycler 480 real-time PCR machine (Roche, Basel, Switzerland), as well as 2 μl of tick DNA. Reaction conditions were 98°C for 30 sec and then 50 cycles of 98°C at 8 sec and 58°C at 30 sec using the primers listed in Table 6. The deer assay had the addition of a melting curve at the end of the cycling. Samples were run in duplicate and considered positive if amplification peaks crossed the background by cycle 44. This cutoff was set at the highest CT that still gave consistently reproducible results. If only one of the duplicates showed a detectable signal in the initial screen, the test was repeated. If no signal was seen on the subsequent amplification, the sample was deemed negative. Because of the extreme sensitivity of this assay, we used strict contamination control procedures. Ticks were collected and sorted with dedicated forceps. All work processing the ticks was physically separated from the PCR machines. PCRs were set up in a dedicated dead-air hood. The hood and the racks were irradiated with UV light between runs. Finally, sham extractions and no-template PCR controls were included in every step of the process to detect in any possible cross-contamination. Verification of a subset of the amplicons was obtained by sequencing (n = 5) (see supplemental information).
To determine whether the age of the ticks affected the results of the assay, we held some of the lab-reared Peromyscus-fed nymphs for 6 months at 9°C before processing them. We also divided our 2018 collection of ticks from Nantucket, processing some within a few weeks of collection and leaving some at 9°C for 6 months before processing.
Data analysis.
The proportion of ticks with detectable mouse or deer DNA as well as the prevalence of infection was calculated for each year and field site and presented with exact binomial 95% confidence intervals. Ticks that had apparently fed on both species (mixed blood meal) were counted in both categories for the statistical calculations. Statistical calculations were done using the two-way contingency table analysis at https://statpages.info/ctab2x2.html. Fisher exact tests were used to analyze categorical data, with a P value of 0.05 set a priori for rejecting the null hypothesis. Odds ratios were calculated to analyze associations of host with infection status. Quantitative data were analyzed within GraphPad Prism (version 5; GraphPad Software Inc.), using unpaired two-tailed t tests to test for differences in mean CTs.
Data availability.
An Excel file containing the data used for the analyses are available at the Open Science Framework (https://osf.io/wcmta/?view_only=991778cbd9e54b14922e1f098b1d957c).
Ethics statement.
Sampling of vertebrates was performed under approved Tufts University IACUC protocols (most recent versions are G2017-122, G2017-123, and G2019-32).
Supplementary Material
ACKNOWLEDGMENTS
Our work is supported, in part, by National Institutes of Health grant R01 AI 130105 and the Rainwater Foundation.
We thank the Nantucket Conservation Foundation for access to field sites on Nantucket Island. Kate Skowyra contributed excellent technical assistance. Eddie Aiduck provided logistical support for work in our Long Island site, and we thank him.
Supplemental material is available online only.
Supplemental material is available online only.
REFERENCES
- 1.Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, Steele FM. 1977. An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum 20:7–17. doi: 10.1002/art.1780200102. [DOI] [PubMed] [Google Scholar]
- 2.Western KA, Benson GD, Gleason NN, Healy GR, Schultz MG. 1970. Babesiosis in a Massachusetts resident. N Engl J Med 283:854–856. doi: 10.1056/NEJM197010152831607. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, Hooks H, Partridge SK, Visser SN, Beard CB, Petersen LR. 2018. Vital signs: trends in reported vector borne disease cases—United States and territories, 2004–2016. MMWR Morb Mortal Wkly Rep 67:496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2018. Nationally notifiable infectious diseases and conditions, United States: annual tables. https://wonder.cdc.gov/nndss/nndss_annual_tables_menu.asp.
- 5.Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. 2017. Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveill Summ 66:1–12. doi: 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Healy GR. 1979. Babesia infections in man. Hosp Pract 14:107–111. doi: 10.1080/21548331.1979.11707564. [DOI] [PubMed] [Google Scholar]
- 7.Healy GR, Speilman A, Gleason N. 1976. Human babesiosis: reservoir of infection on Nantucket Island. Science 192:479–480. doi: 10.1126/science.769166. [DOI] [PubMed] [Google Scholar]
- 8.Levine JF, Wilson ML, Spielman A. 1985. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg 34:355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- 9.Main AJ, Carey AB, Carey MG, Goodwin RH. 1982. Immature Ixodes dammini (Acari: Ixodidae) on small animals in Connecticut, USA. J Med Entomol 19:655–664. doi: 10.1093/jmedent/19.6.655. [DOI] [PubMed] [Google Scholar]
- 10.Mather TN, Wilson ML, Moore SI, Ribeiro JMC, Spielman A. 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am J Epidemiol 130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 11.Spielman A, Etkind P, Piesman J, Ruebush TK, Juranek DD, Jacobs MS. 1981. Reservoir hosts of human babesiosis on Nantucket Island. Am J Trop Med Hyg 30:560–565. doi: 10.4269/ajtmh.1981.30.560. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JF, Johnson RC, Magnarelli LA. 1987. Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. J Clin Microbiol 25:1564–1566. doi: 10.1128/JCM.25.8.1564-1566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piesman J. 1988. Intensity and duration of Borrelia burgdorferi and Babesia microti infectivity in rodent hosts. Int J Parasitol 18:687–689. doi: 10.1016/0020-7519(88)90105-1. [DOI] [PubMed] [Google Scholar]
- 14.Mather TN, Telford SR III, Moore SI, Spielman A. 1990. Borrelia burgdorferi and Babesia microti: efficiency of transmission from reservoirs to vector ticks (Ixodes dammini). Exp Parasitol 70:55–61. doi: 10.1016/0014-4894(90)90085-Q. [DOI] [PubMed] [Google Scholar]
- 15.Piesman J, Spielman A. 1982. Babesia microti: infectivity of parasites from ticks for hamsters and white-footed mice. Exp Parasitol 53:242–248. doi: 10.1016/0014-4894(82)90065-0. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JF. 1989. Epizootiology of Borrelia in Ixodes tick vectors and reservoir hosts. Rev Infect Dis 11:S1451–S1459. doi: 10.1093/clinids/11.Supplement_6.S1451. [DOI] [PubMed] [Google Scholar]
- 17.LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci U S A 100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spielman A. 1988. Prospects for suppressing transmission of Lyme disease. Ann N Y Acad Sci 539:212–220. doi: 10.1111/j.1749-6632.1988.tb31855.x. [DOI] [PubMed] [Google Scholar]
- 19.Spielman A, Wilson ML, Levine JF, Piesman J. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol 30:439–460. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt KA, Ostfeld RS. 2001. Biodiversity and the dilution effect in disease ecology. Ecology 82:609–619. doi: 10.1890/0012-9658(2001)082[0609:BATDEI]2.0.CO;2. [DOI] [Google Scholar]
- 21.Weitz B. 1956. Identification of blood meals of blood-sucking arthropods. Bull World Health Organ 15:473–490. [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman WG, DeFoliart GR, Burkot TR. 1981. Identification of mosquito blood meals by enzyme-linked immunosorbent assay. Am J Trop Med Hyg 30:1336–1341. doi: 10.4269/ajtmh.1981.30.1336. [DOI] [PubMed] [Google Scholar]
- 23.Balashov YS. 1968. Bloodsucking ticks (Ixodoidea)—vectors of disease in man and animals. Nauka, Moscow, Russia. [Google Scholar]
- 24.Cadenas FM, Rais O, Humair P-F, Douet V, Moret J, Gern L. 2007. Identification of host bloodmeal source and Borrelia burgdorferi sensu lato in field-collected Ixodes ricinus ticks in Chaumont (Switzerland). J Med Entomol 44:1109–1117. doi: 10.1603/0022-2585(2007)44[1109:IOHBSA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Collini M, Albonico F, Rosà R, Tagliapietra V, Arnoldi D, Conterno L, Rossi C, Mortarino M, Rizzoli A, Hauffe HC. 2016. Identification of Ixodes ricinus blood meals using an automated protocol with high resolution melting analysis (HRMA) reveals the importance of domestic dogs as larval tick hosts in Italian alpine forests. Parasit Vectors 9:638. doi: 10.1186/s13071-016-1901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collini M, Albonico F, Hauffe HC, Mortarino M. 2015. Identifying the last bloodmeal of questing sheep tick nymphs (Ixodes ricinus L.) using high resolution melting analysis. Vet Parasitol 210:194–205. doi: 10.1016/j.vetpar.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Estrada-Peña A, Osácar JJ, Pichon B, Gray JS. 2005. Hosts and pathogen detection for immature stages of Ixodes ricinus (Acari: Ixodidae) in North-Central Spain. Exp Appl Acarol 37:257–268. doi: 10.1007/s10493-005-3271-6. [DOI] [PubMed] [Google Scholar]
- 28.Honig V, Carolan HE, Vavruskova Z, Massire C, Mosel MR, Crowder CD, Rounds MA, Ecker DJ, Ruzek D, Grubhoffer L, Luft BJ, Eshoo MW. 2017. Broad-range survey of vector-borne pathogens and tick host identification of Ixodes ricinus from Southern Czech Republic. FEMS Microbiol Ecol 93:fix129. doi: 10.1093/femsec/fix129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humair P-F, Douet V, Cadenas FM, Schouls LM, Van De Pol I, Gern L. 2007. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J Med Entomol 44:869–880. doi: 10.1603/0022-2585(2007)44[869:MIOBSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Kirstein F, Gray J. 1999. Blood meal identification in ticks: a promising tool in ecological research on tick-borne diseases. Zentralbl Bakteriol 289:760–764. doi: 10.1016/S0934-8840(99)80052-8. [DOI] [Google Scholar]
- 31.Pichon B, Egan D, Rogers M, Gray J. 2003. Detection and identification of pathogens and host DNA in unfed host-seeking Ixodes ricinus L.(Acari: Ixodidae). J Med Entomol 40:723–731. doi: 10.1603/0022-2585-40.5.723. [DOI] [PubMed] [Google Scholar]
- 32.Wodecka B, Rymaszewska A, Skotarczak B. 2014. Host and pathogen DNA identification in blood meals of nymphal Ixodes ricinus ticks from forest parks and rural forests of Poland. Exp Appl Acarol 62:543–555. doi: 10.1007/s10493-013-9763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wodecka B, Skotarczak B. 2016. Identification of host blood-meal sources and Borrelia in field-collected Ixodes ricinus ticks in north-western Poland. Ann Agric Environ Med 23:59–63. doi: 10.5604/12321966.1196853. [DOI] [PubMed] [Google Scholar]
- 34.Heylen D, Schmidt O, Dautel H, Gern L, Kampen H, Newton J, Gray J. 2019. Host identification in unfed ticks from stable isotope compositions (δ13C and δ15N). Med Vet Entomol 33:360–366. doi: 10.1111/mve.12372. [DOI] [PubMed] [Google Scholar]
- 35.Önder Ö, Shao W, Kemps BD, Lam H, Brisson D. 2013. Identifying sources of tick blood meals using unidentified tandem mass spectral libraries. Nat Commun 4:1746. doi: 10.1038/ncomms2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson SR, Doucet AJ, Kopera HC, Moldovan JB, Garcia-Pérez JL, Moran JV. 2015. The influence of LINE-1 and SINE retrotransposons on mammalian genomes. Microbiol Spectr 3. doi: 10.1128/microbiolspec.MDNA3-0061-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Telford SR., III. 2002. Deer tick transmitted zoonoses in the eastern United States, p 310–324. In Conservation medicine: ecological health in practice. Oxford University Press, New York, NY. [Google Scholar]
- 38.Wilson ML, Telford SR III, Piesman J, Spielman A. 1988. Reduced abundance of immature Ixodes dammini (Acari: Ixodidae) following elimination of deer. J Med Entomol 25:224–228. doi: 10.1093/jmedent/25.4.224. [DOI] [PubMed] [Google Scholar]
- 39.Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F. 2012. Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis 18:1951–1957. doi: 10.3201/eid1812.111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Telford SR III, Mather TN, Moore SI, Wilson ML, Spielman A. 1988. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg 39:105–109. doi: 10.4269/ajtmh.1988.39.105. [DOI] [PubMed] [Google Scholar]
- 41.Piesman J, Spielman A. 1979. Host-associations and seasonal abundance of immature Ixodes dammini in southeastern Massachusetts. Ann Entomol Soc Am 72:829–832. doi: 10.1093/aesa/72.6.829. [DOI] [Google Scholar]
- 42.Telford SR III, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A. 1997. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 3:165. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telford SR III, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A 93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson JF. 1988. Mammalian and avian reservoirs for Borrelia burgdorferi. Ann N Y Acad Sci 539:180–191. doi: 10.1111/j.1749-6632.1988.tb31852.x. [DOI] [PubMed] [Google Scholar]
- 45.Battaly GR, Fish D. 1993. Relative importance of bird species as hosts for immature Ixodes dammini (Acari: Ixodidae) in a suburban residential landscape of southern New York state. J Med Entomol 30:740–747. doi: 10.1093/jmedent/30.4.740. [DOI] [PubMed] [Google Scholar]
- 46.Slajchert T, Kitron UD, Jones CJ, Mannelli A. 1997. Role of the eastern chipmunk (Tamias striatus) in the epizootiology of Lyme borreliosis in northwestern illinois, USA. J Wildl Dis 33:40–46. doi: 10.7589/0090-3558-33.1.40. [DOI] [PubMed] [Google Scholar]
- 47.Donahue J, Piesman J, Spielman A. 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am J Trop Med Hyg 36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 48.Spielman A. 1988. Lyme disease and human babesiosis: evidence incriminating vector and reservoir hosts, p 147–165. In Englund PT, Sher A (ed), The biology of parasitism. Alan R. Liss, New York, NY. [Google Scholar]
- 49.Dolan MC, Schulze TL, Jordan RA, Dietrich G, Schulze CJ, Hojgaard A, Ullmann AJ, Sackal C, Zeidner NS, Piesman J. 2011. Elimination of Borrelia burgdorferi and Anaplasma phagocytophilum in rodent reservoirs and Ixodes scapularis ticks using a doxycycline hyclate-laden bait. Am J Trop Med Hyg 85:1114–1120. doi: 10.4269/ajtmh.2011.11-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mather TN, Ribeiro JM, Spielman A. 1987. Lyme disease and babesiosis: acaricide focused on potentially infected ticks. Am J Trop Med Hyg 36:609–614. doi: 10.4269/ajtmh.1987.36.609. [DOI] [PubMed] [Google Scholar]
- 51.Daniels TJ, Fish D, Falco RC. 1991. Evaluation of host-targeted acaricide for reducing risk of Lyme disease in southern New York state. J Med Entomol 28:537–543. doi: 10.1093/jmedent/28.4.537. [DOI] [PubMed] [Google Scholar]
- 52.Dolan MC, Maupin GO, Schneider BS, Denatale C, Hamon N, Cole C, Zeidner NS, Stafford KC. 2004. Control of Immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J Med Entomol 41:1043–1054. doi: 10.1603/0022-2585-41.6.1043. [DOI] [PubMed] [Google Scholar]
- 53.Hornbostel VL, Ostfeld RS, Benjamin MA. 2005. Effectiveness of Metarhizium anisopliae (Deuteromycetes) against Ixodes scapularis (Acari: Ixodidae) engorging on Peromyscus leucopus. J Vector Ecol 30:91–101. [PubMed] [Google Scholar]
- 54.Richer LM, Brisson D, Melo R, Ostfeld RS, Zeidner N, Gomes-Solecki M. 2014. Reservoir targeted vaccine against Borrelia burgdorferi: a new strategy to prevent Lyme disease transmission. J Infect Dis 209:1972–1980. doi: 10.1093/infdis/jiu005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc Natl Acad Sci U S A 101:18159–18164. doi: 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brisson D, Dykhuizen DE, Ostfeld RS. 2008. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc Biol Sci 275:227–235. doi: 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert L, Norman R, Laurenson KM, Reid HW, Hudson PJ. 2001. Disease persistence and apparent competition in a three-host community: an empirical and analytical study of large-scale, wild populations. J Anim Ecol 70:1053–1061. doi: 10.1046/j.0021-8790.2001.00558.x. [DOI] [Google Scholar]
- 58.Telford SR., III. 2017. Deer reduction is a cornerstone of integrated deer tick management. J Integr Pest Manag 8:25. doi: 10.1093/jipm/pmx024. [DOI] [Google Scholar]
- 59.Shaw MT, Keesing F, McGrail R, Ostfeld RS. 2003. Factors influencing the distribution of larval blacklegged ticks on rodent hosts. Am J Trop Med Hyg 68:447–452. doi: 10.4269/ajtmh.2003.68.447. [DOI] [PubMed] [Google Scholar]
- 60.Levin ML, Fish D. 1998. Density-dependent factors regulating feeding success of Ixodes scapularis larvae (Acari: Ixodidae). J Parasitol 84:36–43. doi: 10.2307/3284526. [DOI] [PubMed] [Google Scholar]
- 61.Arnold E. 2015. Life on a hostile landscape: success, distributions, and consequences of ectoparasites on their hosts. Thesis. University of Illinois at Chicago, Chicago, IL. [Google Scholar]
- 62.Heine KB, DeVries PJ, Penz CM. 2017. Parasitism and grooming behavior of a natural white-tailed deer population in Alabama. Ethol Ecol Evol 29:292–303. doi: 10.1080/03949370.2016.1179683. [DOI] [Google Scholar]
- 63.Jaenson T, Tälleklint L. 1992. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J Med Entomol 29:813–817. doi: 10.1093/jmedent/29.5.813. [DOI] [PubMed] [Google Scholar]
- 64.Kjelland V, Ytrehus B, Stuen S, Skarpaas T, Slettan A. 2011. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks collected from moose (Alces alces) and roe deer (Capreolus capreolus) in southern Norway. Ticks Tick Borne Dis 2:99–103. doi: 10.1016/j.ttbdis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 65.Pacilly FCA, Benning ME, Jacobs F, Leidekker J, Sprong H, Van Wieren SE, Takken W. 2014. Blood feeding on large grazers affects the transmission of Borrelia burgdorferi sensu lato by Ixodes ricinus. Ticks Tick Borne Dis 5:810–817. doi: 10.1016/j.ttbdis.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 66.Kurtenbach K, De Michelis S, Etti S, Schäfer SM, Sewell H-S, Brade V, Kraiczy P. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol 10:74–79. doi: 10.1016/s0966-842x(01)02298-3. [DOI] [PubMed] [Google Scholar]
- 67.Kurtenbach K, Sewell H-S, Ogden NH, Randolph SE, Nuttall PA. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun 66:1248–1251. doi: 10.1128/IAI.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson DR, Rooney S, Miller NJ, Mather TN. 2000. Complement-mediated killing of Borrelia burgdorferi by nonimmune sera from sika deer. J Parasitol 86:1232–1238. doi: 10.2307/3285006. [DOI] [PubMed] [Google Scholar]
- 69.Mühleip JJ, Lin Y-P, Kraiczy P. 2018. Further insights into the interaction of human and animal complement regulator factor H with viable Lyme disease spirochetes. Front Vet Sci 5:346. doi: 10.3389/fvets.2018.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kraiczy P. 2016. Hide and seek: how Lyme disease spirochetes overcome complement attack. Front Immunol 7:385. doi: 10.3389/fimmu.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogden NH, Nuttall PA, Randolph SE. 1997. Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitology 115:591–599. doi: 10.1017/S0031182097001868. [DOI] [PubMed] [Google Scholar]
- 72.Piesman J, Spielman A, Etkind P, Ruebush TK II, Juranek DD. 1979. Role of deer in the epizootiology of Babesia microti in Massachusetts, USA. J Med Entomol 15:537–540. doi: 10.1093/jmedent/15.5-6.537. [DOI] [PubMed] [Google Scholar]
- 73.Brown RN, Lane RS. 1992. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science 256:1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- 74.Smith RP, Rand PW, Lacombe EH, Telford SR, Rich SM, Piesman J, Spielman A. 1993. Norway rats as reservoir hosts for Lyme disease spirochetes on Monhegan Island, Maine. J Infect Dis 168:687–691. doi: 10.1093/infdis/168.3.687. [DOI] [PubMed] [Google Scholar]
- 75.Jordan RA, Schulze TL. 2019. Ability of two commercially available host-targeted technologies to reduce abundance of Ixodes scapularis (Acari: Ixodidae) in a residential landscape. J Med Entomol 56:1095–1101. doi: 10.1093/jme/tjz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tokarz R, Tagliafierro T, Cucura DM, Rochlin I, Sameroff S, Lipkin WI. 2017. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan virus in ticks by a multiplex real-time reverse transcription-PCR assay. mSphere 2:e00151-17. doi: 10.1128/mSphere.00151-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vassetzky NS, Kramerov DA. 2013. SINEBase: a database and tool for SINE analysis. Nucleic Acids Res 41:D83–D89. doi: 10.1093/nar/gks1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An Excel file containing the data used for the analyses are available at the Open Science Framework (https://osf.io/wcmta/?view_only=991778cbd9e54b14922e1f098b1d957c).


