Abstract
Dopamine has been implicated in circadian timing underlying the food entrainable oscillator (FEO) circuitry and overexpression of the dopamine D2 receptor (D2R) in the striatum has been reported to reduce motivation to obtain food rewards in operant tasks. In the present study, we explored both of these mechanisms by examining food anticipatory activity (FAA) in dopamine D2 receptor-overexpressing (D2R-OE) mice under various durations of food availability. First, we noted that at baseline, there were no differences between D2R-OE mice and their littermates in activity level, food intake, and body weight or in circadian activity. Under conditions of very restricted food availability (4 or 6 hr), both genotypes displayed FAA. In contrast, under 8-hr food availability, control mice showed FAA, but D2R-OE mice did not. Normalization of D2R by administration of doxycycline, a tetracycline analogue, rescued FAA under 8-hr restricted food. We next tested for circadian regulation of FAA. When given ad libitum access to food, neither D2R-OE nor controls were active during the daytime. However, after an interval of food restriction, all mice showed elevated locomotor activity at the time of previous food availability in the day, indicating circadian timing of anticipatory activity. In summary, motivation is reduced in D2R-OE mice but circadian timing behavior is not affected. We conclude that an increase in striatal D2R reduces FAA by modulating motivation and not by acting on a clock mechanism.
1 |. INTRODUCTION
Mechanisms have evolved that allow for the temporal regulation of behavior and physiology in time ranges from milliseconds to days (Balsam, Sanchez-Castillo, Taylor, Van Volkinburg, & Ward, 2009; Paton & Buonomano, 2018; Silver & Balsam, 2010; Silver, Balsam, Butler, & LeSauter, 2011). Timing behavior in the second-to-minutes range, termed interval timing, is essential for the timed anticipation of reward availability (Balsam et al., 2009) and depends on cortico-striatal circuits (Emmons et al., 2017; Gu, Kukreja, & Meck, 2018; Meck, Penney, & Pouthas, 2008; Mello, Soares, & Paton, 2015; Merchant, Harrington, & Meck, 2015) which are modulated by dopamine (DA). Thus, DA signaling is implicated in the regulation of interval timing and anticipation.
There are many studies showing that DA receptor agonists and antagonists are able to shift interval timing, and that lesions of mesolimbic and nigrostriatal DA projections impair short interval timing (Meck, 2006). Indeed, direct activation of DA neurons in the ventral tegmental area biases temporal judgments toward shorter durations and inhibition biases toward longer ones (Soares, Atallah, & Paton, 2016).
Of interest, deficits in interval timing are observed in diseases involving pathological dopaminergic signaling including schizophrenia (Allman & Meck, 2012; Ciullo, Spalletta, Caltagirone, Jorge, & Piras, 2016; Ward, Kellendonk, Kandel, & Balsam, 2012; Ward, Simpson, et al., 2012) and Parkinson’s disease (Malapani, Deweer, & Gibbon, 2002; Malapani et al., 1998; Parker, Lamichhane, Caetano, & Narayanan, 2013). In a mouse model of DA depletion, direct stimulation of DA type 1 receptor (D1R) containing neurons in frontal cortex compensates for the effects of striatal DA depletion on timing (Kim et al., 2017).
Additionally, the striatal DA type 2 receptor (D2R) seems to play an especially important role in the modulation of interval timing as drugs and molecular/genetic manipulations that affect D2R signaling alter the timing of anticipatory operant responses (Drew, Fairhurst, Malapani, Horvitz, & Balsam, 2003; Drew et al., 2007; Matell, Bateson, & Meck, 2006; Ward, Kellendonk, et al., 2009; Ward, Tomiya, et al., 2009). Overexpression of the D2R produces deficits in the accuracy and increases the variability of interval timing (Drew et al., 2007; Ward, Kellendonk, et al., 2009; Ward, Tomiya, et al., 2009). While the effects of DA on interval timing in the seconds to minutes range suggest that the D2R influences timing in a broad range of paradigms (Meck, 1986; Teki, Konishi, & Hara, 2017), the role of D2R in longer, circadian time intervals has not been extensively examined.
Striatal D2R signaling has been implicated not only in timing behavior but also in the modulation of motivation and cognition. We previously showed that developmental overexpression of D2R impairs motivation and that normalizing receptor expression in adults restores motivation (Drew et al., 2007; Simpson, Kellendonk et al., 2011; Simpson, Gurr et al., 2011; Ward, Kellendonk, et al., 2012; Ward, Simpson, et al., 2012). In contrast, normalizing receptor expression did not reverse some cognitive impairments (Bach et al., 2008; Kellendonk et al., 2006). With respect to interval timing, rescue of motivation improved performance (Avlar et al., 2015; Ward, Kellendonk, et al., 2009; Ward, Tomiya, et al., 2009). One explanation for the selective effects of Dox on different behaviors is that overexpression of D2R-OE during development leads to some permanent changes in anatomy and/or physiology that are important for some cognitive functions, while concurrent expression of the transgene affects processes that are reversible and involved in motivation. In support of this idea, we found that striatal D2R overexpression reduced both tonic activity and burst firing of midbrain dopamine neurons. Tonic activity, which may play a role in motivation, was rescued by Dox treatment; burst firing, which may be more influential in cognitive tasks, was not rescued (Krabbe et al., 2015).
In the present study, we further examined the role of the D2R on timing behavior and on motivation in a circadian food anticipation paradigm of mice that reversibly overexpress the D2R in the striatum (Kellendonk et al., 2006). In mammals, circadian rhythms of physiology and behavior are orchestrated by a master, light-entrainable clock in the suprachiasmatic nuclei (SCN) of the hypothalamus (Fifel & Cooper, 2014; Huang et al., 2015; Mendoza & Challet, 2014). The SCN are critical for entrainment of daily rhythms to light-dark cycles. Additionally, food-entrainable oscillators (FEOs) of unknown locations can generate daily rhythms of food anticipatory activity (FAA). These emerge when rodents are restricted to one or two regularly timed meals at a fixed time of day. Food anticipatory rhythms have the canonical properties of circadian clock control (Mistlberger, 1994, 2011). They survive ablation of the SCN, indicating they are localized elsewhere, although the location of FEOs driving food anticipatory activity is uncertain (reviewed in Mistlberger, 2011). Furthermore, the molecular basis of circadian oscillation differs between SCN oscillators and FEO, first shown by Pitts, Perone, and Silver (2003). Specifically, the classical Per-Cry feedback loop, a key feature of circadian oscillation, is not required for the functioning of the FEO (reviewed in Mohawk, Green, & Takahashi, 2012). Interestingly, timed anticipatory behavior at short intervals also does not depend on this molecular pathway (Papachristos, Jacobs, & Elgersma, 2011) or intact SCN (Lewis & Miall, 2003).
The localization of FEO’s and the mechanisms mediating food anticipation are of substantial interest. The dopaminergic modulation of striatal reward pathways in circadian food anticipation has been suggested (Mendoza, Angeles-Castellanos, & Escobar, 2005a,b; Smit, Patton, Michalik, Opiol, & Mistlberger, 2013). The amount of weight lost in a restricted food regimen was correlated with a downregulation of D2R and an increase in FAA (Vaanholt, Mitchell, Sinclair, & Speakman, 2015).
Phase shifts in timing of FAA have been suggested, but not proven, to implicate circadian clock mechanisms. In this context, it is relevant that systemic treatment with the D2 agonist quinpirole, resulted in a phase delay of FAA onset prior to the next meal (Smit et al., 2013). However, there was no shift following administration of the D1 agonist SKF81297. In contrast, Gallardo et al., (Gallardo et al., 2014) found that genetic deletion of D1 but not D2 receptors altered the level of FAA.
In the FAA paradigm, mice are fed for a time-limited duration in the daytime, which is their normal inactive or sleep phase. In these conditions, they spontaneously awaken from sleep and become active about 2 hr prior to the availability of food. This is done by redistributing some of their nocturnal activity to the time preceding the regularly scheduled availability of food. This behavior is considered to be a circadian rhythm controlled by FEO. Evidence includes the fact that FAA has circadian limits of entrainment (Boulos, Rosenwasser, & Terman, 1980), and the response recurs at the appropriate time of day following days of ad libitum feeding. Here we asked whether striatal D2R play an important role in FAA as they do in the regulation of timed behavior at shorter intervals. We used the food anticipation paradigm to examine the contribution of the striatal D2R receptor to anticipatory behavior measured by FAA and to circadian timing behavior in D2R-OE mice. As noted above, this mouse shows impairments in interval timing and anticipation behavior (Drew et al., 2007; Ward, Kellendonk, et al., 2009; Ward, Tomiya, et al., 2009).
2 |. MATERIALS AND METHODS
2.1 |. Mice
Male mice (N = 10) overexpressing DA receptor 2 in the striatum (D2R-OE mice) were 5 months old at the start of the study and their littermates (N = 8), carrying a single transgene (Tg-tetO-D2R or Tg-CaMKIIα-tTA) or no transgene (WT), were used as controls. The generation of D2R-OE transgenic mice was achieved by crossing Tg-tetOD2R mice on a C57BL/6J background with Tg-CaMKIIα-tTA mice on a 129SveV background, as previously described (Kellendonk et al., 2006). Briefly, Tg-TetO-D2R mice (Jax stock # 028294) express the long form of the human D2R open reading frame and have been backcrossed to C57BL/6J for over 25 generations. Tg-CaMKIIα-tTA mice (Jax stock # 016198, Mayford et al., 1996) express the tetracycline-regulated transactivator (tTA or “Tet-Off”) gene under a calcium/calmodulin-dependent kinase IIα (CaMKIIα) promoter and have been backcrossed to 129S6/SvEvTac for over 25 Generations. Double transgenic mice (D2R-OE mice) express transgenic D2Rs in the postsynaptic spiny projection neurons of the striatum that can be switched off by administration of tetracycline or doxycycline (Dox, Figure 1a).
FIGURE 1.

(a) Breeding scheme and transgene regulation (modified from Kellendonk et al., 2006). D2R-OE mice are produced crossing Tg-TetO-D2R mice with Tg-CaMKIIα-tTA mice. On a regular diet, the tetracycline transactivator (tTA) drives expression of the transgene (Tg D2R gene on). On a Dox-supplemented diet, the tTA cannot drive transgene expression (Tg D2R gene off). (b) Experimental design. Top bar indicates time of day; light–dark cycle (LD cycle) is indicated by white and black bars respectively. Days are shown on the y axis. Mice are in LD except when indicated on the left (DD:dark–dark) when mice are in constant dark to test for FAA persistence. Gray shading indicates time of food availability (light gray: rodent chow; dark gray: Dox-supplemented chow). During testing for effect of Dox, mice were maintained on 8-hr food access
2.2 |. Housing and food
Mice were individually housed in translucent propylene cages (29 × 19 × 12.5 cm) equipped with a running wheel (11 cm diameter). At the start of the study, rodent chow was provided ad libitum except during food restriction tests, as noted (LabDiet 5012, PMI Nutrition, Brentwood, MO, USA, composed of 23.2% protein, 5% Fat, 3.9% fiber, 6.4% ash, for detail, see www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/~edisp/ducm04_028442.pdf). To establish the effect of reversing the overexpression of D2Rs, mice were given Dox-supplemented chow (40 mg/kg; Mutual Pharmaceutical, Philadelphia, PA, composed of 22.5% protein, 5.4% Fat, 4% fiber, 6.1% ash, see www.labsupplytx.com/wp-content/uploads/2012/10/5P75-5P76.pdf).
Animals had continuous access to tap water. They were adapted to their home cages and to the light-dark (LD) cycle for 2 weeks before the experiments. In keeping with the terminology of circadian protocols, zeitgebers time 12 (ZT12) is the time of lights off and ZT0 is the time of lights on. Light was provided by an array of green LEDs (230 lux, peak wavelength 524 nm, half-maximal width 47 nm, mean dominant wavelength 518 nm, HLMP-AM01-Q00zz; Avago Technologies, San Jose, CA, USA). The rooms were maintained at 21 ± 1°C. Environmental noise was masked by white noise (76 dB SPL). All animals were cared for in accordance with the Columbia University Institutional Animal Care and Use Committee and Animal Welfare regulations.
2.3 |. Experimental design
The experimental design for the entire study, in which hunger motivation, circadian timing and effects of Dox administration were tested, is shown in Figure 1b. First, to evaluate the effect of hunger motivation on anticipatory behavior, three intervals, of restricted feeding (RF) namely 4, then 8 and finally 6 hr of food availability were tested. At the end of each test, ad libitum food access was provided. For the 4-hr restricted food (RF4) schedule, food was removed at ZT12 and food availability was reduced gradually from 6 hr (ZT 6–12) to 5 hr (ZT 6–11) for 2 days each until RF4 h (ZT 6–10) was reached, and then continued for 14 days. For RF8, after the interval of ad libitum feeding, food was removed at ZT14, and RF8 (ZT6–14) was imposed for 10 days. For RF6 (ZT6–12), food was removed at ZT12 and RF6 was imposed for 14 days. Body weight and food intake were measured at ZT6 after a period of ad libitum feeding, the day before the start of RF6 and the day after the end of RF6.
2.3.1 |. Circadian timing test
At the end of RF8, a test for the contribution of the circadian timing system to anticipation was performed. Here food was provided at ZT6 followed by ad libitum feeding with lights off (DD) starting at ZT12. After 3 days, food was removed at ZT12 to test for the possible reappearance of anticipatory behavior the following subjective day at the appropriate circadian time (previously prior to ZT6). Following this test, mice were placed back in LD with ad libitum food availability for 30 days.
2.3.2 |. Dox administration
At the end of the 30-day period of ad libitum feeding described above, animals were placed on RF8 for 21 days to determine baseline FAA. Then all animals were fed Dox-supplemented chow for 7 days to switch off the D2R transgene. Subsequently, regular chow was again provided.
2.3.3 |. Data collection and analysis
Wheel running data were collected continuously in 10 min bins using VitalView (Mini-Mitter Company Inc.) and were quantified by Actiview (Mini-Mitter Company Inc.) and Microsoft Excel. Daily activity was established by measuring total wheel running for 24 hr. FAA was quantified as wheel revolutions in the 2 hr preceding food availability (ZT4–6). FAA was assessed for the last days in each treatment condition namely RF4 and 6 (10 days), RF8 (8 days), and post Dox (9 days). For group data where comparisons between genotypes in FAA were assessed, to account for individual differences in overall activity level and possible differences in wheel resistance, locomotor wheel running was normalized for each animal as follows: number of wheel revolutions per 10 min bin divided by the average daily rotations and multiplied by 144 (the number of 10 min bins/day). This is indicated in the figure legends. Statistical analyses involving T-tests or repeated measure two-way ANOVA were performed using SigmaStat 2.03 (SPSS Inc., Chicago, IL).
3 |. RESULTS
3.1 |. Experiment 1. Effect of D2R overexpression on food anticipation
We first evaluated whether D2R overexpression affects overall activity levels by comparing daily activity level in D2R-OE mice and their littermate controls (Figure 2). Actograms show the amount of locomotor activity of representative individual mice in each experimental group (Figure 2a). The 12:12 LD cycle is shown by the top horizontal bars. The gray areas show the times of food availability. The locomotor activity profiles for each 10 min interval are shown for each individual animal depicted in the actograms in panel A (Figure 2b) and for the group as a whole (left panel Figure 2c). There are no differences between D2R-OE and control mice in daily activity under baseline, ad libitum feeding conditions (t(15) = 0.6, p = 0.5; Figure 2c right panel).
FIGURE 2.

(a) Actograms show the absolute amount of locomotor activity of representative individual control and D2R-OE mice prior to the start of the experiment. The 12:12 light:dark cycle is shown by the top horizontal bars (white denotes day, black denotes night). (b) Absolute levels of locomotor activity are shown for each 10-min bin for the days shown in the actograms of the individual mice in (a). (c) Left panel shows the absolute levels of group activity profiles for all animals in each group, as above. The right panel shows absolute amount of total daily wheel running
We next asked whether D2R overexpression in the striatum has an effect on food anticipation, and so we tested D2R-OE mice and littermate controls under three RF schedules. Under a severe (4 hr) or moderate (6 hr) food restriction schedule, both D2R-OE and control animals show FAA (Figure 3) with no differences between genotypes [RF4 (t(16) = 0.05, p = 0.9), RF6 (t(14) = 0.4, p = 0.7)]. In contrast, with RF8, there is a difference between genotypes in that control animals showed FAA, but D2R-OE did not (t(13) = 2.7, p = 0.02).
FIGURE 3.
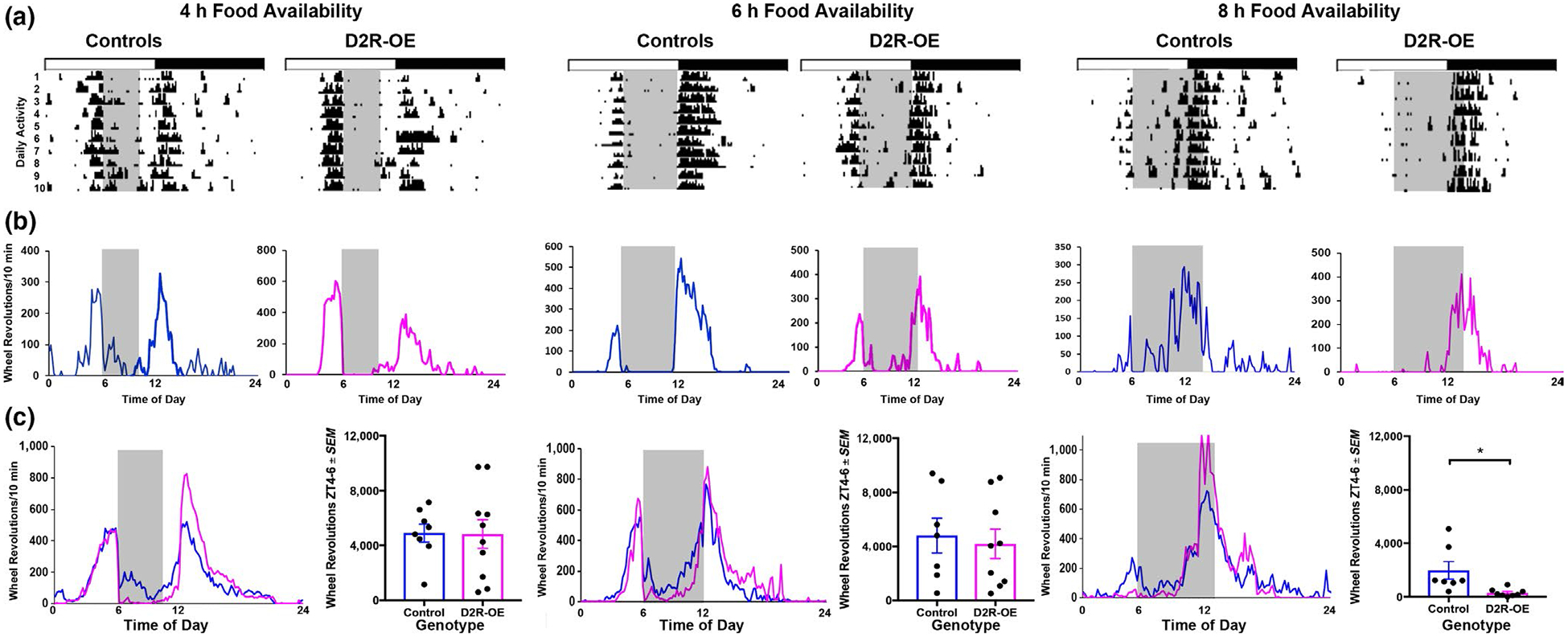
(a) Anticipatory behavior was examined following 4, 6, and 8 hr of restricted food availability. Actograms show the amount of locomotor activity of representative individual mice in each experimental group (4, 6, or 8 hr food availability). The 12:12 LD cycle is shown by the top horizontal bars. The gray areas show the times of food availability. (b) Locomotor activity profiles for each 10-min interval, averaged over the last 10 days of the study, are shown for each individual animal depicted in the actograms in panel a. (c) Locomotor activity profiles (normalized) for each 10-min interval, averaged over 8–10 days are shown for each group of mice. In order to compare activity patterns across individuals, activity levels are expressed as a percent of total daily activity for each 10-min interval. The bar graph shows the amount of anticipatory activity for the 2-hr preceding food availability
Finally, we compared the amount of FAA under each RF condition within each group. The results show a significant decrease in FAA in 8-hr RF compared to 4- and 6-hr RF for the D2R-OE mice (F2,26 = 6.2, p = 0.01) but not for the control mice (F2,21 = 2.6, p = 0.12). This points to a difference between control and D2R-OE mice in sensitivity to the deprivation conditions.
3.2 |. Experiment 2. Memory for circadian time
We next tested the ability of mice to remember the time of food availability as follows:
Mice were kept in constant darkness (DD) for 3 days with ad libitum food availability, and then food deprived for the following 18 hr (in DD), starting at ZT12. The results show that both D2R-OE mice and controls show little activity at ZT4 to 6 on the last day of ad libitum food availability (Figure 4). In contrast, following the 18-hr food deprivation, both D2R-OE and control mice show increased activity prior to the time of previously available meals (F1,29 = 9.16, p < 0.01), with no difference between groups (F1,29 = 0.98, p = 0.33). The results indicate that even though D2R-OE mice did not show FAA during the 8-hr food restriction, they had actually learned the time of food presentation.
FIGURE 4.

(a) Wheel running activity at ZT4–6 during ad libitum feeding (Pretest) and on the following day, after 18-hr food deprivation (Test Day) for control and D2R-OE mice (*pretest vs. test day, Tukey test: controls: p = 0.02, D2R-OE, p = 0.01). (b) Locomotor activity profiles for each 10-min interval on circadian timing test day explores the ability of mice to remember the previous time of food availability. The results show a bout of FAA restricted to the time of previously available food for both control and D2R-OE groups
3.3 |. Experiment 3. Effect of food restriction on body weight and food intake
We next examined whether responses in FAA might have been mediated by differences in body weight or food intake in the two groups of mice. We found no differences in body weight between control and D2R-OE mice on an ad lib diet (Pretest), (F1,37 = 1.9, p = 0.2). We also found no difference in body weight during food restriction (Test Day) conditions (F1,37 = 0.1, p = 0.8). Also, there were no differences in food intake between control and D2R-OE mice (t(12) = 0.1, p = 0.9; Figure 5).
FIGURE 5.
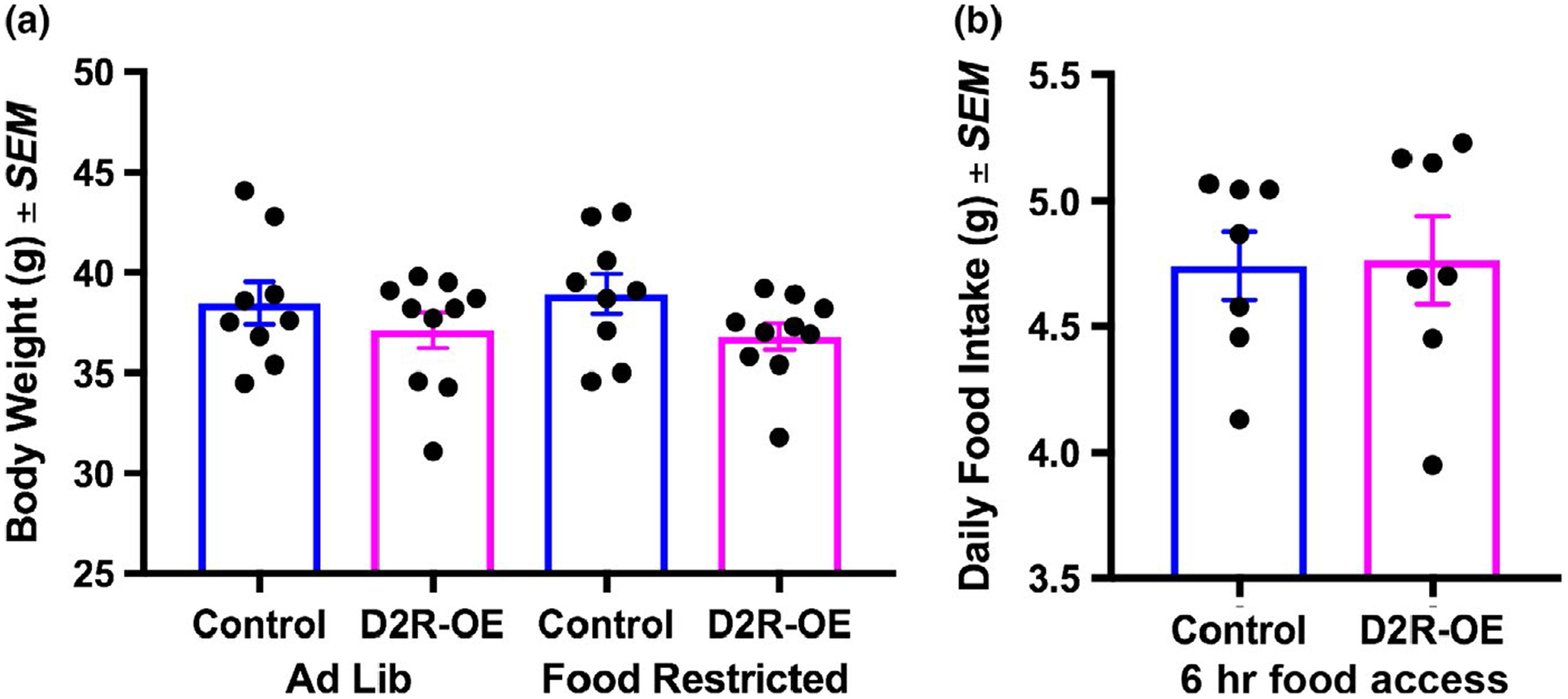
(a) Body weight of control and D2R-OE mice fed ad libitum or restricted to 6 hr food/day. (b) Food intake when mice were restricted to 6 hr food/day
3.4 |. Experiment 4. Rescue of FAA in D2R-OE mice following Dox treatment
The results of Experiment 1 suggest that D2R overexpression interacts with motivation in the failure of D2R-OE mice to show anticipatory behavior in RF8. Previous studies have shown that D2R-OE mice show deficits in motivation and timed anticipation that are rescued when the transgene is switched off by putting the mice on a Dox diet (Drew et al., 2007; Simpson, Kellendonk et al., 2011; Simpson, Gurr et al., 2011; Ward, Kellendonk, et al., 2012; Ward, Simpson et al., 2012, Ward et al., 2015). In the next experiment, we administered Dox and monitored the occurrence of FAA in the RF8 condition as the transgene expression was switched off. The results indicate that during Dox treatment, FAA is progressively established in the D2R-OE mice. Specifically, there are significant differences in anticipatory responses between experimental and control animals on days 1–3 and 4–6 [Days 1–3 of treatment, t(16) = 3.3, p = 0.005; Days 4–6, t(16) = 3.2, p = 0.006]. However, by the last day of treatment (day 7) and the two subsequent days, there are no differences in FAA between D2R-OE mice and their littermate controls (t(16) = 0.6, p = 0.5; Figure 6). The timing of the appearance of FAA corresponds to the timing of loss of transgenic receptors due to Dox administration. Previous studies show loss of transgenic D2R mRNA after 1 week of Dox documented by in situ hybridization (Kellendonk et al., 2006) and by quantitative PCR (Gil-Santos et al., 2017).
FIGURE 6.
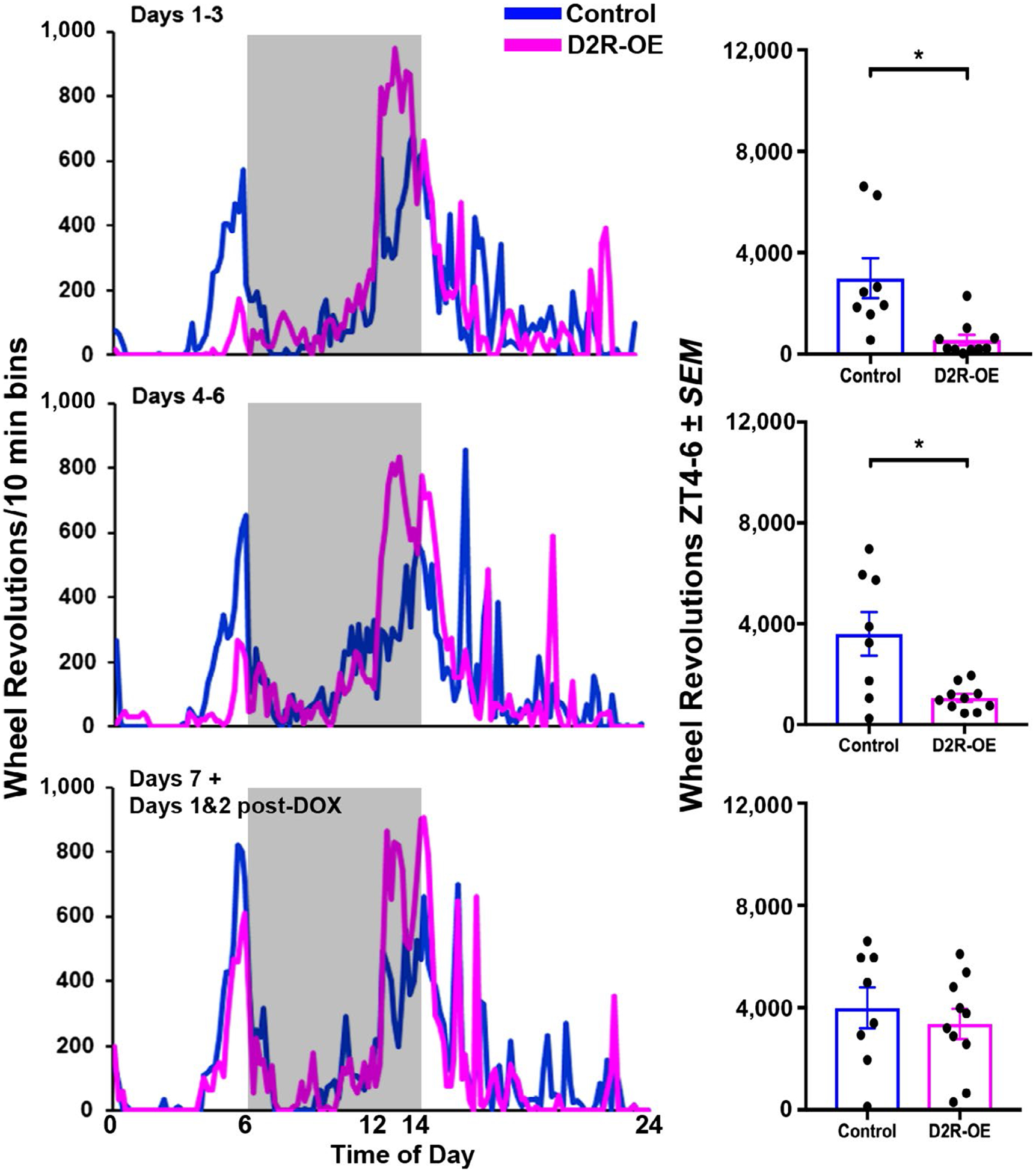
Left: Locomotor activity profiles (normalized) for all animals in each group following Dox treatment. Activity shown is averaged over days 1–3 (upper panels), days 4–6 (middle panels), and days 7–9, [the last days of Dox], and days 1 and 2 [after Dox treatment] (lower panels). Right: The bar graphs show the average wheel running activity for the 2-hr preceding food access (ZT4–6). *p < 0.005
4 |. DISCUSSION
There is substantial interest in understanding how the dopaminergic system and central brain oscillators interact to regulate timing behavior (Lartigue & McDougle, 2018; Mendoza & Challet, 2014; Pendergast & Yamazaki, 2018). The present series of studies examine the effect of striatal D2R overexpression on timing in the circadian range, using the FAA paradigm. The results show that upregulation of striatal D2Rs alters the circadian regulation of food anticipation, while leaving intact the SCN mediated light-entrainable circadian system. This provides further evidence for the independence of the circadian system underlying FAA and the circadian system mediated by the SCN. The results show that there were no differences between control and D2R-OE mice in FAA when animals were highly motivated (i.e., very restricted feeding schedule). Furthermore, the D2R-OE had no learning deficit as they timed just as well as controls about when to expect food when there was restricted availability. When tested after ad libitum access to food and then deprived for 18 hr both controls and D2R-OE showed equal FAA at the previous time of food availability indicating that D2R overexpression did not interfere with the ability to learn about the daily time of food availability. However, compared to their control littermates, the D2R-OE mice show reduced anticipatory behavior prior to food availability when motivational state is relatively low (animals are under a less restricted feeding schedule). These effects of striatal D2R-OE on time anticipation cannot be attributed to differences in body weight or food intake, findings reported elsewhere (Labouesse et al., 2018).
The present results also indicate the overexpression of D2R does not impact the ability of mice to measure circadian time. Instead they point to a motivational deficit in D2R-OE animals. Unlike controls, the D2R-OE mice failed to show anticipatory behavior when access to food was moderately long (at 8 hr), but they were not different from controls in anticipating food when availability was restricted to 4 hr. Furthermore, application of Dox and consequent normalization of D2Rs restored the behavior of these mice to that seen in littermate controls. By reversibly altering striatal D2R’s under an RF schedule, the present work points to the role of striatum in modulating FAA by effects on motivation rather than by altering timing behavior directly.
The interaction between motivational state and circadian FAA mirrors our prior observations of an interaction between motivation and interval timing in the shorter time range. D2R-OE mice are less accurate and show reduced anticipatory behavior in timing tasks in the seconds range (Drew et al., 2007; Ward, Kellendonk, et al., 2009; Ward, Tomiya, et al., 2009). These mice also show reduced motivation in several different operant behavioral tasks (Simpson, Kellendonk et al., 2011; Simpson, Gurr et al., 2011; Ward, Kellendonk, et al., 2012; Ward, Simpson et al., 2012; Ward et al., 2015). Furthermore, interval timing performance in D2R-OE mice is improved by increasing motivation with a variety of manipulations (Avlar et al., 2015; Ward, Kellendonk, et al., 2009; Ward, Tomiya, et al., 2009; Ward, Kellendonk, et al., 2012; Ward, Simpson et al., 2012).
The motivational deficits in D2R-OE mice are not due to a reduced interest in or reactions to consuming food rewards. Instead, it appears that the mice are less willing to expend effort to obtain the anticipated outcome (Filla & Bailey, 2018; Simpson, Kellendonk et al., 2011; Simpson, Gurr et al., 2011; Ward, Kellendonk, et al., 2012; Ward, Simpson et al., 2012; Ward et al., 2015). Moreover, the motivational deficit in D2R-OE mice does not result from an increase in D2Rs during development, but results from acute overexpression because when the transgene is switched off by Dox treatment, the mice show normal motivation, along with the improved temporal anticipation (Drew et al., 2007; Simpson, Kellendonk et al., 2011; Simpson, Gurr et al., 2011; Ward, Kellendonk, et al., 2009; Ward, Tomiya, et al., 2009). Here we show that normalizing D2R expression which normalizes motivation also normalizes timed food anticipation in the circadian domain. This is consistent with an earlier report that altered dopamine signaling affected the level of activity in an FAA paradigm (Gallardo et al., 2014). Thus, we hypothesize that as in the timing of short intervals, the role of D2R in circadian food anticipation is to modulate the willingness to expend effort in anticipation of food access.
Prior studies also suggest a role of striatal D2R in regulating circadian rhythms of food anticipation. Restricted feeding shifts clock gene rhythms in many brain regions, including the striatum (Wakamatsu et al., 2001). Activation of D2 receptors regulates expression of clock genes in the striatum (Imbesi et al., 2009; Sahar, Zocchi, Kinoshita, Borrelli, & Sassone-Corsi, 2010). Blocking striatal D2R, but not D1R, blunts PER2 rhythm. In addition, the regularly scheduled daily activation of D2R, but not D1R, restores and entrains PER2 rhythmicity in the DA-depleted striatum (Hood et al., 2010). In summary, it appears that activation or suppression of DA activity in the striatum affects food anticipation, as both D2R agonists and antagonist, but not D1R agonists or antagonists reduce FAA (Liu et al., 2012; Mistlberger & Mumby, 1992; Smit et al., 2013). Finally, circadian modulation of DA has been demonstrated (Huang et al., 2015; Mendoza & Challet, 2014). Loss of DA disrupts circadian rhythms in mice bearing a progressive degeneration of DA neurons in midbrain structures (Fifel & Cooper, 2014) though DA-deficient mice have very abnormal locomotor behavior making it difficult to assess anticipatory behavior in this animal (Zhou & Palmiter, 1995).
We found that FAA activity was higher in the Dox condition than in the comparable 8-hr RF condition (mean of ~2000 for RF8 vs. ~3000 wheel rotations at the end of Dox treatment). This is consistent with reports that locomotor sensitization develops with sufficient exposure to RF (Opiol et al., 2017).
The striking result of the current experiment is that in control mice, the FAA timing mechanism continues to operate accurately independent of motivational state. This is consistent with our observation that anticipatory activity in dorsomedial hypothalamus, orbitofrontal cortex and perhaps lateral septum, assessed by c-FOS expression, increases during anticipation (for methamphetamine), but no such increase is seen in the dopaminergic striatal system (Juárez-Portilla et al., 2018). Here we find that the level of anticipatory activity is affected by motivational state but the underlying timing is not. The independence of timing and motivation has not been tested in the interval timing domain. This gap occurs because the underlying clock mechanism for interval timing is unknown and therefore, behavioral output is the only way to infer changes in a clock. Clearly, interval timing depends not only on motivation but also on perception, attention, memory, decision-making, and a clock. The locus of motivational effects on timed behavioral output is not likely to be specified until the underlying clock mechanisms are better delineated. Simply put, access to the clock mechanism is necessary to specifically implicate an alteration in timing.
ACKNOWLEDGEMENTS
The work was supported by the National Science Foundation grant 1256105 to R. Silver, the National Institute of Mental Health grant MH068073 to P.D. Balsam and the Lieber Institute for Brain Development for E.H. Simpson.
Funding information
National Institute of Mental Health, Grant/Award Number: MH068073; Division of Integrative Organismal Systems, Grant/Award Number: 125605
Abbreviations:
- D2R
dopamine D2 receptor
- D2R-OE
dopamine D2 receptor overexpressing
- DA
dopamine
- FAA
food anticipatory activity
- FEO
food-entrainable oscillator
- WT
wild type
- ZT
zeitgeber time
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DATA ACCESSIBILITY
We note that the following information is provided in full in the text of the manuscript so that the results are fully reproducible: statistical tools, protocols, software and hardware used reagents, animal strains, and food sources. Every data point is shown in the publication. The following are NA: such as stimuli, computer code, or simulations.
All peer review communications can be found with the online version of the article.
REFERENCES
- Allman MJ, & Meck WH (2012). Pathophysiological distortions in time perception and timed performance. Brain (London, England: 1878), 135, 656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avlar B, Kahn JB, Jensen G, Kandel ER, Simpson EH, & Balsam PD (2015). Improving temporal cognition by enhancing motivation. Behavioral Neuroscience, 129, 576–588. 10.1037/bne0000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, & Kellendonk C (2008). Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proceedings of the National Academy of Sciences of the United States of America, 105(41), 16027–16032. 10.1073/pnas.0807746105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam P, Sanchez-Castillo H, Taylor K, Van Volkinburg H, & Ward RD (2009). Timing and anticipation: conceptual and methodological approaches. European Journal of Neuroscience, 30, 1749–1755. 10.1111/j.1460-9568.2009.06967.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos Z, Rosenwasser AM, & Terman M (1980). Feeding schedules and the circadian organization of behavior in the rat. Behavioural Brain Research, 1, 39–65. 10.1016/0166-4328(80)90045-5 [DOI] [PubMed] [Google Scholar]
- Ciullo V, Spalletta G, Caltagirone C, Jorge RE, & Piras F (2016). Explicit time deficit in Schizophrenia: Systematic review and meta-analysis indicate it is primary and not domain specific. Schizophrenia Bulletin, 42, 505–518. 10.1093/schbul/sbv104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, & Balsam PD (2003). Effects of dopamine antagonists on the timing of two intervals. Pharmacology Biochemistry and Behavior, 75, 9–15. 10.1016/S0091-3057(03)00036-4 [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, … Balsam PD (2007). Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. Journal of Neuroscience, 27, 7731–7739. 10.1523/JNEUROSCI.1736-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons EB, De Corte BJ, Kim Y, Parker KL, Matell MS, & Narayanan NS (2017). Rodent medial frontal control of temporal processing in the dorsomedial striatum. Journal of Neuroscience, 37(36), 8718–8733. 10.1523/JNEUROSCI.1376-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifel K, & Cooper HM (2014). Loss of dopamine disrupts circadian rhythms in a mouse model of Parkinson’s disease. Neurobiology of Disease, 71, 359–369. 10.1016/j.nbd.2014.08.024 [DOI] [PubMed] [Google Scholar]
- Filla I, & Bailey M (2018). Striatal dopamine D2 receptors regulate effort but not value-based decision making and alter the dopaminergic encoding of cost. Neuropsychopharmacology, 43, 2180–2189. 10.1038/s41386-018-0159-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo CM, Darvas M, Oviatt M, Chang CH, Michalik M, Huddy TF, … Steele AD (2014). Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. Elife, 3, e03781 10.7554/eLife.03781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Santos E, Labousse M, Baker C, Goetschy A, Hease W, Gomez C, … Favero I (2017). Light-mediated cascaded locking of multiple nano-optomechanical oscillators. Physical Review Letters, 118, 063605 10.1103/PhysRevLett.118.063605 [DOI] [PubMed] [Google Scholar]
- Gu B-M, Kukreja K, & Meck WH (2018). Oscillation patterns of local field potentials in the dorsal striatum and sensorimotor cortex during the encoding, maintenance, and decision stages for the ordinal comparison of sub- and supra-second signal durations. Neurobiology of Learning and Memory, 153, 79–91. [DOI] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Cossette M-P, Weigl Y, Verwey M, Robinson B, … Amir S (2010). Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. Journal of Neuroscience, 30, 14046–14058. 10.1523/JNEUROSCI.2128-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhong Z, Wang M, Chen X, Tan Y, Zhang S, … Lu H (2015). Circadian modulation of dopamine levels and dopaminergic neuron development contributes to attention deficiency and hyper-active behavior. Journal of Neuroscience, 35, 2572–2587. 10.1523/JNEUROSCI.2551-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbesi M, Yildiz S, Arslan AD, Sharma R, Manev H, & Uz T (2009). Dopamine receptor-mediated regulation of neuronal “clock” gene expression. Neuroscience, 158, 537–544. 10.1016/j.neuroscience.2008.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez-Portilla C, Pitter M, Kim RD, Patel PY, Ledesma RA, LeSauter J, & Silver R (2018). Brain activity during methamphetamine anticipation in a non-invasive self-administration paradigm in mice. eNeuro, 5, 0433–0417.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, … Kandel ER (2006). Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron, 49, 603–615. 10.1016/j.neuron.2006.01.023 [DOI] [PubMed] [Google Scholar]
- Kim Y-C, Han S-W, Alberico SL, Ruggiero RN, De Corte B, Chen K-H, & Narayanan NS (2017). Optogenetic stimulation of frontal D1 neurons compensates for impaired temporal control of action in dopamine-depleted mice. Current Biology, 27, 39–47. 10.1016/j.cub.2016.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbe S, Duda J, Schiemann J, Poetschke C, Schneider G, Kandel ER, … Simpson EH (2015). Increased dopamine D2 receptor activity in the striatum alters the firing pattern of dopamine neurons in the ventral tegmental area. Proceedings of the National Academy of Sciences of the United States of America, 112(12), E1498–E1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse MA, Sartori AM, Weinmann O, Simpson EH, Kellendonk C, & Weber-Stadlbauer U (2018). Striatal dopamine 2 receptor upregulation during development predisposes to diet-induced obesity by reducing energy output in mice. Proceedings of the National Academy of Sciences of the United States of America, 115, 10493–10498. 10.1073/pnas.1800171115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deLartigue G, & McDougle M (2018) Dorsal striatum dopamine oscillations: Setting the pace of food anticipatory activity. Acta Physiologica, June, e13152 10.1111/apha.13152 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, & Miall RC (2003). Brain activation patterns during measurement of sub-and supra-second intervals. Neuropsychologia, 41, 1583–1592. 10.1016/S0028-3932(03)00118-0 [DOI] [PubMed] [Google Scholar]
- Liu Y-Y, Liu T-Y, Qu W-M, Hong Z-Y, Urade Y, & Huang Z-L (2012). Dopamine is involved in food-anticipatory activity in mice. Journal of Biological Rhythms, 27, 398–409. 10.1177/0748730412455913 [DOI] [PubMed] [Google Scholar]
- Malapani C, Deweer B, & Gibbon J (2002). Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. Journal of Cognitive Neuroscience, 14, 311–322. 10.1162/089892902317236920 [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, & Gibbon J (1998). Coupled temporal memories in Parkinson’s disease: A dopamine-related dysfunction. Journal of Cognitive Neuroscience, 10, 316–331. 10.1162/089892998562762 [DOI] [PubMed] [Google Scholar]
- Matell MS, Bateson M, & Meck WH (2006). Single-trials analyses demonstrate that increases in clock speed contribute to the methamphetamine-induced horizontal shifts in peak-interval timing functions. Psychopharmacology (Berl), 188, 201–212. 10.1007/s00213-006-0489-x [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, & Kandel ER (1996). Control of memory formation through regulated expression of a CaMKII transgene. Science, 274(5293), 1678–1683. [DOI] [PubMed] [Google Scholar]
- Meck WH (1986). Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacology Biochemistry and Behavior, 25, 1185–1189. 10.1016/0091-3057(86)90109-7 [DOI] [PubMed] [Google Scholar]
- Meck WH (2006). Neuroanatomical localization of an internal clock: A functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Research, 1109, 93–107. 10.1016/j.brainres.2006.06.031 [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, & Pouthas V (2008). Cortico-striatal representation of time in animals and humans. Current Opinion in Neurobiology, 18, 145–152. 10.1016/j.conb.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Mello GB, Soares S, & Paton JJ (2015). A scalable population code for time in the striatum. Current Biology, 25, 1113–1122. 10.1016/j.cub.2015.02.036 [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, & Escobar C (2005a). Differential role of the accumbens Shell and Core subterritories in food-entrained rhythms of rats. Behavioural Brain Research, 158, 133–142. 10.1016/j.bbr.2004.08.016 [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, & Escobar C (2005b). Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience, 133, 293–303. 10.1016/j.neuroscience.2005.01.064 [DOI] [PubMed] [Google Scholar]
- Mendoza J, & Challet E (2014). Circadian insights into dopamine mechanisms. Neuroscience, 282, 230–242. 10.1016/j.neuroscience.2014.07.081 [DOI] [PubMed] [Google Scholar]
- Merchant H, Harrington DL, & Meck WH (2015). Neural basis of the perception and estimation of time. Annual Review of Neuroscience, 36, 313–336. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE (1994). Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neuroscience & Biobehavioral Reviews, 18, 171–195. 10.1016/0149-7634(94)90023-X [DOI] [PubMed] [Google Scholar]
- Mistlberger RE (2011). Neurobiology of food anticipatory circadian rhythms. Physiology & Behavior, 104, 535–545. 10.1016/j.physbeh.2011.04.015 [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, & Mumby DG (1992). The limbic system and food-anticipatory circadian rhythms in the rat: Ablation and dopamine blocking studies. Behavioural Brain Research, 47, 159–168. 10.1016/S0166-4328(05)80122-6 [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, & Takahashi JS (2012). Central and peripheral circadian clocks in mammals. Annual Review of Neuroscience, 35, 445–462. 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opiol H, de Zavalia N, Delorme T, Solis P, Rutherford S, Shalev U, & Amir S (2017). Exploring the role of locomotor sensitization in the circadian food entrainment pathway. PLoS ONE, 12(3), e0174113 10.1371/journal.pone.0174113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristos EB, Jacobs EH, & Elgersma Y (2011). Interval timing is intact in arrhythmic Cry1/Cry2-deficient mice. Journal of Biological Rhythms, 26, 305–313. 10.1177/0748730411410026 [DOI] [PubMed] [Google Scholar]
- Parker K, Lamichhane D, Caetano M, & Narayanan N (2013). Executive dysfunction in Parkinson’s disease and timing deficits. Frontiers in Integrative Neuroscience, 7, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, & Buonomano DV (2018). The neural basis of timing: Distributed mechanisms for diverse functions. Neuron, 98, 687–705. 10.1016/j.neuron.2018.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast JS, & Yamazaki S (2018). The mysterious food-entrainable oscillator: Insights from mutant and engineered mouse models. Journal of Biological Rhythms 33, 458–474. 10.1177/0748730418789043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts S, Perone E, & Silver R (2003). Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 285, R57–R67. 10.1152/ajpregu.00023.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Zocchi L, Kinoshita C, Borrelli E, & Sassone-Corsi P (2010). Regulation of BMAL1 protein stability and circadian function by GSK3β-mediated phosphorylation. PLoS ONE, 5, e8561 10.1371/journal.pone.0008561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, & Balsam P (2010). Oscillators entrained by food and the emergence of anticipatory timing behaviors. Sleep and Biological Rhythms, 8, 120–136. 10.1111/j.1479-8425.2010.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, Balsam PD, Butler MP, & LeSauter J (2011). Food anticipation depends on oscillators and memories in both body and brain. Physiology & Behavior, 104, 562–571. 10.1016/j.physbeh.2011.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson M, Gurr G, Simmons A, Wratten S, James D, Leeson G, … Orre GUS (2011). Field evaluation of the ‘attract and reward’ biological control approach in vineyards. Annals of Applied Biology, 159, 69–78. 10.1111/j.1744-7348.2011.00477.x [DOI] [Google Scholar]
- Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, … Balsam PD (2011). Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biological Psychiatry, 69, 928–935. 10.1016/j.biopsych.2011.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AN, Patton DF, Michalik M, Opiol H, & Mistlberger RE (2013). Dopaminergic regulation of circadian food anticipatory activity rhythms in the rat. PLoS ONE, 8, e82381 10.1371/journal.pone.0082381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S, Atallah BV, & Paton JJ (2016). Midbrain dopamine neurons control judgment of time. Science, 354, 1273–1277. 10.1126/science.aah5234 [DOI] [PubMed] [Google Scholar]
- Teki H, Konishi K, & Hara N (2017). Amplitude death in a pair of one-dimensional complex Ginzburg-Landau systems coupled by diffusive connections. Physical Review E, 95, 062220 10.1103/PhysRevE.95.062220 [DOI] [PubMed] [Google Scholar]
- Vaanholt LM, Mitchell SE, Sinclair RE, & Speakman JR (2015). Mice that are resistant to diet-induced weight loss have greater food anticipatory activity and altered melanocortin-3 receptor (MC3R) and dopamine receptor 2 (D2) gene expression. Hormones and Behavior (2015), 73, 83–93. 10.1016/j.yhbeh.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, & Shibata S (2001). Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. European Journal of Neuroscience, 13, 1190–1196. 10.1046/j.0953-816x.2001.01483.x [DOI] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Kandel ER, & Balsam PD (2012). Timing as a window on cognition in schizophrenia. Neuropharmacology, 62, 1175–1181. 10.1016/j.neuropharm.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Simpson EH, Lipatova O, Drew MR, Fairhurst S, … Balsam PD (2009). Impaired timing precision produced by striatal D2 receptor overexpression is mediated by cognitive and motivational deficits. Behavioral Neuroscience, 123, 720 10.1037/a0016503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Simpson EH, Richards VL, Deo G, Taylor K, Glendinning JI, … Balsam PD (2012). Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology, 37, 1699 10.1038/npp.2012.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SR, Tomiya A, Regev GJ, Thacker BE, Benzl RC, Kim CW, & Lieber RL (2009). Passive mechanical properties of the lumbar multifidus muscle support its role as a stabilizer. Journal of Biomechanics, 42, 1384–1389. 10.1016/j.jbiomech.2008.09.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RD, Winiger V, Higa KK, Kahn JB, Kandel ER, Balsam PD, & Simpson EH (2015). The impact of motivation on cognitive performance in an animal model of the negative and cognitive symptoms of schizophrenia. Behavioral Neuroscience, 129, 292 10.1037/bne0000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q-Y, & Palmiter RD (1995). Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell, 83, 1197–1209. 10.1016/0092-8674(95)90145-0 [DOI] [PubMed] [Google Scholar]


