Abstract
Steam pop (SP) refers to audible sound related to the intramyocardial explosion when tissue temperatures reach 100 °C. In this case the SP was recorded using intracardiac echocardiography (ICE), using Sound-star probe and Smart-touch catheter with ablation index (AI) module (Biosense-Webster Inc., Diamond-Bar, CA, USA). Guided by the anatomical reconstruction (EAM) and electrograms, we applied radiofrequencies (RF) in a “point-by-point” along the entire line on cavo-tricuspid-isthmus (CTI) using a target of an AI ≥500. The tip-tissue force recorded was 12–18 g and a power of 35 W. ICE imaging was important so that the anatomical position of the catheter tip can be precisely monitored. During RF, ICE showed a growing, hyperechogenic intramyocardial bubble at the catheter-tissue interface. ICE imaging showed a hyperechogenic intramyocardial formation at the moment of occurrence of the SP. ICE imaging showed that the formation suddenly expanded to a sphere over the course of several seconds. After SP we reduced the RF output energy from 35 W to 30 W. After RF line on CTI the patient had no complications and no recurrence of atrial flutter was recorded.
<Learning objective: The use of intracardiac echocardiography during cavo-tricuspid-isthmus ablation permits the detection of the increase of microbubbles before steam pop formation.>
Keywords: Intracardiac echocardiography, Atrial flutter catheter ablation, Transcatether ablation, Steam pop
Introduction
Steam pop (SP) refers to the audible sound produced by intramyocardial explosion when tissue temperature reaches 100 °C, leading to the production of gas [1]. The high endocardial tissue temperatures develop blood/tissue vaporizations and explosion. In addition, discrepancies between monitored catheter tip temperature and actual endocardial tissue temperature may be related to the unanticipated elicitation of SP. SP may induce tissue disruptions that can have important clinical consequences such as cardiac perforation, pericardial effusion, and embolism. In this case we describe an experience of SP during atrial flutter ablation, recorded with intracardiac echocardiography (ICE) from the heating to explosion of atrial tissue.
Case report
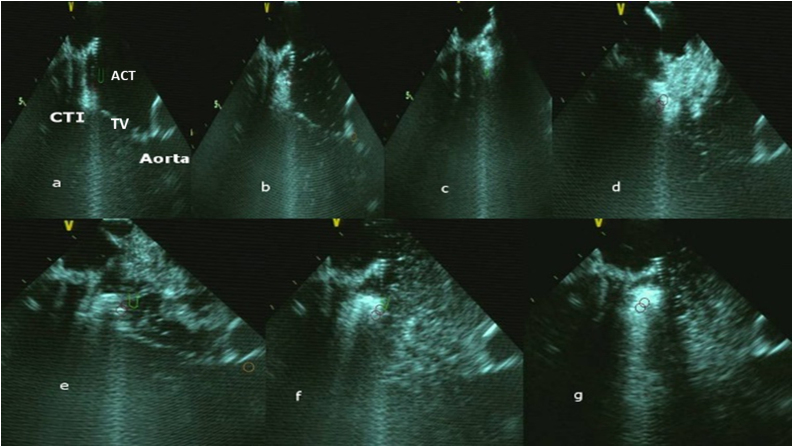
A 65-year-old man underwent atrial fibrillation ablation (AFAB) and cavo-tricuspid-isthmus (CTI) ablation using CARTO mapping and ICE with a force-sensor tip irrigated catheter (Ablation catheter: TC smart-touch, Biosense Webster, Diamond-Bar, CA, USA; ICE-Soundstar, Biosense-Webster Inc.). The procedure was performed under conscious sedation using bolus administration of fentanyl and midazolam. Before the procedure, an accurate cleaning of the area where the magnetic patches were located was performed. The common femoral vein punctures were performed echo-guided to avoid casual puncture of artery or superficial femoral vein. After AFAB, the ICE contours were used to delineate a 3D-map of CTI. Activated clotting time was >250 s. The anatomy of the CTI was accurately drawn using the ablation catheter. A merged image was obtained using Cartosound Module. Guided by the anatomical reconstruction and electrograms, we applied radiofrequencies (RF) in a “point-by-point” along the entire line on CTI using a target of ablation index (AI) ≥500, until we obtained the complete conduction block through the CTI. The tip-tissue force recorded was 12–18 g using a power of 35 W. Real-time automated display of RF applications using CARTO VISITAG (Module, Biosense Webster) was used according to a settings of catheter stability of 5 mm for 3 s and respiration adjustments. During RF an increase of microbubbles could be shown (Fig. 1a–c; Supplementary Video S1). At maximum increased microbubbles visualization, an audible sound with a sudden change in electrical impedance and electrode temperature were present when the visible explosions occurred on ICE (SP; Fig. 1d–g). During RF, ICE showed a growing, hyperechogenic intramyocardial bubble at the catheter-tissue interface without changes of the impedance. ICE imaging showed a hyperechogenic intramyocardial formation at the moment of occurrence of the SP. ICE imaging showed that the formation suddenly expanded to a sphere over the course of several seconds. The volume of this formation slowly decreased and completely disappeared (Supplementary Video S1). The radiofrequency was suddenly stopped. Although SP often occurs without consequences, cardiac perforation, pericardial effusion, and embolism were reported in the literature [2], [3], [4], [5]. After SP pop we reduced the RF output energy from 35 W to 30 W. After RF line on CTI the patient had no complications and no recurrence of atrial flutter was recorded.
Fig. 1.
Steam-pop formation during RF (a–g). (a–d) The microbubbles were observed before steam pop; (e–g) ICE showed the growing, hyperechogenic intramyocardial bubbles and a tissue “explosion”. CTI: cavo-tricuspid-isthmus; ACT: ablation catheter; TV: tricuspid valve.
Discussion
SP is relatively infrequent (0.1%–1.5%) but represents a potentially severe complication of RF ablation. Hence it may be associated with embolic stroke and cardiac perforation. In this case, the only fact that happened was a visual displacement of the catheter tip and/or a rapid change in electrode temperature. The visualization of tissue-tip contact permitted to show the microbubbles that often are released before the elicitation of SPs. If these microbubbles could be detected, the immediate reduction of RF power might reduce the SP formation. The visualization of intramural gas formation could be detected prior to audible pops and increased impedance [6]. The contact force catheter and AI did not reduce or predict SP formation [6]. The irrigated catheters were developed to minimize the risk of thrombus formation at the catheter-electrode during RF. The SPs formation were not always related to precedented impedance change as reported in this case [7]. The AI correlated with lesion depth and lesion dimension [7]. In a recent study, high power setting resulted in a much shorter duration of delivery time to the SP; the AI did not correlate with the contact force at time of the SP [7]. Our case report is a rare case of real time video and images formation of SP from increase of microbubbles to tissue explosion during catheter ablation using AI and contact force catheter. ICE imaging is an important tool during catheter ablation to visualize the anatomical isthmus, to trace CTI point-point ablation line guided by electrogram, and to monitor the tissue-force contact during creation of microbubbles. Detection of these microbubbles may be an early indicator of undesired events. In addition, determination of catheter tip–tissue orientation may help ensure proper lesion formation while importantly minimizing the potential for inducing steam pops. We cannot always prevent SP formation, but we can prevent the consequences of the SP with a rapid and immediate visualization of the tissue and pericardium during and after SPs.
Conflict of interest
All authors have no conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.jccase.2020.08.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
Steam pop formation during radiofrequencies delivery.
References
- 1.Viles-Gonzalez J.F., Berjano E., d’Avila A. Complications of radiofrequency catheter ablation: can we prevent steam pops? JACC Clin Electrophysiol. 2018;4:501–503. doi: 10.1016/j.jacep.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Tokuda M., Tedrow U.B., Stevenson W.G. Silent steam pop detected by intracardiac echocardiography. Heart Rhythm. 2013;10:1558–1559. doi: 10.1016/j.hrthm.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Tokuda M., Kojodjojo P., Epstein L.M., Koplan B.A., Michaud G.F., Tedrow U.B. Outcomes of cardiac perforation complicating catheter ablation of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2011;4:660–666. doi: 10.1161/CIRCEP.111.963413. [DOI] [PubMed] [Google Scholar]
- 4.Seiler J., Roberts-Thomson K.C., Raymond J.M., Vest J., Delacretaz E., Stevenson W.G. Steam pops during irrigated radiofrequency ablation: feasibility of impedance monitoring for prevention. Heart Rhythm. 2008;5:1411–1416. doi: 10.1016/j.hrthm.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Cooper J.M., Sapp J.L., Tedrow U., Pellegrini C.P., Robinson D., Epstein L.M. Ablation with an internally irrigated radiofrequency catheter: learning how to avoid steam pops. Heart Rhythm. 2004;1:329–333. doi: 10.1016/j.hrthm.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Fassini G., Conti S., Pontone G., Pepi M., Tondo C., Dello Russo A. Tissue characteristics and evolution after steam pop. J Interv Card Electrophysiol. 2015;43:313. doi: 10.1007/s10840-015-9997-0. [DOI] [PubMed] [Google Scholar]
- 7.Mori H., Kato R., Sumitomo N., Ikeda Y., Goto K., Tanaka S. Relationship between the ablation index, lesion formation, and incidence of steam pops. J Arrhythm. 2019;35:636–644. doi: 10.1002/joa3.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Steam pop formation during radiofrequencies delivery.