Abstract
Introduction
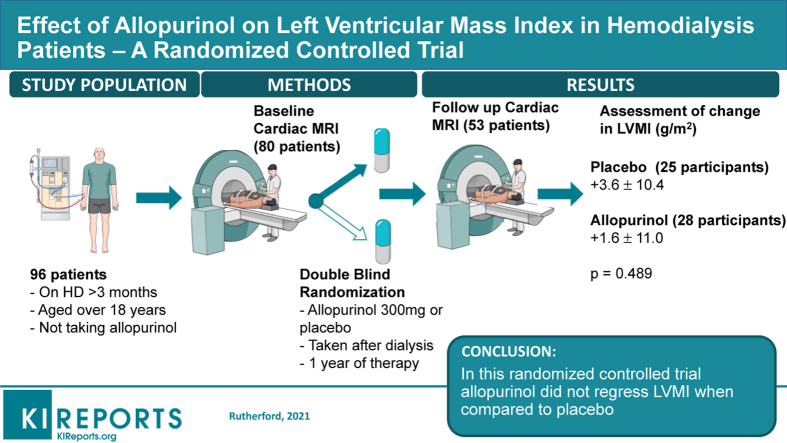
Increased left ventricular mass index (LVMI) is associated with mortality in end-stage renal disease. LVMI regression may improve outcomes. Allopurinol has reduced LVMI in randomized controlled trials in chronic kidney disease, diabetes, and ischemic heart disease. This study investigated whether allopurinol would regress LVMI in hemodialysis patients.
Methods
This was a randomized placebo-controlled double-blind multicenter trial funded by the British Heart Foundation (PG/12/72/29743). A total of 80 patients undergoing regular maintenance hemodialysis were recruited from NHS Tayside, NHS Greater Glasgow and Clyde and NHS Ayrshire and Arran in Scotland, UK. Participants were randomly assigned on a 1:1 ratio to 12 months of therapy with allopurinol 300 mg or placebo after each dialysis session. The primary outcome was change in LVMI, as assessed by cardiac magnetic resonance imaging (CMRI) at baseline and 12 months. Secondary outcomes were change in BP, flow-mediated dilation (FMD), augmentation indices (AIx), and pulse wave velocity (PWV).
Results
A total of 53 patients, with a mean age of 58 years, completed the study and had CMRI follow-up data for analysis. Allopurinol did not regress LVMI (change in LVMI: placebo +3.6 ± 10.4 g/m2; allopurinol: +1.6 ± 11 g/m2; P = 0.49). Allopurinol had no demonstrable effect on BP, FMD, AIx, or PWV.
Conclusion
Compared with placebo, treatment with allopurinol did not regress LVMI in this trial.
Keywords: allopurinol, hemodialysis, left ventricular mass, magnetic resonance imaging, randomized controlled trial
Graphical abstract
Premature cardiovascular death is the leading cause of death among hemodialysis patients. Left ventricular hypertrophy (LVH) is extremely common in these patients, present in 74% of patients commencing dialysis.1 Increased left ventricular mass (LVM) is strongly associated with all-cause and cardiovascular mortality.2,3 Increased LVM in patients with chronic kidney disease (CKD) has consistently been shown to be associated with adverse outcomes2,4; it is therefore plausible that reduction of LVM may reduce cardiovascular morbidity and mortality, although this has been more challenging to demonstrate.5
Multiple randomized trials have focused on reduction of LVM in patients receiving hemodialysis as a potential therapeutic target.6, 7, 8, 9 Although some studies have achieved reduction in LVM, this has often involved a significant lifestyle alteration for patients, for example, nocturnal or frequent hemodialysis.9,10 Some drug therapies, such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and potassium-sparing diuretics, have potential benefit in attenuating LVH11 but have side effects, such as hyperkalemia, which are particularly unwelcome in the hemodialysis population. Therefore, additional and alternative therapeutic strategies to target LVH are required. Allopurinol has been in clinical use for more than 50 years, and although traditionally used to lower uric acid and therefore reduce recurrent gout episodes, it has novel properties that make it a potentially attractive tool in the battle against LVH. Firstly, increased oxidative stress and endothelial dysfunction are hallmarks of end-stage kidney disease (ESKD) and hemodialysis.12,13 Allopurinol has been shown to profoundly reduce oxidative stress and to improve endothelial function in multiple populations.14, 15, 16, 17 Secondly, arterial stiffness is a feature of ESKD that further contributes to LVH and cardiovascular risk, and allopurinol has been shown to ameliorate this.18,19 Furthermore, in a population with CKD stage 3, allopurinol regressed LVMI, improved endothelial function, and improved central AIx when compared to placebo.18
Allopurinol has been shown to reduce LVMI in randomized controlled trials in chronic CKD stage 3,18 diabetes,20 and ischemic heart disease,17 but until now no study had examined its use in hemodialysis. As well as having a more severe stage of kidney disease, patients undergoing hemodialysis are subject to many more complex hemodynamic, metabolic, and inflammatory insults than their counterparts who have early CKD. It was therefore unknown whether allopurinol would still be able to regress LVH in this more extreme condition. We therefore investigated whether allopurinol might regress LVMI in a population undergoing hemodialysis by conducting a multicenter, double-blind, randomized, placebo-controlled trial.
Methods
Study Design
This was a 12-month, placebo-controlled, randomized, double-blind, multicenter, parallel group trial. All patients provided consent to participate. This trial was conducted in adherence to the Declaration of Helsinki and was approved by the East of Scotland Ethics Committee (13/ES/0051) and the UK Medicine and Healthcare Products Regulatory Authority (2013-001436-22). The trial was publicly registered (clinicaltrials.gov NCT01951404).
Study Population
Subjects receiving hemodialysis therapy for ESKD in NHS Ayrshire & Arran, NHS Tayside and NHS Greater Glasgow and Clyde, Scotland, UK, were randomized between January 2014 and June 2015. Participants had been receiving hemodialysis for more than 3 months and were more than 18 years of age.
Patients were excluded if any of the following criteria were present: known heart failure (ejection fraction <45%); already on allopurinol, known to have an adverse reaction to it, or to have active gout; malignancy, severe hepatic disease, or other life-threatening condition; on azathioprine, 6-metacaptopurine, or theophylline; had a planned kidney transplant; in another clinical study (other than observational or registry) within the last 30 days; unable to give informed consent; pregnant or breastfeeding; contraindication to magnetic resonance imaging; or any other serious illness or significant abnormalities that may have compromised their safety or successful participation in the study. To optimize study recruitment without impeding study integrity, a number of amendments were made to these criteria during the course of the study (see Supplementary Methods).
Trial Intervention
After baseline assessments, participants were randomly assigned to receive either an allopurinol 100 mg or placebo capsule 3 times weekly after hemodialysis for 2 weeks. If this dose was tolerated, it was then escalated weekly to 200 mg, 250 mg, then up to the maximum dose of 300 mg which was the optimal dose determined in a dose finding study.21 Participants then continued on this dose, or the maximum tolerated dose, for the duration of the trial. Follow-up visits at dialysis sessions were conducted at 6 weeks, 6 months, and 9 months (Supplementary Figure S1). Cardiac magnetic resonance imaging (CMRI) on a post-dialysis day was performed prior to randomization and following 12 months of treatment.
Study Outcomes
The primary study outcome was to determine whether allopurinol induced a change in CMRI-measured LVMI in patients with ESKD when compared to placebo. Secondary outcomes of this study were as follows: (i) to determine whether there was a change in left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), or left ventricular ejection fraction (LVEF) with allopurinol when compared to placebo; (ii) to determine whether there was a difference in endothelial function with allopurinol compared with placebo, measured by flow-mediated dilation (FMD) and augmentation indices (AIx); and (iii) to assess whether there were changes in blood pressure (BP) control as measured by clinic (dialysis) BP and 24-hour BP monitoring with allopurinol compared with placebo.
Cardiac Magnetic Resonance Imaging Methods
All participants underwent CMRI on a post-dialysis day prior to randomization and following 12 months of treatment on a 3.0 Tesla magnetic resonance imaging scanner (MAGNETOM, Verio/Trio, Siemens Healthcare, Erlangen, Germany). Full CMRI methods are described in the Supplementary Methods.
Other Trial Parameters
Flow-mediated dilation, radio-carotid pulse wave velocity (PWV), and radial AIx were optional components of the trial that were performed at baseline, month 9, and month 12 of the trial. All FMD was carried out in accordance with the International Brachial Artery Reactivity Task Force guidelines.22,23
Blood pressure taken as a part of standard care was available for analysis; participants could also undergo optional 24-hour ambulatory BP monitoring at baseline and after 12 months of treatment.
Blinding and Randomization
This was a double-blind trial; investigators and participants were blinded to treatment allocations. Block randomization was on a 1:1 ratio and was undertaken by Tayside Pharmaceuticals, who used Randomization.com (www.randomization.com) to create randomization blocks of 4. Medication was pre-labeled with a sequential study number and distributed to local clinical trial pharmacies. Medication was then distributed from there in sequence according to order of randomization.
Sample Size Calculation
Grothues et al. proposed that CMRI studies of LVH regression should be powered for a 10-g change in LV mass (4.8g/m2 change in LVMI).24 A previous study using spironolactone in CKD found a reduction in LV mass of 10 ± 12 g.25 Based on these values, 32 patients would be required per group to have 90% power with α = 0.05 to detect this change in LV mass. A 10% death or transplantation rate per year and a 10% dropout rate for the trial were originally expected; therefore, we planned to recruit 76 participants. Withdrawals during the trial were more common than originally anticipated; thus, 4 additional participants were recruited, meaning that 80 participants were randomized.
Statistical Analyses
The statistical analysis plan was recorded prior to data lock and analysis and is included in the Supplementary Statistical Analysis Plan. Normality was assessed using a Kolmogorov−Smirnov test with additional visual inspection of box plots. Data for continuous variables are presented as mean ± SD for normally distributed data and median (interquartile range) for non−normally distributed data. Categorical data are expressed as numbers (%). Continuous variables were compared using analysis of variance (if normally distributed) or Mann−Whitney U test (if not normally distributed), whereas categorical variables were analyzed using the χ2 test or the Fisher exact test if appropriate. Correlation analysis was performed for changes in LVMI. The primary efficacy analysis was analyzed as described above and is presented as the between-group difference in change in LVMI between baseline and 12 months; the 95% confidence interval for the treatment effects have also been presented. Follow-up was on a “modified intention-to-treat” basis, whereby all participants were invited for follow-up and included in the analysis even if study medication had been discontinued unless a participant underwent kidney transplantation or died. Patients without a follow-up CMRI were not included in the final analysis of the primary outcome but were included in a missing-cases sensitivity analysis. The number of patients not completing the second CMRI scan study was unexpectedly high. A further pre-specified analysis was performed using imputed LVMI in patients unable to have a second CMRI scan (5 imputations per completed case). After establishing that the data were missing at random, a further analysis was performed using multiple imputation for the missing values for change in LVMI. The influence of pre-specified covariates (pre-dialysis systolic BP, ultrafiltration volumes, and baseline LVMI) on the primary outcome was assessed using analysis of covariance. Secondary outcome analyses were conducted in a similar manner. Between-group differences in urate, FMD, pulse wave velocity (PWV), and AIx were assessed using a repeated-measures analysis of variance. A 2-sided P value of <0.05 was considered statistically significant for all analyses. All analyses were undertaken in SPSS version 23.0 (IBM Corporation, Armonk, NY).
Results
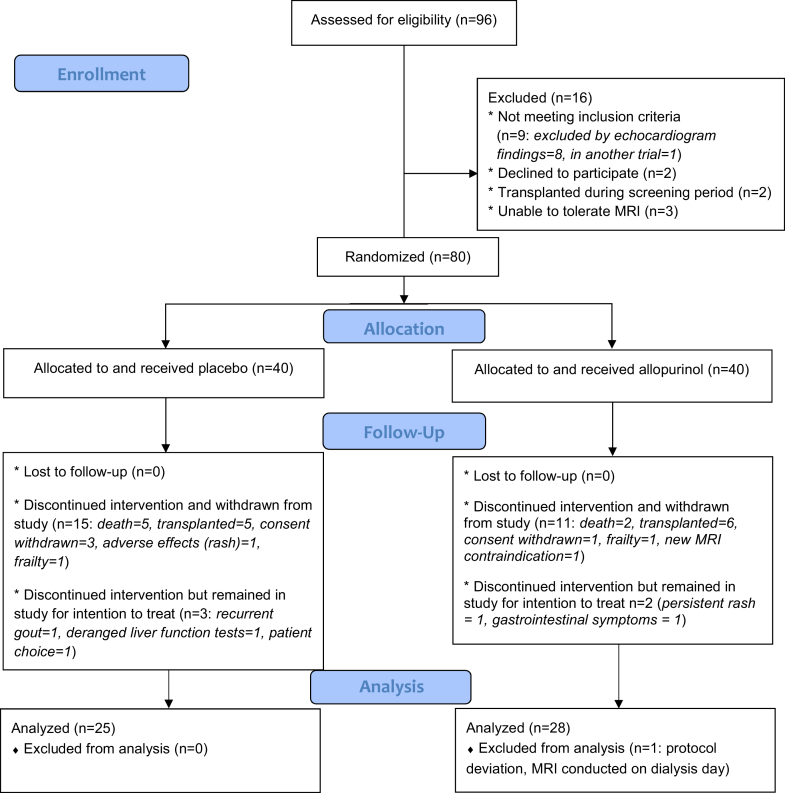
Between January 2014 and June 2015, a total of 96 patients who were undergoing regular hemodialysis consented to participate in this trial. Of those, 16 participants were not eligible for randomization following screening; 80 participants were therefore randomized. Figure 1 shows the study Consolidated Standards of Reporting Trials (CONSORT) diagram. There were 26 participant withdrawals during the trial (15 in the placebo group and 11 in the allopurinol group). This included 4 deaths on the placebo arm and 2 on the allopurinol arm. An additional 5 participants (3 on placebo and 2 on allopurinol) withdrew from trial medication but continued in the trial for the intention-to-treat analysis. One participant on the active treatment arm was excluded from all analysis prior to unblinding because of a protocol deviation whereby the participant’s CMRI was conducted immediately pre-dialysis, rather than on a post-dialysis day.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. MRI, magnetic resonance imaging.
Baseline characteristics for all participants by their treatment allocation are shown in Table 1. Participants were well matched in terms of age, sex, renal replacement therapy history, cause of ESKD, and past medical history. Medication use was similar between groups (Supplementary Table S1). There was no difference in serum urate between study groups at baseline (urate allopurinol group: 365 ± 86 mmol/l; placebo: 365 ± 88 mmol/l; P = 0.9).
Table 1.
Study baseline clinical parameters
| Baseline characteristics | Allopurinol (n = 39) | Placebo (n = 40) | P value |
|---|---|---|---|
| Age, yr | 57.8 ± 11.6 | 58.0 ± 13.0 | 0.90 |
| Sex, % male | 64.1 | 57.5 | 0.55 |
| Ethnicity | |||
| White/Caucasian, % | 95 | 100 | 0.35 |
| Other, % | 5 | 0 | |
| Presystolic BP, mm Hg | 139 ± 21 | 144 ± 25 | 0.301 |
| Prediastolic BP, mm Hg | 68 ± 12 | 75 ± 13 | 0.05 |
| Ultrafiltration volume, L | 1.5 (1.3–2.5) | 1.7 (1.0–2.4) | 0.84 |
| Weight, kg | 73 (60–84) | 78 (67–96) | 0.16 |
| BMI, kg/m2 | 26.1(21.9 - 29.3) | 28.7 (22.6–34.8) | 0.24 |
| RRT, mo | 32 (16–100) | 34 (20–78) | 0.90 |
| Duration hemodialysis, mo | 30 (15–61) | 29 (16–51) | 0.90 |
| Dialysis access, % | |||
| Fistula or graft | 87.2 | 82.5 | 0.18 |
| Line | 12.8 | 17.5 | |
| Primary renal disease, % | |||
| Diabetic nephropathy | 20.5 | 17.5 | 0.54 |
| ADPKD | 15.4 | 17.5 | 0.80 |
| Glomerulonephritis | 23.1 | 17.5 | 0.74 |
| Renovascular disease | 5.1 | 5.0 | 0.90 |
| Chronic pyelonephritis | 0 | 7.5 | 0.05 |
| Other/unknown | 33.4 | 32.5 | 0.90 |
| Hypertension | 2.6 | 2.5 | 0.57 |
| Past medical history, % | |||
| Diabetes | 25.6 | 22.5 | 0.90 |
| Hypertension | 69.2 | 70.0 | 0.90 |
| Cerebrovascular disease | 23.1 | 20.0 | 0.74 |
| Peripheral vascular disease | 23.1 | 12.5 | 0.22 |
| Ischemic heart disease | 17.9 | 22.5 | 0.62 |
| Dyslipidemia | 43.6 | 35.0 | 0.43 |
| Smoking history, % | |||
| Ex/current | 61.5 | 52.5 | 0.45 |
| Never | 38.5 | 47.5 | |
| Hemoglobin, g/dla | 11.8 (11.3–12.6) | 111 (10.5–11.8) | 0.41 |
| URR, %b | 75.8 ± 5.3 | 73.8 ± 7.7 | 0.20 |
| Albumin, g/La | 35 (32–36) | 33 (30–36) | 0.08 |
| Urate, mmol/La | 365 ± 86 | 365 ± 88 | 0.90 |
| Phosphate, mmol/La | 1.61 ± 0.45 | 1.74 ± 0.51 | 0.22 |
| LVM, g | 123.8 ± 45.8 | 121.3 ± 44.5 | 0.80 |
| LVMI, g/m2 | 63.0 (54.2–79.8) | 58.5 (45.8–78.5) | 0.25 |
| EDV, ml | 150.0 (124.7–174.6) | 143.5 (126.6–172.6) | 0.90 |
| ESV, ml | 59.3 (40.6–73.4) | 61.3 (51.5–75.6) | 0.83 |
| Ejection fraction, % | 60.4 ± 8.5 | 59.2 ± 8.8 | 0.53 |
| Post-systolic BP, mm Hg | 126 (113–138) | 123 (110–149) | 0.88 |
| Post-diastolic BP, mm Hg | 65 ± 13 | 69 ± 15 | 0.15 |
| 24-h systolic BP, mm Hgc | 120 ± 18 | 128 ± 23 | 0.35 |
| 24-h diastolic BP, mm Hgc | 71 ± 13 | 74 ± 14 | 0.46 |
| FMD–baseline cuff, % changed | 4.1 ± 2.7 | 3.9 ± 3.8 | 0.87 |
| FMD–baseline GTN, % changed | 11.7 ± 6.0 | 13.8 ± 3.8 | 0.95 |
| PWV, m/se | 7.6 ± 1.6 | 7.7 ± 2.2 | 0.82 |
| AIx, %f | 23.9 ± 10.0 | 24.4 ± 17.0 | 0.92 |
AIx, augmentation indices; ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; BP, blood pressure; EDV, end-diastolic volume; ESV, end-systolic volume; FMD, flow-mediated dilation; GTN, glyceryl trinitrate; PVW, pulse wave velocity; LVM, left ventricular mass; LVMI, left ventricular mass index; RRT, duration of time receiving renal replacement therapy (in months); URR, urea reduction ratio.
Data are presented as mean ± SD or as median (interquartile range) if nonparametric.
Data available for 78 participants.
Data available for 75 participants.
Data available for 26 participants.
Data available for 34 participants.
Data available for 31 participants.
Data available for 35 participants.
Primary Outcome
In this trial, 300 mg of allopurinol after dialysis for 12 months was not associated with a significant reduction in LVMI. In a modified intention-to-treat analysis, in participants who completed both CMRI scans, following 12 months of treatment the mean change in LVMI with allopurinol was +1.6 ± 11.0 g/m2, compared with +3.6 ± 10.4g/m2 with placebo (between-group difference: −2.1g/m2; 95% confidence interval [CI]: −7.9 to 3.8g/m2; P = 0.49). Adjustment for pre-specified factors that may influence LVMI change in this population (pre-dialysis systolic BP, ultrafiltration volumes, and baseline LVMI) did not have a significant impact on these results.
In a further intention-to-treat analysis using imputation to account for missing CMRI data in patients unable to have a second CMRI scan, once again there was no significant difference between treatment groups (change in LVMI placebo: +2.7; 95% CI: −6.0 to +14.1) g/m2; allopurinol: +2.3; 95% CI: −6.0 to +14.4; P = 0.88. Similarly, there was no difference in change in end-diastolic volume (EDV), end-systolic volume (ESV), or ejection fraction between treatment groups (Table 2).
Table 2.
Comparison of the change in parameters by treatment groups
| Parameter change | Allopurinol (n = 28) | Placebo (n = 25) | Between-group difference (95% CI) | P |
|---|---|---|---|---|
| Change in LVMI at 12 mo, g/m2 | 1.6 ± 11.0 | 3.6 ± 10.4 | –2.1 (–7.9 to 3.8) | 0.49 |
| Change in LVM at 12 mo, g | –0.7 –(10.2 to 11.9) | 1.0 (–5.3 to 14.6) | –4.2 (–14.7 to 6.2) | 0.49 |
| Change in LVEF at 12 mo, % | –1.3 ± 5.6 | –1.0 ± 7.2 | –0.3 (–3.9 to 3.3) | 0.86 |
| Change in ESV at 12 mo, ml | 4.7 ± 1 8.2 | 3.0 ± 22.8 | 1.7 (–9.8 to 13.2) | 0.77 |
| Change in EDV at 12 mo, ml | 6.1 ± 28.0 | 0.8 ± 27.7 | 5.3 (–10.1 to 20.7) | 0.49 |
| Change in FMD response to hyperemia at 9 mo, %a | 0.0 ± 2.9 | –2.6 ± 4.0 | 2.6 (–1.0 to 6.3) | 0.12 |
| Change in FMD response to hyperemia at 12 mo, %b | –0.4 (–2.1 to –0.0) | 1.4 (–3.4 to 5.2) | –2.7 (–7.6 to 2.3) | 0.55 |
| Change in FMD response to GTN at 9 mo, %a | –1.5 ± 6.0 | –4.9 ± 4.3 | 3.4 (–1.7 to 8.6) | 0.19 |
| Change in FMD response to GTN at 12 mo, %b | –2.3 ± 5.2 | –0.4 ± 4.9 | –1.5 (–6.7 to 3.6) | 0.54 |
| Change in radial AIx at 9 mo, %c | –4.0 (–12.0 to –2.0 | 0.0 (–7.0 to 12) | –10.5 (–26.9 to 5.8) | 0.09 |
| Change in radial AIx at 12 mo, %c | –1.4 ± 8.8 | 3.6 ± 9.9 | 4.9 (–4.0 to 13.9) | 0.26 |
| Change in PWV at 9 mo, m/sd | 0.9 ± 2.7 | 0.5 ± 1.8 | –0.4 (–2.8 to 2.0) | 0.72 |
| Change in PWV at 12 mo, m/sa | 1.1 ± 1.7 | 0.7 ± 2.0 | –0.3 (–2.3 to 1.7) | 0.72 |
| Change in mean systolic 24-h BP at 12 mo, mm Hge | 8.3 ± 13.3 | 8.8 ± 19.8 | –0.5 (–30.7 to 29.7) | 0.9 |
| Change in mean diastolic 24-h BP at 12 mo, mm Hge | 2.3 ± 5.6 | 1.0 ± 11.6 | –1.5 (–16.3 to 13.2) | 0.85 |
| Change in mean pre-dialysis systolic BP at 12 mo, mm Hg | 3.7 ± 17.1 | 0.9 ± 21.8 | –0.28 (–13.5 to 8.0) | 0.60 |
| Change in mean pre-dialysis diastolic BP at 12 mo, mm Hg | 1.7 ± 10.5 | 0.2 ± 13.3 | –1.5 (–16.3 to 13.2) | 0.65 |
AIx, augmentation indices; BP, blood pressure; EDV, end-diastolic volume; ESV, end-systolic volume; FMD, flow-mediated dilation; GTN, glyceryl trinitrate; PVW, pulse wave velocity; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Results available for 18 participants.
Results available for 19 participants.
Results available for 20 participants.
Results available for 17 participants.
Results available for 8 participants.
In the 18 patients who underwent FMD, there was no statistically significant change in response to hyperemia at 9 or 12 months (9-months change in FMD response to hyperemia: allopurinol: +0.0% (±2.9%); placebo: −2.6% (±4.0%); treatment effect: 2.6%; 95% CI: −1.0% to 6.3%; P = 0.12). Change in response to glyceryl trinitrate at 12 months was negatively correlated with change in LVMI (Pearson’s R = −0.601, P = 0.007). This and other correlations of change in LVMI with other parameters are shown in Table 3.
Table 3.
Correlations between change in LVMI and other parameters
| Parameter | Change in LVMI |
|---|---|
| Δ FMD cuffa |
R = 0.197 P = 0.42 |
| Δ FMD GTNa |
R = −0.601 P = 0.007 |
| Δ AIxb |
R = 0.188 P = 0.39 |
| Δ PWVc |
R = −0.105 P = 0.68 |
| Δ EDVd |
R = 0.525 P < 0.001 |
| Δ Uratee |
R = 0.287 P = 0.046 |
AIx, augmentation index; EDV, end-diastolic volume; FMD, flow-mediated dilation; GTN, glyceryl trinitrate; LVMI, left ventricular mass index; PWV, pulse wave velocity.
Data available for 19 participants.
Data available for 23 participants.
Data available for 18 participants.
Data available for 53 participants.
Data available for 49 participants.
Allopurinol had no statistically significant effect on AIx at 9 or 12 months. There was no difference in change in pulse wave velocity between treatment groups, which changed minimally over the course of the trial (Table 2).
In this trial, there was no evidence that allopurinol had any effect on BP. A total of 26 participants undertook 24-hour ambulatory BP monitoring at baseline, with 8 participants completing follow-up measurements (4 on allopurinol and 4 on placebo). Results from these participants were in line with the changes seen in pre-dialysis BP, which was available for all participants, in which changes in BP between treatment allocation groups were no different from each other (Table 2).
Change in Urate
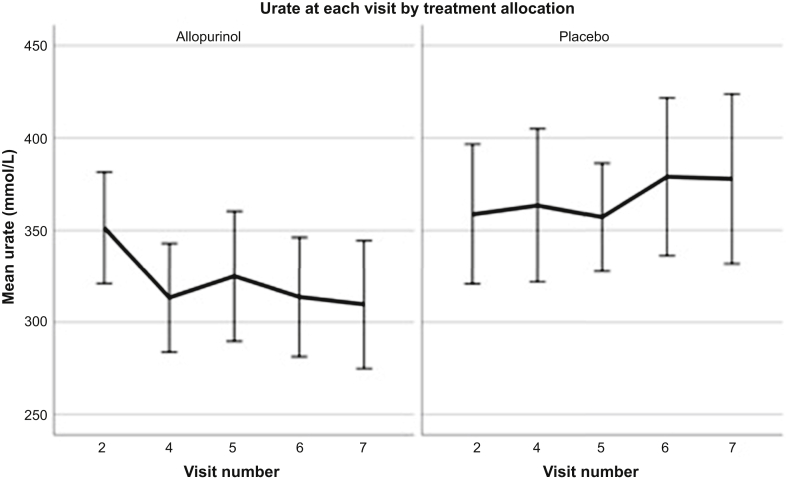
A modest reduction in mean pre-dialysis urate was achieved in the allopurinol group when compared to placebo (Figure 2, Table 4). The difference in mean urate from baseline at month 12 in the placebo arm was + 21.0 ± 110.0 μmol/l, whereas with allopurinol, a mean reduction in urate of −42.6 ± 82.8 μmol/l was achieved (P = 0.013); treatment effect: −63.6; 95% CI: −114.2 to −13.0) μmol/l. This equated to a median reduction in urate of 18.5 mmol/L (interquartile range: −26.8% to 0.0%) in subjects receiving allopurinol.
Figure 2.
Change in serum urate with treatment allocation. Figure shows change in mean urate at visits throughout the study (visit 2 at baseline, visit 4 at 6 weeks, visit 5 at 6 months, visit 6 at 9 months, and visit 7 at 12 months).
Table 4.
Comparison of urate values by treatment groups
| Urate measurement | Placebo | Allopurinol |
|---|---|---|
| Baseline urate, mmol/l | 365; 95% CI = 338−392 n = 40 | 365; 95% CI = 338−392 n = 38 |
| Visit 4 urate, mmol/l | 362; 95% CI = 334−389 n = 37 | 318; 95% CI = 294−342n = 38 |
| Visit 5 urate, mmol/l | 357; 95% CI = 332−382 (n = 30) | 327; 95% CI = 295−360 n = 32 |
| Visit 6 urate, mmol/l | 375; 95% CI = 341−409 (n = 28) | 313; 95% CI = 285−341 n = 32 |
| Visit 7 urate, mmol/l | 376; 95% CI = 336−417 n = 25 | 317; 95% CI = 283−351 n = 30 |
CI, confidence interval; n, number of patients in each group.
Safety
Allopurinol 300 mg was well tolerated. The number of adverse events (AEs) in this trial was in keeping with other trials within the ESKD population, with 93.75% of randomized participants having at least 1 event.26, 27, 28 Table 5 provides a summary of serious AEs and reactions, and a full breakdown of all study AEs is included in Supplementary Table S2. The number of AEs was similar between treatment groups, and there was no significant increase in gastrointestinal or skin and subcutaneous tissue disorders in the allopurinol arm.
Table 5.
Breakdown of unique serious adverse events by treatment group
| MedDRA Coding | Allopurinol | Placebo | Grand totala |
|---|---|---|---|
| 1. Adverse events | 81 | 74 | 155 |
| 2. Adverse reactions | 15 | 15 | 30 |
| 3. Serious adverse events | 16 | 28 | 44 |
| Blood and lymphatic system disorders | 1 | 1 | |
| Cardiac disorders | 1 | 1 | 2 |
| Gastrointestinal disorders | 2 | 4 | 6 |
| Hepatobiliary disorders | 1 | 1 | |
| Immune system disorders | 1 | 1 | |
| Infections and infestations | 3 | 3 | 6 |
| Injury, poisoning, and procedural complications | 1 | 5 | 6 |
| Metabolism and nutrition disorders | 1 | 1 | |
| Musculoskeletal and connective tissue disorders | 1 | 2 | 3 |
| Neoplasms benign, malignant and unspecified | 1 | 1 | 2 |
| Nervous system disorders | 3 | 1 | 4 |
| Respiratory, thoracic, and mediastinal disorders | 2 | 2 | |
| Skin and subcutaneous tissue disorders | 1 | 1 | |
| Surgical and medical procedures | 1 | 4 | 5 |
| Vascular disorders | 1 | 2 | 3 |
| 4. Serious adverse reaction | 1 | 1 | |
| Skin and subcutaneous tissue disorders | 1 | 1 | |
| Grand total | 112 | 118 | 230 |
MedDRA, Medical Dictionary for Regulatory Activities.
Repetitions of the same adverse event for any given participant have been excluded from this table.
Per Protocol Analysis
Five participants completed their second CMRI but were taken off study medication during the course of the study (3 on placebo and 2 on allopurinol). A per protocol analysis of completed cases only was performed with the above participants excluded. In this completed cases analysis, allopurinol was not associated with reduction of LVMI when compared to placebo. Mean change in LVMI with allopurinol +1.6 (± 11.1), compared to + 3.7 (±10.7) with placebo (P = 0.508).
Discussion
This trial is the first clinical trial to consider regression of LVMI with allopurinol in a population undergoing regular hemodialysis. Overall, allopurinol 300 mg after dialysis for 1 year did not regress LVMI when compared with placebo. Furthermore, there was no statistically significant effect of allopurinol 300 mg on BP, FMD, AIx, or pulse wave velocity.
The reduction in urate in this trial was lower than in previously reported studies in other populations in which allopurinol regressed LVMI, including in a population with CKD 3 in which a reduction in urate of approximately 42% was achieved.17,18,20 In this previous work, reduction in LVMI has not been directly correlated with reduction in urate. However, in this trial, perhaps because of the lower reduction in urate achieved, change in LVMI over the trial period was weakly correlated with change in urate from baseline (Spearman R = 0.287, P = 0.046).
It is, worth noting that in this study, for participants undergoing FMD, change in response to GTN at 12 months was negatively correlated with change in LVMI (Pearson R = −0.601, P = 0.007). Although, traditionally, nitrate-mediated dilation has served as a control measure, its association with LVMI in this study is in keeping with work that has shown nitrate-mediated dilation to be an independent predictor of cardiovascular disease.29,30
This trial used the modest dose of 300 mg of allopurinol to minimize potential increased risks of severe rash or drug hypersensitivity syndrome associated with the use of allopurinol in patients with significant kidney function impairment.31 In order to use the minimal potentially effective dose of allopurinol, an open-label dose-finding study was conducted prior to this trial, in which 10 patients undergoing regulat hemodialysis received increasing doses of allopurinol after dialysis up to a maximum of 350 mg, with the intention of stopping escalating doses when a reduction in urate of 50% was achieved.21 In fact, no patient achieved a reduction of 50%, and the greatest mean reduction of urate of around 20% was achieved with allopurinol 300 mg. This is likely to be because allopurinol and its active metabolites are removed by hemodialysis.32 Allopurinol was given after hemodialysis, and at this time urate, which is also removed by dialysis, would be at its lowest. However, a 20% reduction in urate is only a modest reduction. This trial demonstrated the tolerability of this dose, so in a further trial it would be reasonable to use a larger dose of allopurinol, potentially titrated to effect on urate.
This study does have several limitations. The study was classified as an intention-to-treat study, as we included patients in our final analysis who stopped study medication during the course of the study. However, as specified in our study protocol, we were unable to include patients who had undergone transplantation, had died, or did not have a second CMRI in our primary outcome analysis as a result of these missing data. The original power calculation for this study was based on a 20% drop-out during follow-up; however, the drop-out rate was higher than anticipated at 33.8%, which raises the issue of whether the study might have been underpowered. To explore the effect of these withdrawals on the overall outcome, a missing case analysis was performed to confirm that the data were missing at random. There were no significant differences in terms of baseline age, sex, baseline LVMI, ejection fraction, BP, or ultrafiltration volumes between those that withdrew from the study and those that completed it.
The fact that the difference in LVMI between the treatment and placebo groups remained statistically nonsignificant after using pre-specified multiple imputation modeling for the missing change in LVMI values suggests that the lack of treatment effect between treatment groups is a result of a truly negative trial and that it is not a result of a type 2 error. Further supporting the fact that this is a truly negative study and not simply underpowered is the fact that retrospectively re-performing our original power calculation with adjustment for our baseline data suggests that 42 participants were required to complete the study for 90% power and an α of 0.05. This is significantly fewer than the 53 participants who completed the study. In addition, a difference in LVMI was detected in a similarly designed CMRI study in hemodialysis patients in which only 44 participants completed final follow-up.8 This further supports the assertion that the failure of this study to meet its primary outcome was not the result of a type 2 error.
Recruitment to this trial proved challenging. In order to maximize the number of potentially eligible participants, the inclusion criteria were relaxed so that participants without LVH could participate. This was not believed to detract from the value of the trial, as any regression of LVM regardless of any arbitrary cut-offs for LVH may potentially be beneficial to patient outcomes.33 However, it is possible that any potential changes in myocardial mass were smaller and more difficult to detect with a lower starting mass.20 Putting this trial in a wider context, despite observational data consistently associating LVM with reduced survival in patients requiring hemodialysis,34 a meta-analysis published after recruitment to our trial was complete demonstrated no clear association between reduction of LVM and improved survival in this patient group.5 Therefore, it is unclear whether LVM remains a surrogate marker of future cardiovascular mortality that should be targeted in clinical trials to improve outcomes. Furthermore, the CKD-FIX trial has recently reported showing no benefit of allopurinol on cardiovascular or renal outcomes in advanced CKD compared to placebo.35
In addition to the limitations discussed above, the population in this study was not ethnically diverse; allopurinol may have been less well tolerated in a group of patients with a different gene pool,36 and in a more diverse trial population, the effect of the 300 mg dose may have been different. In addition, although regression of absolute myocardial mass is an important therapeutic target, this trial was not designed to quantify any change in character of myocardial tissue associated with allopurinol. Adverse fibrotic myocardial remodeling is well known to contribute to poor outcomes, and, in any future trial, it would be interesting to characterize myocardial tissue using emerging non-contrast imaging techniques.37 A larger trial examining the effect of allopurinol on myocardial structure, composition, and morbidity and mortality would be of potential interest.
In conclusion, in this study, compared with placebo, treatment with allopurinol 300 mg did not regress LVMI in hemodialysis patients over 1 year of therapy.
Disclosure
ADS and the University of Dundee have a patent for the use of allopurinol in the treatment of ischemic heart disease. All the other authors declared no competing interests.
Acknowledgments
The investigators would like to thank all trial participants and our funders the British Heart Foundation (PG/12/72/29743). This trial would not have been possible without support from the renal clinical research nurses in NHS Greater Glasgow and Clyde and NHS Ayrshire and Arran. This trial was prospectively registered with clinicaltrials.gov (NCT01951404). ER is currently funded by a joint NHS Education for Scotland and Chief Scientist Office Clinical Lectureship (PCL/18/03).
Author Contributions
ER recruited patients, arranged follow-up visits, analyzed the study data, and wrote the original manuscript draft. SI assisted with trial management, follow-up visits, and patient recruitment. ER, KM, RW, SJG, GR, and MDW assisted with study data acquisition, safety, and integrity. GAS, MM, and PBM were local site study principal investigators. ADS, PBM, AGJ, and JGH conceived the study and provided senior supervision for the study. MDW assisted with data close-out and study data integrity. PR provided statistical input throughout the planning, conducting, close-out, and analysis phases of the study. All authors contributed to and agreed on the final submitted version of this manuscript and take responsibility for its contents.
Footnotes
Supplementary Statistical Analysis Plan.
Supplementary Methods. Study population. CMRI methods, flow-mediated dilation methods, pulse wave analysis and pulse wave velocity, and safety and trial withdrawals.
Table S1. Breakdown of medication use at study baseline.
Table S2. Breakdown of baseline characteristics for completed cases.
Table S3. Breakdown of all adverse events in the study.
Figure S1. Flowchart of patient visits.
Supplementary Material
Supplementary Statistical Analysis Plan.
Supplementary Methods. Study population. CMRI methods, flow-mediated dilation methods, pulse wave analysis and pulse wave velocity, and safety and trial withdrawals.
Table S1. Breakdown of medication use at study baseline.
Table S2. Breakdown of baseline characteristics for completed cases.
Table S3. Breakdown of all adverse events in the study.
Figure S1. Flowchart of patient visits.
References
- 1.Foley R.N., Parfrey P.S., Harnett J.D. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–192. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 2.Foley R.N., Parfrey P.S., Harnett J.D. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 3.Parfrey P.S., Foley R.N., Harnett J.D. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277–1285. [PubMed] [Google Scholar]
- 4.Zoccali C., Benedetto F.A., Mallamaci F. Left ventricular mass monitoring in the follow-up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65:1492–1498. doi: 10.1111/j.1523-1755.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 5.Badve S.V., Palmer S.C., Strippoli G.F.M. The validity of left ventricular mass as a surrogate end point for all-cause and cardiovascular mortality outcomes in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2016;68:554–563. doi: 10.1053/j.ajkd.2016.03.418. [DOI] [PubMed] [Google Scholar]
- 6.Chan C.T., Greene T., Chertow G.M. Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clin J Am Soc Nephrol. 2013;8:2106–2116. doi: 10.2215/CJN.03280313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thadhani R., Appelbaum E., Pritchett Y. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307:674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 8.Odudu A., Eldehni M.T., McCann G.P., McIntyre C.W. Randomized controlled trial of individualized dialysate cooling for cardiac protection in hemodialysis patients. Clin. J Am Soc Nephrol. 2015;10:1408–1417. doi: 10.2215/CJN.00200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culleton B., Walsh M., Quinn R. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis. J Am Med Assoc. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 10.Rocco M.V., Lockridge R.S., Beck G.J. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannella G., Paoletti E., Delfino R. Prolonged therapy with ACE inhibitors induces a regression of left ventricular hypertrophy of dialyzed uremic patients independently from hypotensive effects. Am J Kidney Dis. 1997;30:659–664. doi: 10.1016/s0272-6386(97)90490-x. [DOI] [PubMed] [Google Scholar]
- 12.Del Vecchio L., Locatelli F., Carini M. What we know about oxidative stress in patients with chronic kidney disease on dialysis—clinical effects, potential treatment, and prevention. Semin Dial. 2011;24:56–64. doi: 10.1111/j.1525-139X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 13.Locatelli F., Canaud B., Eckardt K.-U. Oxidative stress in end-stage renal disease: an emerging threat to patient outcome. Nephrol Dial Transplant. 2003;18:272–280. doi: 10.1093/ndt/gfg074. [DOI] [PubMed] [Google Scholar]
- 14.Butler R., Morris A.D., Belch J.J. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 15.Doehner W., Schoene N., Rauchhaus M. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105:2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 16.Rajendra N.S., Ireland S., George J. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol. 2011;58:820–828. doi: 10.1016/j.jacc.2010.12.052. [DOI] [PubMed] [Google Scholar]
- 17.Rekhraj S., Gandy S.J., Szwejkowski B.R. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol. 2013;61:926–932. doi: 10.1016/j.jacc.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 18.Kao M.P., Ang D.S., Gandy S.J. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382–1389. doi: 10.1681/ASN.2010111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng G., Qiu Z., Li D. Effects of allopurinol on arterial stiffness: a meta-analysis of randomized controlled trials. Med Sci Monit. 2016;22:1389–1397. doi: 10.12659/MSM.898370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szwejkowski B.R., Gandy S.J., Rekhraj S. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol. 2013;62:2284–2293. doi: 10.1016/j.jacc.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford E., Stewart G., Houston J.G. An open-label dose-finding study of allopurinol to target defined reduction in urate levels in hemodialysis patients. J Clin Pharmacol. 2017;57:1409–1414. doi: 10.1002/jcph.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corretti M.C., Anderson T.J., Benjamin E.J. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 23.Thijssen D.H.J., Black M.A., Pyke K.E. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. AJP Heart Circ Physiol. 2011;300:H2–H12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grothues F., Smith G.C., Moon J.C.C. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 25.Edwards N.C., Steeds R.P., Stewart P.M. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 26.Holdaas H., Fellström B., Jardine A.G. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–2031. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 27.Wanner C., Krane V., März W. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 28.Fellström B.C., Jardine A.G., Schmieder R.E. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 29.Kajikawa M., Maruhashi T., Hida E. Combination of flow-mediated vasodilation and nitroglycerine-induced vasodilation is more effective for prediction of cardiovascular events. Hypertension. 2016;67:1045–1052. doi: 10.1161/HYPERTENSIONAHA.115.06839. [DOI] [PubMed] [Google Scholar]
- 30.Maruhashi T., Nakashima A., Matsumoto T. Relationship between nitroglycerine-induced vasodilation and clinical severity of peripheral artery disease. Atherosclerosis. 2014;235:65–70. doi: 10.1016/j.atherosclerosis.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Dalbeth N., Stamp L. Allopurinol dosing in renal impairment: walking the tightrope between adequate urate lowering and adverse events. Semin Dial. 2007;20:391–395. doi: 10.1111/j.1525-139X.2007.00270.x. [DOI] [PubMed] [Google Scholar]
- 32.Allopurinol 100mg tablets—summary of product characteristics (SPC) (eMC) https://www.medicines.org.uk/emc/medicine/25729 Available at:
- 33.Dahlöf B., Devereux R.B., Kjeldsen S.E. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 34.Stack A.G., Saran R. Clinical correlates and mortality impact of left ventricular hypertrophy among new ESRD patients in the United States. Am J Kidney Dis. 2002;40:1202–1210. doi: 10.1053/ajkd.2002.36881. [DOI] [PubMed] [Google Scholar]
- 35.Badve S.V., Pascoe E.M., Tiku A. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382:2504–2513. doi: 10.1056/NEJMoa1915833. [DOI] [PubMed] [Google Scholar]
- 36.Hung S.-I., Chung W.-H., Liou L.-B. HLA-B∗5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–4139. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham-Brown MPM, Rutherford E, Levelt E, et al. Native T1 mapping: inter-study, inter-observer and inter-center reproducibility in hemodialysis patients. J Cardiovasc Magn Reson. 20017;19:21. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.