Abstract
Duchenne muscular dystrophy (DMD) is complicated by an early and progressive left ventricular (LV) dysfunction. Despite the reduction of ejection fraction (EF) usually manifests in the second decade, subtle alterations in LV mechanics can be detected earlier. Longitudinal and circumferential LV deformation, evaluated by speckle tracking echocardiography (STE), are considered sensitive markers of early dysfunction. We retrospectively examined clinical and echocardiographic data of 32 DMD children with preserved LV function. According to the median age, patients were then divided into younger and older than 9 years, and compared to 24 age-matched healthy subjects. Six-minute-walk test (6MWT), North Star Ambulatory Assessment (NSAA), and a comprehensive cardiac evaluation were performed. Although EF was within the normal range, DMD patients had significantly lower values than healthy controls, and the same occurred for the remaining conventional systolic and diastolic indices. Global longitudinal strain (GLS) was reduced in all patients (older and younger, both p < 0.001). Global circumferential strain (GCS) was reduced only in older patients (< 0.001). Both GLS and GCS worsened with age in DMD patients (GLS p = 0.005; GCS p = 0.024). GLS was significantly worse in the apical segments and in the postero-lateral wall. GCS in the antero-septal, anterior and antero-lateral segments was significantly reduced in older patients, with a prevalent involvement of the sole septal wall in the younger boys. 6MWT appeared to be correlated inversely to GLS and directly to EF. A longitudinal evaluation should be scheduled in DMD boys to assess the global cardiac performance over time and to evaluate the impact of therapies.
Key words: Duchenne muscular dystrophy, cardiomyopathy, speckle tracking echocardiography, strain, motor performance
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked genetic disorder caused by a mutation in the gene encoding the dystrophin protein. It affects 1 in every 5000 live male births (20,000 new cases worldwide each year) 1. The lack of dystrophin results in a cascade of events leading to progressive loss of muscle function and to a multisystemic involvement 2. Myocardial dysfunction is part of the natural history of DMD, although timing of onset, progression and severity of myocardial involvement can vary 3. Respiratory failure has been in the past the most common cause of morbidity and mortality in DMD patients. However, with the improvement of the respiratory support and the use of non-invasive ventilation, cardiomyopathy represents nowadays one of the main sources of morbidity and mortality in this clinical setting 4.
The prevalence of cardiomyopathy increases inexorably over the years, and therefore age is a significant predictor of cardiomyopathy in DMD patients. If 5% is the estimated prevalence of myocardial dysfunction in patients aged 2 to 5 years, it is expected to rise over 60% beyond 18 years 4,5. Unfortunately, the progression of cardiac damage is concealed most of the times, due to the severe functional limitations of DMD patients. Clinical diagnosis of cardiac failure is mostly unreliable, since patients with very limited activity do not develop symptoms until severe ventricular dysfunction occurs. For these reasons, DMD multidisciplinary care recommends that cardiovascular assessment, including electrocardiogram (ECG) and echocardiography, has to be performed at diagnosis and then annually 2. The presence of abnormal ventricular function suggests the need for increased surveillance and should prompt initiation of drug therapy, following general recommendations for heart failure treatment 2,6,7.
How can we better define “abnormalities of ventricular function”? Historically, the officially accepted parameter that represents left ventricular (LV) function is the ejection fraction (EF). However, as widely demonstrated, EF as well as other conventional systolic and diastolic parameters fail to reveal subclinical LV dysfunction. A significant reduction of EF below the normal limits occurs when the disease progression has already reached an advanced stage. Therefore, early markers of LV dysfunction have been extensively evaluated, and longitudinal deformation or strain is now considered as one of the most sensitive parameters of LV function, along with circumferential deformation. Myocardial strain represents the change in myocardial fiber length compared with its original length in the plane in which it is measured 8. LV longitudinal function is expression of the contraction of subendocardial fibers, which are known to be particularly vulnerable to damage. Speckle tracking echocardiography (STE) is the most widely used method for the evaluation of longitudinal and circumferential strain of the left ventricle, and nowadays it represents a validated and reproducible technique 8-10.
The aim of our study has been to identify and define the onset of LV abnormalities in DMD children, not reporting cardiological symptoms and with preserved EF, in order to answer the following questions: (1) which parameters should we follow to better define LV dysfunction in the earliest stage? and (2) when are these parameters expected to become altered in the natural history of the disease? We also attempted, for the first time, to evaluate a correlation among cardiological parameters and functional motor performances, assessed with the most used outcome measures in DMD clinical trials.
Methods
We retrospectively examined clinical and echocardiographic data of 45 children with DMD, undergoing routine cardiovascular follow up evaluation in our Institution. We excluded from our analysis 10 patients with manifest LV dysfunction, expressed by a LV EF below 55%. We also excluded 3 cases with unsuitable echocardiographic recordings due to the low quality of patients’ acoustic window. Therefore, we considered a comprehensive assessment, including neuromuscular and cardiovascular evaluation, of 32 DMD patients with preserved LV function (median age 9 years, IQR 6) and not referring cardiological symptoms. The study was approved by the local Ethical Committee (Prot. Number 105/16).
6-minute-walk test (6MWT)
The 6MWT has been chosen as the primary outcome measure in international multicentre investigational drug clinical trials as well as in longitudinal natural history studies in DMD ambulant patients. 6MWT was performed in all DMD ambulant boys older than 5 years using a modified version of the American Thoracic Society guidelines 11,12. Modifications include the addition of continuous encouragement from the testing staff, and a “safety chaser” to walk along behind the subject during testing. The test is generally completed within 15 to 20 minutes. Suitability and inter-rater and intra-rater reliability in DMD for the 6MWT have already been reported 12,13.
North Star Ambulatory Assessment (NSAA)
The functional scale NSAA represents an ideal additional tool to the 6MWT, as it provides information on a wider spectrum of functions that reflect everyday life activities. The scale consists of 17 items, ranging from standing (item 1) to running (item 17) and includes several items assessing abilities that are necessary to remain functionally ambulant. Each item can be scored on a 3-point scale using simple criteria: 2 - Normal, achieves goal without any assistance; 1 - Modified method, but achieves goal independently of physical assistance from another person; 0 - Unable to achieve independently. The score can range from 0, if all the activities are failed, to 34, if all the activities are achieved. The scale is generally completed in a maximum of 15 minutes 12.
Cardiac evaluation
Cardiovascular assessment was based on clinical evaluation, physical examination, 12-leads ECG and bi-dimensional (2D) echocardiography, particularly aimed to study myocardial mechanics. A conventional 2D-echocardiogram was performed with a Vivid 7 echocardiography equipment (GE Vingmed Ultrasound AS, Horten, Norway) in each case, and records were collected in a private local archive. Clear loops of the LV in apical 4-chamber (4-ch), 2-chamber (2-ch) and 3-chamber (3-ch) views, and also parasternal short axis views at the level of mitral valve (MV-Sax), papillary muscles (PM-Sax) and apex (AP-Sax) were available for the majority of patients. Three consecutive end-expiratory cycles, in gray scale (frame rate > 70 frames/s), have been stored twice for each view and were analyzed by two experienced independent operators, unaware of the clinical conditions of the patients. All measures and functional evaluations were processed off-line on the Echopac GE, Vivid 7 workstation. Left ventricular diameters, volumes and mass were calculated. Diastolic function was studied by mitral early inflow Doppler velocity (E wave), early to late (A wave) inflow Doppler velocity ratio (E/A), medial and lateral mitral annular tissue Doppler imaging (TDI), early inflow velocity (E’) and the E/E’ ratio. Systolic function was expressed as global LV function (EF), fractional shortening (FS), longitudinal systolic function by M-Mode echocardiography and TDI, global longitudinal and circumferential strain (GLS, GCS) by STE. EF was obtained with biplane Simpson’s method. FS was calculated on M-mode modality. Longitudinal systolic function was assessed by mitral and tricuspid annular plane systolic excursion by M-mode echocardiography (MAPSE, TAPSE), and by TDI mitral annular systolic velocity (S’). Since lateral MAPSE and S’ were not accurately sampled for the majority of patients, only medial values were considered. Furthermore, systolic longitudinal deformation of the left ventricle was studied by STE, recording both global, partial (3-ch, 4-ch, 2-ch views) and segmental strain values, expressed as percentage. Also, global, partial (at mitral valve [MV]-, papillary muscles [PM]- and apical [AP]-short axis [SAX] views) and segmental circumferential deformation were studied at basal, medial and apical LV level. For longitudinal strain calculation a semi-automated method was used, Automated Function Imaging – AFI (GE Vingmed Ultrasound), in which the operator is required to track manually only three points, medial mitral annulus, lateral mitral annulus and LV apex; then, the software provides automatically the entire endocardial and epicardial border of the left ventricle, that can still be modified by the operator, if necessary. For circumferential strain, manual tracking of the whole endocardial border was performed by the operators.
The 6MWT and the same comprehensive echocardiographic study were performed also in a control group of 24 age-matched healthy children, sent to our hospital for evaluation of sport eligibility.
The intraobserver/interobserver variability was 6.8/8.8% (GLS) and 8.5/10.5% (GCS), respectively.
Statistics
A classic non-parametric approach was used since some numerical variables were not normally distributed, as verified by Kolmogorov Smirnov test, also considering the small sample size. Numerical data are consistently expressed as median with interquartile range (IQR) in brackets, categorical variables as number and percentage. Comparisons between patients and controls were carried out by the Mann-Whitney test. In order to address the aims of the present study, also considering that several parameters may be age-dependent, the DMD population has been then divided into two groups basing on the median age value (9 years), as were also control subjects, thus having four groups for statistical analysis: patients and controls younger than 9 years (DMD 1 and Controls 1, respectively); patients and controls older than 9 years (DMD 2 and Controls 2, respectively). Consistently, the Mann-Whitney test was applied in order to perform between-groups comparisons, including segmental strain analysis. Correlations among the variables were tested by Spearman’s test. Moreover, dependence analysis was carried out by multiple regression models, in order to assess the contribution of any potential predictor on the study response variable(s) (GLS, GCS, EF%, E/A ratio, 6MWT, NSAA). Statistical analyses were performed using SPSS 17.0 for Window package. A two-tailed alpha of 0.05 was used to denote statistical significance.
Results
Motor assessment
The distance covered at 6MWT was significantly lower in DMD patients vs controls (median value 362 m, IQR 220 m, vs a median value of 585 m, IQR 118 m, p < 0.001). NSAA median score was 30 (9.8) in patients, but not comparable with controls, since such a test is not usually administered to healthy individuals.
Cardiological assessment
All patients denied symptoms of cardiovascular involvement, like palpitations, shortness of breath or chest pain. Cardiovascular physical examination was unremarkable for all patients and controls. ECG was nearly normal, except for a mild degree of right bundle branch block in 5 patients and 3 controls. No significant arrhythmias were observed on ECG.
2D-echocardiography
Indexed LV diameters, volumes and mass were similar between patients and healthy children. Table I reports motor performance and conventional echocardiographic parameters in patients and controls, without age stratification. Although EF was within the normal range in both groups, a significant reduction was seen in patients and the same occurred for the remaining conventional systolic (FS, MAPSE, TAPSE, S’) and diastolic (E, E’) indices. E/A ratio also was reduced with respect to healthy controls.
Table I.
Motor performance and conventional echocardiographic parameters. Median (IQR)
| Controls | DMD patients | P-value | |
|---|---|---|---|
| Age (years) | 8.75(5) | 9(6) | 0.256 |
| 6MWT (m) | 585(118) | 362(220) | < 0.001 |
| NSAA | -- | 30 (9.8) | -- |
| BSA (m2) | 1.04 (0.64) | 0.96 (1.04) | 0.956 |
| LV EDD mm/m2 | 59.7 (11.3) | 61.3 (11.3) | 0.315 |
| LV ESD mm/m2 | 37.8(8) | 38.1 (9.3) | 0.471 |
| LV EDV ml/m2 | 88.7 (41.9) | 83.9 (41.9) | 0.826 |
| LV ESV ml/m2 | 30.6 (16.1) | 29.1 (13.7) | 0.427 |
| LV M g/m2 | 54(15) | 55(12) | 0.723 |
| FS% | 37(4) | 35(22) | < 0.001 |
| EF% | 69(5) | 64(8) | < 0.001 |
| MAPSE mm | 12(2) | 10(2) | < 0.001 |
| TAPSE mm | 20.5(5) | 18(3) | < 0.001 |
| E cm/s | 105(20) | 90.5(18) | < 0.001 |
| E’ cm/s | 15(2) | 13.5(3) | < 0.001 |
| S’ cm/s | 8(2) | 7(2) | 0.007 |
| E/A | 2.1 (0) | 1.86(1) | 0.025 |
| E/E’ | 7.3(2) | 7.6(1) | 0.122 |
The same parameters are summarized in Table II, based on age stratification: patients younger and older than 9 years are compared to controls younger and older than 9 years, respectively. All parameters were significantly reduced in older patients with respect to older controls (2 vs 2), although E/E’ ratio has not reached the alpha level, whereas no significant difference was observed between younger patients and younger controls (1 vs 1), except for the E wave velocity that appeared reduced in patients.
Table II.
Motor performance and conventional echocardiographic parameters based on age stratification. Median (IQR)
| Controls 1 |
Controls 2 |
DMD 1 |
DMD 2 |
P-value 1 vs 1 |
P-value 2 vs 2 |
|
|---|---|---|---|---|---|---|
| Age (years) | 6(2) | 12 (3.2) | 6.25 (3.5) | 12.2 (3.5) | 0.760 | 0.488 |
| 6MWT (meters) | 519.5(60) | 630(44) | 385(488) | 160(398) | 0.002 | < 0.001 |
| NSAA | -- | -- | 30 (9.5) | 24(7) | -- | -- |
| FS% | 38.5(7) | 36(3) | 36(3) | 34(6) | 0.294 | < 0.001 |
| EF% BP Simpson | 70(9) | 67(4) | 67(4) | 64(8) | 0.213 | < 0.001 |
| MAPSE mm | 11(2) | 12(3) | 10.5(2) | 10(1) | 0.121 | < 0.001 |
| TAPSE mm | 20(5) | 21(4) | 18(3) | 16 (2.0) | 0.100 | < 0.001 |
| E cm/s | 113(25) | 105(10) | 99(14) | 93(20) | 0.029 | 0.007 |
| E’ cm/s | 15(4) | 16(3) | 15 (4.0) | 12 (3.0) | 0.545 | < 0.001 |
| S’ cm/s | 8(1) | 9(1) | 7(1) | 7(1) | 0.286 | 0.001 |
| E/A | 2.1(1) | 2.0 (0) | 1.9(1) | 1.9(1) | 0.038 | 0.041 |
| E/E’ | 7.6(1) | 6.1(2) | 8.7(3) | 7.3(1) | 0.320 | 0.211 |
Controls 1 and DMD 1 are younger than 9 years; Controls 2 and DMD 2 are older than 9 years.
Myocardial deformation analysis
GLS was significantly reduced in patients vs controls (p < 0.001), without age stratification (a less negative value reflects a more impaired GLS) (Tab. III). The same behavior was maintained if GLS was considered for each partial analysis of 4-ch, 2-ch and 3-ch view. Moreover, also GCS was significantly reduced in patients vs controls (p = 0.013), with preserved difference when considering separated MV-SAX, PM-SAX and AP-SAX views (a less negative value reflects a more impaired GCS).
Table III.
Global longitudinal and circumferential strain. Median (IQR)
| Controls | DMD Patients | P-value | |
|---|---|---|---|
| 3ch GLS | -24.6 (4.1) | -19.7 (3.5) | < 0.001 |
| 4ch GLS | -22.8(3) | -19.8(4) | < 0.001 |
| 2ch GLS | -24.8(4) | -21.5(4) | 0.006 |
| GLS | -24.2(3) | -20.6(3) | < 0.001 |
| MV-sax GCS | -18.8 (2.4) | -16.6 (3.9) | < 0.001 |
| PM-sax GCS | -18.3 (3.4) | -15.5 (5.5) | 0.011 |
| AP-sax GCS | -25.3 (7.8) | -20.3 (11.4) | 0.040 |
| GCS | -21.4 (4.4) | -17.6 (4.6) | 0.013 |
The effect of age stratification on GLS and GCS is illustrated in Table IV. Either global or partial 4-ch, 2-ch and 3-ch GLS were reduced to the same extent in older and in younger patients, compared to their matched controls (p < 0.001 and p < 0.001 respectively). A different behavior was observed for GCS, that was clearly reduced in older patients respect to older controls (p < 0.001), but not equally altered in younger. Although a trend towards reduction was evident also in younger patients, a significant decrease was observed only at the midventricular level (PM-SAX GCS), whereas the global value of GCS, as well as basal and apical partial GCS, were not significantly different as compared to matched controls (GCS p = 0.217). Figure 1 illustrates areas with impaired GLS and GCS in DMD patients compared to age-matched controls. In younger patients, apical and infero-lateral segments show impaired GLS, while in older patients the damage is extended also to mid-septal and basal antero-lateral areas. GCS in younger patients appears to be impaired in the septal region, whereas in older patients reduced GCS involves also the apex, and the anterior and antero-lateral segments, but not the inferior septum.
Table IV.
Global longitudinal and circumferential strain based on age stratification. Median (IQR)
| Controls 1 |
Controls 2 |
DMD 1 |
DMD 2 |
P-value 1 vs 1 |
P-value 2 vs 2 |
|
|---|---|---|---|---|---|---|
| 3ch GLS | -25.2 (3.8) | -23.4(6) | -21.2 (4.6) | -18.9 (3.2) | 0.004 | < 0.001 |
| 4ch GLS | -23.6(3) | -20.7 (3.2) | -20.1(3) | -18.0 (6.0) | < 0.001 | 0.005 |
| 2ch GLS | -24.9(3) | -24.4(8) | -22.1(5) | -19.5 (6.0) | 0.003 | 0.007 |
| GLS | -24.9(3) | -24(6) | -21.2(2) | -19.3 (4.0) | < 0.001 | < 0.001 |
| MV-sax GCS | -19.9 (3.5) | -18.0 (4.8) | -17.7 (5.2) | -15.5 (3.8) | 0.103 | < 0.001 |
| PM-sax GCS | -17.7 (5.6) | -18.3 (3.9) | -14.0 (7.4) | -13.8(4) | 0.032 | < 0.001 |
| AP-sax GCS | -27.9 (6.3) | -24.3 (5.6) | -24.9 (9.5) | -17.5 (9.3) | 0.210 | 0.035 |
| GCS | -22.1 (5.3) | -20.4 (2.6) | -17.9 (3.9) | -15.9 (4.9) | 0.217 | < 0.001 |
Controls 1 and DMD 1 are younger than 9 years; Controls 2 and DMD 2 are older than 9 years.
Figure 1.
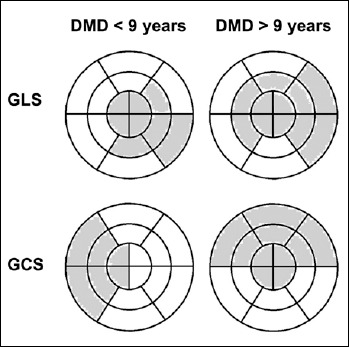
Bulls-eye diagrams illustrating the distribution of abnormal strain values in DMD patients according to age. A 16-segment model of the left ventricle is used. Grey areas depict impaired GLS and GCS segments, compared to age-matched normal controls.
Concerning age stratification, we also found that both GLS and GCS worsened with age in DMD patients (GLS p < 0.005; GCS p = 0.024. DMD 1 vs DMD 2), while no change was observed between control subjects (GLS p = 0.247; GCS p = 0.456). The regressive analyses further confirmed these findings, suggesting the increasing age as the variable most strongly associated with the subtle alterations in LV mechanics found in this population (GLS: Beta = 0.466; T = 3.078; p = 0.009; GCS: Beta = 0.477; T = 2.878; p = 0.008), and also with EF% (Beta = -0.567; T = -3.363; p < 0.001). Moreover, also the physical performance, as assessed by 6MWT, is confirmed to decrease by increasing age (Beta = -0.490; T = -2.978; p = 0.006).
Correlation between motor and cardiological assessment
6MWT appeared to be inversely correlated to GLS (rho: -0.434, p = 0.021) and directly to EF (rho: 0.410, p = 0.035) and S’ (rho: 0.584, p < 0.001). No significant correlations were found with NSAA.
Discussion
Cardiovascular complications are a leading cause of disease-related morbidity and mortality among individuals with DMD. Historically, individuals with DMD have not been referred to a cardiac specialist until late in the disease, contributing to poor clinical outcomes. Furthermore, cardiac management has been challenging because the New York Heart Association classification of heart failure relies on reduced exercise tolerance, a feature that in DMD arises from skeletal muscle and cardiac disease combined. The signs and symptoms of heart failure in the non-ambulatory patients are frequently subtle and overlooked. A proactive strategy of early diagnosis and treatment is essential to maximize quality of life and survival. Involvement of a cardiologist who is integrated into a multidisciplinary care team is recommended, given the complex decision making involved in managing DMD cardiomyopathy. Nowadays, however, LV dysfunction may be still often underdiagnosed and consequently undertreated 2,6-7.
The analysis of our results showed that in children with DMD, although EF is preserved (i.e. > 55%), LV function is definitely not normal. In younger patients (< 9 years), conventional parameters accounting for systolic and diastolic function are still comparable to age-matched control subjects. However, longitudinal function is already impaired, as demonstrated by significantly reduced GLS. At this stage global circumferential deformation appears to be preserved. In older patients (> 9 years), not only longitudinal and circumferential function are reduced, but also conventional parameters accounting for systolic and diastolic function have subtle alterations, that could not be defined “abnormal” if considered by themselves, but still appear significantly reduced when compared to normal subjects. Therefore, there might be a period, that we can define between 3 and 9 years of age, during which longitudinal function begins to decrease, while all the remaining compensatory mechanisms act to maintain a preserved LV systolic and diastolic performance. Beyond 9 years of age, it is expected that the worsening of the disease may involve progressively LV mechanics until the eventual decrease of the EF. Since circumferential strain appears to be globally preserved in younger and reduced in older patients, our hypothesis is that it might be considered a “transitional” parameter, which may help for a better characterization of patients with intermediate age, as those of 7 to 11 years of age. Nonetheless, a progressive trend can be identified for both GLS and GCS, which clearly worsened with age in DMD patients.
At the bivariate analysis we observed that the lower the distance covered at the 6MWT, the worse the EF, the S’ and the GLS. In other words, motor performance and myocardial function are related to each other and both worsen with age, even in young children with DMD. As concerns segmental strain analysis, we noticed that longitudinal deformation of the apical segments and the infero-lateral wall was particularly affected, regardless of the age of patients, since it was equally impaired in either younger or older patients. However, the distribution of circumferential strain impairment was different from GLS, and also different between age groups. In fact, septal wall was the most affected in younger, while antero-septal, anterior and lateral walls showed the worst performance in older patients.
In the past years there has been increasing interest for circumferential strain in children with DMD. Several papers reported that circumferential strain is reduced in young children with normal ejection fraction 14-18. However, in these studies strain analysis was performed with cardiac magnetic resonance imaging or with STE at the sole midventricular level. With respect to this, we also observed that in our population circumferential strain was particularly reduced at the level of the papillary muscles, but the global value was still preserved in patients younger than 9 years. Concerning the regional distribution of circumferential strain alterations, the lateral LV free wall has been reported with the earliest decline in young patients and the greatest involvement in older patients with DMD. Moreover, the lateral wall is also the area in which fibrosis is first recognized by cardiac magnetic resonance, in the advanced stage of the disease 15. Ryan et al. described a different regional pattern of circumferential strain impairment in children with DMD, since the most involved appeared to be antero-septal, inferior and infero-lateral segments 14. Furthermore, Taqatqa et al. reported that different segments could be affected, so that the reduction of circumferential strain cannot be localized to one specific region 19. Since there is a great variability in the results reported by different authors, concerning the distribution of circumferential strain anomalies, it is likely that these might not be confined to one specific area, but are rather the expression of a diffuse myocardial damage. Recently, also longitudinal strain has been described as an early marker of ventricular dysfunction in young DMD patients 5,19. Particularly, the reduction of GLS has been reported to be more pronounced in the apical area, as occurred in our population. It is worth noting that longitudinal strain values are usually higher at the apex in normal subjects, with a base-to-apex gradient 20. We can speculate that in DMD longitudinal dysfunction might involve at first the region with the higher performance.
The awareness about the early natural history of ventricular mechanics in DMD boys is crucial to support the opportunity of cardioprotective therapy, as already attempted by several studies in human and animals 21,22. This is particularly relevant as opinion differs on the use of angiotensin-converting enzyme (ACE) inhibitors in very young (< 10 years) asymptomatic individuals without evidence of abnormality on cardiac magnetic resonance or echocardiogram 2. A reduced GLS could allow clinicians to start ACE inhibitors or beta-blockers even below 10 years of age and to monitor the therapeutic effect, aiming to preserve systolic and diastolic parameters within the normal range as long as possible.
Several drugs have been employed with the aim to contrast the evolution of cardiomyopathy toward stages of severe congestive heart failure, before an invasive approach should be considered, e.g. through the implantation of cardioverter defibrillator or cardiac resynchronization therapy defibrillator 23. It is widely accepted that ACE inhibition could reduce mortality and hospitalization in DMD patients, delaying the onset and progression of cardiac dysfunction and reinforcing the usefulness of an early therapeutic approach 24-28. Also, the adoption of a therapy with beta-blockers, alone or combined with ACE inhibitors, showed results for delaying progression of heart failure in these patients 28-30. On that framework, STE-derived myocardial strain could be considered an additional outcome measure to test the efficacy of new therapeutic approaches, particularly in trials focusing on younger ambulant DMD patients.
Our study is limited by its retrospective nature and by the relatively small sample size. Also, deformation of the chest profile, which is often present in older patients with DMD, may have affected the accuracy of strain analysis, due to suboptimal ultrasound image quality.
Conclusions
Progressive LV dysfunction is a part of the natural history of DMD and begins very early. Despite the reduction of EF usually manifests in the second decade of life, subtle alterations in LV mechanics can be clearly seen as early as before 9 years of age. STE provides a reproducible and reliable evaluation of longitudinal and circumferential LV deformation, which represent sensitive markers of early dysfunction. Our results confirm that cardiac performances could be impaired already in very young DMD patients, below 9 years, apparently with a preferential involvement of the apical segments and the postero-lateral wall. Also, GCS begins to alter at this stage, even if the impairment becomes significant after 9 years of age. Both GLS and GCS show a progressive worsening with age in children with DMD. A longitudinal evaluation should be scheduled to assess the global cardiac performance over time and to evaluate the impact of therapies on the cardiovascular and overall outcome.
Figures and tables
References
- 1.Messina S, Vita GL. Clinical management of Duchenne muscular dystrophy: the state of the art. Neurol Sci 2018;39:1837-45. https://doi.org/10.1007/s10072-018-3555-3 10.1007/s10072-018-3555-3 [DOI] [PubMed] [Google Scholar]
- 2.Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 2018;17:347-61. https://doi.org/10.1016/s1474-4422(18)30025-5 10.1016/s1474-4422(18)30025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourke JP, Bueser T, Quinlivan R. Interventions for preventing and treating cardiac complications in Duchenne and Becker muscular dystrophy and X-linked dilated cardiomyopathy. Cochrane Database Syst Rev 2018;10(10):CD009068 https://doi.org/10.1002/14651858.cd009068.pub3 10.1002/14651858.cd009068.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shih JA, Folch A, Wong BL. Duchenne muscular dystrophy: the heart of the matter. Curr Heart Fail Rep 2020;17:57-66. https://doi.org/10.1007/s11897-020-00456-0 10.1007/s11897-020-00456-0 [DOI] [PubMed] [Google Scholar]
- 5.Spurney C, Shimizu R, Morgenroth LP, et al. Cooperative International Neuromuscular Research Group Duchenne Natural History Study demonstrates insufficient diagnosis and treatment of cardiomyopathy in Duchenne muscular dystrophy. Muscle Nerve 2014;50:250-6. https://doi.org/10.1002/mus.24163 10.1002/mus.24163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Section on Cardiology and Cardiac Surgery. Cardiovascular health supervision for individuals affected by Duchenne or Becker muscular dystrophy. Pediatrics 2005;116:1569-73. https://doi.org/10.1542/peds.2005-2448 10.1542/peds.2005-2448 [DOI] [PubMed] [Google Scholar]
- 7.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. https://doi.org/10.1093/eurheartj/ehw128 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 8.Imbalzano E, Zito C, Carerj S, et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography 2011;28:649-57. https://doi.org/10.1111/j.1540-8175.2011.01410.x 10.1111/j.1540-8175.2011.01410.x [DOI] [PubMed] [Google Scholar]
- 9.Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 2011;12:167-205. https://doi.org/10.1093/ejechocard/jer021 10.1093/ejechocard/jer021 [DOI] [PubMed] [Google Scholar]
- 10.Todaro MC, Khandheria BK, Longobardo L, et al. New diagnostic perspectives on heart failure with preserved ejection fraction: systolic function beyond ejection fraction. J Cardiovasc Med (Hagerstown) 2015;16:527-37. https://doi.org/10.2459/jcm.0000000000000199 10.2459/jcm.0000000000000199 [DOI] [PubMed] [Google Scholar]
- 11.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000;161:309-29. https://doi.org/10.1164/ajrccm.161.1.ats11-99 10.1164/ajrccm.161.1.ats11-99 [DOI] [PubMed] [Google Scholar]
- 12.Mazzone E, Martinelli D, Berardinelli A, et al. North Star Ambulatory Assessment, 6-minute walk test and timed items in ambulant boys with Duchenne muscular dystrophy. Neuromuscul Disord 2010;20:712-6. https://doi.org/10.1016/j.nmd.2010.06.014 10.1016/j.nmd.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 13.Mercuri E, Signorovitch JE, Swallow E, et al. Categorizing natural history trajectories of ambulatory function measured by the 6-minute walk distance in patients with Duchenne muscular dystrophy. Neuromuscul Disord 2016;26:576-83. https://doi.org/10.1016/j.nmd.2016.05.016 10.1016/j.nmd.2016.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan TD, Taylor MD, Mazur W, et al. Abnormal circumferential strain is present in young Duchenne muscular dystrophy patients. Pediatr Cardiol 2013;34:1159-65. https://doi.org/10.1007/s00246-012-0622-z 10.1007/s00246-012-0622-z [DOI] [PubMed] [Google Scholar]
- 15.Hor KN, Kissoon N, Mazur W, et al. Regional circumferential strain is a biomarker for disease severity in Duchenne muscular dystrophy heart disease: a cross-sectional study. Pediatr Cardiol 2015;36:111-9. https://doi.org/10.1007/s00246-014-0972-9 10.1007/s00246-014-0972-9 [DOI] [PubMed] [Google Scholar]
- 16.Hor KN, Wansapura J, Markham LW, et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol 2009;53:1204-10. https://doi.org/10.1016/j.jacc.2008.12.032 10.1016/j.jacc.2008.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagenbuch SC, Gottliebson WM, Wansapura J, et al. Detection of progressive cardiac dysfunction by serial evaluation of circumferential strain in patients with Duchenne muscular dystrophy. Am J Cardiol 2010;105:1451-5. https://doi.org/10.1016/j.amjcard.2009.12.070 10.1016/j.amjcard.2009.12.070 [DOI] [PubMed] [Google Scholar]
- 18.Yu HK, Xia B, Liu X, et al. Initial application of three-dimensional speckle-tracking echocardiography to detect subclinical left ventricular dysfunction and stratify cardiomyopathy associated with Duchenne muscular dystrophy in children. Int J Cardiovasc Imaging 2019;35:67-76. https://doi.org/10.1007/s10554-018-1436-8 10.1007/s10554-018-1436-8 [DOI] [PubMed] [Google Scholar]
- 19.Taqatqa A, Bokowski J, Al-Kubaisi M, et al. The use of speckle tracking echocardiography for early detection of myocardial dysfunction in patients with Duchenne muscular dystrophy. Pediatr Cardiol 2016;37:1422-8. https://doi.org/10.1007/s00246-016-1451-2 10.1007/s00246-016-1451-2 [DOI] [PubMed] [Google Scholar]
- 20.Leitman M, Lysiansky M, Lysyansky P, et al. Circumferential and longitudinal strain in 3 myocardial layers in normal subjects and in patients with regional left ventricular dysfunction. J Am Soc Echocardiogr 2010;23:64-70. https://doi.org/10.1016/j.echo.2009.10.004 10.1016/j.echo.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Ramaciotti C, Heistein LC, Coursey M, et al. Left ventricular function and response to enalapril in patients with Duchenne muscular dystrophy during the second decade of life. Am J Cardiol 2006;98:825-7. https://doi.org/10.1016/j.amjcard.2006.04.020 10.1016/j.amjcard.2006.04.020 [DOI] [PubMed] [Google Scholar]
- 22.Rafael-Fortney JA, Chimanji NS, Schill KE, et al. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation 2011;124:582-8. https://doi.org/10.1161/circulationaha.111.031716 10.1161/circulationaha.111.031716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palladino A, Papa AA, Morra S, et al. Are there real benefits to implanting cardiac devices in patients with end-stage dilated dystrophinopathic cardiomyopathy? Review of literature and personal results. Acta Myol 2019;38:1-7 (http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6598406). [PMC free article] [PubMed] [Google Scholar]
- 24.Politano L, Nigro G. Treatment of dystrophinopathic cardiomyopathy: review of the literature and personal results. Acta Myol 2012;31:24-30 (http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3440799). [PMC free article] [PubMed] [Google Scholar]
- 25.Russo V, Papa AA, Williams EA, et al. ACE inhibition to slow progression of myocardial fibrosis in muscular dystrophies. Trends Cardiovasc Med 2018;28:330-7. https://doi.org/10.1016/j.tcm.2017.12.006 10.1016/j.tcm.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 26.Duboc D, Meune C, Lerebours G, et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol 2005;45:855-7. https://doi.org/10.1016/j.jacc.2004.09.078 10.1016/j.jacc.2004.09.078 [DOI] [PubMed] [Google Scholar]
- 27.Duboc D, Meune C, Pierre B, et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am Heart J 2007;154:596-602. https://doi.org/10.1016/j.ahj.2007.05.014 10.1016/j.ahj.2007.05.014 [DOI] [PubMed] [Google Scholar]
- 28.Ogata H, Ishikawa Y, Ishikawa Y, et al. Beneficial effects of beta-blockers and angiotensin-converting enzyme inhibitors in Duchenne muscular dystrophy. J Cardiol 2009;53:72-8. https://doi.org/10.1016/j.jjcc.2008.08.013 10.1016/j.jjcc.2008.08.013 [DOI] [PubMed] [Google Scholar]
- 29.Rhodes J, Margossian R, Darras BT, et al. Safety and efficacy of carvedilol therapy for patients with dilated cardiomyopathy secondary to muscular dystrophy. Pediatr Cardiol 2008;29:343-51. https://doi.org/10.1007/s00246-007-9113-z 10.1007/s00246-007-9113-z [DOI] [PubMed] [Google Scholar]
- 30.Nigro G, Politano L, Passamano L, et al. Cardiac treatment in neuro-muscular diseases. Acta Myol 2006;25:119-23. [PubMed] [Google Scholar]


